
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 21 August 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1066922
This article is part of the Research TopicEndometrial Thickness as a Risk Factor for Pregnancy ComplicationsView all 6 articles
Background: The relationship between endometrial thickness and pregnancy safety after in vitro fertilization treatment is an important topic that should provoke attention. The aim of this study was to demonstrate the relationship between endometrial thickness on day of embryo transfer and early pregnancy complications, including ectopic pregnancy and early miscarriage, in frozen thawed embryo transfer (FET) cycles.
Methods: Patients undergoing their first FET cycles were included into this study from January 2010 to December 2021. Patients were divided into three groups according to endometrial thickness on day of embryo transfer: Thin, ≤ 7 mm; Medium, 7-14 mm; Thick, ≥ 14 mm. Ectopic pregnancy and early miscarriage were the two primary outcomes. Endometrial thickness was the main measured variable. The risk factors of these two compilations were determined based on univariate analysis and multivariate logistic regression analysis.
Results: A total of 11138 clinical pregnancies were included. The overall ectopic pregnancy and early spontaneous miscarriage rates were 2.62% and 13.40%. The ectopic pregnancy and early spontaneous miscarriage rates were significantly higher in patients with thin endometrium as compared with those in the other two groups (ectopic pregnancy rate: 5.06% vs. 2.62% vs. 1.05%; P < 0.001; early spontaneous miscarriage rate: 15.18% vs. 13.45% vs. 11.53%; P < 0.001). In multivariate logistic regression analysis, thin endometrium was an independent factor to predict ectopic pregnancy [adjusted odds ratio (aOR): 5.62; 95% confidence interval (CI): 2.51–12.58, P < 0.001], and to predict early spontaneous miscarriage rate (aOR: 1.57; 95% CI: 1.21–1.74, P < 0.001).
Conclusion: Thin endometrium on day of embryo transfer in FET cycles is an independent predictor for early pregnancy compilations, including ectopic pregnancy and early spontaneous miscarriage.
Frozen-thawed embryo transfer (FET), as one of the most important related technologies of in vitro fertilization (IVF) and embryo transfer, has been widely used currently, especially after the consistent increasing number of frozen embryos and awareness of ovarian hyper-stimulation syndrome. In fresh embryo transfer cycles after oocyte retrieval, there are several factors, including ovarian stimulation protocols, estrogen and progesterone levels before embryo transfer, and endometrial thickness those affect IVF outcomes and pregnancy complications. Compared to fresh embryo transfer cycles, FET has its own advantages in studying factors affect IVF pregnancy outcomes as many factors can be well controlled.
It is known that embryo quality and endometrial receptivity are two key elements for a successful pregnancy in IVF treatment. As for endometrial receptivity, there are non-invasive and invasive methods to assess it. Endometrial thickness assessment can be performed noninvasively, and has been used as a routine practice in IVF centers. Many previous studies demonstrated that endometrial thickness, which is measured either on day of progesterone administration or on day of embryo transfer, is positively associated with pregnancy outcomes, including clinical pregnancy rate and live birth rate (1, 2). In addition, recent studies also showed that the change of endometrial thickness (endometrium compaction) between day of embryo transfer and day of ovulation in FET cycles impacts pregnancy outcomes (3–5).
The relationship between endometrial thickness and IVF pregnancy rates has been extensively studied. However, the pregnancy safety after IVF treatment is another important topic that should provoke attention. Recently, a large cohort study with more than 10,000 pregnancies after embryo transfer showed that patients with an endometrial thickness > 7.6 mm had a significant lower risk of ectopic pregnancy compared to those with an endometrial thickness < 7.6 mm (6). In addition, data also showed that the incidence of hypertensive disorders of pregnancy in patients with a thin endometrial thickness (< 8 mm) was significantly greater than that in patients with endometrial thickness being 8 mm-12 mm (7). Moreover, the impact of thin endometrial thickness and other pregnancy complications, such as spontaneous miscarriage, preterm birth, and low birth weight has also been explored, but with controversial results (8–10).
As one of the largest IVF centers in China, our center has advantages in cohort study design as the convenience in high quality clinical data collection and management, and has gained wider experience in exploring the impact of endometrial thickness and IVF outcomes. The aim of this study was to demonstrate the relationship between endometrial thickness on day of embryo transfer and early pregnancy complications (ectopic pregnancy and early miscarriage) in FET cycles.
Institutional Review Board approval was not needed for this study, because all patients included underwent the routine clinical treatment in our center and no additional intervention or sampling was performed. Written informed consent was obtained from all patients before IVF treatment for physicians collecting basic information and treatment data. Data in this study were from the Clinical Reproductive Medicine Management System/Electronic Medical Record Cohort Database (CCRM/EMRCD) in Reproductive Medical Center, First Affiliated Hospital of Zhengzhou University.
Patients undergoing their first FET cycles were included this study from January 2010 to December 2021. Included first FET cycles were with complete information regarding basic clinical characteristics and treatment outcome. Exclusion criteria were as follows: uterine malformation; uterine adhesion, tuberculosis, and polyps; sperm/oocyte donation cycles, pre-implantation genetic testing cycles.
Endometrial thickness was measured in the morning on day of embryo transfer using trans-vaginal probe. During endometrial thickness assessment, the ultrasound image of the endometrium from the inner cervical canal to the uterus fundus was firstly displayed. The maximum distance between the myometrium and the endometrium on both sides was then measured. These steps were performed by well-trained physicians according to our standard operation procedure in our center. Endometrial patterns were classified based on the morphology of the endometrium, as follows: pattern A, triple-line or multi-layered type; pattern B, slight-triple-line type; and pattern C, even-and-strong-echoed. On day of embryo transfer, only endometrium with pattern C was included into this study.
Endometrium preparation protocols were natural cycles and estrogen-progesterone cycles. For natural cycles used for patients with regular menstruation, endometrial thickness and follicles were measured on menstrual day 7-9, with blood test of estrogen and progesterone levels if necessary. On day of ovulation, 40 mg progesterone in oil was used before embryo transfer. On day of embryo transfer, 10 mg of oral progesterone (Duphaston; Solvay Pharmaceuticals B.V., Veenendaal, The Netherlands) was used 3 times per day, combined with daily use of 90 mg of vaginal progesterone (Crinone, Merck Serono, Germany). Cleavage stage embryos and blastocyst were transferred 3, or 4 days after ovulation, respectively.
In estrogen-progesterone cycles, oral estradiol (1-2 mg [Progynova]; Bayer, Leverkusen, Germany) twice a day on cycle day 3 was firstly given. This dose was adjusted based on endometrial thickness 7 days later, and the maximum dose was 8 mg in total per day. After 12–18 days, if no leading follicle was present, 60 mg progesterone in oil and 10 mg of oral progesterone (Duphaston; Solvay Pharmaceuticals B.V., Veenendaal, The Netherlands) (this dose will be changed to 30 mg 3 days later) was added for endometrial transformation. Cleavage stage embryos and blastocyst were transferred 5, or 6 days after progesterone administration, respectively.
Patients were divided into three groups according to endometrial thickness: Thin, ≤ 7 mm; Medium, 7-14 mm; Thick, ≥ 14 mm. The diagnosis of ectopic pregnancy, and criteria for tubal infertility were elaborated in our previous studies (11). Clinical pregnancy was defined as a gestational sac or multiple sacs seen via ultrasound 5 weeks after embryo transfer with detection of cardiac activity. The definition of early spontaneous miscarriage was miscarriage that occurred before 12 weeks of gestation.
Firstly, the rates of ectopic pregnancy and early spontaneous miscarriage were compared in different groups according to basic parameters (age, BMI, type of embryos transferred, endometrial thickness, etc) using Chi-square test. Parameters selection was based on our experiences and previous publications (8, 11). In addition, only those parameters were found to be possibly associated with ectopic pregnancy or early spontaneous miscarriage were included further for multivariate logistic regression analysis using SPSS (Statistical Package for Social Science, SPSS Inc, Chicago, IL) 21.0. A P value < 0.05 was considered statistically significant.
A total of 11138 clinical pregnancies were included. Each patient was included only once. The overall ectopic pregnancy and early spontaneous miscarriage rates were 2.62% (292/11138) and 13.40% (1492/11138), respectively. As shown in Figure 1, both ectopic pregnancy rate and early spontaneous miscarriage rate declined with increasing endometrial thickness.
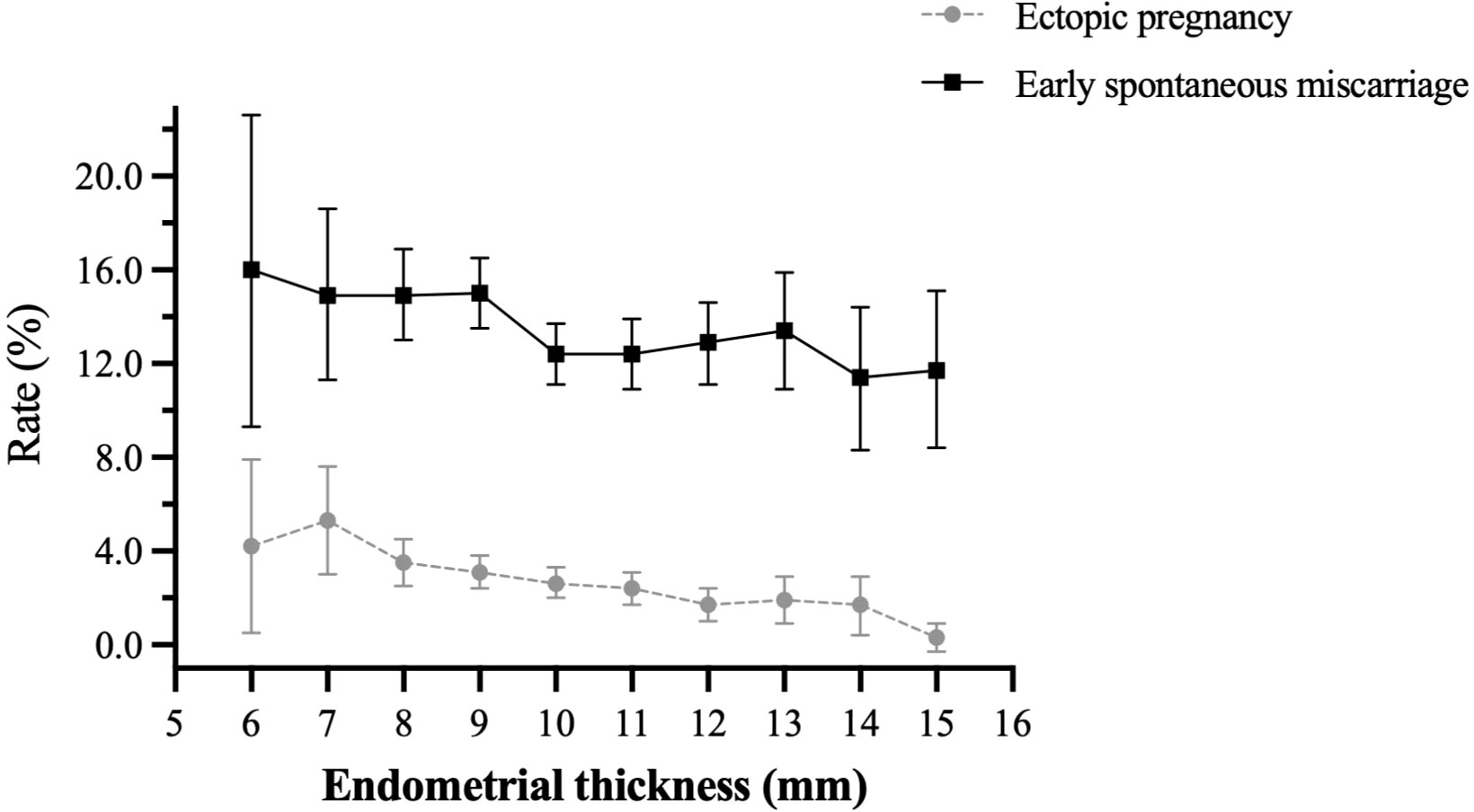
Figure 1 Overall ectopic pregnancy rate and early spontaneous miscarriage rate in patients with different endometrial thickness.
Table 1 shows the basic demographic information of patients with different endometrial thickness. BMI and basic FSH levels were similar in the three groups. However, the differences in patient age were statistically different (31.15 vs. 30.22 vs. 30.72, P < 0.001). In addition, except for the number of embryos transferred, which was comparable among the three groups, patients with a thin endometrium had a higher proportion of blastocyst transfer (59.72% vs. 47.93% vs. 44.04%, P < 0.001) and EP protocol for endometrium preparation (79.35% vs. 67.30% vs. 41.42%; P < 0.001).
The ectopic pregnancy rate is shown in Table 2. It varied in patients with different types and numbers of embryos transferred and in those with or without tubal factor infertility. Moreover, the ectopic pregnancy rate in patients with thin, medium, and thick endometrium was significantly different, at 5.06% (25/494), 2.62% (259/9881), and 1.05% (8/763), respectively. Early spontaneous miscarriage rate was also higher in women with advanced age, those with a higher BMI, and those with blastocyst transfer. Similarly, it was also found that miscarriage rate was significantly higher in women with thin endometrium as compared with that in patients with thick endometrium (15.18% vs. 13.45% vs. 11.53%; P < 0.001). However, neither ectopic pregnancy nor early spontaneous miscarriage rate was found to be different in patients with natural cycles and EP protocols for endometrium preparation.
To control for confounding factors that affect early pregnancy complications, multivariate logistic regression analysis was also performed. As shown in Table 3, after adjusting for tubal factor infertility, number of embryos transferred, and stage of embryos transferred, a thin endometrium was still an independent factor to predict ectopic pregnancy [adjusted odds ratio (aOR): 5.62; 95% confidence interval (CI): 2.51–12.58, P < 0.001]. Interestingly, in multivariate logistic regression analysis, after controlling for female age, BMI, infertility diagnosis, and stage or number of embryos transferred, it was found that, apart from advanced female age or elevated BMI, thin endometrium was also a risk factor for early spontaneous miscarriage rate (aOR: 1.57; 95% CI: 1.21–1.74, P < 0.001).
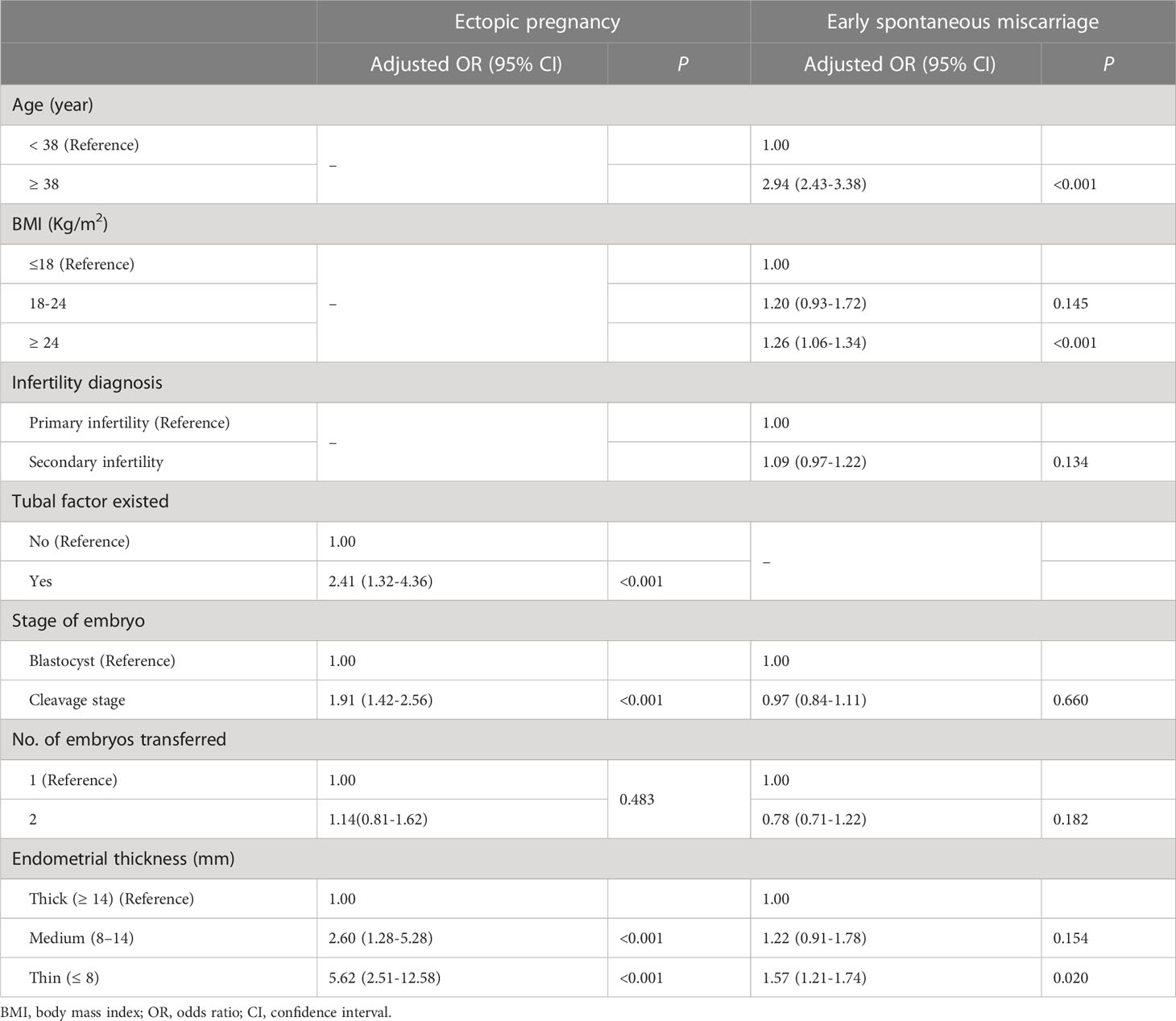
Table 3 Factors associated with ectopic pregnancy and early spontaneous miscarriage rates by logistic regression analysis in frozen-thawed transfer cycles.
Infertility is a global health issue affecting millions of people of reproductive age worldwide. However, compared with infertility itself, pregnancy complications after IVF treatment are more frustrating, especially in ectopic pregnancy and early spontaneous miscarriage cases. As we mentioned earlier, the impact of endometrial thickness on late pregnancy outcomes, such as preterm birth, placenta previa, etc has been explored in a few studies (12, 13). However, these late complications are more complicated and are associated with many other factors that are out of our control, such as diet habit, infection of amniotic fluid or lower genital tract, and trauma.
In the current study, we mainly focused on the impact of endometrial thickness and early pregnancy complication from a large sample of patients, and found that thin endometrium was an independent risk factor for both ectopic pregnancy and early spontaneous miscarriage rates.
During IVF treatment, the occurrence of an ectopic pregnancy is not only a risk factor for recurrent ectopic pregnancy in the next pregnancy, but also brings financial and psychological burden to infertile couples (14). Currently, there was no consensus on the risk factors for ectopic pregnancy after IVF treatment, however, tubal factor infertility; high estrogen levels before embryo transfer seem to increase the incidence of ectopic pregnancy. On the contrast, blastocyst transfer was a protective factor as compared with cleavage stage embryo transfer (11, 15, 16).
As for the association between endometrial thickness and ectopic pregnancy, previous large data showed that a thin endometrium increased ectopic pregnancy rate in both fresh and FET cycles (17–19), which was consistent with results from ours. However, in Rombauts’s study, repeated cycles were presented (17). In the current study, we only included first IVF cycles, and made a thorough analysis of possible ectopic pregnancy related factors. Then, the next question was why thin endometrium increased ectopic pregnancy rate.
One of the possible explanations was that, the uterine peristalsis may change and subsequently affect pregnancy in thin endometrium patients. In 2014, Zhu et al. found that the uterine peristaltic wave frequency before embryo transfer was inversely related to the clinical pregnancy rates, and then a hypothesis was made that intense uterine peristalsis could extrude embryos out of the uterine cavity, leading to implantation failure or ectopic pregnancy (17, 20). Recently, Zhao et al. also suspected that a thin endometrial thickness could be linked with abnormal endometrial peristaltic waves or abnormal endometrial receptivity, and then resulting in ectopic pregnancy (21). Moreover, data also showed that controlled ovarian stimulation cycles with super-physiological estrogen significantly increased the uterine peristaltic waves (22), it was reasonable to found that ectopic pregnancy rate was higher in fresh embryo transfer cycles as compared with that in FET cycles (23); and higher in patients with elevated estrogen as compared with those with lower hormonal milieu (11, 24). In FET cycles, patients with thin endometrium tended to take more pills or other actions to increase estrogen levels and endometrial thickness, which eventually increased ectopic pregnancy rate as well. Secondly, other scholars also speculated that the higher status of oxygen concentration in a thin endometrium was similar with that in the fallopian tube. This could also increase the higher risk of ectopic pregnancy as higher oxygen concentrations inhibit the growth of embryos (25).
Early spontaneous miscarriage is another common pregnancy complication after IVF treatment. Currently, there are no precise data on the incidence of spontaneous miscarriage after IVF, but most studies indicate that it is higher than that in natural conceptions (26, 27).
Firstly, results from the current study were in line with previous ones, in which advanced female age and elevated BMI were risk factors with early spontaneous miscarriage (28–31). However, most previous studies mainly focused on the impact of endometrial thickness on clinical pregnancy or live birth rates, few ones took spontaneous miscarriage rate as a primary outcome. In addition, conflicting results existed in regard to the impact of endometrial thickness on spontaneous miscarriage rate (8, 32). In this large cohort study, data also showed that thin endometrial thickness was also an independent risk factor for early spontaneous miscarriage after adjusting female age and BMI.
It is known that decidualization is an essential prerequisite for implantation. During this process, stromal cells undergo remarkable morphogenetic and vascular changes under the influence of the steroid hormones. Thus, lacking of these receptors leads to a failure of pregnancy and miscarriage (33, 34). A recently study showed that thin endometrium was associated with reduced expression of estrogen receptor in stromal cells both during proliferative and secretory phase (35). This may explain the phenomenon that early spontaneous miscarriage elevated in thin endometrium patients as the existence of compromised decidualization.
One of the strengths in this study was the large sample from a single center. In addition, each patient was included only once. The homogeneity of patient’s data and physician standard operating procedure made the data more reliable. In addition, all patients underwent a detailed ultrasound examination to determine the endometrial thickness and morphology before embryo transfer in our center. Since only patients with Pattern C Endometrium were included, there was no effect of endometrial morphology on the miscarriage rate in this study. Moreover, all patients in this study underwent hysteroscopy examination before each cycle, and only those with normal uterine cavity were included. Severe uterine adhesion, uterine polyps, fibroids, and other lesions were excluded.
However, several limitations also existed. Not all bias could be controlled as the retrospective nature of this study. In patients with thin endometrium, it was obvious that the proportion of E-P cycles was much higher and patient basic demographic data were different. We performed multivariate logistic regression analysis to minimize these side effects. More importantly, hysteroscopy examination mainly detects the form of uterine cavity, the specific etiology for thin endometrium needed further extensive study.
In summary, data from this large cohort study show that, thin endometrium on day of embryo transfer in FET cycles is an independent predictor for early pregnancy compilations, including ectopic pregnancy and early spontaneous miscarriage. This reminds us to pay attention to the association between thin endometrial thickness and IVF safety. Although the treatment of thin endometrium is still a challenge, these data help us provide more comprehensive consultation and make appropriate decisions for patients with thin endometrium.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of First Affiliated Hospital of Zhengzhou University. The patients/participants provided their written informed consent to participate in this study.
LS and ZB contributed to the conception, design, acquisition and interpretation of data, and drafting of the manuscript. YS supervised the study. All authors contributed to the article and approved the submitted version.
The authors would like to thank all the staff from Reproductive Medical Center, First Affiliated Hospital of Zhengzhou University. We also would like to thank Dr. Keyan Wang from Henan Academy of Medical and Pharmaceutical Sciences for her assistance in data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bu Z, Wang K, Dai W, Sun Y. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. GYNECOL Endocrinol (2016) 32:524–8. doi: 10.3109/09513590.2015.1136616
2. Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update (2014) 20:530–41. doi: 10.1093/humupd/dmu011
3. Zilberberg E, Smith R, Nayot D, Haas J, Meriano J, Barzilay E, et al. Endometrial compaction before frozen euploid embryo transfer improves ongoing pregnancy rates. FERTIL STERIL (2020) 113:990–5. doi: 10.1016/j.fertnstert.2019.12.030
4. Jin Z, Li J, Yang E, Shi H, Bu Z, Niu W, et al. Effect of endometrial thickness changes on clinical pregnancy rates after progesterone administration in a single frozen-thawed euploid blastocyst transfer cycle using natural cycles with luteal support for PGT-SR- and PGT-M-assisted reproduction: a retrospective cohort study. Reprod Biol ENDOCRIN (2021) 19:154. doi: 10.1186/s12958-021-00841-x
5. Youngster M, Mor M, Kedem A, Gat I, Yerushalmi G, Gidoni Y, et al. Endometrial compaction is associated with increased clinical and ongoing pregnancy rates in unstimulated natural cycle frozen embryo transfers: a prospective cohort study. J Assist Reprod Gen (2022) 39:1909–16. doi: 10.1007/s10815-022-02544-7
6. Liu J, Kong H, Yu X, Zhou M, Liu X, Liu X, et al. The role of endometrial thickness in predicting ectopic pregnancy after in vitro fertilization and the establishment of a prediction model. Front Endocrinol (2022) 13:895939. doi: 10.3389/fendo.2022.895939
7. Zhang M, Li J, Fu X, Zhang Y, Zhang T, Wu B, et al. Endometrial thickness is an independent risk factor of hypertensive disorders of pregnancy: a retrospective study of 13,458 patients in frozen-thawed embryo transfers. Reprod Biol ENDOCRIN (2022) 20:93. doi: 10.1186/s12958-022-00965-8
8. Bu Z, Hu L, Su Y, Guo Y, Zhai J, Sun YP. Factors related to early spontaneous miscarriage during IVF/ICSI treatment: an analysis of 21,485 clinical pregnancies. Reprod BioMed Online (2020) 40:201–6. doi: 10.1016/j.rbmo.2019.11.001
9. Guo Z, Xu X, Zhang L, Zhang L, Yan L, Ma J. Endometrial thickness is associated with incidence of small-for-gestational-age infants in fresh in vitro fertilization-intracytoplasmic sperm injection and embryo transfer cycles. FERTIL STERIL (2020) 113:745–52. doi: 10.1016/j.fertnstert.2019.12.014
10. Liu X, Wang J, Fu X, Li J, Zhang M, Yan J, et al. Thin endometrium is associated with the risk of hypertensive disorders of pregnancy in fresh IVF/ICSI embryo transfer cycles: a retrospective cohort study of 9,266 singleton births. Reprod Biol ENDOCRIN (2021) 19:55. doi: 10.1186/s12958-021-00738-9
11. Bu Z, Xiong Y, Wang K, Sun Y. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6-year, single-center study. FERTIL STERIL (2016) 106:90–4. doi: 10.1016/j.fertnstert.2016.02.035
12. Hu KL, Kawai A, Hunt S, Li W, Li X, Zhang R, et al. Endometrial thickness in the prediction of neonatal adverse outcomes in frozen cycles for singleton pregnancies. Reprod BioMed Online (2021) 43:553–60. doi: 10.1016/j.rbmo.2021.04.014
13. Huang J, Lin J, Xia L, Tian L, Xu D, Liu P, et al. Decreased endometrial thickness is associated with higher risk of neonatal complications in women with polycystic ovary syndrome. Front Endocrinol (2021) 12:766601. doi: 10.3389/fendo.2021.766601
14. Tang S, Du T, Huang J, Ye H, Zhao M, Lin J, et al. Effect of previous wedge resection for interstitial pregnancy on pregnancy and neonatal outcomes following frozen-thawed embryo transfer (FET) cycles of IVF/ICSI: a retrospective study. Reprod Biol ENDOCRIN (2022) 20:23. doi: 10.1186/s12958-022-00896-4
15. Cheng LY, Lin PY, Huang FJ, Kung FT, Chiang HJ, Lin YJ, et al. Ectopic pregnancy following in vitro fertilization with embryo transfer: A single-center experience during 15 years. TAIWAN J OBSTET GYNE (2015) 54:541–5. doi: 10.1016/j.tjog.2015.08.004
16. Fang C, Huang R, Wei LN, Jia L. Frozen-thawed day 5 blastocyst transfer is associated with a lower risk of ectopic pregnancy than day 3 transfer and fresh transfer. FERTIL STERIL (2015) 103:655–61. doi: 10.1016/j.fertnstert.2014.11.023
17. Rombauts L, McMaster R, Motteram C, Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod (2015) 30:2846–52. doi: 10.1093/humrep/dev249
18. Liu H, Zhang J, Wang B, Kuang Y. Effect of endometrial thickness on ectopic pregnancy in frozen embryo transfer cycles: an analysis including 17,244 pregnancy cycles. FERTIL STERIL (2020) 113:131–9. doi: 10.1016/j.fertnstert.2019.09.003
19. Fang T, Chen M, Yu W, Ma T, Su Z, Chan D, et al. The predictive value of endometrial thickness in 3117 fresh IVF/ICSI cycles for ectopic pregnancy. J Gynecol Obstet Hum Reprod (2021) 50:102072. doi: 10.1016/j.jogoh.2021.102072
20. Zhu L, Che HS, Xiao L, Li YP. Uterine peristalsis before embryo transfer affects the chance of clinical pregnancy in fresh and frozen-thawed embryo transfer cycles. Hum Reprod (2014) 29:1238–43. doi: 10.1093/humrep/deu058
21. Zhao Y, Liu D, Liu N, Li Y, Yao Z, Tian F, et al. An endometrial thickness < 8 mm was associated with a significantly increased risk of EP after freeze-thaw transfer: an analysis of 5,960 pregnancy cycles. Front Endocrinol (2022) 13:884553. doi: 10.3389/fendo.2022.884553
22. Zhu L, Li Y, Xu A. Influence of controlled ovarian hyperstimulation on uterine peristalsis in infertile women. Hum Reprod (2012) 27:2684–9. doi: 10.1093/humrep/des257
23. Huang B, Hu D, Qian K, Ai J, Li Y, Jin L, et al. Is frozen embryo transfer cycle associated with a significantly lower incidence of ectopic pregnancy? An analysis of more than 30,000 cycles. FERTIL STERIL (2014) 102:1345–9. doi: 10.1016/j.fertnstert.2014.07.1245
24. Wang J, Wei Y, Diao F, Cui Y, Mao Y, Wang W, et al. The association between polycystic ovary syndrome and ectopic pregnancy after in vitro fertilization and embryo transfer. Am J OBSTET GYNECOL (2013) 209:131–9. doi: 10.1016/j.ajog.2013.05.007
25. Casper RF. It's time to pay attention to the endometrium. FERTIL STERIL (2011) 96:519–21. doi: 10.1016/j.fertnstert.2011.07.1096
26. Wang A, Kort J, Westphal L. Miscarriage history association with euploid embryo transfer outcomes. Reprod BioMed Online (2019) 39:617–23. doi: 10.1016/j.rbmo.2019.05.011
27. Kling C, Magez J, Hedderich J, von Otte S, Kabelitz D. Two-year outcome after recurrent first trimester miscarriages: prognostic value of the past obstetric history. Arch GYNECOL OBSTET (2016) 293:1113–23. doi: 10.1007/s00404-015-4001-x
28. Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol ENDOCRIN (2015) 13:35. doi: 10.1186/s12958-015-0028-x
29. Cai H, Mol BW, Gordts S, Wang H, Wang T, Li N, et al. Early and late pregnancy loss in women with polycystic ovary syndrome undergoing IVF/ICSI treatment: a retrospective cohort analysis of 21 820 pregnancies. BJOG-INT J OBSTET GY (2021) 128:1160–9. doi: 10.1111/1471-0528.16590
30. Dahan MH, Zeadna A, Dahan D, Son WY, Steiner N. The biochemical pregnancy loss rate remains stable up irrespective of age and differs in pattern from clinical miscarriages. GYNECOL Endocrinol (2021) 37:61–4. doi: 10.1080/09513590.2020.1807931
31. Bellver J. BMI. and miscarriage after IVF. Curr Opin OBSTET GYN (2022) 34:114–21. doi: 10.1097/GCO.0000000000000778
32. Gao G, Cui X, Li S, Ding P, Zhang S, Zhang Y. Endometrial thickness and IVF cycle outcomes: a meta-analysis. Reprod BioMed Online (2020) 40:124–33. doi: 10.1016/j.rbmo.2019.09.005
33. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med (2010) 28:17–26. doi: 10.1055/s-0029-1242989
34. Ochoa-Bernal MA, Fazleabas AT. Physiologic events of embryo implantation and decidualization in human and non-human primates. Int J Mol Sci (2020) 21:1973. doi: 10.3390/ijms21061973
Keywords: endometrial thickness, frozen thawed embryo transfer, ectopic pregnancy, early spontaneous miscarriage, pregnancy complications
Citation: Song L, Bu Z and Sun Y (2023) Endometrial thickness and early pregnancy complications after frozen-thawed embryo transfers. Front. Endocrinol. 14:1066922. doi: 10.3389/fendo.2023.1066922
Received: 11 October 2022; Accepted: 02 March 2023;
Published: 21 August 2023.
Edited by:
Tom Kelsey, University of St Andrews, United KingdomReviewed by:
Caroline E. Dunk, Toronto General Research Institute (TGRI), CanadaCopyright © 2023 Song, Bu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingpu Sun, c3lwMjAwOEB2aXAuc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.