- 1Department of General Surgery, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Department of Gynecology, The Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 3Emergency Department, East China Hospital affiliated to Fudan University, Shanghai, China
- 4Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, Ürümqi, China
- 5Department of Ophthalmology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 6Department of Cardiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 7Department of Phase I Clinical Trial Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 8Clinical Medical School, the Southwest Medical University, Luzhou, Sichuan, China
- 9Department of General Surgery, the Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 10Department of Thyroid and Breast Surgery, the Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 11Division of Oral and Maxillofacial Surgery, Columbia University Irving Medical Center, New York, NY, United States
- 12College of Osteopathic Medicine, Kansas City University, Kansas City, MO, United States
- 13UK Program Site, American University of the Caribbean School of Medicine, Preston, United Kingdom
- 14Bronxcare Health System, The Bronx, NY, United States
Objectives: The aim of this study was to investigate the association between diabetes status and the risk of breast cancer among adult Americans, exploring the impact of BMI, age, and race on this relationship.
Methods: A cross-sectional analysis of 8,249 individuals from the National Health and Nutrition Examination Survey (NHANES) was conducted. Diabetes was categorized as type 2 diabetes and prediabetes, with both conditions diagnosed according to the ADA 2014 guidelines. The association between diabetes status and breast cancer risk was explored using multiple logistic regression analysis.
Results: Patients with diabetes had higher odds of breast cancer (OR: 1.51; 95% CI 1.00 to 2.28), Using the two-piecewise linear regression model, it was observed that there is a threshold effect in the risk of breast cancer occurrence at the age of 52 years. Specifically, the risk of breast cancer is relatively low before the age of 52 but increases significantly after this age.
Conclusions: This study identified a significant association between diabetes status and breast cancer risk among adult Americans. We also found a threshold effect in breast cancer occurrence at the age of 52. Age was significantly associated with breast cancer risk in both Non-Hispanic White and Non-Hispanic Black individuals. These findings underscore the importance of diabetes management, maintaining a healthy BMI, and age-related risk considerations in reducing breast cancer risk.
Introduction
There are more than 40 million cases of breast cancer in women worldwide and it is the second most common cancer among women in the United States (1, 2). The American Cancer Society indicates that approximately 42,000 women will die from breast cancer in 2020, with 276,000 newly diagnosed cases (3). Breast cancer affects women of all ages. However, the incidence of breast cancer increases with age, with a peak incidence at 45-64 years (4). There are many factors associated with the risk of breast cancer (5, 6). The prevalence of diabetes is increasing at an alarming rate and has become one of the most serious public health problems in the world. Diabetes is also considered to be the most common endocrine disease. The American Diabetes Association (ADA) shows that diabetes is the fourth leading cause of death in the United States (7).
There is a growing recognition that type 2 diabetes mellitus (T2DM) and breast cancer (BC) occur together in the same patient population with high mortality rates (8). Overall survival and disease-specific survival are significantly worse in diabetic BC patients compared to non-diabetic BC patients, suggesting a correlation between T2DM and cancer progression (9). Hardefeldt et al. showed that diabetes mellitus is an independent risk factor for breast cancer (10). According to the results of a meta-analysis, women with diabetes had a 23% higher risk of future breast cancer than women without diabetes (11). A meta-analysis showed that women with diabetes had a significantly higher risk (~20%) of breast cancer than those without diabetes (12). T2DM and hyperinsulinemia were independently associated with postmenopausal breast cancer (13). In addition, a growing body of data suggests that diabetes and its complications adversely affect cancer treatment (14) and increase mortality (15), thereby affecting the prognosis of breast cancer patients (16, 17). Studies have suggested that the higher risk of breast cancer among the diabetes patients can be resulted from detection bias or potential confounders (18, 19); and that the use of antidiabetic drugs might affect the risk of breast cancer.
Patients with prediabetes have higher than normal blood glucose levels, but not high enough to be considered asT2DM. However, this is often seen as a warning sign. Prediabetes is characterized by impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or an HbA1c of 39 mmol/mol (5.7%) to 46 mmol/mol (6.4%) (20). The significance of prediabetes lies in the risk associated with progression to T2DM, which is disproportionately higher at the upper end of the prediabetes range and in the combined presence of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (20). Prediabetes and T2DM are parts of a continuum of spectrum that share pathophysiology and are associated with typical phenotypes including obesity, hypertension (HTN) and dyslipidemia (DLP) (21). Despite extensive research on the association between diabetes and breast cancer, many aspects of the relationship and underlying mechanisms remain unclear. Therefore, further research in this area is necessary.The aim of this study was to investigate the relationship between diabetes status and breast cancer in United States adults using data from the National Health and Nutrition Examination Survey (NHANES) from 2011-2016. Specifically, the objectives of this study were to: 1) examine the distribution of diabetes status (T2DM, prediabetes, and non-diabetes) in the study population; 2) determine the correlation between diabetes status and breast cancer; 3) determine the relationship between race and breast cancer; and 4) determine the relationship between BMI and breast cancer. By analyzing these factors, we aimed to gain a better understanding of the risk factors associated with breast cancer in relation to diabetes status.
Materials and methods
Data source
NHANES is a cross-sectional, population-based survey that assesses the health and nutritional status of the United States civilian, noninstitutional population through interviews, physical examinations, and laboratory tests. It is publicly available, and data is released every two years on a nationally representative sample using a multistage probability sampling design and weights (22). The NHANES program is reviewed annually by the National Center for Health Statistics Ethics Review Committee to ensure its ethical and scientific standards (23).
Study population
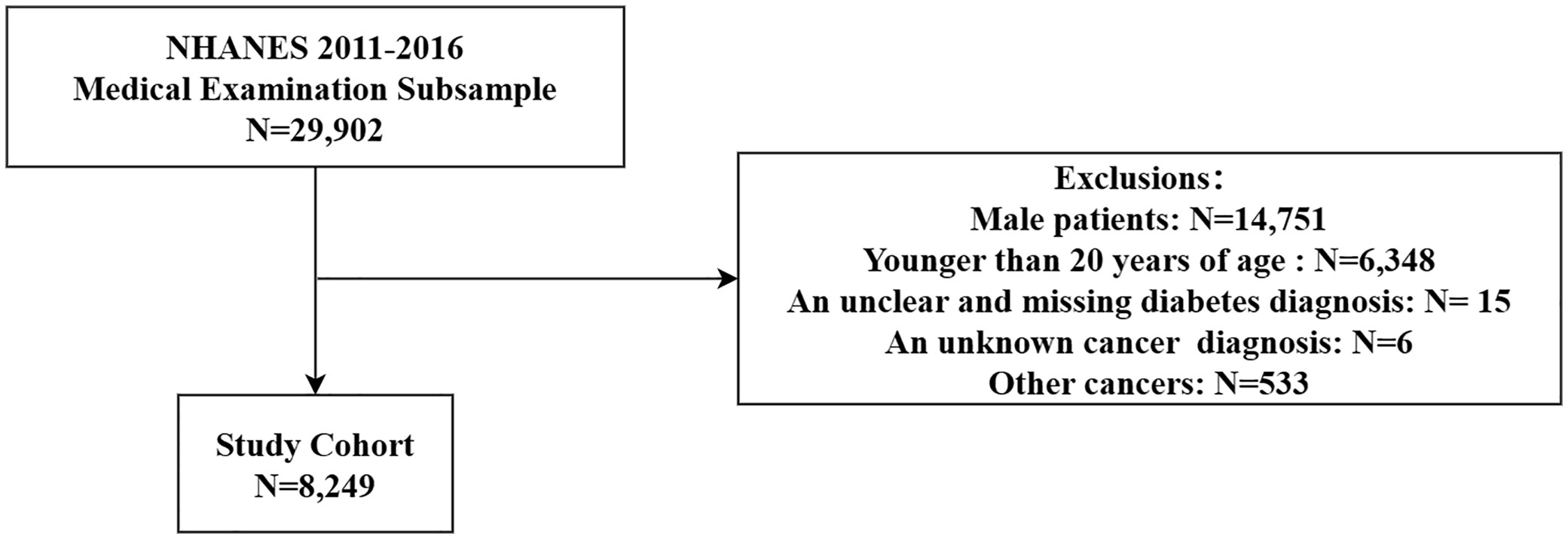
The data used in this study were obtained from the 2011-2016 survey cycle (24). This provides information on all the variables that have been used to determine the risk factors and determinants of type 2 diabetes in recent years. The process for study selection is shown in the flow diagram in Figure 1. Multiple interpolation was used for missing data.
Diagnostic criteria for diabetes and prediabetes
The diagnostic criteria for type 2 diabetes mellitus (T2DM) and prediabetes are shown in Supplement Table 1 and the study population had to meet the diagnostic criteria or have a clear diagnosis of diabetes in NHANES.
Statistical analysis
Data were presented as weighted mean ± standard error (SE) for continuous variables and weighted percentages (95% confidence interval) for categorical variables. The associations between diabetes status and breast cancer, as well as race and breast cancer, were examined using logistic regression models. Three models were employed for the analysis: Model 1 as the crude model with no adjustments, Model 2 adjusted for age, race, and body mass index (BMI), and Model 3 adjusted for age, race, BMI, educational level, serum creatinine, cholesterol, triglycerides, glycohemoglobin, serum cotinine, estradiol, marital status, serum glucose, and reproductive health. The threshold effect analysis of BMI and age on breast cancer was assessed using two-piecewise linear regression models. The inflection points for BMI and age were determined, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for both below and above these inflection points. The log-likelihood ratio was also reported to evaluate the goodness-of-fit of the models. Additionally, the threshold effect analysis of BMI and age for different racial groups was performed using the standard linear model, and the adjusted ORs with 95% CIs were reported for each racial group. The statistical software package R (http://www.R-project.org) was used for statistical analyses. Statistical significance was considered when the P value was < 0. 05.
Results
Characteristics of the participants
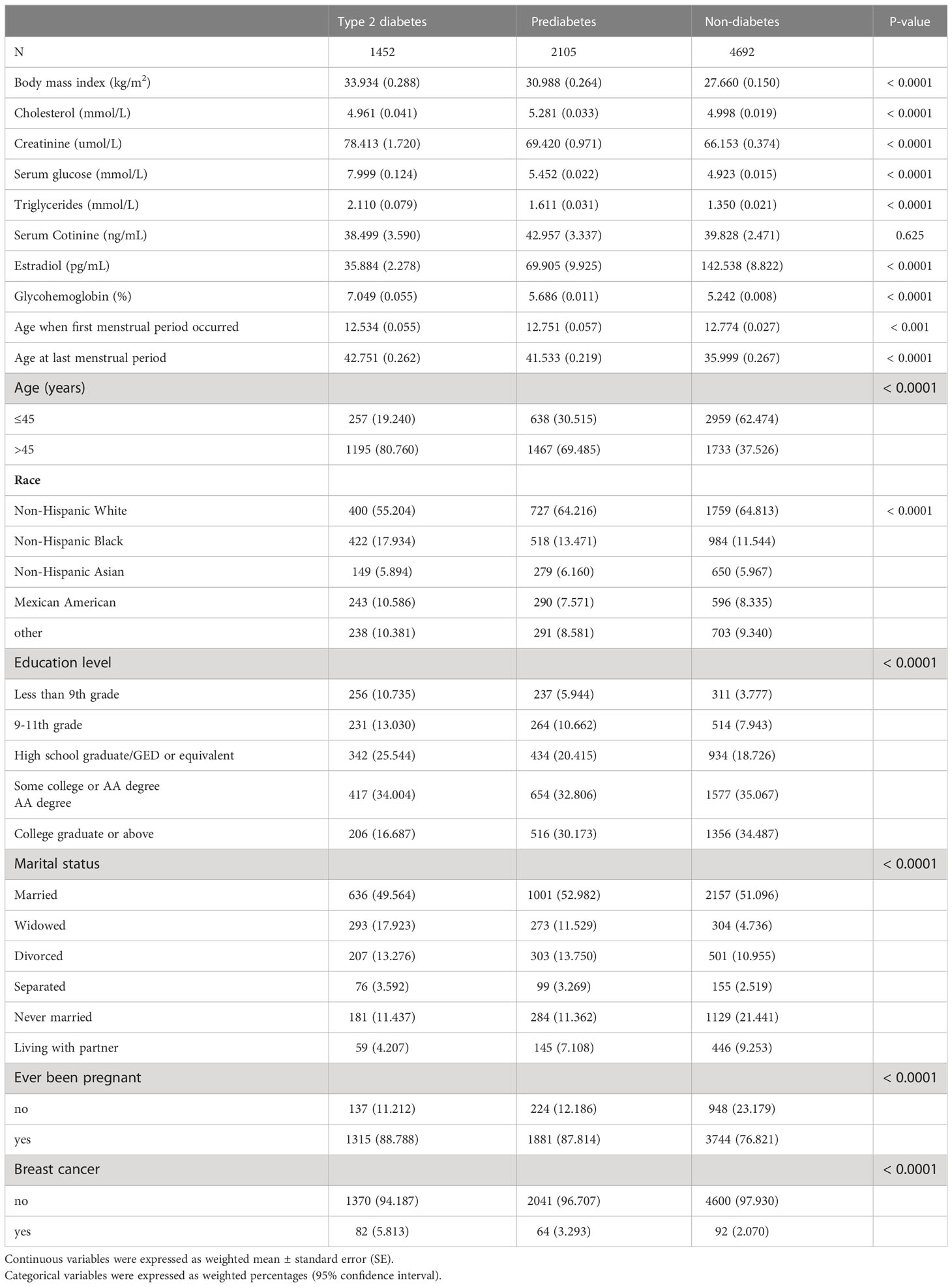
Table 1 presents the weighted characteristics of the study sample, which consisted of 8,249 participants classified by NHANES and grouped by diabetes status (Type 2 diabetes, prediabetes, and non-diabetes). Our findings showed that the Type 2 diabetes group had a significantly higher BMI (33.934 kg/m2) than both the Prediabetes group (30.988 kg/m2) and Non-diabetes group (27.660 kg/m2) (p < 0.0001). Additionally, there was no significant difference in serum nicotine levels between the Type 2 diabetes group and the Non-diabetes group (p = 0.625). However, the estradiol level in the Type 2 diabetes group was significantly lower (35.884 pg/mL) than the Prediabetes group (69.905 pg/mL) and Non-diabetes group (142.538 pg/mL) (p < 0.0001). Furthermore, the age of menarche in the Type 2 diabetes group (12.534 years) was significantly lower than the Prediabetes group (12.751 years) and Non-diabetes group (12.774 years) (p < 0.001). Lastly, the age of menopause in the Type 2 diabetes group (42.751 years) was significantly higher than the Prediabetes group (41.533 years) and Non-diabetes group (35.999 years) (p < 0.0001). More detailed results can be found in Table 1.
Associations between diabetes status and breast cancer
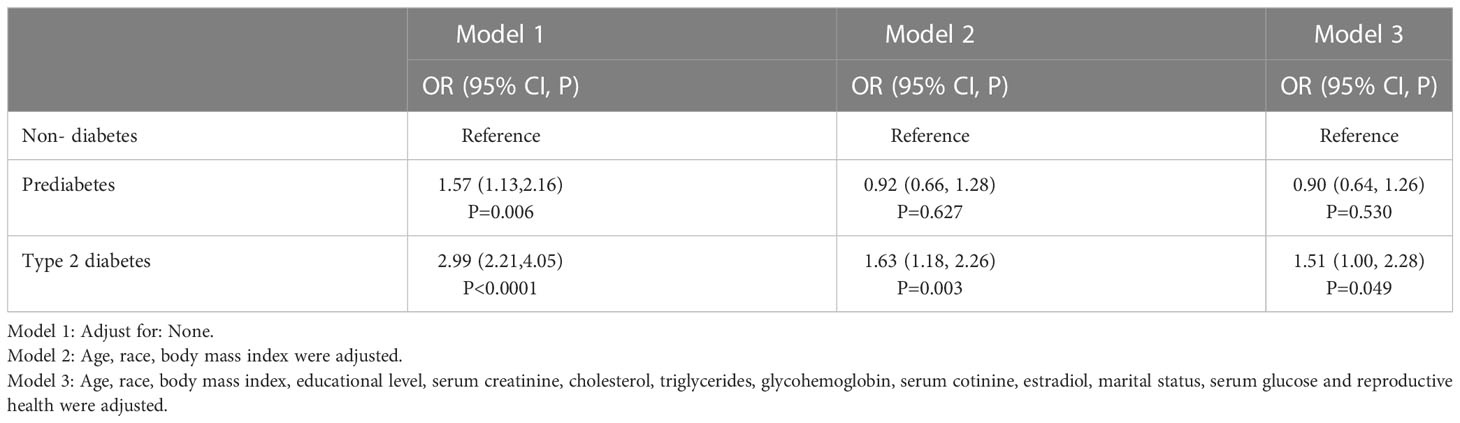
Table 2 shows the results of multiple logistic regression analysis of the association between diabetes status (non-diabetes, prediabetes, and Type 2 diabetes) and breast cancer, with odds ratios (ORs) and 95% confidence intervals (CIs) for three different models. Model 1 does not adjust for any covariates, Model 2 adjusts for age, race, and body mass index (BMI), and Model 3 adjusts for age, race, BMI, educational level, serum creatinine, cholesterol, triglycerides, glycohemoglobin, serum cotinine, estradiol, marital status, serum glucose, and reproductive health. For the non-diabetes group, the ORs in all three models are considered the reference group. For the prediabetes group, the OR in Model 1 is 1.57 (95% CI, 1.13-2.16, P=0.006), in Model 2 is 0.92 (95% CI, 0.66-1.28, P=0.627), and in Model 3 is 0.90 (95% CI, 0.64-1.26, P=0.530). For the Type 2 diabetes group, the OR in Model 1 is 2.99 (95% CI, 2.21-4.05, P<0.0001), in Model 2 is 1.63 (95% CI, 1.18-2.26, P=0.003), and in Model 3 is 1.51 (95% CI, 1.00-2.28, P=0.049). Overall, these findings suggest that Type 2 diabetes is significantly associated with an increased risk of breast cancer, even after adjusting for multiple covariates. Results are detailed in Table 2.
Associations between prediabetes/diabetes and breast cancer by race
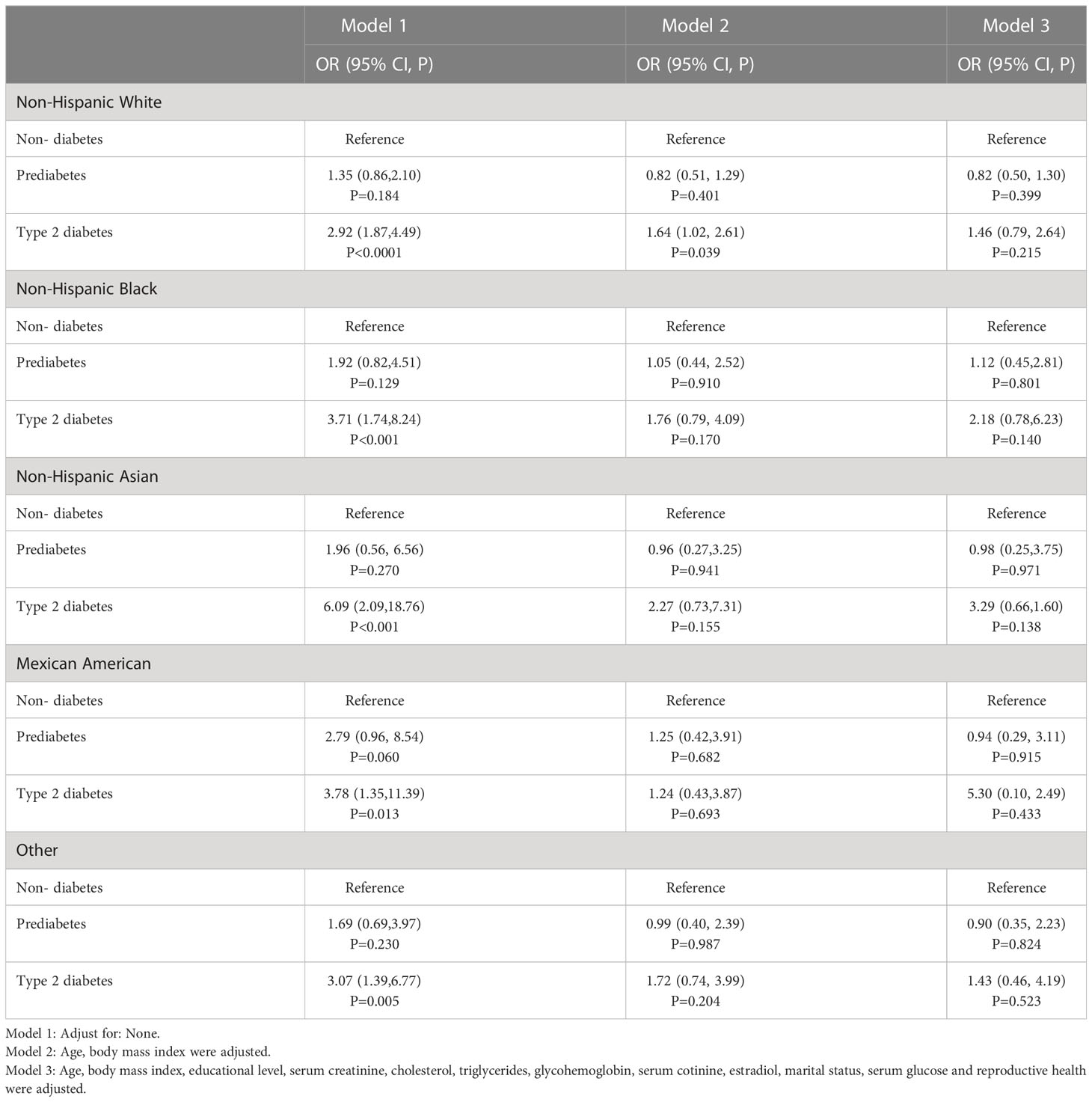
Table 3 shows the results of multiple logistic regression analysis testing the relationship between race and breast cancer. The unadjusted model (Model 1) was first examined, followed by Model 2 adjusted for age and body mass index, and finally Model 3 adjusted for additional covariates, including educational level, serum creatinine, cholesterol, triglycerides, glycohemoglobin, serum cotinine, estradiol, marital status, serum glucose, and reproductive health. For each racial group and diabetes status, the odds ratios (ORs) with 95% confidence intervals (CIs) and p-values were calculated, with the non-diabetes group as the reference. For example, Model 1 showed that among non-Hispanic White individuals, those with type 2 diabetes had an increased risk of breast cancer, with an OR of 2.92 (95% CI, 1.87-4.49, P < 0.0001). The results are shown in Table 3.
Analysis of the effect of BMI threshold on female breast cancer using a two-part linear regression model
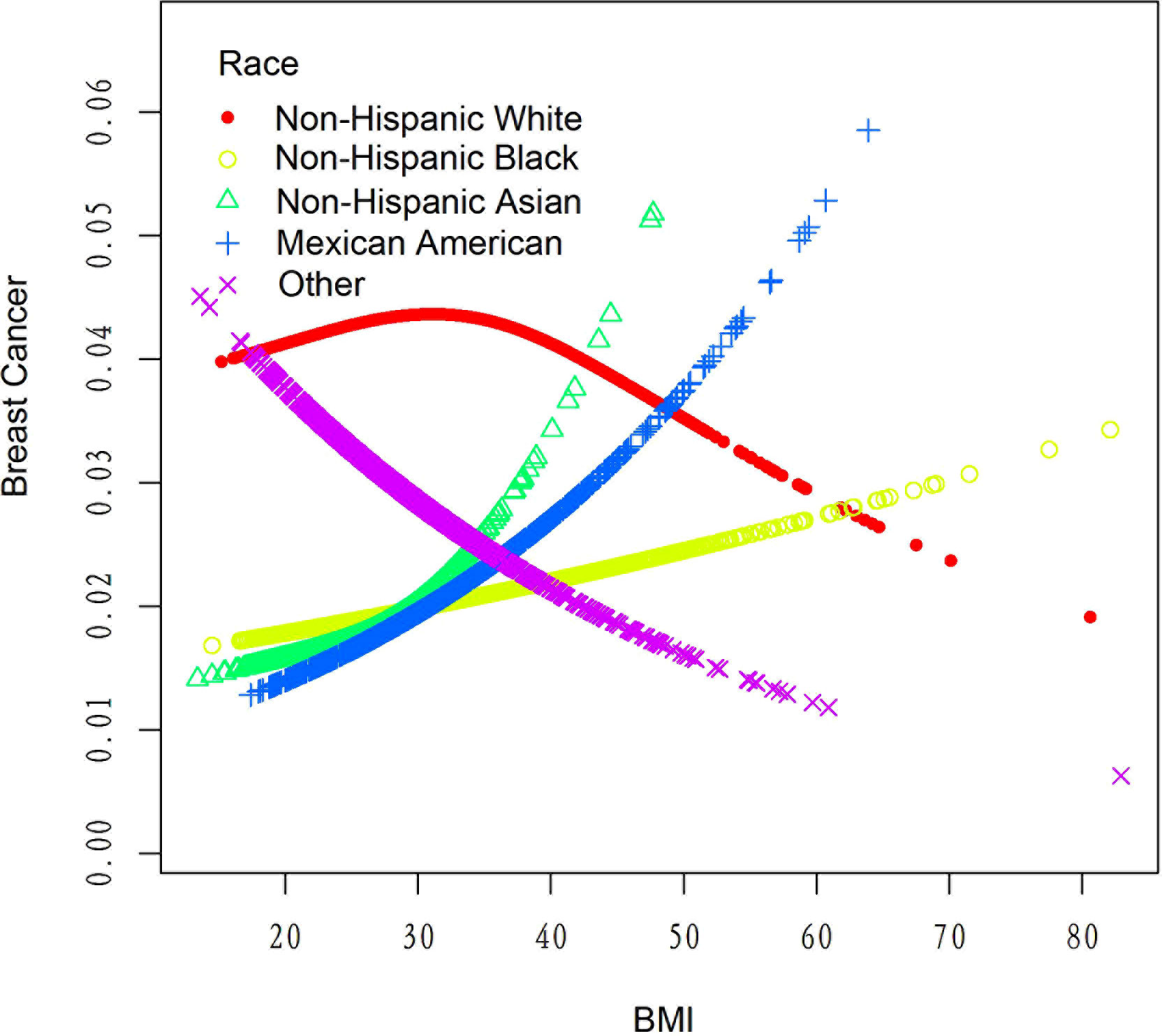
Table 4 displays the results of a threshold effect analysis examining the relationship between body mass index (BMI) and breast cancer risk in women using a two-piecewise linear regression model. The adjusted odds ratios (ORs) with 95% confidence intervals (CIs) are presented. The table compares the results of fitting the standard linear model with those of the two-piecewise linear model. The inflection point is at 21 kg/m2. For individuals with BMI less than 21 kg/m2, the adjusted OR for breast cancer is 0.88 (95% CI: 0.69, 1.11). For individuals with BMI greater than 21 kg/m2, the adjusted OR for breast cancer is 1.01 (95% CI: 0.98, 1.03). The log-likelihood ratio is 0.297. Results are detailed in Table 4; Figure 2. Figure 3 shows the relationship between BMI and breast cancer among different racial/ethnic groups. These findings suggest that there may be a threshold effect of BMI on breast cancer risk in women.
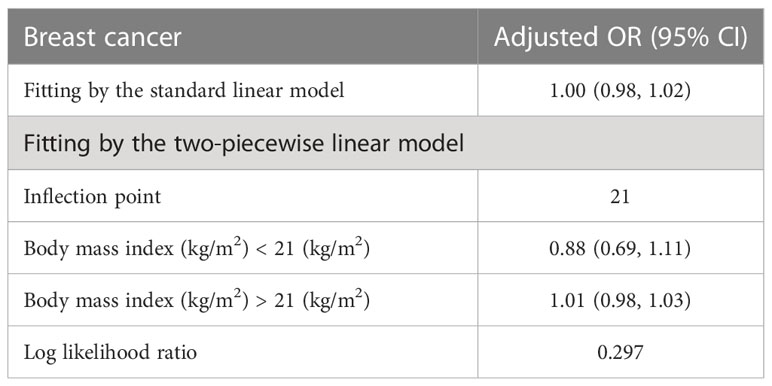
Table 4 Threshold effect analysis of BMI on breast cancer in female using the two piecewise linear regression model.
Threshold effect analysis of age on breast cancer in female using the two piecewise linear regression model
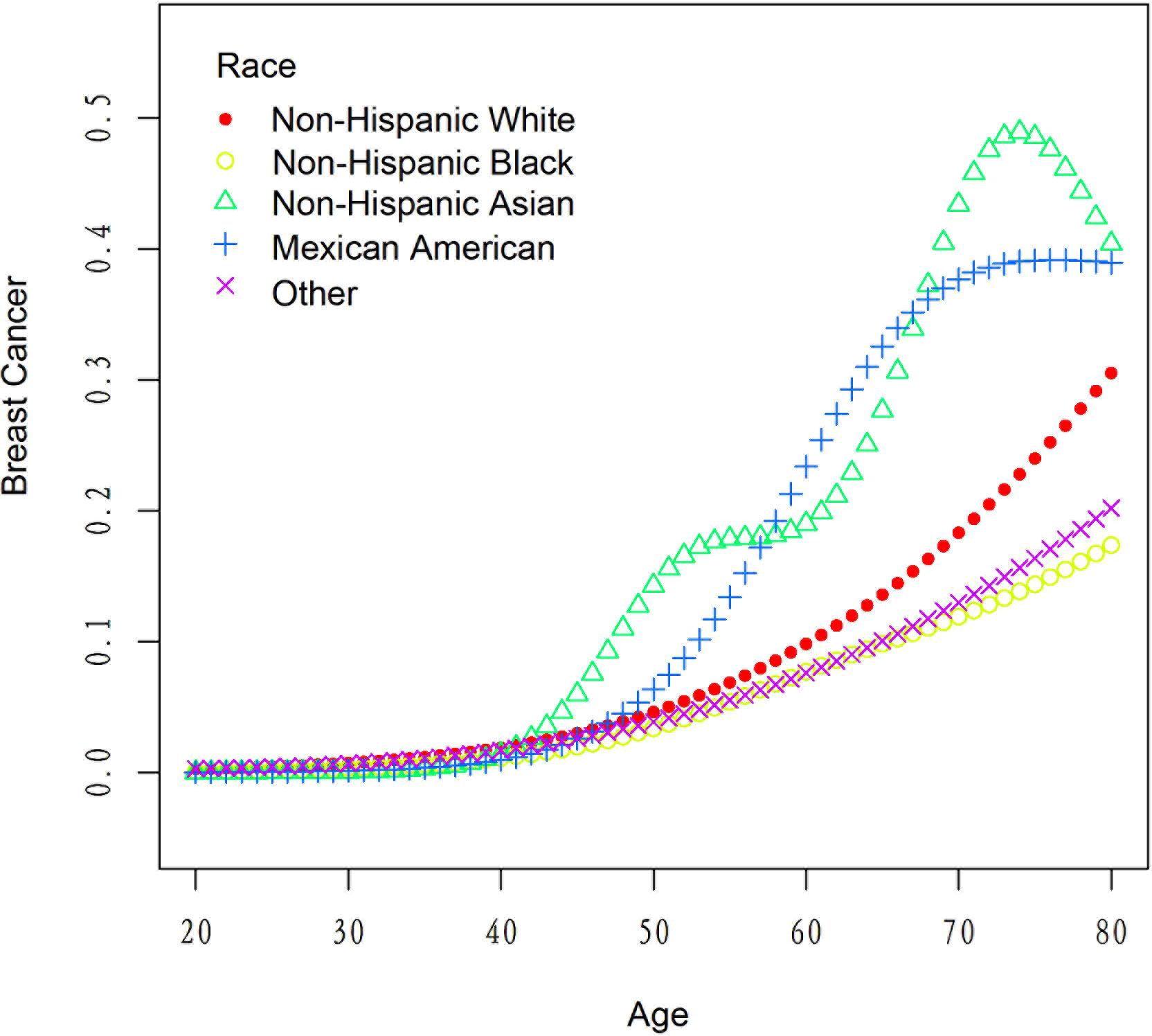
Table 5 presents the results of the threshold effect analysis of age on breast cancer in females using the two-piecewise linear regression model. The table shows the adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for both the standard linear model and the two-piecewise linear model. The standard linear model yielded an adjusted OR of 1.08 (95% CI: 1.06, 1.09). However, the two-piecewise linear model identified an inflection point at age 52 years. Among females aged less than 52 years, the adjusted OR was 1.18 (95% CI: 1.12, 1.26), while for those aged over 52 years, the adjusted OR was 1.06 (95% CI: 1.04, 1.08). The log-likelihood ratio was less than 0.001, indicating that the two-piecewise linear model was a better fit for the data than the standard linear model. These findings suggest that age has a threshold effect on the risk of breast cancer in females, with the risk increasing significantly after age 52 years. The results are presented in Table 5; Figure 4. Figure 5 shows the relationship between Age and breast cancer among different racial/ethnic groups.
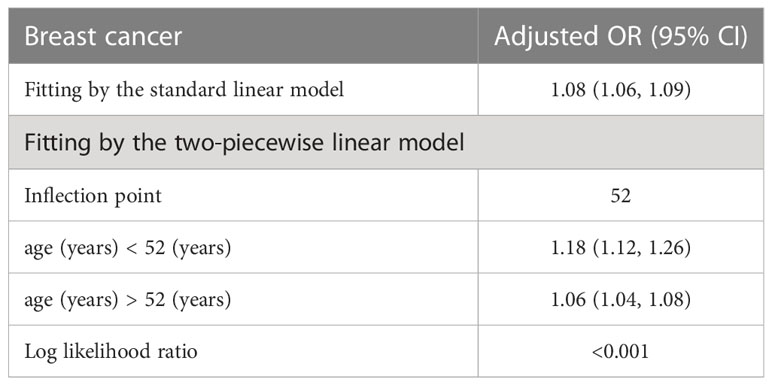
Table 5 Threshold effect analysis of age on breast cancer in female using the two piecewise linear regression model.
Threshold effect analysis of BMI/Age using the standard linear model across different racial/ethnic groups
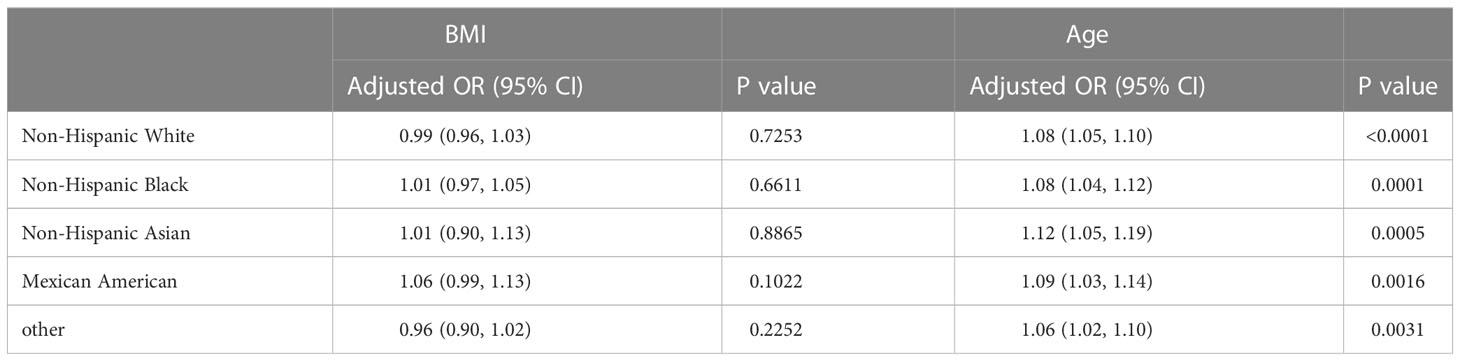
Table 6 presents the results of the threshold effect analysis of BMI/age using the standard linear model for different racial/ethnic groups. The adjusted odds ratios (ORs) with 95% confidence intervals (CIs) and p-values are shown for each group. For Non-Hispanic White individuals, the ORs for BMI and age were 0.99 (95% CI, 0.96-1.03, P=0.7253) and 1.08 (95% CI, 1.05-1.10, P<0.0001), respectively. Similarly, for Non-Hispanic Black individuals, the ORs were 1.01 (95% CI, 0.97-1.05, P=0.6611) for BMI and 1.08 (95% CI, 1.04-1.12, P=0.0001) for age. For Non-Hispanic Asian individuals, the ORs were 1.01 (95% CI, 0.90-1.13, P=0.8865) for BMI and 1.12 (95% CI, 1.05-1.19, P=0.0005) for age. For Mexican American individuals, the ORs were 1.06 (95% CI, 0.99-1.13, P=0.1022) for BMI and 1.09 (95% CI, 1.03-1.14, P=0.0016) for age. For individuals from other racial/ethnic groups, the ORs were 0.96 (95% CI, 0.90-1.02, P=0.2252) for BMI and 1.06 (95% CI, 1.02-1.10, P=0.0031) for age. The results indicate that the association between BMI/age and breast cancer risk varies across different racial/ethnic groups. Table 6; Figures 3, 5 display the results.
Discussion
The present study investigated the associations between diabetes status, BMI, age, and breast cancer risk in a representative sample of US adults, using data from the NHANES. Our analysis revealed significant relationships between diabetes status, BMI, and age with breast cancer risk, with varying associations observed across different racial groups. Our results demonstrated that individuals with Type 2 diabetes had a significantly higher risk of breast cancer compared to those without diabetes. This association persisted even after adjusting for multiple covariates, such as age, race, BMI, and other potential confounders. These findings are in line with previous research indicating that Type 2 diabetes may be associated with an increased risk of breast cancer (25). Possible explanations for this relationship include hyperinsulinemia, insulin resistance, and chronic inflammation, which have been suggested to contribute to breast cancer development and progression (26). In addition, our study showed that individuals with prediabetes had no significant increase in breast cancer risk compared to those without diabetes. This finding emphasizes the need for further research to understand the role of glycemic control and potential interventions to reduce breast cancer risk among individuals with diabetes.
Our threshold effect analysis revealed an inflection point at 21 kg/m² in the relationship between BMI and breast cancer risk. For individuals with a BMI greater than 21 kg/m², the risk of breast cancer increased, whereas those with a BMI less than 21 kg/m² had no significant change in risk. These findings are consistent with previous research demonstrating that higher BMI is associated with an increased risk of postmenopausal breast cancer (27). Several mechanisms have been proposed to explain this relationship, including increased estrogen production in adipose tissue, altered adipokine and insulin signaling, and increased inflammation (28). Our analysis also identified a threshold effect of age on breast cancer risk, with a significant increase in risk observed after the age of 52 years. This finding is in line with existing literature, which has consistently reported that breast cancer risk increases with age, particularly after menopause (29). The increased risk at older ages may be attributed to the accumulation of genetic and epigenetic changes over time, as well as age-related changes in hormone levels and immune function (30).
In our study, we discovered that the relationships between BMI, age, and breast cancer risk exhibited variations across different racial groups. However, it is important to note that the differences in the association between BMI and breast cancer risk among various racial groups were not statistically significant. This finding highlights the complexity of the relationship between BMI and breast cancer risk, and suggests that further research is necessary to better understand the underlying factors that may contribute to these variations, such as differences in body fat distribution, hormone levels, and genetic factors (31). On the other hand, age was found to be significantly associated with breast cancer risk across all racial groups, emphasizing the importance of age as a universal risk factor for breast cancer (32).
Our study has several limitations that should be considered when interpreting the findings. First, the cross-sectional nature of the data precludes establishing causal relationships between diabetes status, BMI, age, and breast cancer risk. Longitudinal studies are needed to confirm these associations and investigate potential underlying mechanisms. Second, the reliance on self-reported data may introduce recall bias, particularly for variables such as age at menarche and age at menopause. Future studies could benefit from objective measures to minimize potential biases. Third, although we adjusted for multiple covariates, residual confounding cannot be ruled out. There may be additional unmeasured factors, such as genetic predisposition, environmental exposures, and lifestyle factors, that contribute to the observed associations.
Despite these limitations, our study provides valuable insights into the relationships between diabetes status, BMI, age, and breast cancer risk in a diverse US population. Our findings highlight the importance of considering these factors in breast cancer prevention strategies and suggest that targeted interventions for individuals with Type 2 diabetes may be beneficial in reducing breast cancer risk. Moreover, our results underscore the need for further research to understand the mechanisms underlying the associations between diabetes status, BMI, age, and breast cancer risk, as well as the potential differences in these relationships across racial groups.
Future research should aim to replicate our findings in larger, prospective cohorts and investigate the biological pathways linking diabetes, obesity, and age to breast cancer development. Additionally, intervention studies targeting glycemic control, weight management, and other modifiable risk factors could help determine the effectiveness of such strategies in reducing breast cancer risk among individuals with diabetes and those with higher BMI. Finally, understanding the racial differences in the relationships between these factors and breast cancer risk may contribute to the development of more targeted and effective prevention strategies for different populations.
Conclusion
In conclusion, our study demonstrates significant associations between diabetes status, BMI, age, and breast cancer risk in a representative US population. These findings highlight the importance of considering these factors in breast cancer prevention efforts and suggest that targeted interventions may be warranted to reduce breast cancer risk among individuals with Type 2 diabetes and those with higher BMI. Further research is needed to elucidate the underlying mechanisms and identify effective prevention strategies for diverse populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
XS, QZ, and KK: They were responsible for drafting the work and critically revising it for important intellectual content. PH, XL, JL, YY, CS, VY, SL, and MM: They contributed to the acquisition, analysis, and interpretation of data for the work. ZL and MZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1059303/full#supplementary-material
References
1. World Cancer Research Fund. Breast cancer statistics . World Cancer Research Research Fund International. Available at: https://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics (Accessed August 17, 2020).
2. Centers for Disease Control and Prevention. Breast cancer statistics . Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/cancer/breast/statistics/index.htm (Accessed August 17, 2020).
3. The American Cancer Society medical and editorial content team. About breast cancer (2020). AmericanCancerSociety,Inc. Available at: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (Accessed August 17, 2020).
4. Nagy TR, Maistro S, Encinas G, Katayama MLH, Pereira GFL, Gaburo-Júnior N, et al. Germline and somatic mutations in postmenopausal breast cancer patients. Clinics (Sao Paulo). (2021) 76:e2837. doi: 10.6061/clinics/2021/e2837
5. Paths to prevention the California breast cancer primary prevention plan . Available at: https://escholarship.org/uc/item/1v2745z0 (Accessed 1 December 2020).
6. Hiatt RA, Engmann NJ, Balke K, Rehkopf DH. Paradigm II multidisciplinary panel. a complex systems model of breast cancer etiology: the paradigm II conceptual model. Cancer Epidemiol Biomarkers Prev (2020) 29(9):1720–30. doi: 10.1158/1055-9965.EPI-20-0016
7. Mahmoud MF, El Ashry FE, El Maraghy NN, Fahmy A. Studies on the antidiabetic activities of momordica charantia fruit juice in streptozotocin-induced diabetic rats. Pharm Biol (2017) 55(1):758–65. doi: 10.1080/13880209.2016.1275026
8. Zhang X, Zhang Y, Yu Y, Liu J, Yuan Y, Zhao Y, et al. Convergence and divergence of genetic and modular networks between diabetes and breast cancer. J Cell Mol Med (2015) 19(5):1094–102. doi: 10.1111/jcmm.12504
9. Chou PC, Choi HH, Huang Y, Fuentes-Mattei E, Velazquez-Torres G, Zhang F, et al. Impact of diabetes on promoting the growth of breast cancer. Cancer Commun (Lond). (2021) 41(5):414–31. doi: 10.1002/cac2.12147
10. Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer (2012) 19(6):793–803. doi: 10.1530/ERC-12-0242
11. De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg (2013) 100(11):1421–9. doi: 10.1002/bjs.9229
12. Vona-Davis L, Rose DP. Type 2 diabetes and obesity metabolic interactions: common factors for breast cancer risk and novel approaches to prevention and therapy. Curr Diabetes Rev (2012) 8(2):116–30. doi: 10.2174/157339912799424519
13. Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, Morgan A, et al. Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer (2010) 17(2):539–51. doi: 10.1677/ERC-09-0221
14. Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE. The impact of diabetes on survival following breast cancer. Breast Cancer Res Treat (2008) 109(2):389–95. doi: 10.1007/s10549-007-9654-0
15. Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol (2013) 24(10):2506–14. doi: 10.1093/annonc/mdt224
16. Hou G, Zhang S, Zhang X, Wang P, Hao X, Zhang J. Clinical pathological characteristics and prognostic analysis of 1,013 breast cancer patients with diabetes. Breast Cancer Res Treat (2013) 137(3):807–16. doi: 10.1007/s10549-012-2404-y
17. American Diabetes Association. 2. classification and diagnosis of diabetes. Diabetes Care (2017) 40(Suppl 1):S11–24. doi: 10.2337/dc17-S005
18. Tseng CH, Chong CK, Tai TY. Secular trend for mortality from breast cancer and the association between diabetes and breast cancer in Taiwan between 1995 and 2006. Diabetologia (2009) 52(2):240–6. doi: 10.1007/s00125-008-1204-8
19. Tseng CH. Diabetes and breast cancer in Taiwanese women: a detection bias? Eur J Clin Invest (2014) 44(10):910–7. doi: 10.1111/eci.12323
20. Ferrannini E, Gastaldelli A, Iozzo P. Pathophysiology of pre-diabetes. Med Clin North Am (2011) 95(2):327–39. doi: 10.1016/j.mcna.2010.11.005
21. Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The national health and nutrition examination survey: sample design, 1999-2006. Vital Health Stat 2. (2012) 155):1–39.
22. CDC’s National Center for Health Statistics. NCHS research ethics review board (ERB) approval. CDC/National Center for Health Statistics. Available at: https://www.cdc.gov/nchs/nhanes/irba98.htm.
23. NHANES-national health and nutrition examination survey homepage. Available at: https://www.cdc.gov/nchs/nhanes/ (Accessed April 20, 2017).
24. Torres MDT, Andrade GP, Sato RH, Pedron CN, Manieri TM, Cerchiaro G, et al. Natural and redesigned wasp venom peptides with selective antitumoral activity. Beilstein J Org Chem (2018) 14:1693–703. doi: 10.3762/bjoc.14.144
25. Tharakan S, Zimmerman B, Ru M, Blanter J, Cascetta K, Tiersten A. Diabetes and metformin association with recurrence score in a Large oncotype database of breast cancer patients. Oncology (2020) 98(8):589–92. doi: 10.1159/000506076
26. Mazidi M, Kengne AP, Mikhailidis DP, Toth PP, Ray KK, Banach M. Dietary food patterns and glucose/insulin homeostasis: a cross-sectional study involving 24,182 adult americans. Lipids Health Dis (2017) 16(1):192. doi: 10.1186/s12944-017-0571-x
27. Martinez JA, Chalasani P, Thomson CA, Roe D, Altbach M, Galons JP, et al. Phase II study of metformin for reduction of obesity-associated breast cancer risk: a randomized controlled trial protocol. BMC Cancer (2016) 16:500. doi: 10.1186/s12885-016-2551-3
28. Provatopoulou X, Georgiou GP, Kalogera E, Kalles V, Matiatou MA, Papapanagiotou I, et al. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer (2015) 15:898. doi: 10.1186/s12885-015-1898-1
29. Tapia KA, Garvey G, Mc Entee M, Rickard M, Brennan P. Breast cancer in Australian indigenous women: incidence, mortality, and risk factors. Asian Pac J Cancer Prev (2017) 18(4):873–84. doi: 10.22034/APJCP.2017.18.4.873
30. Canin B, Freund KM, Ganz PA, Hershman DL, Paskett ED. Disparities in breast cancer care and research: report from a breast cancer research foundation sponsored workshop, 9-10 October 2014. NPJ Breast Cancer (2015) 1:15013. doi: 10.1038/npjbcancer.2015.13
31. Ooi BNS, Loh H, Ho PJ, Milne RL, Giles G, Gao C, et al. The genetic interplay between body mass index, breast size and breast cancer risk: a mendelian randomization analysis. Int J Epidemiol (2019) 48(3):781–94. doi: 10.1093/ije/dyz124
Keywords: diabetes status, prediabetes, type 2 diabetes, obesity, breast cancer, NHANES
Citation: Sun X, Zhang Q, Kadier K, Hu P, Liu X, Liu J, Yan Y, Sun C, Yau V, Lowe S, Meng M, Liu Z and Zhou M (2023) Association between diabetes status and breast cancer in US adults: findings from the US National Health and Nutrition Examination Survey. Front. Endocrinol. 14:1059303. doi: 10.3389/fendo.2023.1059303
Received: 01 October 2022; Accepted: 16 May 2023;
Published: 21 June 2023.
Edited by:
Michaela Ruth Reagan, MaineHealth, United StatesReviewed by:
Loredana Mauro, University of Calabria, ItalyXiao Yang, Peking University People’s Hospital, China
Copyright © 2023 Sun, Zhang, Kadier, Hu, Liu, Liu, Yan, Sun, Yau, Lowe, Meng, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meirong Zhou, em1yMjQ3QGNzdS5lZHUuY24=
†These authors have contributed equally to this work
 Xingyu Sun
Xingyu Sun Qiangsong Zhang3†
Qiangsong Zhang3† Pengcheng Hu
Pengcheng Hu Xiaozhu Liu
Xiaozhu Liu Chenyu Sun
Chenyu Sun Vicky Yau
Vicky Yau Scott Lowe
Scott Lowe Muzi Meng
Muzi Meng