- 1Zhejiang Chinese Medical University, Fourth Clinical Medical College, Hangzhou, China
- 2Hangzhou Traditional Chinese Medicine Hospital Affiliated to Zhejiang Chinese Medical University, The Department of General Surgery, Hangzhou, China
- 3Affiliated Hangzhou First People’s Hospital Zhejiang University School of Medicine, Department of Oncological Surgery, Hangzhou, China
Objective: To explore the clinical significance of blood immune indexes in predicting lateral lymph node metastasis (LLNM) of thyroid papillary carcinoma (PTC).
Methods: The pathological data and preoperative blood samples of 713 patients that underwent thyroid surgery at affiliated Hangzhou First People’s Hospital Zhejiang University School of Medicine from January 2013 to June 2021 were collected as the model group. The pathological data and preoperative blood samples of 177 patients that underwent thyroid surgery in the same hospital from July 2021 to October 2021 were collected as the external validation group. Univariate and multivariate logistic regression analyses were used to determine the independent risk factors of LLNM in PTC patients. A predictive model for assessing LLNM in PTC patients was established and externally validated using the external data.
Results: According to univariate and multivariate logistic regression analyses, tumor diameter (P < 0.001, odds ratios (OR): 1.205, 95% confidence interval (CI): 1.162–1.249) and the preoperative systemic immune-inflammation index (SII) (P = 0.032, OR: 1.001, 95% CI: 1.000–1.002) were independent risk factors for distinguishing LLNM in PTC patients. When the Youden index was the highest, the area under the curve (AUC) was 0.860 (P < 0.001, 95% CI: 0.821–0.898). The externally validated AUC was 0.827 (P < 0.001, 95% CI: 0.724–0.929), the specificity was 86.4%, and the sensitivity was 69.6%. The calibration curve and the decision curve indicated that the model had good diagnostic value.
Conclusion: Blood immune indexes can reflect the occurrence of LLNM and the biological behavior of PTC. The predictive model established in combination with SII and tumor diameter can effectively predict the occurrence of LLNM in PTC patients.
Introduction
According to the latest global cancer data released by the World Health Organization (GLOBOCAN 2018), in 2018, more than 567,000 new cases of thyroid cancer were diagnosed in 20 regions in the world, and the incidence of thyroid cancer in women was as high as 10.2/100,000, approximately three times that of men (1). Papillary thyroid carcinoma (PTC) is the most common type of thyroid cancer. Although the prognosis is good, 9.1–38% of patients with PTC develop lateral lymph node metastasis (LLNM), which seriously affects the prognosis (2). We found that 5.6% of PTC patients with tumor diameter <10 mm had lateral neck lymph node metastasis (3). A recent single-sequencing study has found that tumor ecosystems and immune microenvironments can affect the occurrence and development of PTC (4). Meanwhile, other studies have reported that preoperative blood immune indexes, such as the neutrophil/lymphocyte ratio (NLR), lymphocyte/monocyte ratio (LMR), platelet/lymphocyte ratio (PLR), and preoperative systemic immune-inflammatory index (SII), can be used to assess immune function and diagnose tumors, and all have a good effect on tumor prediction (5, 6). However, the correlation of LLNM in PTC with systemic immunity and blood immune indexes is not clear. The purpose of this study is to investigate the clinical significance of preoperative blood immune indexes on LLNM in PTC patients, as well as to establish and verify the clinical predictive model and to guide the diagnosis and treatment of metastatic PTC.
Materials and methods
General information
The relevant data of 713 patients that underwent thyroid surgery at Affiliated Hangzhou First People’s Hospital Zhejiang University School of Medicine (Hangzhou, China) from January 2013 to June 2021 were collected for retrospective analysis. The relevant data of 177 patients that underwent thyroid surgery at the same hospital from July 2021 to October 2021 were collected for external verification analysis. The inclusion criteria were as follows: (1) postoperative pathological diagnosis of PTC and (2) unilateral gland lobe plus isthmus or full gland lobectomy, coupled with dissection of the lateral cervical lymph nodes, with evidence of lymph node metastasis in the lateral cervical region. The exclusion criteria were as follows: (1) evidence of other malignancies, (2) evidence of blood system diseases, (3) evidence of autoimmune system diseases, (4) evidence of preoperative acute or chronic inflammation or other diseases that could affect routine blood tests, and (5) incomplete clinical information.
Data collection
Fasting peripheral blood was collected from the patients in the morning one week before thyroid surgery and processed by the Mindary BC-6800 automatic blood cell analyzer (Shenzhen Mairui Biomedical Electronics Co., Ltd., Shenzhen, China) and supporting reagents. Peripheral blood cells were classified and counted by the sheath flow impedance method, laser light scattering method, and flow cytometry combined with fluorescent staining. The absolute value of neutrophils, absolute value of lymphocytes, absolute value of monocytes, and absolute value of platelets were obtained. The absolute value of neutrophils/absolute value of lymphocytes (NLR), absolute value of platelets/absolute value of lymphocytes (PLR), absolute value of lymphocytes/absolute value of monocytes (LMR), and absolute value of platelets × absolute value of neutrophils/absolute value of lymphocytes (SII) were calculated.
Color doppler ultrasound machines equipped with a real-time, high-frequency (5-10 MHZ) linear-array probes were used in this study. The maximum diameter of thyroid tumors was interpreted by two sonographers with 3-5 years of experience in ultrasonic diagnosis before surgery. If the results of the two patients were inconsistent, the third sonographer with 3-5 years of experience in ultrasonic diagnosis would interpret and compare the results to determine the final preoperative maximum diameter of the thyroid tumor.
The general information of the patients, including gender and age, and the tumor information, including evidence of unilateral or bilateral tumors, multifocality and evidence of postoperative pathology confirmed LLNM, were collected.
Statistical methods
SPSS software (version 26; IBM, Armonk, NY, USA) was used for the statistical analysis of the data. For measurement data, a normality test was carried out, expressed as mean ± standard deviation (± S), and a t-test was used for comparisons between two independent groups. A nonparametric test was used for comparisons between groups. The count data were expressed as frequency and percentage, and comparisons between groups were carried out with the chi-square (X2) test. By the t-test and X2 test, the influencing factors showing statistically significant differences were taken as the risk factors related to LLNM in PTC patients.
Logistic multivariate regression analysis was used to analyze the risk factors identified by univariate regression analysis. Differences in the preoperative blood inflammatory indexes and pathological characteristics of LLNM were analyzed, and the independent risk factors affecting LLNM in PTC patients were identified. The odds ratios (OR) and 95% confidence intervals (95% CI) were determined.
According to the results of logistic multivariate regression analysis, a predictive model was constructed, and receiver operator characteristic (ROC) curve analysis was used to evaluate the area under the curve (AUC) and its 95% CI, calculate the Youden index (sensitivity + specificity -1), and determine the sensitivity and specificity when the Youden index was at its highest. The predictive model was constructed, and the model was validated by random sampling 1000 times using the bootstrap method. The models were fitted using the external validation data, and AUC, 95% CI, sensitivity, and specificity were assessed. Lastly, a calibration plot was constructed. The performance of the predictive model was evaluated by the Hosmer–Lemeshow test, AUC, and goodness-of-fit. Decision curve analysis (DCA) was used to validate the clinical net benefit rate of the predictive model. Statistical analysis was performed using R studio (version 4.1.0). P < 0.05 was considered statistically significant.
Results
General information
A total of 713 patients were enrolled in this study. According to the inclusion and exclusion criteria, 702 patients with PTC were enrolled in the final model group, including 173 males (24.6%) and 529 females (75.4%), with an average age of 46.4 years. In the model group, there were 106 patients (15.1%) with LLNM and 596 (84.9%) without LLNM. The external validation group was comprised of 171 patients, including 46 males (26.9%) and 125 females (73.1%), with an average age of 45.5 years. In the external validation group, there were 22 patients (12.9%) with LLNM and 149 (87.1%) without LLNM. The general characteristics of the patients are shown in Table 1.
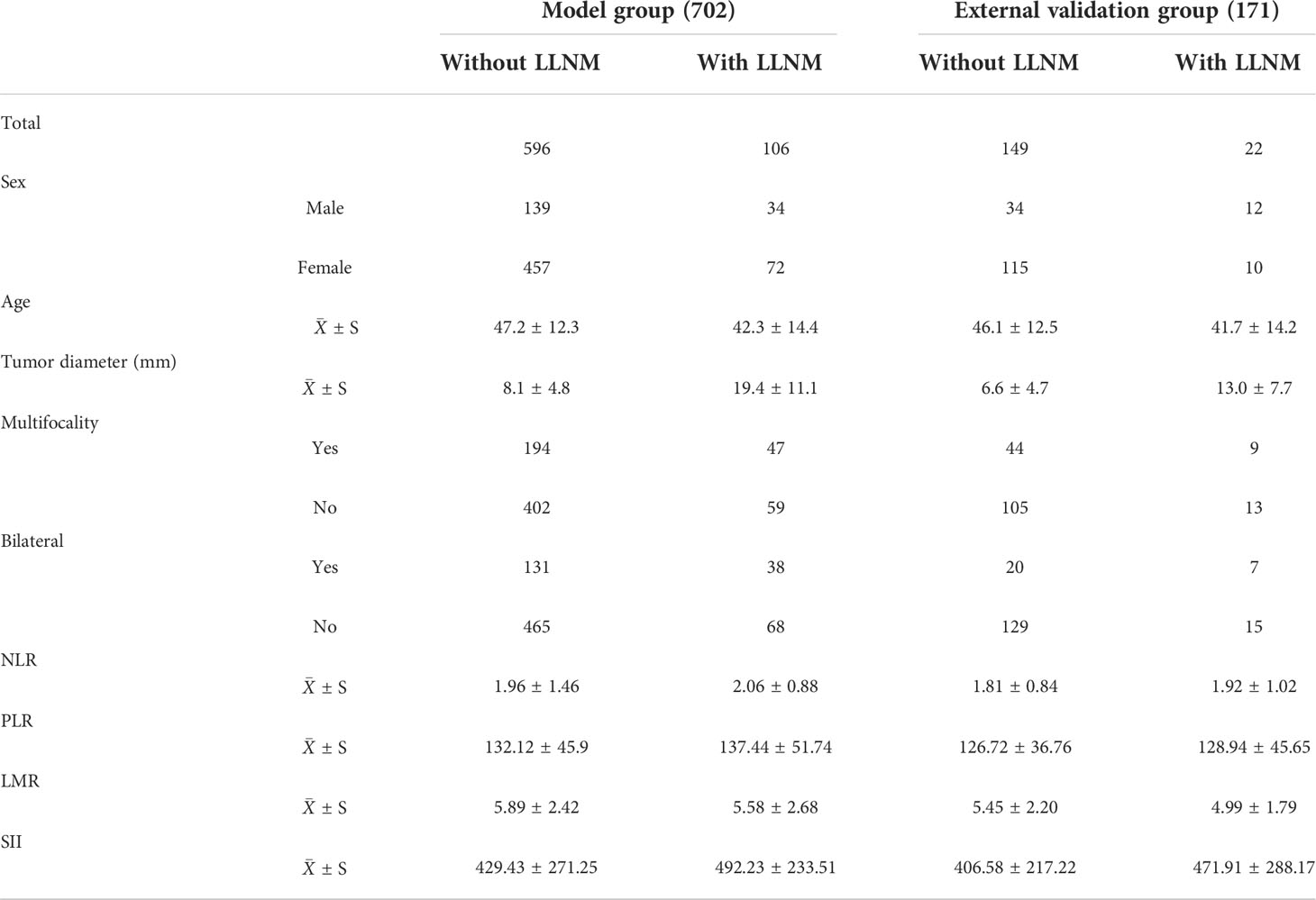
Table 1 General information of papillary thyroid carcinoma patients in model and external validation groups.
Screening of predictive model variables
Gender, age, tumor diameter, multifocality, bilateral tumor, NLR, PLR, LMR, and SII were included in the analyses that employed the t-test, nonparametric test, and X2 test. The results of univariate regression analysis showed that age, tumor diameter, SII, bilateral tumor, and multifocality were significantly associated with LLNM in PTC (P < 0.05). However, there were no significant differences between gender, PLR, NLR, LMR, and LLNM in PTC (Table 2).
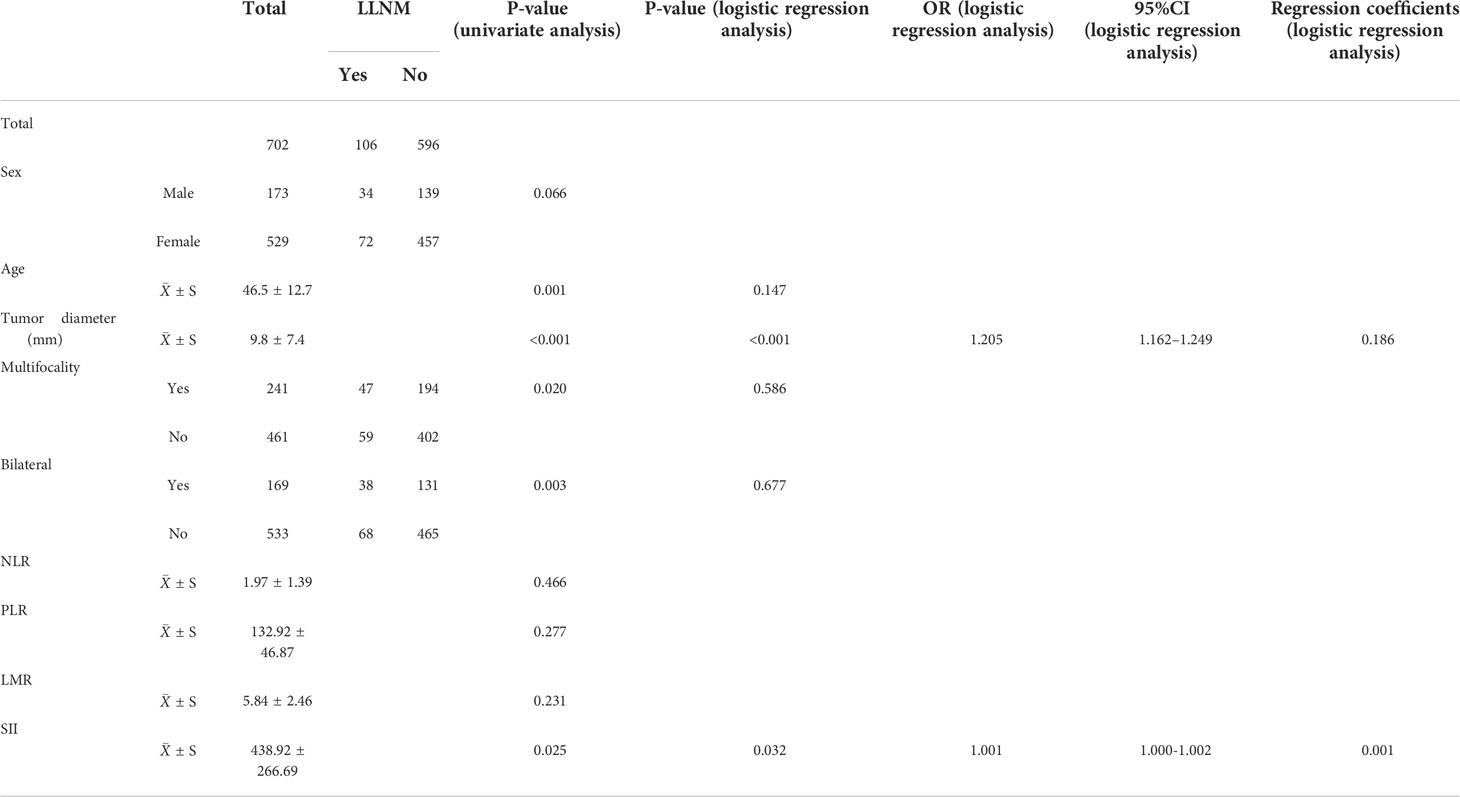
Table 2 General information and results of univariate logistic regression analysis of 702 papillary thyroid carcinoma patients.
Age, tumor diameter, SII, bilateral tumor, and multifocality were included in logistic multivariate regression analysis. The results showed that tumor diameter (P < 0.001, OR: 1.205, 95% CI: 1.162–1.249) and SII (P = 0.032, OR: 1.001, 95% CI: 1.000–1.002) were independent risk factors for LLNM in PTC.
Model establishment
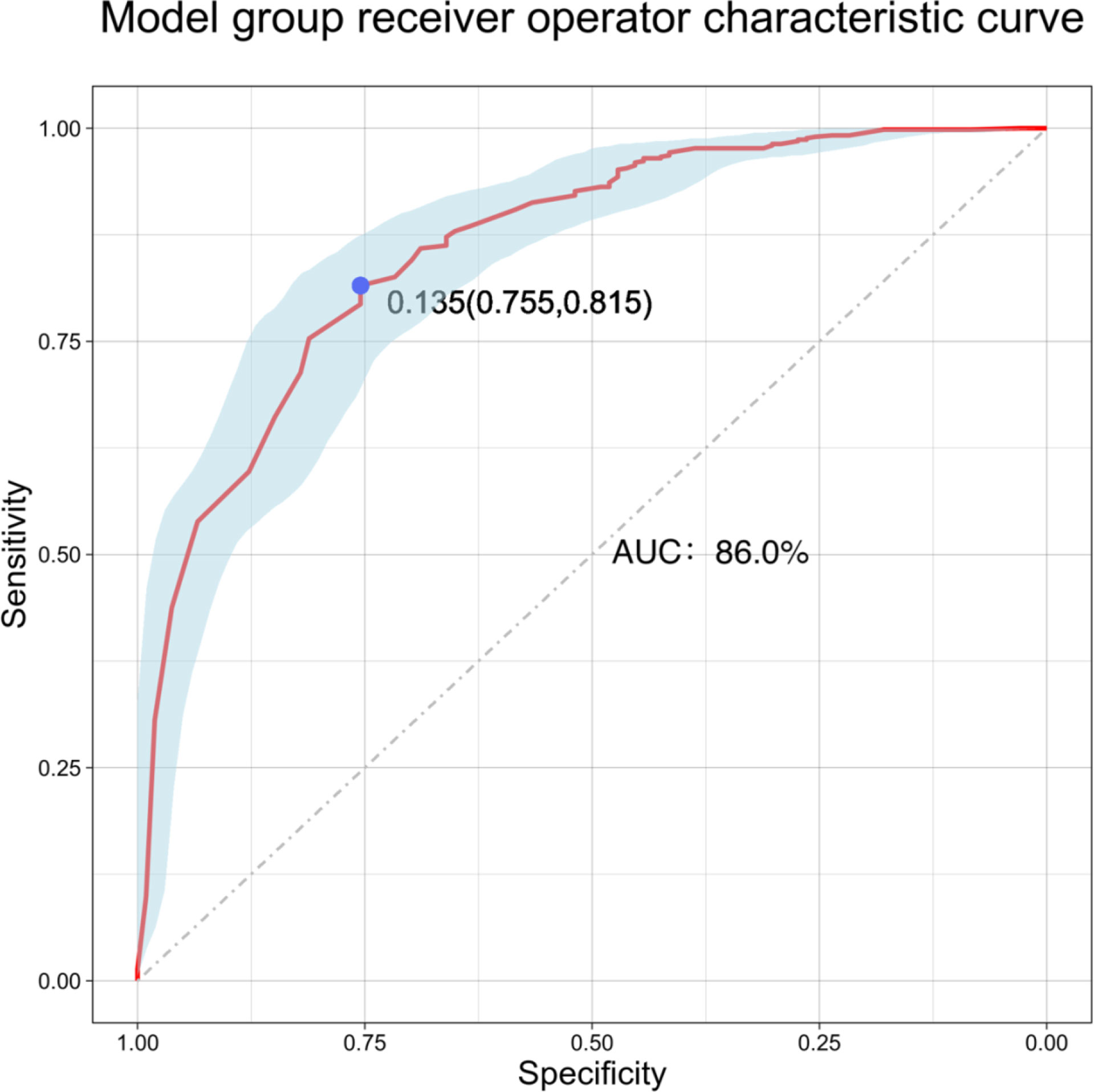
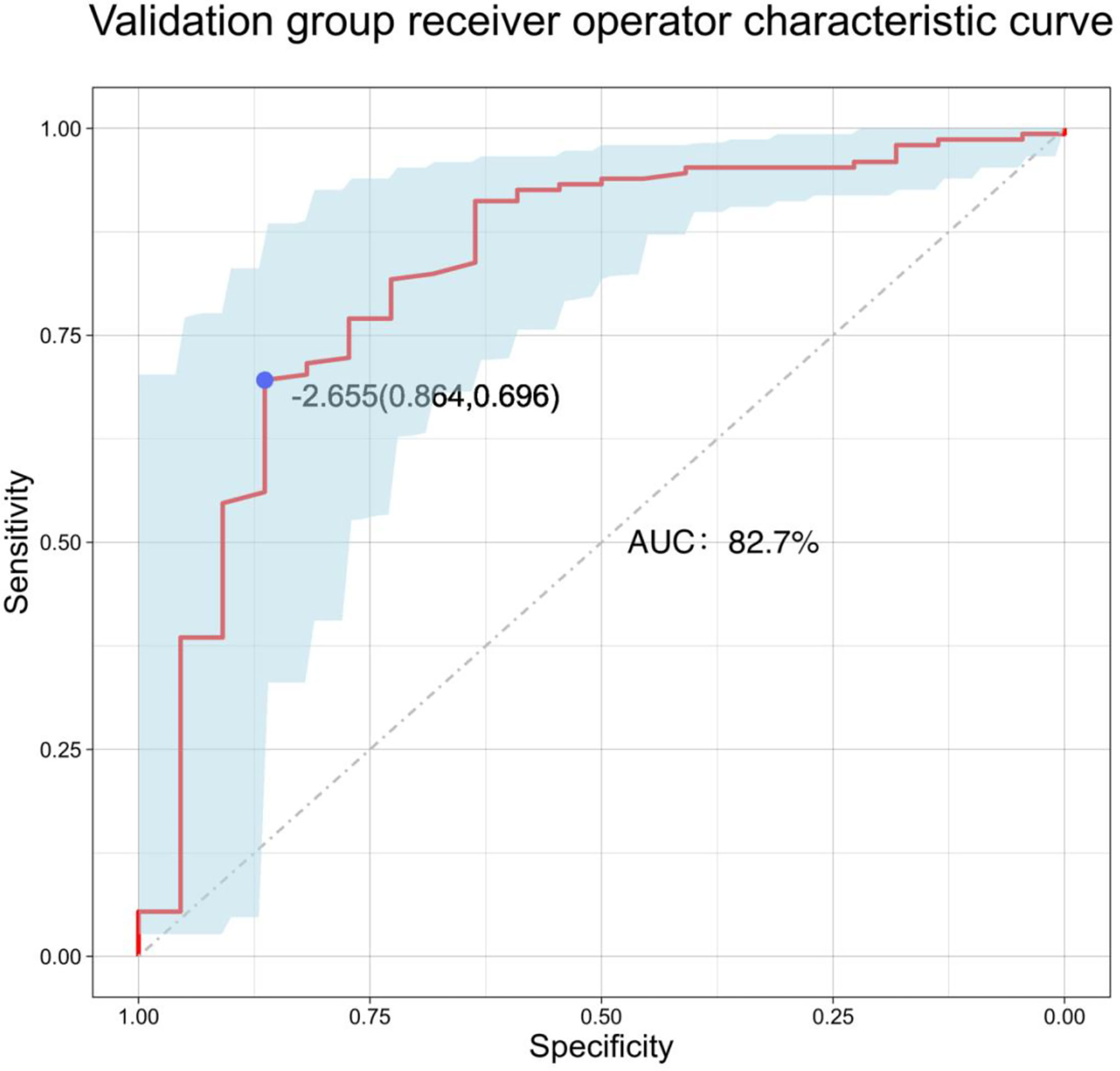
The results of logistic multivariate regression analysis were included in the construction of the predictive model, and the independent risk factors of LLNM in PTC included tumor diameter and SII. Thus, these independent predictors were combined to establish a predictive model to distinguish LLNM in PTC (Figure 1). The ROC curve was established according to the logistic multivariate regression results. The results showed that the AUC of the two risk factors, namely, tumor diameter and SII, for predicting LLNM in PTC patients was 0.860 (P < 0.001, 95% CI: 0.821–0.898). When the Youden index was the highest, the specificity was 75.5% and the sensitivity was 81.5%, which showed good discrimination (Figure 2).
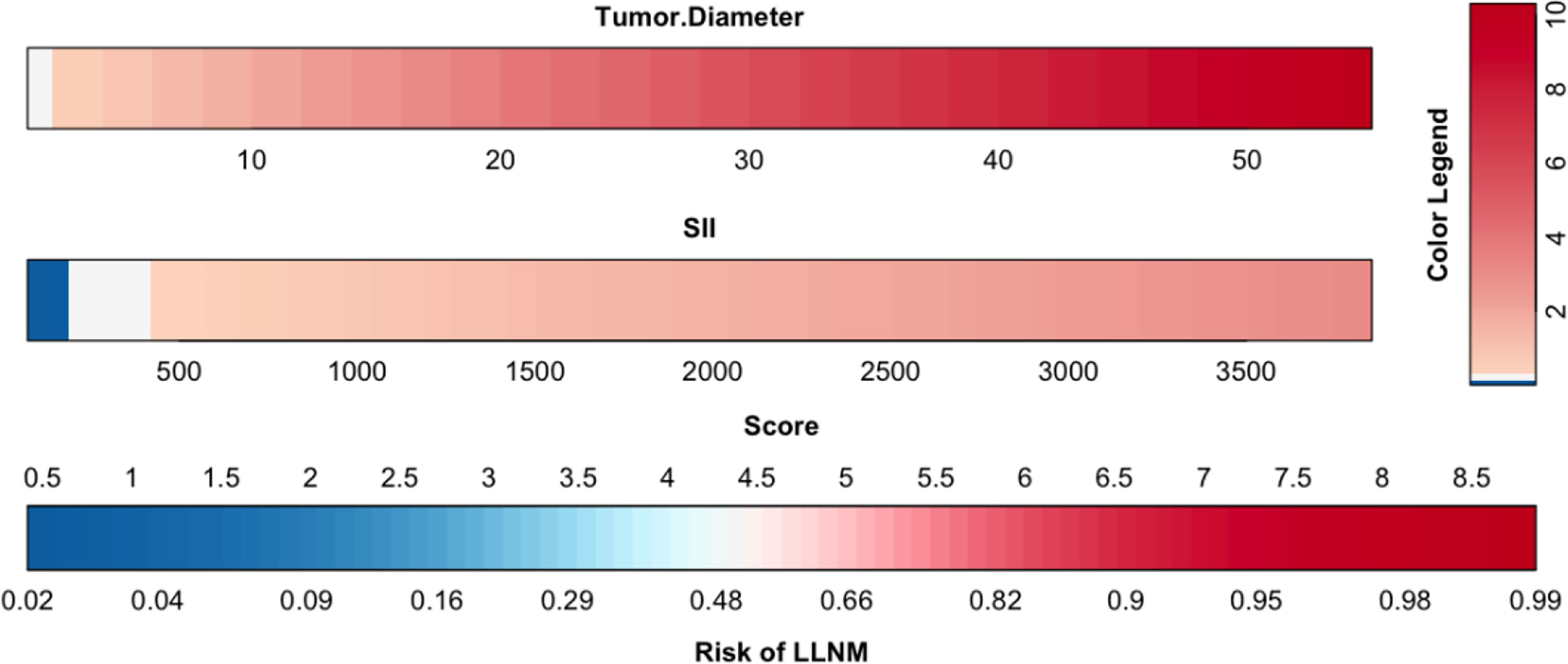
Figure 1 Clinical predictive model of lateral lymph node metastasis in papillary thyroid carcinoma patients.
Model checking
Model internal inspection
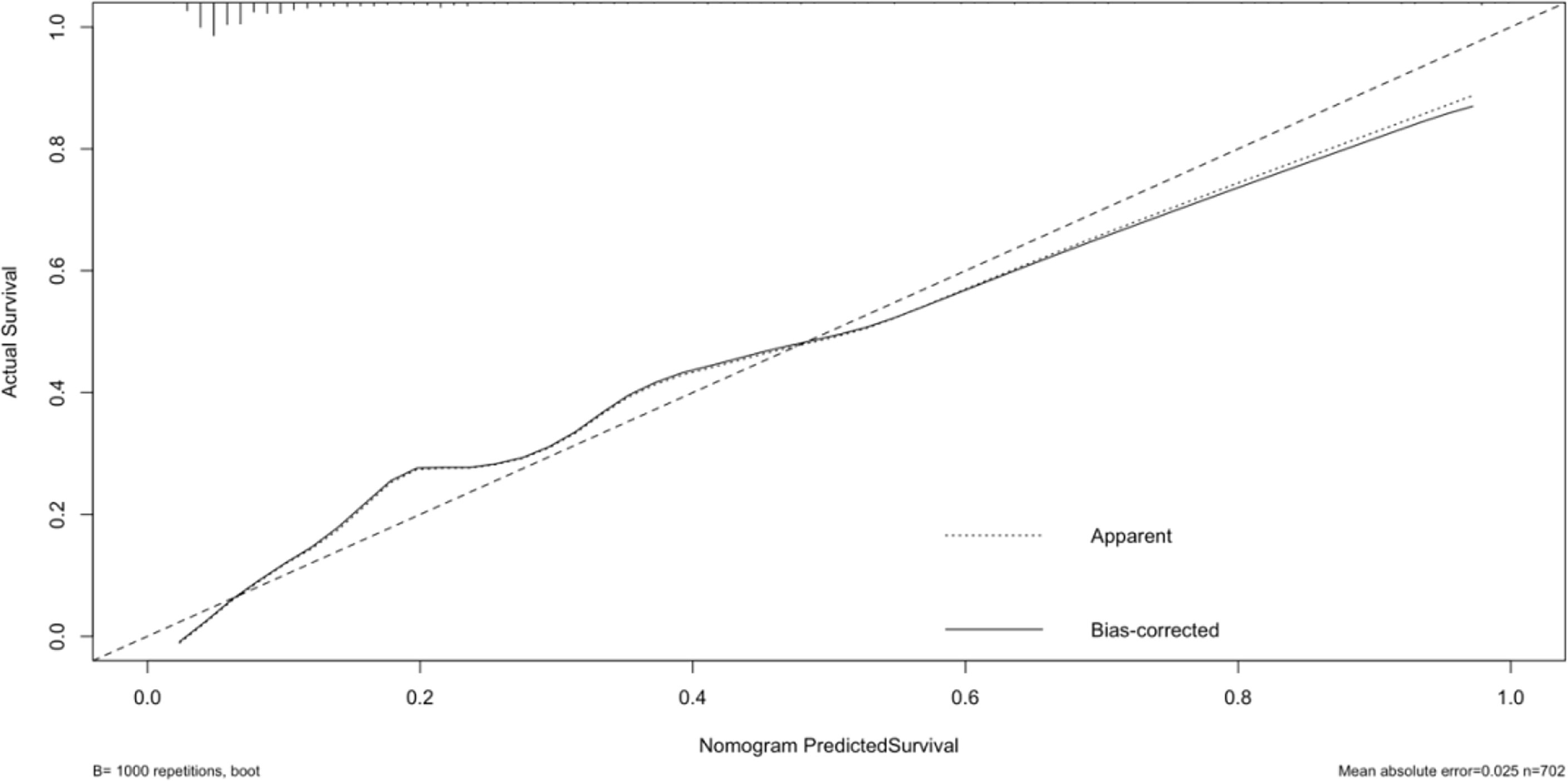
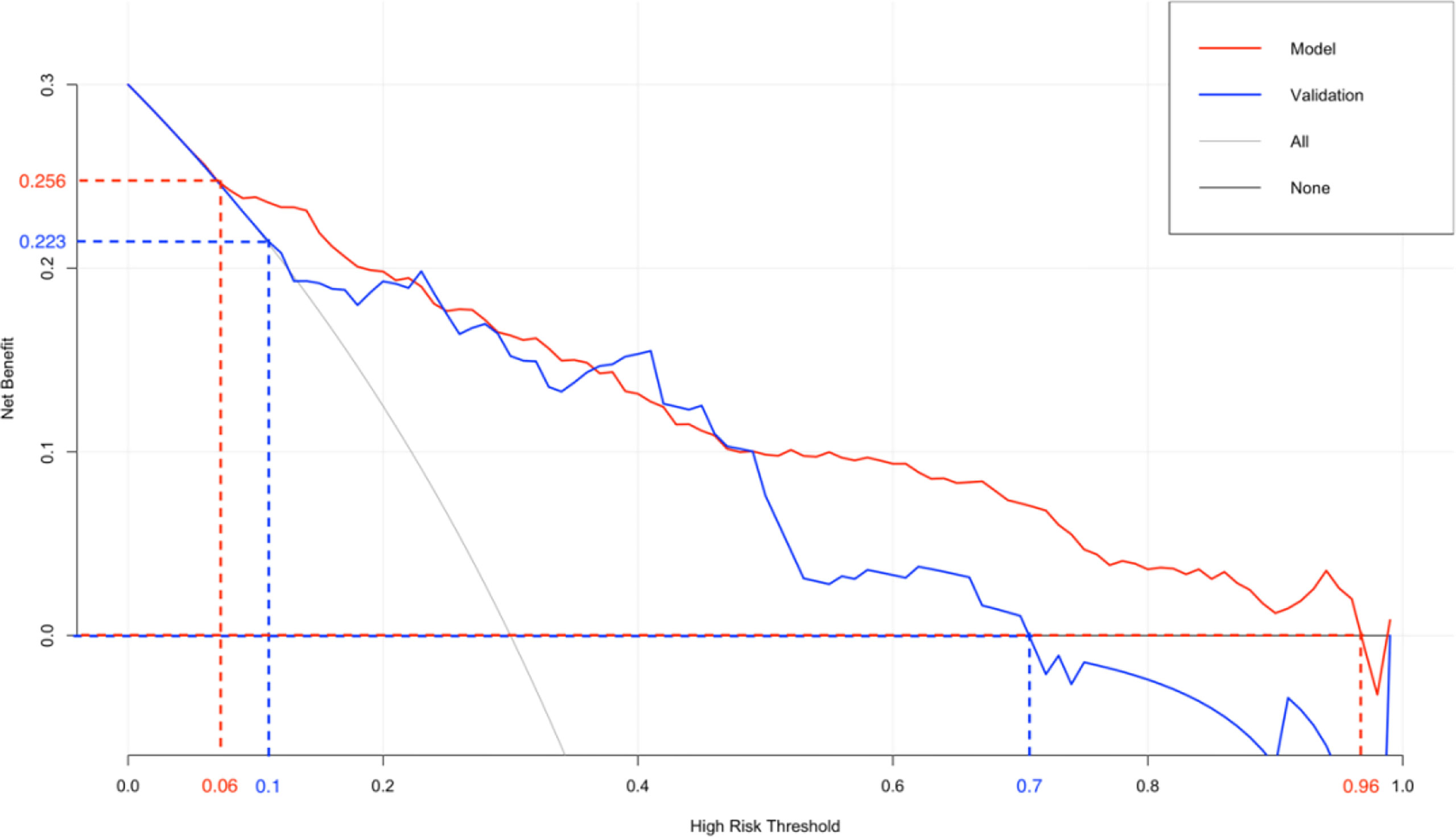
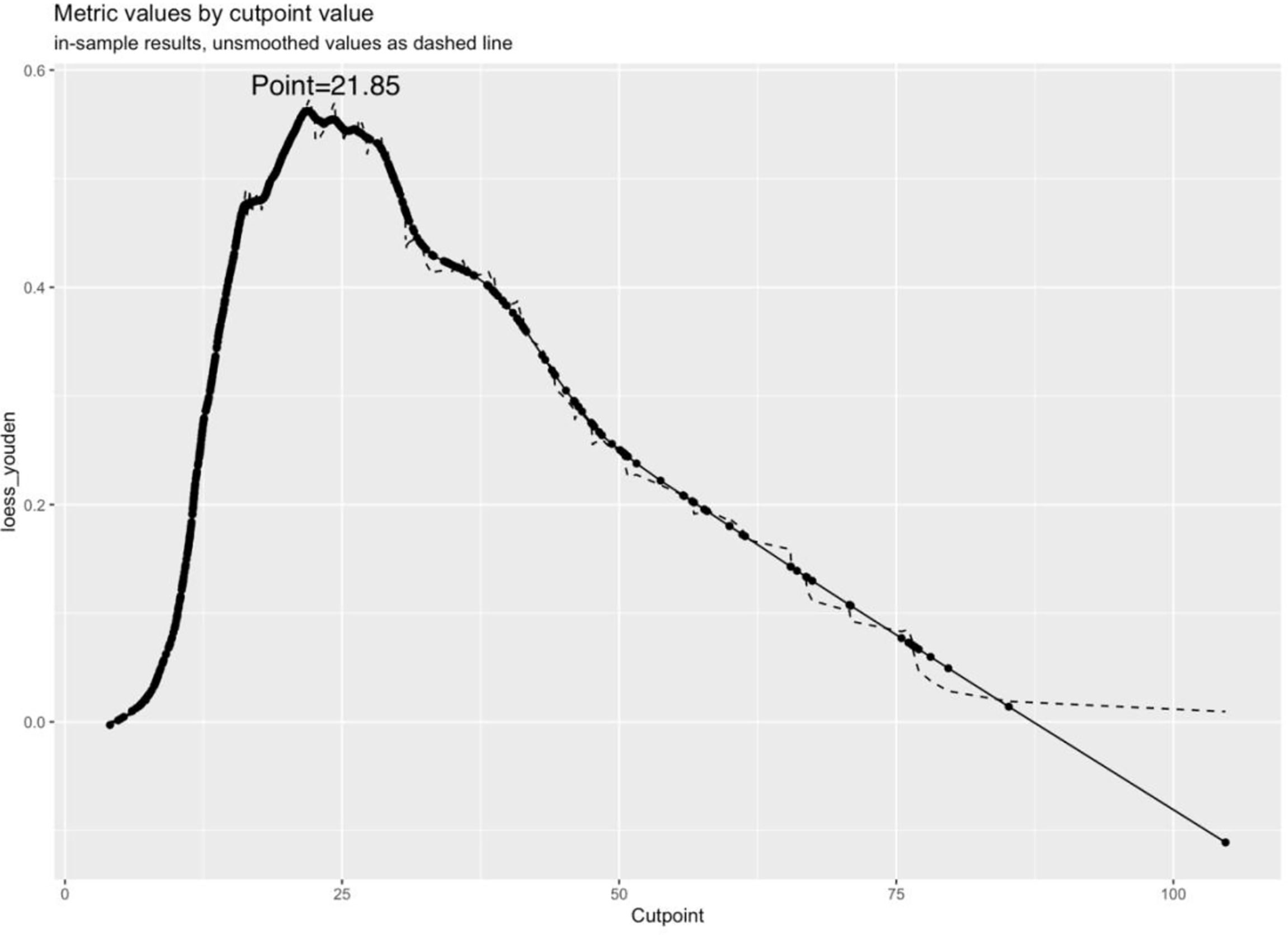
The original dataset was re-sampled 1000 times by the bootstrap method to create a simulated dataset. The calibration curve showed that the predictive model had good agreement between the predicted discrimination rate and the actual discrimination rate of LLNM, with a mean absolute error of 0.025 (Figure 3). The Hosmer–Lemeshow test showed good goodness-of-fit (P = 0.448), indicating that the predictive model had good calibration ability. By DCA, no patient required further intervention, that is, no patient underwent lateral neck lymph node dissection, and the net benefit was 0. All represented clinical intervention, that is, all patients underwent lateral neck lymph node dissection, and the net benefit was the reverse of the backslash. When the predictive model curve was higher than the All, the predictive model had a net clinical benefit. Figure 4 shows that in the net benefit range of 0–0.3, when the model risk threshold was in the range of 6–96%, the benefit of the intervention was significantly higher than that of no intervention or complete intervention, suggesting that the model has certain clinical utility (Figure 4). The Youden index of the nomogram was also calculated, and the cut-off point of the nomogram to predict LLNM in PTC was 21.85 (Figure 5).
Model external inspection
A total of 171 external validation data were included to fit the model. The results showed that the AUC of the validation group was 0.827, and the 95% CI was 0.724–0.929 (P < 0.001). When the Youden index was at its highest, the specificity was 86.4% and the sensitivity was 69.6% (Figure 6). The Hosmer–Lemeshow test showed that the external validation data test model had better calibration (P = 1). By DCA, within a net benefit range of 0–0.3, when the model risk threshold was in the range of 10–70%, this predictive model yielded better clinical benefits (Figure 4).
Discussion
Several studies have reported that PTC patients with LLNM have a higher risk of local recurrence and a poorer prognosis than those without LLNM (7, 8). The American Thyroid Association in 2015 (9) and the Chinese Society of Clinical Oncology in 2021 recommended dissection in cases of definite LLNM, that is, therapeutic lymph node dissection. For PTC patients without LLNM, lateral neck lymph node dissection does not reduce the risk of recurrence. In addition, it increases the risk of postoperative complications such as lymphatic leakage, damage to accessory nerves, and impaired neck muscle function. Presently, the preoperative assessment of the presence or absence of LLNM and the specific classification of metastatic lymph nodes are mainly based on imaging studies such as ultrasonography and computed tomography (CT). However, the diagnostic performance of both methods is limited because they often fail to detect metastatic lymph nodes, thus affecting the prognosis of patients (10). Therefore, a more convenient and efficient method was examined to assess LLNM in PTC patients before surgery and to formulate an appropriate method of cervical lymph node dissection. On the premise of ensuring the survival of patients, reducing the risk of postoperative complications, as well as recurrence, is critical in the diagnosis and treatment of PTC.
In recent years, many studies have demonstrated that immune function, inflammation, and tumor occurrence are closely related. For instance, immune-related factors can affect the tumor microenvironment and promote tumorigenesis (11, 12). Based on preoperative blood values detected by standard laboratory tests, preoperative immune indicators, including LMR, PLR, and NLR, are used because they can predict the presence of malignant tumors, such as lung cancer and prostate cancer, and predict the survival of patients (13, 14). In this study, several preoperative blood immune indexes, including LMR, PLR, NLR, and SII, reflected the immune function of PTC patients. Ceylan et al. studied the significance of NLR and PLR on PTC and showed that both parameters were associated with the clinicopathological invasiveness of PTC, and both could be used as markers for the risk assessment of PTC patients (5). However, in this study, LMR, PLR, and NLR had no significant effect on LLNM in PTC.
The absolute value of platelets × absolute value of neutrophils/absolute value of lymphocytes is a comprehensive index of platelets, neutrophils, and lymphocytes, and it can reflect immune function to a certain extent. Zheng et al. analyzed different indicators of 2415 patients with malignant tumors and reported that a high SII was a predictor of a worse prognosis of patients with malignancies (15). In a study of PTC patients, Muzza et al. reported the elevated expression of inflammatory molecules (16), while Zhang et al. studied 406 patients and showed that SII was a marker for predicting central lymph node metastasis (17). A high SII may indicate an impaired immune response, thus affecting the biological behavior of PTC. However, there is no study using SII to predict LLNM in PTC patients. This study is the first to find that a high SII is of great significance in PTC patients with LLNM. Combined with the results of previous studies, this may be related to the following points: (1) PTC patients show increased platelet counts. Platelets can induce endothelial cell proliferation, while increased levels of platelet factor and platelet endothelial growth factor can stimulate tumor cell proliferation and differentiation. In a study of thyroid diseases, Martin et al. reported that preoperative platelet counts were higher in patients with PTC than in those with benign thyroid lesions (18). Moreover, studies by Zhang et al. and Adewuyi et al. demonstrated that platelet-derived growth factor receptor α could up-regulate the mitogen-activated protein kinase/extracellular regulated protein kinase pathway, which promotes lymph node metastasis and invasion in PTC patients (19, 20). In other words, thrombocytosis is associated with the invasive ability of PTC. (2) PTC patients show increased neutrophil counts. Increased neutrophil counts associate with the release of several inflammatory mediators, which promote tumor development and changes in the tumor microenvironment. He et al. performed RNA sequencing and methylated RNA immunoprecipitation sequencing on PTC tissues and demonstrated that a reduction in the expression of METTL3, an RNA methyltransferase, can induce the accumulation of interleukin 8 and the recruitment of tumor-associated neutrophils, thus affecting the growth of papillary thyroid tumors (21), while Kucuk et al. reported that PTC with neutrophil infiltration was more aggressive, associating with a shorter disease-free survival time (22). In other words, with the increase in the number of neutrophils, the invasiveness of PTC increases. (3) PTC patients show decreased lymphocyte counts. In some solid tumors, as the number of lymphocytes decreases, the body’s ability to fight tumors also decreases (23). Pu et al. performed single-cell sequencing on PTC cells and demonstrated that CD8+ T cells, natural killer cells, and other lymphocytes interacted with tumor cells, indicating that immune regulation plays important roles in the cellular ecology and development of PTC (4). Zhu et al. confirmed that PTC was accompanied by marked T lymphocyte depletion, in which the number of lymphocytes in the blood of PTC patients was reduced (24). Therefore, a high SII has a certain relationship with the increased invasive ability of PTC, which can be used as a marker to predict LLNM in PTC patients.
Feng et al. studied the predictors of lymph node metastasis in the central and lateral cervical regions and observed that tumor T stage, that is, tumor diameter, was an independent risk factor of LLNM that affected the recurrence-free survival of PTC patients (25). Several studies have reported that the larger the tumor diameter, the higher the risk of LLNM in PTC (26, 27), which is consistent with the results of this study. These findings suggest that PTC patients with a larger tumor diameter should undergo rigorous preoperative examinations, together with an evaluation of the status of LLNM to avoid misdiagnosis and reduce the risk of local recurrence after surgery.
The nomogram is based on logistic multivariate regression analysis, and it integrates the influencing factors and converts the regression coefficients into line segments in proportion to the graph. Scores are assigned according to the value of each influencing factor, and the total scores are determined to obtain the predicted value of the individual events through a conversion relationship between the total score and the outcome probability. In this study, for the first time, preoperative blood immune indexes were used to predict LLNM in PTC, and it was confirmed that tumor diameter and SII were independent risk factors for LLNM in PTC. At the same time, a predictive model for assessing LLNM in PTC patients was constructed and verified. The AUC of the predictive model was 0.860, and a nomogram was constructed (Figure 1). The external validation data of our research center fit the model. The AUC was 0.827, and the model prediction effect was good. The specificity (80.2% vs 86.4%) and sensitivity (77.0% vs 69.6%) were also higher in the model and external validation groups. The bootstrap method (Figure 3) indicated that the calibration curve was close to the ideal curve, indicating that the model had a good predictive ability. The Hosmer–Lemeshow test confirmed the internal and external verification, and indicated that the predictive model had good discrimination and calibration. In recent years, several investigators have used DCA to evaluate the clinical benefit rate. This study shows that in the model group, with a risk threshold of 6–96%, the predictive model showed a significant clinical benefit, and in the external validation group, with a risk threshold of 10–70%, there was also a certain clinical benefit (Figure 4). In summary, this predictive model can better predict LLNM in PTC patients, and it is convenient for use in clinical settings, with high accuracy and repeatability. It can be used as a preoperative assessment method for LLNM in PTC patients to formulate individualized cervical lymph node dissection protocols.
The shortcomings of this study are as follows: (1) The newly established predictive model consisted of a single-center study, and subsequent studies should include more centers, involve large-sample verification, and improve the accuracy of the predictive model. (2) Several preoperative blood indicators were not examined, and follow-up studies should incorporate additional indicators to improve the predictive accuracy of the model. (3) This study predicted LLNM in PTC patients but did not examine the specific lateral neck regions. In the future, a prediction method for specific classifications should be devised.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Affiliated Hangzhou First People’s Hospital Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
DL had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Concept and design: all authors. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: LZ and TZ. Critical revision of the manuscript for important intellectual content: LZ, TZ, and DL. Statistical analysis: LZ and TZ. Supervision: YZ and DL.
Funding
This work was supported by the Project of Medical Scientific and Technology Program in Hangzhou (grant number: A20200432), and Zhejiang Province Public Welfare Technology Application Research Project (grant number: GF22H165705).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Huang Y, Yin Y, Zhou W. Risk factors for central and lateral lymph node metastases in patients with papillary thyroid micro-carcinoma: Retrospective analysis on 484 cases. Front Endocrinol (Lausanne) (2021) 12:640565. doi: 10.3389/fendo.2021.640565
3. Luo Y, Zhao Y, Chen K, Shen J, Shi J, Lu S, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest (2019) 42(2):227–36. doi: 10.1007/s40618-018-0908-y
4. Pu W, Shi X, Yu P, Zhang M, Liu Z, Tan L, et al. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun (2021) 12(1):6058. doi: 10.1038/s41467-021-26343-3
5. Ceylan Y, Kumanlioglu K, Oral A, Ertan Y, Ozcan Z. The correlation of clinicopathological findings and neutrophil-to-Lymphocyte and platelet-to-Lymphocyte ratios in papillary thyroid carcinoma. Mol Imaging Radionucl Ther (2019) 28(1):15–20. doi: 10.4274/mirt.galenos.2018.60490
6. Tokumaru Y, Oshi M, Murthy V, Tian W, Yan L, Angarita FA, et al. Low intratumoral genetic neutrophil-to-lymphocyte ratio (NLR) is associated with favorable tumor immune microenvironment and with survival in triple negative breast cancer (TNBC). Am J Cancer Res (2021) 11(11):5743–55.
7. Kim SK, Park I, Hur N, Rayzah M, Lee JH, Choe J.H, et al. Patterns, predictive factors, and prognostic impact of contralateral lateral lymph node metastasis in N1b papillary thyroid carcinoma. Ann Surg Oncol (2017) 24(7):1943–50. doi: 10.1245/s10434-016-5761-7
8. Chereau N, Buffet C, Tresallet C, Tissier F, Leenhardt L, Menegaux F, et al. Recurrence of papillary thyroid carcinoma with lateral cervical node metastases: Predictive factors and operative management. Surgery (2016) 159(3):755–62. doi: 10.1016/j.surg.2015.08.033
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Lee Y, Kim JH, Baek JH, Jung SL, Park SW, Kim J, et al. Value of CT added to ultrasonography for the diagnosis of lymph node metastasis in patients with thyroid cancer. Head Neck (2018) 40(10):2137–48. doi: 10.1002/hed.25202
11. Jang JH, Park D, Park GS, Kwak DW, Park J, Yu DY, et al. Leukotriene B4 receptor-2 contributes to KRAS-driven lung tumor formation by promoting interleukin-6-mediated inflammation. Exp Mol Med (2021) 1559–1568. doi: 10.1038/s12276-021-00682-z
12. Park S, Zhu J, Altan-Bonnet G, Cheng SY. Monocyte recruitment and activated inflammation are associated with thyroid carcinogenesis in a mouse model. Am J Cancer Res (2019) 9(7):1439–53.
13. Qi J, Zhang J, Ge X, Wang X, Xu L, Liu N, et al. The addition of peripheral blood inflammatory indexes to nomogram improves the predictive accuracy of survival in limited-stage small cell lung cancer patients. Front Oncol (2021) 11:713014. doi: 10.3389/fonc.2021.713014
14. Shui Y, Li M, Su J, Chen M, Gu X, Guo W, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging (Albany NY) (2021) 13(16):20585–97. doi: 10.18632/aging.203449
15. Zheng K, Liu X, Ji W, Lu J, Cui J, Li W, et al. The efficacy of different inflammatory markers for the prognosis of patients with malignant tumors. J Inflammation Res (2021) 14:5769–85. doi: 10.2147/JIR.S334941
16. Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf) (2010) 72(5):702–8. doi: 10.1111/j.1365-2265.2009.03699.x
17. Zhang Z, Xia F, Wang W, Huang Y, Li X. The systemic immune-inflammation index-based model is an effective biomarker on predicting central lymph node metastasis in clinically nodal-negative papillary thyroid carcinoma. Gland Surg (2021) 10(4):1368–73. doi: 10.21037/gs-20-666
18. Martin S, Mustata T, Enache O, Ion O, Chifulescu A, Sirbu A, et al. Platelet activation and inflammation in patients with papillary thyroid cancer. Diagnostics (Basel) (2021) 11(11):1959. doi: 10.3390/diagnostics11111959
19. Zhang J, Wang P, Dykstra M, Gelebart P, Williams D, Ingham R, et al. Platelet-derived growth factor receptor-alpha promotes lymphatic metastases in papillary thyroid cancer. J Pathol (2012) 228(2):241–50. doi: 10.1002/path.4069
20. Adewuyi EE, Deschenes J, Lopez-Campistrous A, Kattar MM, Ghosh S, McMullen TPW, et al. Autocrine activation of platelet-derived growth factor receptor alpha in metastatic papillary thyroid cancer. Hum Pathol (2018) 75:146–53. doi: 10.1016/j.humpath.2018.01.025
21. He J, Zhou M, Yin J, Wan J, Chu J, Jia J, et al. METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Mol Ther (2021) 29(5):1821–37. doi: 10.1016/j.ymthe.2021.01.019
22. Kucuk S, Oltulu P. The relationship between types of inflammatory cells located at the micro-environment of papillary thyroid microcarcinoma prognostic factors. J buon (2021) 26(5):2157–68.
23. Waidhauser J, Nerlinger P, Arndt TT, Schiele S, Sommer F, Wolf S, et al. Alterations of circulating lymphocyte subsets in patients with colorectal carcinoma. Cancer Immunol Immunother (2021) 1937–1947. doi: 10.1007/s00262-021-03127-8
24. Zhu C, Dai Y, Zhang H, Ruan Y, Zhou Y, Dai Y, et al. T Cell exhaustion is associated with the risk of papillary thyroid carcinoma and can be a predictive and sensitive biomarker for diagnosis. Diagn Pathol (2021) 16(1):84. doi: 10.1186/s13000-021-01139-7
25. Feng JW, Yang XH, Wu BQ, Sun DL, Jiang Y, Qu Z, et al. Predictive factors for central lymph node and lateral cervical lymph node metastases in papillary thyroid carcinoma. Clin Transl Oncol (2019) 21(11):1482–91. doi: 10.1007/s12094-019-02076-0
26. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
Keywords: papillary thyroid carcinoma, lateral cervical lymph node, lymph node metastasis, systemic immune-inflammation index, nomogram
Citation: Zhao L, Zhou T, Zhang W, Wu F, Jiang K, Lin B, Zhan S, Hu T, Tang T, Zhang Y and Luo D (2022) Blood immune indexes can predict lateral lymph node metastasis of thyroid papillary carcinoma. Front. Endocrinol. 13:995630. doi: 10.3389/fendo.2022.995630
Received: 16 July 2022; Accepted: 15 August 2022;
Published: 31 August 2022.
Edited by:
Salvatore Sorrenti, Department of Surgical Sciences, Sapienza University of Rome, ItalyReviewed by:
Gregorio Scerrino, University of Palermo, ItalyRoberto Cirocchi, University of Perugia, Italy
Copyright © 2022 Zhao, Zhou, Zhang, Wu, Jiang, Lin, Zhan, Hu, Tang, Zhang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dingcun Luo, bGRjNjVAMTYzLmNvbQ==
 Lingqian Zhao
Lingqian Zhao Tianhan Zhou
Tianhan Zhou Wenhao Zhang
Wenhao Zhang Fan Wu
Fan Wu Kecheng Jiang
Kecheng Jiang Bei Lin1
Bei Lin1