- 1Institute of Gerontology, Department of Geriatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Gerontology Center of Hubei Province, Wuhan, China
Background: The impact of lifestyle factors on circulating fibroblast growth factor 21 (cFGF21) remains unclear. We conducted this systematic review and meta-analysis to evaluate the association between lifestyle factors and cFGF21 levels.
Methods: We included studies that evaluated the effects of different lifestyles on cFGF21 concentration in adults, which included smoking, exercise, diets, alcohol consumption and weight loss. Random effects models or fixed effects models were used for meta-analysis to calculate the standardized mean difference (SMD) and 95% confidence interval according to the heterogeneity among studies. Study quality was assessed using the Newcastle–Ottawa Scale for cohort studies, the Joanna Briggs Institution Checklist for cross-sectional studies, and the PEDro scale for experimental studies.
Results: A total of 50 studies with 1438 individuals were included. Overall, smoking, a hypercaloric carbohydrate-rich diet, a hypercaloric fat-rich diet, amino acid or protein restriction, excessive fructose intake and alcohol consumption significantly upregulated cFGF21 levels (p<0.05), whereas fish oil intake and calorie restriction with sufficient protein intake significantly decreased cFGF21 (p<0.05). Compared to the preexercise cFGF21 level, the cFGF21 level significantly increased within 3 hours postexercise (p<0.0001), while it significantly decreased in the blood sampled >6 h postexercise (p=0.01). Moreover, higher exercise intensity resulted in higher upregulation of cFGF21 at 1-hour post exercise (p=0.0006).
Conclusion: FGF21 could serve as a potential biomarker for the assessment of different lifestyle interventions. When it is used for this purpose, a standard study protocol needs to be established, especially taking into consideration the intervention types and the sampling time post-intervention.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021254758, identifier CRD42021254758.
Introduction
Lifestyle is an important factor affecting individual health, which has attracted increasing attention from medical researchers. According to the data of the World Health Organization, 60% of individual health-related factors are associated with lifestyle (1). Thus, early lifestyle interventions could be highly important and effective to promote health, prevent diseases and alleviate unhealthy lifestyle-caused medical and social burdens.
Fibroblast growth factor 21 (FGF21) was first discovered by Nishimura et al. from mouse embryos in 2000 (2). In humans, FGF21 is expressed in many tissues including liver, adipose tissue, pancreas, muscle, and central nervous system. However, FGF21 is most abundantly expressed in the liver (3). As FGF21 lacks the canonical heparan-binding domain that defines the nonendocrine FGFs, it could escape from the extracellular matrix into the blood and act as an endocrine factor (4). Circulating FGF21 (cFGF21) binds to the receptor complex on the cell surface, which consists of coreceptor protein β-klotho (KLB) and FGF receptor (FGFR), especially FGFR1c, to activate FGFR signaling and regulate a variety of processes including glucose, lipid and energy metabolism, macronutrient preference and anti-inflammatory process (4–7). Therefore, long-acting analogs of FGF21 or FGF21 receptor agonists have been created to treat diabetes. The elevation of FGF21 by long-acting FGF21 analogs shows significant improvements in dyslipidemia and hepatic fat fractions in nonalcoholic steatohepatitis patients (7, 8).
cFGF21 levels have been shown to be elevated in subjects with metabolic syndrome, nonalcoholic fatty liver, hyperlipidemia, and cardiovascular diseases (9, 10). Thus, cFGF21 is regarded as a useful biomarker of metabolic disorders. In this study, lifestyles were referred to daily behaviors that are known to affect health, including smoking, alcohol consumption, diet, weight loss and exercise. Lifestyles play a major role in affecting metabolic syndrome, suggesting a possible link between cFGF21 and lifestyles. In recent years, the number of studies investigating associations between cFGF21 and different lifestyles has grown, but the results remain elusive and controversial. Therefore, this systematic review and meta-analysis was performed to summarize the findings of previous studies and to evaluate the association between cFGF21 and different lifestyles including smoking, alcohol consumption, diet, weight loss and exercise.
Methods
Study design
This work was executed in accordance with the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) guidelines (11). It was also registered in the International Prospective Register of Systematic Reviews (PROSPERO) before screening studies for inclusion (ID : CRD42021254758) (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021254758).
Literature search
We conducted a systematic literature search by searching PubMed, Web of Science, EMBASE and Cochrane Library in June 2021. Studies that assessed the association between serum FGF21 concentration and different lifestyles were identified. The following terms and their combinations were employed: “fibroblast growth factor 21”, “FGF21”, “lifestyle”, “smoking”, “drinking”, “alcohol drinking”, “ethanol”, “exercise”, “physical activity”, “weight loss”, “diet”, “fasting”, “sleep”, “sedentary”. The same medical subject headings (MeSH) and keywords in various combinations were used in the mentioned electronic databases. The detail search strategy in PubMed was fully described in the Supplementary Method 1.
Selection criteria
The inclusion criteria were as follows (1): Studies assessed the association between serum or plasma FGF21 concentrations and lifestyles, including smoking, physical activity, diets, alcohol drinking and weight loss (2); Studies reported the outcomes as the mean with standard deviation or median with quartile of serum FGF21 concentration in patients with or without different lifestyles; and (3) Adult clinical studies published in English.
The exclusion criteria were as follows: (1) reviews, letters, meeting abstracts, case reports, comments and editorials; (2) duplicate studies with overlapping data; (3) studies reporting invalid data; and (4) studies on pregnant women or children.
According to the selection criteria, the initial screening of studies was based on the titles and abstracts. Then, the full texts of the potential studies were assessed. An additional manual search of references from identified studies was also performed. All studies were independently screened by two reviewers (QZH and ZYC). A third researcher (YN) was consulted to resolve disagreements.
Data extraction and quality assessment
Two reviewers independently extracted data from the included studies. Basic information and patient baseline characteristics of all studies were extracted. To assess the association between cFGF21 and lifestyle factors, the following data were extracted: mean with standard deviation or median with quartile of cFGF21 concentration. In studies with different groups of participants, such as male and female participants, groups were extracted separately. In addition, we generally chose to extract cFGF21 level data with the longest interval period following lifestyle intervention in each study. For example, for the studies that provided both 1- and 2-day sampling points after lifestyle intervention, only the data at the 2-day sampling point were used. However, for studies studying the relationships between exercise and cFGF21, when the cFGF21 level was measured within 3 hours, we chose data of the maximum or the minimum to best reflect the temporary effect of exercise, while after 3 hours, we also extracted cFGF21 level data with the longest interval period following exercise. If there was any missing information about the eligible study, we contacted the corresponding authors and the first authors. The authors of eight included studies provided the mean ± SD or raw data (Tables 1–5, labeled with *). However, eleven studies did not provide specific data, and we extracted the data from image figures using WebPlotDigitizer (Tables 1–5, labeled with #) (https://apps.automeris.io/wpd/index.zh_CN.html).
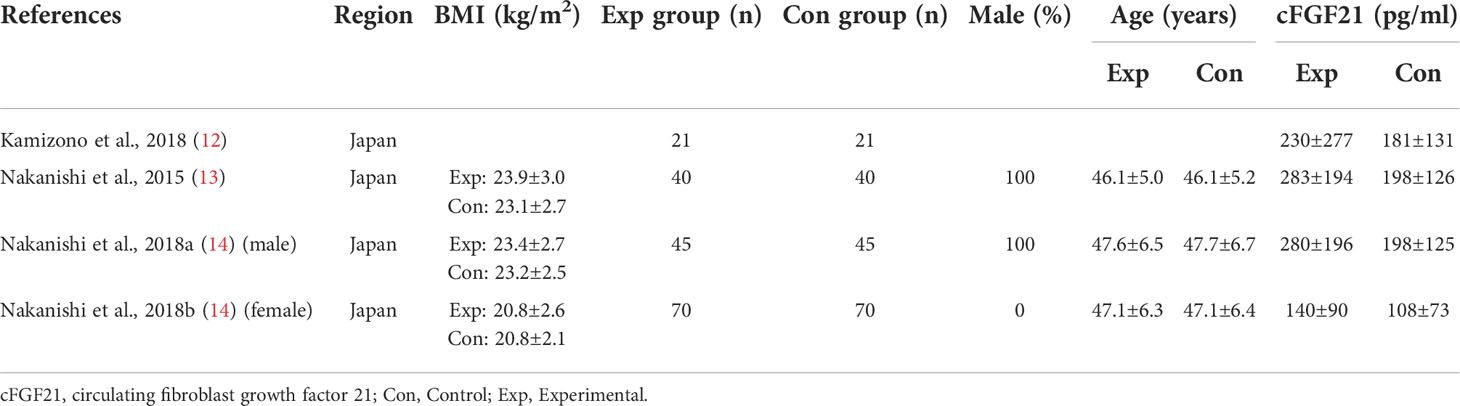
Table 1 Baseline characteristics of studies reported cFGF21 concentration in subjects with or without smoking.
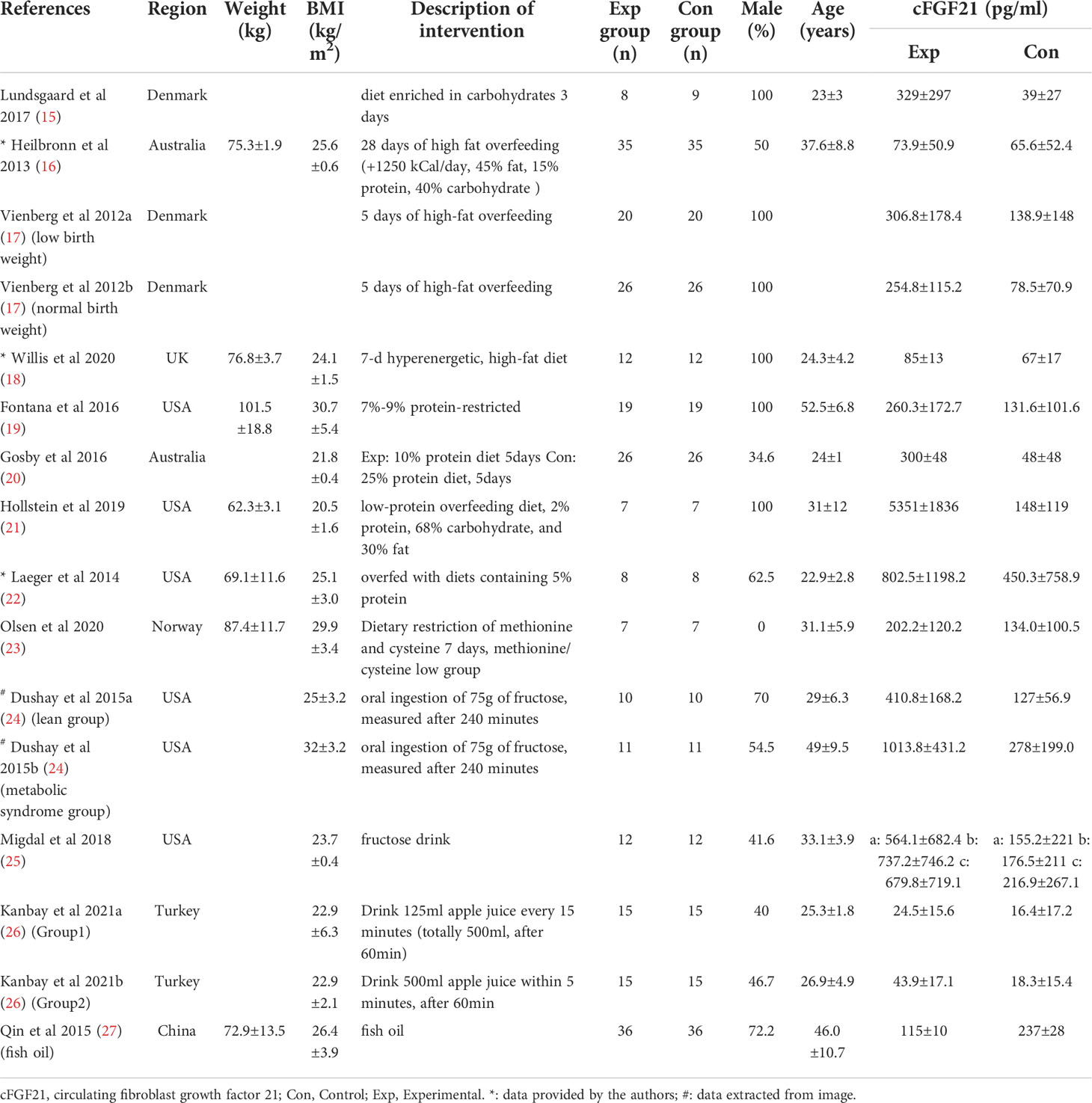
Table 2 Baseline characteristics of studies reported cFGF21 concentration in subjects with diet intervention.
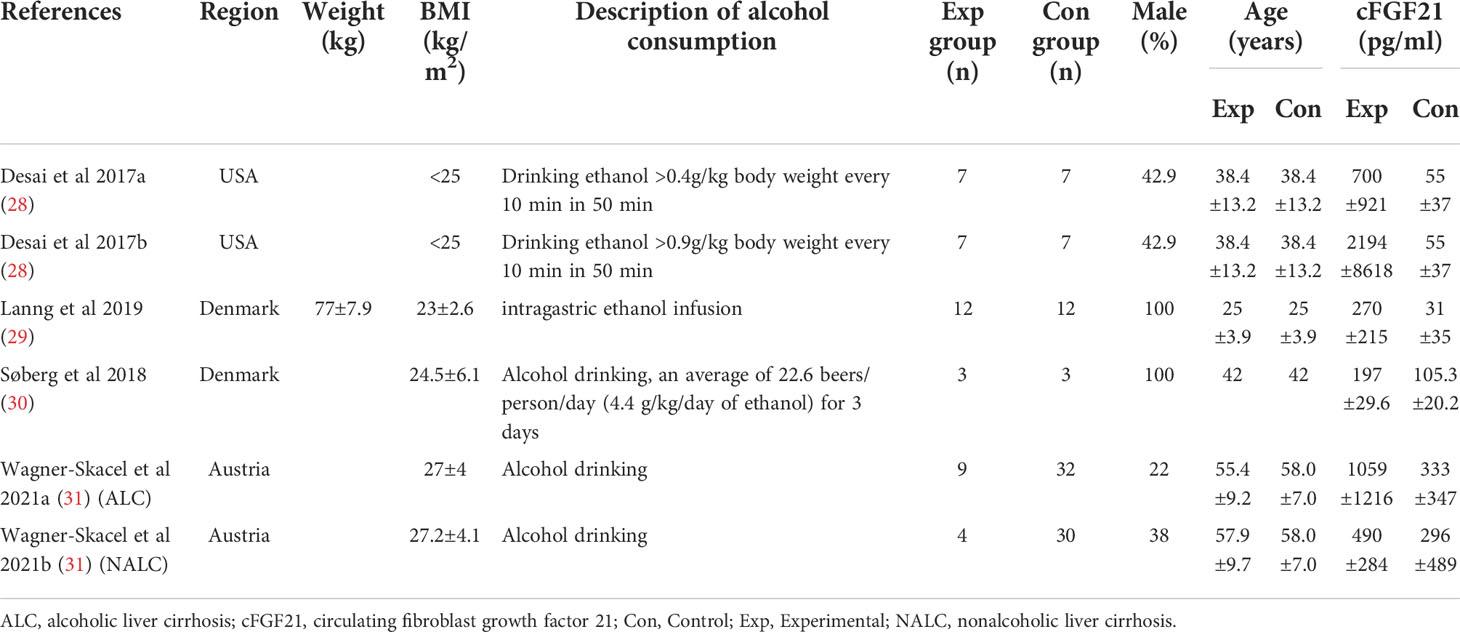
Table 3 Baseline characteristics of studies reported cFGF21 concentration in subjects with or without alcohol consumption.
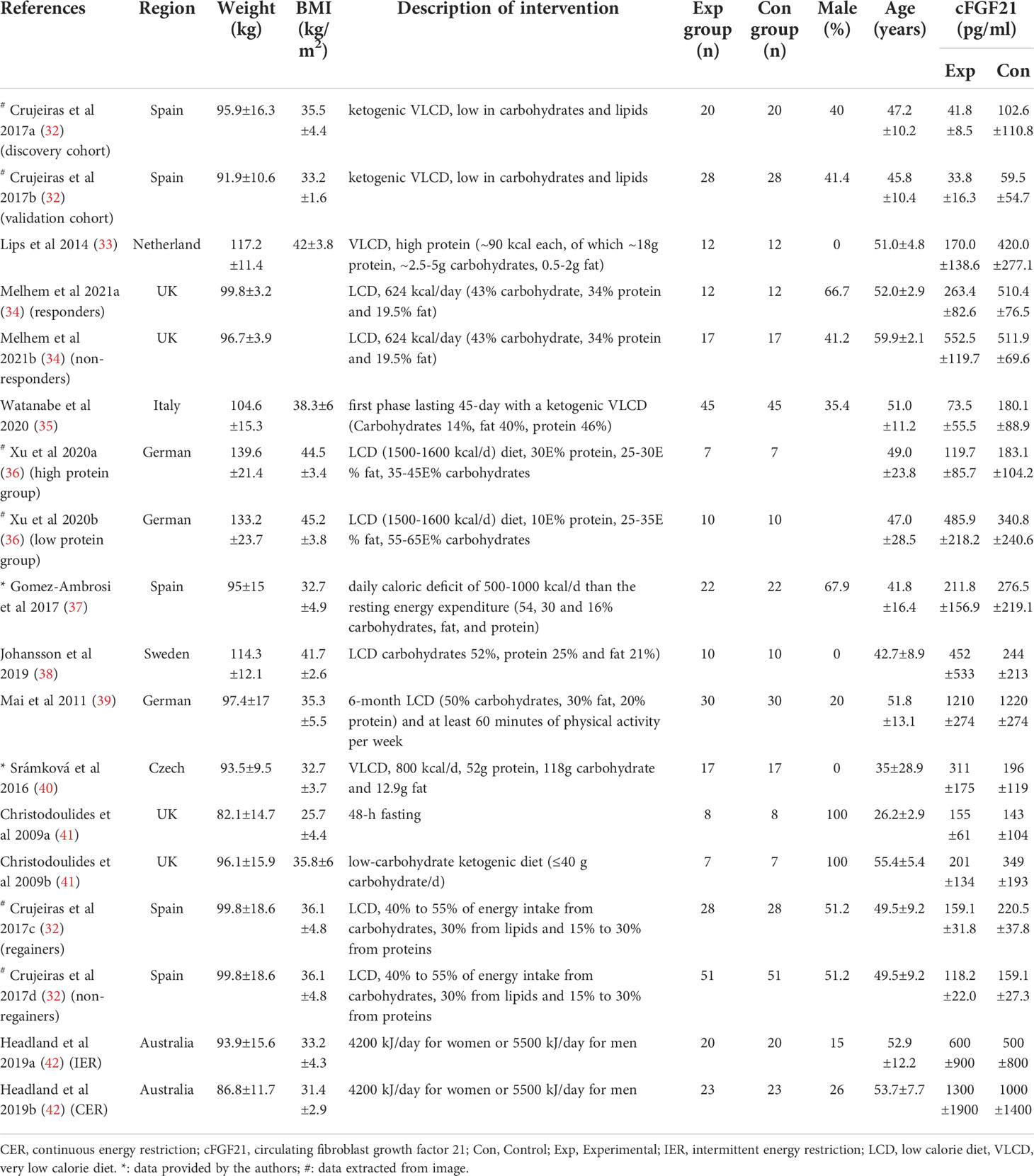
Table 4 Baseline characteristics of studies reported cFGF21 concentration in subjects with weight loss intervention.
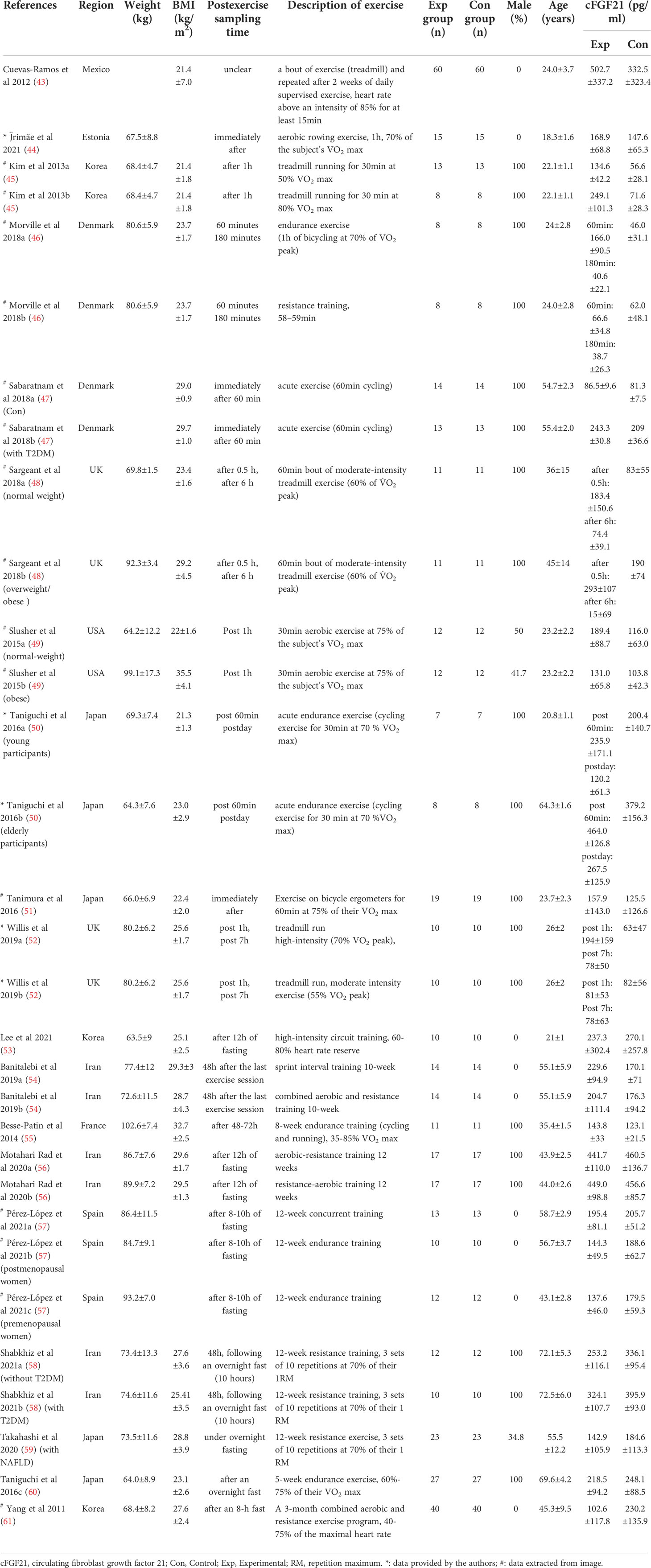
Table 5 Baseline characteristics of studies reported cFGF21 concentration in subjects with or without physical activity intervention.
Quality assessment was independently performed by two reviewers (NH and YZ). Discrepancies were resolved by discussion with a third reviewer (LPC). The quality of cohort studies was assessed by using the Newcastle–Ottawa Quality Assessment Scale (NOS) (62), as reported in Supplementary Table 1. Studies scored > 5 were considered to be high-quality. The quality of the cross-sectional studies was assessed by using the Joanna Briggs Institution (JBI) Checklist for Analytical Cross-Sectional Study (https://jbi.global/critical-appraisal-tools), as reported in Supplementary Table 2. A score of 4 to 6 indicated moderate quality, whereas as score of 7 or more indicated high quality (63). The methodological quality of the included experimental studies was rated using the PEDro scale (https://pedro.org.au/english/resources/pedro-scale/), as reported in Supplementary Table 3. Publication bias was assessed by funnel plots if the number of included cohorts was ≥10. Publication bias was considered to be significant if the funnel plot was asymmetric.
Data analysis
This meta-analysis was performed by using RevMan 5.3 (the Nordic Cochrane Center, Copenhagen, Denmark). Heterogeneity was tested by using the Chi-squared test and I2 statistic. p<0.05 or I2 >50% indicated that the heterogeneity was significant. A fixed-effects model was used to calculate the pooled estimates if no significant heterogeneity was identified (I2<50%); otherwise, a random-effects model was used. The overall effects were determined by the Z-test and p<0.05 was considered as statistically significant. Subgroup analysis was conducted according to specific lifestyle.
Medians with quartiles were transformed into means with standard deviations for pooled estimates by using the webpage tool in the BOX-COX manner developed by McGrath et al. (64).
Results
Study selection, characteristics and quality assessment
The flow diagram of the search and screening process is shown in Figure 1. After removing duplicate articles, 2221 articles were identified in the initial database search. After screening titles and abstracts, 69 articles remained for further full-text evaluation. Finally, a total of 50 studies with 1438 individuals were included in this meta-analysis.
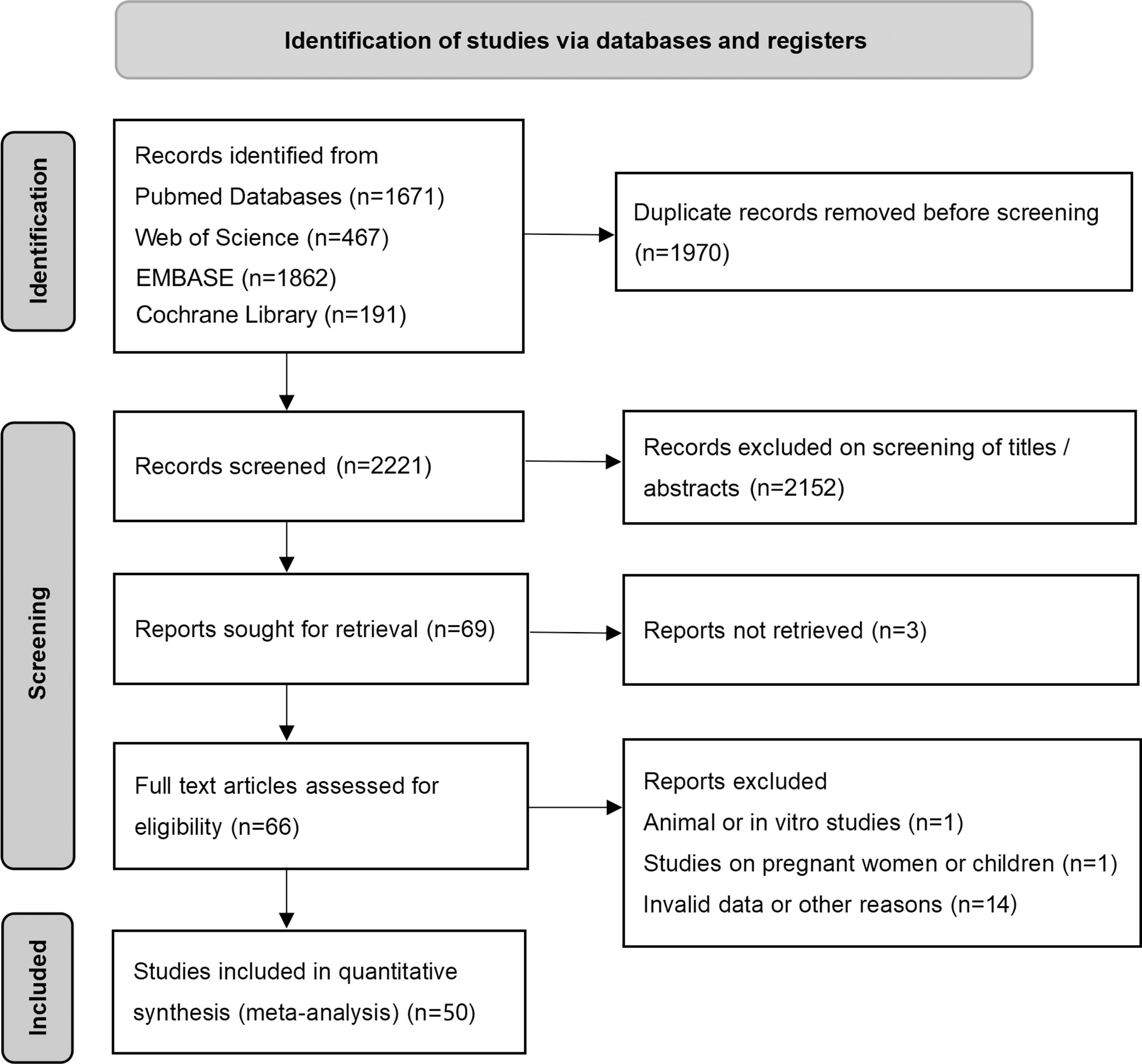
Figure 1 Preferred reporting items for systemic reviews and meta-analysis flow diagram of literature screening. cFGF21, circulating fibroblast growth factor 21.
Tables 1–5 present the characteristics of all included studies. These studies were published between 2011 and 2021. Patients were from 17 countries including Japan, USA, Austria, Australia, Iran, France, Spain, Korea, Mexico, UK, China, German, Czech, Denmark, Norway, Turkey and Netherland. According to the NOS, the cohort study was considered to be high quality (Supplementary Tables 1). According to the JBI checklist, all cross-sectional studies were also considered to be high quality (Supplementary Table 2). For the PEDro scale, a quality of study scoring less than 4 is considered ‘poor’, 4 to 5 is considered ‘fair’, and 6 to 9 is considered ‘good’ (65). Therefore, 18 studies were rated as fair quality, while 29 studies were rated as good quality (Supplementary Table 3).
The association between smoking and cFGF21 concentration
Three studies containing 176 individuals were involved in the analysis of the association between smoking and cFGF21 concentration (12–14) (Table 1). Overall, the cFGF21 concentration was significantly higher in smokers (Experimental) than in nonsmokers (Control) (SMD=0.42 pg/ml, 95% CI: 0.21-0.64, p<0.0001) (Figure 2). The heterogeneity among these studies was insignificant (p=0.86).
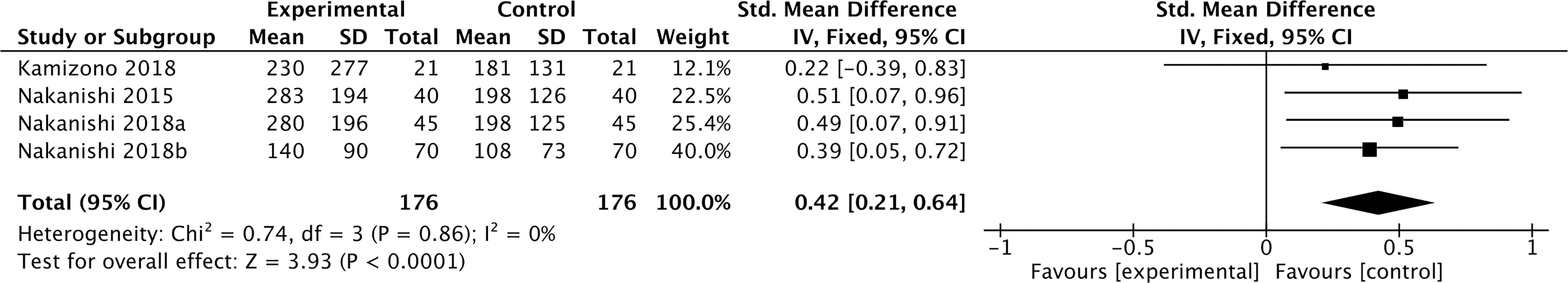
Figure 2 Forest plot of cFGF21 concentration in individuals with or without smoking. Fixed effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
The association between diet and cFGF21 concentration
Thirteen studies containing 300 individuals were involved in the analysis of the association between diets and cFGF21 concentration (15–27) (Table 2). Overall, the cFGF21 concentration was significantly higher in individuals with different diets (SMD=1.03 pg/ml, 95%CI: 0.28-1.78, p=0.007) (Experimental) compared to their cFGF21 levels before indicated diet interventions (Control), largely contributed by the significant elevation of cFGF21 in individuals with a hypercaloric carbohydrate-rich diet (15) (p=0.01), hypercaloric fat-rich diet (16–18) (p=0.01), amino acid- or protein-restricted diets (19–23) (p=0.02) and fructose intake (24, 25) (p<0.0001) (Figure 3). While a quick and excessive drink of apple juice significantly increased the cFGF21 level, the overall effect of apple juice consumption on the cFGF21 level was insignificant (26) (p=0.06), due to a nonsignificant increase in cFGF21 after a slow drink of apple juice. Only consuming fish oil significantly downregulated the cFGF21 concentration (27) (p<0.00001) without affecting the overall effects of diets on the cFGF21 concentration (p=0.007). The heterogeneity among these studies was significant (p<0.00001). Publication bias was assessed by a funnel plot, which indicated high publication bias (Supplementary Figure 1).
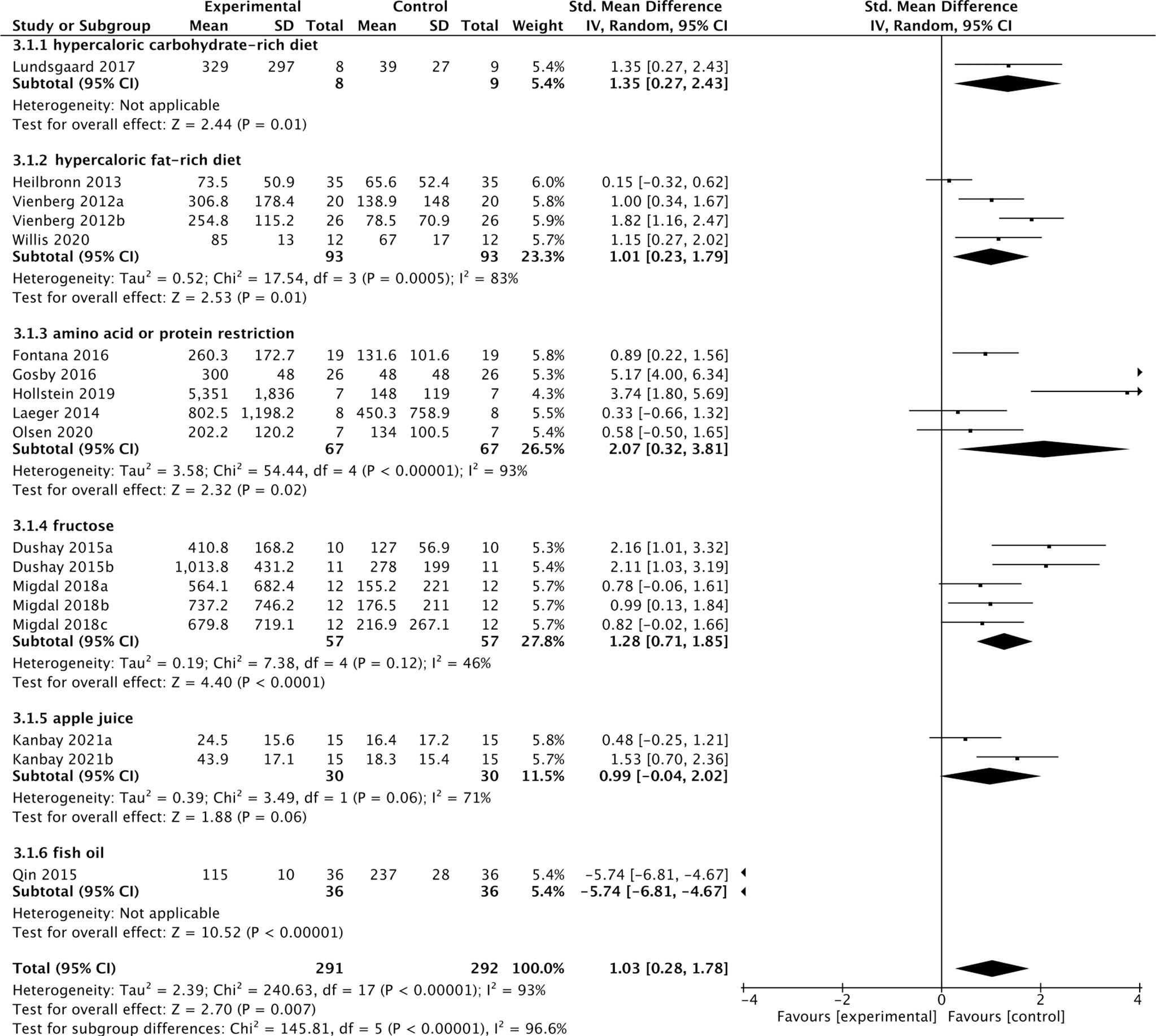
Figure 3 Forest plot of cFGF21 concentration in individuals before and after different diet interventions. Random effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
The association between alcohol consumption and cFGF21 concentration
Four studies containing 104 individuals were involved in the analysis of the association between alcohol consumption and cFGF21 concentration (28–31) (Table 3). The cFGF21 levels were significantly elevated in individuals after both acute alcohol intake (cFGF21 levels measured within 4h post alcohol consumption) (28, 29) (p<0.00001) and subchronic alcohol intake (cFGF21 levels measured in the last 12-72h post-alcohol consumption) (30, 31) (p=0.003) (Experimental) compared to their cFGF21 levels before alcohol consumption (Control), resulting in an overall elevation of cFGF21 by alcohol consumption (SMD=1.22 pg/ml, 95%CI: 0.77-1.67, p<0.00001) (Figure 4). In addition, the alcohol consumption-induced cFGF21 elevation was higher in patients with alcohol liver cirrhosis (ALC) than in those without (NALC) (31). The heterogeneity among these studies was insignificant (p=0.10).
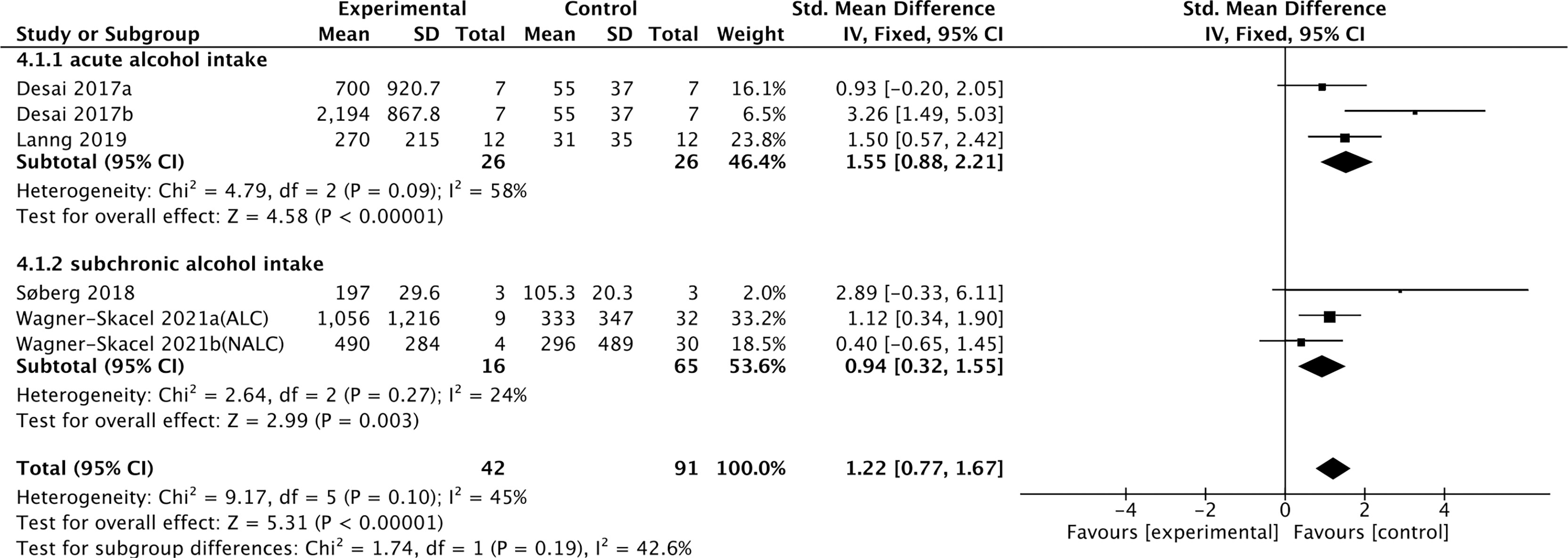
Figure 4 Forest plot of cFGF21 concentration in individuals before and after alcohol consumption. Fixed effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
The association between calorie restriction-induced weight loss and cFGF21 concentration
Eleven studies containing 367 individuals were involved in the analysis of the association between calorie restriction (CR)-induced weight loss (CRIWL) and cFGF21 concentration (32–42) (Table 4). Overall, CRIWL significantly decreased the cFGF21 level (SMD=-0.51 pg/ml, 95%CI: -0.92 to -0.09, p=0.02) (Figure 5). Among them, cFGF21 concentrations were significantly lower in individuals who underwent hypocaloric diets with relatively high protein contents (≥30% diet calorie from protein, ≥30E% protein) than their cFGF21 levels before the diets (32–36) (p=0.003); while CR with <30E% protein (36–40) or CR with unspecified protein content (32, 41, 42) did not significantly affected the cFGF21 level (Figure 5, p=0.30 and p=0.12 respectively). The heterogeneity among these studies was significant (p<0.00001). Publication bias was assessed by a funnel plot, which indicated moderate publication bias (Supplementary Figure 2).
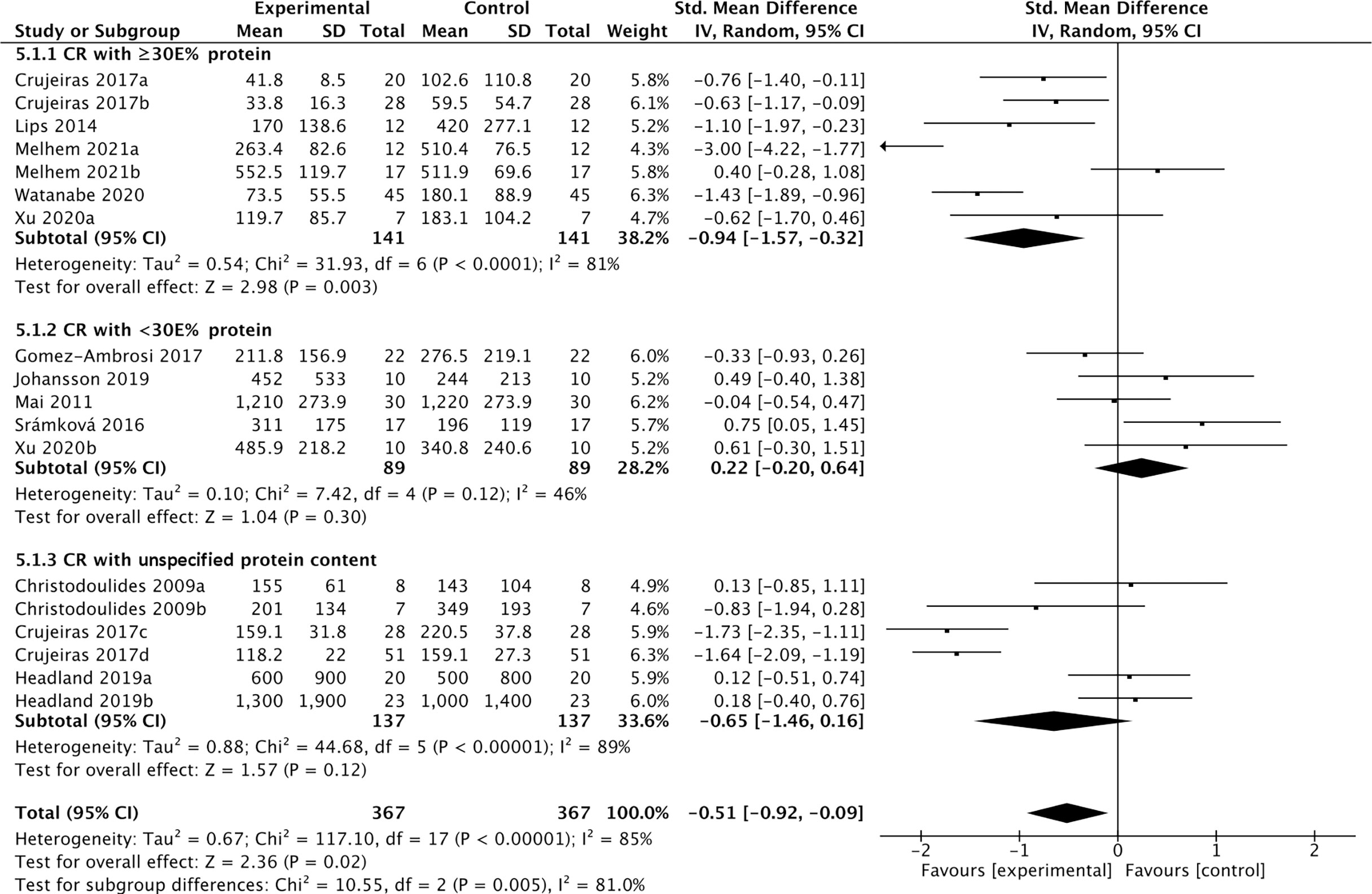
Figure 5 Forest plot of cFGF21 concentration in individuals before and after calorie restriction-induced weight loss. Random effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
The association between acute exercise and cFGF21 concentration
Nineteen studies containing 491 individuals were involved in the analysis of the association between physical activity and the cFGF21 concentration (43–61) (Table 5). Among them, 10 studies containing 239 individuals measured cFGF21 levels before (Control) and within 3 hours postexercise (Experimental) (43–52). They were then used to evaluate the effect of acute exercise on the cFGF21 level (Figure 6). Overall, the cFGF21 concentrations were significantly higher in the blood sampled within 3 hours postexercise than in that sampled preexercise (SMD=0.69 pg/ml, 95%CI: 0.38-1.00, p<0.0001) (Figure 6). The heterogeneity among these studies was significant (p=0.001). Publication bias was assessed by a funnel plot, which indicated moderate publication bias (Supplementary Figure 3).
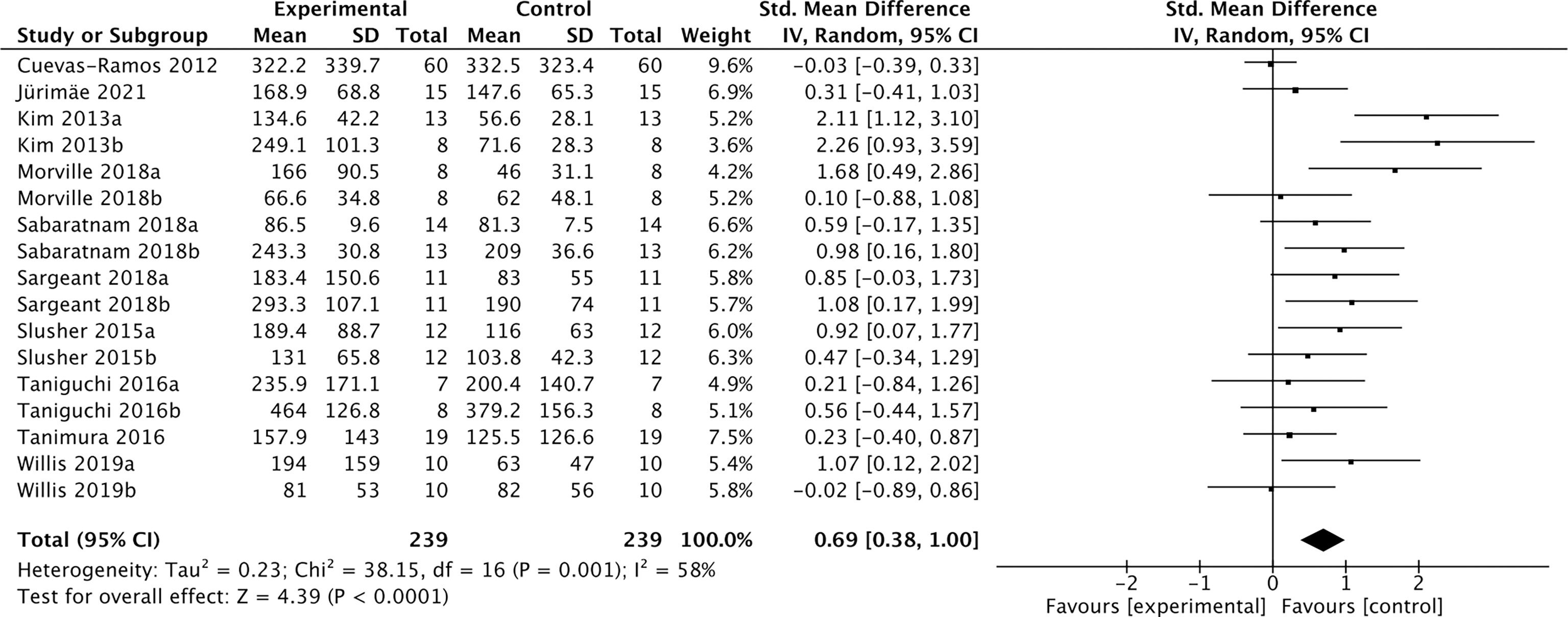
Figure 6 Forest plot of acute exercise effects on cFGF21 concentration sampled within 3 hours postexercise. Random effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
The association between exercise intensity and cFGF21 concentration
Among the previous 10 studies related to the effects of acute exercise on cFGF21 (Figure 6), 2 studies containing 31 individuals were involved in the analysis of the association between exercise intensity and the cFGF21 concentration 1-hour postexercise of different intensities were compared in both studies (45, 52) (Table 5). Overall, the 1-hour postexercise cFGF21 concentration was significantly higher in individuals who underwent relatively higher intensity exercises than in those who underwent relatively lower intensity exercises (SMD=1.21 pg/ml, 95%CI: 0.52-1.90, p=0.0006) (Figure 7). The heterogeneity among these studies was insignificant (p=0.35).
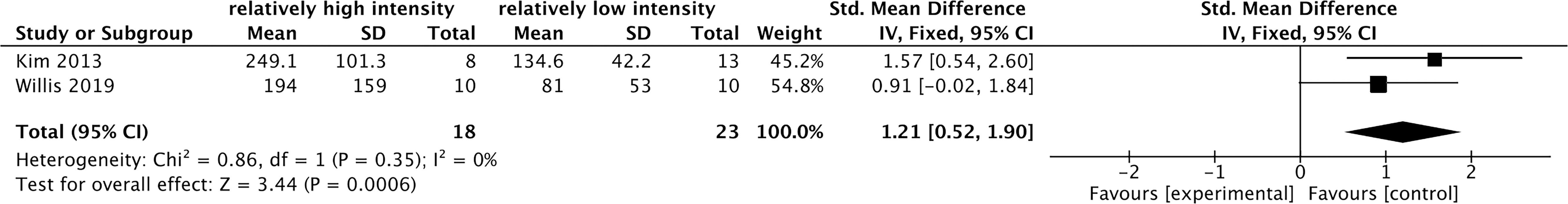
Figure 7 Forest plot of cFGF21 concentration in individuals with different exercise intensities. Fixed effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
Effect of exercise after more than 6 hours of recovery on the cFGF21 level
Among the 19 studies of the effects of exercise (Table 5), 12 studies containing 287 individuals measured cFGF21 levels in the blood sampled beyond 3 hours postexercise (48, 50, 52–61) (Table 5). Among these 12 studies, 4 studies (48, 50, 52, 53) measured cFGF21 concentration of individuals at least 6 hours after one exercise session, while the other 8 studies (54–61) evaluated the effects of chronic training (at least longer than 5 weeks) on the cFGF21 level at least 8 hours after the last exercise session of the whole training program. In both subgroups, the cFGF21 levels tended to decrease after exercise (Experimental) with limited significance compared to those before exercise (Control) (Figure 9, p=0.17 and p=0.07 respectively). Overall, the cFGF21 level was significantly lower in the blood sampled more than 6 hours postexercise (Experimental) than that of preexercise (Control) (SMD=-0.27 pg/ml, 95%CI: -0.49 to -0.05, p=0.01) (Figure 8). The heterogeneity among these studies was significant (p=0.04). Publication bias was assessed by a funnel plot, which indicated moderate publication bias (Supplementary Figure 4).
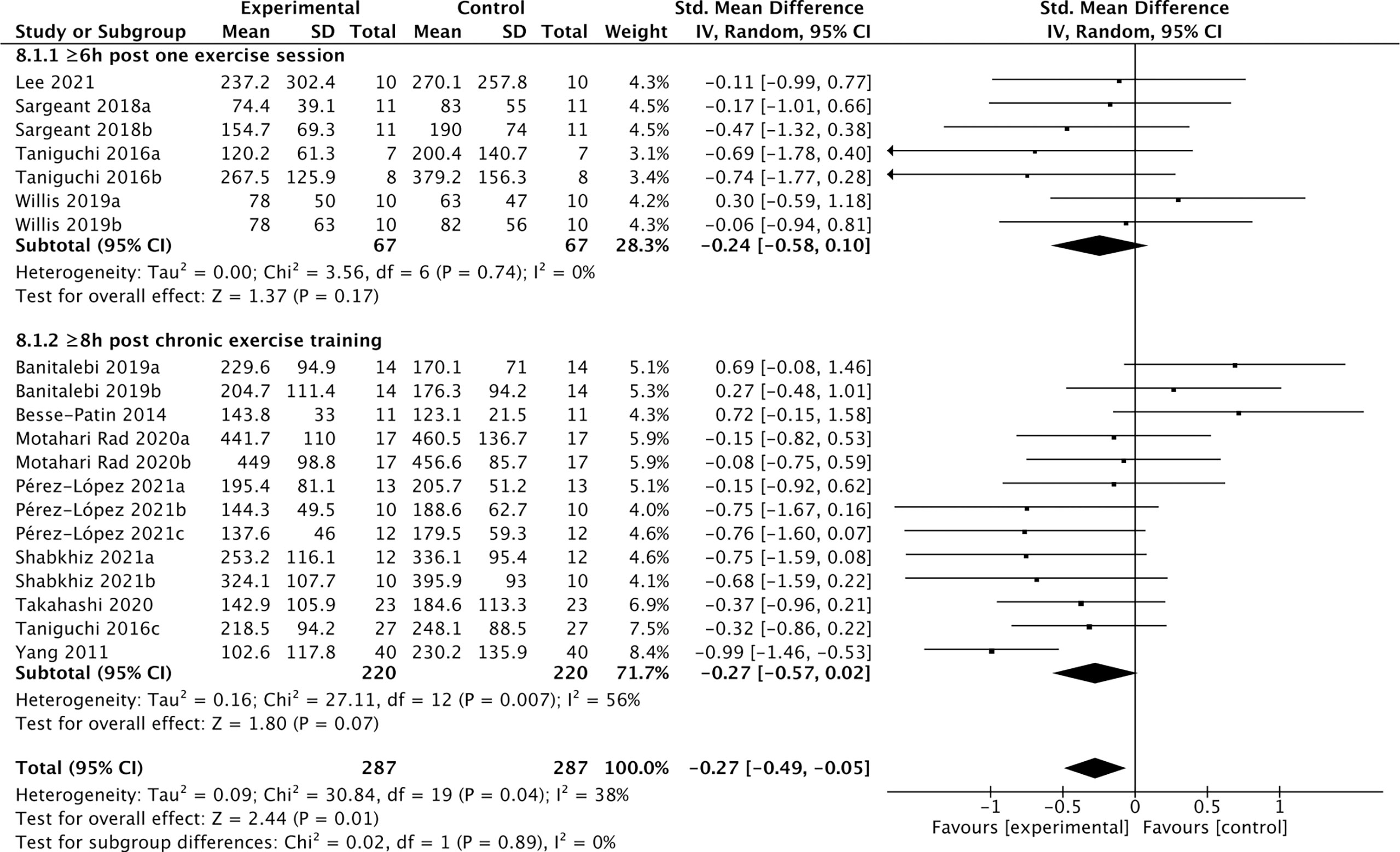
Figure 8 Forest plot of exercise effects on cFGF21 concentration sampled more than 6 hours postexercise. Random effect models were used as the pooling method. The mean value refers to the mean cFGF21 concentration in each group. cFGF21, circulating fibroblast growth factor 21.
Discussion
FGF21 has multiple metabolic regulatory effects, including stimulating hepatic oxidation of fatty acids, decreasing hepatic triglyceride accumulation, increasing insulin sensitivity and suppressing preference for alcohol and sugar intake through the central nervous system (4, 66). Even though the long-acting FGF21 analogs did not decrease plasma glucose levels in humans, they successfully decreased serum lipids, increased serum adiponectin levels, and showed several effects on weight loss (7, 8). Therefore, the elevation of cFGF21 may benefit metabolism regulation and be considered as a useful biomarker to assess the effects of metabolism interactions.
The present systematic literature review and meta-analysis showed significant impacts of different lifestyles on the cFGF21 level. The elevation of the cFGF21 level may be an excellent indicator for lifestyle intervention-induced global metabolic and cardiovascular systems and may be a protective and/or compensatory response to inflammation and stress under stressed conditions.
Smoking and cFGF21
Smoking is the leading preventable cause of mortality and morbidity in modern society. It is well known that smoking is a pivotal risk factor for various diseases, such as cardiovascular disease and metabolic disease. The anti-inflammatory property of FGF21 has been revealed in many studies (67–69). It is known that smoking promotes inflammation by stimulating the release of proinflammatory cytokines such as interleukin-6 (70). A study demonstrated that interleukin-6 concentrations were significantly higher among smokers than never smokers (14). As is also shown in this meta-analysis, smoking significantly upregulates cFGF21 levels, suggesting that it might be a compensatory response to inflammatory stress induced by smoking.
Hypercaloric diet and cFGF21
FGF21, initially identified as a fasting hormone, could be increased by prolonged fasting, hypercaloric fat-rich or carbohydrate-rich diets through different mechanisms (66). Prolonged fasting leads to the release of free fatty acids (FFAs) as an energy source through fatty acid oxidation, while a hypercaloric fat-rich ketogenic diet causes hepatic and plasma triglyceride accumulation. Both ways converge to increase circulating FFAs which is also a signal leading to transcriptional upregulation of FGF21, and in turn promotes FFA consumption as well as the disposal of the excessive fat load (66, 71). Hypercaloric carbohydrate-rich diets could activate the hepatic transcription factor carbohydrate response element binding protein (ChREBP), which binds directly to the FGF21 promoter and enhances the expression of FGF21 (6, 72), which in turn acts directly in the brain to suppress sugar appetite and consumption by targeting hypothalamic networks (73). Our meta-analysis is in line with these previous studies by showing significant increases in cFGF21 levels following acute hypercaloric carbohydrate-rich (15) and hypercaloric fat-rich feeding (16–18) (Figure 3). The limited number of studies included for the meta-analyses of these 2 subgroups could be due to limited studies for ethical reasons. In both cases, elevation of cFGF21 is thought to be a defensive response for maintenance of glucose and lipid homeostasis and regulation of macronutrient preference (6, 66).
Protein or amino acid restriction and cFGF21
FGF21 as an endocrine signal of protein restriction, could be highly upregulated by a low-protein diet and in turn shifts macronutrient preference to increase protein appetite and intake through neural circuits in a β-klotho-dependent manner (6, 22). This was confirmed by this meta-analysis, which showed that both decreased consumption of amino acids (AAs), such as methionine/cysteine (23) or branched-chain amino acids (BCAAs: leucine/isoleucine/valine) (19) and dietary protein restriction (20–22) significantly increased the cFGF21 level of individuals (Figure 3). Classic endoplasmic reticulum stress response pathways (GCN2, PERK, ATF4, etc.) were reported to mediate the restriction-induced cFGF21 elevation, with the involvement of amino acid response elements (AAREs) in the FGF21 promoter (6). Moreover, AA restriction is thought to contribute to lipid and fatty acid metabolism, including lipolysis and fatty acid oxidation, thus elevating cFGF21 (19, 23). It has been reported that the maximal elevation of FGF21 occurs when protein restriction is coupled with high carbohydrate intake in mice (74); however, direct evidence for such a synergistic effect still needs to be further investigated in humans.
Fructose consumption and cFGF21
Excessive and quick fructose consumption acutely stimulates cFGF21 levels (Figure 3) (24, 25) even with a dose as low as 20 g fructose for lean and healthy individuals (25). Natural fruits having a high fructose-to-glucose ratio (≥2:1) include apples, pears, watermelons and mangoes (75, 76). Apple juice, fruit drinks, regular soda and high-fructose-corn-syrup were associated with a 4-5 times higher likelihood of childhood asthma via fructose-induced inflammation and respiratory distress, than never/seldom consumers (p=0.035/p=0.005) (76, 77). Multiple mechanisms are thought to be involved in fructose-triggered FGF21 upregulation. First, free glucose may also stimulate FGF21 expression and secretion in a hepatic ChREBPβ-dependent manner (78). Second, unabsorbed excess-free-fructose may contribute to enteral formation of immunogens, the proinflammatory advanced glycation end products, via reaction with partially digested dietary proteins and enhance cFGF21 levels through increased inflammation (76, 79). Third, the fructose-dietary protein interaction in the gut may also produce a temporary decrease in dietary protein levels (76), which is in turn a strong signal for FGF21 upregulation (6, 22). This could be the reason why an excessive and quick consumption of apple juice (500 mL of apple juice drunk over 5 min) stimulated much higher FGF21 responses than a slow drink of the same amount apple juice (500 mL over an hour by drinking 125 mL every 15 min) (Figure 3, Table 2). Thus, cFGF21 could serve as an exemplary biomarker for the evaluation of excessive fructose intake from foods and/or drinks.
Alcohol consumption and cFGF21
Alcohol consumption is another preventable major cause of morbidity and mortality globally (80). Studies have reported a dose-response relationship between cFGF21 and liver fat content in nonalcoholic fatty liver disease (81). In the liver, chronic alcohol use can cause hepatic fat accumulation and inflammation that are associated with a broad spectrum of liver diseases from simple liver steatosis to liver fibrosis, cirrhosis, and cancer (82), all of which could lead to an increase in cFGF21 levels in patients with liver and metabolic disorders. Moreover, acute alcohol intake can elevate cFGF21, which could in turn cross the blood–brain barrier and suppress further alcohol intake through a specific subpopulation of β-klotho-expressing neurons in the basolateral amygdala in alcohol-preferring nonhuman primates (83). In this meta-analysis, cFGF21 levels were elevated by both acute (within 4 hours) (28, 29) and subchronic (in the last 12-72h) (30, 31) alcohol intake (Figure 4), without significant changes in BMI (30, 31), fasting circulating glucose, triglycerides and liver stiffness (30). Thus, the acute or subchronic alcohol consumption-induced cFGF21 elevations are more likely associated with the feedback of the liver-brain endocrine circuit and act as a liver-derived inhibitor of alcohol preference and intake (83). We also noticed that, elevation of cFGF21 tends to be higher in individuals with alcoholic liver cirrhosis than in those with nonalcoholic liver cirrhosis (Figure 4) (31), suggesting that chronic alcohol consumption may create a more severe “FGF21 resistance” in the liver where more cFGF21 is required to act efficiently as an alcohol preference-suppressing endocrine.
Calorie restriction-induced weight loss and cFGF21
Obesity is associated with elevated levels of cFGF21, and the expected beneficial effects of FGF21 for improving glucose tolerance and reducing plasma glucose and triglyceride levels are attenuated or even lost in obesity, suggesting the existence of an FGF21-resistant state (84). The effects of CRIWL on the cFGF21 level have been conflicting, with many factors involved, such as the number and type of participants, type of dietary intervention, time frame following intervention type and outcome measurement, and amount of weight loss (42). In this meta-analysis, 11 studies containing 373 individuals were involved in the analysis of the association between CRIWL and cFGF21 concentration (Table 4). Overall, CRIWL significantly decreased the cFGF21 level (p=0.02) (Figure 5). Among all the factors that could affect the CRIWL-induced cFGF21 change, we found that the protein content in the diet could be a key factor with a 30% calorie from protein as a cutoff value. Most of the CRIWL-decreased cFGF21 results were obtained with relatively high dietary protein contents (≥30E% protein) (32–36), while controversial results were obtained with relatively low dietary protein contents (<30E% protein) (36–40) or with diets with unspecified protein contents (32, 41, 42).
An elegant review has recently pointed out that CR without malnutrition can reduce disease burden and prolong lifespan in humans and animals, but extreme CR can impair immunity (85). Our analysis is in line with this statement and further indicated that FGF21 could serve as a nutrition marker for monitoring the quality of CR. Numerous studies have shown that obesity and liver fat content are associated with elevated cFGF21 levels (66, 81, 84, 86); thus, a decrease in cFGF21 levels could well reflect the reduction in body fat following CRIWL without malnutrition. On the other hand, protein restriction upregulates the cFGF21 level (6, 22). Thus, a CR with protein shortage could affect the cFGF21 level in two ways: downregulation of cFGF21 via CR-induced fat loss and upregulation of cFGF21 via CR-caused protein shortage.
This could explain the different observations between the study of Crujeiras et al. (32) and that of Šrámková et al. (40) (Figure 5). cFGF21 significantly decreased in obese patients after one month of very low-calorie diet (VLCD, 600–800 kcal/d) with a very high protein content (~58.2E% protein) in Crujeiras et al. (32), while cFGF21 significantly increased in obese women followed 800 kcal/d VLCD with relatively lower protein content (~26.1E% protein) for 28 days in Šrámková et al. (40). The VLCD-caused protein shortage could be the dominant factor that drove this cFGF21 upregulation in Šrámková et al. (40), while the very high protein content could have compensated the VLCD-caused protein shortage, leaving the CR-induced fat loss as the dominant factor that drived this cFGF21 downregulation in Crujeiras et al. (32). We also notice that, with diets of similar or lower protein content, a moderate low calorie restriction (LCD, 1400–1850 kcal/d, 15-30E% protein) could still achieve a significant cFGF21 decrease (32), while the VLCD (800 kcal/d, ~26.1% calorie from protein) could not (40), suggesting that the increased calorie intake [LCD (32) vs. VLCD (40)] at the same time rescued the VLCD-induced protein shortage and attenuated the protein shortage-caused cFGF21 upregulation, resulting an overall decreased cFGF21 level, even with a higher overall calorie intake. Ultimately, the works of Xu et al. (36) showed that with the same level of calorie restriction (LCD, 1500-1600 kcal/d) for 3 weeks, 30E% protein could still significantly decrease cFGF21 in obese patients with lowered liver fat content and inflammation and increased lipid metabolism, whereas the 10E% protein group exhibited a significant increase in cFGF21 with deteriorated lipid metabolism, underlining the importance of protein content in the diet for successful CRIWL.
In addition, among the 10 studies included in this meta-analysis, 2 studies indicated that omega-3 fatty acids were supplemented in their VLCD (800 kcal/d) (32, 35). Fish oil is rich in omega-3 polyunsaturated fatty acids, which have been reported to have myriad health benefits, including reductions in the circulating levels of lipids, glucose and inflammatory cytokines such as TNF-α and nuclear factor-κB (87). All these factors could lead to the marked down-regulation of cFGF21 levels in the patients with nonalcoholic fatty liver disease (NAFLD) associated with hyperlipidemia after consumption of fish oil 4 g per day for 3 months (n=36) (Figure 3) (27). Thus, fish oil supplement could also contribute to the decrease in cFGF21 even after VLCD in these 2 CRIWL studies (32, 35).
Taken together, FGF21 could be an excellent nutrition marker for monitoring the quality of CRIWL, and its downregulation after CRIWL could serve to evaluate the fat loss effect of CRIWL, while an unaffected or upregulated cFGF21 level after CRIWL could warn of CRIWL with protein shortage or malnutrition.
Exercise and cFGF21
Regular exercise is an effective lifestyle intervention for the prevention and treatment of many chronic metabolic diseases, including obesity, diabetes, and NAFLD (88, 89). As cFGF21 changes in response to exercise, FGF21 has been identified as an exercise-mediated hormone (45). However, there are controversial results for the impact of exercises on cFGF21, with some studies revealing that exercise increased cFGF21 levels (43, 44, 46, 47, 49, 51, 52, 54, 55), while other studies reported opposite results (50, 53, 56–61).
Exercise promotes lipolysis of adipose tissue, which is similar to fasting, and the subsequently released FFAs are utilized as an energy source through fatty acid oxidation (90). As the increased circulating FFA is also a signal leading to transcriptional upregulation of FGF21 (43, 60, 66, 71), the beneficial effects of acute exercise in improving the lipid metabolism could be partly contributed by the exercise-caused temporary FFA release, followed by the elevations of the cFGF21 levels and in turn promoting the FFA’s own consumption and fat burning (66, 90). In the case of chronic training, repeatedly enhanced lipid metabolism and fat burning by daily exercise often result in improved lipid metabolic flexibility and decreased body fat (90), and, consequently, an eventual decrease in cFGF21 levels.
With the guidance of the above theoretical predictions, we dissected our meta-analysis of the exercise-cFGF21 association according to the blood-sampling time postexercise. As expected, 10 studies containing 239 individuals showed overall significantly increased cFGF21 levels within 3 hours postexercise compared with preexercise levels (43–52) (p<0.0001) (Figure 6). Five of 10 studies measured cFGF21 concentrations with blood sampled at multiple time points postexercise. Among them, 4 studies showed a cFGF21 peak at 1 h postexercise (46, 49, 50, 52), one peaked at 2h postexercise (48), and all returned to baseline thereafter. This result is in line with the fact that serum FGF21 constantly undergoes proteolytic degradation with a half-life of approximately 0.5-1 h (91). Moreover, a higher intensity of acute exercise resulted in a higher cFGF21 level 1 h postexercise, which is in line with the fact that exercise with a higher intensity requires a higher availability of FFA and higher fatty acid oxidation to match the energy demands, thus resulting in a higher level of FFA-enhanced cFGF21 upregulation compared to exercise with a lower intensity (Figure 7).
On the other hand, 12 studies that measured cFGF21 levels in the blood sampled beyond 3 hours postexercise (48, 50, 52–61) showed an overall reduction in cFGF21 (p=0.01) (Figure 8). After a long postexercise recovery time, the acute exercise-induced temporary lipolysis and FFA release, as well as the FFA-induced cFGF21, can be largely eliminated. Therefore, the cFGF21 level beyond 3 hours postexercise would mainly be associated with the healthy benefits of exercise, including reduced obesity and liver fat content, decreased obesity-induced FGF21 resistance, and improved glucose and lipid metabolism. All these factors could converge to lead to a reduced cFGF21 level (66, 90) (Figure 8).
Taken together, it is important to differentiate the acute effect of exercise from the chronic effect of training (Figure 9). While acute exercise transiently increases the cFGF21 within hours postexercise, mostly with a peak at approximately 1 hour postexercise, the increase induced by a high intensity exercise displays a higher and longer ascending phase followed by a much extended descent to the baseline level compared to that induced by a relatively lower intensity exercise. Thus, the cFGF21 level at 1 h postexercise could serve as an excellent biomarker for evaluating the intensity of acute exercise, as well as the exercise-promoted fat burning. After a longer recovery time, commonly after an overnight fast (>10 hours) to exclude any temporary effects of exercise, the beneficial effects of chronic training on global glucose and lipid homeostasis could be assessed by the lowered blood FGF21 level.
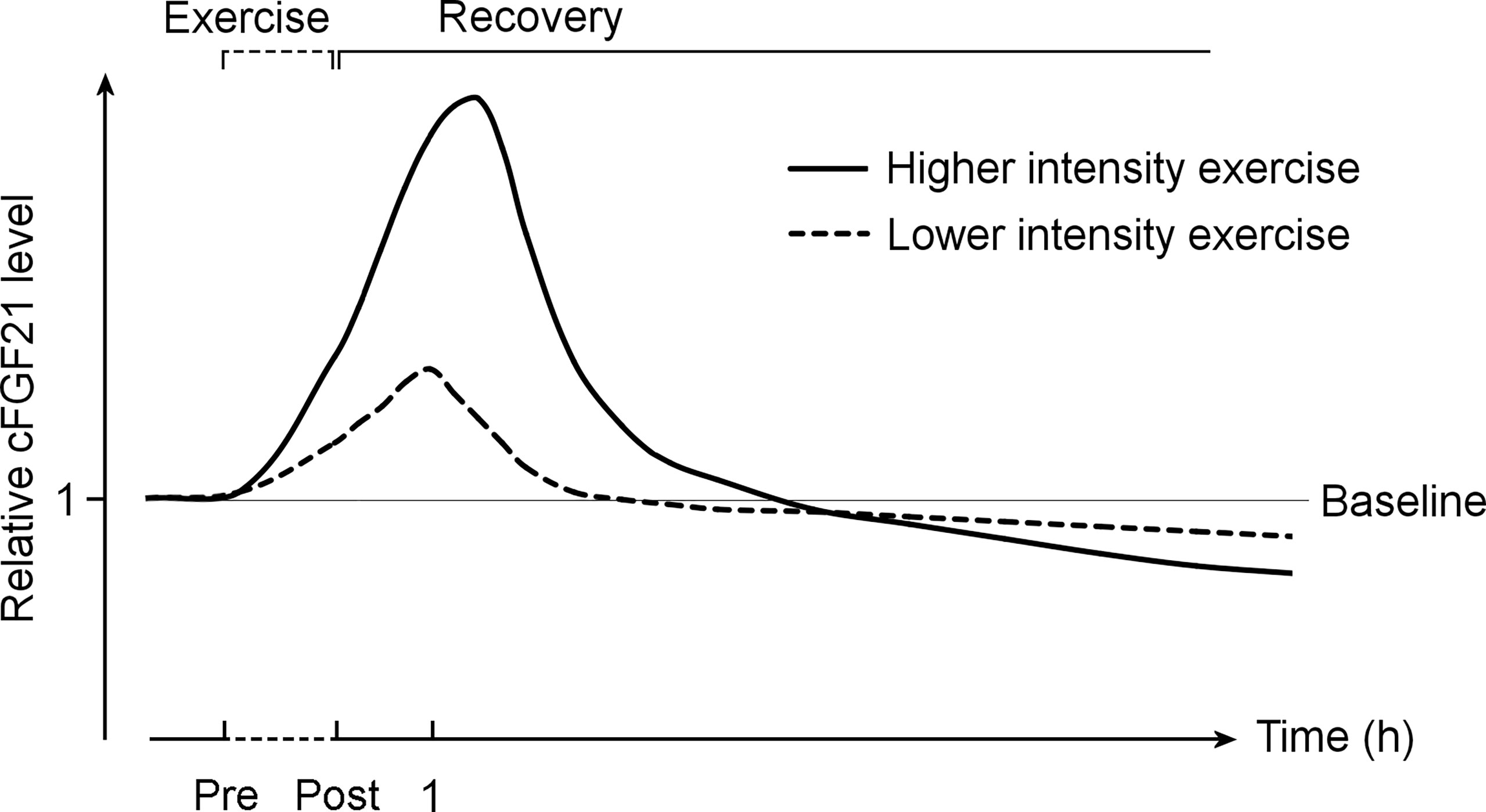
Figure 9 The exercise-induced cFGF21 level changes over time and with different exercise intensities. The figure includes two curves depicting changes in relative cFGF21 levels in subjects undergoing relatively higher- and lower-intensity exercises during the pre-exercise (Pre) and post-exercise (Post) periods. cFGF21, circulating fibroblast growth factor 21.
Overall, future studies focusing on the relationship between exercise intervention and cFGF21 levels should particularly take into consideration the blood-sampling times postexercise. In addition, a standard protocol needs to be established, including exercise types, intensity, duration and timing of blood sampling following exercise, when studying the relationship between FGF21 and exercise.
Several limitations of our meta-analysis must be taken into consideration. First, significant heterogeneity was observed in the studies included in our meta-analysis. The discrepancies between studies may be due to differences in the number and types of participants, as well as specific interventional types and durations, also possibly influenced by the timing of the measurement of circulating FGF21 following intervention. Second, our meta-analysis only focused on smoking, physical activity, diet, alcohol consumption and weight loss. Studies on other lifestyles, such as sleep habits, were not included due to the limited number of relevant studies. Third, some original data reported in articles were transformed for meta-analysis, especially for data of median value with quartile. Those original data with skewed distributions may not be suitable for meta-analysis. Fourth, on account of the limited number of relevant clinical studies and sample sizes of individuals, which may be due to ethical restrictions, the observations of some studies were discussed in the light of known FGF21 biology summarized with cell and animal experiments. Therefore, the fact that the included articles were few in number may have introduced bias, and further studies are needed. Fifth, we only included articles published in English and also did not include unpublished articles, thus the publication bias may exist in this study.
Several modalities were applied to reduce these limitations. First, we conducted a systematic, comprehensive search in four databases. Second, we strictly stipulated the inclusion criteria, eliminating the bias caused by some potential confounding factors, and the data were independently extracted by two reviewers. Third, we conducted subgroup analyses of specific lifestyles.
Conclusion
Serum FGF21 levels are closely associated with lifestyle interventions. They can be elevated by smoking, a hypercaloric carbohydrate-rich diet, a hypercaloric fat-rich diet, amino acid or protein restriction, excessive fructose intake and alcohol consumption, whereas fish oil supplementation and calorie restriction with sufficient protein intake significantly decreased cFGF21. Acute exercise significantly elevated cFGF21 levels within 3 hours postexercise, and stronger exercise intensity resulted in higher cFGF21 upregulation, while cFGF21 levels significantly decreased in the blood sampled 6 hours postexercise. FGF21 could serve as a potential biomarker for assessment of the effects of different lifestyle interventions. When it is used for this purpose, a standard study protocol needs to be established, especially taking into consideration the intervention types and sampling time post-intervention.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
ZQ, YZ and NY designed the study, performed the literature search and data extraction, analyzed the data, and drafted the manuscript; HN,ZY and PL performed the quality assessment of all included studies, advised on interpretation of the data and critically revised the manuscript; XW, YG, YH, JY and LR critically revised the manuscript; LZ and CZ conceived the idea for the review, performed the searches and data extraction, and critically revised the manuscript content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China [grant number 2020YFC2008000] to Cuntai Zhang; the National Natural Science Foundation of China [grant numbers 81873533] to Le Zhang.
Acknowledgments
The authors would like to thank the subjects included in the studies we reviewed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.984828/full#supplementary-material
References
1. Ziglio E, Currie C, Rasmussen VB. The who cross-national study of health behavior in school-aged children from 35 countries: Findings from 2001-2002. J Sch Health (2004) 74(6):204–6. doi: 10.1111/j.1746-1561.2004.tb07933.x
2. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel fgf, fgf-21, preferentially expressed in the liver. Biochim Biophys Acta (2000) 1492(1):203–6. doi: 10.1016/s0167-4781(00)00067-1
3. Staiger H, Keuper M, Berti L, Hrabe de Angelis M, Haring HU. Fibroblast growth factor 21-metabolic role in mice and men. Endocrine Rev (2017) 38(5):468–88. doi: 10.1210/er.2017-00016
4. Kliewer SA, Mangelsdorf DJ. A dozen years of discovery: Insights into the physiology and pharmacology of Fgf21. Cell Metab (2019) 29(2):246–53. doi: 10.1016/j.cmet.2019.01.004
5. Kuro OM. Ageing-related receptors resolved. Nature (2018) 553(7689):409–10. doi: 10.1038/d41586-017-09032-4
6. Hill CM, Qualls-Creekmore E, Berthoud H-R, Soto P, Yu S, McDougal DH, et al. Fgf21 and the physiological regulation of macronutrient preference. Endocrinology (2020) 161(3):bqaa019. doi: 10.1210/endocr/bqaa019
7. Szczepańska E, Gietka-Czernel M. Fgf21: A novel regulator of glucose and lipid metabolism and whole-body energy balance. Hormone Metab Res = Hormon- und Stoffwechselforschung = Hormones metabolisme (2022) 54(4):203–11. doi: 10.1055/a-1778-4159
8. Geng L, Lam KSL, Xu A. The therapeutic potential of Fgf21 in metabolic diseases: From bench to clinic. Nat Rev Endocrinol (2020) 16(11):654–67. doi: 10.1038/s41574-020-0386-0
9. Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, et al. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arteriosclerosis thrombosis Vasc Biol (2013) 33(10):2454–9. doi: 10.1161/ATVBAHA.113.301599
10. Zhang Y, Yan J, Yang N, Qian Z, Nie H, Yang Z, et al. High-level serum fibroblast growth factor 21 concentration is closely associated with an increased risk of cardiovascular diseases: A systematic review and meta-analysis. Front Cardiovasc Med (2021) 8:705273. doi: 10.3389/fcvm.2021.705273
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700
12. Kamizono Y, Shiga Y, Suematsu Y, Imaizumi S, Tsukahara H, Noda K, et al. Impact of cigarette smoking cessation on plasma a-klotho levels. Medicine (2018) 97(35):e11947. doi: 10.1097/md.0000000000011947
13. Nakanishi K, Nishida M, Harada M, Ohama T, Kawada N, Murakami M, et al. Klotho-related molecules upregulated by smoking habit in apparently healthy men: A cross-sectional study. Sci Rep (2015) 5:14230. doi: 10.1038/srep14230
14. Nakanishi K, Nishida M, Yamamoto R, Koseki M, Moriyama T, Yamauchi-Takihara K. An implication of klotho-related molecules in different smoking-related health outcomes between men and women. Clinica chimica acta; Int J Clin Chem (2018) 476:44–8. doi: 10.1016/j.cca.2017.11.007
15. Lundsgaard AM, Fritzen AM, Sjøberg KA, Myrmel LS, Madsen L, Wojtaszewski JFP, et al. Circulating Fgf21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab (2017) 6(1):22–9. doi: 10.1016/j.molmet.2016.11.001
16. Heilbronn LK, Campbell LV, Xu A, Samocha-Bonet D. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS One (2013) 8(10):e78864. doi: 10.1371/journal.pone.0078864
17. Vienberg SG, Brøns C, Nilsson E, Astrup A, Vaag A, Andersen B. Impact of short-term high-fat feeding and insulin-stimulated Fgf21 levels in subjects with low birth weight and controls. Eur J Endocrinol (2012) 167(1):49–57. doi: 10.1530/eje-12-0039
18. Willis SA, Sargeant JA, Yates T, Takamura T, Takayama H, Gupta V, et al. Acute hyperenergetic, high-fat feeding increases circulating Fgf21, Lect2, and fetuin-a in healthy men. J Nutr (2020) 150(5):1076–85. doi: 10.1093/jn/nxz333
19. Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep (2016) 16(2):520–30. doi: 10.1016/j.celrep.2016.05.092
20. Gosby AK, Lau NS, Tam CS, Iglesias MA, Morrison CD, Caterson ID, et al. Raised fgf-21 and triglycerides accompany increased energy intake driven by protein leverage in lean, healthy individuals: A randomised trial. PLoS One (2016) 11(8):e0161003. doi: 10.1371/journal.pone.0161003
21. Hollstein T, Ando T, Basolo A, Krakoff J, Votruba SB, Piaggi P. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: Further evidence for spendthrift and thrifty metabolic phenotypes. Am J Clin Nutr (2019) 110(3):593–604. doi: 10.1093/ajcn/nqz062
22. Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, et al. Fgf21 is an endocrine signal of protein restriction. J Clin Invest (2014) 124(9):3913–22. doi: 10.1172/JCI74915
23. Olsen T, Øvrebø B, Haj-Yasein N, Lee S, Svendsen K, Hjorth M, et al. Effects of dietary methionine and cysteine restriction on plasma biomarkers, serum fibroblast growth factor 21, and adipose tissue gene expression in women with overweight or obesity: A double-blind randomized controlled pilot study. J Trans Med (2020) 18(1):122. doi: 10.1186/s12967-020-02288-x
24. Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E. Fructose ingestion acutely stimulates circulating Fgf21 levels in humans. Mol Metab (2015) 4(1):51–7. doi: 10.1016/j.molmet.2014.09.008
25. Migdal A, Comte S, Rodgers M, Heineman B, Maratos-Flier E, Herman M, et al. Fibroblast growth factor 21 and fructose dynamics in humans. Obes Sci Pract (2018) 4(5):483–9. doi: 10.1002/osp4.295
26. Kanbay M, Guler B, Ertuglu LA, Dagel T, Afsar B, Incir S, et al. The speed of ingestion of a sugary beverage has an effect on the acute metabolic response to fructose. Nutrients (2021) 13(6):1916. doi: 10.3390/nu13061916
27. Qin Y, Zhou Y, Chen SH, Zhao XL, Ran L, Zeng XL, et al. Fish oil supplements lower serum lipids and glucose in correlation with a reduction in plasma fibroblast growth factor 21 and prostaglandin E2 in nonalcoholic fatty liver disease associated with hyperlipidemia: A randomized clinical trial. PLoS One (2015) 10(7):e0133496. doi: 10.1371/journal.pone.0133496
28. Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, et al. Fibroblast growth factor 21 (Fgf21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab (2017) 6(11):1395–406. doi: 10.1016/j.molmet.2017.08.004
29. Lanng AR, Gasbjerg LS, Bergmann NC, Bergmann S, Helsted MM, Gillum MP, et al. Gluco-metabolic effects of oral and intravenous alcohol administration in men. Endocrine connections (2019) 8(10):1372–82. doi: 10.1530/EC-19-0317
30. Søberg S, Andersen ES, Dalsgaard NB, Jarlhelt I, Hansen NL, Hoffmann N, et al. Fgf21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol Metab (2018) 11:96–103. doi: 10.1016/j.molmet.2018.03.010
31. Wagner-Skacel J, Horvath A, Grande P, Wenninger J, Matzer F, Fazekas C, et al. Association of fibroblast growth factor 21 with alcohol consumption and alcohol liver cirrhosis. Neuropsychiatr (2021) 35(3):140–6. doi: 10.1007/s40211-020-00380-8
32. Crujeiras AB, Gomez-Arbelaez D, Zulet MA, Carreira MC, Sajoux I, de Luis D, et al. Plasma Fgf21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: A marker of metabolic stress? Int J Obes (Lond) (2017) 41(10):1570–8. doi: 10.1038/ijo.2017.138
33. Lips MA, de Groot GH, Berends FJ, Wiezer R, van Wagensveld BA, Swank DJ, et al. Calorie restriction and roux-En-Y gastric bypass have opposing effects on circulating Fgf21 in morbidly obese subjects. Clin Endocrinol (2014) 81(6):862–70. doi: 10.1111/cen.12496
34. Melhem S, Steven S, Taylor R, Al-Mrabeh A. Effect of weight loss by low-calorie diet on cardiovascular health in type 2 diabetes: An interventional cohort study. Nutrients (2021) 13(5):1465. doi: 10.3390/nu13051465
35. Watanabe M, Risi R, Camajani E, Contini S, Persichetti A, Tuccinardi D, et al. Baseline homa ir and circulating Fgf21 levels predict nafld improvement in patients undergoing a low carbohydrate dietary intervention for weight loss: A prospective observational pilot study. Nutrients (2020) 12(7):2141. doi: 10.3390/nu12072141
36. Xu C, Markova M, Seebeck N, Loft A, Hornemann S, Gantert T, et al. High-protein diet more effectively reduces hepatic fat than low-protein diet despite lower autophagy and Fgf21 levels. Liver Int (2020) 40(12):2982–97. doi: 10.1111/liv.14596
37. Gómez-Ambrosi J, Gallego-Escuredo JM, Catalán V, Rodríguez A, Domingo P, Moncada R, et al. Fgf19 and Fgf21 serum concentrations in human obesity and type 2 diabetes behave differently after diet- or surgically-induced weight loss. Clin Nutr (Edinburgh Scotland) (2017) 36(3):861–8. doi: 10.1016/j.clnu.2016.04.027
38. Johansson H-E, Edholm D, Kullberg J, Rosqvist F, Rudling M, Straniero S, et al. Energy restriction in obese women suggest linear reduction of hepatic fat content and time-dependent metabolic improvements. Nutr Diabetes (2019) 9(1):34. doi: 10.1038/s41387-019-0100-2
39. Mai K, Schwarz F, Bobbert T, Andres J, Assmann A, Pfeiffer AFH, et al. Relation between fibroblast growth factor-21, adiposity, metabolism, and weight reduction. Metabolism: Clin Exp (2011) 60(2):306–11. doi: 10.1016/j.metabol.2010.02.016
40. Šrámková V, Rossmeislová L, Krauzová E, Kračmerová J, Koc M, Langin D, et al. Comparison of early (2 days) and later (28 days) response of adipose tissue to very low-calorie diet in obese women. J Clin Endocrinol Metab (2016) 101(12):5021–9. doi: 10.1210/jc.2016-2161
41. Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab (2009) 94(9):3594–601. doi: 10.1210/jc.2009-0111
42. Headland ML, Clifton PM, Keogh JB. Effects of weight loss on fgf-21 in human subjects: An exploratory study. Int J Environ Res Public Health (2019) 16(23):4877. doi: 10.3390/ijerph16234877
43. Cuevas-Ramos D, Almeda-Valdés P, Meza-Arana CE, Brito-Córdova G, Gómez-Pérez FJ, Mehta R, et al. Exercise increases serum fibroblast growth factor 21 (Fgf21) levels. PLoS One (2012) 7(5):e38022. doi: 10.1371/journal.pone.0038022
44. Jürimäe J, Vaiksaar S, Purge P, Tillmann V. Irisin, fibroplast growth factor-21, and follistatin responses to endurance rowing training session in female rowers. Front Physiol (2021) 12:689696. doi: 10.3389/fphys.2021.689696
45. Kim KH, Kim SH, Min YK, Yang HM, Lee JB, Lee MS. Acute exercise induces Fgf21 expression in mice and in healthy humans. PLoS One (2013) 8(5):e63517. doi: 10.1371/journal.pone.0063517
46. Morville T, Sahl RE, Trammell SA, Svenningsen JS, Gillum MP, Helge JW, et al. Divergent effects of resistance and endurance exercise on plasma bile acids, Fgf19, and Fgf21 in humans. JCI Insight (2018) 3(15):e122737. doi: 10.1172/jci.insight.122737
47. Sabaratnam R, Pedersen AJT, Kristensen JM, Handberg A, Wojtaszewski JFP, Højlund K. Intact regulation of muscle expression and circulating levels of myokines in response to exercise in patients with type 2 diabetes. Physiol Rep (2018) 6(12):e13723. doi: 10.14814/phy2.13723
48. Sargeant JA, Aithal GP, Takamura T, Misu H, Takayama H, Douglas JA, et al. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and Overweight/Obese men. Appl Physiol Nutr Metab (2018) 43(5):482–90. doi: 10.1139/apnm-2017-0639
49. Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj M, Huang CJ. Attenuated fibroblast growth factor 21 response to acute aerobic exercise in obese individuals. Nutrition metabolism Cardiovasc Dis NMCD (2015) 25(9):839–45. doi: 10.1016/j.numecd.2015.06.002
50. Taniguchi H, Tanisawa K, Sun X, Higuchi M. Acute endurance exercise lowers serum fibroblast growth factor 21 levels in Japanese men. Clin Endocrinol (2016) 85(6):861–7. doi: 10.1111/cen.13162
51. Tanimura Y, Aoi W, Takanami Y, Kawai Y, Mizushima K, Naito Y, et al. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Physiol Rep (2016) 4(12):e12828. doi: 10.14814/phy2.12828
52. Willis SA, Sargeant JA, Thackray AE, Yates T, Stensel DJ, Aithal GP, et al. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Appl Physiol Nutr Metab (2019) 44(10):1065–72. doi: 10.1139/apnm-2018-0818
53. Lee JS, Yoon ES, Jung SY, Yim KT, Kim DY. Effect of high-intensity circuit training on obesity indices, physical fitness, and browning factors in inactive female college students. J Exerc Rehabil (2021) 17(3):207–13. doi: 10.12965/jer.2142260.130
54. Banitalebi E, Kazemi A, Faramarzi M, Nasiri S, Haghighi MM. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci (2019) 217:101–9. doi: 10.1016/j.lfs.2018.11.062
55. Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int J Obes (Lond) (2014) 38(5):707–13. doi: 10.1038/ijo.2013.158
56. Motahari Rad M, Bijeh N, Attarzadeh Hosseini SR, Raouf Saeb A. The effect of two concurrent exercise modalities on serum concentrations of Fgf21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch Physiol Biochem (2020) 12:1–10. doi: 10.1080/13813455.2020.1829649
57. Pérez-López A, Gonzalo-Encabo P, Pérez-Köhler B, García-Honduvilla N, Valadés D. Circulating myokines il-6, il-15 and Fgf21 response to training is altered by exercise type but not by menopause in women with obesity. Eur J Sport Sci (2021) 5:1–10. doi: 10.1080/17461391.2021.1939430
58. Shabkhiz F, Khalafi M, Rosenkranz S, Karimi P, Moghadami K. Resistance training attenuates circulating fgf-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: A randomised controlled clinical trial. Eur J Sport Sci (2021) 21(4):636–45. doi: 10.1080/17461391.2020.1762755
59. Takahashi A, Abe K, Fujita M, Hayashi M, Okai K, Ohira H. Simple resistance exercise decreases cytokeratin 18 and fibroblast growth factor 21 levels in patients with nonalcoholic fatty liver disease: A retrospective clinical study. Medicine (2020) 99(22):e20399. doi: 10.1097/md.0000000000020399
60. Taniguchi H, Tanisawa K, Sun X, Kubo T, Higuchi M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J Clin Endocrinol Metab (2016) 101(1):191–8. doi: 10.1210/jc.2015-3308
61. Yang SJ, Hong HC, Choi HY, Yoo HJ, Cho GJ, Hwang TG, et al. Effects of a three-month combined exercise programme on fibroblast growth factor 21 and fetuin-a levels and arterial stiffness in obese women. Clin Endocrinol (2011) 75(4):464–9. doi: 10.1111/j.1365-2265.2011.04078.x
62. Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess (2003) 7(27):iii–x, 1-173. doi: 10.3310/hta7270
63. Luctkar-Flude M, Groll D. A systematic review of the safety and effect of neurofeedback on fatigue and cognition. Integr Cancer Ther (2015) 14(4):318–40. doi: 10.1177/1534735415572886
64. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res (2020) 30:962280219889080. doi: 10.1177/0962280219889080
65. Cashin AG, McAuley JH. Clinimetrics: Physiotherapy evidence database (Pedro) scale. J Physiother (2020) 66(1):59. doi: 10.1016/j.jphys.2019.08.005
66. Fisher FM, Maratos-Flier E. Understanding the physiology of Fgf21. Annu Rev Physiol (2016) 78:223–41. doi: 10.1146/annurev-physiol-021115-105339
67. Li X, Zhu Z, Zhou T, Cao X, Lu T, Liang Y, et al. Early increases in serum Fgf21 levels predict mortality of septic patients. Cytokine (2018) 111:428–33. doi: 10.1016/j.cyto.2018.05.020
68. Gariani K, Drifte G, Dunn-Siegrist I, Pugin J, Jornayvaz FR. Increased Fgf21 plasma levels in humans with sepsis and sirs. Endocrine connections (2013) 2(3):146–53. doi: 10.1530/ec-13-0040
69. Li X, Zhu Z, Zhou T, Cao X, Lu T, He J, et al. Predictive value of combined serum Fgf21 and free T3 for survival in septic patients. Clinica chimica acta; Int J Clin Chem (2019) 494:31–7. doi: 10.1016/j.cca.2019.03.005
70. Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, et al. Cigarette smoke induces proinflammatory cytokine release by activation of nf-kappab and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol (2006) 291(1):L46–57. doi: 10.1152/ajplung.00241.2005
71. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by pparalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab (2007) 5(6):426–37. doi: 10.1016/j.cmet.2007.05.002.
72. Iizuka K, Takeda J, Horikawa Y. Glucose induces Fgf21 mrna expression through chrebp activation in rat hepatocytes. FEBS Lett (2009) 583(17):2882–6. doi: 10.1016/j.febslet.2009.07.053
73. von Holstein-Rathlou S, Gillum MP. Fibroblast growth factor 21: An endocrine inhibitor of sugar and alcohol appetite. J Physiol (2019) 597(14):3539–48. doi: 10.1113/JP277117
74. Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, et al. Defining the nutritional and metabolic context of Fgf21 using the geometric framework. Cell Metab (2016) 24(4):555–65. doi: 10.1016/j.cmet.2016.09.001
75. Dennison BA. Fruit juice consumption by infants and children: A review. J Am Coll Nutr (1996) 15(5 Suppl):4S–11S. doi: 10.1080/07315724.1996.10720475
76. DeChristopher LR, Tucker KL. Excess free fructose, apple juice, high fructose corn syrup and childhood asthma risk - the national children's study. Nutr J (2020) 19(1):60. doi: 10.1186/s12937-020-00578-0
77. DeChristopher LR, Uribarri J, Tucker KL. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in us children aged 2-9 years. Public Health Nutr (2016) 19(1):123–30. doi: 10.1017/S1368980015000865
78. Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, et al. A critical role for chrebp-mediated Fgf21 secretion in hepatic fructose metabolism. Mol Metab (2017) 6(1):14–21. doi: 10.1016/j.molmet.2016.11.008
79. DeChristopher LR, Tucker KL. Excess free fructose, high-fructose corn syrup and adult asthma: The framingham offspring cohort. Br J Nutr (2018) 119(10):1157–67. doi: 10.1017/S0007114518000417
80. Schuckit MA. Alcohol-use disorders. Lancet (2009) 373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X
81. Xiao F, Shi X, Huang P, Zeng X, Wang L, Zeng J, et al. Dose-response relationship between serum fibroblast growth factor 21 and liver fat content in non-alcoholic fatty liver disease. Diabetes Metab (2021) 47(6):101221. doi: 10.1016/j.diabet.2020.101221
82. Fuster D, Samet JH. Alcohol use in patients with chronic liver disease. New Engl J Med (2018) 379(13):1251–61. doi: 10.1056/NEJMra1715733
83. Flippo KH, Trammell SAJ, Gillum MP, Aklan I, Perez MB, Yavuz Y, et al. Fgf21 suppresses alcohol consumption through an amygdalo-striatal circuit. Cell Metab (2022) 34(2):317–28.e6. doi: 10.1016/j.cmet.2021.12.024
84. Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, et al. Obesity is a fibroblast growth factor 21 (Fgf21)-resistant state. Diabetes (2010) 59(11):2781–9. doi: 10.2337/db10-0193
85. O'Leary K. Health benefits of calorie restriction. Nat Med (2022). doi: 10.1038/d41591-022-00036-w
86. Jimenez V, Jambrina C, Casana E, Sacristan V, Muñoz S, Darriba S, et al. Fgf21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol Med (2018) 10(8):e8791. doi: 10.15252/emmm.201708791
87. Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol (2018) 9:345–81. doi: 10.1146/annurev-food-111317-095850
88. Myers J, Kokkinos P, Nyelin E. Physical activity, cardiorespiratory fitness, and the metabolic syndrome. Nutrients (2019) 11(7):1652. doi: 10.3390/nu11071652
89. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of nafld with diet, physical activity and exercise. J Hepatol (2017) 67(4):829–46. doi: 10.1016/j.jhep.2017.05.016
90. Fritzen AM, Lundsgaard A-M, Kiens B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat Rev Endocrinol (2020) 16(12):683–96. doi: 10.1038/s41574-020-0405-1
Keywords: circulating FGF21, lifestyle, biomarker, diet, exercise
Citation: Qian Z, Zhang Y, Yang N, Nie H, Yang Z, Luo P, Wei X, Guan Y, Huang Y, Yan J, Ruan L, Zhang C and Zhang L (2022) Close association between lifestyle and circulating FGF21 levels: A systematic review and meta-analysis. Front. Endocrinol. 13:984828. doi: 10.3389/fendo.2022.984828
Received: 02 July 2022; Accepted: 03 August 2022;
Published: 25 August 2022.
Edited by:
Roger Gutiérrez-Juárez, National Autonomous University of Mexico, MexicoReviewed by:
Endalamaw Tesfa, Bahir Dar University, EthiopiaTamer Coskun, Lilly Research Laboratories, United States
Copyright © 2022 Qian, Zhang, Yang, Nie, Yang, Luo, Wei, Guan, Huang, Yan, Ruan, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Zhang, bGVfemhhbmdAZm94bWFpbC5jb20=; Cuntai Zhang, Y3R6aGFuZzA0MjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zonghao Qian
Zonghao Qian Yucong Zhang
Yucong Zhang Ni Yang1,2†
Ni Yang1,2† Hao Nie
Hao Nie Zhen Yang
Zhen Yang Pengcheng Luo
Pengcheng Luo Yuqi Guan
Yuqi Guan Cuntai Zhang
Cuntai Zhang Le Zhang
Le Zhang