- 1Department of General Surgery, Xiangya Hospital Central South University, Changsha, China
- 2Clinical Research Center for Thyroid Disease in Hunan Province, Changsha, China
- 3Hunan Provincial Engineering Research Center for Thyroid and Related Diseases Treatment Technology, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, China
Purpose: Hashimoto’s thyroiditis often leads to reactive hyperplasia of the central compartment lymph nodes in papillary thyroid carcinoma (PTC) patients. However, the effect and clinical significance of Hashimoto’s thyroiditis (HT) on ultrasonography evaluation for cervical lymph node (LN) lesions remain unknown. This study aims to investigate the effect of Hashimoto’s thyroiditis on the diagnostic efficacy of preoperative ultrasonography on cervical lymph node lesions in PTC patients.
Patients and methods: This study consecutively enrolled 1,874 PTC patients who underwent total thyroidectomy and radical cervical lymph node dissection between January 2010 and December 2021. Eligible patients were categorized as with HT and without HT. The diagnostic performance of preoperative ultrasonography for cervical LN lesions (including central LNs and lateral LNs) was evaluated between PTC patients with HT and those without HT, respectively.
Results: Among the 1,874 PTC patients, 790 (42.1%) had central cN+ and 1,610 (85.9%) had lateral cN+. Compared with PTC patients without HT, the preoperative US for central LNs displays a higher false-positive rate (27.9% vs. 12.2%, p <0.001) and a lower specificity (72.1% vs. 87.8%, p < 0.001) in PTC patients with HT. Moreover, in PTC patients with HT, the ratio of the absence of fatty hilum in central LNs without metastasis was higher than in PTC patients without HT (13.02% vs. 7.46%, p = 0.013). However, no such differences were observed in lateral LNs.
Conclusion: HT will interfere with the preoperative US evaluation for central LNs and increase the incidence of the absence of fatty hilum in central benign LNs. When PTC patients have concomitant HT, clinicians should thoroughly evaluate the central LNs, thereby decreasing the incidence of misdiagnosis and unnecessary surgery.
1. Introduction
Thyroid carcinoma (TC) is one of the most frequent malignancies with a rapidly rising incidence in the past 40 years worldwide (1, 2). Papillary thyroid carcinoma (PTC) is the most common histologic variant of TC, accounting for approximately 80%–90% (3, 4). Despite PTC being considered a low-grade malignant tumor, lymph node metastasis (LNM) is a common biological behavior and exists in approximately 30%–80% of PTC patients (5–7). Adequate preoperative evaluation and tailoring of surgical resections for primary and metastatic lesions are the most important for PTC patients to minimize the possibility of metastatic lesion residual and recurrence.
In recent years, with the continuous improvement of diagnostic technology, high-resolution ultrasonography (US) has been the most important measure for the preoperative assessment of cervical lymph node metastatic lesions in PTC patients (5, 8, 9). Of note, central lymph node dissection is recommended only for PTC patients with clinically apparent metastatic disease to the nodes (clinical N1) detected by physical examination or preoperative imaging studies or with advanced primary tumors (T3 or T4), according to the 2015 American Thyroid Association (ATA) Management Guidelines (8). However, the diagnostic efficacy of preoperative US on cervical (especially central compartments) lymph node (LN) metastatic lesion is not obvious (10–12).
Hashimoto’s thyroiditis (HT) is the most common autoimmune disease and is frequently accompanied by a humoral immune response and inflammatory manifestations (13–16). Since PTC patients often have concomitant Hashimoto’s thyroiditis (HT), reactive hyperplasia is often observed in the neck LNs, especially central LNs, which may result in some abnormal ultrasonographic features in preoperative imaging examinations (13, 17, 18). However, the effect and clinical significance of HT on ultrasonography evaluation for cervical LN lesions remain unknown.
In the present study, we comprehensively investigate the diagnostic performance of preoperative US on cervical lymph node metastasis lesions and aim to determine the effect of Hashimoto’s thyroiditis on preoperative US for cervical lymph node lesions in papillary thyroid cancer.
2. Materials and methods
2.1. Patients and study design
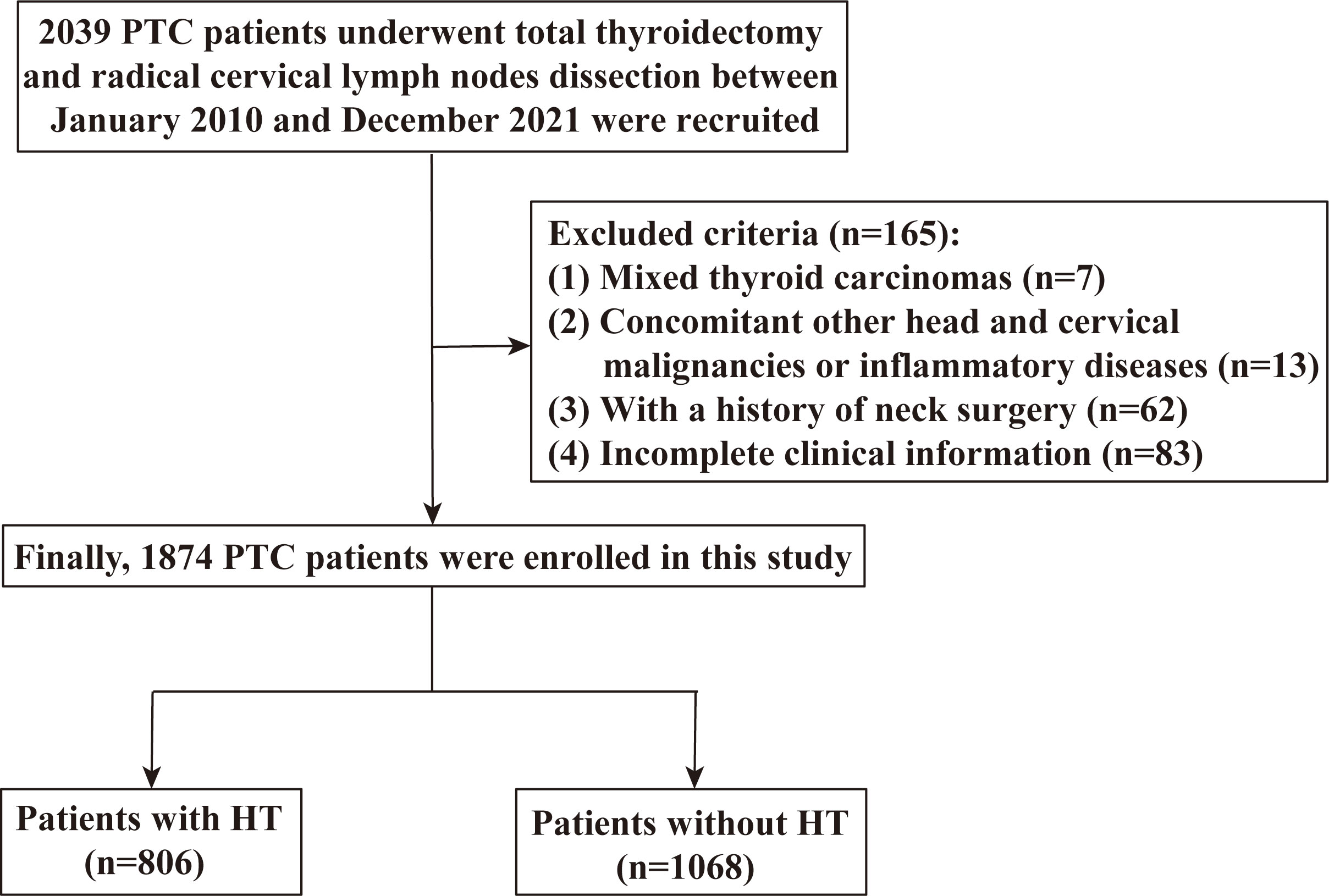
This study was approved by the Ethics Review Committee of Xiangya Hospital, Central South University (20211245) and performed in accordance with the Declaration of Helsinki. The informed consent was waived because of the retrospective and anonymous nature of the study. We enrolled patients who underwent total thyroidectomy and radical cervical lymph node dissection between January 2010 and December 2021 at the Xiangya Hospital, Central South University. All patients underwent physical cervical examination or high-resolution US imaging before surgery. The inclusion criteria were pathologically proven PTC. Patients were excluded if they have any of the following conditions: mixed thyroid carcinomas, PTC combined with other head and cervical malignancies or inflammatory diseases, a history of cervical surgery, and incomplete clinical information. Finally, a total of 1,874 patients with PTC (including 806 patients with HT) were enrolled in this study (Figure 1).
2.2. Surgical strategy
All patients underwent total thyroidectomy and prophylactic central compartment cervical dissection. Therapeutic lateral lymph node dissection (LLND) was performed exclusively for patients with clinically suspicious lateral LN metastasis, which was confirmed by fine-needle aspiration biopsy and/or US and CT before surgery (8). Prophylactic LLND may be performed for PTC patients with intermediate to high recurrence risk based on the surgeon’s decision and/or the patient’s preferences.
2.3. Evaluation of ultrasonography features
All patients received preoperative neck high-resolution ultrasonography examination to evaluate the status of thyroid nodules and cervical central and lateral compartment LNs. Lymph node compartments on ultrasound were separated into levels based on the 2015 ATA management guidelines (7, 8). The abnormal features on ultrasonography suggestive of suspicious LN metastasis included hyperechogenicity, microcalcificationcations, absence of fatty hilum, cystic components, round shape, and peripheral vascularity (8, 19–21). A round shape is defined as a lymph node aspect ratio >0.5. Clinically node-positive (cN+) was defined as with any abnormal ultrasonography features (8), and clinically node-negative (cN−/cNx) was defined as without any abnormal ultrasonography features or LNs undetected on preoperative neck ultrasonography examination (8). The diagnostic accuracy of ultrasonography for LN detection and examination was calculated based on the final pathological findings.
2.4. Evaluation of clinicopathological characteristics
The clinicopathological characteristics including age, gender, primary tumor size, extrathyroidal extension, and lymph node metastatic status were collected. Central lymph node metastasis (CLNM) and lateral lymph node metastasis (LLNM) were diagnosed by postoperative histologic examination. Serum antithyroglobulin (TGAb) and antithyroid peroxidase (TPOAb) levels were measured by electrochemiluminescence immunoassay within 7 days before surgery, and the range for normal values was 0–115 IU/ml for TGAb and 0–34 IU/ml for TPOAb. The results were considered positive when TGAb and TPOAb levels exceeded 115 and 34 IU/ml, respectively. HT was diagnosed by postoperative histologic examination or positive preoperative TGAb, TPOA, and diffuse lesions on preoperative ultrasonography (22–25).
2.5. Statistical analysis
GraphPad Prism 8.3.1 for Windows (GraphPad Software, San Diego, CA, USA) was used for all statistical analyses. Continuous variables are presented as the mean ± standard deviation (SD), whereas categorical variables are presented as the number of cases with percentages (%). The independent t-test was performed for the comparison of continuous variables, and a chi-square test or Fisher’s exact test was conducted for categorical variables. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), false-positive rate (FPR), and false-negative rate (FNR) for lymph node metastasis diagnosed on ultrasound were calculated based on the patients with and without Hashimoto’s thyroiditis, respectively. The chi-square test was used to compare the results of diagnostic tests (sensitivity, specificity, PPV, NPV, FPR, and FNR) between patients with and without Hashimoto’s thyroiditis. In addition, to further determine the effect of HT in the diagnostic performance of preoperative US for cervical LNs, we analyzed the differences in each ultrasonography feature of LNs between PTC patients with HT and those without HT by the chi-square test. A p-value <0.05 was considered statistically significant.
3. Results
3.1. Compared with patients without HT, abnormal lymph nodes in the central compartment were more easily detected in PTC patients with HT
A total of 1,874 PTC patients were included in the study, consisting of 1,266 women (67.6%) and 608 men (32.4%) (female:male ratio, 2.1:1). In this study, 1,673 patients (89.3%) were younger than 55 years, and 499 (26.6%) patients had a tumor size ≤10 mm. Moreover, 806 (43.0%) patients had concomitant HT, and compared with male PTC patients, HT is prone to occur in female PTC patients (p < 0.001).
Among the 1,874 PTC patients, 790 (42.1%) patients had central cN+ and 1,608 (85.9%) patients had lateral cN+, but 709 (89.7%) and 1,363 (84.8%) of them had CLNM and LLNM by histological examination, respectively. In addition, central cN−/cNx and lateral cN−/cNx were found in 1,084 (57.9%) and 266 (14.1%) patients, but 736 (67.9%) and 39 (14.7%) of them had CLNM and LLNM, respectively. Although there was no significant difference in the proportion of CLNM in pathological findings between the patients with HT and without HT (p = 0.867), the proportion of CLNM detected on ultrasound (central cN+) was higher in the patients with HT compared with the patients without HT (p < 0.001) (Table 1).
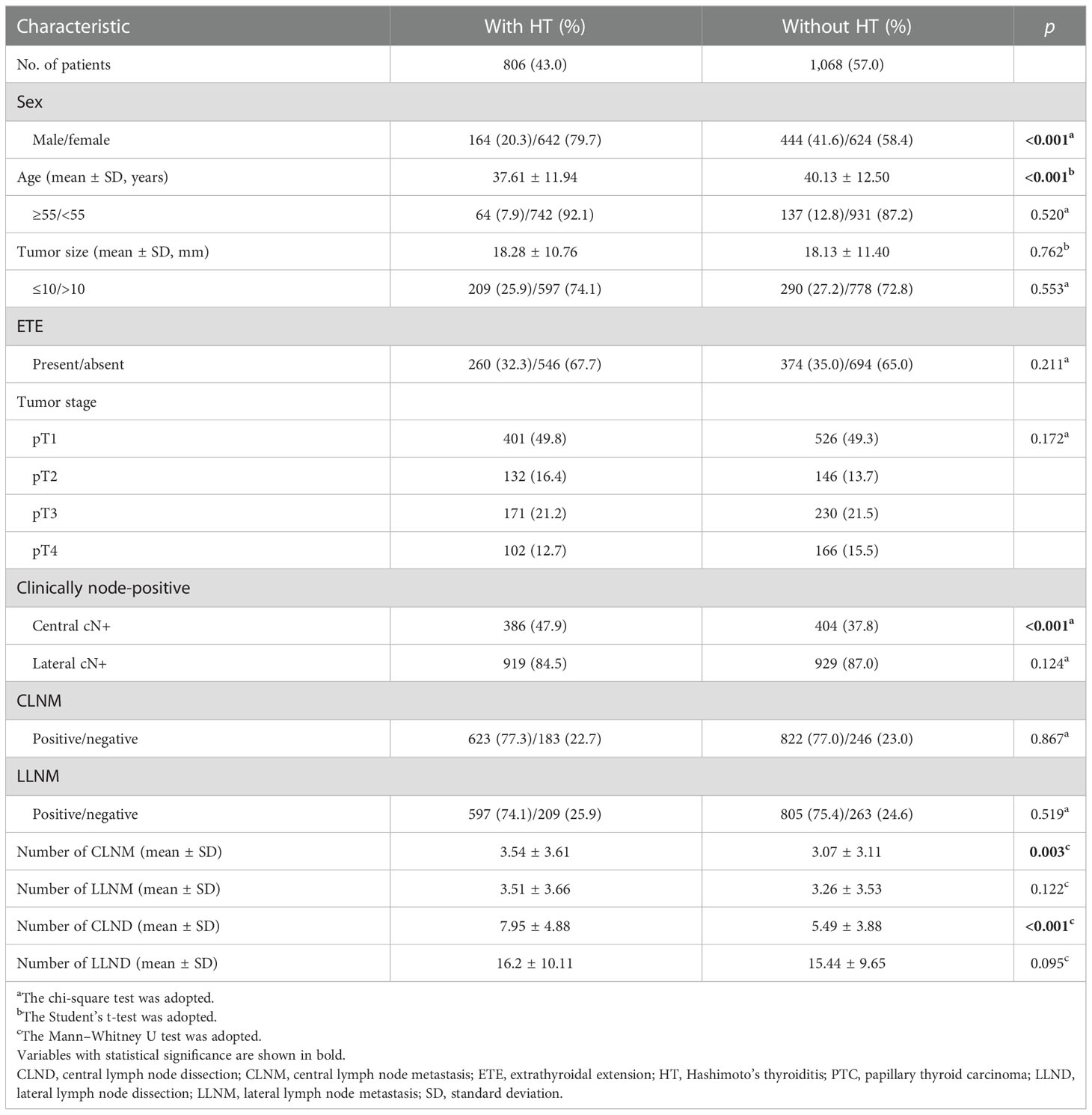
Table 1 Comparison of the clinicopathological characteristics of PTC patients with HT and without HT.
3.2. HT decreased the diagnostic efficacy of preoperative US for central metastatic LNs
Furthermore, we compared the diagnostic value of preoperative US for cervical LNs (including central LNs and lateral LNs) between PTC patients with HT and those without HT. Interestingly, compared with those without HT, we found increased sensitivity and FPR of the preoperative US for central LNs in PTC patients with HT (sensitivity, 53.8% vs. 45.5%, p = 0.002; FPR, 27.9% vs. 12.2%, p < 0.001), but lower specificity, PPV, and FNR (specificity, 72.1% vs. 87.8%, p < 0.001; PPV, 86.8% vs. 92.6%, p = 0.007; FNR, 46.3% vs. 54.5%, p = 0.002) (Table 2). Moreover, this difference was also observed when the patients were divided into two groups by tumor size (cutoff = 10 mm) (Tables S1, S2). Of note, no differences related to the diagnostic efficacy of the US were observed in lateral LNs (p > 0.05) (Table 3).
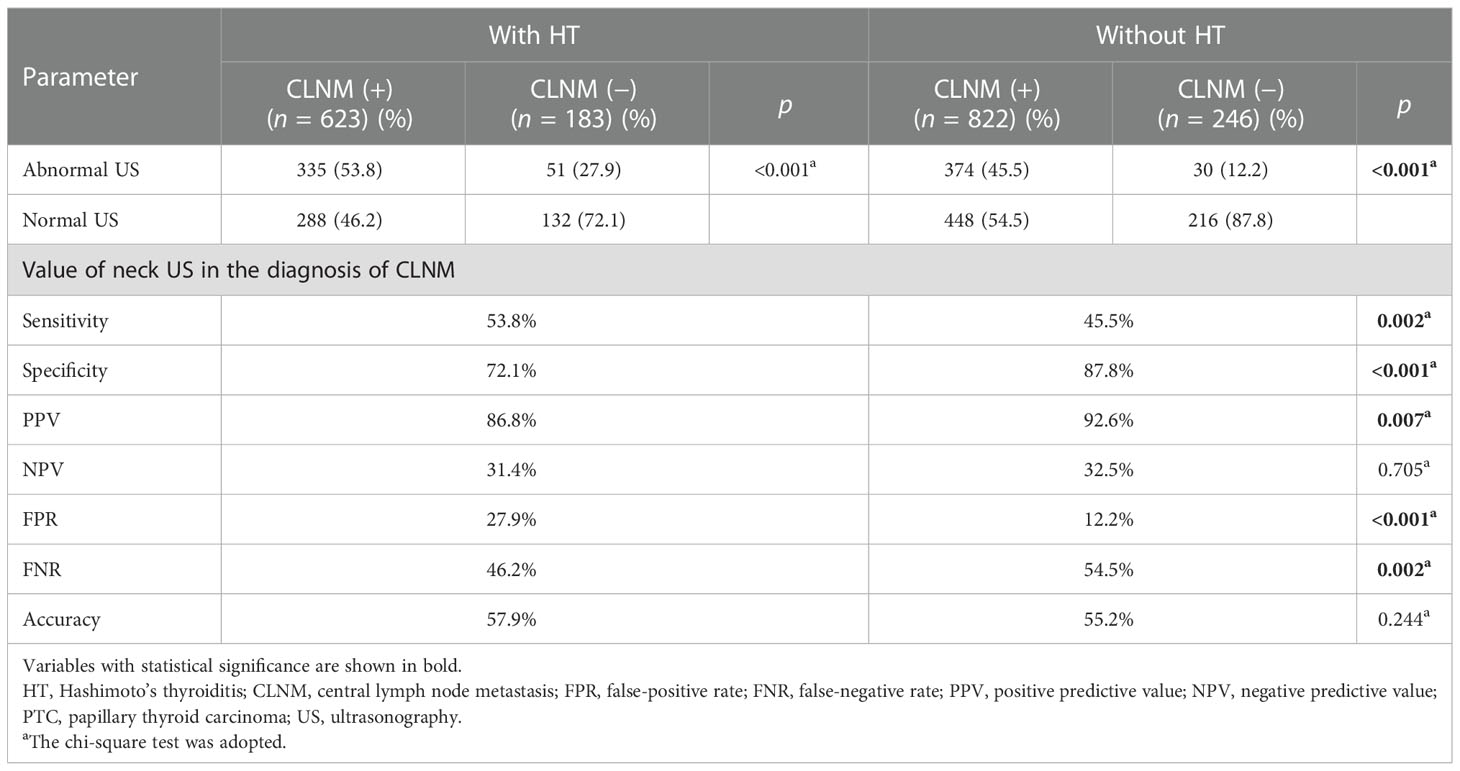
Table 2 The diagnostic value for central compartment lymph node metastases in PTC patients with or without HT on neck US.
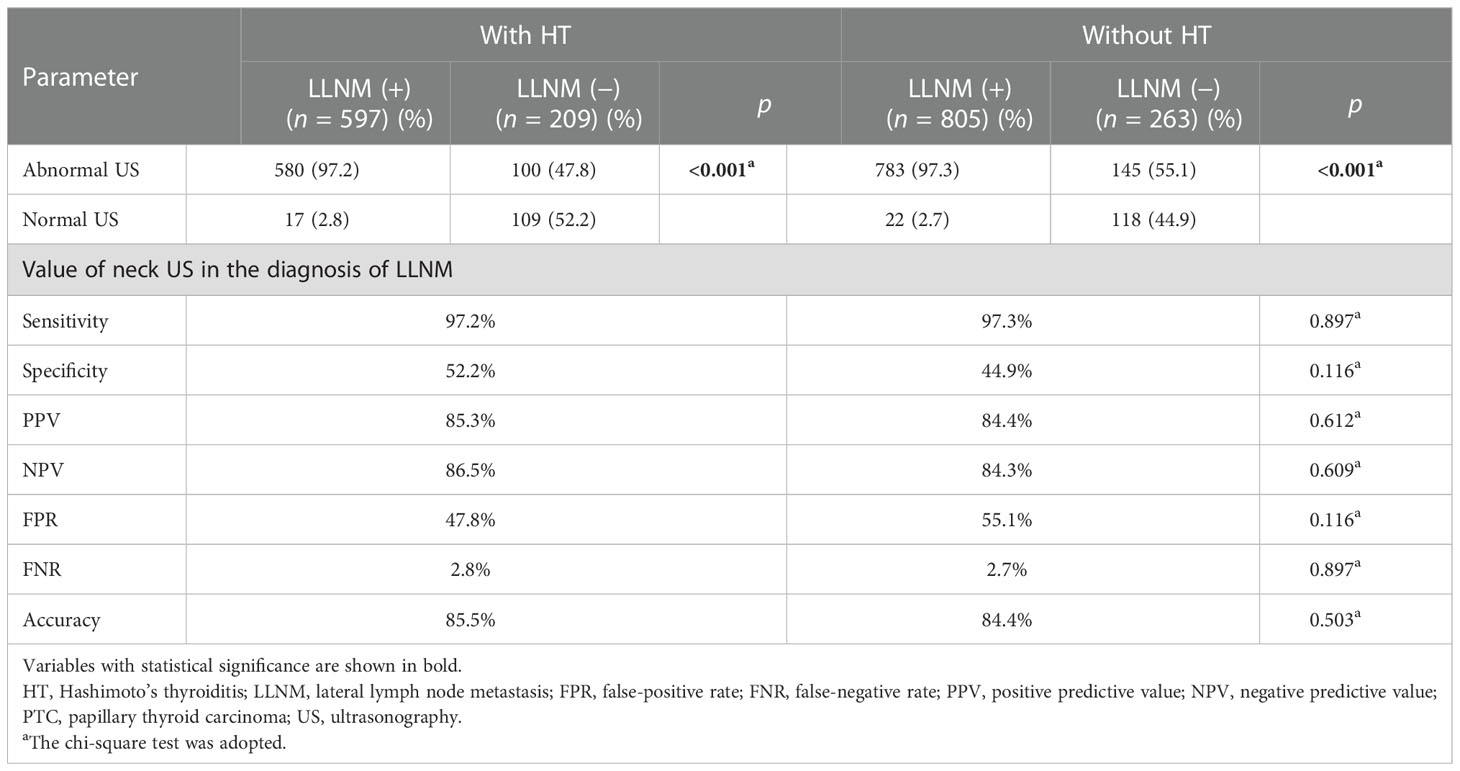
Table 3 The diagnostic value for lateral compartment lymph node metastases in PTC patients with or without HT on neck US.
3.3. HT increased the incidence of the absence of fatty hilum in central LNs without metastasis
In addition, we also analyzed the differences in each ultrasonography feature of LNs between PTC patients with HT and those without HT and observed that the absence of fatty hilum of central LNs was more prevalent in the non-metastatic central LNs of PTC patients with HT than in those without HT (13.02% vs. 7.46%, p = 0.013). Otherwise, no differences in other ultrasonography features including hyperechogenicity, microcalcifications, cystic components, round shape, and peripheral vascularity were observed (p > 0.05) (Figure 2). Furthermore, we also compared the lateral lymph node ultrasonography features of PTC patients with HT and without HT, and no significant differences were observed (p > 0.05) (Figure 3).
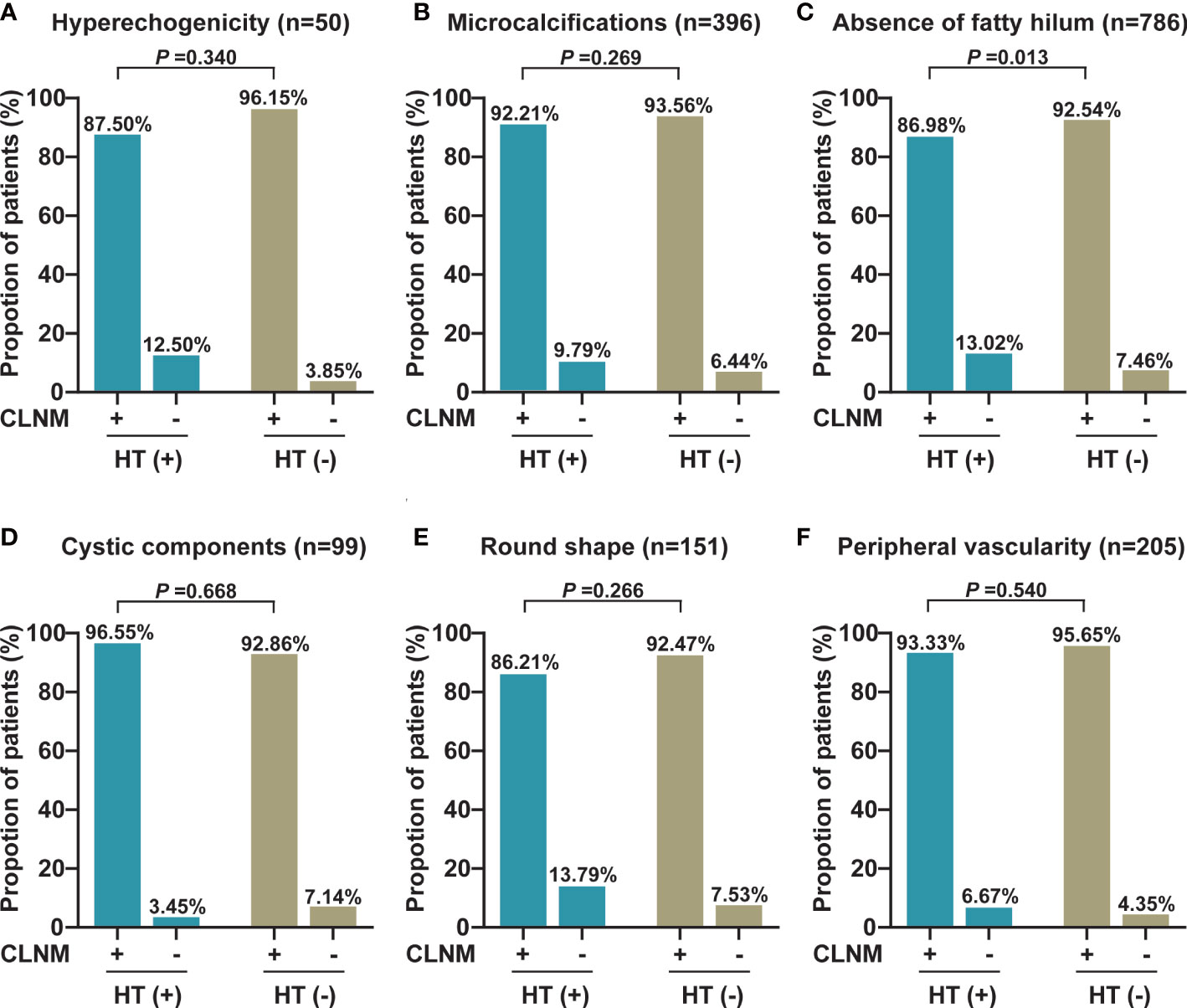
Figure 2 The proportion of each abnormal US feature between malignant and benign central LNs in PTC patients with or without HT. (A) The proportion of hyperechogenicity (n = 50). (B) The proportion of microcalcifications (n = 396). (C) The proportion of the absence of fatty hilum (n = 786). (D) The proportion of cystic components (n = 99). (E) The proportion of round shape (n = 151). (F) The proportion of peripheral vascularity (n = 205). CLNM, central lymph node metastasis; HT, Hashimoto’s thyroiditis.

Figure 3 The proportion of each abnormal US feature between malignant and benign lateral LNs in PTC patients with or without HT. (A) The proportion of hyperechogenicity (n = 158). (B) The proportion of microcalcifications (n = 980). (C) The proportion of the absence of fatty hilum (n = 1,586). (D) The proportion of cystic components (n = 317). (E) The proportion of round shape (n = 278). (F) The proportion of peripheral vascularity (n = 439). LLNM, lateral lymph node metastasis; HT, Hashimoto’s thyroiditis.
4. Discussion
This study is the first to comprehensively investigate the effect of HT on the diagnostic efficacy of preoperative US on cervical LNs in PTC patients. Our results indicate that HT will reduce the diagnostic efficacy of preoperative US and increase the misdiagnosis rate of preoperative US for central LNs.
In reality, PTC patients frequently have concomitant HT which manifests as reactive hyperplasia involving the neck LNs especially central LNs, which is frequently observed intraoperatively (17, 18, 26–28). This may interfere with the evaluation of preoperative US for cervical LNs. However, the effect and clinical significance of HT on ultrasonography evaluation for cervical LN lesions remain unknown. Therefore, we comprehensively analyzed the diagnostic efficacy of preoperative US for cervical LNs between PTC patients with HT and those without HT. The results indicate a significantly higher false-positive rate of preoperative US for central LNs in PTC patients with HT as compared with PTC patients without HT (27.9% vs. 12.2%), but this was not observed in lateral LNs. These data suggest an increased misdiagnosis rate of preoperative US for central LNs in PTC patients with HT, which may lead to a surgeon’s misjudgment for central LNs and increase the extent of surgery and the incidence of surgery-related complications.
Preoperative high-resolution US is the primary means for the evaluation of cervical LN lesions by the imaging features of LNs (8). Malignant LNs can be distinguished from normal LNs based on size, shape, echogenicity, hypervascularity, loss of fatty hilum, and calcifications of imaging features (21). Of note, abnormally enlarged LNs are often observed during the preoperative US and intraoperative exploration in PTC patients with HT; however, we often cannot accurately judge the nature of enlarged LNs. Therefore, we compared the differences of each US imaging feature of cervical LNs (including central and lateral compartment LNs) in PTC patients with or without HT. Compared with those PTC patients without HT, we found that the absence of fatty hilum was prone to present on central benign LNs in PTC patients with HT (13.02% vs. 7.46%), but this difference was not observed in lateral compartment LNs. These results denote that clinicians should cautiously evaluate the characteristic of central LNs, when the fatty hilum of central LN was undetected during the US examination in PTC patients with HT, thereby decreasing the possibility of misdiagnosis.
There are some limitations to this study that should be noted. Firstly, although this study found that HT interferes with the evaluation of preoperative US for central LNs in PTC patients, we are currently unable to accurately identify the metastatic status of central LNs in PTC patients with HT before surgery. Secondly, this study lacks external validation, and further studies from multiple centers are needed in the future.
5. Conclusion
HT will interfere with the preoperative US evaluation for central LNs and increase the incidence of the absence of fatty hilum in central benign LNs. When PTC patients had concomitant HT, clinicians should thoroughly evaluate the central LNs, thereby decreasing the incidence of misdiagnosis and unnecessary surgery.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Study concepts: H-LT and PH. Study design: H-LT and S-LD. Data acquisition: H-LT and AN. Quality control of data and algorithms: Y-XZ and PC. Data analysis and interpretation: QH and Z-JZ. Statistical analysis: H-LT and Z-JZ. Manuscript preparation: H-LT and PH. Manuscript editing: H-LT, AN, and PH. Manuscript review: H-LT, SC, and PH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81974423, 81902729), the Special Funding for the Construction of Innovative Provinces in Hunan (2020SK4003), the China Postdoctoral Science Foundation (2020M672517, 2021T140749), the Natural Science Foundation of Hunan Province (2020JJ5904, 2020JJ4925), and the Project Program of National Clinical Research Center for Geriatric Disorders (2021KFJJ03).
Acknowledgments
We wish to thank all the patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.987906/full#supplementary-material
Abbreviations
ATA, American Thyroid Association; CLNM, central lymph node metastasis; cN+, clinically node-positive; cN−, clinically node-negative; cNx, clinically node-undetected; CT, computed tomography; ETE, extrathyroidal extension; FPR, false-positive rate; FNR, false-negative rate; HT, Hashimoto’s thyroiditis; LLNM, lateral lymph node metastasis; LND, lymph node dissection; NPV, negative predictive value; PPV, positive predictive value; PTC, papillary thyroid carcinoma; TC, thyroid carcinoma; TT, total thyroidectomy; US, ultrasonography.
References
1. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: A population-based study. Lancet Diabetes Endocrinol (2021) 9(4):225–34. doi: 10.1016/S2213-8587(21)00027-9
2. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, Vecchia LC, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol (2022) 10(4):264–72. doi: 10.1016/S2213-8587(22)00035-3
3. LeClair K, Bell KJL, Furuya-Kanamori L, Doi SA, Francis DO, Davies L, et al. Evaluation of gender inequity in thyroid cancer diagnosis: Differences by sex in US thyroid cancer incidence compared with a meta-analysis of subclinical thyroid cancer rates at autopsy. JAMA Intern Med (2021) 181(10):1351–8. doi: 10.1001/jamainternmed.2021.4804
4. McLeod DSA, Zhang L, Durante C, Cooper DS. Contemporary debates in adult papillary thyroid cancer management. Endocr Rev (2019) 40(6):1481–99. doi: 10.1210/er.2019-00085
5. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6
6. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol (2019) 30(12):1856–83. doi: 10.1093/annonc/mdz400
7. Stack BC Jr., Ferris RL, Goldenberg D, Haymart M, Shaha A, Sheth S, et al. American Thyroid association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid (2012) 22(5):501–8. doi: 10.1089/thy.2011.0312
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2015) 26(1):1–133. doi: 10.1089/thy.2015.0020
9. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope (2011) 121(3):487–91. doi: 10.1002/lary.21227
10. Lee DW, Ji YB, Sung ES, Park JS, Lee YJ, Park DW, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol (2013) 39(2):191–6. doi: 10.1016/j.ejso.2012.07.119
11. Khokhar MT, Day KM, Sangal RB, Ahmedli NN, Pisharodi LR, Beland MD, et al. Preoperative high-resolution ultrasound for the assessment of malignant central compartment lymph nodes in papillary thyroid cancer. Thyroid (2015) 25(12):1351–4. doi: 10.1089/thy.2015.0176
12. Alabousi M, Alabousi A, Adham S, Pozdnyakov A, Ramadan S, Chaudhari H, et al. Diagnostic test accuracy of ultrasonography vs computed tomography for papillary thyroid cancer cervical lymph node metastasis: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg (2022) 148(2):107–18. doi: 10.1001/jamaoto.2021.3387
13. Ehlers M, Schott M. Hashimoto's thyroiditis and papillary thyroid cancer: are they immunologically linked? trends endocrinol metab. Trends Endocrinol Metab (2014) 25(12):656–64. doi: 10.1016/j.tem.2014.09.001
14. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol (2013) 168(3):343–9. doi: 10.1530/EJE-12-0903
15. Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto's thyroiditis: Celebrating the centennial through the lens of the johns Hopkins hospital surgical pathology records. Thyroid (2013) 23(2):142–50. doi: 10.1089/thy.2012.0554
16. Roh JL, Park JY, Kim JM, Song CJ. Use of preoperative ultrasonography as guidance for neck dissection in patients with papillary thyroid carcinoma. J Surg Oncol (2009) 99(1):28–31. doi: 10.1002/jso.21164
17. Vita R, Ieni A, Tuccari G, Benvenga S. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma. Rev Endocr Metab Disord (2018) 19(4):301–9. doi: 10.1007/s11154-018-9474-z
18. Cappellacci F, Canu GL, Lai ML, Lori E, Biancu M, Boi F, et al. Association between hashimoto thyroiditis and differentiated thyroid cancer: A single-center experience. Front Oncol (2022) 12:959595. doi: 10.3389/fonc.2022.959595
19. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Cailou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab (2007) 92(9):3590–4. doi: 10.1210/jc.2007-0444
20. Lee JY, Yoo RE, Rhim JH, Lee KH, Choi KS, Hwang I, et al. Validation of ultrasound risk stratification systems for cervical lymph node metastasis in patients with thyroid cancer. Cancers (Basel) (2022) 14(9):2106. doi: 10.3390/cancers14092106
21. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid association statement on preoperative imaging for thyroid cancer surgery. Thyroid (2015) 25(1):3–14. doi: 10.1089/thy.2014.0096
22. Ralli M, Angeletti D, Fiore M, Aguanno VD, Lambiase A, Artico M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev (2020) 19(10):102649. doi: 10.1016/j.autrev.2020.102649
23. Min Y, Huang Y, Wei M, Wei X, Chen H, Wang X, et al. Preoperatively predicting the central lymph node metastasis for papillary thyroid cancer patients with hashimoto's thyroiditis. Front Endocrinol (Lausanne) (2021) 12:713475. doi: 10.3389/fendo.2021.713475
24. Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, et al. Prevalence of hashimoto thyroiditis in adults with papillary thyroid cancer and its association with cancer recurrence and outcomes. JAMA Netw Open (2021) 4(7):e2118526. doi: 10.1001/jamanetworkopen.2021.18526
25. Benvenga S, Trimarchi F. Changed presentation of hashimoto's thyroiditis in north-Eastern Sicily and calabria (Southern Italy) based on a 31-year experience. Thyroid (2008) 18(4):429–41. doi: 10.1089/thy.2007.0234
26. Konturek A, Barczynski M, Wierzchowski W, Stopa M, Nowak W. Coexistence of papillary thyroid cancer with hashimoto thyroiditis. Langenbecks Arch Surg (2013) 398(3):389–94. doi: 10.1007/s00423-012-1021-x
27. Xu J, Ding K, Mu L, Huang J, Ye F, Peng Y, et al. Hashimoto's thyroiditis: A "Double-edged sword" in thyroid carcinoma. Front Endocrinol (Lausanne). (2022) 13:801925. doi: 10.3389/fendo.2022.801925
Keywords: Hashimoto’s thyroiditis, papillary thyroid carcinoma, lymph node metastasis, ultrasonography, diagnostic efficacy
Citation: Tan H-L, Nyarko A, Duan S-l, Zhao Y-X, Chen P, He Q, Zhang Z-J, Chang S and Huang P (2023) Comprehensive analysis of the effect of Hashimoto’s thyroiditis on the diagnostic efficacy of preoperative ultrasonography on cervical lymph node lesions in papillary thyroid cancer. Front. Endocrinol. 13:987906. doi: 10.3389/fendo.2022.987906
Received: 06 July 2022; Accepted: 19 December 2022;
Published: 12 January 2023.
Edited by:
Mario Vitale, University of Salerno, ItalyReviewed by:
Donald Bodenner, University of Arkansas, United StatesChang-Mo Oh, Kyung Hee University, South Korea
Copyright © 2023 Tan, Nyarko, Duan, Zhao, Chen, He, Zhang, Chang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Huang, eGlhbmd5YWhwQGNzdS5lZHUuY24=
 Hai-Long Tan
Hai-Long Tan AdolphusOsei Nyarko
AdolphusOsei Nyarko Sai-li Duan
Sai-li Duan Ya-Xin Zhao
Ya-Xin Zhao Pei Chen
Pei Chen Qiao He1
Qiao He1 Shi Chang
Shi Chang Peng Huang
Peng Huang