
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 15 September 2022
Sec. Neuroendocrine Science
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.994545
Background: Restless Legs Syndrome (RLS) is closely related to poorer sleep quality. Vitamin D can regulate sleep regulation, cell proliferation, and differentiation. To measure whether vitamin D has predictive value for poor sleep quality in RLS was our aim in this study.
Methods: To analyze the serum levels of 25-hydroxyvitamin D [25(OH)D] in 95 RLS patients. We used the Pittsburgh Sleep Quality Index (PSQI) to measure sleep quality. Subjects had been divided into a normal and poor-sleeper groups according to the PSQI score. Using correlation and regression analysis to explore underlying etiologies that affect sleep disorder in RLS patients.
Results: Patients in the poor-sleeper group had significantly lower vitamin D levels in comparison to the normal group. The serum vitamin D levels were negative correlate with PSQI scores after adjusting for confounding factors. In addition, regression analysis showed that vitamin D could act as a predictor for sleep disorders in RLS patients (odds ratio [OR] = 0.008, p = 0.004). The area under the curve (AUC), cut-off value, sensitivity, and specificity of serum vitamin D was 0.967 (95% CI 0.935–0.998), 16.84 ng/ml, 87.5%, and 93.7% by receiver operating characteristic (ROC) analysis.
Conclusion: Our study confirmed the relationship between poorer sleep quality and vitamin D in RLS. However, the causal relationship between vitamin D deficiency and RLS is currently inconclusive. The effect of vitamin D supplementation is needed to confirm as the therapeutic strategies for sleep disorders in RLS patients in future work.
Restless legs syndrome (RLS) is a sleep-related movement disorder (1), which manifests clinically as a strong desire to move both lower extremities, with symptoms often worsening at night (2). The global prevalence of RLS is 0.03% to 24.2% (3–5). RLS can be divided into two categories: primary and secondary forms. Additionally, previous studies had found that secondary RLS was associated with chronic renal failure (6), type 2 diabetes (7), etc. However, the pathogenesis of primary RLS has not been fully established, but genetic factors, impaired dopaminergic neuron function and brain iron deficiency are recognized to be associated with the development of primary RLS (8).
It has been reported that RLS patients also suffer from sleep disturbance and depression (9), which can strongly impact the quality of life (10). Additionally, previous studies had demonstrated that vitamin D can be able to regulate dopamine levels and its metabolites (11, 12). However, vitamin D deficiency is common in RLS patients (13, 14), which may participate pathogenesis and progress of the disease (13, 15). Furthermore, lower serum vitamin D level is related to worse sleep quality, and the severity of disease in RLS patients (12, 13), suggesting that vitamin D may have predictive value for pooter sleep quality in RLS patients. Indeed, vitamin D deficiency is associated with the risk of sleep disorders (16). Apart from that, vitamin D supplementation also is related to improving mood and quality of sleep (17). Therefore, based on these findings, we hypothesized that vitamin D can affect the sleep quality of RLS patients.
To our knowledge, the association of vitamin D with poorer sleep quality in RLS patients remains unclear. Therefore, in the present study, to investigate the predictive efficacy of vitamin D for poorer sleep quality in RLS patients was our aim, which may be contributing to the development of therapeutic strategies to diagnose and prevent RLS with poorer sleep quality.
Our study was conducted from January 2018 to October 2021, including 95 patients with RLS who derived from the Henan Provincial People’s Hospital.
The Research Ethics Committee of Henan Provincial People’s Hospital approved our study. Additionally, all the participants signed the written informed consent forms.
Diagnosis of RLS according to International Classification of Sleep Disorder (ICSD-3) (18) and the International Restless Legs Syndrome Study Group (IRLSSG) (2, 19).
The following patients were excluded: 1) combined with endocrine and metabolic Diseases; 2) Patients with secondary RLS, for instance, Parkinson’s disease (PD), drug-induced factors (for instance: antidepressant treatment with SSRI, etc.), iron deficiency, chronic renal failure; 3) combined with other sleep disorders such as narcolepsy, or obstructive sleep apnea (OSA); 4) patients taking drugs that affected serum vitamin D levels; 5) history of mental disorders; 6) pregnant and lactating women.
To measure the disease severity and quality of life in RLS, we used The International Restless Legs Scales (IRLS) (20), and The Restless Legs Syndrome Quality of Life Questionnaire (QoL-RLS) (21), respectively. Additionally, to measure depression and anxiety, the 24-item Hamilton Depression Rating Scale (HAMD24), and the 14-item Hamilton Anxiety Scale (HAMA14) were used.
To measure the sleep quality, the Pittsburgh sleep quality index (PSQI) was used (22). Participants were classified into non-sleep disorders (normal, PSQI ≤ 5) and sleep disorders (poor-sleeper, PSQI > 5) groups, due to the PSQI score (22).
To measure serum 25-hydroxyvitamin D (25(OH)D) levels, we used the Enzyme-Linked Immunosorbent Assay (ELISA). Additionally, in our hospital, insufficient vitamin D level has been defined as < 20 ng/ml respectively.
The SPSS Statics 22.0 software for analysis. Representation of non-normal date in interquartile range (M, P25, P75) and normal date in mean ± standard deviation (SD). The student’s-t test for normally distributed continuous data, while The Mann-Whitney U test for the non-normally date. Categorical data are expressed in amount (%). Spearman or Pearson to analyze the clinical characteristics and vitamin D in RLS. To determine the predictive value of serum vitamin D levels for RLS sleep disorders, receiver operating characteristic (ROC) curve analysis was used for evaluation. P < 0.05 was deemed statistically significant.
According to the PSQI score, subjects can be divided into the poor-sleeper group (n=63) and normal group (n=32). The baseline data of all subjects are summarized in Table 1. Compared to the normal group, the serum vitamin D level was lower in the poor-sleepers. In addition, no difference was found between both groups in age, sex, BMI, SBP, DBP, IRLS, QoL-RLS, disease duration, education duration, and sunlight exposure (p > 0.05) (Figure 1).
Correlation analysis showed that serum vitamin D was negatively correlated with PSQI, HAMA14, HAMD24, and IRLS (p < 0.05). The partial correlation analysis was used to show that serum vitamin D was still positively associated with PSQI, HAMA14, HAMD24, and IRLS (p < 0.05), after adjustment for the factors of age, gender, BMI, disease duration, education duration, and sunlight exposure. There was no significant association between vitamin D levels and sex, age, BMI, disease duration, education duration, and sunlight exposure among the two groups (p > 0.05). The correlation analysis was shown in Table 2.
Three regression models of regression analysis were shown in Table 3. In Model 1, serum vitamin D was an independent risk predictor for poorer sleep quality in RLS (p < 0.001; odds ratio=0.141; 95% confidence interval: 0.058–0.338). In Model 2, after further adjustment for age, sex and BMI, serum vitamin D was still an independent risk predictor for poorer sleep quality in RLS (p < 0.001; OR=0.046, 95%CI: 0.009). In Model 3, after adjustment for age, sex, BMI, disease duration, education duration and, sunlight exposure, serum vitamin D was also an independent risk predictor for poorer sleep quality in RLS (p = 0.004; OR=0.008, 95%CI:0.000,0.215).
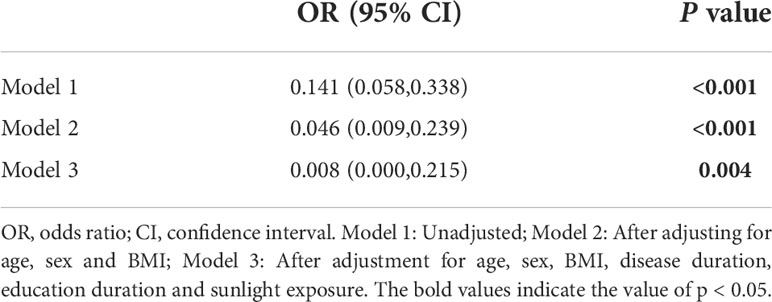
Table 3 Regression analysis of serum vitamin D and the risk of developing poorer sleep quality in RLS.
ROC analysis was performed to determine the predictive value of serum vitamin D for poorer sleep quality in RLS patients, which was shown in Figure 2. The area under the curve (AUC) was 0.967 (95% CI 0.935–0.998). In addition, the cut-off value of serum vitamin D was 16.84 ng/ml with a sensitivity of 87.5% and specificity of 93.7%.
Our study mainly found that serum vitamin D levels were significantly lower in poor-sleeper patients. Additionally, correlation regression analysis found that serum vitamin D can act as an independent predictor for sleep disorders in RLS. According to the ROC analysis, we can hypothesize that serum vitamin D may be a potential biomarker for predicting sleep disorders in RLS patients with high sensitivity and specificity.
The exact pathophysiology of RLS is not fully established. Previous neuroimaging studies had demonstrated that dopaminergic dysfunction was associated with the pathogenesis of RLS (23–25). In addition, growing evidence suggests that iron deficiency plays an important role in the pathologic features and symptoms of RLS, influenced by a combination of environmental factors and genetic inheritance (26, 27). A growing body of evidence had demonstrated that iron can be a first-line treatment option for RLS (28–30). Moreover, there is also evidence of the link between iron deficiency and nigrostriatal dopamine dysfunction (31), which contributes to understanding the pathophysiology of the disease. However, brain iron deficiency associated with vitamin D has not been adequately studied.
The prevalence of the poor-sleepers was found to be about 66% in our study, which is in line with most previous reports (9, 32). Previous studies confirmed that most RLS patients experience sleep disorders, mainly complaining of difficulty falling asleep (33). In addition, previous studies have reported that vitamin D deficiency was correlated with worse sleep quality in RLS patients (12, 13), and our results also suggested a statistically significant negative correlation between serum vitamin D level and PSQI points. Additionally, numerous clinical and epidemiological studies have shown that sleep disorder was a risk factor for depression in RLS patients (34, 35), which was consistent with our finding that the HAMA14 and HAMD24 values were significantly higher in the poor-sleepers of RLS.
Vitamin D is involved in the synthesis and release of neurotransmitters and acts as a neuroprotective role (36). Recent studies have found that vitamin D deficiency plays a role in the pathogenesis and development of RLS (13, 15). Previous studies have reported that the serum vitamin D level was correlated with the disease severity in RLS patients (12, 13), and our results also suggested a statistically significant negative correlation between serum vitamin D level and IRLS points. Previous studies confirmed that vitamin D deficiency was an independent risk factor associated with RLS (37). The incidence of RLS is significantly higher in patients with vitamin D deficiency (15). In contrast to these findings, Jimenez-Jimenez et al. (38) found that compared with HCs, serum levels of vitamin D were significantly higher in idiopathic RLS patients.
Currently, randomized clinical trials on vitamin D supplementation and RLS are not only small in the number of studies, but also have conflicting results (39, 40). In future studies, multi-center, large-sample clinical studies are needed to further illustrate the therapeutic effects of vitamin D supplementation on sleep quality and symptoms of RLS patients.
There are increasing evidence that vitamin D can regulate the circadian rhythm of sleep (41, 42). Several magnetic resonance imaging (MRI) studies had revealed an increased glutamic acid in the thalamus of RLS patients, which also was associated with sleep quality (43–45), providing insight into the role of glutamate and dopamine in regulating sleep–wake disorder (46). The pathogenesis of the regulatory role of vitamin D in sleep disorder with RLS patients is largely unknown. Interestingly, the results of a study revealed the death of dopaminergic neurons can be caused by decreased glutathione content (47). Furthermore, vitamin D can upregulate glial cell line-derived neurotrophic factor (GDNF) expression (48), which plays an essential role in the development of dopaminergic neuron (49). We hypothesized that vitamin D can induce and promote the growth, development and differentiation of dopaminergic neurons, which may affect mood and sleep-wake cycles.
Several studies have shown that physiological levels of vitamin D can inhibit the production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and IL-10 (50). A recent study on RLS has revealed increased circulating levels of inflammatory cytokines (51). A large number of studies have investigated the neuroinflammation was related to sleep disorders (46). The anti-inflammatory effects of vitamin D may be another explanation for vitamin D deficiency that can lead to sleep disorder in the disease. Additionally, the production of melatonin can be regulated by vitamin D (13), which is the primary sleep-regulating hormone (52). Several lines of evidence reveal that melatonin can benefit patients with sleep disorders related to various neurological disorders (53, 54). However, more research is needed on the more complex relationship between vitamin D and sleep disorders.
There are some limitations. First, our study was unable to show a causal relationship between vitamin D deficiency and RLS and further prospective investigation is needed. Second, our study participants were only primary RLS patients. Finally, in vitro and in vivo studies on vitamin D development and prevention in RLS patients with sleep disorders are lacking.
Our study is the first to confirm the relationship between vitamin D and poor sleep status in RLS, and it could act as a useful biomarker for the development of therapeutic strategies to diagnose and prevent RLS with poor sleep quality. To confirm the effect of vitamin D supplementation as the therapeutic strategy for sleep disorders in RLS patients is needed in a larger population in future work.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The study was approved by the Research Ethics Committee of Henan Provincial People’s Hospital according to the principles of the Helsinki Declaration. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CG: wrote first draft and statistics. ZY, PX, and XK: statistics and data collection. HZ: conceptualization, resources and supervision. All authors approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ito E, Inoue Y. The international classification of sleep disorders, third edition. American academy of sleep medicine. includes bibliographies and index. Nihon Rinsho (2015) 73(6):916–23.
2. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med (2014) 15(8):860–73. doi: 10.1016/j.sleep.2014.03.025
3. Koo BB. Restless leg syndrome across the globe: Epidemiology of the restless legs Syndrome/Willis-ekbom disease. Sleep Med Clin (2015) 10(3):189–205. doi: 10.1016/j.jsmc.2015.05.004
4. Didriksen M, Rigas AS, Allen RP, Burchell BJ, Di Angelantonio E, Nielsen MH, et al. Prevalence of restless legs syndrome and associated factors in an otherwise healthy population: results from the Danish blood donor study. Sleep Med (2017) 36:55–61. doi: 10.1016/j.sleep.2017.04.014
5. Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev (2012) 16(4):283–95. doi: 10.1016/j.smrv.2011.05.002
6. Merlino G, Lorenzut S, Gigli GL, Romano G, Montanaro D, Moro A, et al. A case-control study on restless legs syndrome in nondialyzed patients with chronic renal failure. Mov Disord (2010) 25(8):1019–25. doi: 10.1002/mds.23010
7. Merlino G, Fratticci L, Valente M, Del Giudice A, Noacco C, Dolso P, et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep (2007) 30(7):866–71. doi: 10.1093/sleep/30.7.866
8. Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J (1053) 2013:402–10:89. doi: 10.1136/postgradmedj-2012-131634
9. Xu Y, Wen H, Li J, Yang J, Luo K, Chang L. The relationship between sleep disorders, anxiety, depression, and cognitive function with restless legs syndrome (RLS) in the elderly. Sleep Breath (2021). doi: 10.1007/s11325-021-02477-y
10. Didato G, Di Giacomo R, Rosa GJ, Dominese A, de Curtis M, Lanteri P. Restless legs syndrome across the lifespan: Symptoms, pathophysiology, management and daily life impact of the different patterns of disease presentation. Int J Environ Res Public Health (2020) 17(10). doi: 10.3390/ijerph17103658
11. Cui X, Pelekanos M, Liu PY, Burne TH, McGrath JJ, Eyles DW. The vitamin d receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain. Neuroscience (2013) 236:77–87. doi: 10.1016/j.neuroscience.2013.01.035
12. Balaban H, Yildiz OK, Cil G, Senturk IA, Erselcan T, Bolayir E, et al. Serum 25-hydroxyvitamin d levels in restless legs syndrome patients. Sleep Med (2012) 13(7):953–7. doi: 10.1016/j.sleep.2012.04.009
13. Liu HM, Chu M, Liu CF, Zhang T, Gu P. Analysis of serum vitamin d level and related factors in patients with restless legs syndrome. Front Neurol (2021) 12:782565. doi: 10.3389/fneur.2021.782565
14. Mansourian M, Rafie N, Khorvash F, Hadi A, Arab A. Are serum vitamin d, calcium and phosphorous associated with restless leg syndrome? a systematic review and meta-analysis. Sleep Med (2020) 75:326–34. doi: 10.1016/j.sleep.2020.08.022
15. Oran M, Unsal C, Albayrak Y, Tulubas F, Oguz K, Avci O, et al. Possible association between vitamin d deficiency and restless legs syndrome. Neuropsychiatr Dis Treat (2014) 10:953–8. doi: 10.2147/NDT.S63599
16. Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The association between vitamin d deficiency and sleep disorders: A systematic review and meta-analysis. Nutrients (2018) 10(10). doi: 10.3390/nu10101395
17. Huiberts LM, Smolders K. Effects of vitamin d on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep Med Rev (2021) 55:101379. doi: 10.1016/j.smrv.2020.101379
18. Sateia ML. International classification of sleep disorders-third edition: Highlights and modifications. Chest (2014) 146(5):1387–94. doi: 10.1378/chest.14-0970
19. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. a report from the restless legs syndrome diagnosis and epidemiology workshop at the national institutes of health. Sleep Med (2003) 4(2):101–19. doi: 10.1016/S1389-9457(03)00010-8
20. Sharon D, Allen RP, Martinez-Martin P, Walters AS, Ferini Strambi L, Hogl B, et al. Validation of the self-administered version of the international restless legs syndrome study group severity rating scale - the sIRLS. Sleep Med (2019) 54:94–100. doi: 10.1016/j.sleep.2018.10.014
21. Walters AS, Frauscher B, Allen R, Benes H, Chaudhuri KR, Garcia-Borreguero D, et al. Review of quality of life instruments for the restless legs syndrome/Willis-ekbom disease (RLS/WED): critique and recommendations. J Clin Sleep Med (2014) 10(12):1351–7. doi: 10.5664/jcsm.4300
22. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res (1989) 28(2):193–213. doi: 10.1016/0165-1781(89)90047-4
23. Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol (2002) 249(2):164–70. doi: 10.1007/PL00007859
24. Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology (1999) 52(5):932–7. doi: 10.1212/WNL.52.5.932
25. Ruottinen HM, Partinen M, Hublin C, Bergman J, Haaparanta M, Solin O, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology (2000) 54(2):502–4. doi: 10.1212/WNL.54.2.502
26. Allen RP, Earley CJ. The role of iron in restless legs syndrome. Mov Disord (2007) 22 Suppl 18:S440–448. doi: 10.1002/mds.21607
27. Cakir T, Dogan G, Subasi V, Filiz MB, Ulker N, Dogan SK, et al. An evaluation of sleep quality and the prevalence of restless leg syndrome in vitamin d deficiency. Acta Neurol Belg (2015) 115(4):623–7. doi: 10.1007/s13760-015-0474-4
28. Auerbach M. The role of intravenous iron for the treatment of restless legs syndrome. Am J Hematol (2014) 89(10):1016. doi: 10.1002/ajh.23820
29. Allen RP, Picchietti DL, Auerbach M, Cho YW, Connor JR, Earley CJ, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-ekbom disease in adults and children: an IRLSSG task force report. Sleep Med (2018) 41:27–44. doi: 10.1016/j.sleep.2017.11.1126
30. Trenkwalder C, Allen R, Hogl B, Clemens S, Patton S, Schormair B, et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol (2018) 17(11):994–1005. doi: 10.1016/S1474-4422(18)30311-9
31. Lozoff B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J Nutr (2011) 141(4):740S–6S. doi: 10.3945/jn.110.131169
32. Cartwright RD. Alcohol and NREM parasomnias: evidence versus opinions in the international classification of sleep disorders, 3rd edition. J Clin Sleep Med (2014) 10(9):1039–40. doi: 10.5664/jcsm.4050
33. Broman JE, Mallon L, Hetta J. Restless legs syndrome and its relationship with insomnia symptoms and daytime distress: epidemiological survey in Sweden. Psychiatry Clin Neurosci (2008) 62(4):472–5. doi: 10.1111/j.1440-1819.2008.01825.x
34. Li Y, Mirzaei F, O'Reilly EJ, Winkelman J, Malhotra A, Okereke OI, et al. Prospective study of restless legs syndrome and risk of depression in women. Am J Epidemiol (2012) 176(4):279–88. doi: 10.1093/aje/kws016
35. Bogan RK, Cheray JA. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med (2013) 125(3):99–111. doi: 10.3810/pgm.2013.05.2636
36. Wang M, Cui C, Jin F. Research progress on the correlation between vitamin d and neurological disorders. Turk Neurosurg (2021).
37. Wali S, Alsafadi S, Abaalkhail B, Ramadan I, Abulhamail B, Kousa M, et al. Alama m et al: The association between vitamin d level and restless legs syndrome: A population-based case-control study. J Clin Sleep Med (2018) 14(4):557–64. doi: 10.5664/jcsm.7044
38. Jimenez-Jimenez FJ, Amo G, Alonso-Navarro H, Calleja M, Diez-Fairen M, Alvarez-Fernandez I, et al. Turpin-Fenoll l et al Vitamin d receptor and binding protein genes polymorphisms in restless legs syndrome. J Neurol (2021) 268(4):1461–72. doi: 10.1007/s00415-020-10312-9
39. Wali SO, Abaalkhail B, Alhejaili F, Pandi-Perumal SR. Efficacy of vitamin d replacement therapy in restless legs syndrome: a randomized control trial. Sleep Breath (2019) 23(2):595–601. doi: 10.1007/s11325-018-1751-2
40. Tutuncu M, Tutuncu M. The effect of vitamin d on restless legs syndrome: prospective self-controlled case study. Sleep Breath (2020) 24(3):1101–6. doi: 10.1007/s11325-019-01984-3
41. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. D'Agostino d et al: Vitamin d supplements and prevention of cancer and cardiovascular disease. N Engl J Med (2019) 380(1):33–44. doi: 10.1056/NEJMoa1809944
42. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin d in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev (2019) 18(9):102350. doi: 10.1016/j.autrev.2019.102350
43. Sforza E, Roche F, Pichot V. Determinants of nocturnal cardiovascular variability and heart rate arousal response in restless legs syndrome (RLS)/Periodic limb movements (PLMS). J Clin Med (2019) 8(10). doi: 10.3390/jcm8101619
44. Kim TJ, Cha KS, Lee S, Yang TW, Kim KT, Park BS, et al. Sunwoo JS et al: Brain regions associated with periodic leg movements during sleep in restless legs syndrome. Sci Rep (2020) 10(1):1615. doi: 10.1038/s41598-020-58365-0
45. Tuovinen N, Stefani A, Mitterling T, Heidbreder A, Frauscher B, Gizewski ER, et al. Functional connectivity and topology in patients with restless legs syndrome: a case-control resting-state functional magnetic resonance imaging study. Eur J Neurol (2021) 28(2):448–58. doi: 10.1111/ene.14577
46. Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology (2013) 80(22):2028–34. doi: 10.1212/WNL.0b013e318294b3f6
47. Nakamura K, Wang W, Kang UJ. The role of glutathione in dopaminergic neuronal survival. J Neurochem (1997) 69(5):1850–8. doi: 10.1046/j.1471-4159.1997.69051850.x
48. Pertile RAN, Cui X, Hammond L, Eyles DW. Vitamin d regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J (2018) 32(2):819–28. doi: 10.1096/fj.201700713R
49. Luan W, Hammond LA, Cotter E, Osborne GW, Alexander SA, Nink V, et al. Developmental vitamin d (DVD) deficiency reduces Nurr1 and TH expression in post-mitotic dopamine neurons in rat mesencephalon. Mol Neurobiol (2018) 55(3):2443–53. doi: 10.1007/s12035-017-0497-3
50. Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin d inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol (2012) 188(5):2127–35. doi: 10.4049/jimmunol.1102412
51. Uslu FI, Demir E, Guler EM, Kocyigit A. Circulating levels of cytokines are increased in restless legs syndrome. Sleep Breath (2021) 25(3):1581–5. doi: 10.1007/s11325-020-02218-7
52. Prodhan A, Cavestro C, Kamal MA, Islam MA. Melatonin and sleep disturbances in alzheimer's disease. CNS Neurol Disord Drug Targets (2021) 20(8):736–54. doi: 10.2174/1871527320666210804155617
53. Esposito S, Laino D, D'Alonzo R, Mencarelli A, Di Genova L, Fattorusso A, et al. Pediatric sleep disturbances and treatment with melatonin. J Transl Med (2019) 17(1):77. doi: 10.1186/s12967-019-1835-1
Keywords: Restless Legs Syndrome, vitamin D, sleep disorder, mechanism, case- control study
Citation: Geng C, Yang Z, Kong X, Xu P and Zhang H (2022) Correlation between vitamin D and poor sleep status in restless legs syndrome. Front. Endocrinol. 13:994545. doi: 10.3389/fendo.2022.994545
Received: 29 July 2022; Accepted: 29 August 2022;
Published: 15 September 2022.
Edited by:
Spyridon N. Karras, Aristotle University of Thessaloniki, GreeceReviewed by:
Sara Marelli, San Raffaele Hospital (IRCCS), ItalyCopyright © 2022 Geng, Yang, Kong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongju Zhang, aG9uZ2p1ekBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.