- 1Department of Endocrinology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2Department of Thyroid Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background: BRAF mutation is one of the most common genetic alterations contributing to the initiation and progression of papillary thyroid carcinoma (PTC). However, the prognostic value of BRAF mutation for PTC is limited. Novel markers are needed to identify BRAF-mutant patients with poor prognosis.
Methods: Transcriptional expression data were downloaded from the Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) datasets. Pathway enrichment was performed by Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis and gene set enrichment analysis (GSEA). Protein-protein interaction networks were predicted by the GeneMANIA. The correlation between STRA6 expression and immune infiltration was analyzed by tumor immune estimation resource (TIMER) and tumor-immune system interaction database (TISIDB). Immunohistochemistry was used to detect the STRA6 protein expression level of PTC. Infiltration of regulatory T cells (Tregs) and CD8+ T cells in tumor samples were analyzed by fluorescent multiplex immunohistochemistry.
Results: In BRAF-mutant PTC, STRA6 was extremely upregulated and predicted unfavorable survival, which was an independent risk factor for increased mortality risk. Bioinformatic analyses indicated that STRA6 might activate the MAPK pathway synergistically with BRAFV600E. The expression of STRA6 was associated with immune infiltrates and T cell exhaustion. Fluorescent multiplex immunohistochemistry showed that STRA6 increased Tregs abundance and decreased CD8+ T cells infiltration in PTC. Moreover, STRA6 promoted epithelial-mesenchymal transition via increased cancer-associated fibroblasts infiltration.
Conclusions: Our study demonstrates STRA6 may serve as a prognostic marker for BRAF-mutated PTC, which may drive thyroid carcinogenesis via activation of oncogenic pathway and regulation of tumor immunosuppressive microenvironment.
Introduction
Thyroid carcinoma (TC) is the most common endocrine malignancy with an increasing incidence worldwide (1). Most of TCs arise from thyroid follicular epithelial cells, among which 80% are papillary thyroid carcinoma (PTC) (2). Initiation and progression of TC involve multiple genetic alterations, of which the most common oncogenic mutation is BRAFV600E (3, 4). Although BRAFV600E mutation is associated with poorer recurrence-free probability (5), it was suggested that not all of the PTCs with BRAFV600E mutation belonged to high-risk recurrence in American Thyroid Association Management Guidelines (6) or even were associated with aggressive clinicopathological outcomes (7, 8). Thus, novel markers are needed to identify BRAF-mutant PTC patients with poor prognosis.
STRA6, functions as a Vitamin A transporter and cytokine receptor (9), has been reported in multiple cancers. STRA6 activated the Wnt/β-catenin signaling in gastric cancer and served as a prognostic biomarker (10, 11). Upregulation of STRA6 in colon cancer was correlated with poor oncologic prognosis and transduced JAK2-STAT signaling, which contributes to colon tumorigenesis (12, 13). Moreover, STRA6 polymorphisms were correlated with clinical characteristics and might be potential markers in locally-advanced and metastatic non-small cell lung cancer (14). However, the prognostic value of STRA6 in PTC remains unknown.
Tumor microenvironment, composed of tumor cells, immune cells, cytokines, etc., plays an essential role in cancer metastasis, progression and recurrence. The escape of tumor cells often occurs in an immunosuppressive microenvironment (15). It was reported that regulatory T cells (Tregs) were enriched in primary thyroid tumors (16) and had higher infiltration in lymph node metastases (17). Besides, the density of tumor-associated macrophages was increased in advanced thyroid cancer, which correlated with decreased survival rate of patients (18). Recent study revealed that STRA6 was associated with infiltration of antigen-presenting cells in stomach adenocarcinoma, which could serve as a potential mRNA vaccine candidate (19). Nevertheless, the engagement of STRA6 in tumor immune microenvironment of TC remains elusive.
In this study, we demonstrate STRA6 is upregulated in BRAF-mutant PTC. High STRA6 expression is associated with unfavorable prognosis for individuals with BRAFV600E. Immune infiltration and pathway enrichment were investigated in samples with high STRA6 expression. STRA6 may contribute to the progression of BRAF-mutant PTC via regulation of immunosuppressive tumor microenvironment and activation of oncogenic pathway.
Materials and methods
Transcriptional data
Transcriptional expression data and clinicopathological information of the Cancer Genome Atlas (TCGA) cohort were downloaded from UCSC Xena (https://xena.ucsc.edu/). GSE199023 was downloaded from Gene Expression Omnibus (GEO) datasets (https://www.ncbi.nlm.nih.gov/). The RNA sequencing data of the FAH-SYSU cohort was obtained from our previous study (20).
Tumor immune estimation
Tumor immune estimation resource (TIMER) 2.0 (http://timer.cistrome.org/) is a web server for comprehensive analysis of immune infiltrates with diverse algorithms. It was applied to analyze STRA6 expression level in pan-cancer based on the TCGA dataset. The correlation of STRA6 expression between BRAF mutation status was further explored by Timer 2.0. It was also used to analyze the correlation of immune infiltrates with STRA6 expression and BRAF mutation status. The relation between immunomodulators and STRA6 expression was evaluated via tumor-immune system interaction database (TISIDB) (http://cis.hku.hk/TISIDB/index.php).
Differential expression analysis and functional annotations
NetworkAnalyst (https://www.networkanalyst.ca/) is a platform for comprehensive gene expression profiling analysis. We divided the BRAF-mutant PTC from the TCGA cohort into two groups according to STRA6 expression. After uploading a gene expression table, differently expressed genes (DEGs) were analyzed by the platform and followed with Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. Additionally, principal component analysis (PCA) between two groups was performed on Clustvis website (https://biit.cs.ut.ee/clustvis/). Protein-protein interaction (PPI) was predicted by GeneMANIA (http://genemania.org/).
Gene set enrichment analysis
GSEA software was downloaded from the website (http://www.gsea-msigdb.org/gsea/index.jsp). We performed analysis in 50 patients with high STRA6 expression and 50 patients with low STRA6 expression of the TCGA cohort. Hallmark gene sets were used to explore the mechanism of STRA6 in thyroid carcinoma progression.
Immunohistochemistry
The study was approved by the Institutional Research Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University (no. [2021] 109). 30 pairs of PTC tissues were collected from the First Affiliated Hospital of Sun Yat-sen University with informed consent from patients. Paraffin embedded tissues were deparaffinized with xylene for two times and dehydrated with a series of ethanol. Antigen retrieval was performed by boiling the slides in EDTA buffer (C1034, Solarbio). After cooling, the slides were blocked with 20% goat serum at room temperature for 30 min and incubated with STRA6 primary antibody (diluted 1:200, H00064220-D01P, Novus) at 4°C overnight. Followed by rinse with PBS for 3 times, the slices were incubated with secondary antibody at room temperature for 30 min. Detection of protein expression was performed with a 3,3’-diaminobenzidine (DAB) peroxidase substrate kit (K5007; Dako) under a microscope. Subsequently, the slides were counterstained with Harris hematoxylin, followed by dehydration in graded alcohol and incubation in xylene. The immunohistochemistry (IHC) staining score was calculated with the following formula: IHC score = extent score × intensity score. The staining extent was scored as 0 (1%–4% positive cells), 1(5%–25% positive cells), 2 (26%–50% positive cells), 3 (51%–75% positive cells), 4 (≥75% positive cells). The staining intensity was scored as 0-3, corresponding to no staining, weak staining, intermediate staining and strong staining, respectively.
Fluorescent multiplex immunohistochemistry
Fluorescent multiplex immunohistochemistry (mIHC) was applied to characterize immune infiltration in PTC. After incubation with primary antibody, the slides were incubated with an HRP-conjugated secondary antibody and fluorophore-conjugated Tyramide Signal Amplification reagent (Cat. 10003100100, Panovue). Multiplex panel design was as follows: FoxP3 (diluted 1:100, ab215206, abcam), Opal 570 TSA (diluted 1:200); CD8 (diluted 1:100, 70306S, CST), Opal 690 TSA (diluted 1:200). Finally, the slides were stained with DAPI (4’, 6-diamidino-2-phenylindole) and mounted with anti-fated reagent.
Statistical analysis
Experimental data was analyzed with GraphPad Prism 8.0 and presented as mean ± standard deviation (SD). Comparison between two groups was analyzed with Mann-Whitney U test or Student’s t test. The correlation between STRA6 expression and clinicopathological features in the TCGA cohort was analyzed with a chi-squared test. Kaplan–Meier analysis with a log-rank test was used to evaluate the prognostic value of STRA6 in BRAF-mutant PTC. Univariate Cox regression analysis was performed to investigate the correlation between variables and survival. After multivariate adjustment for age, gender, tumor size, multifocality, extrathyroidal extension and lymph node metastasis, multivariate Cox regression analysis further accessed whether high STRA6 expression was an independent risk factor for recurrence and mortality. A P value of <0.05 was considered statistically significant.
Results
STRA6 is upregulated in BRAF-mutated PTC.
To unveil the expression pattern of STRA6 across different cancers, we analyzed the mRNA expression level of STRA6 in the TCGA cohort. STRA6 was upregulated in most cancers including thyroid carcinoma (Figure 1A). Notably, the expression of STRA6 was positively correlated with BRAF mutation in TC and melanoma (Figure S1A). Compared with BRAF wild-type (WT) PTC, STRA6 was significantly upregulated in BRAF-mutated samples (Figure 1B). Based on a public dataset GSE199023, zebrafish thyroid tumors transfected with BRAFV600E oncogenes showed higher expression of STRA6 as compared to those transfected with CCDC6-RET fusion (Figure 1C). We further analyzed the mRNA expression in our cohort based on distinct molecular subtypes. STRA6 was remarkably upregulated in BRAF subtype compared with other PTC subtypes and benign nodules (Figure 1D). Overexpression of STRA6 protein level in BRAFV600E PTC was also confirmed by IHC (Figure 1E). Taken together, STRA6 is upregulated in thyroid cancer and exhibits extremely higher expression in BRAF-mutant PTC.
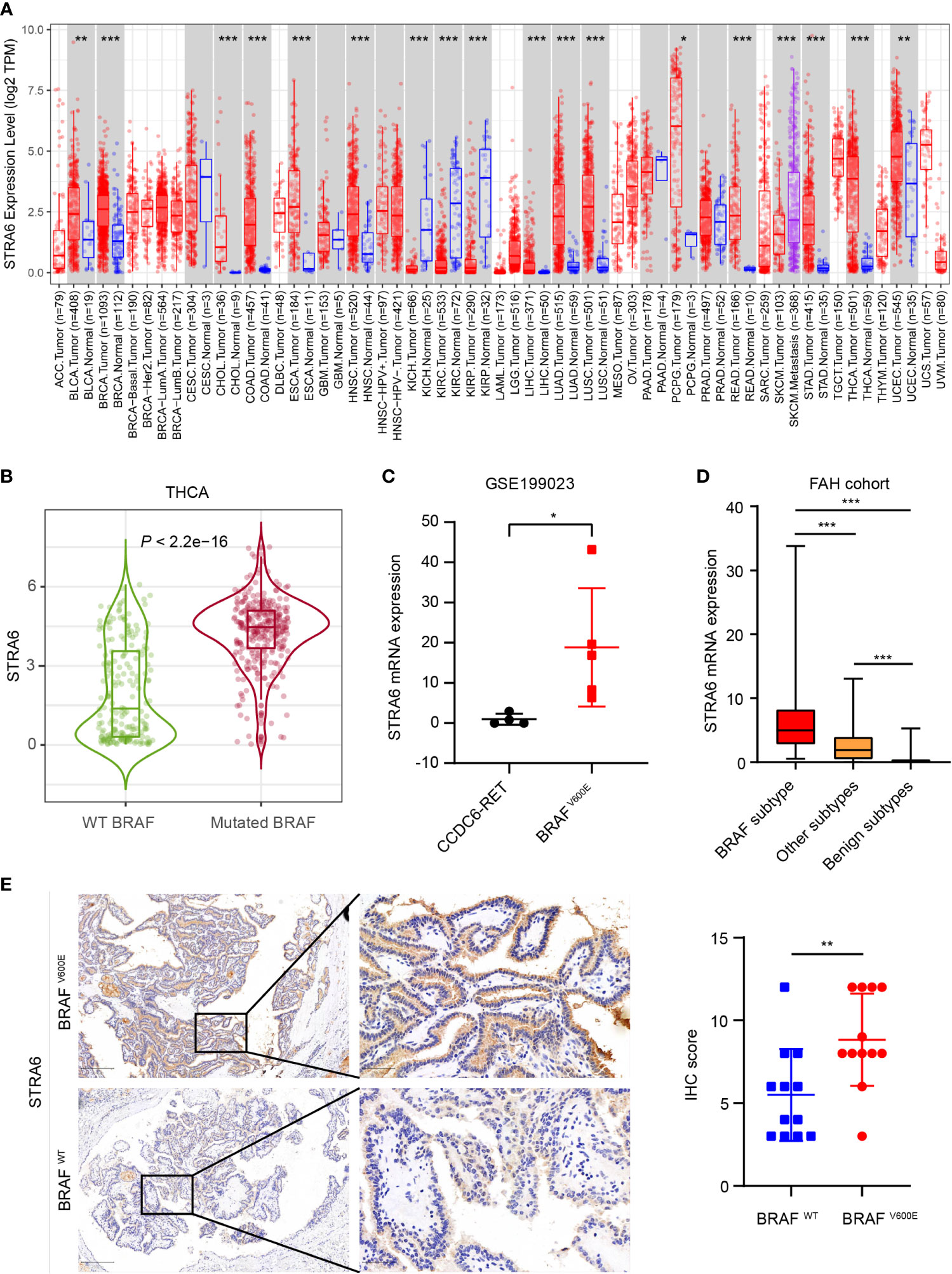
Figure 1 STRA6 is upregulated in BRAF-mutated PTC. (A) mRNA expression of STRA6 in various cancers according to the TCGA cohort. (B) STRA6 mRNA expression based on BRAF genotype in THCA cohort. (C) STRA6 mRNA expression of zebrafish thyroid tumors with oncogenic transfection gene in GSE199023. (D) mRNA abundance in FAH cohort with respect to different molecular subtypes. (E) Immunohistochemistry (IHC) staining of STRA6 protein level based on the BRAF mutation status in PTC samples. (*p < 0.05, **p < 0.01, ***p < 0.001. The data are shown as the mean ± SD).
Upregulation of STRA6 predicts aggressive outcomes and unfavorable prognosis for BRAF-mutant PTC
We investigated the relationship between STRA6 expression and clinicopathological features in the TCGA cohort. High STRA6 expression was associated with BRAF mutation (P = 0.001) and distant metastasis (P = 0.019) (Table 1). Then we divided PTC patients into two groups according to the genotype of BRAF. Intriguingly, high STRA6 expression was significantly associated with aggressive clinicopathological outcomes in BRAF-mutant PTC, but not in BRAF WT groups. For individuals with BRAF mutation, rates of distant metastasis were 2/130 (1.5%) vs. 3/26 (11.5%) (P = 0.033) in low-STRA6 group vs. high-STRA6 group, respectively. The recurrence rates were significantly higher in high-STRA6 PTC than low-STRA6 PTC [11/40 (27.5%) vs. 20/212 (9.4%), P = 0.003]. As for mortality, it was robustly higher in PTC with high STRA6 expression than those with low STRA6 expression [5/40 (12.5%) vs. 4/212 (1.9%), P = 0.004] (Table 2). Kaplan-Meier analysis showed higher STRA6 expression significantly predicted poorer recurrence free survival (RFS) (P = 0.0007) and overall survival (OS) (P = 0.0006) (Figures 2A, B).
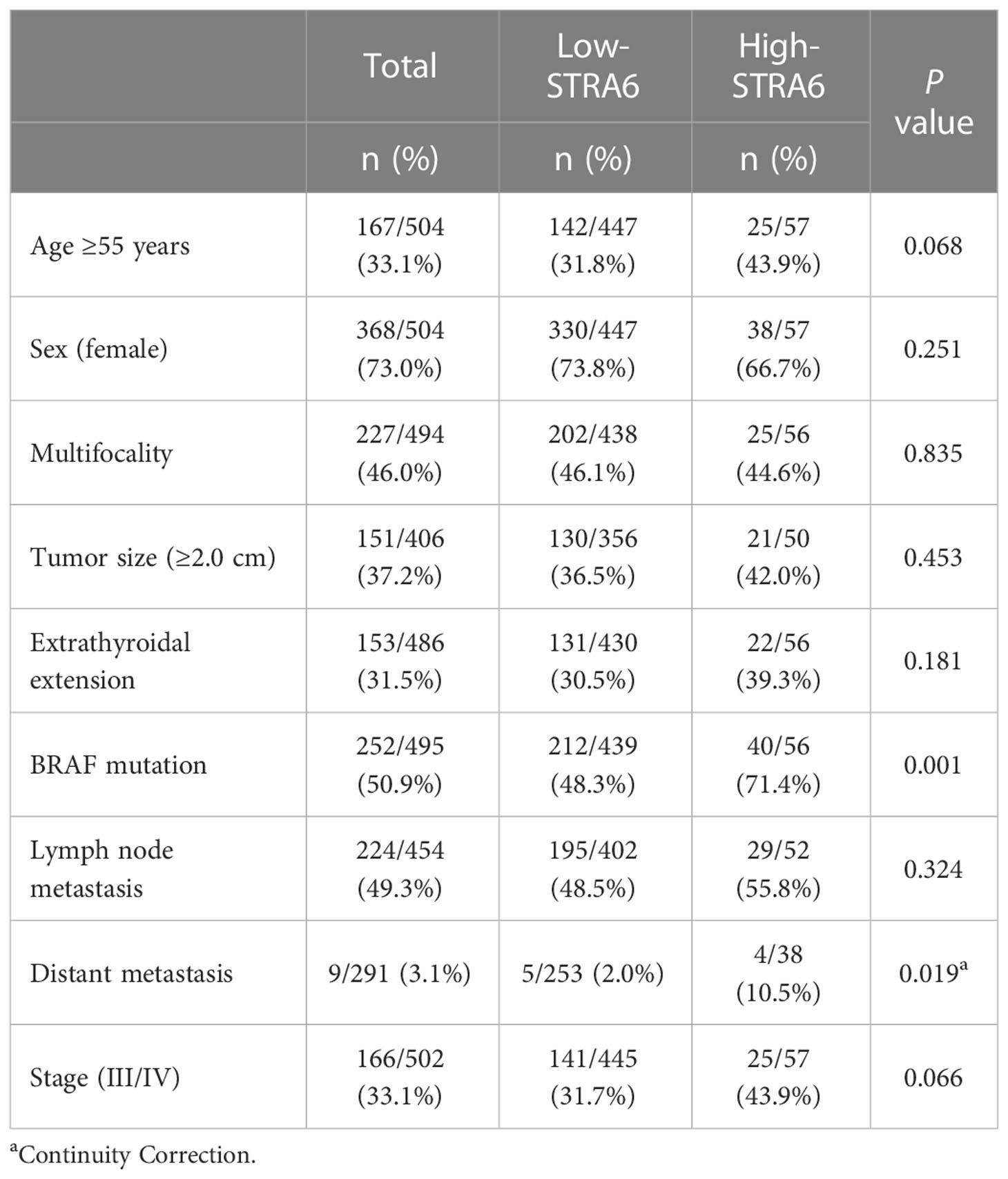
Table 1 The association between STRA6 expression and clinicopathologic characteristics in the TCGA cohort.
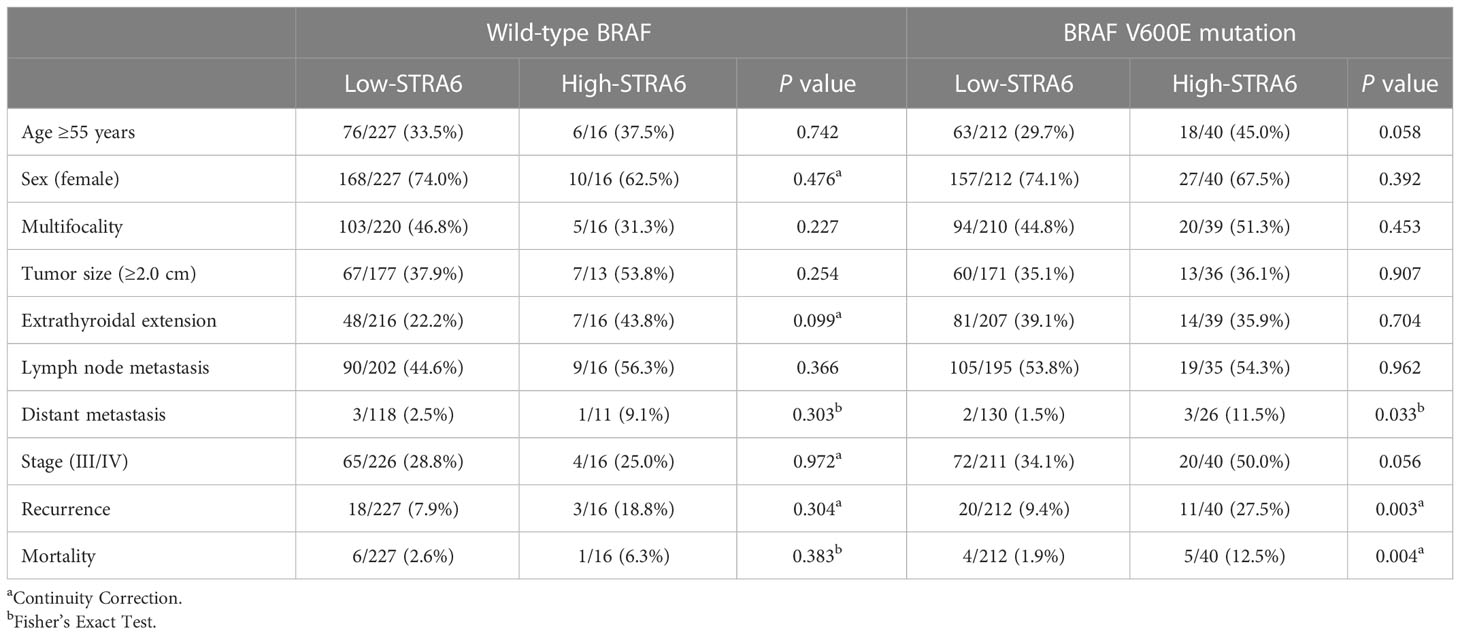
Table 2 Correlation between STRA6 expression and clinicopathological characteristics of PTC in the TCGA cohort based on the BRAF genotypes.
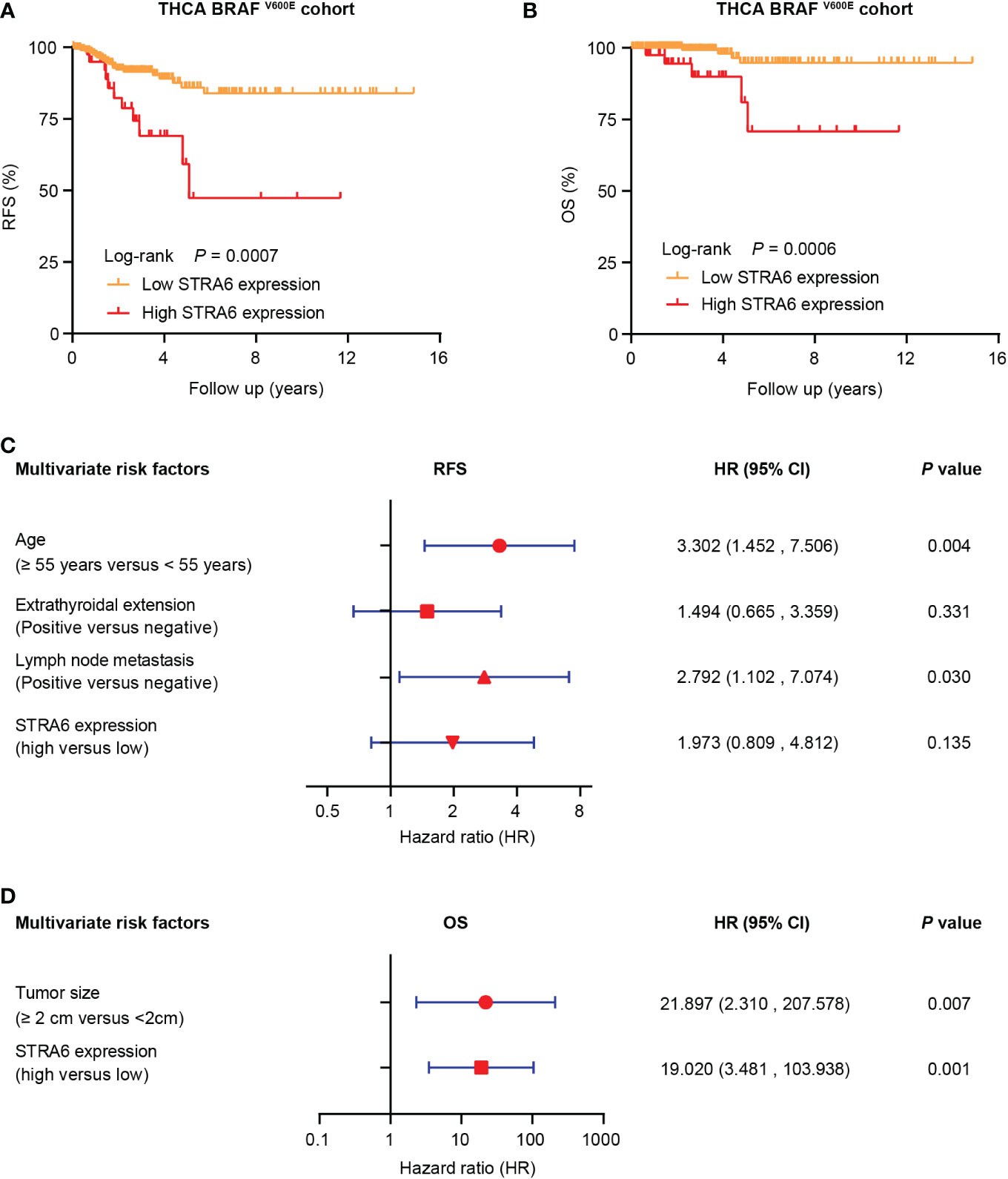
Figure 2 STRA6 predicts poor prognosis for BRAF-mutant PTC. (A) Kaplan–Meier survival curves of recurrence-fee survival (RFS) according to STRA6 expression in BRAF-mutant PTC. Prognostic cutoff value was identified as 10.3 using X-Tile. (B) Kaplan-Meier analysis of overall survival (OS) based on STRA6 expression in PTC patients with BRAF mutation. (C) Multivariate Cox regression analysis of the risk factors for recurrence in BRAF-mutant PTC. All bars represented 95% confidence intervals (95% CI). (D) Multivariate Cox regression analysis of the risk factors for mortality in BRAF-mutant PTC. All bars corresponded to 95% CI.
High STRA6 expression is an independent risk factor for mortality in BRAF-mutated PTC
Furthermore, we explored the hazard ratios (HRs) of STRA6-related risk (Table 3). In the entire TCGA cohort, univariate Cox regression analysis showed that upregulation of STRA6 was an independent risk factor for recurrence and mortality, with HRs being 2.98 (1.62-5.48) (P < 0.001) and 4.67 (1.69-12.87) (P = 0.003), respectively. After multivariate adjustment for age, gender, tumor size, multifocality, extrathyroidal extension and lymph node metastasis, HRs of recurrence and mortality remained significantly (P = 0.013 and P = 0.017). Then we divided patients into two groups according to BRAF mutation status. In BRAF wild-type patients, neither recurrence rates nor mortality rates was associated with STRA6 expression. In BRAF-mutant patients, STRA6-related recurrence risk was 3.32 (1.59-6.96) (P =0.001) and became 1.97 (0.81-4.81) (P = 0.135) after multivariate adjustment (Figure 2C). STRA6 overexpression was a risk factor for mortality with an HR being 7.12 (1.91-26.56) (P = 0.003), which became more significant with an HR being 19.02 (3.48-103.94) (P = 0.001) after multivariate adjustment (Figure 2D). Therefore, individuals with high STRA6 expression show increased mortality risk in BRAF-mutated PTC.
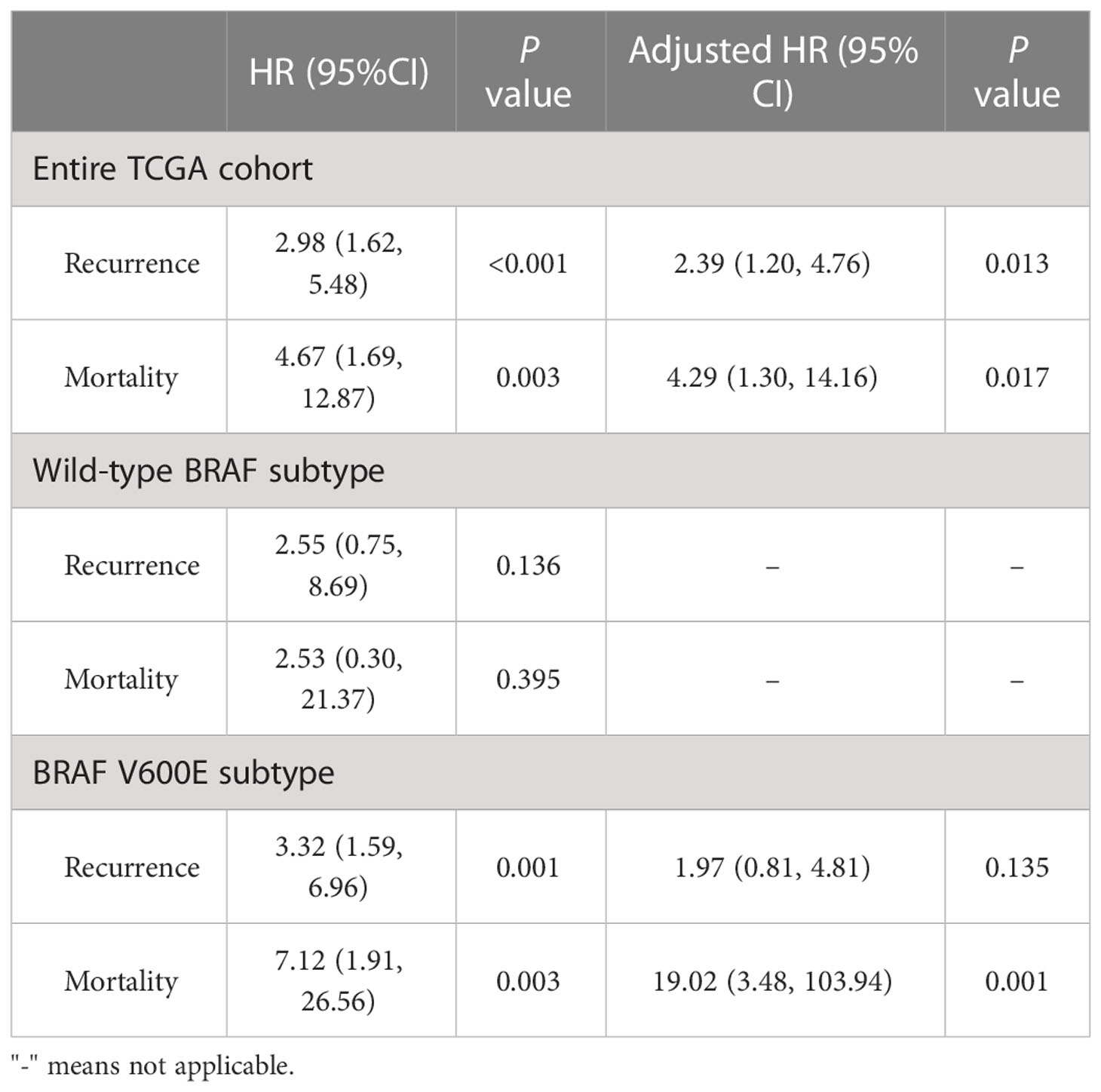
Table 3 Hazard ratios of high STRA6 expression vs low STRA6 expression in risk of recurrence and mortality in the THCA cohort with respect to BRAF status.
STRA6 plays oncogenic and immunoregulatory roles in BRAF-mutated PTC progression
To explore the mechanism of STRA6 contributing to the poor prognosis in BRAF-mutant PTC, we divided patients into two groups according to STRA6 expression. Principal component analysis (PCA) showed separation between high-STRA6 PTC and low-STRA6 PTC (Figure 3A). Differential expression genes (DEGs) between two groups were shown in Figure 3B. Of which, most genes were involved in malignant tumor progression, such as OBP2B, TNNT1, KLK6, KRT17 etc. Moreover, chemokine signaling pathway and cytokine-cytokine receptor interaction were enriched with the upregulated genes in high-STRA6 groups, indicating STRA6 may engage in the regulation of the immune interaction (Figure 3C). We also found that STRA6 expression was associated with immunomodulators in the TCGA cohort (Figures S1B, C), which indicated that STRA6 may function as an immunoregulator in TC progression. Additionally, the MAPK signaling pathway, which has emerged as one of the frequently activated pathways in thyroid tumorigenesis, was also remarkably enriched with the upregulated genes in high-STRA6 PTC. Downregulation of genes enriched in thyroid hormone synthesis further suggested the oncogenic role of STRA6 in TC progression (Figure 3C). PPI networks of STRA6 were constructed by GeneMANIA. It was predicted that 20 proteins were binding to STRA6. Of note, STRA6 interacted with several proteins modulated by the MAPK signaling pathway, including DIO3, BMP4 and DUSP6 (Figure 3D). Therefore, our results demonstrate that STRA6 may contribute to BRAF-mutated PTC progression by regulating oncogenic pathways and immune microenvironment.
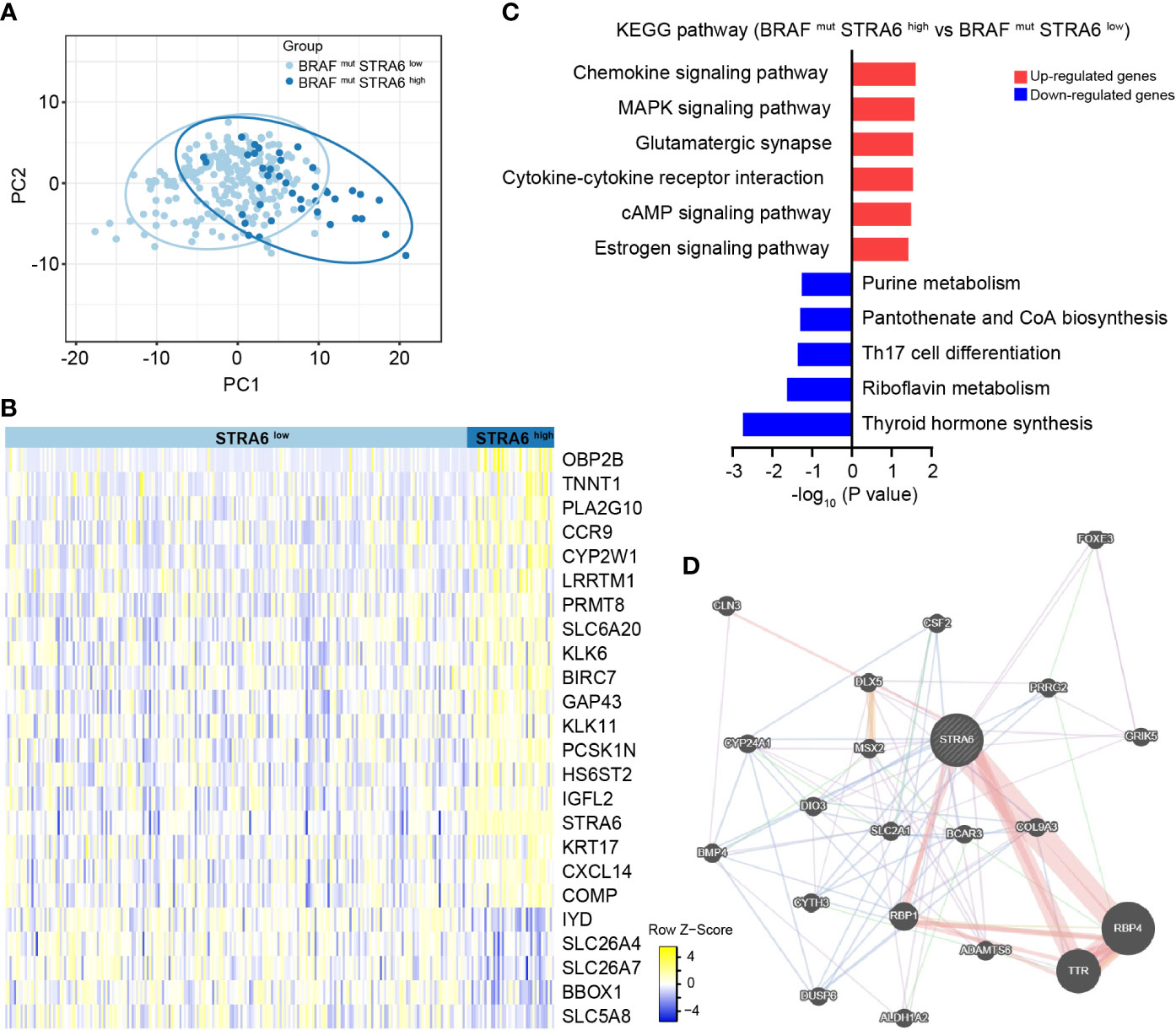
Figure 3 STRA6 plays immunoregulatory and oncogenic roles in BRAF-mutated PTC. (A) Principal component analysis (PCA) plot of BRAF-mutated samples based on STRA6 expression. (B) Differently expressed genes (DEGs) between high-STRA6 group and low-STRA6 group. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis based on upregulated genes and downregulated genes. (D) Proteins interacted with STRA6 were predicted by GeneMANIA.
STRA6 is correlated with immune infiltration in thyroid carcinoma
Given immune infiltration takes part in tumor progression, we performed Gene Set Enrichment Analysis (GSEA) with transcriptome data from the TCGA cohort. The results showed that samples with high STRA6 expression enriched in the IL2-STAT5 signaling pathway (Figure 4A). STRA6 expression was positively correlated with the mRNA level of IL2, STAT5a and STAT5b (Figure 4B). IL2 is a critical immunomodulatory cytokine, which increases Tregs and induces CD8+ T cell exhaustion via activation of STAT5. Thus, we evaluated the association between STRA6 expression and immune cell infiltration in PTC samples. After purity adjustment, upregulation of STRA6 was correlated with abundance of Tregs. However, STRA6 expression was negatively correlated with the infiltration of CD8+ T cells (Figure 4C). We also assessed the expression of exhausted T cells markers, including LAG3, PDCD1G2, CTLA4, HAVCR2, PRDM1 and GZMB in the TCGA cohort. STRA6 was positively correlated with most T cell exhaustion markers (Figure 4D). Pan-caner analysis also revealed the role of STRA6 in inducing T cell exhaustion (Figure 4E). Tregs were significantly abundant in high-STRA6 PTC, while CD8+ T cells had a lower infiltration level (Figure 4F). The infiltration of other immune cells was also correlated with STRA6 expression, including NK cells, neutrophils, MDSCs, macrophages, myeloid dendritic cells, monocytes and B cells (Figure S2A).
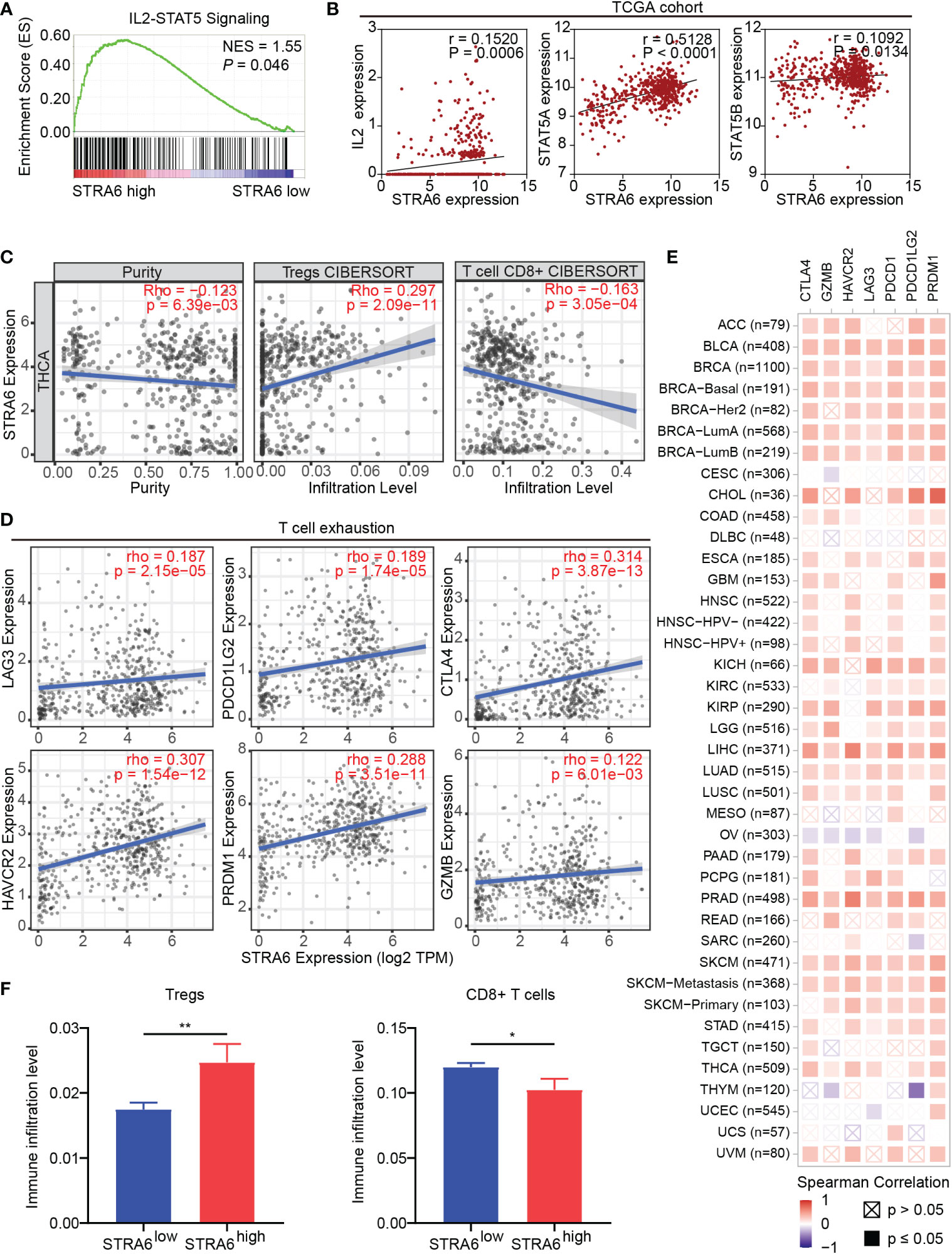
Figure 4 STRA6 regulates immunosuppressive microenvironment via the IL2-STAT5 signaling pathway. (A) Gene set enrichment analysis (GSEA) of the IL2-STAT5 signaling pathway enriched in samples with high-STRA6 expression. (B) Correlation of the expression of STRA6 and key molecules in the IL2-STAT5 pathway. (C) The correlation of STRA6 expression with infiltration of Tregs and CD8+ T cells according to the TCGA datasets. (D) Scatterplots of correlations between STRA6 expression and T cell exhaustion markers in the TCGA cohort. (E) Spearman correlations between STRA6 expression and T cell exhaustion markers in pan-cancer. (F) Immune infiltration level of Tregs and CD8+ T cells in PTC based on STRA6 expression. (*p < 0.05, **p < 0.01. The data are shown as the mean ± SEM).
To confirm the role of STRA6 in Tregs and CD8+ T cells infiltration, we performed fluorescent multiplex immunohistochemistry (mIHC) in PTC with different STRA6 expression. Samples with high STRA6 expression were mainly infiltrated with FoxP3+ Tregs while CD8+ T cells were mainly observed in samples with low STRA6 expression (Figures 5A, B). Adjacent thyroid tissues were less infiltrated with immune cells (Figure 5C). Upregulation of STRA6 was associated with lower CD8+ cells and higher Tregs infiltrates (Figure 5D), which was consistent with the infiltration level of immune cells in PTC with BRAF mutation (Figures S3A, B). Therefore, STRA6 may limit antitumor immunity and induce immunosuppressive tumor microenvironment in TC.
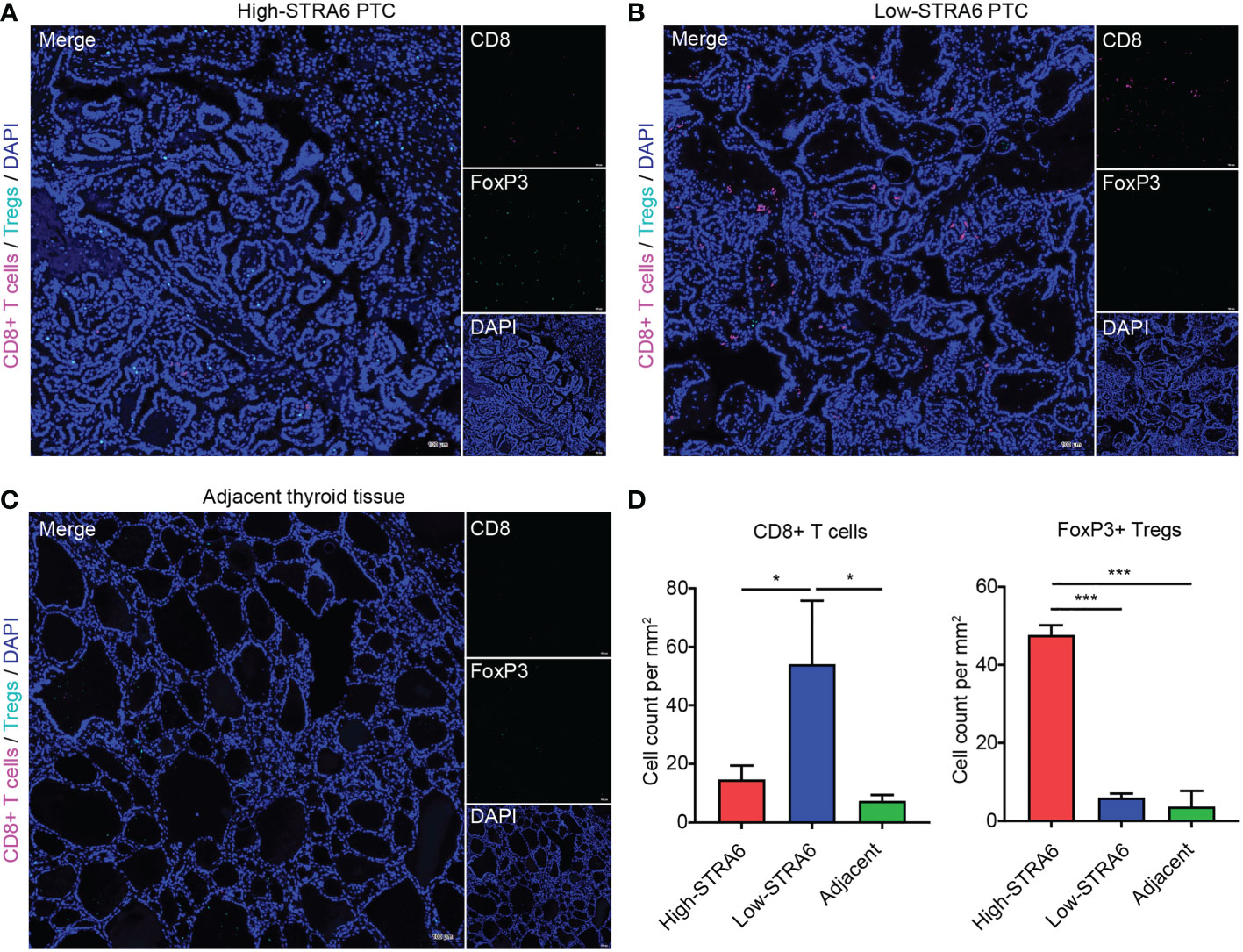
Figure 5 STRA6 increases Tregs abundance and inhibits CD8+ T cells infiltration in PTC. (A–C) Representative images of fluorescent multiplex immunohistochemistry (mIHC) in high-STRA6 PTC, low-STRA6 PTC and adjacent thyroid tissue. CD8+ T cells were stained with CD8α and Tregs were stained with FoxP3. Nuclei were stained with DAPI. (D) Positive Tregs and CD8+ T cells were counted in each group. (*p < 0.05, ***p < 0.001. The data are shown as the mean ± SD).
STRA6 promotes epithelial-mesenchymal transition via increased cancer-associated fibroblasts infiltration in thyroid carcinoma
(CAF) Cancer-associated fibroblast is one of the important components in tumor microenvironment. CAFs infiltration was positively associated with the expression of STRA6 (Figure 6A). We also analyzed the expression level of CAFs markers in PTC. COLA1, COLA2 and COL3A1 were all upregulated in high-STRA6 samples (Figure 6B). Compared with low-STRA6 group, CAFs were abundant in high-STRA6 group (Figure 6C). Higher infiltration level of CAFs was also observed in BRAF-mutant PTC (Figure S3C). CAFs play important roles in cancer development via regulation of metastasis. The results of GSEA showed that the TNFα/NF-κB signaling pathway and Epithelial-Mesenchymal Transition (EMT) hallmark gene set was significantly enriched in high-STRA6 PTC (Figures 6D, E). Consistently, the mRNA expression of STRA6 was positively correlated with the expression of EMT markers, including Vimentin (r = 0.1446), MMP7 (r = 0.5345), MMP9 (r = 0.3594), MMP14 (r = 0.4984) (Figure 6F). Taken together, STRA6 may promote TC metastasis via CAF-mediated EMT process.
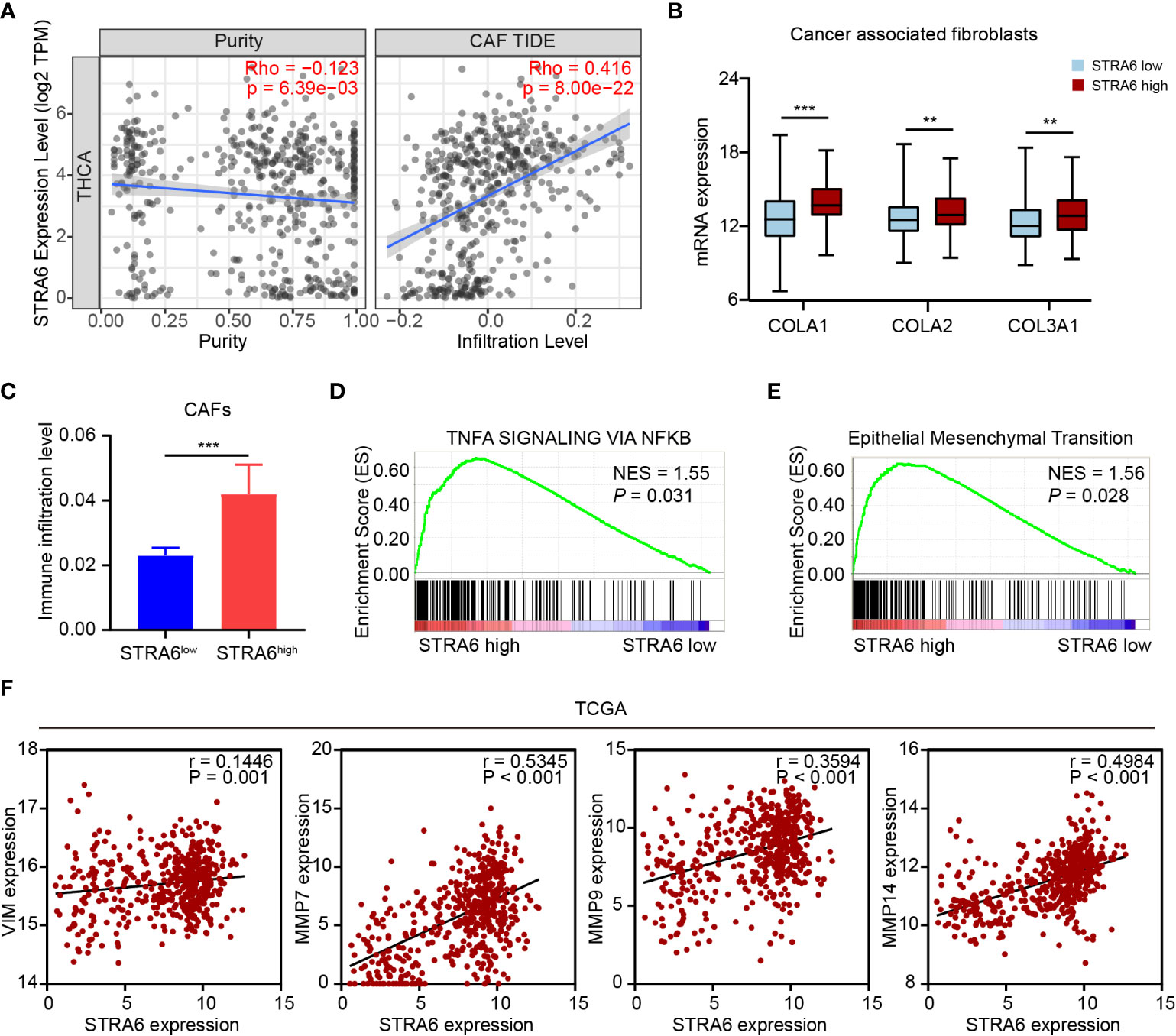
Figure 6 STRA6 promotes EMT process via increased CAFs infiltration. (A) The correlation of STRA6 expression with CAFs infiltration after purity adjustment. (B) mRNA expression of CAFs markers in PTC based on STRA6 expression. (C) Infiltration level of CAFs in low-STRA6 PTC and high-STRA6 PTC. (D, E) GSEA results of TNFα/NF-κB signaling pathway and epithelial mesenchymal transition enrichment according to the TCGA database. (F) Scatter plot analysis of correlation between STRA6 mRNA expression and EMT markers. (**p < 0.01, ***p < 0.001. The data are shown as the mean ± SEM).
Discussion
As the most common genetic alteration in PTC, the prevalence of BRAF mutation differs in races. Compared with western populations (3), the incidence of BRAF mutation was much higher in Chinese population (4, 21), which was detected in about 75% PTC patients. However, BRAF mutation may not be a single and independent prognostic marker for PTC. Studies revealed that BRAF mutation may not totally associate with clinicopathological characteristics or survival (22, 23). In our study, we found STRA6 is associated with BRAF genotype. Upregulation of STRA6 predicts poor RFS and OS in BRAF-mutant PTC, which is an independent risk factor for mortality. Previous study showed that BRAFV600E mutation was significantly correlated with PTC-related mortality in an unadjusted analysis and the association was no longer significant after adjusting for multiple clinical factors (24). Thus, our study indicated that STRA6 may act as a prognostic marker, which may provide individualized diagnosis and therapeutic strategy for BRAF-mutant PTC.
We further explored the mechanism of STRA6 in BRAF-mutant PTC progression. Interestingly, when we divided BRAF-mutant PTC into two groups according to STRA6 expression, the MAPK signaling pathway is significantly enriched with the upregulated genes. STRA6 also interacts with several proteins regulated by the MAPK pathway, including BMP4, DIO3, and DUSP6. It was reported that BMP4 can inhibit heat-induced apoptosis by enhancing the activation of ERK (25). DIO3 is increased in PTC through activation of the MAPK pathway (26, 27). DUSP6 is a member of the MAPK phosphatase family and plays pro-tumorigenic role in TC (28, 29). Moreover, BRAF mutation drives aberrant activation of the MAPK pathway (30) and promotes TC tumorigenesis. Dual activation of the MAPK pathway by STRA6 and BRAF mutation, may contribute to the poor survival of BRAF-mutant PTC with high STRA6 expression.
Pathway analyses showed that STRA6 involves in the regulation of the immune interaction and activation of the IL2-STAT5 pathway. Our study also revealed that STRA6 expression is correlated with the infiltration of multiple immune cells. Notably, upregulation of STRA6 results in higher Tregs abundance and lower CD8+ T cells infiltration. Additionally, STRA6 is correlated with T cell exhaustion. In support of our results, IL-2 is mainly produced by activated CD4+ T cells and induces the expansion of Tregs (31). Persistent activation of the IL2-STAT5 pathway triggers CD8+ T cells into exhausted status (32). Decreased infiltration of CD8+ T cells and a low CD8/regulatory T cell ratio in tumors are critical for unfavorable prognosis of cancers (33, 34). Our results also showed that high STRA6 expression is associated with increased CAFs and promotes EMT process, which further explains the worse prognosis in BRAF-mutant PTC. CAFs are one of the most abundant stromal components in the tumor microenvironment. By secreting TGF-β, CCL2, CCL5, IL-6 etc., CAFs recruit immunosuppressive cells into the tumor stroma, which contributes to cancer metastasis and progression (35). The secretion of various cytokines promotes aggressive phenotypes in tumor cells via the activation of EMT process (36). We also confirmed BRAFV600E mutation was associated with suppressive immune cell infiltration (37). In hence, immune suppression mediated by STRA6 and BRAF contributes to PTC progression synergistically.
Taken together, STRA6 is overexpressed in TC and associated with BRAF mutation. Upregulation of STRA6 predicts unfavorable prognosis for BRAF-mutant PTC. High STRA6 expression is correlated with decreased CD8+ T cells as well as abundant Tregs and CAFs, which induces immunosuppressive microenvironment in TC. Moreover, STRA6 may play an oncogenic role via the MAPK pathway and EMT process. In conclusion, STRA6 might be a prognostic marker for BRAF-mutant PTC and individualized therapeutic strategies for the patients are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Research Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
HX, SH and WH conceived and designed the study. WH, JG, and XW performed the bioinformatic analysis. WH and YS performed immunohistochemistry and fluorescent multiplex immunohistochemistry. WH analyzed the data and wrote the manuscript with the approval of all other authors. BL collected tissue samples. SY and YL gave critical comments. HX and SH supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the General Program of the National Natural Science Foundation of China (No. 82072956) and the Youth Program of the National Natural Science Foundation of China (No. 81802677).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1076640/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Zaballos MA, Santisteban P. Key signaling pathways in thyroid cancer. J Endocrinol (2017) 235(2):R43–61. doi: 10.1530/JOE-17-0266
3. Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell (2014) 159(3):676–90. doi: 10.1016/j.cell.2014.09.050
4. Wang Z, Tang P, Hua S, Gao J, Zhang B, Wan H, et al. Genetic and clinicopathologic characteristics of papillary thyroid carcinoma in the Chinese population: High BRAF mutation allele frequency, multiple driver gene mutations, and RET fusion may indicate more advanced TN stage. Onco Targets Ther (2022) 15:147–57. doi: 10.2147/OTT.S339114
5. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol (2015) 33(1):42–50. doi: 10.1200/JCO.2014.56.8253
6. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
7. Li X, Kwon H. The impact of BRAF mutation on the recurrence of papillary thyroid carcinoma: A meta-analysis. Cancers (Basel) (2020) 12(8):2056. doi: 10.3390/cancers12082056
8. Niederer-Wust SM, Jochum W, Forbs D, Brandle M, Bilz S, Clerici T, et al. Impact of clinical risk scores and BRAF V600E mutation status on outcome in papillary thyroid cancer. Surgery (2015) 157(1):119–25. doi: 10.1016/j.surg.2014.07.015
9. Zhong M, Kawaguchi R, Costabile B, Tang Y, Hu J, Cheng G, et al. Regulatory mechanism for the transmembrane receptor that mediates bidirectional vitamin a transport. Proc Natl Acad Sci U S A. (2020) 117(18):9857–64. doi: 10.1073/pnas.1918540117
10. Lin L, Xiao J, Shi L, Chen W, Ge Y, Jiang M, et al. STRA6 exerts oncogenic role in gastric tumorigenesis by acting as a crucial target of miR-873. J Exp Clin Cancer Res (2019) 38(1):452. doi: 10.1186/s13046-019-1450-2
11. Nakamura S, Kanda M, Shimizu D, Sawaki K, Tanaka C, Hattori N, et al. STRA6 expression serves as a prognostic biomarker of gastric cancer. Cancer Genomics Proteomics (2020) 17(5):509–16. doi: 10.21873/cgp.20207
12. Berry DC, Levi L, Noy N. Holo-retinol-binding protein and its receptor STRA6 drive oncogenic transformation. Cancer Res (2014) 74(21):6341–51. doi: 10.1158/0008-5472.CAN-14-1052
13. Karunanithi S, Levi L, DeVecchio J, Karagkounis G, Reizes O, Lathia JD, et al. RBP4-STRA6 pathway drives cancer stem cell maintenance and mediates high-fat diet-induced colon carcinogenesis. Stem Cell Rep (2017) 9(2):438–50. doi: 10.1016/j.stemcr.2017.06.002
14. Muniz-Hernandez S, Velazquez-Fernandez JB, Diaz-Chavez J, Mondragon-Fonseca O, Mayen-Lobo Y, Ortega A, et al. STRA6 polymorphisms are associated with EGFR mutations in locally-advanced and metastatic non-small cell lung cancer patients. Front Oncol (2020) 10:579561. doi: 10.3389/fonc.2020.579561
15. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
16. French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab (2010) 95(5):2325–33. doi: 10.1210/jc.2009-2564
17. French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC Jr., Klopper JP, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab (2012) 97(6):E934–43. doi: 10.1210/jc.2011-3428
18. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer (2008) 15(4):1069–74. doi: 10.1677/ERC-08-0036
19. You W, Ouyang J, Cai Z, Chen Y, Wu X. Comprehensive analyses of immune subtypes of stomach adenocarcinoma for mRNA vaccination. Front Immunol (2022) 13:827506. doi: 10.3389/fimmu.2022.827506
20. Hong S, Xie Y, Cheng Z, Li J, He W, Guo Z, et al. Distinct molecular subtypes of papillary thyroid carcinoma and gene signature with diagnostic capability. Oncogene (2022) 41(47):5121–5132. doi: 10.1038/s41388-022-02499-0
21. Liang J, Cai W, Feng D, Teng H, Mao F, Jiang Y, et al. Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol (2018) 244(2):215–26. doi: 10.1002/path.5005
22. Al-Masri M, Al-Shobaki T, Al-Najjar H, Iskanderian R, Younis E, Abdallah N, et al. BRAF V600E mutation in papillary thyroid carcinoma: it’s relation to clinical features and oncologic outcomes in a single cancer centre experience. Endocr Connect (2021) 10(12):1531–7. doi: 10.1530/EC-21-0410
23. Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J (2009) 56(1):89–97. doi: 10.1507/endocrj.K08E-208
24. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309(14):1493–501. doi: 10.1001/jama.2013.3190
25. Deng H, Ravikumar TS, Yang WL. Bone morphogenetic protein-4 inhibits heat-induced apoptosis by modulating MAPK pathways in human colon cancer HCT116 cells. Cancer Lett (2007) 256(2):207–17. doi: 10.1016/j.canlet.2007.06.008
26. Romitti M, Wajner SM, Ceolin L, Ferreira CV, Ribeiro RV, Rohenkohl HC, et al. MAPK and SHH pathways modulate type 3 deiodinase expression in papillary thyroid carcinoma. Endocr Relat Cancer (2016) 23(3):135–46. doi: 10.1530/ERC-15-0162
27. Romitti M, Wajner SM, Zennig N, Goemann IM, Bueno AL, Meyer EL, et al. Increased type 3 deiodinase expression in papillary thyroid carcinoma. Thyroid (2012) 22(9):897–904. doi: 10.1089/thy.2012.0031
28. Degl’Innocenti D, Romeo P, Tarantino E, Sensi M, Cassinelli G, Catalano V, et al. DUSP6/MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr Relat Cancer (2013) 20(1):23–37. doi: 10.1530/ERC-12-0078
29. Lee JU, Huang S, Lee MH, Lee SE, Ryu MJ, Kim SJ, et al. Dual specificity phosphatase 6 as a predictor of invasiveness in papillary thyroid cancer. Eur J Endocrinol (2012) 167(1):93–101. doi: 10.1530/EJE-12-0010
30. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer (2013) 13(3):184–99. doi: 10.1038/nrc3431
31. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol (2012) 12(3):180–90. doi: 10.1038/nri3156
32. Liu Y, Zhou N, Zhou L, Wang J, Zhou Y, Zhang T, et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat Immunol (2021) 22(3):358–69. doi: 10.1038/s41590-020-00850-9
33. Piersma SJ, Jordanova ES, van Poelgeest MI, Kwappenberg KM, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res (2007) 67(1):354–61. doi: 10.1158/0008-5472.CAN-06-3388
34. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. (2005) 102(51):18538–43. doi: 10.1073/pnas.0509182102
35. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discovery (2019) 18(2):99–115. doi: 10.1038/s41573-018-0004-1
36. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer (2019) 18(1):70. doi: 10.1186/s12943-019-0994-2
Keywords: papillary thyroid carcinoma (PTC), BRAF mutation, STRA6, prognosis, tumor immune microenvironment
Citation: He W, Sun Y, Ge J, Wang X, Lin B, Yu S, Li Y, Hong S and Xiao H (2023) STRA6 regulates tumor immune microenvironment and is a prognostic marker in BRAF-mutant papillary thyroid carcinoma. Front. Endocrinol. 14:1076640. doi: 10.3389/fendo.2023.1076640
Received: 21 October 2022; Accepted: 31 January 2023;
Published: 10 February 2023.
Edited by:
Jose Federico Carrillo, National Institute of Cancerology (INCAN), MexicoReviewed by:
Xiaopei Shen, Fujian Medical University, ChinaMinghua Ge, Zhejiang Provincial People’s Hospital, China
Copyright © 2023 He, Sun, Ge, Wang, Lin, Yu, Li, Hong and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haipeng Xiao, eGlhb2hwQG1haWwuc3lzdS5lZHUuY24=; Shubin Hong, aG9uZ3NoYjNAbWFpbC5zeXN1LmVkdS5jbg==
 Weiman He
Weiman He Yijia Sun1
Yijia Sun1 Yanbing Li
Yanbing Li Shubin Hong
Shubin Hong Haipeng Xiao
Haipeng Xiao