- Department of Nuclear Medicine, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College (PUMC) Hospital, Chinese Academy of Medical Sciences & PUMC, Beijing, China
Radioiodine (131I) therapy (RAI) has been utilized for treating differentiated thyroid cancer (DTC) for decades, and its uses can be characterized as remnant ablation, adjuvant therapy (RAT) or treatment for known diseases. Compared with the definite 131I treatment targets for remnant ablation and known disease, 131I adjuvant therapy (RAT) aims to reduce the risk of recurrence by destroying potential subclinical disease. Since it is merely given as a risk with no imaging confirmation of persistence/recurrence/metastases, the evidence is uncertain. With limited knowledge and substance, the indication for RAT remains poorly defined for everyday clinical practice, and the benefits of RAT remain controversial. This ambiguity results in a puzzle for clinicians seeking clarity on whether patients should receive RAT, and whether patients are at risk of recurrence/death from undertreatment or adverse events from overtreatment. Herein, we clarified the RAT indications in terms of clinicopathological features, postoperative disease status and response to therapy evaluation, and retrospectively examined the clinical outcomes of RAT as reported in current studies and guidelines. Furthermore, given the evolution of nuclear medicine imaging techniques, it can be expected that the future of RAT may be advanced by nuclear medicine theranostics (i.e., 131I whole-body scan, PET/CT) by accurately revealing the biological behaviors, as well as the underlying molecular background.
1. Introduction
As the most common endocrine cancer, thyroid cancer’s incidence keeps rising worldwide (1). Differentiated thyroid cancer (DTC), mainly consisting of papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), accounts for 94% (2) of thyroid cancer and it generally carries a favorable clinical outcome under surgery followed by radioiodine (RAI) therapy and thyroid stimulating hormone (TSH) suppressive therapy.
Currently, management of RAI can be characterized as RAI for remnant ablation, adjuvant therapy (RAT) and treatment for known disease (3). As for clinical practice, RAI for remnant ablation is aimed at destroying residual thyroid tissue, thereby increasing the sensitivity of long-term monitoring by using serum thyroglobulin(Tg) and diagnostic radioiodine-131 whole-body scan (Dx-WBS). RAI for known disease is aimed at destroying persistently locoregional or distant metastases, in order to reduce recurrence and mortality or for palliation. Unlike the other two goals of RAI treatment that target remnant thyroid tissue or known disease, RAT is given to treat subclinical tumors that may or may not actually be present after prior adequate treatment (4).
As we noticed, the concept of RAT has been evolved over decades. In 1957, RAT was initially embodied in “ablation”, which intended to destroy both normal residual thyroid tissue and concealed microscopic tumor foci (5, 6). In 1996, the role of routine “ablation” was further specified by the 1996 American Thyroid Association Management Guidelines (the 1996 ATA Guidelines) as decreasing the risk of recurrent locoregional disease and facilitating long-term surveillance (7). It was not until 2009 that “adjuvant therapy” (RAT) was separated from remnant ablation with an aim to destroy subclinical lesions and this definition has been used ever since (3, 8). Since the existence of subclinical lesions cannot be accurately confirmed by current imaging modalities, the indication of RAT remains controversial, elusive and ambiguous, making it a dilemma during RAI decision-making.
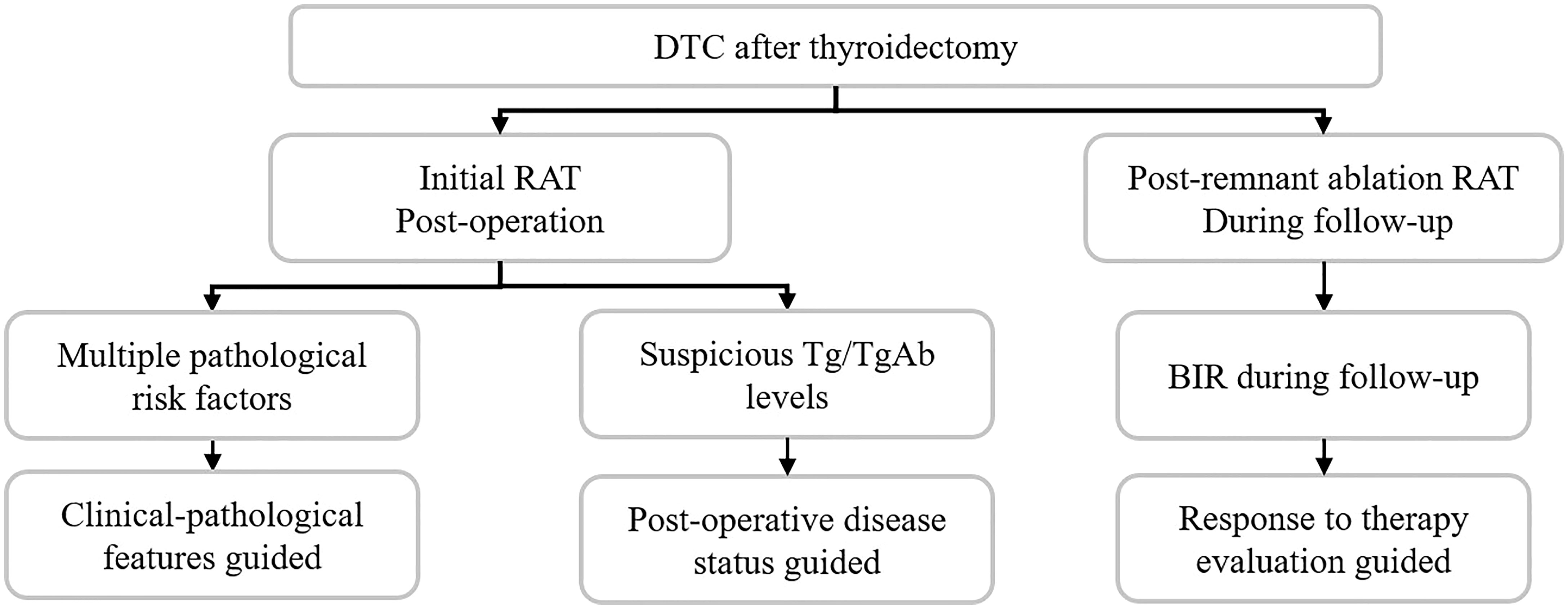
Thus, this review intends to offer insights into clarifying RAT by integrating recently published evidence, and the indications and benefits of RAT were summarized with interpretations of three aspects: clinicopathological features, postoperative disease status, and response to therapy evaluation as follows.
1.1. Clinicopathological features guided RAT
1.1.1. Pros and cons of clinicopathological features guided RAT
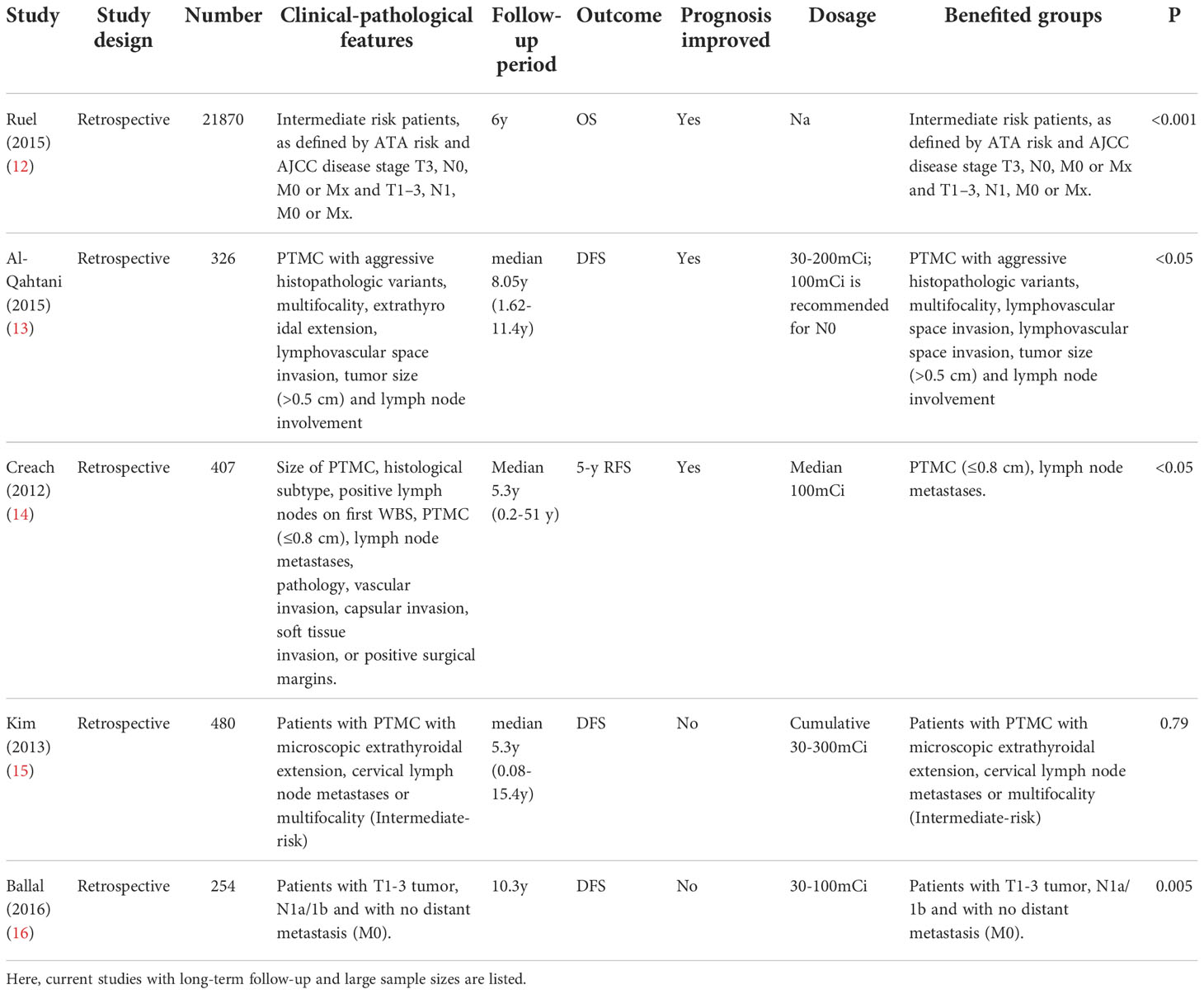
Some tumor features have a profound impact on prognosis. Current studies have indicated that patients with highly suspicious clinicopathologic findings including aggressive tumor histology, large primary tumor size, local invasion, vascular invasion and gene mutations, carry a higher risk of recurrence and are potential targets/candidates for RAT (9–11). However, the clinical benefit of RAT in these patients remains debatable (Table 1).
Multivariate adjusted analyses from Surveillance, Epidemiology, and End Results Program (SEER) indicated that RAT in DTC patients with aggressive histologies such as diffuse sclerosing, tall cell, and insular variants were associated with improved overall survival (OS) (9, 10). A meta-analysis found that RAT can improve the cause-specific survival in high-risk DTC patients that were aged >45 years with primary tumors >4 cm, microscopic extrathyroidal invasion, and/or lymph node metastases (6th UICC/AJCC TNM stage III/IV), yet patients aged <45 years with microscopic central compartment lymph node metastases are however, failed to benefit from RAT (17). The clinical outcome was also not clear in lateral or macroscopic lymph node metastatic patients (17). Recently, a retrospective study focused on the optimal timing, demonstrated that a prolonged RAT interval (time between thyroidectomy and 1st RAT ≥3 months) may cause an incomplete response (categorized by the 2015 ATA Guidelines), as well as soft tissue invasion (18). Thus, in terms of patients with aggressive histology, large primary tumor size and local/vascular invasion, definite benefits can be seen from RAT. Moreover, Ruel et al. studied 21,870 adult PTC patients who underwent total thyroidectomy with/without RAT in the National Cancer Database (NCDB) from 1998–2006, involving microscopic soft tissue invasion, positive lymph nodes, aggressive histology, vascular invasion, RAI uptake outside the thyroid gland on WBS, or T3N0M0/Mx, T3N0M0/Mx and T1–3 N1M0/Mx by 6th UICC/AJCC TNM. During the median follow-up of 6 years, RAT was indicated to improve the OS, with a 29% reduction in the risk of death with a hazard ratio (HR) of 0.71, 95% confidence interval (95% CI) 0.62–0.86, p<0.001. For age < 45 years, the reduction in death risk associated with RAT was up to 36%, HR 0.64, p=0.016 (12). Moreover, fewer patients developed distant metastases (P < 0.002) after RAT, yet this effect is observed only in patients whose tumor size was more than 1.5cm (19). Even for papillary microcarcinoma (PTMC) patients, a retrospective study involving 326 patients also indicated RAT was associated with improved disease-free survival (DFS) or in those with aggressive histopathologic variants, multifocality, extrathyroidal extension, lymphovascular space invasion, tumor size (>0.5 cm) and lymph node involvement (13). Another study focused on PTMC patients with lymph node metastasis also demonstrated longer 5-year recurrence-free survival (5y-RFS) in those who underwent RAT than that in those who didn’t (93.2%vs. 42.9%) (14). In contrast, Kim et al. studied PTMC patients of complete tumor resection, no extrathyroidal extension, no cervical lymph node metastasis, no distant metastasis, microscopic extrathyroidal extension, no cervical lymph node metastases or multifocality, and suggested that those patients were unlikely to benefit from RAT (15).
Over the recent years, BRAFV600E mutation has been valued as an aggressive clinicopathological feature with a high recurrence rate and tumor-specific mortality (11), Jiao Li et al. found a noninferior response to RAT in nonmetastatic BRAFV600E-mutated PTC patients, which may suggest a possible effect of RAT in improving the general clinical outcome in this patient group (20).
However, some studies suggested that RAT guided only by clinicopathological features may lead to overtreatment in patients who have been treated sufficiently by their primary surgery. Ballal et al. studied 254 postoperative DTC patients with age >45 years, vascular invasion, microscopic extrathyroidal extension, the presence of cervical lymph node metastases and the presence of aggressive histological variants who were stratified as intermediate-risk. By integrating nuclear medicine modalities (e.g. Dx-WBS) and biochemical markers(e.g. Tg), patients showing RAI uptake ≤0.2% in the first Dx-WBS along with postoperative stimulated Tg levels (ps-Tg) ≤10 ng/ml can be spared from RAT, with similar disease-free survival rate compared with RAT group(92% vs 90%), suggesting that not all intermediate-risk patients need RAT (16).
1.1.2. Recommendations from guidelines concerning the clinicopathological features guided RAT
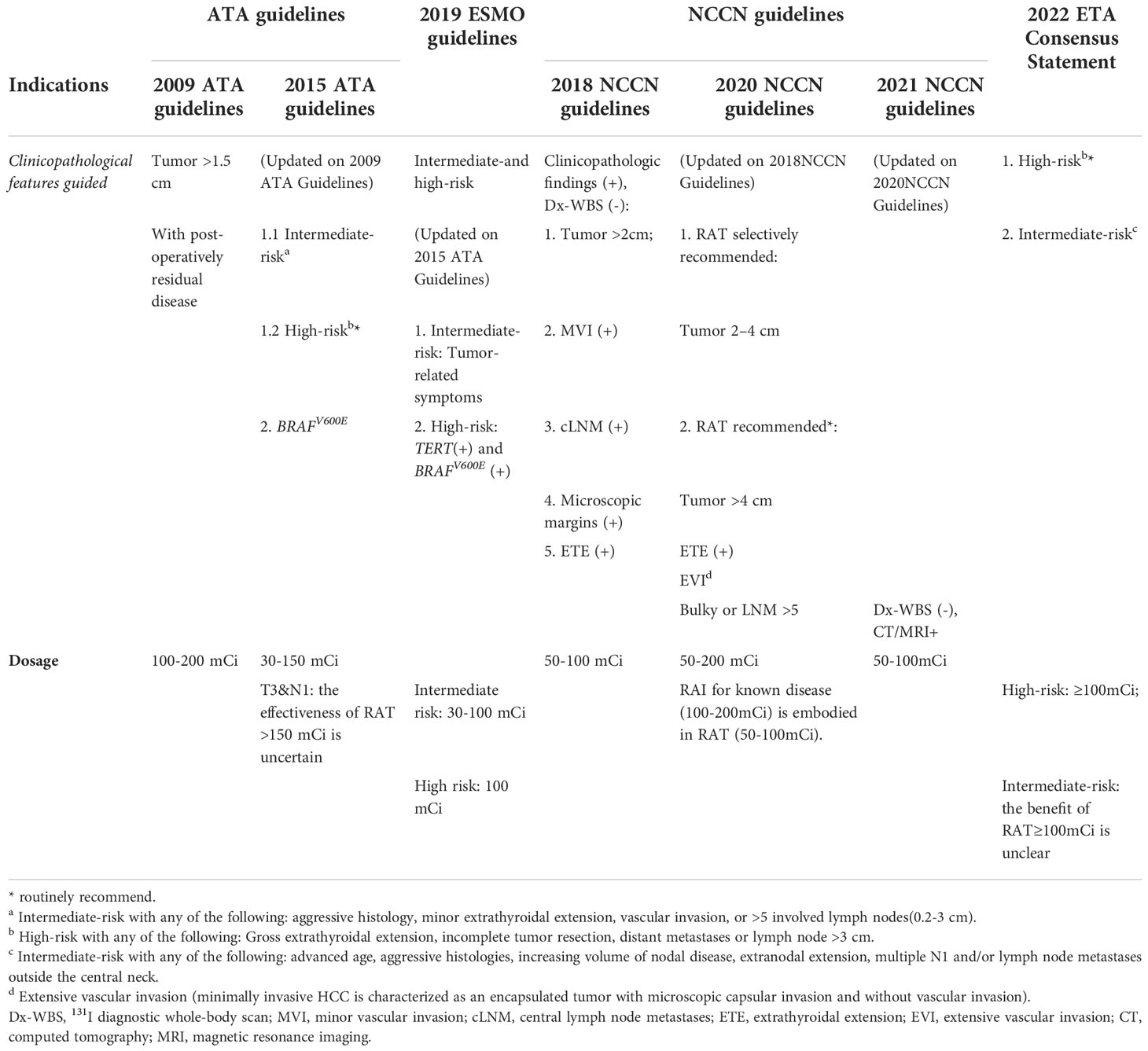
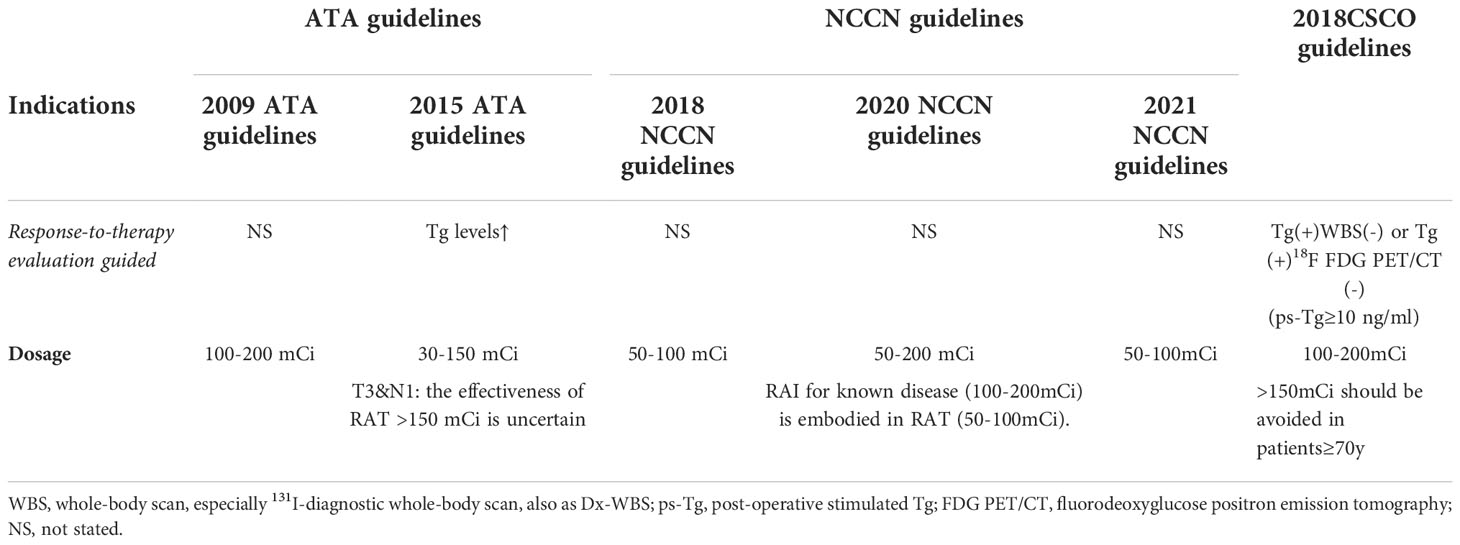
Based on the evidence mentioned above, the role of clinicopathological features in directing RAT consideration has been highlighted by multiple guidelines (Table 2), including the ATA Guidelines, 2019 European Society for Medical Oncology Clinical Practice Guidelines for Thyroid Cancer (2019ESMO Guidelines), 2022 European Thyroid Association consensus statement(2022ETA Consensus Statement) and the National Comprehensive Cancer Network in Oncology Guidelines (NCCN Guidelines) (3, 21–23). From which we can see the evolution of RAT in different guidelines. For instance, the ATA Guidelines have gradually specified the clinicopathological features, and adjusted the dosage recommendations accordingly, from 100-200 mCi to 30-150 mCi, which may due to the uncertain benefit from large dosage of RAT (3). Molecular pathological features, especially BRAFV600E, were selectively suggested for RAT in the 2015 ATA Guidelines, whereas the 2019 ESMO Guidelines underscored the significance of the coexistence of the BRAFV600E and TERT mutations (3, 23). The 2022 ETA Consensus Statement suggested patients with advanced age, aggressive histologies, increasing volume of nodal disease, extranodal extension, multiple N1 and/or lymph node metastases outside the central neck should receive RAT. Of note, the NCCN Guidelines provided more specified indications of RAT and appeared more practical in clinical use, including suspicious clinicopathologic findings, postoperative unstimulated Tg levels and radiological examination and/or Dx-WBS (3, 21, 23, 24). From these recommendations of guidelines, an area of controversy can be seen, making it too sticky for clinicians to follow.
By reviewing the relevant literature above, it is not difficult to perceive that clinicopathological features have been the major consideration in RAT decision-making among guidelines. However, clinicopathological features alone may not be enough to accurately predict the recurrent/metastatic risks of patients, as the real-time status of patients may be altered by initial therapy, urging the consideration of postoperative disease status.
1.2. Postoperative disease status-guided RAT
1.2.1. Pros and cons of postoperative disease status guided RAT
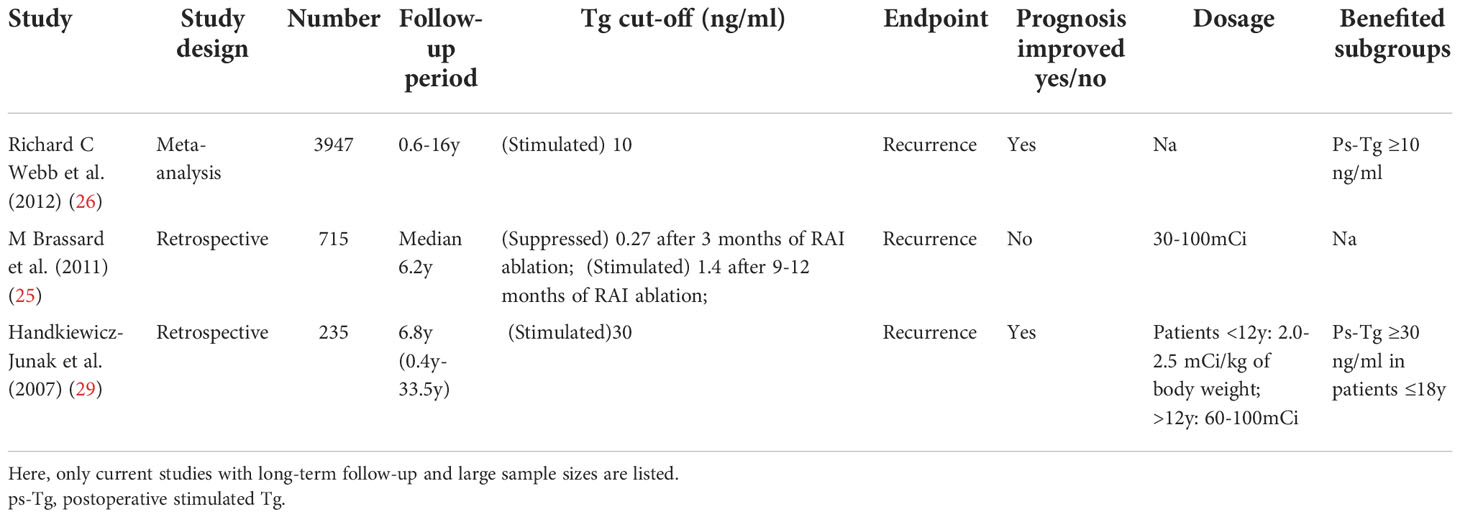
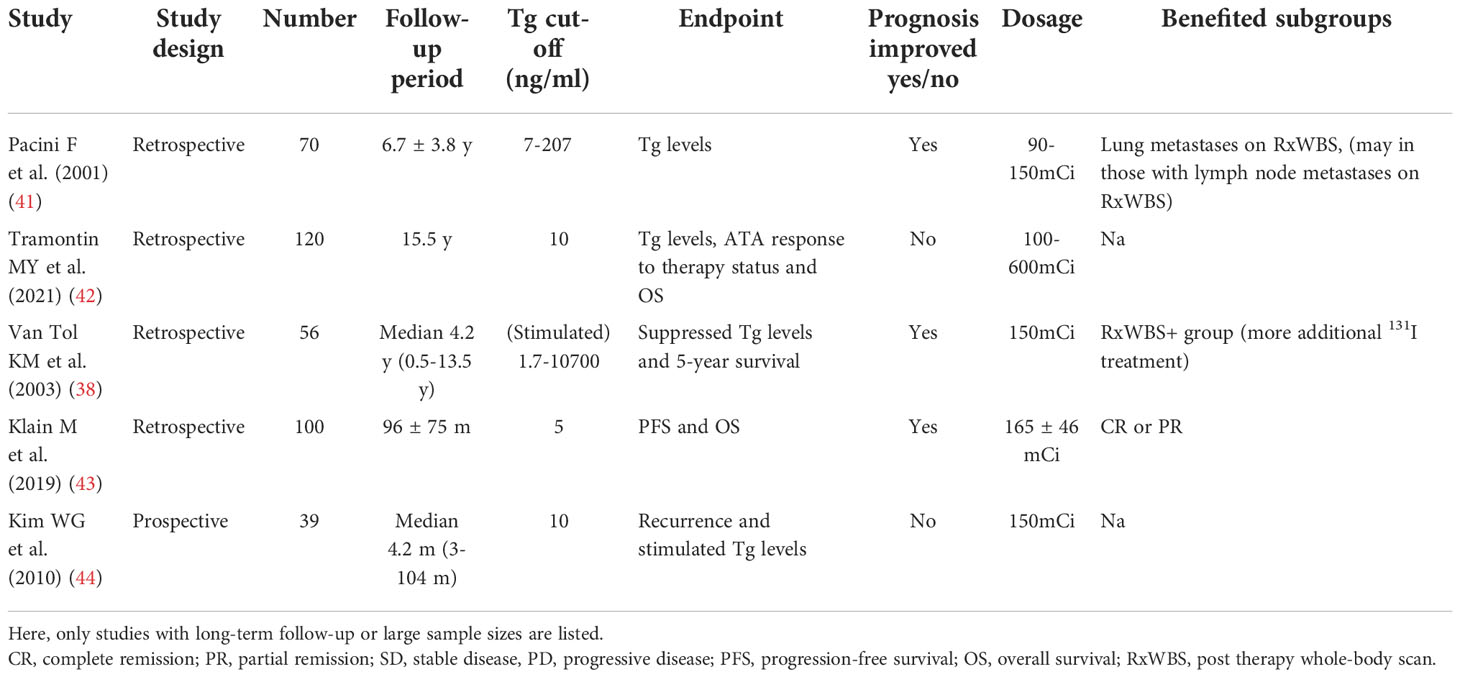
Integrated with real-time biochemical and Dx-WBS evaluation,postoperative disease status appears more essential for clinicians to determine if RAT is needed. Serum Tg or Tg-antibody (TgAb) levels, as sensitive organ-specific markers, are part of early postoperative disease status evaluation to identify patients who need more aggressive therapy. Studies have confirmed that patients with higher postoperative TSH-stimulated Tg (ps-Tg) (>1–2 ng/mL) at the time of RAI ablation have an increased risk of recurrence (25, 26), in contrast to ps-Tg <1–2 ng/mL as indicators of remission (27, 28). A few studies have been conducted in a large sample with long-term follow-up regarding the correlation between postoperative Tg levels and the clinical benefit of RAT (Table 3). A meta-analysis involving 3947 DTC patients in fifteen studies demonstrated that DTC patients with ps-Tg levels less than 10 ng/ml without the influence of TgAb levels are ideally linked to a better prognosis through RAT, and merely 6% of those patients had persistent disease (26). In a multicentre prospective study, 80% DTC patients with ps-Tg levels ≥10 ng/ml, as well as worse clinicopathological features, kept in a non-structurally incomplete response after 5.55 GBq (150 mCi) of RAT, with a median follow-up of 10.6 months (30). Therefore, higher ps-Tg levels (≥10 or 30 ng/ml) which can reflect unsatisfying real-time postoperative disease status, are likely to guide the consideration of RAT (26, 29). While in patients with a lower Tg cut-off value of 0.27 and 1.4 ng/ml (suppressive vs stimulated), RAT failed to improve patients’ prognosis. Therefore, a definite Tg cut-off remains hard to define, and more evidence is needed to determine the optimal Tg levels of suspicious globulinemia which could benefit from RAT (25).
Also, Dx-WBS was considered as an indispensable modality in evaluating postoperative disease status, for it can provide supplementary information by detecting functional local regional and distant metastatic lesions (31, 32). Accidental findings on DxWBS could lead to changes in clinical management in about 29.4%-53% of the patients, for whom the intention of RAI upgraded from ablation/adjuvant to treatment of know disease (32, 33).
1.2.2. Recommendations from guidelines concerning postoperative disease status guided RAT
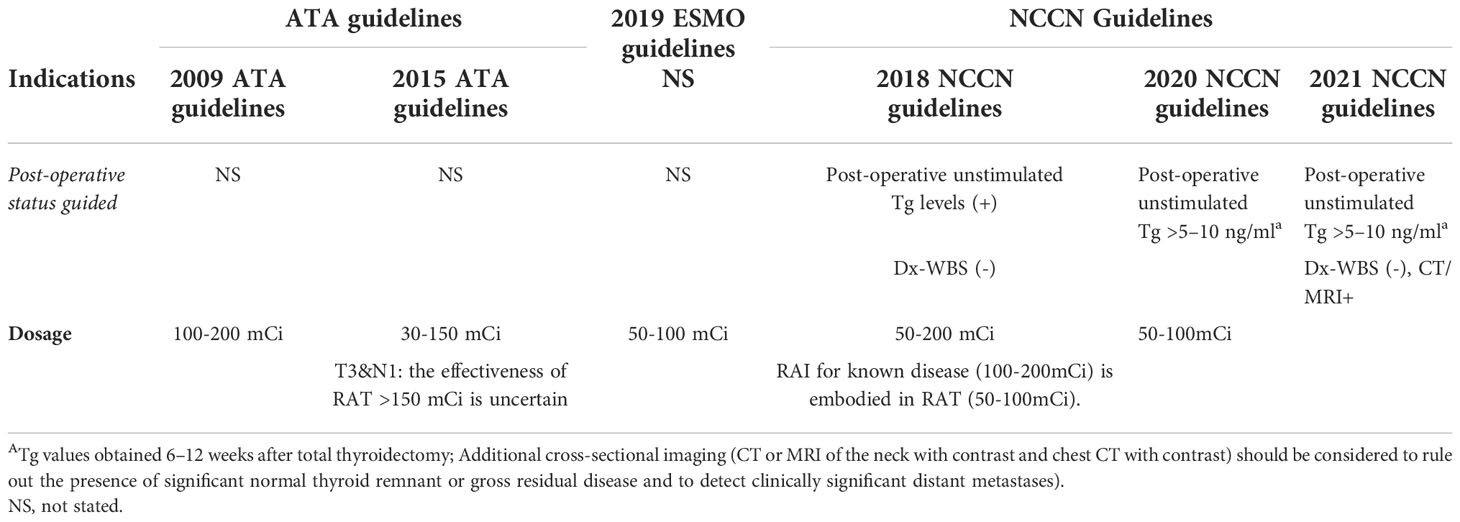
The NCCN Guidelines suggested patients who had elevated postoperative unstimulated Tg >5-10 ng/ml but no positive imaging evidence and patients whose postoperative unstimulated Tg levels were normal but with a high risk of recurrence undergo RAT (22). Besides, the significance of Dx-WBS was also emphasized and was recommended by both the 2015 ATA Guidelines and the NCCN Guidelines (3, 22) (Table 4).
Therefore, postoperative disease status guided RAT seems mainly based on unexplained postoperatively hyperthyroglobulinemia, with assistance of Dx-WBS to exclude functional lesions. Obviously, it could be a complement to clinicopathological features guided RAT.
1.3. Response to therapy evaluation guided RAT
1.3.1. Pros and cons of response to therapy evaluation guided RAT
As far as we are concerned, RAT during follow-up is a kind of “empiric therapy”, which is given to patients with an incomplete biochemical response (BIR, by ATA response to therapy evaluation) to the previous 1st RAI therapy. So far, the precise level of serum Tg which to trigger RAT has been uncertain, and most studies take 10 ng/mL as the cut-off value for its proven high predictive value for recurrence. BIR patients usually have good clinical outcomes, and approximately 56-68% of them will be downstaged to no evidence of disease (NED) during follow-up (34), while 8%–17% of BIR patients still develop structurally identifiable disease over 5–10 years of follow-up (34, 35). In addition, if a patient presents a rapidly rising serum Tg level, it is more likely for them to progress to distant metastases and locoregional recurrence (36). The 2015 ATA Guidelines proposed that the purpose of empirical therapy is to locate possible disease and even to treat it, since a wide range (25%-94%) of these patients may reveal uptake on RxWBS (37–40).
Considering the increased diagnostic efficacy under a therapeutic dose, the poor prognosis of distant metastatic patients may be improved by the early detection of unexpected lesions. Regarding the therapeutic efficacy of RAT, taking Tg as a marker to reflect tumor burden, more than half of such patients present a decreasing pattern after RAI administration. Van Tol et al. reported that half of the patients could even achieve a complete remission (CR) (40, 41). However, it should be noted that a spontaneous decline of Tg is not uncommon. Vaisman et al. demonstrated that 34% of BIR patients could transition to NED status without any additional RAI therapy (34). Moreover, the therapeutic effect of RAT for BIR patients seems to be restricted to the biochemical level, and no persuasive evidence for improved survival has been found (38, 41, 42). In addition, Klain M et al. found that the response to empirical treatment at 12 months seems to be related to the clinical outcome. Distant metastases at RxWBS, CR and objective response rate were predictors of both progression-free survival and overall survival (43) (Table 5). Thus, the benefit from response to therapy evaluation guided RAT such as “empiric therapy” remains debatable.
1.3.2. Recommendations from guidelines concerning response to therapy evaluation guided RAT
As for the recommended RAI administered activity, an “empirical RAI therapy” of 100-200 mCi may be considered in patients with elevated ps-Tg ≥10 ng/mL or rapidly rising serum Tg levels with negative imaging in the 2015 ATA guidelines. Similarly, the 2018 Chinese Society of Clinical Oncology Guidelines (the 2018CSCO Guidelines) suggested that patients with persistent/recurrent/metastatic disease whose elevated levels were >10 ng/ml without WBS evidence may benefit from 131I empirical therapy with weak recommendations (45). The 2021 NCCN guidelines suggested consideration of RAT ≥100 mCi in patients with progressively rising Tg (basal or stimulated) and negative scans, including positron emission tomography (PET) (22). Notably, it emphasized the application of PET scans because they have shown great value in detecting incidental lesions and therefore improving restaging (46). However, there was no treatment instruction for BIR patients in the 2019 ESMO guidelines; only a RxWBS after “therapeutic” activity was suggested if the PET scan was normal (23) (Table 6).
Therefore, response to therapy evaluation guided RAT is mainly based on the undesirable BIR to prior RAI therapy, meanwhile, it should be performed with caution only when the expected efficacy will outweigh its side effects. We can expect that, it could be further clarified along with the evolving imaging techniques.
2. Future directions of RAT
Along with the rapidly evolving techniques, the theranostic value of nuclear medicine molecular imaging has been highlighted as an indispensable modality in accurate pre-RAI assessment and therapeutic response of RAI prediction, thus assisting in refining the aim of RAT (47).
2.1. Promising value of theranostic imaging
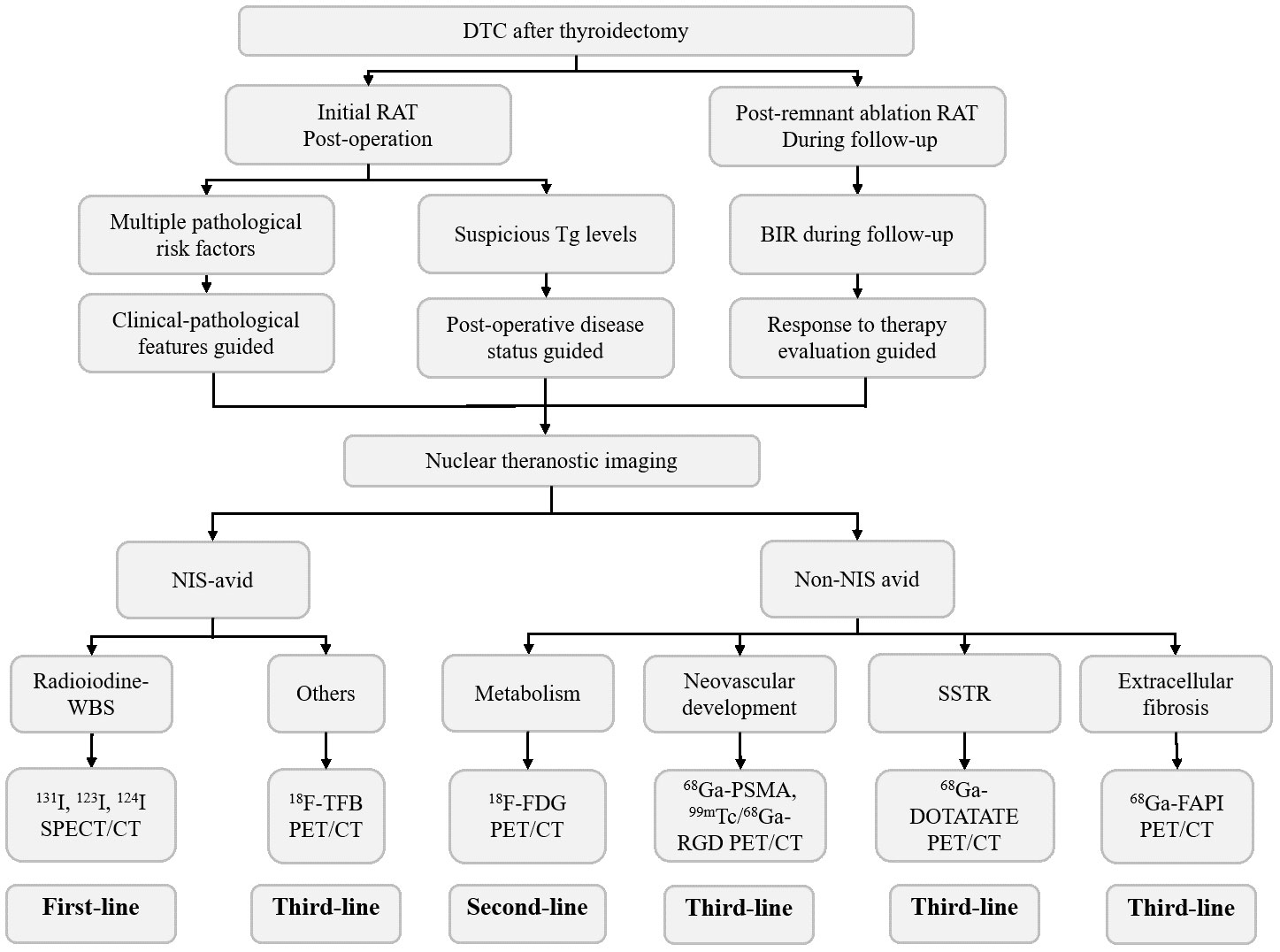
Radioiodine-WBS (131I,123I,124I), which is the best example of the so-called “theranostics”, can reflect sodium/iodide symporter (NIS) expression and iodine metabolism from both diagnostic and therapeutic perspectives and will play a complementary role in the abovementioned 3-factor guided RAT. Of note, despite the informative value of demonstrating the risk of persistence/recurrence/metastasis, a single clinicopathologic feature itself may not be enough to dominantly direct the strategy of RAT. For instance, by integrating 131I theranostics as a dynamic assessment modality, those with high pathological risk features but downstaged by adequate prior therapy may be spared from unnecessary aggressive RAT, while those with a low risk stratified by clinicopathologic features may be upstaged and be more likely to benefit from RAT (Figure 1). Likewise, although postoperative serum Tg levels are predictive for evaluating postoperative disease status, they might be confused by either residual thyroid cancer or remnant tissue and TSH/TgAb levels during measurement (48, 49).
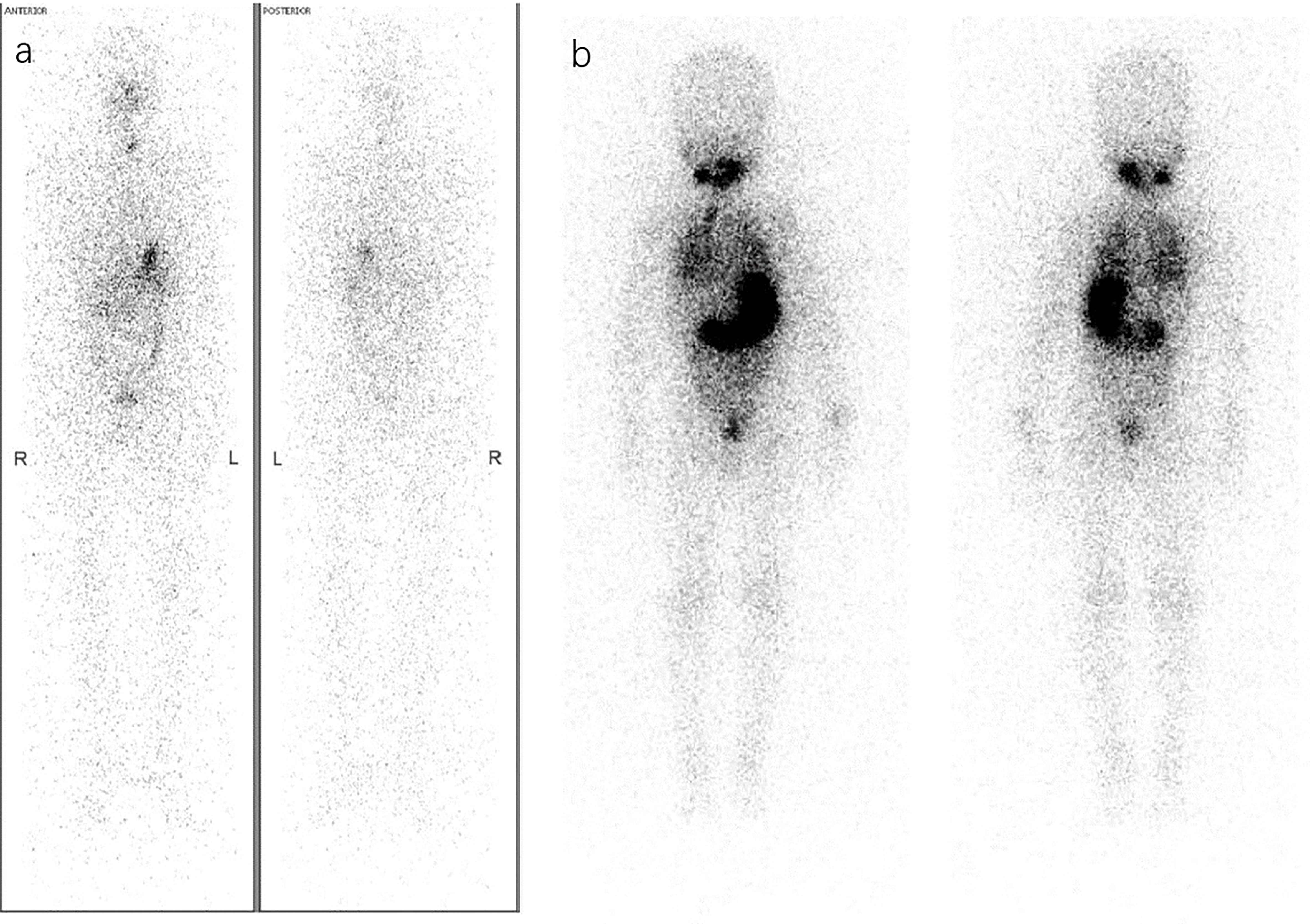
Figure 1 Different RAT considerations via Dx-WBS. (A) A 36-year-old woman was diagnosed with PTC (pT1bN1bM0, ATA intermediate-risk) by pathology after thyroidectomy and lymph node dissection. Considering both the postoperative disease status of ps-Tg<0.04 ng/ml and A-Tg 111 IU/ml with a decreasing trend and Dx-WBS findings that did not indicate suspicious concealed lesions, she may not benefit from RAT. Therefore, she received TSH suppression therapy instead of RAT. (B) A 4-year-old boy under thyroidectomy was confirmed pathologically to have PTC (pT1aN1aM0, ATA low-risk) in 2008. Surprisingly, Dx-WBS revealed wide uptake of 131I in the lungs, whereas preoperative CT did not. Through 3 times of 60 mCi RAI, he obtained and has maintained an excellent response (ER) in recent years (1st RAT: TSH >150 μIU/mL, Tg 91 ng/ml, A-Tg 13.25 IU/ml; 2nd RAT: TSH >150 μIU/mL, Tg 9.4 ng/ml, A-Tg 10 IU/ml; 3rd RAT: TSH >150 μIU/mL, Tg 3.4 ng/ml, A-Tg 10 IU/ml; the latest follow-up: TSH 0.244 μIU/mL, Tg<0.04 ng/ml, A-Tg 15 IU/ml).
As a result, the traditional factor-guided RAT may be further refined by incorporating theranostic imaging evidence, including Dx-WBS, for identifying NIS-avid lesions and other modalities for confirming the actual evidence of tumor existence (Figure 2). Recent studies suggested that apart from iodine, other NIS-targeted imaging modalities, such as 18F-tetrafluoroborate (18F-TFB), may provide incremental value for sensitive detection of RAI-avid lesions (50). Under such circumstances, the conception of RAT may be altered from suspected lesions to known lesions. When it comes to non-NIS-avid lesions, they may be detected by 18F-FDG PET/CT at the level of tumor metabolism and reflected by 68Ga-prostate-specific membrane antigen (68Ga-PSMA) PET/CT as well as 99mTc/68 Ga-arginine-glycine-aspartic acid (68Ga-RGD) PET/CT at the level of neovascular development 68.Ga-DOTATATE and 68Ga-fibroblast-activation-protein inhibitors (68Ga-FAPI) PET/CT can demonstrate the expression of somatostatin receptors (SSTRs) and extracellular fibrosis of tumors, respectively (51–55). In clinical practice, lesions with adequate NIS expression (NIS-avid) will inspire clinicians to prescribe a therapeutic rather than a merely adjuvant dosage of 131I, while others (non-NIS-avid) may be transferred to other treatments based on their individual molecular characteristics.
Notably, the theranostics mentioned above are merely focused on the diagnostic superiority of molecular nuclear theranostic imaging. Those who are diagnosed as BIR by traditional anatomical modalities may be additionally identified as having a structural incomplete response (SIR) by means of nuclear molecular theranostics. The more thorough the imaging studies are, the fewer BIR patients are left. Thus, comprehensive structural and functional imaging studies could be expected in the future.
2.2. Genetic background of theranostics
The genetic background plays a vital role in the underlying tumor behavior and theranostic imaging results. Studies have confirmed that the expression of NIS can predict responsiveness to RAI therapy to some degree (56, 57), and Xing et al. proved that once DTC carries the BRAFV600E mutation, it may manifest a relatively low level of NIS expression, thus leading to unsatisfactory radioiodine uptake (non-RAI avidity) and a high risk of recurrence (56). The authors also previously reported that if BRAFV600E mutation was concomitant with other oncogenic mutations such as TERT mutations, it would have a robust synergistic impact on the aggressiveness of DTC, and the benefit from RAI therapy was very limited (57, 58). Moreover, coexisting BRAFV600E mutation and PIK3CA, TP53, and AKT1 mutations were also identified as predictors of a less favourable clinical outcome by recent studies (59–62). Thus, the heterogeneous mutations of genes can explain the differences in tumor morphology, gene expression and clinical features of every individual, which are expected to become prognostic molecular markers for RAT decision-making. Therefore, the impact of mutations on disease evolution remains to be further explored.
Nevertheless, no accurate therapeutic response evaluation system for RAT has been established owing to a lack of strong evidence, and few data can help in forming a consensus on the detailed indications and clinical outcomes of RAT. Well-designed, prospective randomized controlled trials are urgently needed to offer an accurate therapeutic response evaluation system and avoid confusing decision-making based on observational and low-quality studies with diverse outcomes.
3. Conclusions
In this review, we have summarized three aspects of RAT indications (Figure 3), which can help advance our understanding of RAT in a complementary way. Although consensus is not yet reached on certain RAT guiding features, it offers a new perspective in clinical RAT decision-making. Nuclear medicine imaging and genetic background may help refine the RAT conception and clinical practice in the future. Furthermore, well-designed studies with strong evidence are urgently needed.
Author contributions
Y-QS, DS, and Y-SL contributed to conception and design of the study. Y-QS gathered evidence and wrote the first draft of manuscript. DS wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2020-I2M-2-003); Project on Inter-Governmental International Scientific and Technological Innovation Cooperation in National Key Projects of Research and Development Plan (Grant No. 2019YFE0106400); National High Level Hospital Clinical Research Funding; National Natural Science Foundation of China (No. 81771875 ); the CSCO-Hengrui Research Foundation (No. Y-HR2018-143, Y-HR2018-144.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.994288/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. New Engl J Med (2016) 375(11):1054–67. doi: 10.1056/NEJMra1501993
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
4. Tuttle RM, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Daniels GH, et al. Controversies, consensus, and collaboration in the use of (131)I therapy in differentiated thyroid cancer: A joint statement from the American thyroid association, the European association of nuclear medicine, the society of nuclear medicine and molecular imaging, and the European thyroid association. Thyroid (2019) 29(4):461–70. doi: 10.1089/thy.2018.0597
5. Pochin EE. Radioiodine therapy of thyroid cancer. Semin Nucl Med (1971) 1(4):503–15. doi: 10.1016/S0001-2998(71)81043-7
6. DeGroot LJ, Kaplan EL, McCormick M, Straus FH. Natural history, treatment, and course of papillary thyroid carcinoma. J Clin Endocrinol Metab (1990) 71(2):414–24. doi: 10.1210/jcem-71-2-414
7. Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Am Thyroid Assoc Arch Internal Med (1996) 156(19):2165–72. doi: 10.1001/archinte.1996.00440180017002
8. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules andDifferentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009) 19(11):1167–214. doi: 10.1089/thy.2009.0110
9. Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol (2012) 19(6):1874–80. doi: 10.1245/s10434-011-2129-x
10. Kazaure HS, Roman SA, Sosa JA. Insular thyroid cancer: a population-level analysis of patient characteristics and predictors of survival. Cancer (2012) 118(13):3260–7. doi: 10.1002/cncr.26638
11. Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. J Clin Endocrinol Metab (2012) 97(12):4559–70. doi: 10.1210/jc.2012-2104
12. Ruel E, Thomas S, Dinan M, Perkins JM, Roman SA, Sosa JA. Adjuvant radioactive iodine therapy is associated with improved survival for patients with intermediate-risk papillary thyroid cancer. J Clin Endocrinol Metab (2015) 100(4):1529–36. doi: 10.1210/jc.2014-4332
13. Al-Qahtani KH, Al Asiri M, Tunio MA, Aljohani NJ, Bayoumi Y, Fatani H, et al. Adjuvant radioactive iodine 131 ablation in papillary microcarcinoma of thyroid: Saudi Arabian experience [corrected]. J Otolaryngol Head Neck Surg (2015) 44:51. doi: 10.1186/s40463-015-0108-0
14. Creach KM, Siegel BA, Nussenbaum B, Grigsby PW. Radioactive iodine therapy decreases recurrence in thyroid papillary microcarcinoma. ISRN Endocrinol (2012) 2012:816386. doi: 10.5402/2012/816386
15. Kim HJ, Kim NK, Choi JH, Kim SW, Jin SM, Suh S, et al. Radioactive iodine ablation does not prevent recurrences in patients with papillary thyroid microcarcinoma. Clin Endocrinol (Oxf) (2013) 78(4):614–20. doi: 10.1111/cen.12034
16. Ballal S, Soundararajan R, Garg A, Chopra S, Bal C. Intermediate-risk differentiated thyroid carcinoma patients who were surgically ablated do not need adjuvant radioiodine therapy: long-term outcome study. Clin Endocrinol (2016) 84(3):408–16. doi: 10.1111/cen.12779
17. Sacks W, Fung CH, Chang JT, Waxman A, Braunstein GD. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: a systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid (2010) 20(11):1235–45. doi: 10.1089/thy.2009.0455
18. Yu F, Li X, Ji Y, Tan J, Zhang G, Wang P, et al. Delayed initial radioiodine adjuvant therapy does affect biochemical response in intermediate- to high-risk differentiated thyroid cancer. Front Endocrinol (Lausanne) (2021) 12:743310. doi: 10.3389/fendo.2021.743310
19. Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid (1997) 7(2):265–71. doi: 10.1089/thy.1997.7.265
20. Li J, Liang J, Zhao T, Lin Y. Noninferior response in BRAF(V600E) mutant nonmetastatic papillary thyroid carcinoma to radioiodine therapy. Eur J Nucl Med Mol Imaging. (2016) 43(6):1034–9. doi: 10.1007/s00259-015-3305-1
21. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN guidelines insights thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw. (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089.
22. NCCN clinical practice guidelines in oncology: thyroid carcinoma (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
23. Filetti S, Durante C, Hartl D, ESMO Guidelines Committee. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
24. NCCN clinical practice guidelines in oncology: thyroid carcinoma (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf.
25. Brassard M, Borget I, Edet-Sanson A, THYRDIAG Working Group. Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab (2011) 96(5):1352–9. doi: 10.1210/jc.2010-2708
26. Webb RC, Howard RS, Stojadinovic A, Gaitonde DY, Wallace MK, Ahmed J, et al. The utility of serum thyroglobulin measurement at the time of remnant ablation for predicting disease-free status in patients with differentiated thyroid cancer: a meta-analysis involving 3947 patients. J Clin Endocrinol Metab (2012) 97(8):2754–63. doi: 10.1210/jc.2012-1533
27. Kim TY, Kim WB, Kim ES, Ryu JS, Yeo JS, Kim SC, et al. Serum thyroglobulin levels at the time of 131I remnant ablation just after thyroidectomy are useful for early prediction of clinical recurrence in low-risk patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab (2005) 90(3):1440–5. doi: 10.1210/jc.2004-1771
28. Piccardo A, Arecco F, Puntoni M, Foppiani L, Cabria M, Corvisieri S, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med (2013) 38(1):18–24. doi: 10.1097/RLU.0b013e318266d4d8
29. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med (2007) 48(6):879–88. doi: 10.2967/jnumed.106.035535
30. Cheng L, Sa R, Luo Q, Fu H, Jin Y, Tang L, et al. Unexplained hyperthyroglobulinemia in differentiated thyroid cancer patients indicates radioiodine adjuvant therapy: A prospective multicenter study. (2021) 62(1):62–8. doi: 10.2967/jnumed.120.243642
31. Avram AM, Fig LM, Frey KA, Gross MD, Wong KK. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab (2013) 98(3):1163–71. doi: 10.1210/jc.2012-3630
32. Avram AM, Esfandiari NH, Wong KK. Preablation 131-I scans with SPECT/CT contribute to thyroid cancer risk stratification and 131-I therapy planning. J Clin Endocrinol Metab (2015) 100(5):1895–902. doi: 10.1210/jc.2014-4043
33. Van Nostrand D, Aiken M, Atkins F, Moreau S, Garcia C, Acio E, et al. The utility of radioiodine scans prior to iodine 131 ablation in patients with well-differentiated thyroid cancer. Thyroid (2009) 19(8):849–55. doi: 10.1089/thy.2008.0419
34. Vaisman F, Momesso D, Bulzico DA, Pessoa CH, Dias F, Corbo R, et al. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin Endocrinol (2012) 77(1):132–8. doi: 10.1111/j.1365-2265.2012.04342.x
35. Vaisman F, Tala H, Grewal R, Tuttle RM. In differentiated thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than only an incomplete thyroglobulin response. Thyroid (2011) 21(12):1317–22. doi: 10.1089/thy.2011.0232
36. Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Takamura Y, et al. Prognostic impact of serum thyroglobulin doubling-time under thyrotropin suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid (2011) 21(7):707–16. doi: 10.1089/thy.2010.0355
37. Fatourechi V, Hay ID, Javedan H, Wiseman GA, Mullan BP, Gorman CA. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab (2002) 87(4):1521–6. doi: 10.1210/jcem.87.4.8373
38. van Tol KM, Jager PL, de Vries EG, Piers DA, Boezen HM, Sluiter WJ, et al. Outcome in patients with differentiated thyroid cancer with negative diagnostic whole-body scanning and detectable stimulated thyroglobulin. Eur J Endocrinol (2003) 148(6):589–96. doi: 10.1530/eje.0.1480589
39. Pineda JD, Lee T, Ain K, Reynolds JC, Robbins J. Iodine-131 therapy for thyroid cancer patients with elevated thyroglobulin and negative diagnostic scan. J Clin Endocrinol Metab (1995) 80(5):1488–92. doi: 10.1210/jcem.80.5.7744991.
40. Ma C, Xie J, Kuang A. Is empiric 131I therapy justified for patients with positive thyroglobulin and negative 131I whole-body scanning results? J Nucl Med (2005) 46(7):1164–70.
41. Pacini F, Agate L, Elisei R, Capezzone M, Ceccarelli C, Lippi F, et al. Outcome of differentiated thyroid cancer with detectable serum tg and negative diagnostic (131)I whole body scan: comparison of patients treated with high (131)I activities versus untreated patients. J Clin Endocrinol Metab (2001) 86(9):4092–7. doi: 10.1210/jcem.86.9.7831
42. Tramontin MY, Nobre GM, Lopes M, Carneiro MP, Alves PAG, de Andrade FA, et al. High thyroglobulin and negative whole-body scan: no long-term benefit of empiric radioiodine therapy. Endocrine (2021) 73(2):398–406. doi: 10.1007/s12020-021-02647-8
43. Klain M, Pace L, Zampella E, Mannarino T, Limone S, Mazziotti E, et al. Outcome of patients with differentiated thyroid cancer treated with 131-iodine on the basis of a detectable serum thyroglobulin level after initial treatment. Front Endocrinol (2019) 10:146. doi: 10.3389/fendo.2019.00146
44. Kim WG, Ryu JS, Kim EY, Lee JH, Baek JH, Yoon JH, et al. Empiric high-dose 131-iodine therapy lacks efficacy for treated papillary thyroid cancer patients with detectable serum thyroglobulin, but negative cervical sonography and 18F-fluorodeoxyglucose positron emission tomography scan. J Clin Endocrinol Metab (2010) 95(3):1169–73. doi: 10.1210/jc.2009-1567
45. Lin YS, Huang HQ, Guo Y, Jentzen W, Rosenbaum SJ, Kühl H, et al. Chinese Society of clinical oncology (CSCO) diagnosis and treatment guidelines for persistent/recurrent and metastatic differentiated thyroid cancer 2018. Chin J Cancer Res (2019) 31(1):99–116. doi: 10.21147/j.issn.1000-9604.2019.01.06
46. Freudenberg LS, Antoch G, Frilling A, Bernet VJ, Bourguet P, Draganescu C, et al. Combined metabolic and morphologic imaging in thyroid carcinoma patients with elevated serum thyroglobulin and negative cervical ultrasonography: role of 124I-PET/CT and FDG-PET. Eur J Nucl Med Mol Imaging. (2008) 35(5):950–7. doi: 10.1007/s00259-007-0634-8
47. Gulec SA, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Draganescu Cc , et al. A joint statement from the American thyroid association, the European association of nuclear medicine, the European thyroid association, the society of nuclear medicine and molecular imaging on current diagnostic and theranostic approaches in the management of thyroid cancer. Thyroid (2021) 31(7):1009–19. doi: 10.1089/thy.2020.0826
48. Giovanella L, Clark PM, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol (2014) 171(2):R33–46. doi: 10.1530/EJE-14-0148
49. Giovanella L, Feldt-Rasmussen U, Verburg FA, Grebe SK, Plebani M, Clark PM. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin Chem Lab Med (2015) 53(9):1301–14. doi: 10.1515/cclm-2014-0813
50. Liu M, Chai L, Luo Q, Ruan M, Cheng L, Lv Z, et al. 99mTc-pertechnetate-avid metastases from differentiated thyroid cancer are prone to benefit from 131I therapy: A prospective observational study. Medicine (Baltimore) (2017) 96(33):e7631. doi: 10.1097/MD.0000000000007631
51. Alam MS, Kasagi K, Misaki T, Miyamoto S, Iwata M, Iida Y, et al. Diagnostic value of technetium-99m methoxyisobutyl isonitrile (99mTc-MIBI) scintigraphy in detecting thyroid cancer metastases: a critical evaluation. Thyroid (1998) 8(12):1091–100. doi: 10.1089/thy.1998.8.1091
52. Lawhn-Heath C, Yom SS, Liu C, Villanueva-Meyer JE, Aslam M, Smith R, et al. Gallium-68 prostate-specific membrane antigen ([(68)Ga]Ga-PSMA-11) PET for imaging of thyroid cancer: a feasibility study. EJNMMI Res (2020) 10(1):128. doi: 10.1186/s13550-020-00720-3
53. Gao R, Zhang GJ, Wang YB, Liu Y, Wang F, Jia X, et al. Clinical value of (99m)Tc-3PRGD2 SPECT/CT in differentiated thyroid carcinoma with negative (131)I whole-body scan and elevated thyroglobulin level. Sci Rep (2018) 8(1):473. doi: 10.1038/s41598-017-19036-9
54. Almeida LS, Araújo MC, Zantut-Wittmann DE, Assumpção LV, Souza TF, Silva CM, et al. Effect of thyroid-stimulating hormone in 68Ga-DOTATATE PET/CT of radioiodine-refractory thyroid carcinoma: a pilot study. Nucl Med Commun (2018) 39(5):441–50. doi: 10.1097/MNM.0000000000000823
55. Fu H, Fu J, Huang J, Su X, Chen H. 68Ga-FAPI PET/CT in thyroid cancer with thyroglobulin elevation and negative iodine scintigraphy. Clin Nucl Med (2021) 46(5):427–30. doi: 10.1097/RLU.0000000000003569
56. Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol (2015) 33(1):42–50. doi: 10.1200/JCO.2014.56.8253
57. Zhang N, Liang J, Lin YS. Unfavorable efficacy to (131)I ablation in BRAFV600E mutant papillary thyroid carcinoma with positive TgAb. Oncotarget (2017) 8(57):97407–15. doi: 10.18632/oncotarget.22129
58. Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocrine-related cancer. (2016) 23(3):R143–155. doi: 10.1530/ERC-15-0533
59. Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab (2014) 99(5):E754–765. doi: 10.1210/jc.2013-3734
60. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol (2014) 32(25):2718–26. doi: 10.1200/JCO.2014.55.5094
61. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res (2009) 69(11):4885–93. doi: 10.1158/0008-5472.CAN-09-0727
Keywords: differentiated thyroid cancer, radioiodine therapy (RAI), 131I adjuvant therapy, nuclear theranostics, 131I whole-body scan
Citation: Sun Y-q, Sun D, Zhang X, Zhang Y-q and Lin Y-s (2022) Radioiodine adjuvant therapy in differentiated thyroid cancer: An update and reconsideration. Front. Endocrinol. 13:994288. doi: 10.3389/fendo.2022.994288
Received: 14 July 2022; Accepted: 07 November 2022;
Published: 30 November 2022.
Edited by:
Trevor Edmund Angell, University of Southern California, United StatesReviewed by:
Emese Mezosi, University of Pécs, HungaryChentian Shen, Shanghai Sixth People’s Hospital, China
Copyright © 2022 Sun, Sun, Zhang, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-song Lin, bGlueWFuc29uZzE5NjhAMTYzLmNvbQ==
†These authors share first authorship
 Yu-qing Sun
Yu-qing Sun Di Sun
Di Sun Xin Zhang
Xin Zhang Ying-qiang Zhang
Ying-qiang Zhang Yan-song Lin
Yan-song Lin