
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 18 August 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.984157
 Canxiao Li1†
Canxiao Li1† Jingting Li1†
Jingting Li1† Shijie Li1
Shijie Li1 Yishen Zhao1
Yishen Zhao1 Guandong Liu1
Guandong Liu1 Rui Du1
Rui Du1 Gianlorenzo Dionigi2,3
Gianlorenzo Dionigi2,3 Nan Liang1*‡
Nan Liang1*‡ Hui Sun1*‡
Hui Sun1*‡Background: Lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR), mean platelet volume (MPV) and fibrinogen (FIB) have been identified as predictive biomarkers in several malignancies. The aim of this study was to explore the association between inflammatory index with clinicopathologic features as well as recurrence risk in intermediate-to high-risk papillary thyroid carcinoma (PTC).
Methods: Retrospective evaluation of 212 patients diagnosed with intermediate-to high-risk PTC who underwent surgery at China-Japan Union Hospital between 2015 and 2016. Logistic regression and receiver operating curves (ROC) were used to explore possible risk factors.
Results: LMR was predictive of capsular invasion (AUC=0.595, P=0.017), FIB was predictive of lymph node metastasis (LN) (AUC=0.714, P=0.002), MPV was predictive of largest LN size ≥1cm (AUC=0.639, P=0.002), PLR and MPV were predictive of recurrence (AUC=0.616, P=0.032; AUC=0.626, P=0.020). In addition, FIB ≤ 2.6 (OR=6.440, 95%CI:1.777-23.336, P=0.005) and capsular invasion (OR=3.773, 95%CI:1.171-12.159, P=0.026) were identified as independent risk factors for lymph node metastasis by multivariate analysis. In addition, LN metastasis (P=0.048), largest LN size ≥ 1 cm (P=0.032), MPV > 9.4 (P=0.046), and PLR ≤ 128.1 (P=0.032) were significantly related with recurrence. Further multivariate regression analysis revealed that PLR ≤ 128.1 was a potentially independent risk factor for recurrence. Specifically, the risk of recurrence was 2.951 times higher in patients with a PLR ≤ 128.1 compared with patients with a PLR > 128.1 (OR=2.951, 95% CI:1.238-7.037, P=0.015).
Conclusion: In intermediate-to high-risk PTC, LMR, PLR, MPV, and FIB could predict clinicopathologic features and recurrence, with lower PLR being the potential risk factors for recurrence.
More and more studies show that the systemic inflammatory response has a significant impact on the clinicopathological features and prognosis of malignancies (1). Tumor-related inflammatory responses play a crucial role in the formation and development of tumors., In lung cancer, pancreatic cancer, breast cancer, and other cancers, common inflammatory indices have been shown to be safe (2–5), such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR). Moreover, some new inflammatory markers, such as platelet volume (MPV) and fibrinogen (FIB), have been shown not only to represent the nutritional status of patients but also to assess their prognosis (6). In general, the above inflammatory indices are practical and reliable, and they are all obtained from routine hospital examinations, which does not impose any additional burden on patients. Therefore, inflammatory index has a good application prospect as a potential tumor marker.
Thyroid cancer (TC) accounts for almost 3% of all types of cancers and is the most common endocrine malignant tumor (7). In recent decades, the incidence of thyroid cancer gradully increased. It is of vital therapeutic importance to explore the occurrence and development of thyroid cancer and to find appropriate and reliable tumor markers to reduce the burden of the disease. According to the and the 2015 American Thyroid Association (ATA) guidelines and 2012 Chinese guidelines (8, 9), the clinicopathological features of intermediate-and high-risk DTC are worse compared with low-risk DTC, and the prognosis is poorer. Therefore, it is of great importance to search for tumor markers that can predict the clinicopathological features and prognosis of intermediate-to high-risk DTC to optimize the surgical plan and further improve the prognosis of DTC.
In this study, we aim to evaluate the diagnostic value of various inflammatory indices and to analyze the correlation between the inflammatory indices and the prognosis of intermediate-high risk PTC patients, further providing the basis for individualized and accurate diagnosis and treatment of intermediate-high risk PTC.
The inclusion criteria were 1) sequential enrollment and an initial diagnosis of primary PTC, and 2) patients at intermediate and high risk of recurrence as determined by histological examination and imaging.
The exclusion criteria were as followed: patients who had obtained prior anti-tumor therapy, such as chemotherapy, radiotherapy, and immunotherapy; patients diagnosed with other primary cancers, coinfections, or inflammatory disorders (excluding autoimmune thyroid diseases, such as chronic lymphocytic thyroiditis); and the absence of relevant information. Throughout the study period, we enrolled all patients who met the inclusion criteria. In addition, a follow-up of clinical characteristics and outcomes was performed. No interventions wereconducted during treatment.
A total of 300 intermediate-high risk PTC patients were enrolled. Preoperative complete blood count, clinical and histological data, and follow-up data were available for 212 patients (Figure 1).
This study was approved by the Ethic Review Board of China-Japan Union Hospital(2021081001). Informed consent was obtained from the participants or their guardians.
All patients provided tumor tissue samples for histological examination or surgical excision. The diagnosis of PTC was made using the guidelines (10). Tumor size was identified as the largest tumor diameter detected on postoperative pathologic report.
Platelet-to-lymphocyte ratio (PLR) is the ratio of platelets to lymphocytes, while lymphocyte-to-monocyte ratio (LMR) is the ratio of lymphocytes to monocytes. Preoperative complete blood count was used to calculate the above inflammatory indicators. All the blood examination were completed in the same laboratory of China-Japan Union Hospital of Jilin University.
By January 31, 2022, follow-up ranged from 7 to 83 months, with a median of 68 months.
Patient assessment periods can be divided into one month, three months, and six months, depending on the different tumor burden of each patient. If no disease progression or adverse events occur, routine postoperative examinations are performed. Initial follow-up examinations occur every six months, and if the patient’s condition is relative stable, the interval is eventually increased to one time per year.
According to the 2015 guidelines from ATA (9), recurrence was evaluated according to both biochemical incomplete response and structural incomplete response. Biochemical incomplete response was identified as: negative imaging and suppressed Tg ≥ 1 ng/ml or stimulated Tg ≥ 10 ng/ml or rising anti-Tg antibody levels. Structural incomplete response was identified as: structural or functional evidence of disease with any Tg levels with or without anti-Tg antibodies.
All the data analysis were conducted upon the SPSS package version 23.0 (SPSS). All data are expressed as mean, standard deviation (SD), interquartile range (IQR) and median accordingly. Categorical variables were compared and by rank sum test and Fisher’s exact test. The logistic regression models were applied to explore the risk factors of the prognosis of PTC. The threshold for statistical significance was set as P < 0.05. The receiver-operating characteristic curve (ROC curve) is applied to evaluate the diagnostic capabilities.
As illustrated in Table 1, a total of 212 patients were included in this study, including 57 men (26.9%) and155 women (72.1%), and a mean age of 39.3 ± 9.5 years. Postoperative pathology confirmed that 109 cases (51.4%) were reported with capsular invasion. Lymph node metastases were found in 193 (91.0%), of which 45 (21.2%) had central lymph node metastases and 148 (69.8%) with lateral neck lymph node metastases. 117 cases (48.5%) were at intermediate risk and 124 cases (51.5%) were at high risk. After a median follow-up of 68 months, 34 (16%) patients were observed to have recurrence.
As expressed in Figure 2, we further evaluated the predictive performance of each inflammatory index for capsular invasion, lymph node metastasis (LN), largest LN size, and recurrence using the ROC curve. The respective AUC values are shown in Table 2. First, LMR was found to have significant discriminatory power for predicting capsular invasion (AUC=0.595, P=0.017). However, PLR, MPV, and FIB had more modest discriminatory power. The predictive value of each inflammatory index in LN metastases and the largest LN size was then assessed, and we discovered that patients with a lower FIB were more likely to have lymph node metastases (P=0.002), and when the cut-off value of FIB was 2.6, the AUC was 0.714, the specificity was 0.895, and the sensitivity was 0.544. Further analysis showed that the largest LN size in patients with a higher MPV (AUC=0.639, P=0.002) was closer to 1cm. Finally, we examined how well inflammatory indices predicted recurrence. We found that both PLR and MPV were significantly predictive of recurrence, with AUC values of 0.616 (P=0.032) for PLR and 0.626 (P=0.020) for MPV, respectively. As for PLR, the cut-off was 128.1, the specificity and sensitivity were 48.3% and 51.7%, respectively. As for MPV, the cut-off value was 9.4, the specificity was 61.8% while sensitivity was 63.5%.
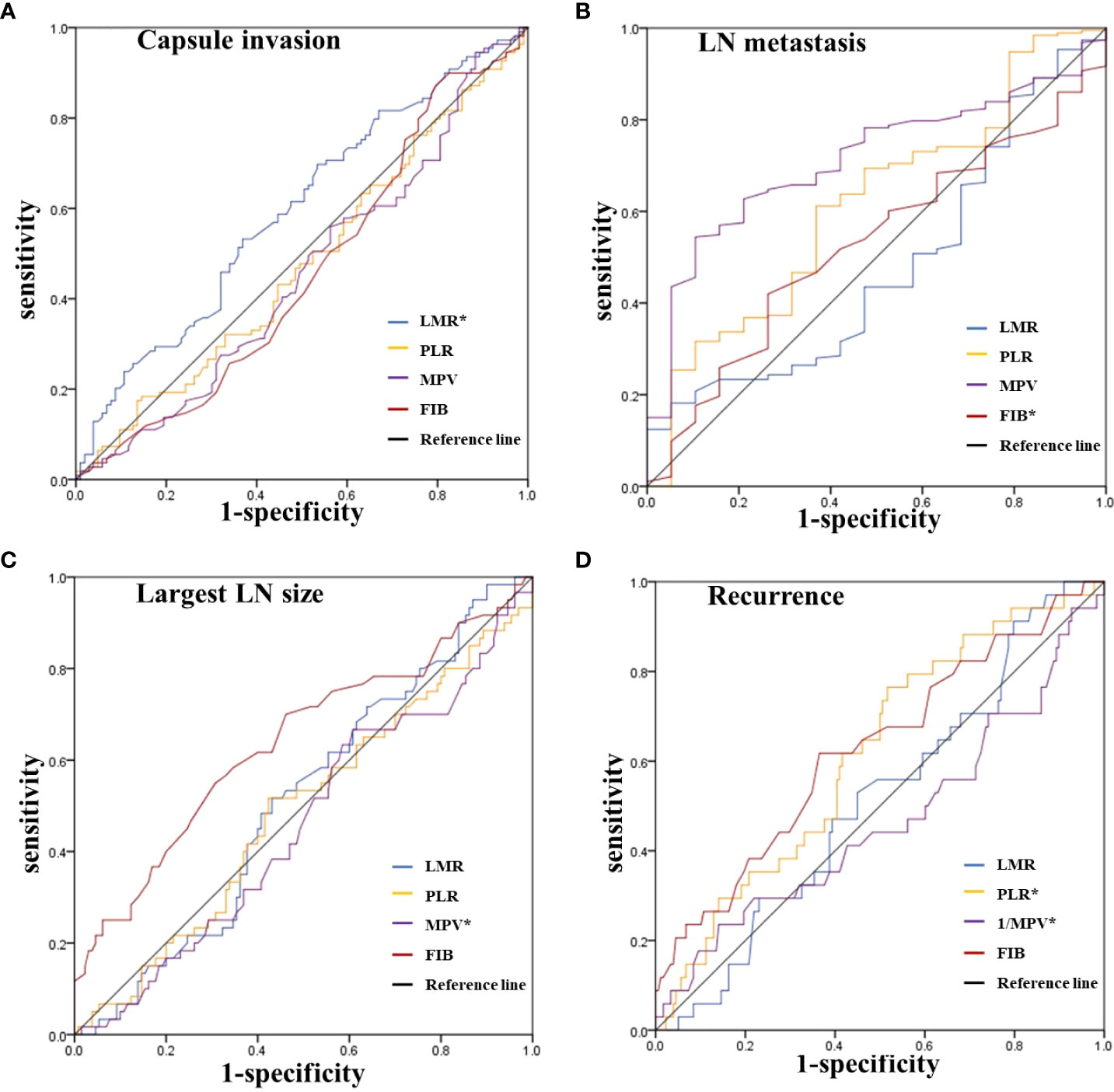
Figure 2 ROC curves for the preoperative LMR, PLR, MPV and FIB to predict (A) Capsule invasion, (B) LN metastasis, (C) Largest LN size, and (D) Recurrence in intermediate-high risk PTC patients. *Statistically significant P-values (P < 0.05). LMR, lymphocyte-to-monocyte ratio; PLR, platelet-to-lymphocyte ratio; MPV, average platelet volume; FIB, fibrinogen; LN, lymph node; PTC, Papillary thyroid carcinoma.
To further discuss the risk factors for LN metastasis, the univariate and multivariate regression were applied(Table 3). According to univariate analysis, FIB ≤ 2.6 (P=0.001), age ≤ 55 (P=0.036), and capsular invasion (P=0.012) were significantly associated with lymph node metastasis. Further multivariate analysis revealed that FIB ≤ 2.6 and capsular invasion were potentially independent risk factors for lymph node metastasis. Specifically, the risk of lateral LN metastasis was increased 6.440-fold in patients with FIB ≤ 2.6 compared with patients with FIB > 2.6 (OR =6.440, 95% CI: 1.777-23.336, P=0.005). Patients with capsular invasion had a 3.773-fold higher risk of lateral LN metastasis than those without capsular invasion (OR =3.773, 95%CI: 1.171-12.159, P=0.026).
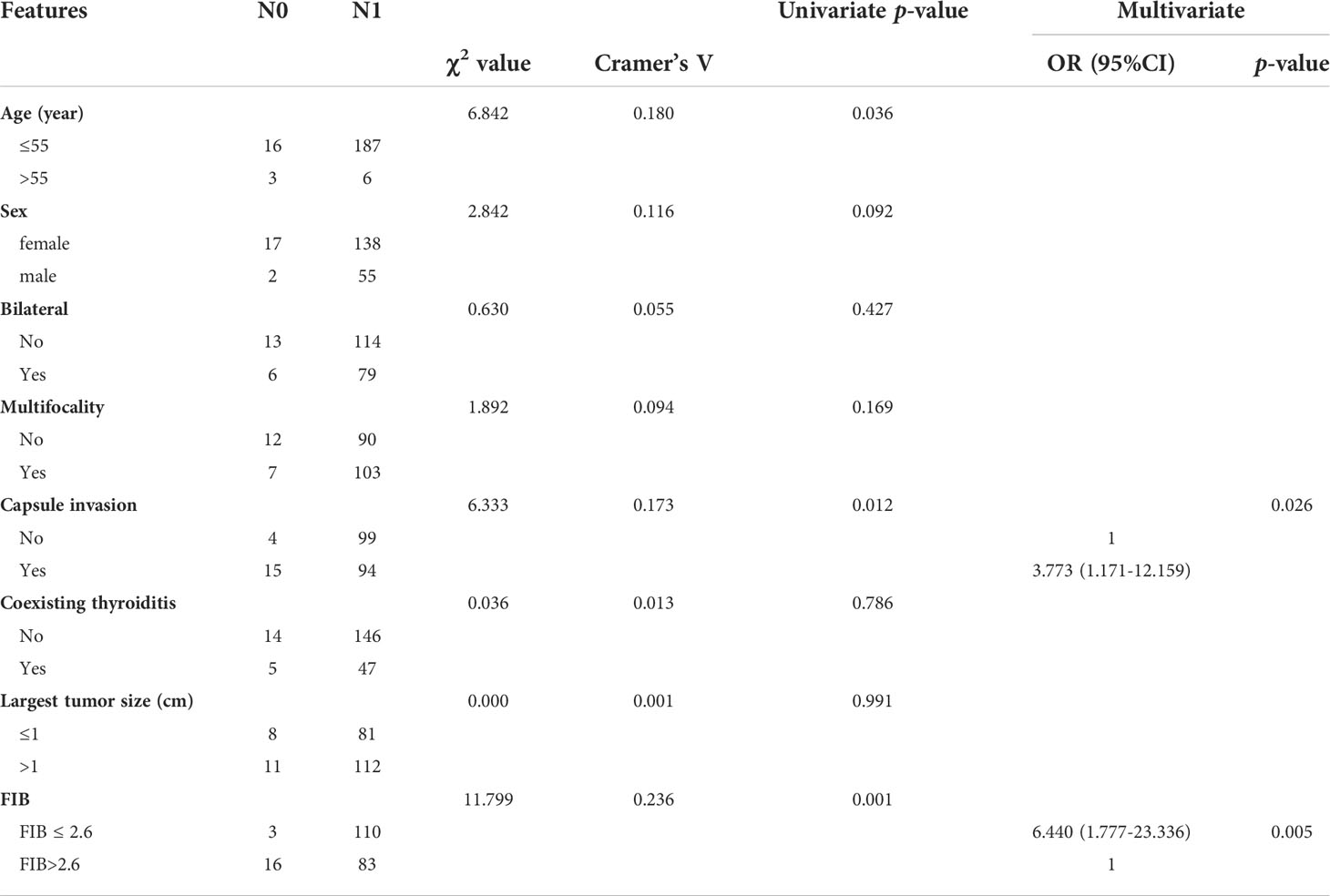
Table 3 Univariate and multivariate regression analyses of the possible risk factors for LN metastasis.
Similarly, we analyzed the possible risk factors for recurrence (Table 4). According to univariate analysis, LN metastases (P=0.048), largest LN size ≥ 1cm (P=0.032), MPV > 9.4 (P=0.046), and PLR ≤ 128.1(P=0.032) were significantly related with recurrence. The multivariate analysis further suggested that PLR ≤ 128.1 was a potentially independent risk factor for recurrence. Specifically, the risk of recurrence was 2.951 times higher in patients with a PLR ≤ 128.1 than in those with a PLR > 128.1 (OR =2.951, 95% CI: 1.238-7.037, P=0.015).
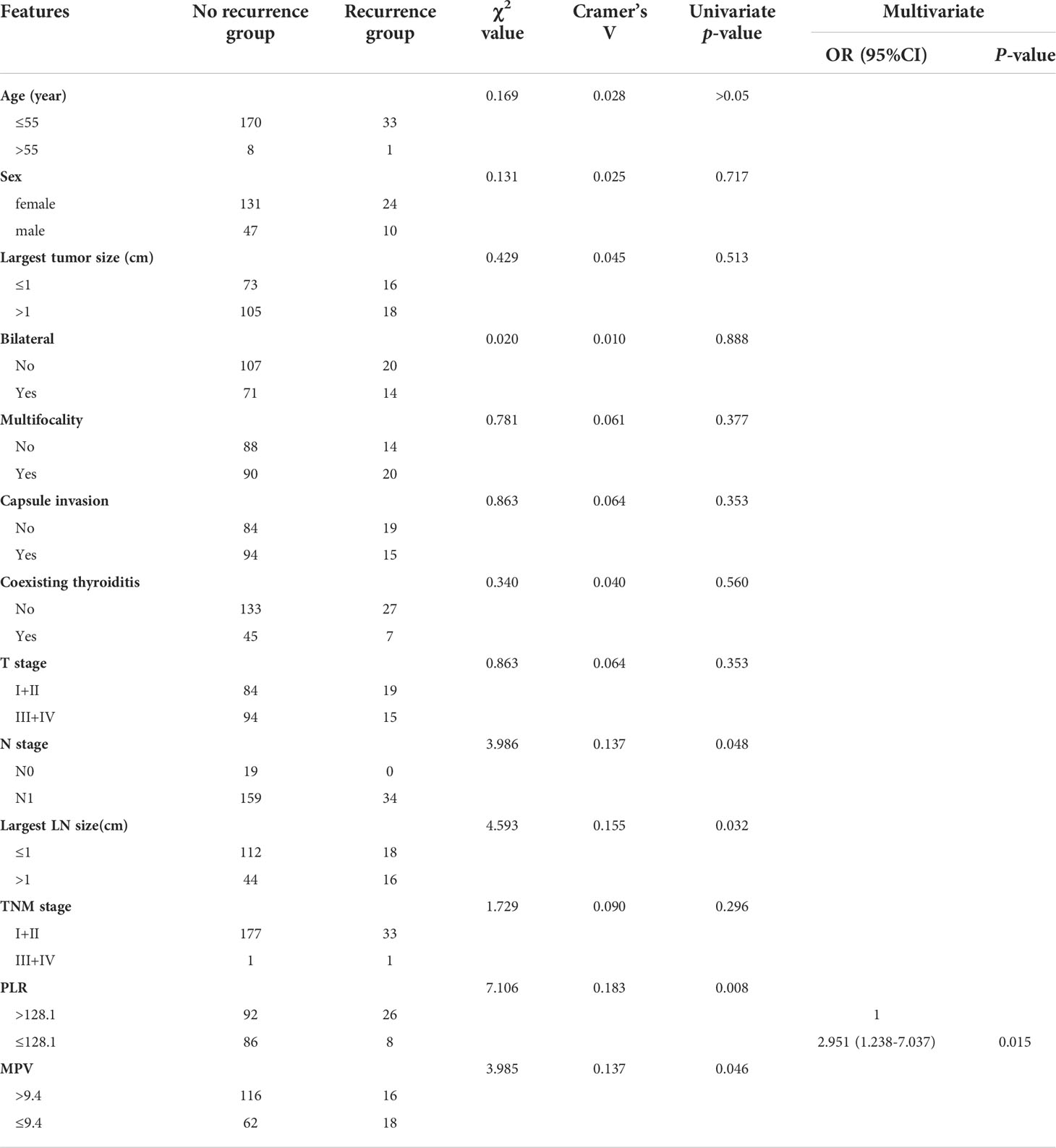
Table 4 Univariate and multivariate regression analyses of the possible risk factors for recurrence.
In the tumor microenvironment, the inflammatory response is a double-edged sword that contributes to both the progression and suppression of tumor growth (11). In recent years, inflammatory index has been identified as a new tumor marker based on host inflammatory response (12). According to studies, there is a relationship between inflammatory index, clinicopathological features as well as prognosis in several cancers, such as pancreatic cancer, lung cancer, and thyroid cancer (13–15). The incidence of thyroid cancer is increasing annually, but there are few studies on the association between thyroid tumors and inflammatory index. Therefore, this study aims to clarify and evaluate the application potential of inflammatory index as a PTC tumor marker. Compared with other studies, this study is aimed to explore the association between inflammatory index (LMR, PLR, MPV, and FIB) and recurrence risk in intermediate-to high-risk PTC.
Several previous studies have suggested the association between invasion and inflammatory index, however the conclusions inconsistent (16–18). In this study, LMR was found to be predictive of capsular invasion for the first time (AUC=0.595, P=0.017). The exact mechanism is currently unclear. Possible reasons include: circulating monocytes may develop into tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) (19, 20). They contribute to tumor cell growth, invasion, and metastasis by accelerating the transformation between epithelium and stroma, while lymphocytes could promote cytotoxins production, such as perforin, and the release of various inflammatory mediators that directly or indirectly play antitumor roles (21, 22). Therefore, LMR may reflect the state of the host immune system to some extent and is likely to be a tumor marker in PTC patients.
Patients often have impaired coagulation and fibrinolysis systems, and FIB is the highest coagulation factor in plasma. Therefore, it is worthwhile to investigate the potential of FIB as a tumor marker in PTC patients. Our study suggests that FIB is predictive of LN metastases (AUC=0.714, P=0.002), and further multivariate analysis showed that FIB ≤ 2.6 (OR =6.440, 95%CI: 1.777-23.336, P=0.005) is an independent risk factor for LN metastases. Similarly, FIB (23–25)also shows a good application prospect in other tumors such as breast cancer, renal cancer, urothelial carcinoma, etc.
In this study, ROC analysis revealed a significant association between PLR and recurrence, and further multivariate regression analysis implied that PLR ≤ 128.1 was a possible independent risk factor for recurrence. Notably, the risk of recurrence was 2.951-fold higher compared with patients with PLR ≤ 128.1 than that of patients with PLR > 128.1 (OR =2.951, 95%CI:1.238-7.037, P=0.015). This may be due to the fact that platelets may be increased by the release of numerous inflammatory mediators. Activated platelets can release platelet-activating factor (PAF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), as well as other cytokines, which could enhance tumor-induced blood vessel development and promote extracellular matrix degradation, increasing tumor growth and distant metastasis (26).
However, in recent years, it is reported that platelet volume is more closely related to platelet activation compared with platelet number (27). The MPV, i.e., average platelet volume, provides information about the average platelet size, which indicates the platelet generation rate and stimulation (28). According to a number of studies, larger platelets seems to have higher metabolic and enzymatic activity than smaller ones (29). Therefore, more and more attention has been paid to the critical role of MPV in tumor evaluation. Osada (30) found that the MPV of gastric cancer patients was higher than that of 20 healthy control subjects. Similarly, we also discovered that higher MPV was predictive of the largest LN size ≥ 1cm (AUC=0.639, P=0.002).
This study has several limitations. First, this was a retrospective analysis. Second, though we had excluded patients with diseases that could affect complete blood count, there still might be some other possible affective factors undetectable or predictable. Finally, this study is based on one single center data, and the sample size might be limited, so it is necessary to conduct a large-scale prospective study to further clarify the scope and mechanism.
In summary, our study preliminarily investigated the potential application of inflammatory indices (LMR, PLR, MPV, and FIB) in moderate-to high-risk PTC. Specifically, LMR was predictive of capsular invasion, lower FIB could predict the possibility of lymph node metastasis, lower MPV was predictive of largest LN size ≥ 1 cm, and PLR was predictive of recurrence. Remarkably, the risk of recurrence was higher in patients with lower PLR (≤128.1). This suggests that LMR, PLR, MPV, and FIB may be possible potential biomarkers for evaluating the clinicopathologic features as well as prognosis of intermediate-and high-risk PTC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the China-Japan Union Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HS, NL, CL and JL contributed to the study design and conception. CL, SL, YZ, GL, RD contributed to the data collection and analysis. All authors proposed many professional suggestions when data analysis. The first draft of the manuscript was completed by CL and JL. NL and GD contributed much effort to manuscript reviewing and editing. HS was responsible for study supervision. CL and JL contributed equally to this study and shared first authorship. All authors approved for the publication of manuscript.
This study was sponsored by National Nature Science Foundation of China [81972499]; the Jilin Province Science and Technology Development Project [20210402011GH; 20210101342JC]; the Project of Jilin Provincial Finance Department [2020SCZ03; 2021SCZ23]; Jilin University Bethune Project [2020B14].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moore MM, Chua W, Charles KA, Clarke SJ. Inflammation and cancer: Causes and consequences. Clin Pharmacol Ther (2010) 87(4):504–8. doi: 10.1038/clpt.2009.254
2. Chen Y, Wang W, Zhang X, Yu X, Xi K, Wen Y, et al. Prognostic significance of combined preoperative platelet-to-Lymphocyte ratio and lymphocyte-to-Monocyte ratio in patients undergoing surgery with stage ib non-Small-Cell lung cancer. Cancer Manag Res (2018) 10:5411–22. doi: 10.2147/cmar.s177320
3. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver (2018) 12(3):342–52. doi: 10.5009/gnl17216
4. Ahn J, Song E, Oh HS, Song DE, Kim WG, Kim TY, et al. Low lymphocyte-to-Monocyte ratios are associated with poor overall survival in anaplastic thyroid carcinoma patients. Thyroid (2019) 29(6):824–9. doi: 10.1089/thy.2018.0684
5. Moldoveanu D, Pravongviengkham V, Best G, Martínez C, Hijal T, Meguerditchian AN, et al. Dynamic neutrophil-to-Lymphocyte ratio: A novel prognosis measure for triple-negative breast cancer. Ann Surg Oncol (2020) 27(10):4028–34. doi: 10.1245/s10434-020-08302-2
6. Li C, Zhang H, Li S, Zhang D, Li J, Dionigi G, et al. Prognostic impact of inflammatory markers plr, lmr, pdw, mpv in medullary thyroid carcinoma. Front Endocrinol (Lausanne) (2022) 13:861869. doi: 10.3389/fendo.2022.861869
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
8. Ya M. Interpretation of the management guidelines for patients with thyroid nodules and differentiated thyroid cancer (2012 Chinese edition). Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zh (2013) 27(16):917–20.
9. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
10. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. Nccn guidelines insights: Thyroid carcinoma, version 2.2018. J Natl Compr Canc Netw (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089
11. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
12. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou F-S, Bifulco C, et al. International validation of the consensus immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet (London England) (2018) 391(10135):2128–39. doi: 10.1016/S0140-6736(18)30789-X
13. Mandaliya H, Jones M, Oldmeadow C, Nordman II. Prognostic biomarkers in stage iv non-small cell lung cancer (Nsclc): Neutrophil to lymphocyte ratio (Nlr), lymphocyte to monocyte ratio (Lmr), platelet to lymphocyte ratio (Plr) and advanced lung cancer inflammation index (Ali). Transl Lung Cancer Res (2019) 8(6):886–94. doi: 10.21037/tlcr.2019.11.16
14. Kim JY, Jung EJ, Kim JM, Lee HS, Kwag SJ, Park JH, et al. Dynamic changes of neutrophil-to-Lymphocyte ratio and platelet-to-Lymphocyte ratio predicts breast cancer prognosis. BMC Cancer (2020) 20(1):1206. doi: 10.1186/s12885-020-07700-9
15. Park J, Park J, Shin JH, Oh YL, Jung HA, Chung MK, et al. Prognostic value of the neutrophil-to-Lymphocyte ratio before and after radiotherapy for anaplastic thyroid carcinoma. Cancers (Basel) (2021) 13(8):1913. doi: 10.3390/cancers13081913
16. Manatakis DK, Tseleni-Balafouta S, Balalis D, Soulou VN, Korkolis DP, Sakorafas GH, et al. Association of baseline neutrophil-to-Lymphocyte ratio with clinicopathological characteristics of papillary thyroid carcinoma. Int J Endocrinol (2017) 2017:8471235. doi: 10.1155/2017/8471235
17. Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-Lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol (2013) 107(5):493–7. doi: 10.1002/jso.23270
18. Zhang L, Luo H, Wang L, Liu Y, Rui S, Wu Z, et al. Diagnostic and prognostic value of preoperative systemic inflammatory markers in anaplastic thyroid cancer. J Surg Oncol (2020) 122(5):897–905. doi: 10.1002/jso.26089
19. Katoh H, Watanabe M. Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediators Inflammation (2015) 2015:159269. doi: 10.1155/2015/159269
20. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: Expect the unexpected. J Clin Invest (2015) 125(9):3356–64. doi: 10.1172/jci80005
21. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity (2004) 21(2):137–48. doi: 10.1016/j.immuni.2004.07.017
22. Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res (2009) 69(13):5383–91. doi: 10.1158/0008-5472.can-08-3845
23. Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, et al. An elevated preoperative plasma fibrinogen level is associated with poor disease-specific and overall survival in breast cancer patients. Breast (2015) 24(5):667–72. doi: 10.1016/j.breast.2015.08.003
24. Tanaka N, Kikuchi E, Matsumoto K, Hayakawa N, Ide H, Miyajima A, et al. Prognostic value of plasma fibrinogen levels in patients with localized upper tract urothelial carcinoma. BJU Int (2013) 111(6):857–64. doi: 10.1111/j.1464-410X.2012.11353.x
25. Obata J, Tanaka N, Mizuno R, Kanao K, Mikami S, Matsumoto K, et al. Plasma fibrinogen level: An independent prognostic factor for disease-free survival and cancer-specific survival in patients with localised renal cell carcinoma. BJU Int (2016) 118(4):598–603. doi: 10.1111/bju.13414
26. Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis Via P2y2 receptor. Cancer Cell (2013) 24(1):130–7. doi: 10.1016/j.ccr.2013.05.008
27. Wen W, Wu P, Li J, Wang H, Sun J, Chen H. Predictive values of the selected inflammatory index in elderly patients with papillary thyroid cancer. J Transl Med (2018) 16(1):261. doi: 10.1186/s12967-018-1636-y
28. Threatte GA. Usefulness of the mean platelet volume. Clin Lab Med (1993) 13(4):937–50. doi: 10.1016/S0272-2712(18)30418-9
29. Mangalpally KK, Siqueiros-Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S. Platelet activation patterns in platelet size Sub-populations: Differential responses to aspirin in vitro. J Thromb Thrombolysis (2010) 30(3):251–62. doi: 10.1007/s11239-010-0489-x
Keywords: papillary thyroid carcinoma, inflammation index, clinicopathological characteristics, recurrence, biomarkers
Citation: Li C, Li J, Li S, Zhao Y, Liu G, Du R, Dionigi G, Liang N and Sun H (2022) Prognostic significance of inflammatory markers LMR, PLR, MPV, FIB in intermediate-and high-risk papillary thyroid carcinoma. Front. Endocrinol. 13:984157. doi: 10.3389/fendo.2022.984157
Received: 01 July 2022; Accepted: 27 July 2022;
Published: 18 August 2022.
Edited by:
Akira Sugawara, Tohoku University, JapanReviewed by:
Laura Giacomelli, Sapienza University of Rome, ItalyCopyright © 2022 Li, Li, Li, Zhao, Liu, Du, Dionigi, Liang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Sun, c19oQGpsdS5lZHUuY24=; Nan Liang, bGlhbmduYW4yMDA2QCBqbHUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Hui Sun, orcid.org/0000-0001-8348-4933
Nan Liang, orcid.org/0000-0002-7733-862X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.