- 1Non-Communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 2School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3Western Sydney University, Translational Health Research Institute, Sydney, NSW, Australia
- 4Social Determinants of Health Research Center, Alborz University of Medical Sciences, Karaj, Iran
- 5School of Medicine, Faculty of Health, Deakin University, Geelong, VIC, Australia
- 6Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Background: Prevalence and subsequent conditions of childhood and adolescent obesity are increasing. It has been seen that obesity in youth is associated with adulthood cancer. This systematic review and meta-analysis aimed to determine the pooled association of childhood obesity with cancers in adulthood.
Methods: In this systematic review, international electronic databases such as Scopus, PubMed, Web of Science, and EMBASE were searched using relevant keywords until February 2022. All Cohort studies assessing the association of childhood and adolescent obesity (under 18 years old) with the incidence and mortality of all types of cancers were included. Two independent reviewers screened and carried out the quality assessment of included studies. Between-studies heterogeneity was assessed using the I squared and Cochran’s Q tests. Random/fixed-effect meta-analyses were used to pool the appropriate effect sizes (Hazard ratios (HR)).
Results: Overall, 46 studies were found to be relevant and were included in this study. Based on the random-effects model meta-analysis, childhood obesity increased the hazard of cancer incidence and mortality in adulthood by 33% (HR: 1.33, 95%CI (1.25, 1.41)) and by 28% (HR: 1.28, 95%CI (1.13, 1.42)), respectively. In the subgroups meta-analysis, the HR of childhood obesity and adulthood cancer incidence mortality in women was higher than in men (HR=1.39, 95%CI (1.25, 1.53) vs HR= 1.20, 95%CI (1.07, 1.32)) and (HR= 1.40, 95%CI (1.10, 1.69) vs HR=1.20, 95%CI (1.04, 1.36)) respectively.
Conclusion: This study found that obesity in childhood and adolescence is associated with a significant increase in the incidence and mortality of cancers in adulthood. Prevention of childhood obesity, in addition to its short-term beneficial effects, can reduce the burden of cancer in adulthood. The data sets of this study are present in the Tables of the current manuscript. Moreover this study was registered online in PROSPERO (registration code: CRD42022331958).
Systemic review registration: https://www.crd.york.ac.uk/Prospero/, identifier CRD42022331958.
Background
Obesity is a condition notorious for its associated morbidity and mortality (1); and plays a part in the prevalence of the leading causes of death and disability worldwide [e.g. Hypertension, diabetes, cancer and so on (1)]. The association between obesity and many of these conditions are well-studied and established; however, its association with cancer is intriguing and complex.
Cancer has resulted in millions of deaths and morbidities throughout the years, thus earning its place among the most significant causes of mortality and morbidities worldwide (2). Despite impressive breakthroughs in Cancer treatment in the past decades, which reduced various cancers’ morbidities and mortalities, it still is a major foe of human health (3). Hence, due to cancers’ resilience to treatment, researchers have also studied the genetics and epigenetics of cancer to prevent it. Although cancer genetic factors are primarily un-adjustable, some epigenetic factors are changeable, and interventions in environmental and epigenetic factors have been introduced to reduce cancer risk. Nevertheless, implementing these interventions requires a thorough knowledge of the epigenetic risk factors of cancer (4, 5).
Similar to epigenetic factors, obesity is a modifiable cancer risk factor (6). Numerous studies have shown that obesity is a significant risk factor for cancer incidence (7–10); moreover, some have stated that it has a protective effect against certain malignancies (11). To better illustrate this association, cohort studies have been designed to determine the association between obesity and cancer (12). Furthermore, preventive measures are being carried out; however, despite our efforts, within the past few decades, the prevalence of both obesity and cancer has been increasing dramatically (13, 14). This increasing trend has also been seen in children and adolescents (15–17). This fact is worrisome since cancer and obesity are associated, and obesity is a chronic condition that tends to persist (18); the longer it persists, the longer the obese individual is at risk of its associated conditions such as cancer (19). Hence obesity in children and adolescents is of particular importance. Nonetheless, despite this importance, few systematic reviews have evaluated the association between obesity in children and adolescents and cancer in adulthood; these studies either are based on a single malignancy incidence (20, 21), or the studies included adults in their analysis as well (22). Hence unlike previous studies, this study aimed to assess the association of obesity in children and adolescents with the incidence of various types of malignancies and malignancy-related mortality.
Methods
In conducting this study, we adopted the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Search strategy
A systematic search of the available literature was conducted across electronic databases (Scopus, PubMed, Web of Science, and EMBASE till the end of February 2022. One of the researchers conducted the search, and another researcher reviewed the results. The searched terms were “cancer”, “ malignancy”, “neoplasm”, “obesity” and their equivalent terms (based on MesH terms). The search strategy is presented in full within the supplementary Table 1.
Eligibility criteria and selection study
All Cohort studies with an English full text assessing the relationship between obesity in children and adolescents and cancers in adulthood were included in this study. All studies must have represented the targeted population in exposure (obesity in children and adolescents) and outcome (cancer incidence and/or mortality in adults). Studies must have appropriately determined cancer incidence and mortality and had proper documentation and measures regarding childhood and adolescent weight, height, and BMI. Studies without the aforementioned criteria were excluded (e.g. studies based on self-reported BMI values, etc.)
After duplicate removal with EndNote X9, two investigators independently assessed the titles and abstracts and, lastly, the full texts of the remaining articles. Moreover, the reference lists of the included studies were hand-searched to find other relevant studies. Discrepancies were referred to the third investigator for resolution.
Data extraction strategy
A pre-designed data extraction data sheet consisting of the first author’s name, year of the study, age range or mean sex, the number of participants, type of malignancy, obesity definition, and measure (qualitative or quantitative) was used. Hazard ratios (HR), Odds ratios (OR), or Risk ratio (RR) (otherwise known as relative risk) alongside their 95% confidence interval (CI) as the effect size and studies’ adjustments for potential confounders were recorded. Two researchers extracted the data, and possible discrepancies were referred to the third researcher for resolution.
Quality assessment (QA)
The Newcastle-Ottawa Scale for cohort studies was used for quality assessment. We scored the studies based on selection, outcome, and comparability with this seven-item scale. The total score, which is the sum of the scores of its items, ranges from 0 to 9. We categorized the studies based on their scores (below 5: unsatisfactory, 5-7: satisfactory, and 8-9: high-quality studies). Two investigators scored the studies independently, and discrepancies were referred to the third researcher for resolution.
Statistical analysis
The I squared, and Cochran’s Q tests were adapted to assess heterogeneity between studies. If the heterogeneity P-value was less than 0.1, a random-effect model (DerSimonian–Laird) was used to pool the effect sizes. Otherwise, a fixed model was adopted. Meta-analysis was performed for outcomes with at least two records with identical measurements. Only the HRs and RRs were combined and pooled in the Meta-analysis (23, 24).
Binary BMI measurement was considered as the highest to the normal measure based on the study reports (highest quartile or quantile or obese individuals to the normal or non-obese population). Continuous BMI measure was considered as a one unit change in BMI Z-score or standard deviation (SD). The HRs or RRs of studies with two and above identical measures were pooled, and then a singular HR or RR for that study was used in the meta-analysis.
Sub-group analysis was performed according to cancer types and sex. Egger’s test for each malignancy determined the level of publication bias. In cases where publication bias was present, trim fill analysis was performed to impute the missing data.
STATA (Stata Corporation, College Station, Texas, USA) version 17 was used for data analyses.
Results
Search results
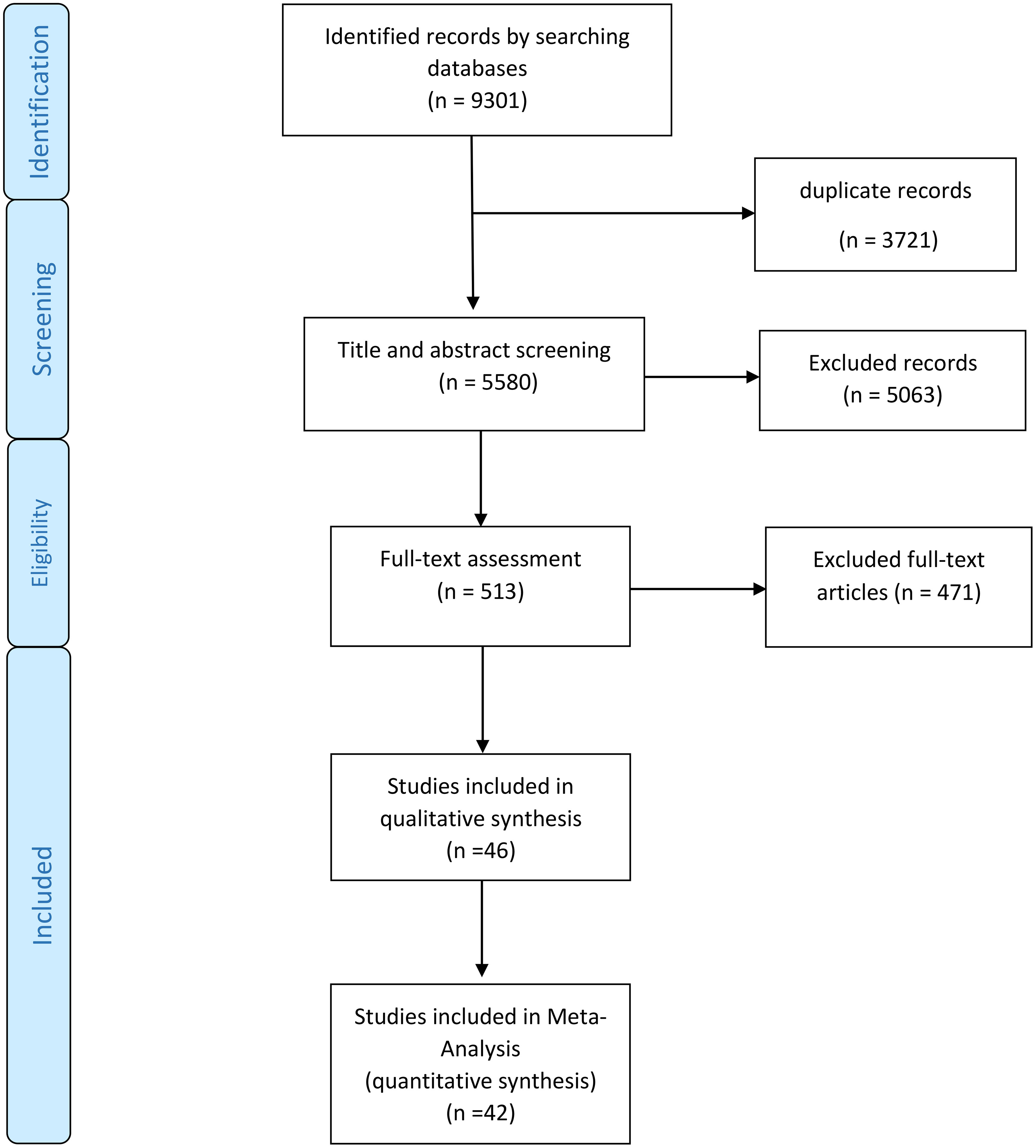
Of the initial 9301 studies found in the initial search (plus hand-searched studies), 3721 were duplicates, the titles or title and abstracts of the remaining 5580 articles were evaluated, and 5063 were found to be irrelevant to this study. The full texts of the 513 remained articles were assessed, and 471 were excluded. Lastly, 46 articles remained in the study and were used for qualitative, and 42 were included in the quantitative data synthesis. The entire illustration of this process can be seen in Figure 1.
The majority of the found studies were conducted in Europe (Denmark, Sweden, Norway, the United Kingdom (UK), and Finland). Other studied countries included Israel, the USA, and Australia. Denmark and Israel had the most studies (17 studies); USA, Finland and Australia had the least number of studies, with only one study assessing them all together.
The largest sample size was from Israel, with 19567635 participants. The smallest was from the UK, with an overall participant number of 6842. Of all the 46 studies,4 focused on cancer mortality/survival; the other 42 assessed cancer incidence. Moreover, out of these 42 articles, most studies assessed gastrointestinal (GI) related malignancies (18 studies) (Esophagus, Stomach, Liver, Pancreas, Colorectal). These data, alongside other primary findings, are shown in Supplementary Table 2. Regarding quality assessment, 4 of the included studies scored 7 (satisfactory), and the other 42 scored 8 or 9 (high quality); the scorings can be found in the Supplementary Table 3.
Qualitative synthesis
The association of childhood and adolescence obesity-related indices with cancers in adulthood in included studies is shown in Supplementary Table 4. Nearly half of the reported effect sizes are statistically significant. The reported HR ranges for the association of childhood and adolescent obesity and cancer incidence in adulthood based on cancer types were as follows (HR, (95%CI)): Prostate cancer: 1.02 (0.96-1.09) to 1.17 (0.97-1.41), endometrial cancer: 1.00 (0.78-1.27) to 1.39 (1.22-1.58), ovarian cancer: 0.75 (0.48-1.16) to 3.71 (1.63-8.46), Breast cancer: 0.69 (0.48-1.00) to 1.05 (0.95-1.14) in females and 2.01 (1.14-3.54) to 4.97 (2.14-11.53) in males, liver cancer: 1.15 (0.95-1.40) to 3.59 (1.85-6.99), renal cell carcinoma: 1.45 (0.63-3.34) to 2.87 (1.32-6.25), Testicular Cancer: 0.88 (0.46-1.67) to 1.29 (0.84-1.98), Hematologic malignancies: 0.89 (0.60-1.32) to 1.81 (1.13-2.92), colorectal cancer: 0.85 (0.50-1.44) to 2.62 (1.62-4.25), stomach cancer: 0.55 (0.24-1.24) to 2.91 (1.15-7.37), Esophageal cancer: 1.10 (0.94-1.29) to 3.11 (1.68-5.76), thyroid cancer: 0.99 (0.74-1.33) to 1.30 (1.08-1.55), pancreatic cancer: 0.47 (0.67-3.33) to 3.90 (1.70-8.92) and cancer mortality: 0.6 (0.3-1.4) to 2.1 (1.1-4.1). The reported effect sizes for other cancers, by sub-type (histology) of cancer, sex, age, etc., can be found in Supplementary Table 4
Quantitative synthesis
The pooled association between obesity in children and adolescents and the incidence and mortality of cancers in adulthood is shown in Table 1. Significant heterogeneity was observed among the included studies. Based on the random effect analysis, in binary (highest (in terms of BMI, obesity, etc.) to the reference group ratio) and continuous data, obesity significantly increased the hazard of all-cause cancer incidence and mortality in adulthood in comparison with the normal weight participants by 33% (HR: 1.33, 95%CI (1.25, 1.41)) and by 28% (HR: 1.28, 95%CI (1.13, 1.42)), respectively.
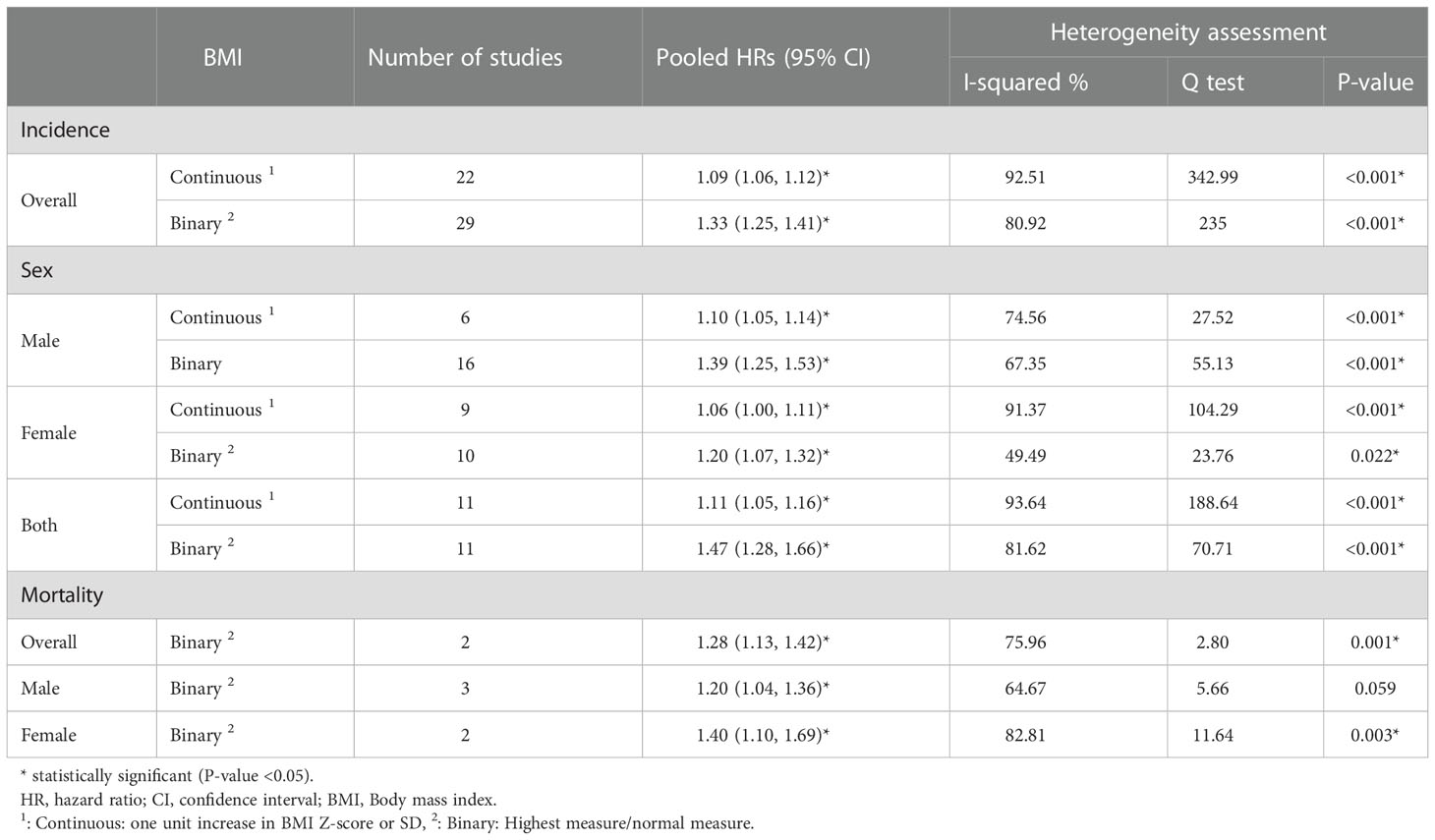
Table 1 Meta-analysis of the association between obesity –related indices in children and adolescents with the incidence and mortality of all- cause cancer in adulthood.
Sex stratified results among Women and men were (HR:1.20, 95%CI (1.07, 1.32), HR:1.39, 95%CI (1.25, 1.53)) for cancer incidence and (HR:1.40, 95%CI (1.10, 1.69), HR:1.20, 95%CI (1.04, 1.36)) for cancer mortality, respectively. Also, in the continuous data (per 1 Z-score/SD increase in BMI), the hazard of cancer incidence in obese individuals significantly increased by 9% (HR:1.09, 95%CI (1.06, 1.12)); Moreover, the hazard ratio based on continuous data in both men and women were statistically significant (HR:1.10, 95%CI (1.05, 1.14)) (HR:1.06, 95%CI (1.00, 1.13) respectively).
The pooled associations of BMI in the youth and cancers in adulthood sub-grouped by type of malignancy can be seen in Figure 2. The highest significant pooled hazard of cancer incidence by type in men was renal cell carcinoma (RCC) (HR: 2.50 95%CI (1.56, 3.44)) and in women was ovarian cancer (HR: 1.31, 95%CI (1.18, 1.43)). Also, in both sexes, the highest and lowest significant hazard of cancer incidence by type was observed in pancreatic (HR:1.96 95%CI (1.10,2.82) and colorectal cancer (HR:1.09 95%CI (1.05,1.13) respectively.
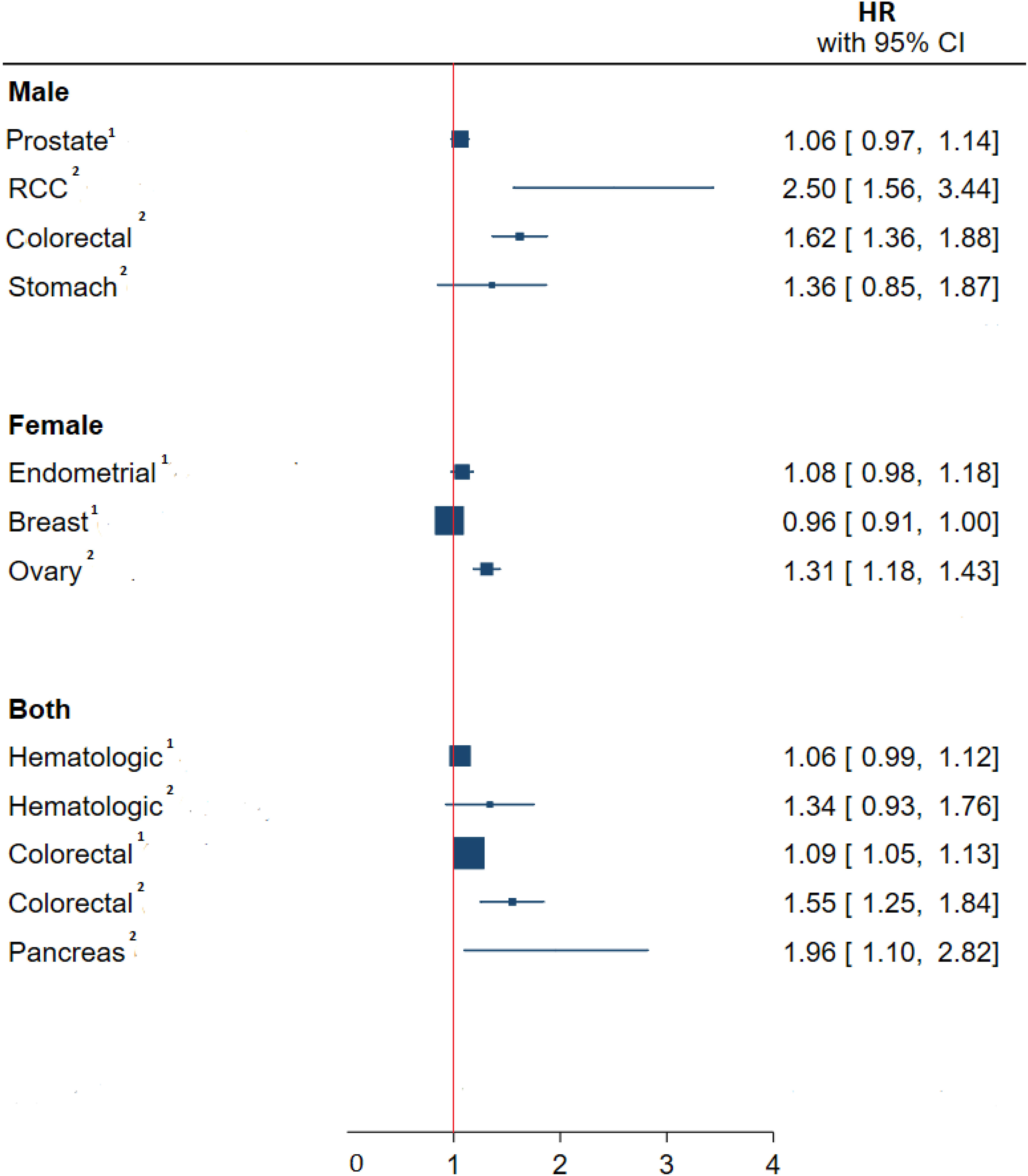
Figure 2 Meta-analysis of the association between obesity –related indices in children and adolescents with type of cancer in adulthood. HR, hazard ratio; CI, confidence interval; RCC, renal cell carcinoma. 1, Continuous, one unit increase in BMI Z-score or SD; 2, Binary, Highest measure/normal measure.
Meta regression
To assess the possible causes of heterogeneity among studies meta-regression was performed for the binary and continuous overall incidence of cancer with sex, type of cancer and the country in which the study was conducted in as moderators. It was found that neither of the aforementioned variables were the underlying cause of the heterogeneity seen among studies (the β (SE) for continuous and binary overall incidence of cancer for the aforementioned variables respectively are as follow. sex: 0.04 (0.03) and 0.06 (0.07), country of study conduction: 0.003 (0.01) and 0.02 (0.04), cancer type: -0.001 (0.005) and -0.001 (0.01))
Publication bias
Publication bias was assessed using Egger’s test and was present across the included studies in our analyses. However, after trim fill analysis, only cancer incidence based on continuous measure changed from (HR:1.09 95%CI (1.06, 1.12)) to (HR:1.26 95%CI (1.17, 1.34)), while other pooled HRs remained unchanged indicating that the meta-analysis results were not substantially affected by publication bias.
Discussion
This systematic review and meta-analysis showed a significant association between childhood and adolescent obesity and malignancies’ overall incidence and mortality in adulthood. Moreover, most cancer incidences, stratified by malignancy type, were directly and significantly associated with obesity under 18 years of age. These findings were in line with other studies as they also reported a significant association between cancer incidence and mortality with obesity in the youth (25, 26). Our findings were in line with other systematic reviews and meta-analyses studying specific malignancies, indicating that obesity in early life is associated with a greater risk of colorectal cancer in adulthood (RR:1.39, 95%CI (1.20, 1.62)) (20), higher risk of ovarian cancer that tends to increase with the increment of BMI (RR:1.19, 95% CI (1.11, 1.28) (21). It should be kept in mind that regardless of the association of obesity with the incidence and mortality of various cancers in adulthood, childhood obesity can persist into adulthood and adulthood obesity itself is an important risk factor for cancer and other morbidities (27). Furthermore, the overall pooled effect size of obesity on all cancer incidences should be interpret cautiously (Table 1), and despite the seen significant association, it is advised to consider the effect sizes sub grouped by type of cancer instead (Figure 2)
The exact mechanisms by which obesity in the youth affects cancer incidence and mortality in adulthood are not well understood. Some studies suggested the role of chronic inflammation and oxidative DNA damage, hormonal and cytokine dysregulation in obesity as possible underlying causes (25, 26). Indicating the need for studies evaluating possible underlying pathways by which obesity contributes to cancer incidence and mortality.
In a systematic review and meta-analysis similar to this study on those aged under 30, a significant association between obesity and colorectal, endometrial, ovarian, thyroid, and pancreas malignancies were found. Furthermore the aforementioned study found that pre and post-menopausal obesity reduces the risk of breast cancer by 12 and 17 percent respectively (RR:0.88 95%CI (0.81, 0.95) and RR:0.83 95%CI (0.77, 0.89)) (20); our findings were similar to the aforementioned study, however, in our study childhood and adolescent did not have a significant protective effect on breast cancer, probably due to the difference of the targeted population; other studies have also stated that obesity in the youth can be protective for adulthood breast cancer (28, 29). Nonetheless, some other studies argue that obesity can increase the risk and worsen the outcome of breast cancer (30, 31). Since many studies on this subject are retrospective cohorts, possible confounding variables such as predisposing genetic factors, nutritional indices, etc. could be the source of this controversy. Moreover, although height (which is also used in the calculation of BMI) has been associated with the incidence of various cancers in numerous studies (12, 32), all of the included studies adjusted their findings for height and BMI to address its potential confounding properties. Nonetheless, The BMI system has its flaws and inadequacies. As new definitions are offered to represent obesity, the inadequacies and weaknesses within the BMI system become more highlighted. Some types of obesity, such as normal weight obesity (NWO), despite having a normal weight and BMI, are at increased risk of cardiometabolic diseases due to their high-fat proportion (33). Individuals with these types of obesity might also have an increased risk of cancer incidence and mortality as well; however, studies have not addressed this issue yet, indicating the need for cohort studies assessing body fat proportion, metabolic obesity, etc.
Although understanding the pathways by which obesity increases the risk of cancer incidence and mortality is essential in fighting it; regardless of the underlying pathologic pathways, the outcome is quite clear and the fact is that many cohort studies have shown that obesity in the youth increases the risk of malignancies in adulthood (34–36). This is worrisome since the prevalence of obesity is increasing worldwide, especially among the young. Since obesity is a major cardiometabolic risk factor as well, with consideration of its long-lasting effects and contribution to the prevalence of cancers, this increase in the prevalence of obesity needs major attention and intervention (25).
This indicates that more thorough preventive measures must be taken to prevent the upcoming rise in cancer incidence. Moreover, the necessity of proper education cannot be ignored. Since not only education on obesity is needed, but in order to prevent it, we should educate people on how to get rid of obesity; as even now, many minors do suffer from obesity, and governments must take action to prevent and treat obese individuals, as soon as possible to prevent the conditions that arise from obesity.
Limitations and strengths
In this thorough systematic review and meta-analysis, only cohort studies with documented measurements for BMI were evaluated (37–69), their aggregated data were pooled, and a precise evaluation of the association between obesity indices in the youth and cancers (all-cause and type of cancer incidence and cancer mortality) in adulthood was drawn. However, there were some limitations. As can be seen, these studies were conducted in a handful of countries; hence the findings of this study may differ across other nations and races as many regions and many countries lacked proper cohort studies to evaluate this subject. Moreover, the number of studies on cancer subtypes was relatively low and more studies are needed to determine the association of obesity with various cancer subtypes. Lastly, many of these studies were retrospective cohorts based on registry data, without proper adjustment for confounding variables and such as predisposing genetic factors, nutritional status and socio-economical status.
Conclusion
This study showed a clear association between childhood and adolescent obesity and cancer incidence and mortality in adulthood. Obesity is associated with an increased prevalence of various types of malignancies and a higher death rate due to cancer in adulthood. With regard to these findings, we must increase our efforts to battle obesity, and interventions in childhood and youth need to be balanced with the need to tackle adult obesity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The Ethic Committee of Alborz university of Medical Sciences (ABZUMS) approved and all methods of the study were carried out by relevant guidelines and regulations.
Author contributions
NMK conceived the study, participated in study design, data collection (searching the databases, extraction of the data, and assessing the qualities of the manuscripts) and wrote the manuscript. MQ, SA and ES aided in data collection (searching the databases, extraction of the data, and assessing the qualities of the manuscripts), SM, LS, BHZ and AE helped in manuscript preparation. And interpreted the results. All authors contributed to the article and approved the submitted version.
Funding
Alborz university of Medical Sciences funded this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1069164/full#supplementary-material
References
1. Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Trans Med (2017) 5(7). doi: 10.21037/atm.2017.03.107
2. Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Global Health (2019) 9(4):217. doi: 10.2991/jegh.k.191008.001
3. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA: Cancer J Clin (2019) 69(5):363–85. doi: 10.3322/caac.21565
4. Dawson MA, Kouzarides T. Cancer epigenetics: from mechanism to therapy. Cell (2012) 150(1):12–27. doi: 10.1016/j.cell.2012.06.013
5. Virani S, Colacino JA, Kim JH, Rozek LS. Cancer epigenetics: a brief review. Ilar J (2012) 53(3-4):359–69. doi: 10.1093/ilar.53.3-4.359
6. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncol (2010) 15(6):556–65. doi: 10.1634/theoncologist.2009-0285
7. Celind J, Ohlsson C, Bygdell M, Martikainen J, Lewerin C, Kindblom JM. Childhood body mass index is associated with the risk of adult hematologic malignancies in men-the best gothenburg cohort. Int J Cancer (2020) 147(9):2355–62. doi: 10.1002/ijc.33015
8. Jensen BW, Meyle KD, Madsen K, Sørensen TIA, Baker JL. Early life body size in relation to risk of renal cell carcinoma in adulthood: a Danish observational cohort study. Eur J Epidemiol (2020) 35(3):251–8. doi: 10.1007/s10654-020-00605-8
9. Aarestrup J, Trabert B, Ulrich LG, Wentzensen N, Sørensen TIA, Baker JL. Childhood overweight, tallness, and growth increase risks of ovarian cancer. Cancer Epidemiol Biomarkers Prev (2019) 28(1):183–8. doi: 10.1158/1055-9965.EPI-18-0024
10. Landberg A, Fält A, Montgomery S, Sundqvist P, Fall K. Overweight and obesity during adolescence increases the risk of renal cell carcinoma. Int J Cancer (2019) 145(5):1232–7. doi: 10.1002/ijc.32147
11. Ahlgren M, Melbye M, Wohlfahrt J, Sørensen TI. Growth patterns and the risk of breast cancer in women. Int J Gynecol Cancer (2006) 16 Suppl 2:569–75. doi: 10.1111/j.1525-1438.2006.00698.x
12. Farfel A, Kark JD, Derazne E, Tzur D, Barchana M, Lazar L, et al. Predictors for thyroid carcinoma in Israel: a national cohort of 1,624,310 adolescents followed for up to 40 years. Thyroid (2014) 24(6):987–93. doi: 10.1089/thy.2013.0173
13. Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer (2013) 132(5):1133–45. doi: 10.1002/ijc.27711
14. Arroyo-Johnson C, Mincey KD. Obesity epidemiology worldwide. Gastroenterol Clin (2016) 45(4):571–9. doi: 10.1016/j.gtc.2016.07.012
15. Han JC, Lawlor DA, Kimm SYS. Childhood obesity. Lancet (2010) 375(9727):1737–48. doi: 10.1016/S0140-6736(10)60171-7
16. Kumar S, Kelly AS. Review of childhood obesity: From epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc (2017) 92(2):251–65. doi: 10.1016/j.mayocp.2016.09.017
17. Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev (2010) 36(4):277–85. doi: 10.1016/j.ctrv.2010.02.003
18. Lo JC, Chandra M, Sinaiko A, Daniels SR, Prineas RJ, Maring B, et al. Severe obesity in children: prevalence, persistence and relation to hypertension. Int J Pediatr Endocrinol (2014) 2014(1):3. doi: 10.1186/1687-9856-2014-3
19. Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, et al. Duration of adulthood overweight, obesity, and cancer risk in the women’s health initiative: a longitudinal study from the united states. PloS Med (2016) 13(8):e1002081. doi: 10.1371/journal.pmed.10020815
20. Garcia H, Song M. Early-life obesity and adulthood colorectal cancer risk: a meta-analysis. Rev Panam Salud Publica (2019) 43:e3. doi: 10.26633/RPSP.2019.3
21. Ding N, Zhan J, Shi Y, Qiao T, Li P, Zhang T. Obesity in children and adolescents and the risk of ovarian cancer: A systematic review and dose-response meta-analysis. PloS One (2022) 7(12):e0278050. doi: 10.1371/journal.pone.0278050
22. Hidayat K, Du X, Shi BM. Body fatness at a young age and risks of eight types of cancer: systematic review and meta-analysis of observational studies. Obes Rev (2018) 19(10):1385–94. doi: 10.1111/obr.12705
23. Stare J, Maucort-Boulch D. Odds ratio, hazard ratio and relative risk. Adv Method Statistics (2016) 13(1):59–67-59–67. doi: 10.51936/uwah2960
24. George A, Stead TS, Ganti L. What’s the risk: differentiating risk ratios, odds ratios, and hazard ratios? Cureus (2020) 12(8). doi: 10.7759/cureus.10047
25. Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism (2019) 92:147–52. doi: 10.1016/j.metabol.2018.12.001
26. Weihe P, Spielmann J, Kielstein H, Henning-Klusmann J, Weihrauch-Blüher S. Childhood obesity and cancer risk in adulthood. Curr Obes Rep (2020) 9(3):204–12. doi: 10.1007/s13679-020-00387-w
27. Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess (Winchester England) (2015) 19(43):1–336. doi: 10.3310/hta19430
28. De Stavola BL, dos Santos Silva I, McCormack V, Hardy RJ, Kuh DJ, Wadsworth ME. Childhood growth and breast cancer. Am J Epidemiol (2004) 159(7):671–82. doi: 10.1093/aje/kwh097
29. Keinan-Boker L, Levine H, Derazne E, Molina-Hazan V, Kark JD. Measured adolescent body mass index and adult breast cancer in a cohort of 951,480 women. Breast Cancer Res Treat (2016) 158(1):157–67. doi: 10.1007/s10549-016-3860-6
30. Andersen ZJ, Baker JL, Bihrmann K, Vejborg I, Sørensen TI, Lynge E. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res (2014) 16(1):R4. doi: 10.1186/bcr3596
31. Bjørge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol (2008) 168(1):30–7. doi: 10.1093/aje/kwn096
32. Green J, Cairns BJ, Casabonne D, Wright FL, Reeves G, Beral V, et al. Height and cancer incidence in the million women study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol (2011) 12(8):785–94. doi: 10.1016/S1470-2045(11)70154-1
33. Khonsari NM, Khashayar P, Shahrestanaki E, Kelishadi R, Nami SM, Heidari-Beni M, et al. Normal weight obesity and cardiometabolic risk factors: A systematic review and meta-analysis. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.857930
34. Furer A, Afek A, Sommer A, Keinan-Boker L, Derazne E, Levi Z, et al. Adolescent obesity and midlife cancer risk: a population-based cohort study of 2.3 million adolescents in Israel. Lancet Diabetes Endocrinol (2020) 8(3):216–25. doi: 10.1016/S2213-8587(20)30019-X
35. Leiba M, Leiba A, Keinan-Boker L, Avigdor A, Derazne E, Levine H, et al. Adolescent weight and height are predictors of specific non-Hodgkin lymphoma subtypes among a cohort of 2,352,988 individuals aged 16 to 19 years. Cancer (2016) 122(7):1068–77. doi: 10.1002/cncr.29792
36. Levi Z, Kark JD, Shamiss A, Derazne E, Tzur D, Keinan-Boker L, et al. Body mass index and socioeconomic status measured in adolescence, country of origin, and the incidence of gastroesophageal adenocarcinoma in a cohort of 1 million men. Cancer (2013) 119(23):4086–93. doi: 10.1002/cncr.28241
37. Aarestrup J, Gamborg M, Cook MB, Sørensen TI, Baker JL. Childhood body mass index and the risk of prostate cancer in adult men. Br J Cancer (2014) 111(1):207–12. doi: 10.1038/bjc.2014.266
38. Aarestrup J, Gamborg M, Tilling K, Ulrich LG, Sørensen TI, Baker JL. Childhood body mass index growth trajectories and endometrial cancer risk. Int J Cancer (2017) 140(2):310–5. doi: 10.1002/ijc.30464
39. Aarestrup J, Gamborg M, Ulrich LG, Sørensen TI, Baker JL. Childhood body mass index and height and risk of histologic subtypes of endometrial cancer. Int J Obes (Lond) (2016) 40(7):1096–102. doi: 10.1038/ijo.2016.56
40. Berentzen TL, Gamborg M, Holst C, Sørensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol (2014) 60(2):325–30. doi: 10.1016/j.jhep.2013.09.015
41. Bjørge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol (2004) 160(12):1168–76. doi: 10.1093/aje/kwh345
42. Bjørge T, Tretli S, Lie AK, Engeland A. The impact of height and body mass index on the risk of testicular cancer in 600,000 Norwegian men. Cancer Causes Control (2006) 17(7):983–7. doi: 10.1007/s10552-006-0032-8
43. Célind J, Ohlsson C, Bygdell M, Nethander M, Kindblom JM. Childhood body mass index is associated with risk of adult colon cancer in men: An association modulated by pubertal change in body mass index. Cancer Epidemiol Biomarkers Prev (2019) 28(5):974–9. doi: 10.1158/1055-9965.EPI-18-1077
44. Cook MB, Freedman ND, Gamborg M, Sørensen TI, Baker JL. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br J Cancer (2015) 112(3):601–7. doi: 10.1038/bjc.2014.646
45. Engeland A, Tretli S, Bjørge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. J Natl Cancer Inst (2003) 95(16):1244–8. doi: 10.1093/jnci/djg010
46. Hagström H, Tynelius P, Rasmussen F. High BMI in late adolescence predicts future severe liver disease and hepatocellular carcinoma: a national, population-based cohort study in 1.2 million men. Gut (2018) 67(8):1536–42. doi: 10.1136/gutjnl-2016-3136225
47. Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D. Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer (2004) 112(2):348–51. doi: 10.1002/ijc.20423
48. Jensen BW, Bjerregaard LG, Ängquist L, Gögenur I, Renehan AG, Osler M, et al. Change in weight status from childhood to early adulthood and late adulthood risk of colon cancer in men: a population-based cohort study. Int J Obes (Lond) (2018) 42(10):1797–803. doi: 10.1038/s41366-018-0109-y
49. Jensen BW, Gamborg M, Gögenur I, Renehan AG, Sørensen TIA, Baker JL. Childhood body mass index and height in relation to site-specific risks of colorectal cancers in adult life. Eur J Epidemiol (2017) 32(12):1097–106. doi: 10.1007/s10654-017-0289-0
50. Kantor ED, Udumyan R, Signorello LB, Giovannucci EL, Montgomery S, Fall K. Adolescent body mass index and erythrocyte sedimentation rate in relation to colorectal cancer risk. Gut (2016) 65(8):1289–95. doi: 10.1136/gutjnl-2014-309007
51. Katz LH, Levi Z, Twig G, Kark JD, Leiba A, Derazne E, et al. Risk factors associated with gastroenteropancreatic neuroendocrine tumors in a cohort of 2.3 million Israeli adolescents. Int J Cancer (2018) 143(8):1876–83. doi: 10.1002/ijc.31589
52. Keinan Boker L, Twig G, Klaitman-Meir V, Derazne E, Shina A, Levine H, et al. Adolescent characteristics and incidence of pre-malignant disease and invasive tumors of the cervix. Int J Gynecol Cancer (2020) 30(7):959–68. doi: 10.1136/ijgc-2019-000884
53. Keinan-Boker L, Levine H, Leiba A, Derazne E, Kark JD. Adolescent obesity and adult male breast cancer in a cohort of 1,382,093 men. Int J Cancer (2018) 142(5):910–8. doi: 10.1002/ijc.31121
54. Kitahara CM, Gamborg M, Berrington de González A, Sørensen TI, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res (2014) 74(1):235–42. doi: 10.1158/0008-5472.CAN-13-2228
55. Kitahara CM, Gamborg M, Rajaraman P, Sorensen TIA, Baker JL. A prospective study of height and body mass index in childhood, birth weight, and risk of adult glioma over 40 years of follow-up. Am J Epidemiol (2014) 180(8):821–9. doi: 10.1093/aje/kwu203
56. Leiba A, Duek A, Afek A, Derazne E, Leiba M. Obesity and related risk of myeloproliferative neoplasms among israeli adolescents. Obes (Silver Spring) (2017) 25(7):1187–90. doi: 10.1002/oby.21863
57. Leiba A, Kark JD, Afek A, Derazne E, Barchana M, Tzur D, et al. Adolescent obesity and paternal country of origin predict renal cell carcinoma: a cohort study of 1.1 million 16 to 19-year-old males. J Urol (2013) 189(1):25–9. doi: 10.1016/j.juro.2012.08.1845
58. Leiba A, Kark JD, Afek A, Levi Z, Barchana M, Tzur D, et al. Overweight in adolescence is related to increased risk of future urothelial cancer. Obes (Silver Spring) (2012) 20(12):2445–50. doi: 10.1038/oby.2012.83
59. Levi Z, Kark JD, Afek A, Derazne E, Tzur D, Furman M, et al. Measured body mass index in adolescence and the incidence of pancreatic cancer in a cohort of 720,000 Jewish men. Cancer Causes Control (2012) 23(2):371–8. doi: 10.1007/s10552-011-9886-5
60. Levi Z, Kark JD, Barchana M, Liphshitz I, Zavdy O, Tzur D, et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. cancer epidemiol biomarkers prev. Cancer Epidemiol Biomarkers Prev (2011) 20(12):2524–31. doi: 10.1158/1055-9965.EPI-11-0531
61. Levi Z, Kark JD, Katz LH, Twig G, Derazne E, Tzur D, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer (2017) 123(20):4022–30. doi: 10.1002/cncr.30819
62. Levi Z, Kark JD, Twig G, Katz L, Leiba A, Derazne E, et al. Body mass index at adolescence and risk of noncardia gastric cancer in a cohort of 1.79 million men and women. Cancer (2018) 124(2):356–63. doi: 10.1002/cncr.31049
63. Meyle KD, Gamborg M, Sørensen TIA, Baker JL. Childhood body size and the risk of malignant melanoma in adulthood. Am J Epidemiol (2017) 185(8):673–80. doi: 10.1093/aje/kww128
64. Nogueira L, Stolzenberg-Solomon R, Gamborg M, Sørensen TIA, Baker JL. Childhood body mass index and risk of adult pancreatic cancer. Curr Dev Nutr (2017) 1(10). doi: 10.3945/cdn.117.001362
65. Nuotio J, Laitinen TT, Sinaiko AR, Woo JG, Urbina EM, Jacobs DR Jr., et al. Obesity during childhood is associated with higher cancer mortality rate during adulthood: the i3C consortium. Int J Obes (Lond) (2021) 46(2):393–9. doi: 10.1038/s41366-021-01000-3
66. Petrick JL, Jensen BW, Sørensen TIA, Cook MB, Baker JL. Overweight patterns between childhood and early adulthood and esophageal and gastric cardia adenocarcinoma risk. Obes (Silver Spring) (2019) 27(9):1520–6. doi: 10.1002/oby.22570
67. Shamriz O, Leiba M, Levine H, Derazne E, Keinan-Boker L, Kark JD. Higher body mass index in 16-19 year-old Jewish adolescents of north African, middle Eastern and European origins is a predictor of acute myeloid leukemia: a cohort of 2.3 million israelis. Cancer Causes Control (2017) 28(4):331–9. doi: 10.1007/s10552-017-0863-5
68. Sørensen KK, Jensen BW, Thomas PE, Madsen K, Eriksson F, Aarestrup J, et al. Early life body size and its associations with adult bladder cancer. Ann Hum Biol (2020) 47(2):166–72. doi: 10.1080/03014460.2019.1707873
Keywords: cancer, childhood, adolescence, obesity, malignancy, adulthood
Citation: Mohammadian Khonsari N, Shahrestanaki E, Ehsani A, Asadi S, Sokoty L, Mohammadpoor Nami S M, Hakak-Zargar B and Qorbani M (2023) Association of childhood and adolescence obesity with incidence and mortality of adulthood cancers. A systematic review and meta-analysis. Front. Endocrinol. 14:1069164. doi: 10.3389/fendo.2023.1069164
Received: 13 October 2022; Accepted: 02 January 2023;
Published: 19 January 2023.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Alexis Llewellyn, University of York, United KingdomAgnieszka Zubkiewicz-Kucharska, Wroclaw Medical University, Poland
Copyright © 2023 Mohammadian Khonsari, Shahrestanaki, Ehsani, Asadi, Sokoty, Mohammadpoor Nami, Hakak-Zargar and Qorbani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mostafa Qorbani, TXFvcmJhbmkxMzc5QHlhaG9vLmNvbQ==; Nami Mohammadian Khonsari, TmFtaS5tLmtoQGdtYWlsLmNvbQ==
 Nami Mohammadian Khonsari
Nami Mohammadian Khonsari Ehsan Shahrestanaki
Ehsan Shahrestanaki Amir Ehsani
Amir Ehsani Sara Asadi3
Sara Asadi3 Leily Sokoty
Leily Sokoty Sahar Mohammadpoor Nami
Sahar Mohammadpoor Nami Benyamin Hakak-Zargar
Benyamin Hakak-Zargar Mostafa Qorbani
Mostafa Qorbani