- 1Department of Thyroid Surgery, Affiliated Hospital of Jining Medical University, Jining, Shandong, China
- 2Qingdao Medical College, Qingdao University, Qingdao, Shandong, China
- 3Department of Cardiovascular Surgery, Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Background: The frequency of thyroid cancer has rapidly increased in recent years globally. Thus, more papillary thyroid microcarcinoma (PTMC) patients are being diagnosed, including clinical lymph node-negative (cN0) patients. Our study attempted to develop a prediction model for assessing the probability of central lymph node metastasis (CLNM) in cN0 PTMC patients.
Methods: A total of 595 patients from the Affiliated Hospital of Qingdao University (training cohort: 456 patients) and the Affiliated Hospital of Jining Medical University (verification cohort: 139 patients) who underwent thyroid surgery between January 2020 and May 2022 were enrolled in this study. Their clinical and molecular pathology data were analyzed with multivariate logistic regression to identify independent factors, and then we established a prediction model to assess the risk of CLNM in cN0 PTMC patients.
Results: Multivariate logistic regression analysis revealed that sex, Hashimoto’s thyroiditis (HT), tumor size, extrathyroidal extension, TERT promoter mutations and NRAS mutation were independent factors of CLNM. The prediction model demonstrated good discrimination ability (C-index: 0.757 and 0.753 in the derivation and validation cohorts, respectively). The calibration curve of the model was near the optimum diagonal line, and decision curve analysis (DCA) showed a noticeably better benefit.
Conclusion: CLNM in cN0 PTMC patients is associated with male sex, tumor size, extrathyroidal extension, HT, TERT promoter mutations and NRAS mutation. The prediction model exhibits good discrimination, calibration and clinical usefulness. This model will help to assess CLNM risk and make clinical decisions in cN0 PTMC patients.
Introduction
The most prevalent endocrine malignancy is thyroid carcinoma, and papillary thyroid carcinoma (PTC) accounts for nearly 90%, including probably 50% microcarcinoma (PTMC) (1, 2). PTC with a maximal diameter of less than 1 cm is referred to PTMC. In the past, most PTMCs were diagnosed after the excision of benign thyroid tumors, but now with the popularity of health checkups and widespread use of ultrasound-guided fine needle aspiration biopsy (US-FNAB), the diagnosis rate of PTMC is on the rise (3).
Papillary thyroid carcinoma itself has relatively indolent biological characteristics and superadd the small tumor burden; PTMC patients usually have a good prognosis after treatment (4). The 2022 National Comprehensive Cancer Network (NCCN) advises against utilizing prophylactic CLND to treat T1 and T2 cN0 PTC (5). Nevertheless, the sensitivity of preoperative detection, such as ultrasound, is low for assessing the central lymph node (CLN) state (6); thus, the high probability of occult CLNM cannot be ignored even in cN0 PTMCs (7).
Numerous studies have analyzed the risk factors for CLNM in cN0 PTMC. Our study aimed to create a validated predictive model including preoperatively determinable clinical data and molecular markers, hoping to provide valuable information for clinicians about facilitating the individualized prediction of CLNM in cN0 PTMC patients and guide precise treatment.
Materials and methods
Study design and population
Our study enrolled 2 independent sets of patients from 2 independent medical centers. A total of 456 patients diagnosed with cN0 PTMC in the Thyroid Surgery Department of the Affiliated Hospital of Qingdao University from January 2020 to May 2022 were included as the training cohort. A total of 139 patients were diagnosed with cN0 PTMC in the Thyroid Surgery Department of the Affiliated Hospital of Jining Medical University at the same time as the validation cohort. All operations were performed by surgeons who completed more than 400 surgeries per year. This study was approved by the Affiliated Hospital of Jining Medical University ethics committee (2022C103). Consent has been obtained from each patient or subject after a full explanation of the purpose and nature of all procedures used.
The inclusion criteria were as follows: (1) patients were diagnosed with cN0 PTMC through preoperative ultrasonography (US), computed tomography (CT) and US-FNAB; (2) patients underwent total thyroidectomy with CLND, unilateral thyroid lobectomy and isthmectomy with CLND; and (3) patients underwent BRAF V600E, TERT, RET, HRAS, KRAS, and NRAS genetic testing.
The exclusion criteria were as follows: (1) patients diagnosed with recurrent thyroid tumors (only primary thyroid carcinoma patients were included); (2) patients who had previously undergone neck radiation therapy or other types of surgery; and (3) patients with incomplete medical records.
Data collection
Data collected included sex, age, surgical method, thyroid peroxidase antibody (TPOAb), thyroglobulin antibody (TGAb), thyroid hormone receptor antibody (TRAb), microcalcification foci, positive lymph node number, BRAF gene mutation(V600E), TERT promoter mutation(C228, C250), RET/PTC chromosomal rearrangement(RET/PTC1, RET/PTC3), HRAS gene mutation(Q61), KRAS gene mutation(G12, G13), and NRAS gene mutation(Q61). All data from preoperative examinations and postoperative pathological reports, such as cervical ultrasonic and CT, laboratory examination, and gene test results, were evaluated.
All preoperative examinations were confirmed by a radiologist and chief surgeon, who confirmed the number, size, and calcification of carcinoma and evaluated the lymph node state. Metastatic lymph nodes were suspected when lymph nodes showed increased size (>0.5 cm), demarcation of the cortex and medulla was unclear or the structure of the medulla disappeared, gravel-like calcification or cystic degeneration, rounded bulging shape, and abnormal blood flow or vascularity. Hashimoto’s thyroiditis (HT) was diagnosed when the thyroid gland was diffusely enlarged with hypoechogenicity, and the gland parenchyma was inhomogeneous with grid-like or compartment-like changes or appeared abnormal TPOAb, TGAb, TRAb results (8, 9). A gene mutation detection kit (Shanghai Anjia Biological Technology Co., Ltd.) was used to detect BRAF, TERT, HRAS, KRAS, NRAS mutations and RET/PTC rearrangements according to the manufacturer’s instructions. The scope of CLND was superior to the hyoid bone, lateral to the carotid sheath, inferior to the sternum notch or the innominate artery, medial to the trachea and dorsal to the prevertebral fascia.
Statistical analysis
The t test (normally distributed data) and Mann−Whitney test (nonnormally distributed data) were used to compare continuous data, and the χ2 test was used to compare categorical data. Multivariate analysis was carried out with logistic regression; therefore, independent predictors were used to construct a predictive model to evaluate the CLNM risk of cN0 PTMC.
Then, we developed a nomogram as a pictorial representation of the model. The nomogram featured a scoring range at the top from 0 to 100 for each factor variable. Predictor factors are shown below, and bars scaled their effects, displaying the importance of each factor clearly and enabling the awarding of points for each significant clinical feature. Each predictor point’s overall value and the corresponding probability of CLNM can be read from the bottom 2 rows.
The predicted accuracy and conformity of the model were assessed using the calibration curve and receiver operating characteristic (ROC) curve. The goodness of fit of the model was evaluated using the Hosmer−Lemeshow test. The net benefit for patients was shown by decision curve analysis (DCA), and 1000 bootstrap repetitions were performed in both discrimination and calibration. In the statistical analyses, P<0.05 was considered significant. Statistical analyses were performed using SPSS (version 22.0) and R (version 3.4.1).
Results
Baseline characteristics
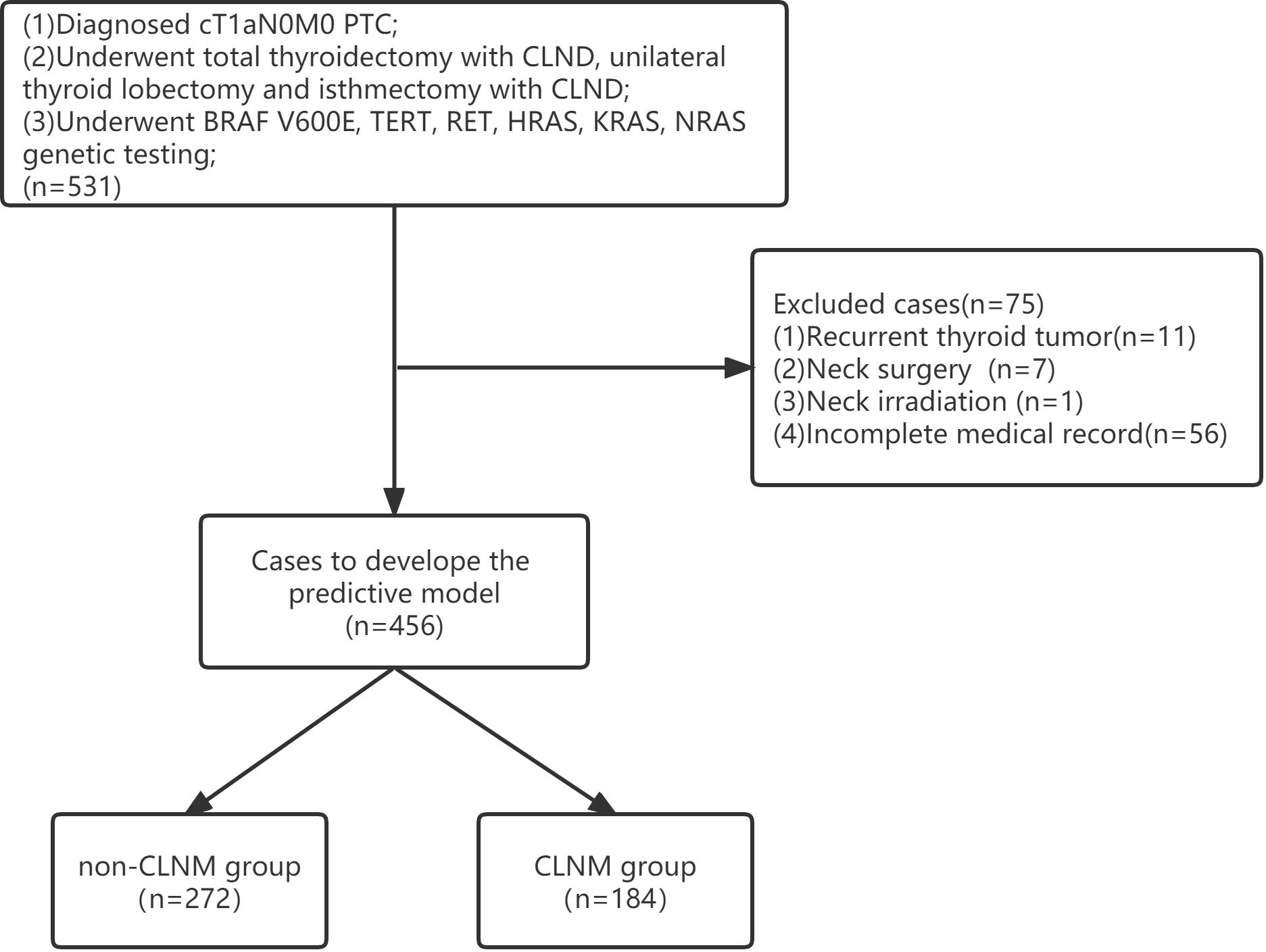
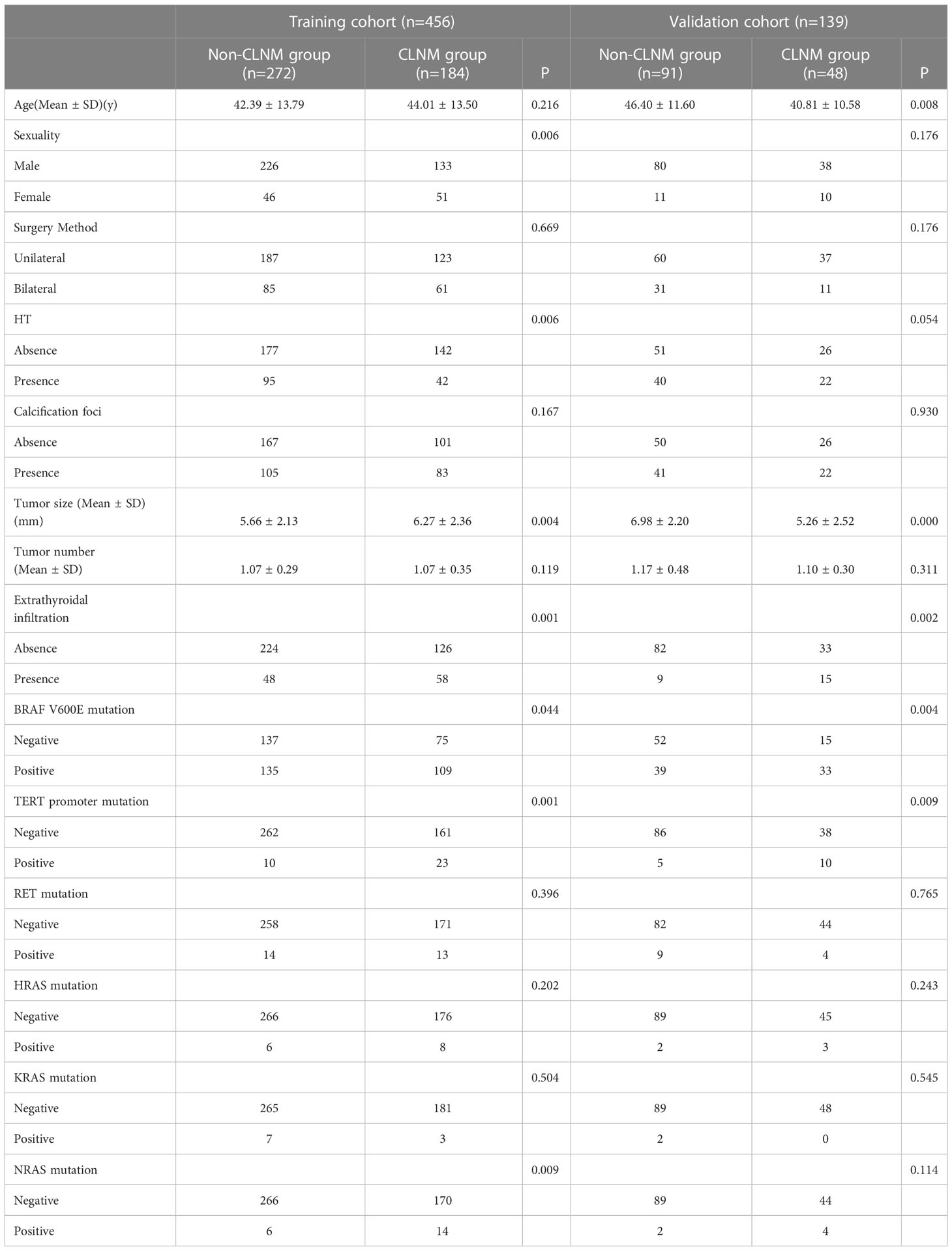
Clinical information from a total of 531 patients was acquired from the Affiliated Hospital of Qingdao University for the training cohort, and 75 met the exclusion criteria due to the absence of medical records and recurrent thyroid tumors (Figure 1). Therefore, 456 patients were enrolled in this cohort (97 males, 359 females; mean age 43.04 ± 13.68 years). Among them, 184 patients (40.35%) had CLNM. Clinical information from a total of 139 patients was acquired from the Affiliated Hospital of Jining Medical University for the validation cohort (21 males, 118 females; mean age 44.47 ± 11.54 years). Among them, 48 patients (34.53%) had CLNM. Finally, 595 patients were included in this study. The patients’ clinical, pathology and molecular characteristics are listed in Table 1.
Univariate and multivariate analysis
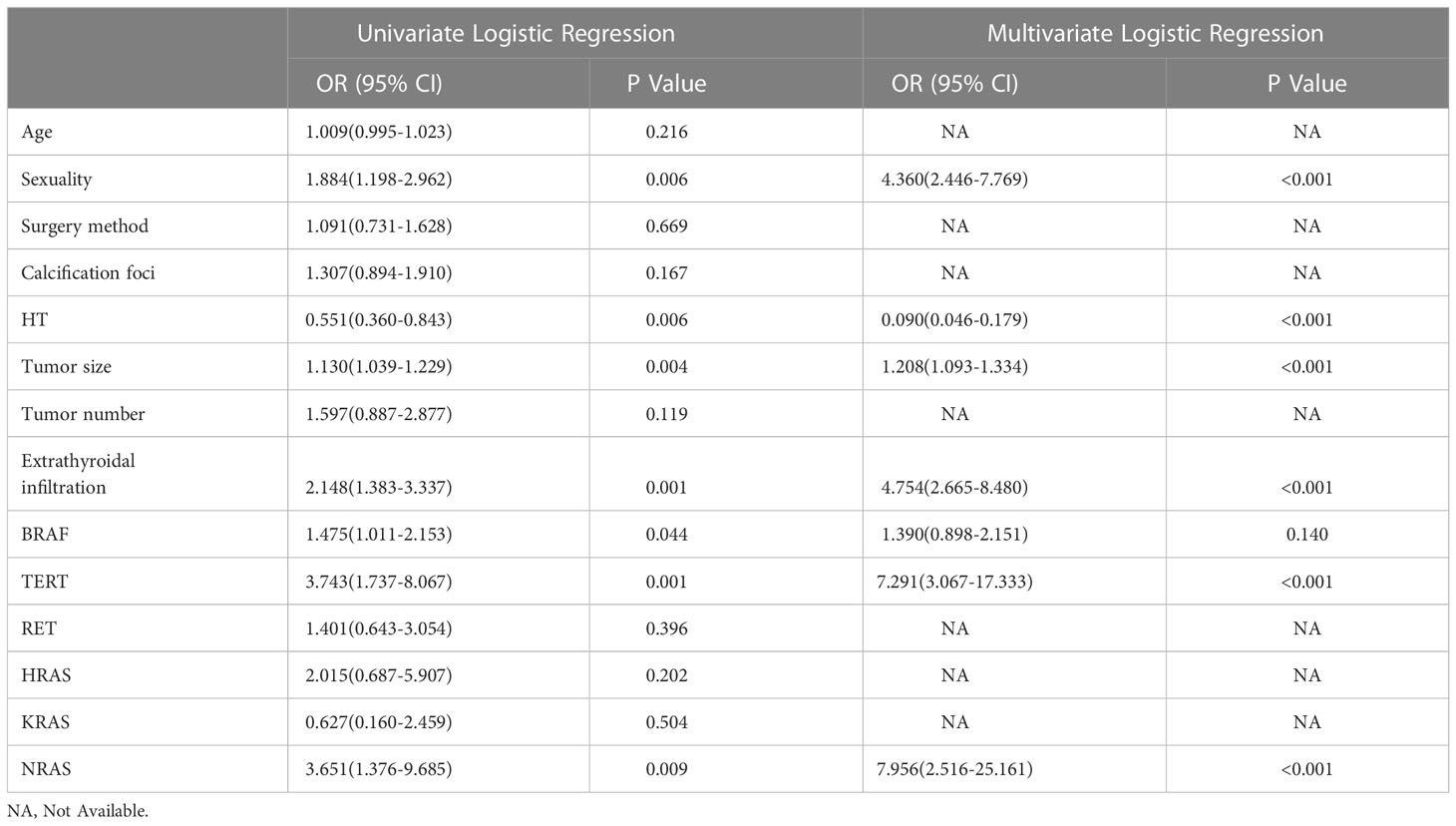
Within the training cohort, univariable analysis demonstrated that sex, HT, tumor size, tumor number, extrathyroidal extension, BRAF mutation, TERT promoter mutations and NRAS mutations were relative influencing factors of CLNM (P<0.05). These factors were included in multivariate logistic regression analysis. As shown in Table 2, the results revealed six independent factors, of which male sex, increased tumor size, extraglandular infiltration, TERT promoter mutations and NRAS mutation increased the risk of CLNM, while HT was a protective factor (P<0.05). Accordingly, a prediction model was built.
The nomogram for predicting PTC recurrence
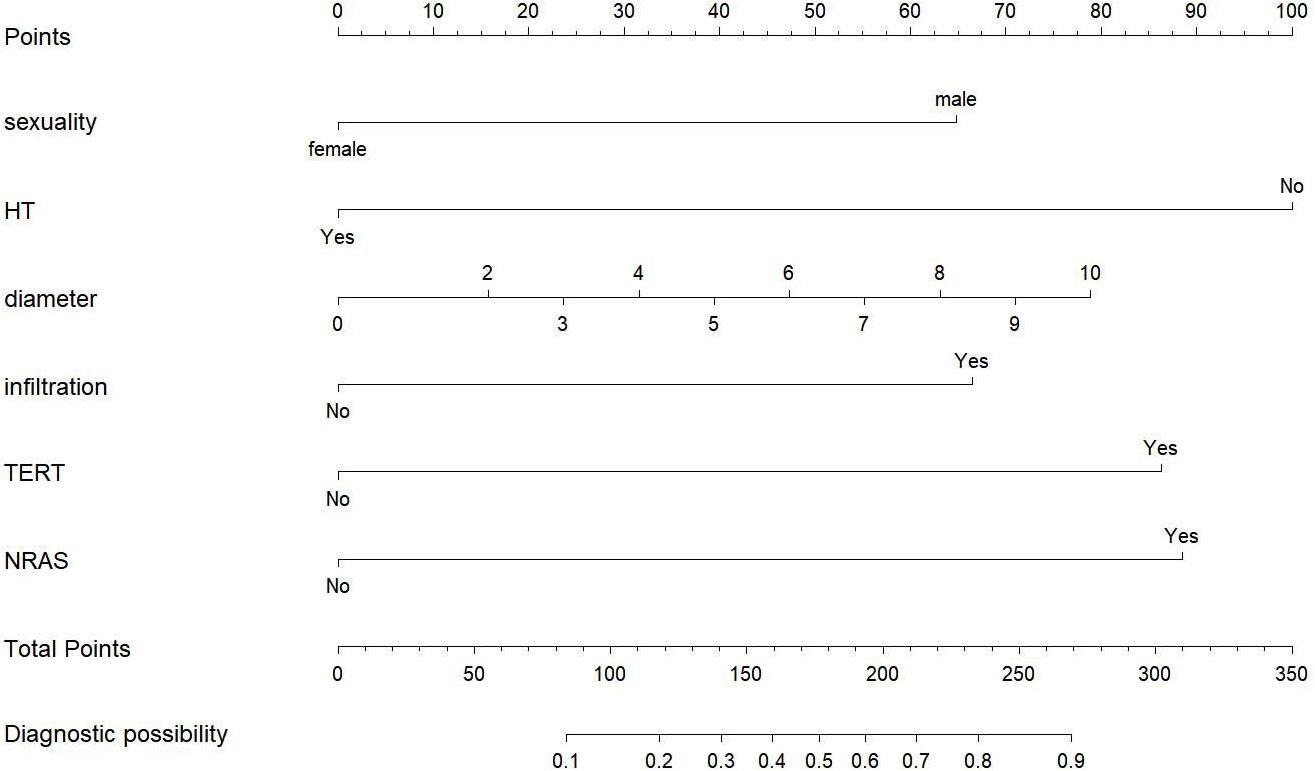
To visualize the model, we plotted a nomogram based on six independent factors (sex, HT, tumor size, extrathyroidal extension, TERT promoter mutations and NRAS mutation) in the training cohort. For each risk factor, an ascending line was drawn in the nomogram to obtain a point value in Line 1. The risk possibility of CLNM in cN0 PTMC patients might be estimated by plotting the total of all six points on the final risk axis (Line 8), as shown in Figure 2.
Subsequently we drew the ROC curve to assess the discriminating ability of the model. The area under the ROC was 0.757 (95% CI, 0.711-0.802) and 0.753 (95% CI, 0.682-0.851) in the training cohort and validation cohort, respectively. The model demonstrated a strong capacity to discriminate CLNM in cN0 PTMC (this result is shown in Figure 3).
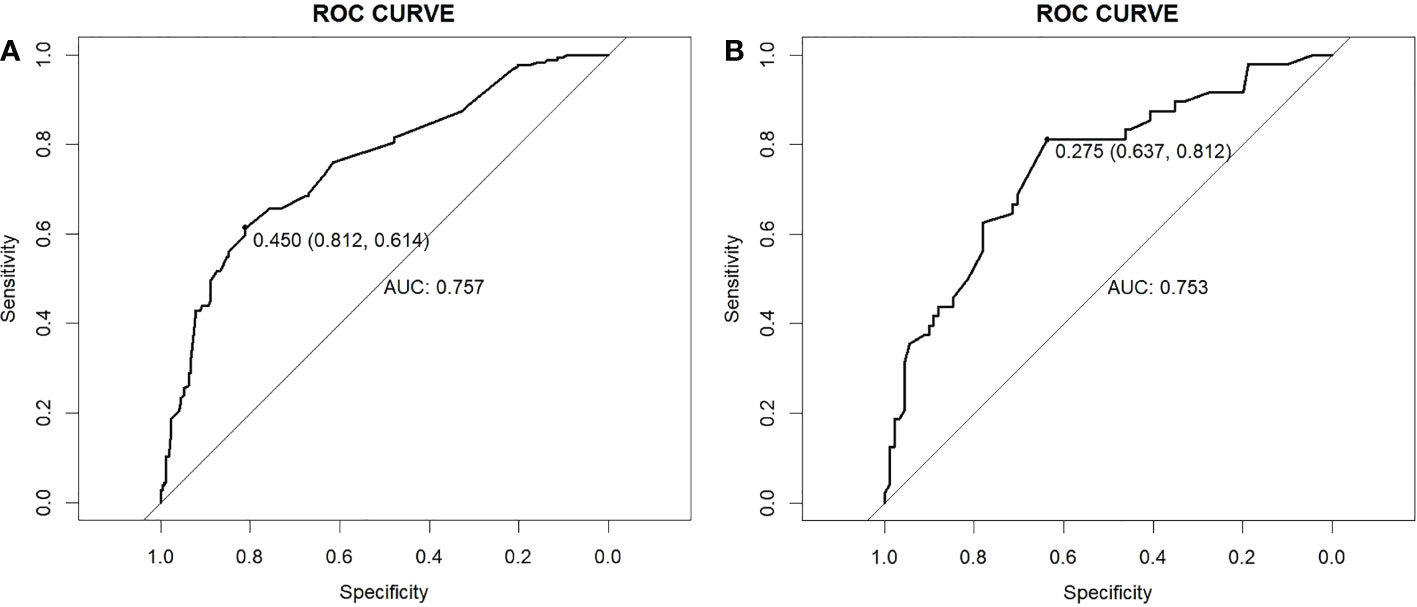
Figure 3 ROC curves. (A) Training cohort. (B) Validation cohort. ROC, receiver operating characteristic; AUC, area under the ROC curve.
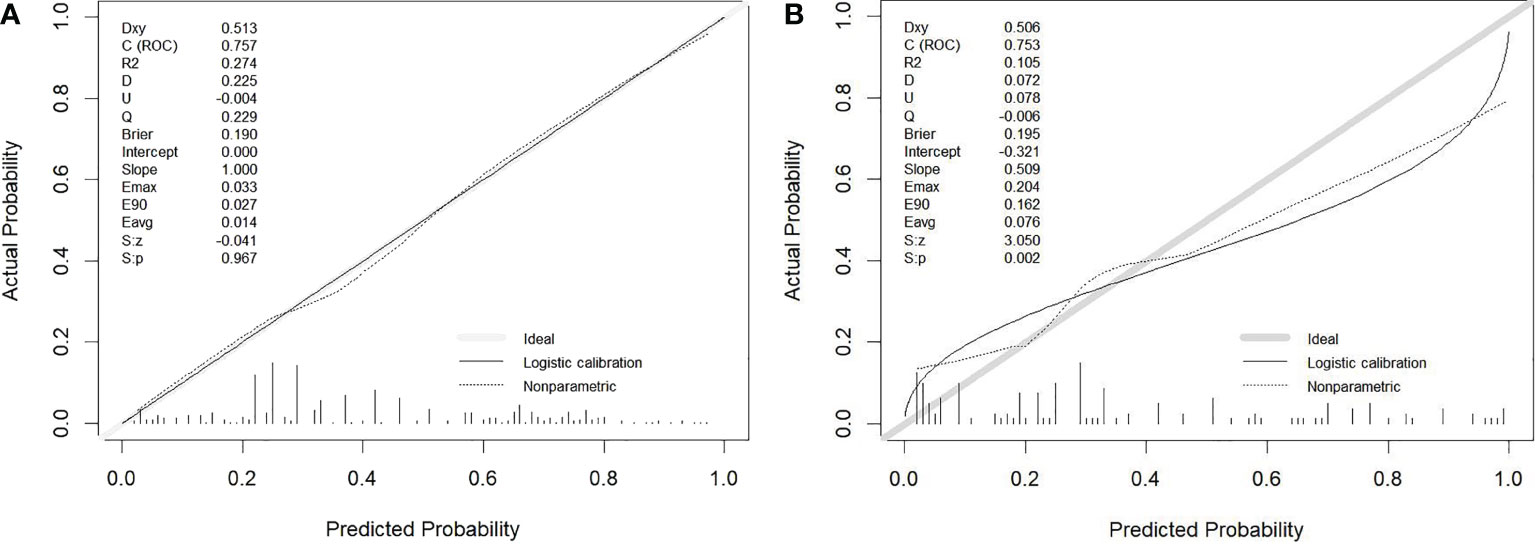
The calibration curve illustrated the model’s potent capacity to calibrate (Figure 4). In both the training cohort and the validation group, the model’s predicted values and the observed variables showed good agreement. According to the Hosmer−Lemeshow test, the model’s goodness of fit was 0.243.
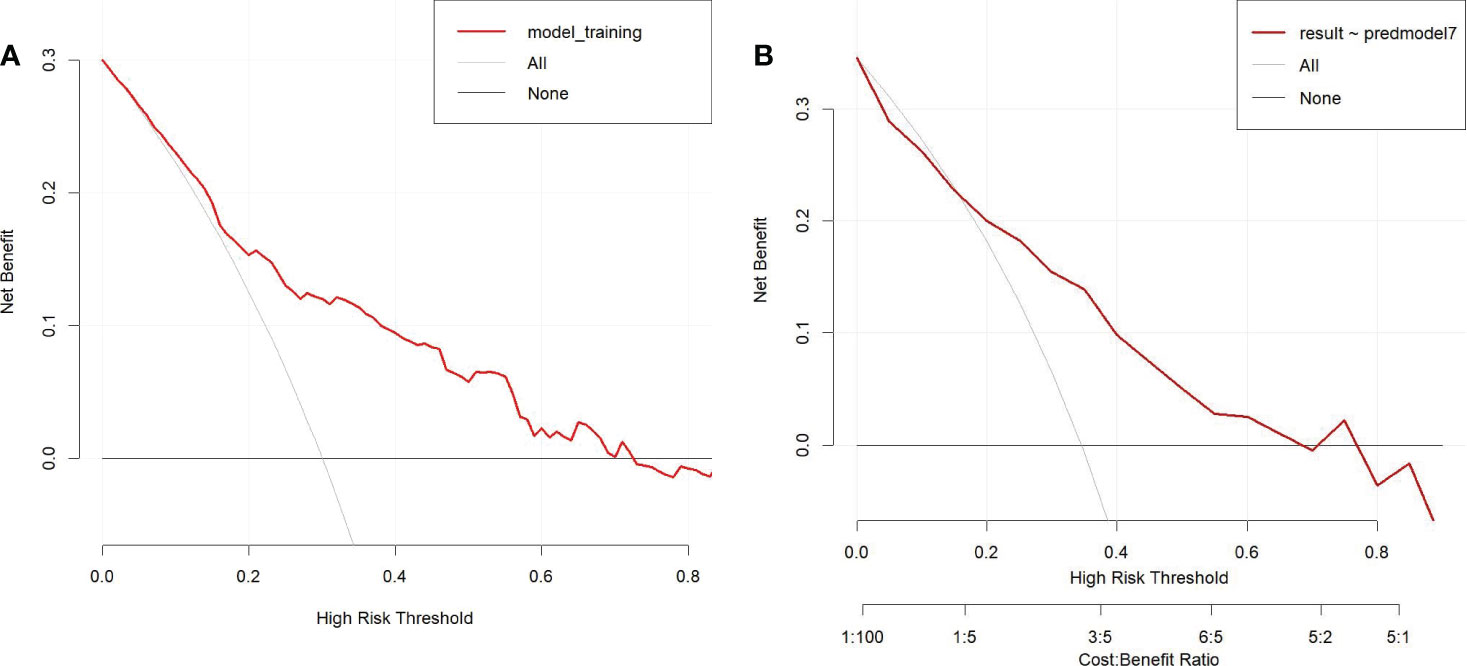
To determine the net benefit of the nomogram, we built a decision curve analysis (DCA) (Figure 5). The curve demonstrated that the nomogram could be useful when the threshold probability of the patients was between 0.15 and 0.70. In the prediction model, the DCA’s net benefit was noticeably higher.
Discussion
The most frequent pathological type of thyroid carcinoma is PTC (10), which is characterized by high differentiation and low malignancy. Most patients have a good prognosis, especially PTMC patients, whose cancer-related mortality rate is merely 0.34% after treatment (11, 12). Whereas PTMC patients have a high CLNM rate, studies have pointed out that 28% of PTMC patients are diagnosed with CLNM (13, 14). Thyroid cancer diagnosis and treatment guidelines (2022) published by the National Health Commission of the People’s Republic of China recommended prophylactic CLND should be performed for PTC patients as long as the parathyroid gland and recurrent laryngeal nerve can be protected (15). Moreover, the 2021 Chinese Society of Clinical Oncology (CSCO) Guidelines of Differentiated Thyroid Cancer recommended that PTMC patients with metastatic lymph nodes should undergo CLND (16). However, some studies still show that preventive dissection does not improve patient survival (17), and patients cannot benefit from it; conversely, it will increase the risk of nerve injury and hypoparathyroidism (18). How can metastasis be discriminated? Currently, the most common preoperative diagnostic method is ultrasound, the sensitivity of which for CLNM diagnosis is only 10.9%-38% (19). No practical way was found to raise the CLNM detection rate before surgery, which means that a large number of cN0 PTMC patients actually belong to pN1. Accordingly, identifying the risk factors for CLNM and evaluating the preoperative lymph node state accurately are of major clinical importance in cN0 PTMC patients, and some unnecessary prophylactic CLND can be avoided.
We conducted a large retrospective study including 595 patients from 2 independent medical centers and established a prediction model to assess the risk of CLNM in cN0 PTMC. The results showed that sex, HT, tumor size, extrathyroidal extension, TERT promoter mutations and NRAS mutation were independent factors.
Previous studies have shown that the incidence of PTC in males is lower than that in females, but the incidence of CLNM is higher (20). This is consistent with our findings that males showed a significant trend of CLNM (P < 0.001).
Tumor size reflects the stage and prognosis, such as lymph node status (5). In our study, the risk of CLNM in PTC increased with the enlargement of tumor size (P < 0.001), similar to other studies.
Extraglandular infiltration refers to the thyroid capsule being invaded by the tumor, and the tumor is more prone to infect lymph nodes through the thyroid capsule’s surface lymphatic channels after it has been breached (21). This result is consistent with our findings (P < 0.001).
HT is characterized by enrichment of inflammatory factors, also known as autoimmune thyroiditis. It produces a significant amount of lymphocytes and macrophages, both of which naturally possess antitumor activity, and the autoimmune antibodies released by HT-related lymphocytes can also destroy the follicles, contributing to their shrinkage and fibrosis (22, 23). Therefore, PTC tissues with HT usually exhibit a microscopic fibrous cell layer wrapping around the primary lesion, indicating that the combination of HL has a limiting effect on the progression of the primary lesion, which can significantly impede the spread and infiltration of tumor cells (24), reduce the invasiveness of the primary lesion (25), and decrease CLNM chance (26). Kim’s studies have shown that PTC patients with HT have lower clinical stage, lower incidence of vascular invasion, lower incidence of CLNM and better prognosis (27). Jara found that the incidence of CLNM in PTC patients with HT was 39% lower than that of PTC alone (28). Zhu analyzed 763 PTMC patients and showed that the presence of HT reduced the lymph node metastasis rate in PTMC patients (29). These results coincide with our study (P < 0.001). It is worth mentioning that the effect of HT may vary depending on the criteria used to define HT, Konturek found that the CLNM rate was four times higher in PTC patients coexisting with HT than non-HT patients (30).
The rapid development of fine-needle aspiration techniques makes preoperative genetic testing possible. Thus, surgeons can obtain preoperative genetic test results, such as BRAF gene mutation, TERT gene mutation, HRAS gene mutation, KRAS gene mutation, NRAS gene mutation and RET/PTC rearrangement.
BRAF, one of the three serine-threonine kinase RAF genes (the others being ARAF and CRAF), is crucial to the mitogen-activated protein kinase (MAPK) pathway (31). BRAF V600E is reported to be the most common mutation in PTC, with a probability of 29-69% (32). Although some studies revealed that BRAF genetic testing was valuable in PTC diagnosis (33), its utility in PTC prognosis remains controversial (34). According to several researchers, BRAF mutation is related to aggressiveness, such as lymph node metastases (35). In our study, the mutation incidence in the CLNM group (59.24%) was higher than that in the non-CLNM group (49.63%), but BRAF V600E mutation was not a relative influencing factor in CLNM (P=0.140). Probably because some BRAF V600E mutation testing was conducted preoperatively when patients could not be definitively diagnosed with PTC by US-FNAB pathology testing, which perhaps increased the proportion of BRAF V600E mutations patients inadvertently and affected its efficacy in CLNM diagnosis.
TERT promoter mutation has been deeply studied in various cancers, such as nervous system tumors, hepatocellular carcinoma, and bladder cancer (36). Recently, research on thyroid carcinoma has increased. Some articles have reported that the incidence of TERT promoter mutations is approximately 7.5-27% in PTC (37), and the mutation is correlated with particularly aggressive clinicopathological parameters, such as extrathyroidal extension, larger tumor size, and lymph node metastases (38–40). A meta-analysis study including 173 TERT promoter mutant and 1587 TERT promoter wild-type thyroid carcinoma patients showed that the LNM probability ratios were 53.18% and 37.30%, respectively, and a significant association existed between TERT promoter mutation and LNM (41). In Yang’s study, the TERT promoter mutation rate was 10.6% (1027/9653), which was significantly associated with PTC LNM (42). And Liu demonstrated that TERT promoter mutations occurred frequently in follicular derived PTC and associated with aggressive disease and poor outcome (43). These studies suggest that TERT promoter mutations can promote lymph node metastasis, which is consistent with our results. Nevertheless, most of the studies focus on advanced thyroid carcinoma (44), and studies on PTMC, especially CLNM, have rarely been conducted. In our study, TERT promoter mutation played an important role in diagnostic value and was an independent predictor of CLNM (p<0.001).
The second most frequent mutation is a RAS mutation in preoperative thyroid FNA. RAS mutations include 3 subtypes: HRAS, KRAS, and NRAS, the most common of which is NRAS (45). RAS mutations promote invasion mainly in follicular tumors, relate to local lymph node metastasis and distant metastasis (46). However, the encapsulated follicular type of papillary carcinoma, which is typically indolent and has a good prognosis, also frequently exhibits RAS mutations (47, 48). RAS mutations may only be present in thyroid carcinomas that are well-differentiated and prone to dedifferentiation and metastasis, but it is unlikely that this point mutation will serve as a general prognostic indicator for all varieties of thyroid carcinoma (49). In our study, NRAS was an independent factor for cN0 CLNM (p<0.001), perhaps just because of a specific subset, such as follicular papillary carcinoma.
There were many predictive models for assessing CLNM possibility in cN0 PTMC, but their models only incorporated clinical baseline data such as tumor characteristics, ultrasound and CT examination features or just included BRAF mutation result (50–52). Our study built the first predictive model that incorporates such molecular markers and included a large sample size. Finally, we identified 6 independent factors from preoperatively determinable clinical data and molecular markers and then built a predictive model to quantify the likelihood of CLNM in cN0 PTMC patients. The model exhibits good discrimination, calibration and clinical usefulness.
Nevertheless, it also had several limitations. First, our data were all obtained in one province, selection bias is inevitable, and the distribution of pathological subtypes and clinical features may differ in other regions. Second, the molecular pathology markers that were included in this study were limited, and important markers such as PTEN mutation, TRK rearrangement, and PAX8/PPARγ rearrangement were not involved. Third, our study requires further validation in prospective studies, and we are proceeding with a larger-scale, multicenter study in follow-up research. We hope our predictive model can provide valuable information for clinicians about facilitating the individualized prediction of CLNM in cN0 PTMC and guiding precise treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
TM designed the study, analyzed the data, and commented on the manuscript at all stages. LW and XZ collected the data. YS provided the research direction. All authors contributed to the article and approved the submitted version.
Funding
Our research was funded by Academician He Lin Research Foundation of Affiliated Hospital of Jining Medical University (JYHL2022FMS10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Davies L, Welch HG. Increasing incidence of thyroid cancer in the united states, 1973-2002. JAMA (2006) 295(18):2164–7. doi: 10.1001/jama.295.18.2164
3. Slijepcevic N, Zivaljevic V, Diklic A, Jovanovic M, Oluic B, Paunovic I. Risk factors associated with intrathyroid extension of thyroid microcarcinomas. Langenbecks Arch Surg (2018) 403(5):615–22. doi: 10.1007/s00423-018-1680-3
4. Tagliabue M, Giugliano G, Mariani MC, Rubino M, Grosso E, Chu F, et al. Prevalence of central compartment lymph node metastases in papillary thyroid micro-carcinoma: A retrospective evaluation of predictive preoperative features. Cancers (2021) 13(23):6028. doi: 10.3390/cancers13236028
5. Haddad RI, Bischoff L, Ball D, Bernet V, Blomain E, Busaidy NL, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20(8):925–51. doi: 10.6004/jnccn.2022.0040
6. Liu Z, Wang R, Zhou J, Zheng Y, Dong Y, Luo T, et al. Ultrasound lymphatic imaging for the diagnosis of metastatic central lymph nodes in papillary thyroid cancer. Eur Radiol (2021) 31(11):8458–67. doi: 10.1007/s00330-021-07958-y
7. Shu X, Tang L, Hu D, Wang Y, Yu P, Yang Z, et al. Prediction model of pathologic central lymph node negativity in cN0 papillary thyroid carcinoma. Front Oncol (2021) 11:727984. doi: 10.3389/fonc.2021.727984
8. Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, et al. Hashimoto thyroiditis: Part 1, sonographic analysis of the nodular form of hashimoto thyroiditis. AJR Am J Roentgenol (2010) 195(1):208–15. doi: 10.2214/AJR.09.2459
9. Anderson L, Middleton WD, Teefey SA, Reading CC, Langer JE, Desser T, et al. Hashimoto thyroiditis: Part 2, sonographic analysis of benign and malignant nodules in patients with diffuse hashimoto thyroiditis. AJR Am J Roentgenol (2010) 195(1):216–22. doi: 10.2214/AJR.09.3680
10. Shirley LA, Jones NB, Phay JE. The role of central neck lymph node dissection in the management of papillary thyroid cancer. Front Oncol (2017) 7:122. doi: 10.3389/fonc.2017.00122
11. Huang Y, Yin Y, Zhou W. Risk factors for central and lateral lymph node metastases in patients with papillary thyroid micro-carcinoma: Retrospective analysis on 484 cases. Front Endocrinol (2021) 12:640565. doi: 10.3389/fendo.2021.640565
12. Aliyev E, Ladra-González MJ, Sánchez-Ares M, Abdulkader-Nallib I, Piso-Neira M, Rodríguez-Carnero G, et al. Thyroid papillary microtumor. Am J Surg Pathol (2020) 44(9):1161–72. doi: 10.1097/PAS.0000000000001522
13. Gao R, Jia X, Liang Y, Fan K, Wang X, Wang Y, et al. Papillary thyroid micro carcinoma: The incidence of high-risk features and its prognostic implications. Front Endocrinol (2019) 10:74. doi: 10.3389/fendo.2019.00074
14. Fu GM, Wang ZH, Chen YB, Li CH, Zhang YJ, Li XJ, et al. Analysis of risk factors for lymph node metastases in elderly patients with papillary thyroid micro-carcinoma. Cancer Manag Res (2020) 12:7143–9. doi: 10.2147/CMAR.S248374
15. Health Commission Of The People's Republic Of China National. National guidelines for diagnosis and treatment of thyroid cancer 2022 in China (English version). Chin J Cancer Res (2022) 34(3). doi: 10.21147/j.issn.1000-9604.2022.03.01
16. Guidelines of Chinese Society of Clinical Oncology (CSCO). Differentiated thyroid Cancer(2021). J Cancer Control And Treat (2021) 34(12):1164–201. doi: 10.3969/j.issn.1674-0904.2021.12.013
17. De Napoli L, Matrone A, Favilla K, Piaggi P, Galleri D, Ambrosini CE, et al. Role of prophylactic central compartment lymph node dissection on the outcome of patients with papillary thyroid carcinoma and synchronous ipsilateral cervical lymph node metastases. Endocrine Pract (2020) 26(8):807–17. doi: 10.4158/EP-2019-0532
18. Mulla M, Schulte K. Central cervical lymph node metastases in papillary thyroid cancer: a systematic review of imaging-guided and prophylactic removal of the central compartment. Clin Endocrinol (2012) 76(1):131–6. doi: 10.1111/j.1365-2265.2011.04162.x
19. Xue T, Liu C, Liu JJ, Hao YH, Shi YP, Zhang XX, et al. Analysis of the relevance of the ultrasonographic features of papillary thyroid carcinoma and cervical lymph node metastasis on conventional and contrast-enhanced ultrasonography. Front Oncol (2021) 11:794399. doi: 10.3389/fonc.2021.794399
20. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and Male sex are predictors of Large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid (2017) 27(10):1285–90. doi: 10.1089/thy.2017.0250
21. Seifert R, Schäfers M, Heitplatz B, Kerschke L, Riemann B, Noto B. Minimal extrathyroidal extension in papillary thyroid microcarcinoma is an independent risk factor for relapse through lymph node and distant metastases. J Nucl Med (2021) 62(12):1702–9. doi: 10.2967/jnumed.121.261898
22. Zhang QY, Ye XP, Zhou Z, Zhu CF, Li R, Fang Y, et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in hashimoto's thyroiditis. Nat Commun (2022) 13(1):775. doi: 10.1038/s41467-022-28120-2
23. Ajjan RA, Weetman AP. The pathogenesis of hashimoto's thyroiditis: Further developments in our understanding. Horm Metab Res (2015) 47(10):702–10. doi: 10.1055/s-0035-1548832
24. Donangelo I, Walts AE, Bresee C, Braunstein GD. Lymphocytic thyroiditis is associated with increased number of benign cervical nodes and fewer central neck compartment metastatic lymph nodes in patients with differentiated thyroid cancer. Endocrine Pract (2016) 22(10):1192–8. doi: 10.4158/E151078.OR
25. Wen X, Wang B, Jin Q, Zhang W, Qiu M. Thyroid antibody status is associated with central lymph node metastases in papillary thyroid carcinoma patients with hashimoto’s thyroiditis. Ann Surg Oncol (2019) 26(6):1751–8. doi: 10.1245/s10434-019-07256-4
26. Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of coexistent hashimoto's thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (2018) 88(1):123–8. doi: 10.1111/cen.13475
27. Kim SJ, Lee SE, Kim YI, Nam-Goong IS, Jung HW, Kim ES. Papillary thyroid cancer with hashimoto's thyroiditis attenuates the tumour aggressiveness through the up-regulation of e-cadherin and TGF-beta expression. Clin Exp Med (2022). doi: 10.1007/s10238-022-00857-6
28. Jara SM, Carson KA, Pai SI, Agrawal N, Richmon JD, Prescott JD, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in north American patients with papillary thyroid carcinoma. Surgery (2013) 154(6):1272–1280, 1280-1282. doi: 10.1016/j.surg.2013.07.021
29. Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The effects of hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: A retrospective Chinese cohort study. Med (Baltimore) (2016) 95(6):e2674. doi: 10.1097/MD.0000000000002674
30. Konturek A, Barczynski M, Wierzchowski W, Stopa M, Nowak W. Coexistence of papillary thyroid cancer with hashimoto thyroiditis. Langenbecks Arch Surg (2013) 398(3):389–94. doi: 10.1007/s00423-012-1021-x
31. Chu Y, Wirth LJ, Farahani AA, Nosé V, Faquin WC, Dias-Santagata D, et al. Clinicopathologic features of kinase fusion-related thyroid carcinomas: An integrative analysis with molecular characterization. Modern Pathol (2020) 33(12):2458–72. doi: 10.1038/s41379-020-0638-5
32. Melo M, Gaspar DRA, Batista R, Vinagre J, Martins MJ, Costa G, et al. TERT, BRAF, and NRAS in primary thyroid cancer and metastatic disease. J Clin Endocrinol Metab (2017) 102(6):1898–907. doi: 10.1210/jc.2016-2785
33. Choi JE, Bae JS, Lim DJ, Jung SL, Jung CK. Atypical histiocytoid cells and multinucleated giant cells in fine-needle aspiration cytology of the thyroid predict lymph node metastasis of papillary thyroid carcinoma. Cancers (2019) 11(6):816. doi: 10.3390/cancers11060816
34. Zhan J, Zhang L, Yu Q, Li CL, Chen Y, Wang WP, et al. Prediction of cervical lymph node metastasis with contrast-enhanced ultrasound and association between presence of BRAFV600E and extrathyroidal extension in papillary thyroid carcinoma. Ther Adv Med Oncol (2020) 12:386355740. doi: 10.1177/1758835920942367
35. Liu S, Liu C, Zhao L, Wang K, Li S, Tian Y, et al. A prediction model incorporating the BRAFV600E protein status for determining the risk of cervical lateral lymph node metastasis in papillary thyroid cancer patients with central lymph node metastasis. Eur J Surg Oncol (2021) 47(11):2774–80. doi: 10.1016/j.ejso.2021.08.033
36. Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun (2013) 4:2185. doi: 10.1038/ncomms3185
37. Panebianco F, Nikitski AV, Nikiforova MN, Nikiforov YE. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med (2019) 8(13):5831–9. doi: 10.1002/cam4.2467
38. Landa I, Pozdeyev N, Korch C, Marlow LA, Smallridge RC, Copland JA, et al. Comprehensive genetic characterization of human thyroid cancer cell lines: A validated panel for preclinical studies. Clin Cancer Res (2019) 25(10):3141–51. doi: 10.1158/1078-0432.CCR-18-2953
39. Yang T, Huang L, Chen C, Luo H, Jiang Y. Comparison between clinicopathological characteristics, BRAF V600E and TERT promoter mutation of familial non-medullary thyroid carcinomas, and sporadic case. Front Oncol (2021) 11:616974. doi: 10.3389/fonc.2021.616974
40. Geng J, Liu Y, Guo Y, Wang H, Tai J, Jin Y, et al. Correlation between TERT C228T and clinic-pathological features in pediatric papillary thyroid carcinoma. Sci China Life Sci (2019) 62(12):1563–71. doi: 10.1007/s11427-018-9546-5
41. Yin DT, Yu K, Lu RQ, Li X, Xu J, Lei M, et al. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: A systematic review and meta-analysis. Clin Endocrinol (Oxf) (2016) 85(2):299–305. doi: 10.1111/cen.13017
42. Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: a systematic review and meta-analysis. Endocrine (2020) 67(1):44–57. doi: 10.1007/s12020-019-02117-2
43. Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene (2014) 33(42):4978–84. doi: 10.1038/onc.2013.446
44. Yoo SK, Song YS, Lee EK, Hwang J, Kim HH, Jung G, et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun (2019) 10(1):2764. doi: 10.1038/s41467-019-10680-5
45. Carneiro T, Bim LV, Buzatto VC, Galdeno V, Asprino PF, Lee EA, et al. Evidence of cooperation between hippo pathway and RAS mutation in thyroid carcinomas. Cancers (Basel) (2021) 13(10). doi: 10.3390/cancers13102306
46. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest (2016) 126(3):1052–66. doi: 10.1172/JCI85271
47. Hernandez-Prera JC, Valderrabano P, Creed JH, de la Iglesia JV, Slebos RJC, Centeno BA, et al. Molecular determinants of thyroid nodules with indeterminate cytology and RAS mutations. Thyroid (2021) 31(1):36–49. doi: 10.1089/thy.2019.0650
48. Angell TE. RAS-positive thyroid nodules. Curr Opin Endocrinol Diabetes Obes (2017) 24(5):372–6. doi: 10.1097/MED.0000000000000354
49. Marotta V, Bifulco M, Vitale M. Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer. Cancers (Basel) (2021) 13(15). doi: 10.3390/cancers13153785
50. Wang Y, Guan Q, Xiang J. Nomogram for predicting central lymph node metastasis in papillary thyroid microcarcinoma: A retrospective cohort study of 8668 patients. Int J Surg (2018) 55:98–102. doi: 10.1016/j.ijsu.2018.05.023
51. Luo QW, Gao S, Lv X, Li SJ, Wang BF, Han QQ, et al. A novel tool for predicting the risk of central lymph node metastasis in patients with papillary thyroid microcarcinoma: a retrospective cohort study. BMC Cancer (2022) 22(1):606. doi: 10.1186/s12885-022-09655-5
Keywords: papillary thyroid microcarcinoma, central lymph node metastasis, molecular pathology markers, nomogram, prediction model
Citation: Ma T, Wang L, Zhang X and Shi Y (2023) A clinical and molecular pathology prediction model for central lymph node metastasis in cN0 papillary thyroid microcarcinoma. Front. Endocrinol. 14:1075598. doi: 10.3389/fendo.2023.1075598
Received: 20 October 2022; Accepted: 23 January 2023;
Published: 02 February 2023.
Edited by:
Erivelto Martinho Volpi, Centro de referencia no ensino do diagnóstico por imagem (CETRUS), BrazilReviewed by:
Andrii Dinets, Taras Shevchenko National University of Kyiv, UkraineGloria Angélica González Villaseñor, Instituto Mexicano del Seguro Social, Mexico
Copyright © 2023 Ma, Wang, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Shi, anlzeWZAbWFpbC5qbm1jLmVkdS5jbg==
 Teng Ma
Teng Ma Lulu Wang3
Lulu Wang3