
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 25 August 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.984561
Objectives: The relationship between renal function and diabetic retinopathy has been controversial. This study is to investigate the influence of renal function on the complex and surgical outcomes of proliferative diabetic retinopathy (PDR).
Methods: This was a post hoc analysis of the CONCEPT clinical trial. A total of 45 eyes with PDR underwent vitrectomy were included. Based on the estimated glomerular filtration rate (eGFR), they were divided into abnormal renal function group (ARF group) and normal renal function group (NRG group). Baseline PDR complex, intraoperative outcomes (Intraoperative bleeding, frequency of endodiathermy, surgical time, iatrogenic hole, and tamponade) and postoperative outcomes (logMAR best-corrected visual acuity, vitreous re-hemorrhage, and macular edema, follow up at postoperative 1 month and 3 months) were estimated. Vitreous, aqueous humor and serum were collected at the vitrectomy day and Vascular endothelia growth factor-A levels were quantified for all included patients using liquid chip method.
Results: There was no significant difference in baseline PDR complex, intraoperative and postoperative outcomes between ARF group and NRG group (all P > 0.05). At the vitrectomy day, there was also no difference of Vascular endothelia growth factor-A levels in vitreous, aqueous humor and serum between the two groups (all P > 0.05).
Conclusion: Our results showed that the renal function seems not parallel to the severity of PDR, neither to the surgical outcomes. This might be interpreted by the similar Vascular endothelia growth factor-A levels in vitreous, aqueous humor and serum between the two groups.
Globally, more than 415 million people suffer from diabetes mellitus and its complications such as diabetic retinopathy (DR) and diabetic nephropathy (DN). These microvascular complications put a huge burden on health-care systems and decrease patients’ life quality (1, 2). DR is known to be the leading cause of vision loss and even blindness in the working-age population. it has been estimated that the number of people with DR will increase from 126.6 million in 2010 to 191.0 million in 2030, and that 30% of affected individuals require close monitoring or treatment (3). According to American Diabetes Association for type 1 diabetes, the fundus examination should be performed within 5 years after the diagnosis, while for type 2 diabetes, it should be performed at diagnosis. If there is no evidence of diabetic retinopathy for one or more annual eye examination and glycaemia is well controlled, screening can be performed every 1 - 2 years (4). Covid 19 pandemic has made it hard to perform screening programmer, though the role of telemedicine has been further enhanced in particular for retinopathy screening, also using new tools (5, 6). DR begins with asymptomatic retinal abnormalities such as microaneurysms and progresses to proliferative diabetic retinopathy (PDR), the advanced stage which is characterized by neovascularization and preretinal or vitreous hemorrhages (VH) (7). Patients with persistent vitreous hemorrhage (VH), extensive fibrovascular proliferations, or tractional retinal detachment (TRD) are generally indications for pars plana vitrectomy (PPV). Careful evaluation of both the systemic and ocular conditions is of importance for the prognosis of PDR patients with necessary of PPV surgery.
DN, with persistent albuminuria, hypertension, and progressive renal failure (8), is associated with increased morbidity and mortality in diabetic patients (9). The incidence and prevalence of DN have also increased dramatically over the past decade (10). The association between DR and diabetic nephropathy, though has been previously investigated, the relationship between the two diabetic complications has been controversial (11). However, few studies have focused on the influence of renal function on the severity of PDR and the surgical outcomes.
This study aimed to explore the relationship between renal dysfunction and the surgical outcomes of PDR. Besides, we also measured the levels of VEGF-A, the key factor of proangiogenic factor in PDR, in order to interpret our main findings.
This was a post hoc analysis of the CONCEPT clinical trial, which was registered at https://clinicaltrials.gov/(ID NCT03506750). The study adhered to the tenets of the Declaration of Helsinki, and was approved by Ethic Committee of First Affiliated Hospital of Nanjing Medical University (2017-SR-283). Informed written consent was obtained from all patients prior to enrollment.
In this retrospective study, 45 consecutive eyes of 45 type 2 diabetic patients with PDR between June 2017 and January 2018 were included. Diabetes were diagnosed based on plasma glucose criteria, either the fasting plasma glucose (FPG) value or the 2-h plasma glucose (2-h PG) value during a 75-g oral glucose tolerance test (OGTT), or A1C criteria (12). Criteria for the diagnosis of diabetes included FPG ≥ 126 mg/dL (7.0 mmol/L) or 2-h PG ≥ 200 mg/dL (11.1 mmol/L) during OGTT or A1C ≥ 6.5% (48 mmol/mol) or in a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random plasma glucose ≥ 200 mg/dL (11.1 mmol/L) (13). The main inclusion criteria were patients (1) with PDR requiring surgical intervention such as unabsorbed vitreous hemorrhage, tractional retinal detachment; (2) with sufficient intro- and post-operative clinical data for analysis; and (3) with measurement of VEGF-A in serum, aqueous humor, and vitreous. The main exclusion criteria were patients (1) with other neovascular diseases such as retinal vein occlusion, iris neovascularization, or other diseases such as glaucoma, age-related macular degeneration, pathological myopia; (2) with previous intraocular surgery history; (3) receiving preoperative anti-inflammation therapy.
Data collected at baseline included age, gender, duration of diabetes mellitus (DM), PRP history best-corrected visual acuity (BCVA), hemoglobin A1C, and estimated glomerular filtration rate (eGFR). The PDR patients were allocated into two groups, abnormal renal function group and normal renal function group, based on the estimated glomerular filtration rate (eGFR), with the value of 90 mL/min/1.73 m2 as cutoff.
Outcome measures were the PDR complexity, intraoperative and postoperative complications, and the VEGF levels in aqueous humor, vitreous, and serum at vitrectomy day. PDR complexity were defined as previously reported (14). Intraoperatively, we recorded surgical time, bleeding and endodiathermy frequency, iatrogenic hole, and type of tamponade used. Postoperatively, we recorded BCVA, BCVA at 1 week, 1 months and 3 months, early VH or macular edema (7 days after surgery), and late or macular edema VH (occurred later than 7 days after surgery).
SD-OCT Cirrus (Carl Zeiss, Meditec, Germany) was used to determine the presence or absence of ME, which manifests as diffuse retinal thickening, cystoid macular oedema and serous retinal detachment.
Visual acuities were recorded using a logMAR scale, and visual acuity levels of counting fingers (CF), hand motion (HM), light perception (LP), and no light perception (NLP) were given the following logMAR scores 1.6 logMAR, 2.0 logMAR, 2.5 logMAR, and 3.0 logMAR respectively. Poor vision was defined as a BCVA ≥ 1.6 logMAR (CF) (15).
For all included patients, 0.2 mL undiluted aqueous humor (AQH) was obtained at the start of vitrectomy. Approximately 0.5 mL undiluted vitreous fluid samples were collected with a vitreous cutter at the start of vitrectomy before intraocular infusion. For all patients, serum samples were obtained at the vitrectomy day. Samples were collected into sterile tubes, immediately placed on ice, centrifuged at 1500 rpm for 5 minutes to remove the cells and debris, and then stored at - 80°C until analyzed.
The commercial kits used were Cytometric Bead Array 1 (Bio-Rad) for VEGF-A. The Cytometric Bead Array experiment was contract service offered by Wayen Biotechnologies, Inc (Shanghai, China) with Bio-Plex MAGPIX System (Bio-Rad) in accordance with the manufacturer’s instructions.
Statistical analyses of the data were carried out using SPSS software version 19.0 (SPSS, Inc., Chicago, IL, USA). Values were expressed as means ± standard deviations. Independent t test was used to determine baseline differences in age, duration of PDR and DM, PDR grade, PPV time, and pre-, postoperative BCVA between two groups. Chi-square test was used to determine baseline differences in gender, tamponade, and central macular edema (CME) rate between two groups. Fisher’s exact test was used to determine baseline differences in VH grade, Coagulation frequency and PDR complex score between two groups. The significance of the differences of VEGF-A levels in vitreous, aqueous humor and serum between renal dysfunction group and normal renal function group at the vitrectomy day was calculated by independent t test. P<0.05 was considered statistically significant.
Forty-five eyes from 45 PDR patients were included in the study. As shown in Table 1, there was no difference between the two groups on mean age (p = 0.131), gender (p = 0.408), PDR duration (p = 0.949), DM duration (p = 0.598), Diabetes therapy (Insulin: p = 0.577; Oral hypoglycemic agent: p = 0.620), PDR complex score (p = 0.908) and intravitreal anti-VEGF rate (p = 0.547).
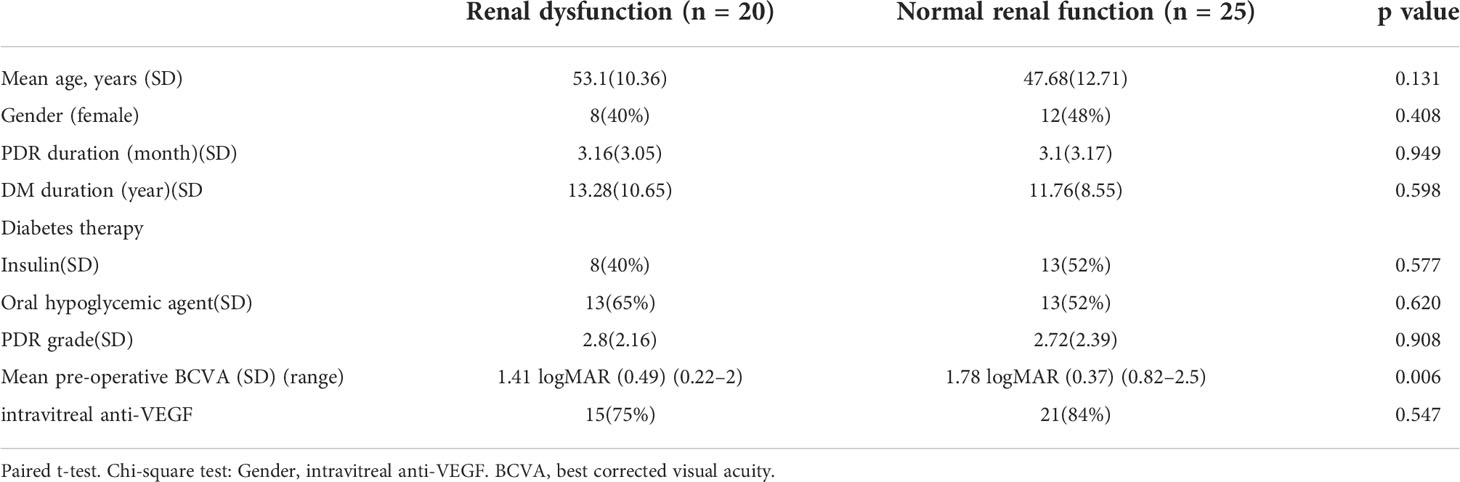
Table 1 Baseline characteristics according to renal function category (Renal dysfunction eGFR < 90 mL/min per 1.73 m2, normal eGFR > 90 mL/min per 1.73 m2).
All 45 eyes underwent vitrectomy with segmentations and/or delamination. As shown in Table 2, there was no difference between the NRF group and ARF group on intraoperative bleeding mild (p = 1.0), intraoperative bleeding severe (p = 0.767), Frequency of endodiathermy (p = 0.557), PPV time (p = 0.516), iatrogenic hole (p = 0.109) and the tamponade used. For NRF group, the preferred tamponade used was silicone oil in 60% (15 eyes) followed by air 40% (10 eyes). For ARF group, the preferred tamponade used was silicone oil in 65% (13 eyes) followed by air 35% (7 eyes) (p = 0.767).
As shown in Table 3, there was no difference between the NRF group and ARF group on mean BCVA at 1 postoperative week (p = 0.615), 1 month (p = 0.404) and 3 months (p = 0.115).
Visual improvement by at least 0.3 logMAR units occurred in 33 eyes (73.3%), 4 (8.9%) improved by less than 0.3 logMAR units, and 4 (8.9%) worsened by at least 0.3 logMAR units. 20% of eyes (6.7% were in the ARF group and 13.3% were in the NRF group) were recorded with BCVA of 6/12 (0.3 logMAR) or better at 3 months.
VH occurred in 15.6% (n = 7) of all included patients. 11.1% (n=5) were with early and 4.4% were with late hemorrhage (n=2). The presence of early or late VH postoperatively was not different between the ARF group and NRG group (Table 4).
CME occurred overall in 42.2% (n = 19) at postoperative 1 month and 60% (n = 27) at postoperative 3 month. The presence of neither early nor late CME was associated with the renal function category (Table 4).
Intravitreal anti-VEGF was used in 36 eyes (80%). For the 36 eyes, there was no statistically significant difference of VEGF-A level in vitreous, aqueous humor and serum between renal dysfunction group and normal renal function group at the vitrectomy day (Table 5).

Table 5 VEGF-A levels in vitreous, aqueous humor and serum between renal dysfunction group and normal renal function group at the vitrectomy day.
The present study shows that there was no significant difference in baseline PDR complexity, intraoperative and postoperative outcomes between abnormal renal function and normal renal function groups (all P > 0.05). The presence of renal dysfunction seems not affect surgical complexity and the prognosis of the PDR patients undergoing vitrectomy for PDR, which can be explained by the similar expression level of VEGF-A in patients’ vitreous, aqueous humor, and serum.
Diabetic retinopathy (DR) is a major global health burden due to its high prevalence and associated high risk of vision impairment (16). Chronic kidney disease (CKD), also known as one of the most common complication of diabetes, shows certain similar pathogenesis with DR. Chronic exposure to hyperglycaemia affects the microvasculature, eventually leading to diabetic nephropathy and retinopathy (17). Both the two types of diabetic microangiopathy involve thickening of basement membrane and muscular layers and increased leakage. Furthermore, a recent study has suggested common inherited susceptibilities to retinopathy and CKD in diabetic patients (18).
The association between CKD and DR has been extensively investigated in previous studies (19). A number of studies provide evidence that DR may be independently associated with the development of micro albuminuria and, hence, be a powerful predictor for the progression of renal damage in DM patients (20). Albumin excretion rate (AER) was significantly higher among subjects with either PDR or severe NPDR as compared with mild NPDR (21). But most were about DR at early stage or without staged. Other investigations show that the progression of DR and DN for patients with T2D was discordant. The Renal Insufficiency and Cardiovascular Events study found that the progression of DN did not affect 41.4% of T2D patients with advanced DR (22). However, few studies have investigated the influence of renal function on the severity of PDR and the surgical outcomes.
Postoperative VH and CME are common complications after vitrectomy for PDR (23, 24). Gupta et al. found, in a similar cohort of patients, a prevalence of VH of 31.9% in patients with TRD (25). In our series, 15.6% of patients developed postoperative VH. CME occurred overall in 42.2% (n = 19) at postoperative 1 month and 60% (n = 27) at postoperative 3 month. Early or late VH and CME were not significantly different among the 2 renal categories. Our visual results also show that there was no significant difference in mean BCVA postoperative 3 months between renal dysfunction group and normal renal function group. All above can be explained by the similar expression level of VEGF-A in patients’ vitreous, aqueous humor, and serum.
Chronic kidney disease has been implicated in the production of vascular endothelial growth factors (VEGF) that are normally detected in podocytes in glomeruli and tubular epithelial cells (26, 27). A marked expression of VEGF secondary to glomerular injury has been observed in experimental diabetic rats with early nephropathy (28). Local production of VEGF may be further released into the systemic circulation. Our results showed that the renal function seems not parallel to the severity of PDR, neither to the surgical outcomes. This might be interpreted by the similar VEGF-A levels in vitreous, aqueous humor and serum between the two groups at the vitrectomy day. Previous study has shown that the humoral VEGF-A level was significantly lower at vitrectomy day than injection day, indicating that anti-VEGF injections preoperatively are a useful surgical tool to reduce postoperative vitreous cavity hemorrhage (29).
Limitations of the study include its retrospective nature and the inherent difficulties of retrospective data collection including the identification of confounders, which cannot always be adjusted for in the analysis. Secondly, the sample size of this study is relatively small. Prospective studies on a larger number of eyes with a longer follow-up period are required. Thirdly, the correlation between the severity of renal and retinal damage is much closer in type 1 diabetes than in type 2. All patients in our study were type 2 diabetic patients with PDR. The PDR patients were allocated into two groups, abnormal renal function group and normal renal function group, based on the estimated glomerular filtration rate (eGFR), with the value of 90 mL/min/1.73 m2 as cutoff, which might be improper. Fourthly, other evaluation indices of renal function such as urine protein were absent. Lastly, as for diabetes therapy, there was no significant difference in either insulin or oral hypoglycemic agents between the two groups. The oral hypoglycemic agents the patients used in this study included biguanide, sulfonylureas, acarbose and glinides. Some antidiabetic drugs such as sodium-dependent glucose transporters 2 (SGLT-2) inhibitor may have a nephroprotective role that could influence the correlation between the severity of the two types of diabetic microangiopathy. The patients in the current study did not use this drug mainly because SGLT-2 inhibitor has been on the market in China since the end of 2017, while our research was carried out between June 2017 and January 2018. The influence of nephroprotective antidiabetic drugs on the relationship between renal function and diabetic retinopathy should be further investigated.
In conclusion, there was no difference of pre- and post-operative outcomes between PDR patients with and without renal dysfunction, which can be induced by no significant difference in VEGF-A level in vitreous, aqueous humor and serum between the two groups. Renal function alone should not be considered a prognostic marker for PPV surgery for patients with PDR.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethic Committee of First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
We declare that all authors have made substantial contributions. JL, WZ, PX, SY, LJ, QL, and ZH conceived the study, developed the protocol, and supervised the study. JL, WZ, and PX collected the data. SY and LJ performed the preliminary data analysis. JL, WZ, and SY performed the final data analysis. All authors contributed to the conduct of the study and interpretation of results. JL, WZ, QL, and ZH drafted the manuscript and all authors contributed to critical revisions of the manuscript. All authors read and approved the final manuscript.
This study was supported by Natural Science Foundation of Jiangsu Province (No. BK20191059) and National Key Research and Development Program of China (No. 2017YFA0104100 to QL).
We thank all the patients who participated in our investigation and those who have helped us in the research but not mentioned as co-authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet (2017) 389(10085):2239–51. doi: 10.1016/S0140-6736(17)30058-2
2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol (2018) 14(2):88–98. doi: 10.1038/nrendo.2017.151
3. Vujosevic .S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T, et al. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol (2020) 8(4):337–47. doi: 10.1016/S2213-8587(19)30411-5
4. American Diabetes Association Professional Practice Committee. 12. retinopathy, neuropathy, and foot care: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S185–94. doi: 10.2337/dc22-S012
5. Galiero R, Pafundi PC, Nevola R, Rinaldi L, Acierno C, Caturano A, et al. The importance of telemedicine during COVID-19 pandemic: A focus on diabetic retinopathy. J Diabetes Res (2020) 2020:9036847. doi: 10.1155/2020/9036847
6. Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, et al. Telemedicine for screening diabetic retinopathy: The NO BLIND Italian multicenter study. Diabetes Metab Res Rev (2019) 35(3):e3113. doi: 10.1002/dmrr.3113
7. Nawaz IM, Rezzola S, Cancarini A, Russo A, Costagliola C, Semeraro F, et al. Human vitreous in proliferative diabetic retinopathy: Characterization and translational implications. Prog Retin Eye Res (2019) 72:100756. doi: 10.1016/j.preteyeres.2019.03.002
8. Valk EJ, Bruijn JA, Bajema IM. Diabetic nephropathy in humans: pathologic diversity. Curr Opin Nephrol Hypertens (2011) 20:285–9. doi: 10.1097/MNH.0b013e328345bc1c
9. Samsu N. Diabetic nephropathy: Challenges in pathogenesis, diagnosis, and treatment. BioMed Res Int (2021) 2021:1497449. doi: 10.1155/2021/1497449
10. Zhang L, Long J, Jiang W. Trends in chronic kidney disease in China. N Engl J Med (2016) 375(9):905–6. doi: 10.1056/NEJMc1602469
11. Min JW, Kim HD, Park SY, Lee JH, Park JH, Lee A, et al. Relationship between retinal capillary nonperfusion area and renal function in patients with type 2 diabetes. Invest Ophthalmol Vis Sci (2020) 61(14):14. doi: 10.1167/iovs.61.14.14
12. International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care (2009) 32:1327–34. doi: 10.2337/dc09-9033
13. American Diabetes Association Professional Practice Committee. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
14. Rizzo S, Genovesi-Ebert F, Emanuele Di B. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol (2008) 246(6):837–42. doi: 10.1007/s00417-008-0774-y
15. Arroyo JG, Postel EA, Stone T, McCuen BW, Egan KM. A matched study of primary scleral buckle placement during repair of posterior segment open globe injuries. Br J Ophthalmol (2003) 87(1):75–8. doi: 10.1136/bjo.87.1.75
16. Pascolini D, Mariotti SP. Global estimates of visual impairment:2010. Br J Ophthalmol (2012) 96(5):614–8. doi: 10.1136/bjophthalmol-2011-300539
17. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol (2020) 18(2):117–24. doi: 10.2174/1570161117666190502103733
18. Grunwald JE, Alexander J, Ying GS, Maguire M, Daniel E, Whittock-Martin R, et al. Retinopathy and chronic kidney disease in the Chronic Renal Insufficiency Cohort (CRIC) study. Arch Ophthalmol (2012) 130(9):1136–44. doi: 10.1001/archophthalmol.2012.1800
19. Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, Casellas A, Barrot-De la Puente JF, Franch-Nadal J, et al. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PloS One (2016) 11(2):1–10. doi: 10.1371/journal.pone.0149448
20. Saini DC, Kochar A, Poonia R. Clinical correlation of diabetic retinopathy with nephropathy and neuropathy. Indian J Ophthalmol (2021) 69:3364–8. doi: 10.4103/ijo.IJO_1237_21
21. Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, et al. Relationship between albuminuric CKD and diabetic retinopathy in a real-world setting of type 2 diabetes: Findings from no blind study. Nutr Metab Cardiovasc Dis (2019) 29(9):923–30. doi: 10.1016/j.numecd.2019.05.065
22. Li Yu, Su X, Ye Q, Guo X, Xu B, Guan T, et al. The predictive value of diabetic retinopathy on subsequent diabetic nephropathy in patients with type 2 diabetes: a systematic review and meta-analysis of prospective studies. Ren Fail (2021) 43(1):231–40. doi: 10.1080/0886022X.2020.1866010
23. Khuthaila MK, Hsu J, Chiang A, DeCroos FC, Milder EA, Setlur V, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol (2013) 155(4):757–63. doi: 10.1016/j.ajo.2012.11.004
24. Park D, Shin J, Kim S. Comparison of clinical outcomes between 23-gauge and 20-gauge vitrectomy in patients with proliferative diabetic retinopathy. Retina (2010) 30(10):1662–70. doi: 10.1097/IAE.0b013e3181d95261
25. Gupta B, Sivaprasad S, Wong R, Laidlaw A, Jackson TL, McHugh D, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye (2012) 26(4):510–6. doi: 10.1038/eye.2011.321
26. Maharaj AS, Saint-Geniez M, Maldonado AE, D'Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol (2006) 168(2):639–48. doi: 10.2353/ajpath.2006.050834
27. Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int (2004) 65(6):2003–17. doi: 10.1111/j.1523-1755.2004.00621.x
28. Cha DR, Kang YS, Han SY, Jee YH, Han KH, Han JY, et al. Vascular endothelial growth factor is increased during earlystage of diabetic nephropathy in type II diabetic rats. J Endocrinol (2004) 183(1):183–94. doi: 10.1677/joe.1.05647
29. Li B, Li MD, Ye JJ, Chen Z, Guo ZJ, Di Y. Vascular endothelial growth factor concentration in vitreous humor of patients with severe proliferative diabetic retinopathy after intravitreal injection of conbercept as an adjunctive therapy for vitrectomy. Chin Med J (Engl) (2020) 133(6):664–9. doi: 10.1097/CM9.0000000000000687
Keywords: proliferative diabetic retinopathy, renal dysfunction, vitrectomy, surgical outcomes, Vascular endothelia growth factor-A (VEGF-A)
Citation: Liu J, Zhang W, Xie P, Yuan S, Jiang L, Liu Q and Hu Z (2022) The relationship between renal function and surgical outcomes of patients with proliferative diabetic retinopathy. Front. Endocrinol. 13:984561. doi: 10.3389/fendo.2022.984561
Received: 06 July 2022; Accepted: 09 August 2022;
Published: 25 August 2022.
Edited by:
Ferdinando Carlo Sasso, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Alfredo Caturano, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Liu, Zhang, Xie, Yuan, Jiang, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghuai Liu, bGl1cWhAbmptdS5lZHUuY24=; Zizhong Hu, aHV6aXpob25nQG5qbXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.