- 1Department of Endocrinology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Department of Radiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
- 3Department of Urology, The Third Xiangya Hospital, Central South University, Changsha, Hunan, China
Introduction: This study aimed to explore the possible pathogenesis of a rare case of co-existing Cushing’s syndrome (CS) and primary aldosteronism (PA) caused by bilateral adrenocortical adenomas secreting aldosterone and cortisol, respectively.
Methods: A 41-year-old Chinese woman with severe hypertension and hypokalemia for 5 and 2 years, respectively, was referred to our hospital. She had a Cushingoid appearance. Preoperative endocrinological examinations revealed autonomous cortisol and aldosterone secretion. Computed tomography revealed bilateral adrenal adenomas. Subsequently, adrenal vein sampling and sequential left and right partial adrenalectomy indicated the presence of a left aldosterone-producing tumor and a right cortisol-producing tumor. Pathological examination included immunohistochemical analysis of the resected specimens. Secretions of aldosterone and cortisol were observed both in vivo and in vitro. Further, whole-exome sequencing was performed for DNA that was extracted from peripheral blood leukocytes and bilateral adrenal adenomas in order to determine whether the patient had relevant variants associated with PA and CS.
Results: Immunohistochemical staining revealed that the left adenoma primarily comprised clear cells expressing CYP11B2, whereas the right adenoma comprised both eosinophilic compact and clear cells expressing CYP11B1. The mRNA levels of steroidogenic enzymes (including CYP11B1 and CYP17A1) were high in the right adenoma, whereas CYP11B2 was highly expressed in the left adenoma. A novel somatic heterozygous missense variant—KCNJ5 c.503T > G (p.L168R)—was detected in the left adrenal adenoma, but no other causative variants associated with PA and CS were detected in the peripheral blood or right adrenocortical adenoma. In the primary cell culture of the resected hyperplastic adrenal adenomas, verapamil and nifedipine, which are two calcium channel blockers, markedly inhibited the secretion of both aldosterone and cortisol.
Conclusion: We present an extremely rare case of bilateral adrenocortical adenomas with distinct secretion of aldosterone and cortisol. The heterogeneity of the tumor cell compositions of aldosterone- and cortisol-producing adenoma (A/CPA) and somatic mutation of KCNJ5 may have led to different hormone secretions in the bilateral adrenal adenomas.
Introduction
Primary aldosteronism (PA) is considered the most common cause of secondary hypertension, and it has been reported to be present in at least 5%–10% of patients with hypertension (1). PA is caused by excessive production of aldosterone and inhibition of renin activity, resulting in hypertension and hypokalemic alkalosis. Moreover, PA is commonly caused by aldosterone-producing adenomas (APAs), idiopathic adrenal hyperplasia, or rare glucocorticoid-remediable aldosteronism. In 1977, Hogan et al. reported the first case of PA with significant cortisol auto-secretion, which was considered to be caused by an aldosterone- and cortisol-producing adenoma (A/CPA) (2). Currently, A/CPA is recognized as a subtype of PA because it is frequently detected when screening for PA. Recent studies have reported that the prevalence of subclinical Cushing’s syndrome (CS) may be high (21%–26.8%) in patients with APA (3, 4), and 5%–21% of adrenal tumors are A/CPA (3, 5, 6). As reported previously, PA associated with cortisol autonomous secretion can be classified into the following types: 1) a single ipsilateral adrenal adenoma secreting both cortisol and aldosterone simultaneously; 2) two adenomas on ipsilateral adrenal glands secreting aldosterone and cortisol separately; and 3) two adenomas, one on each side of the adrenal gland, secreting aldosterone and cortisol separately (3). Most patients had adenomas secreting both cortisol and aldosterone simultaneously, and the cases of bilateral adenomas secreting different hormones independently are extremely rare.
Over the past few years, somatic mutations have been reported to be associated with the development of A/CPA. To date, more than eight genes have been reported to be associated with APAs, including KCNJ5, CACNA1D, ATP1A1, ATP2B3, CACNA1H, CLCN2, CTNNB1, and/or GNAQ/11 (7, 8), representing >50% of sporadic APAs. Of these, mutations in KCNJ5 are the most common, with the KCNJ5 mutation rate in APA reportedly being approximately 40% in Western countries (9) and 60%–70% in Asian countries (10, 11). A recent study reported that among Chinese patients with APAs, the mutation rates of KCNJ5, ATP1A1, ATP2B3, and CACNA1D are 77%, 2%, 0.5%, and 0.5%, respectively Page: 4 (11).
Similarly, somatic gene mutations have been identified in approximately 50% of the cortisol-producing adenomas (CPAs). The affected genes include PRKACA, GNAS, PRKAR1A, and CTNNB1, with PRKACA being the most frequently mutated gene (12, 13). Furthermore, somatic KCNJ5 and PRKACA mutations have been found in patients with A/CPA (14, 15).
In this study, we aimed to explore the possible pathogenesis of a patient with bilateral adrenal adenomas secreting aldosterone and cortisol, respectively.
Materials and methods
Ethics statement
This study was approved by the Institutional Ethics Committee of the Third Xiangya Hospital. After obtaining written informed consent, we collected peripheral blood samples as well as resected adrenocortical adenomas from the patient.
Adrenal vein sampling (AVS)
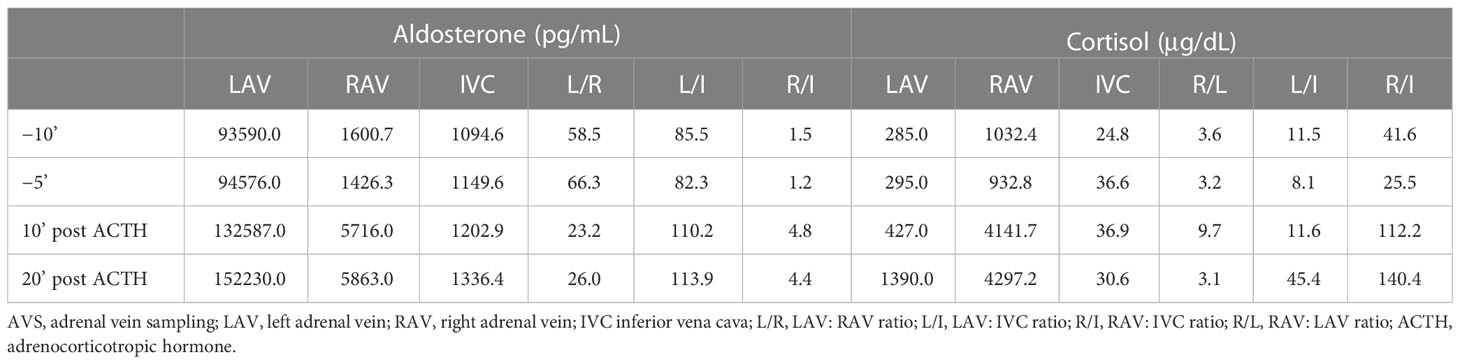
Bilaterally simultaneous AVS techniques with cosyntropin (synthetic adrenocorticotropic hormone 1–24) stimulation were performed as described in a previous study (16). The detailed steps were as follows: after successful placement of bilateral adrenal veins, blood samples were collected at baseline and at 10 minutes and 5 minutes before cosyntropin stimulation as well as at 10 minutes and 20 minutes after the stimulation. Overall, 125 μg of cosyntropin was intravenously injected; subsequently, 125 μg of cosyntropin was infused continuously for 1 hour. Further, cortisol and aldosterone levels were measured using the blood samples.
Whole-exome sequencing
To determine whether the patient had relevant gene variants associated with PA and CS, we performed whole-exome sequencing of DNA extracted from peripheral blood leukocytes and resected bilateral adrenocortical adenomas. The isolated DNA was sheared on a Bioruptor UCD-200 (Diagenode) with a size distribution peak of approximately 200 bp. The samples were diluted, loaded, and sequenced on the HiSeq2500 platform (Illumina, San Diego, CA). Further, exome data processing and variant annotation were performed as described in a previous study (17). The variants were interpreted according to the standards of the American College of Medical Genetics (ACMG) and categorized as follows: pathogenic, likely pathogenic, variants of uncertain significance, likely benign, or benign.
In silico analysis
The effects of single nucleotide variants were predicted using SIFT (http://sift.jcvi.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), MutationTaster (http://www.mutationtaster.org), and PROVEAN (http://provean.jcvi.org/index.php) programs. Further, KCNJ5 amino acids across different species were aligned using AlignX software (Invitrogen).
Immunohistochemical analysis
Immunohistochemical analysis was performed using an En Vision detection kit (Dako). The antibodies and dilutions were as follows: Ki-67 (1:500, OriGene), CYP11B1 (1:200, EMD Millipore), and CYP11B2 (1:500, EMD Millipore). Normal adrenal medulla was used as negative tissue control.
Primary cell culture of the adrenal tumors
The resected bilateral adrenal adenoma tissues (wet weight, 1 g) were washed twice with Dulbecco’s modified eagle medium (DMEM), cut into small pieces, and dispersed by incubation in DMEM containing 2% collagenase I for 20 minutes at 37°C. Primary cell culture of the adrenal adenomas was performed as described previously (18). On day 4, when the cells grew to 80% confluence, they were treated with verapamil (10 μM, Sigma-Aldrich) and nifedipine (10 μM, Sigma-Aldrich) or with the vehicle for 24 hours. The culture medium was collected for measuring aldosterone and cortisol, and the concentrations of aldosterone (Sinbe Diagnostic, China) and cortisol (Roche Diagnostics GmbH, Germany) in the medium were directly measured via chemiluminescence.
RNA isolation and real-time quantitative PCR
To clarify the expression of steroidogenic enzymes for the synthesis of aldosterone and cortisol in bilateral adrenal adenomas, we examined the mRNA levels of steroidogenic enzymes, including steroidogenic acute regulatory protein (STAR), 17α-hydroxylase (CYP17A1), 11β-hydroxylase (CYP11B1), and aldosterone synthase (CYP11B2), in the bilateral adrenal adenomas and other adrenal adenomas, including
five APAs, five CPAs, and three nonfunctional adenomas. Further, the cells and tissues were lysed using TRIzol Reagent (Sigma-Aldrich), and total RNA was isolated in accordance with the standard protocol provided by the manufacturer. The cDNA was synthesized from 1 μg of total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems). PCR amplification for the expression of STAR, CYP17A1, CYP11B1, and CYP11B2 was conducted using the LightCycler 480 SYBR Green I Master (Roche Applied Science) on a LightCycler 480 PCR system. Moreover, relative gene expression levels were determined with the cycle threshold value and were normalized against the expression of the human glyceraldehyde-3-phosphate de-hydrogenase (GAPDH) gene. The primers were used in accordance with a previous study (18).
Statistical analysis
The data were presented as mean ± standard deviation for primary cell culture and qPCR tests. Two groups were compared using the Student t-test, and multiple groups were analyzed by one-way analysis of variance (GraphPad Prism, La Jolla, CA, USA). P < 0.05 was considered statistically significant.
Results
Clinical characteristics
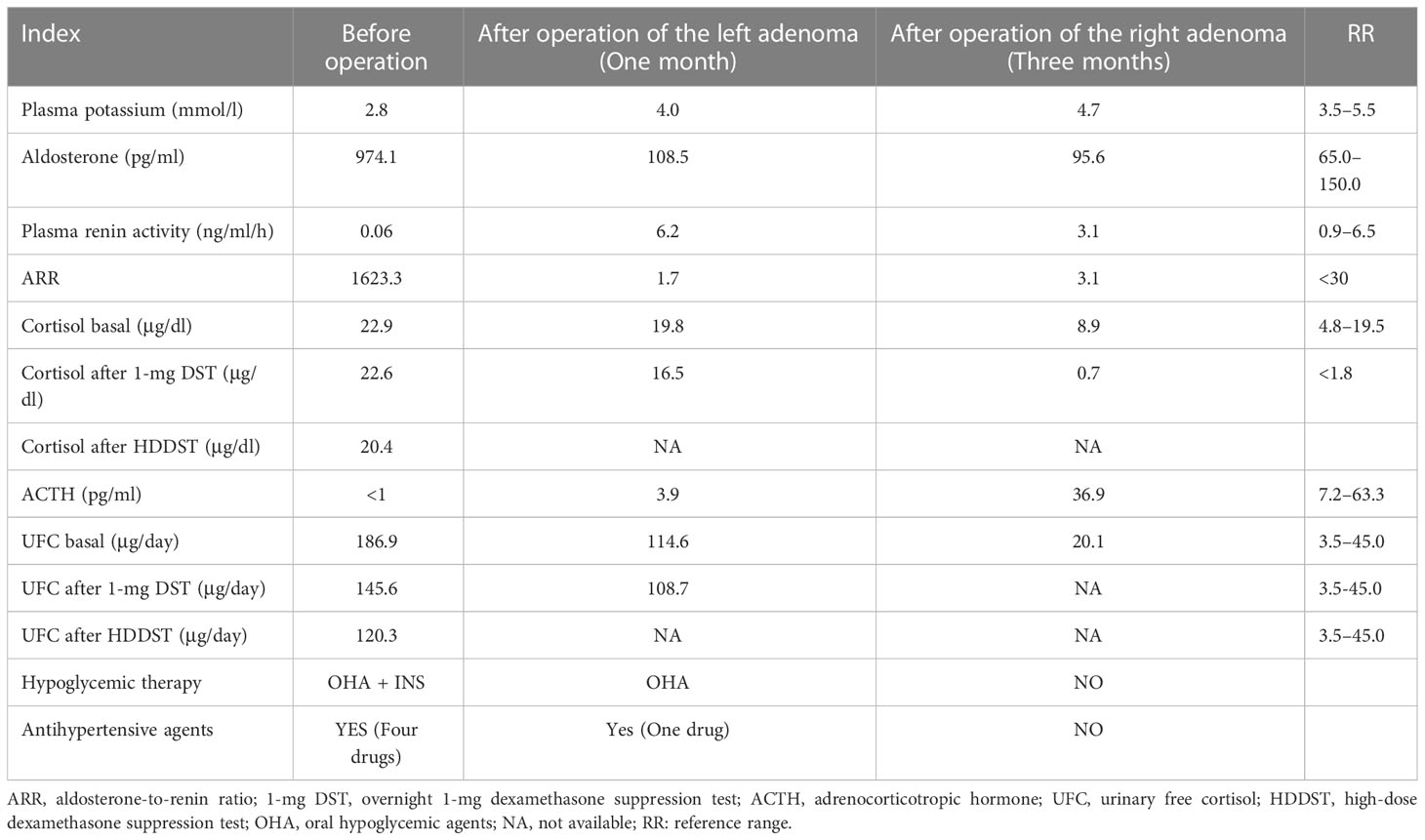
A 41-year-old Chinese woman with severe hypertension and hypokalemia for 5 and 2 years, respectively, was referred to our hospital. She was diagnosed with hypertension 5 years ago and was administered telmisartan (40 mg qd) and amlodipine besylate (5 mg qd), but her blood pressure remained poorly controlled. Two years ago, she was diagnosed with hypokalemia (2.1 mmol/L) because of fatigue and was administered potassium intermittently. She was also diagnosed with new-onset diabetes during that time, but she was not taking any medication. Ten days ago, she was referred to our hospital because of severe shortness of breath after experiencing cold. Based on her physical examination, her blood pressure (BP) was 187/130 mmHg, height was 148 cm, weight was 64 kg, and body mass index was 29.2 kg/cm2. Notably, she had a Cushingoid appearance with a moon face, a buffalo hump, and centripetal obesity; however, no purplish abdominal striae were found. Laboratory examination at admission revealed marked hypokalemia with a high urinary potassium level (80.25 mmol/24 h). Moreover, she had elevated levels of aldosterone, suppressed renin activity, and a significantly high aldosterone-to-renin ratio (ARR) of 1623.3 (Table 1). Furthermore, the captopril challenge test failed to suppress aldosterone secretion. Additionally, she presented with elevated levels of cortisol and 24-hour urinary free cortisol (UFC), loss of normal diurnal rhythm, and suppressed levels of adrenocorticotropic hormone (ACTH) (Table 1). Moreover, serum cortisol remained unsuppressed after overnight 1-mg dexamethasone suppression test (DST) and high-dose (8 mg) dexamethasone suppression test (Table 1). Based on these results, the patient was diagnosed with co-existing ACTH-independent CS and primary aldosteronism. Moreover, serum catecholamine, metanephrine, methoxynorepinephrine, follicle-stimulating hormone, luteinizing hormone, estradiol, testosterone, and dehydroepiandrosterone sulfate levels were normal. Furthermore, glycated hemoglobin (HbA1c) level was 7.7%, creatinine level was 113 μmol/L (RR, 41–85 μmol/L), urinary microalbumin-to-creatinine ratio was 590.7 mg/g (RR, 0–30 mg/g), and brain natriuretic peptide level was 6850.8 pg/ml (RR, 0–500 pg/ml). ECG findings indicated sinus tachycardia with left ventricular high voltage. Chest X-ray revealed an enlarged heart, and the cardiothoracic ratio was 0.74. Fundus examination demonstrated 1:2 arteriovenous pressure traces and a small amount of fundus exudates, indicating changes in the fundus of patients with hypertension. Further, the results of echocardiography indicated a large left atrium and thickened left ventricular wall; moreover, the percentages of ejection fraction and fractional shortening were 50% and 32%, respectively. Computerized tomography scan revealed bilateral adrenocortical adenomas, with the left adenoma of 38 × 28 mm and the right adenoma of 25 × 21 mm (Figure 1A).
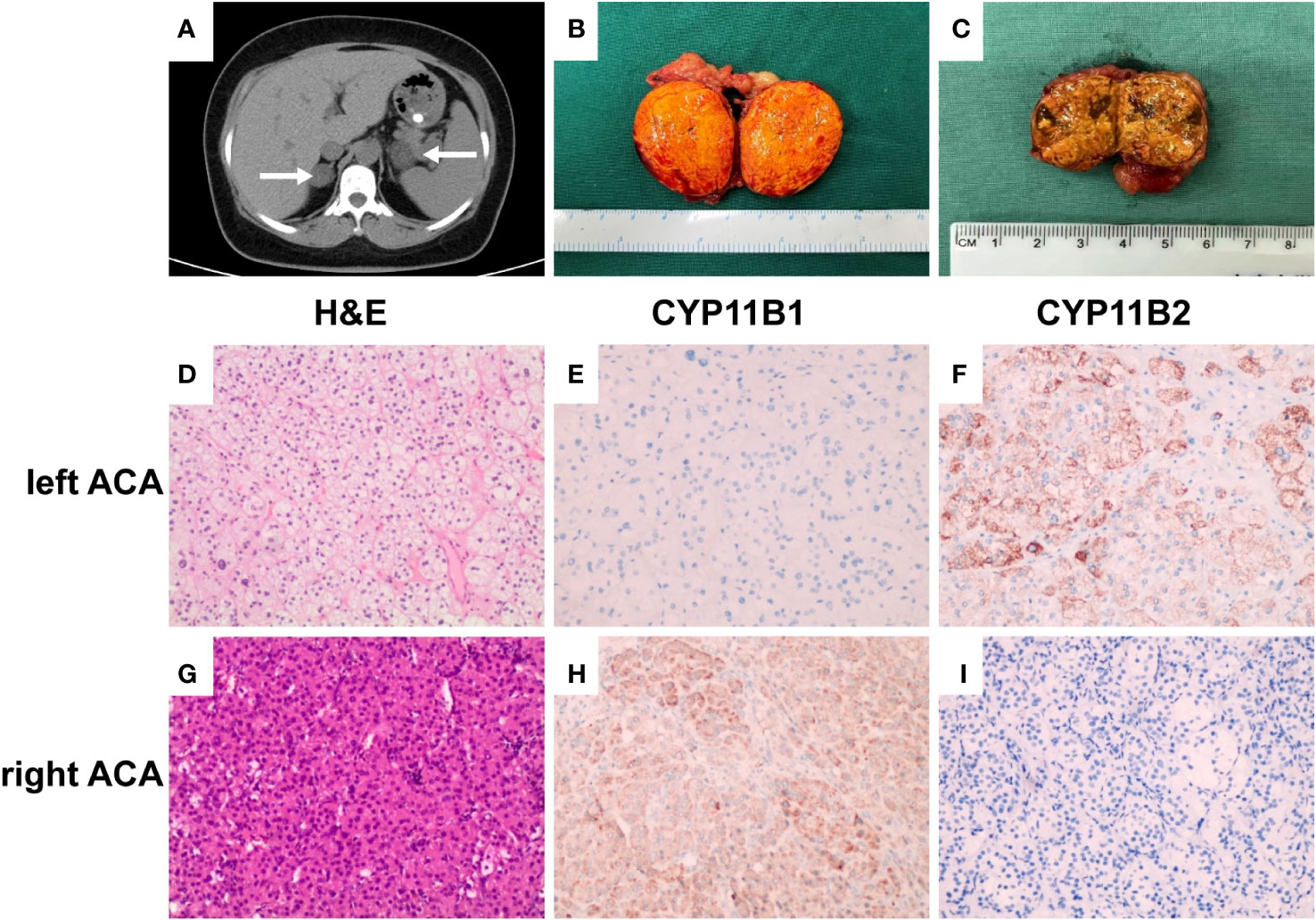
Figure 1 Enhanced computerized tomography (CT), pathological and immunohistochemical findings. (A) CT scan revealed bilateral adrenocortical adenomas, with the left adenoma of 38 × 28 mm and the right adenoma of 25 × 21 mm (white arrows). (B) Gross pathology of the resected left adrenal tumor showing a bright yellow well-circumscribed mass arising from the cortex. (C) Gross pathology of the resected right adrenal tumor showing a brownish yellow, relatively solid area. (D, G) H&E stain results, (×200); (E, F, H, I) immunostaining (×200). Left adrenocortical adenoma (left ACA, predominately secreted aldosterone) consisting primarily of clear cells, CYP11B1 (−) and CYP11B2 (+). Right adrenocortical adenoma (right ACA, predominately secreting cortisol) was composed of both eosinophilic compact cells and clear cells, with the former accounting for 60%, CYP11B1 (+) and CYP11B2 (−).
Bilateral AVS revealed that the aldosterone concentration in the left adrenal vein (LAV) was remarkably higher than that in the right adrenal vein (RAV) and the inferior vena cava (IVC), indicating aldosterone overproduction from the left side. Moreover, the cortisol concentration in RAV was 3.1–9.7 times higher than that in the LAV, indicating cortisol overproduction in the left side (Table 2). Furthermore, significant adrenal-to-IVC gradient of cortisol enabled successful catheterization on both sides.
Treatment and follow-up
The patient was treated with antihypertensive agents (spironolactone 100 mg qd, nifedipine 60 mg qd, metoprolol 47.5 mg qd, prazosin 2 mg q8h, and sacubitril valsartan sodium 50 mg bid) and hypoglycemic therapy (insulin degludec and insulin aspart injection 16 IU qd, linagliptin 5 mg qd, and acarbose 50 mg tid). As the left adenoma was larger than the right adenoma and there was a possibility of hypersecretion of aldosterone from the left tumor, the patient underwent left laparoscopic adrenalectomy. One month after the left partial adrenalectomy, her BP decreased significantly, and she only needed to take 5 mg amlodipine daily to maintain BP at 130–140/90 mmHg. Meanwhile, she stopped receiving insulin therapy and took only one oral hypoglycemic agent. Further, her aldosterone level significantly decreased, renin activity increased, and ARR and serum potassium levels normalized (Table 1). However, serum cortisol and 24-hour UFC still exceeded the normal range. Further, serum cortisol remained unsuppressed after 1-mg DST, indicating that cortisol over-secretion from the right adrenal adenoma persisted. Subsequently, she underwent right laparoscopic adrenalectomy. One month after the second surgery, her BP, blood glucose level, and serum potassium level were found to be normalized without any medication. Replacement therapy with prednisone (5 mg daily) was provided to avoid adrenal insufficiency. Three months later, the patient stopped taking prednisone, her BP and blood glucose levels remained normal, cortisol and ACTH levels returned to the normal ranges, and her condition could be suppressed with a low-dose DST (Table 1).
Immunohistochemical analysis
Pathological examination revealed that the left adrenal mass was a golden-yellow adenoma (Figure 1B) mainly composed of clear cells on hematoxylin/eosin stained sections (Figure 1D). Immunohistochemical analysis revealed cytoplasm immunoreactivity for aldosterone synthase (CYP11B2) with no expression of 11beta-hydroxylase 1 (CYP11B1; Figures 1E, F). Pathological examination revealed that the right adrenal tumor was brownish yellow and relatively solid (Figure 1C). This mass was composed of both eosinophilic compact and clear cells, with the former accounting for 60% (Figure 1G). Immunohistochemical analysis also revealed cytoplasm immunoreactivity for CYP11B1 with no expression of CYP11B2 (Figures 1H, I). These results confirmed that the patient had a co-existing left aldosterone-producing adenoma and right cortisol-producing adrenal adenoma, which was consistent with the results of AVS.
mRNA expression of STAR, CYP17A1, CYP11B1, and CYP11B2
The mRNA level of the steroidogenic enzyme STAR was high in both adenomas. CYP11B1 and CYP17A1 were highly expressed in the right adenoma and CPAs, whereas CYP11B2 was highly expressed in the left adenoma and APAs compared with other adrenal diseases and nonfunctional adenoma (Figure 2).
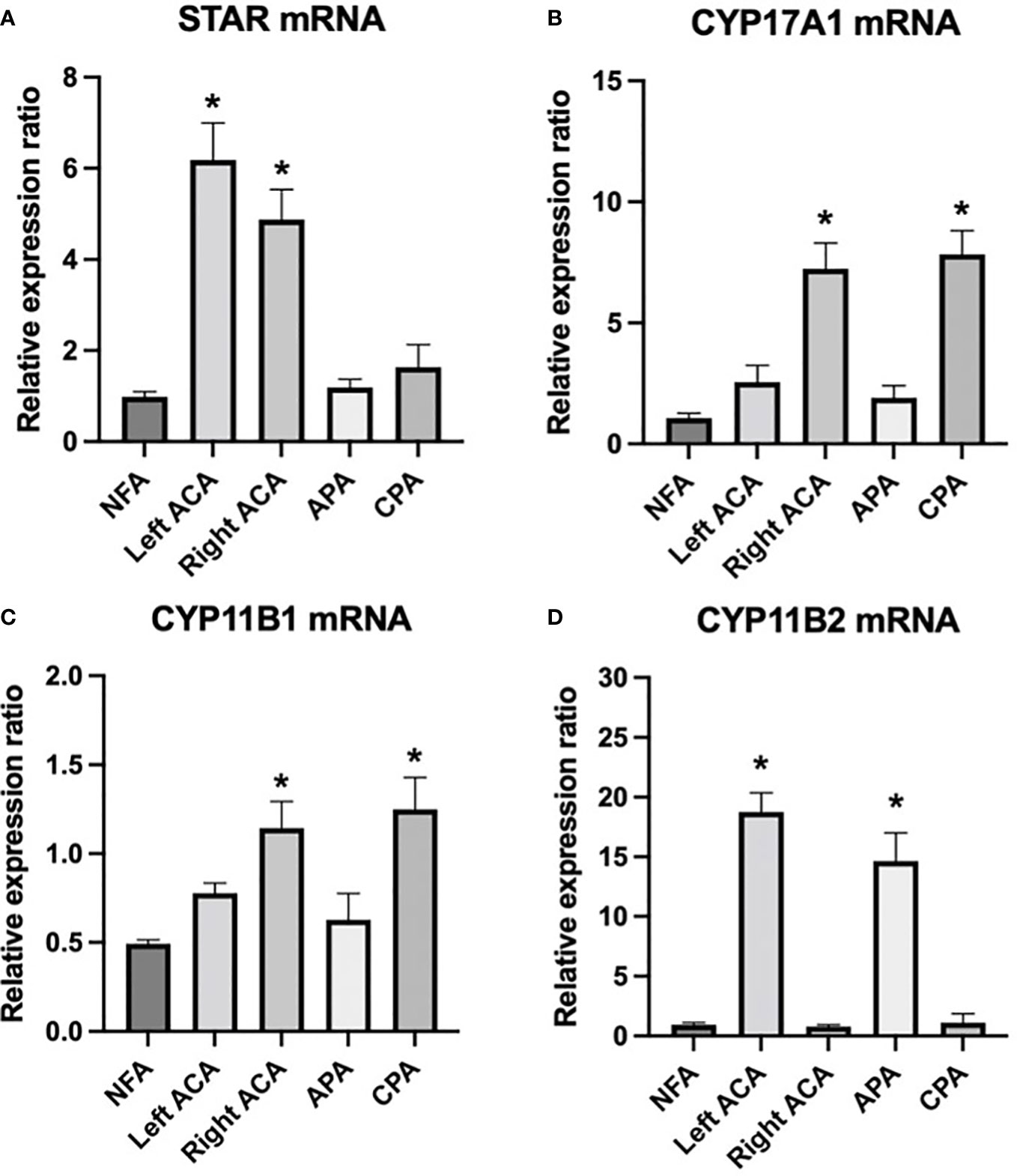
Figure 2 mRNA expression of steroidogenic enzymes STAR, CYP17A1, CYP11B1, and CYP11B2 in the bilateral adrenal adenomas of the patient and other adrenal adenomas. (A) STAR was highly expressed in both bilateral adenomas, (B, C) CYP11B1 and CYP17A1 were highly expressed in right adenoma and CPAs, (D) CYP11B2 was highly expressed in the left adenoma and APAs compared with other adrenal diseases and nonfunctional adenoma. * P < 0.05 compared with other groups. NFA, nonfunctional adenomas; left ACA, left adrenocortical adenoma of the index patient; right ACA, right adrenocortical adenoma of the index patient; APA, aldosterone-producing adenomas; CPA, cortisol-producing adenomas.
Calcium channel involved in the secretion of aldosterone and cortisol
The bilateral adenoma cells were cultured in vitro, and the calcium channel blockers nifedipine and verapamil were administered as an intervention (classified into three groups: control, nifedipine, and verapamil groups). We further detected the concentrations of aldosterone and cortisol in the supernatant of the cultured cells. Aldosterone secreted by cultured cells of the left adenoma was significantly higher than that secreted by the cells of the right side. In contrast, cortisol secreted by the cultured cells of the right adenoma was significantly higher than that secreted by the cells of the left side. Moreover, verapamil and nifedipine remarkably inhibited the secretion of bilateral aldosterone and cortisol (Figure 3).
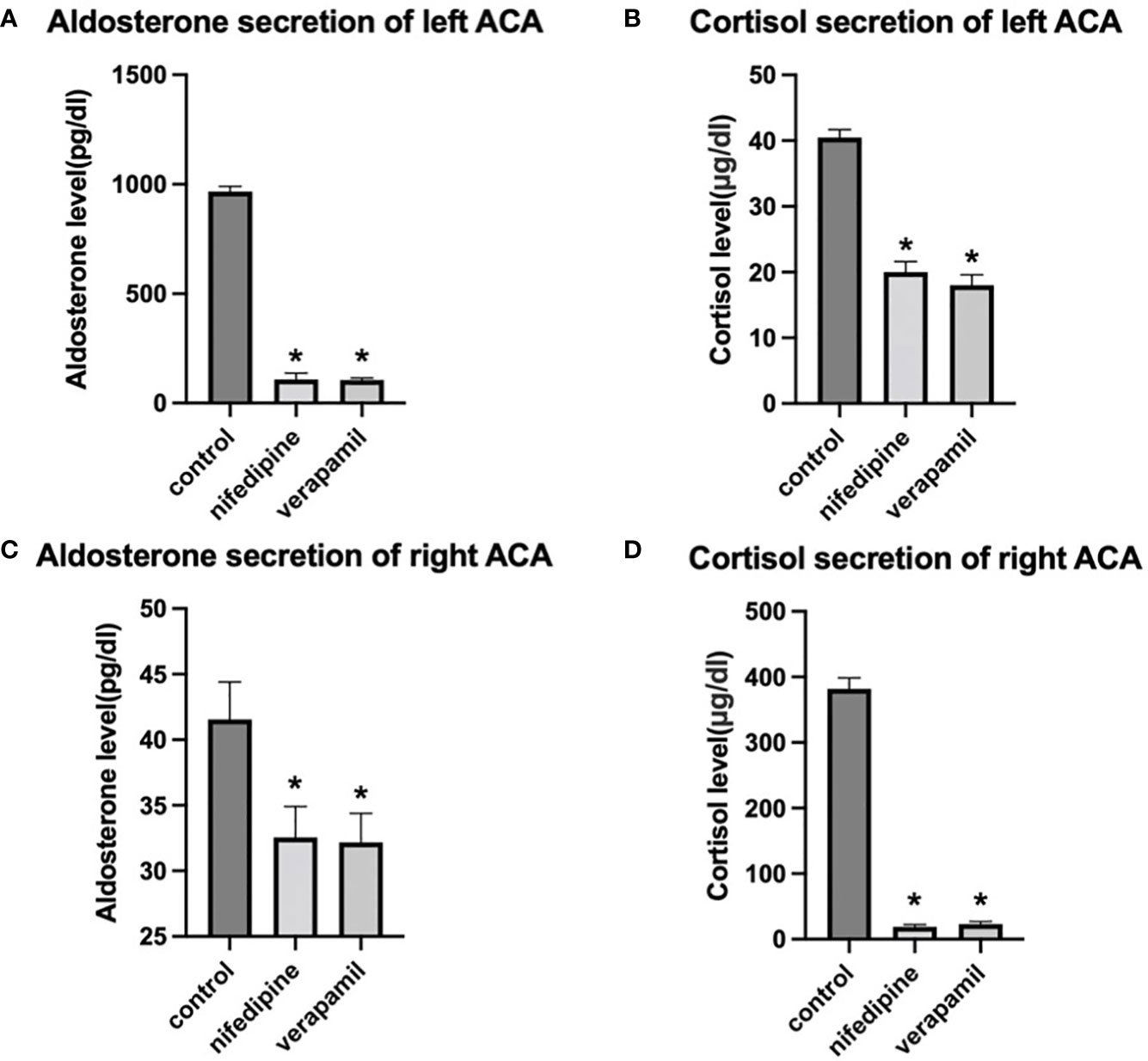
Figure 3 Effects of nifedipine and verapamil on aldosterone and cortisol secretions, respectively, in the primary cells of bilateral adenomas. (A, B) Effects of verapamil and nifedipine on the secretion of aldosterone and cortisol in the left adenoma. (C, D). Effects of verapamil and nifedipine on the secretion of aldosterone and cortisol in right adenoma. * P < 0.05 compared with the control. left ACA, left adrenocortical adenoma of the index patient; right ACA, right adrenocortical adenoma of the index patient.
Germline and somatic variant analysis
A novel somatic heterozygous missense variant, KCNJ5 c.503T > G (p.L168R), was detected in the left adrenal adenoma, whereas no germline and somatic variants associated with PA and CS were found in the peripheral blood samples or right adrenocortical adenoma (Figures 4A–C). The amino acid alignment of KCNJ5 across different species revealed that the leucine residue mutated in our case was conserved across all examined species (Figure 4D). According to the ACMG guidelines, the KCNJ5 c.503T > G variant is considered pathogenic because: 1) this variant was found in an extremely low frequency (PM2), 2) it was predicted to be damaging and disease-causing by SIFT, PolyPhen-2, and MutationTaster (PP3), and 3) it was consistent with the patient’s phenotype (PP4).
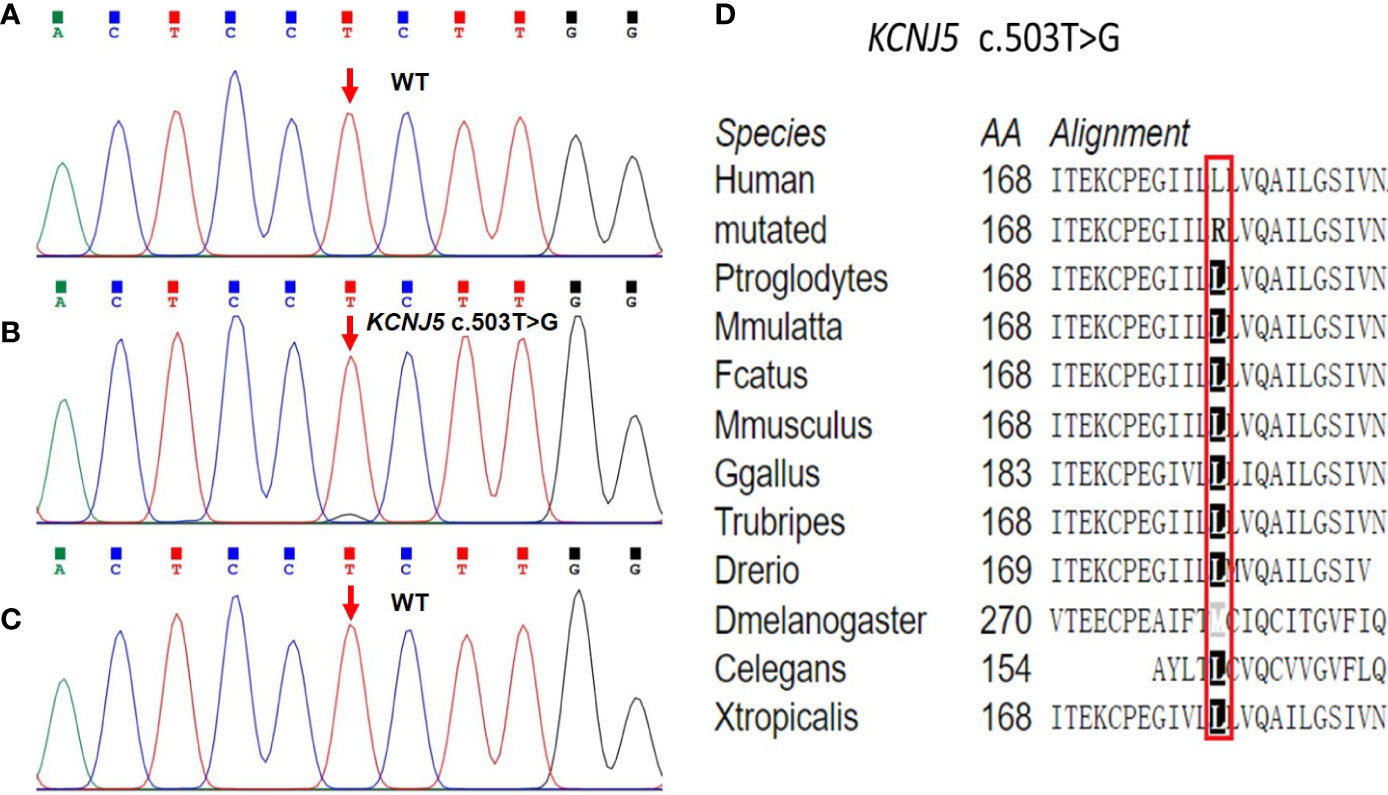
Figure 4 Sequencing chromatograms of the KCNJ5 variant identified in peripheral blood samples and bilateral adenomas. (A) The germline KCNJ5 variant was not found in peripheral blood samples. (B) Heterozygous KCNJ5 c.503T > G somatic variant was identified in the left adenoma. (C) Normal sequence of the KCNJ5 gene in the right adenoma. (D) Multiple alignment of the KCNJ5 protein sequence in different species, indicating conservation of the residues KCNJ5 c.503T > G affected by this variant.
Discussion
PAs are rarely associated with subclinical or clinical CS. Spath et al. reviewed the clinical features of 34 patients with A/CPA and reported that the average age of participants was 52 years and more than two-thirds of the participants were females (19). The majority of patients with A/CPA presented with severe hypertension and electrolyte disturbances, 14% of A/CPA patients presented with typical symptoms of hypercortisolism, and 74% presented with preclinical CS. Among the 34 A/CPA cases, 29 had a single adenoma and only 5 had multiple lesions. In our study, the female patient had co-existing PA and clinical Cushing syndrome as well as bilateral adrenocortical adenomas of >2 cm. She developed diabetes mellitus and multiple target organ damage (including cardiomegaly, heart failure, proteinuria, and impaired renal function). This finding was consistent with that of previous reports on patients with A/CPA presenting with an increased risk for cardiovascular events, glucose intolerance, and postoperative adrenal insufficiency (19, 20). Given the adverse metabolic risk in A/CPA, the presence of an aldosterone and cortisol co-secreting adrenocortical tumor should be considered if a patient has PA and an adenoma of >2 cm or cortisol that is non-suppressible with overnight 1-mg DST.
The majority of the reported A/CPA tumors secreted aldosterone and cortisol simultaneously in the same tumor. Here we report an extremely rare case of bilateral adrenocortical adenomas secreting cortisol and aldosterone from each side. To date, only six cases of bilateral adenomas with different functions have been reported (Table 3) (21–26). Moreover, it is still challenging to detect the lateralization of A/CPA. Currently, the AVS lateralization index (aldosterone/cortisol of bilateral adrenal vein) has been widely used for the lateralizing functioning of primary aldosteronism. However, the secretion of cortisol is uneven in A/CPA tumors, which may lead to false-negative aldosterone-to-cortisol ratios. Thus, epinephrine levels in bilateral adrenal vein and peripheral vein were recommended to be used as the denominator for correcting aldosterone from the predominant side. Among the six reported cases of bilateral functioning adenomas (Table 3), only two cases underwent testing for epinephrine for correction in AVS. The remaining four cases did not undergo testing for epinephrine during AVS and were further confirmed by postoperative clinical manifestations, changes in hormone levels, immunohistochemical staining for steroidogenic enzymes, etc. The limitation of our study was the absence of epinephrine correction in interpretation the results of AVS. Nevertheless, bilateral adenomas with different function were confirmed by subsequent immunohistochemical analysis and in vitro experiments after the sequential removal of adrenal adenomas. Therefore, further studies with larger samples are required for more accurate localization.
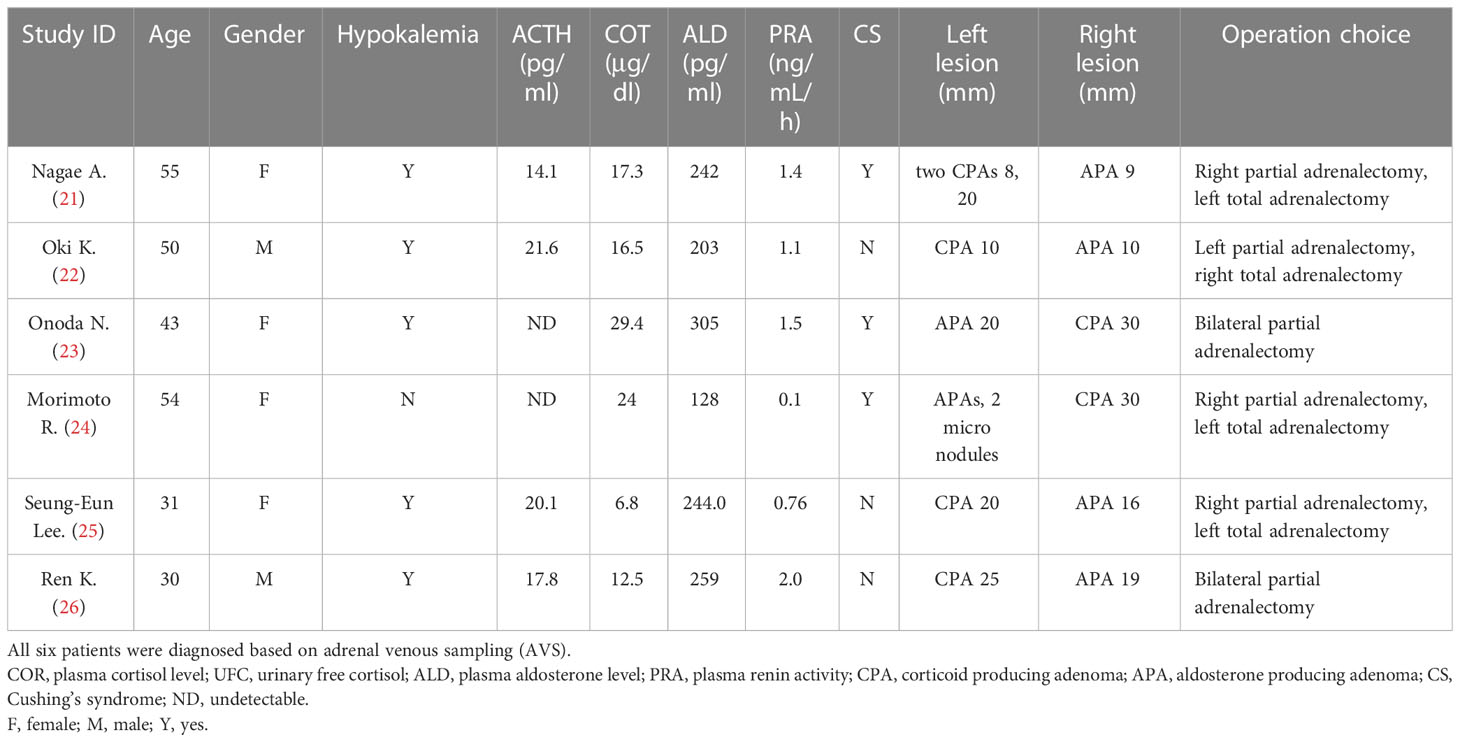
Table 3 Clinical features of case reports published with bilateral adrenocortical adenomas secreting cortisol or aldosterone independently.
The exact mechanisms of tumorigenesis in A/CPA remain to be fully elucidated. As reported previously, APAs composed of pure adrenal cortical zonal cells are very rare, and most APAs are composed of different cell types. Thus, APA tumors have the potential to co-secrete aldosterone and cortisol. Previous studies have reported the capacity of APA cells to produce cortisol (27, 28). Spath et al. reported two different morphological cell types in A/CPA tumors: zona glomerulosa-like cells and zona fasciculate-like cells. Immunohistochemical analysis indicated that A/CPA tumors could express CYP11B1 and CYP11B2—the key enzymes for synthesizing aldosterone and cortisol—simultaneously, but the composition ratio of these two cells and the expression of the two synthases were different in addition to the presence of marked heterogeneity in the tumors (19). In our case, immunohistochemical analysis and mRNA levels of steroidogenic enzymes revealed a left adenoma that primarily consisted of clear cells and mainly expressed CYP11B2, whereas the right adenoma was composed of both eosinophilic compact and clear cells and mainly expressed CYP11B1. The different cell composition of the bilateral adrenocortical adenomas may be an important reason for the secretion of different hormones in each adrenal gland.
A recent Chinese study reported that the KCNJ5 mutation was detected in 9 of 11 A/CPA cases, and PRKACA gene mutation was detected in two A/CPA cases. Another study detected 17 samples harboring KCNJ5 mutations among the 22 patients with A/CPA, but PRKACA, CACNA1D, CACNA1H, ATP1A1, or ATP2B3 mutations were not detected (5). Thus, the authors of that study suggested that A/CPA was more similar to APA than CPA (5, 29). In our case, we identified a novel somatic KCNJ5 c.503T > G mutation in the left adrenocortical adenoma. However, no germline and somatic variants associated with PA and CS were found in the peripheral blood samples or right adrenal adenoma. This observation may also explain significantly higher aldosterone levels in the left adrenal adenoma than those in the right adrenal adenoma.
Elevated cytoplasmic Ca2+ concentrations have been reported to be critical in the pathogenesis of APA with a mutant KCNJ5, and Ca2+ channel blockers can reduce aldosterone secretion in H295R cells expressing mutant KCNJ5 (30, 31). Meanwhile, blockers of voltage-gated Ca2+ channels can inhibit ACTH-stimulated Ca2+ influx and consequent cortisol secretion (32). In the present study, bilateral adenoma cells were cultured in vitro, and the L-type Ca2+ channels blockers nifedipine and verapamil were administered for intervention. We found that both verapamil and nifedipine reduced the secretion of bilateral aldosterone and cortisol, suggesting that hypersecretion of aldosterone and cortisol in our patient was mediated by voltage-gated Ca2+ channels.
Conclusions
We report an extremely rare case of bilateral adrenocortical adenomas with distinct secretion of aldosterone and cortisol, as confirmed by clinical findings and pathological studies. The heterogeneity of the tumor cell compositions of A/CPAs and somatic mutation of KCNJ5 may have led to different hormone secretions in the bilateral adrenal adenomas.
Data availability statement
The data presented in the study are deposited in the BioProject repository, and the BioProject ID is PRJNA923486.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of Third Xiangya Hospital, Central South University, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Study concepts were prepared by PJ. The study was designed by PJ and LZ. Data acquisition was performed by LZ, JJW, and YW. Statistical analysis was done by LZ and WY. Data analysis and interpretation was done by LZ and PJ. AVS was performed by QL. Bilateral partial adrenal resection was performed by JRW. The manuscript was prepared and edited by LZ and PJ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81670730), Natural Science Foundation of Hunan Province (2021JJ31007), Research project of Hunan Health Committee (202103031081).
Acknowledgments
We are grateful to the patient included in this study for her invaluable participation and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1068335/full#supplementary-material
References
1. Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population-based studies–a review of the current literature. Horm Metab Res (2012) 44(3):157–62. doi: 10.1055/s-0031-1295438
2. Hogan MJ, Schambelan M, Biglieri EG. Concurrent hypercortisolism and hypermineralocorticoidism. Am J Med (1977) 62(5):777–82. doi: 10.1016/0002-9343(77)90883-X
3. Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, et al. Clinicopathological features of primary aldosteronism associated with subclinical cushing's syndrome. Endocr J (2011) 58(7):543–51. doi: 10.1507/endocrj.K10E-402
4. Peng KY, Liao HW, Chan CK, Lin WC, Yang SY, Tsai YC, et al. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension (2020) 76(5):1537–44. doi: 10.1161/HYPERTENSIONAHA.120.15328
5. Tang L, Li X, Wang B, Ma X, Li H, Gao Y, et al. Clinical characteristics of aldosterone- and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int J Endocrinol (2018) 2018:4920841. doi: 10.1155/2018/4920841
6. Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, et al. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin Endocrinol (Oxf). (2009) 71(6):772–8. doi: 10.1111/j.1365-2265.2009.03551.x
7. Scholl UI. Genetics of primary aldosteronism. Hypertension (2022) 79(5):887–97. doi: 10.1161/HYPERTENSIONAHA.121.16498
8. Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Somatic and inherited mutations in primary aldosteronism. J Mol Endocrinol (2017) 59(1):R47–r63. doi: 10.1530/JME-17-0035
9. Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension (2014) 64(2):354–61. doi: 10.1161/HYPERTENSIONAHA.114.03419
10. Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, et al. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab (2012) 97(4):1311–9. doi: 10.1210/jc.2011-2885
11. Zheng FF, Zhu LM, Nie AF, Li XY, Lin JR, Zhang K, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension (2015) 65(3):622–8. doi: 10.1161/HYPERTENSIONAHA.114.03346
12. Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, et al. Activating hotspot L205R mutation in PRKACA and adrenal cushing's syndrome. Science (2014) 344(6186):913–7. doi: 10.1126/science.1249480
13. Hernández-Ramírez LC, Stratakis CA. Genetics of cushing's syndrome. Endocrinol Metab Clin North Am (2018) 47(2):275–97. doi: 10.1016/j.ecl.2018.02.007
14. Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, et al. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr J (2012) 59(8):735–41. doi: 10.1507/endocrj.EJ12-0247
15. Nanba K, Omata K, Tomlins SA, Giordano TJ, Hammer GD, Rainey WE, et al. Double adrenocortical adenomas harboring independent KCNJ5 and PRKACA somatic mutations. Eur J Endocrinol (2016) 175(2):K1–6. doi: 10.1530/EJE-16-0262
16. Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension (2014) 63(1):151–60. doi: 10.1161/HYPERTENSIONAHA.113.02097
17. Hu WM, Zhang Q, Huang LH, Mo ZH, Long XD, Yang YB, et al. Identification of novel variants in MEN1: A study conducted with four multiple endocrine neoplasia type 1 patients. Horm Metab Res (2020) 52(11):788–95. doi: 10.1055/a-1147-1375
18. Tong A, Liu G, Wang F, Jiang J, Yan Z, Zhang D, et al. A novel phenotype of familial hyperaldosteronism type III: Concurrence of aldosteronism and cushing's syndrome. J Clin Endocrinol Metab (2016) 101(11):4290–7. doi: 10.1210/jc.2016-1504
19. Späth M, Korovkin S, Antke C, Anlauf M, Willenberg HS. Aldosterone- and cortisol-co-secreting adrenal tumors: The lost subtype of primary aldosteronism. Eur J Endocrinol (2011) 164(4):447–55. doi: 10.1530/EJE-10-1070
20. Akehi Y, Yanase T, Motonaga R, Umakoshi H, Tsuiki M, Takeda Y, et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: A Large, multicenter cohort study in Japan. Diabetes Care (2019) 42(5):938–45. doi: 10.2337/dc18-1293
21. Nagae A, Murakami E, Hiwada K, Kubota O, Takada Y, Ohmori T. Primary aldosteronism with cortisol overproduction from bilateral multiple adrenal adenomas. Jpn J Med (1991) 30(1):26–31. doi: 10.2169/internalmedicine1962.30.26
22. Oki K, Yamane K, Sakashita Y, Kamei N, Watanabe H, Toyota N, et al. Primary aldosteronism and hypercortisolism due to bilateral functioning adrenocortical adenomas. Clin Exp Nephrol. (2008) 12(5):382–7. doi: 10.1007/s10157-008-0064-3
23. Onoda N, Ishikawa T, Nishio K, Tahara H, Inaba M, Wakasa K, et al. Cushing's syndrome by left adrenocortical adenoma synchronously associated with primary aldosteronism by right adrenocortical adenoma: Report of a case. Endocr J (2009) 56(3):495–502. doi: 10.1507/endocrj.K08E-268
24. Morimoto R, Kudo M, Murakami O, Takase K, Ishidoya S, Nakamura Y, et al. Difficult-to-control hypertension due to bilateral aldosterone-producing adrenocortical microadenomas associated with a cortisol-producing adrenal macroadenoma. J Hum Hypertens (2011) 25(2):114–21. doi: 10.1038/jhh.2010.35
25. Lee SE, Kim JH, Lee YB, Seok H, Shin IS, Eun YH, et al. Bilateral adrenocortical masses producing aldosterone and cortisol independently. Endocrinol Metab (Seoul). (2015) 30(4):607–13. doi: 10.3803/EnM.2015.30.4.607
26. Ren K, Wei J, Liu Q, Zhu Y, Wu N, Tang Y, et al. Hypercortisolism and primary aldosteronism caused by bilateral adrenocortical adenomas: a case report. BMC Endocr Disord (2019) 19(1):63. doi: 10.1186/s12902-019-0395-y
27. Stowasser M, Tunny TJ, Klemm SA, Gordon RD. Cortisol production by aldosterone-producing adenomas in vitro. Clin Exp Pharmacol Physiol (1993) 20(5):292–5. doi: 10.1111/j.1440-1681.1993.tb01686.x
28. Rácz K, Fehér J, Csomós G, Varga I, Kiss R, Gláz E. An antioxidant drug, silibinin, modulates steroid secretion in human pathological adrenocortical cells. J Endocrinol (1990) 124(2):341–5. doi: 10.1677/joe.0.1240341
29. Rhayem Y, Perez-Rivas LG, Dietz A, Bathon K, Gebhard C, Riester A, et al. PRKACA somatic mutations are rare findings in aldosterone-producing adenomas. J Clin Endocrinol Metab (2016) 101(8):3010–7. doi: 10.1210/jc.2016-1700
30. Monticone S, Bandulik S, Stindl J, Zilbermint M, Dedov I, Mulatero P, et al. A case of severe hyperaldosteronism caused by a de novo mutation affecting a critical salt bridge Kir3. 4 residue. J Clin Endocrinol Metab (2015) 100(1):E114–8. doi: 10.1210/jc.2014-3636
31. Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology (2014) 155(4):1353–62. doi: 10.1210/en.2013-1944
Keywords: primary aldosteronism, Cushing’s syndrome, KCNJ5 gene, adrenal vein sampling, CYP11B1, CYP11B2, aldosterone- and cortisol-producing adenoma
Citation: Zhao L, Wan J, Wang Y, Yang W, Liang Q, Wang J and Jin P (2023) Different cell compositions and a novel somatic KCNJ5 variant found in a patient with bilateral adrenocortical adenomas secreting aldosterone and cortisol. Front. Endocrinol. 14:1068335. doi: 10.3389/fendo.2023.1068335
Received: 12 October 2022; Accepted: 22 February 2023;
Published: 07 March 2023.
Edited by:
Alfredo Scillitani, Home for Relief of Suffering (IRCCS), ItalyReviewed by:
Maniselvan Kuppusamy, University of Virginia, United StatesFukang Sun, Shanghai Jiao Tong University, China
Juilee Rege, University of Michigan, United States
Copyright © 2023 Zhao, Wan, Wang, Yang, Liang, Wang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Jin, cGluZy5qaW4wNkBjc3UuZWR1LmNu
 Liling Zhao1
Liling Zhao1 Jinjing Wan
Jinjing Wan Qi Liang
Qi Liang Ping Jin
Ping Jin