
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 03 November 2022
Sec. Cancer Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.983160
This article is part of the Research TopicObesity and Cancer: Update on Etiology, Molecular Biomarkers and Biotargets, Clinical Strategies, and EpidemiologyView all 10 articles
Background: The close association of abdominal obesity rather than general obesity with colorectal cancer (CRC) risk might be mediated by IR and inflammation, which has never been systematically explored in large-scale prospective studies.
Methods: We prospectively examined the mediation effects of the fasting triglyceride-glucose (TyG) index and C-reactive protein (CRP) on the associations of obesity (general and abdominal) with CRC risk among 93,659 participants. We used the Cox proportional hazards regression models and subgroup analyses to evaluate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) of CRC. The CAUSALMED procedure was used to perform the mediation analyses.
Results: During 13.02 years of follow-up, a total of 586 CRC cases were verified. Male participants with general obesity and abdominal obesity had a 1.29-fold and a 1.28-fold increased risk of CRC. However, a significant association was only observed among female individuals with abdominal obesity. Both TyG index and CRP were associated with an elevated risk of CRC, and A significant interaction between the TyG index and CRP was found for the risk of CRC (P for interaction<0.05). CRP and the TyG index significantly mediated the positive association between abdominal obesity and CRC risk.
Conclusion: CRP and TyG index increased the risk of CRC independently and synergistically. Mediation effects of CRP and the TyG index were found for the association between abdominal obesity and CRC risk.
Colorectal cancer (CRC) is deadly and expensive to treat (1). Previous epidemiologic studies have reported a possible association between body size and the risk of CRC (2–6). Body mass index (BMI, in kg/m2) is positively correlated with CRC risk in men, while women have weaker correlations. In addition, abdominal obesity [as assessed by waist circumference (WC, in cm)] is closely associated with CRC risk in both sexes. One possible explanation for the disparity is that men and women have distinct body compositions, with fat constituting a more significant percentage of body mass in women (30%) than in men (20%) (7). Another explanation might be that abdominal obesity plays a crucial role in metabolic abnormalities, leading to chronic diseases, including cancer (8, 9).
The insulin resistance (IR) and inflammation hypotheses postulate that there is a relationship between abdominal obesity and CRC risk since the buildup of visceral fat is a significant predictor of IR and inflammation (10, 11). IR and inflammation have increased the risk of CRC incidence in experimental and epidemiologic investigations (12, 13). The fasting triglyceride-glucose (TyG) index is a simple and cost-effective way to detect IR (14) compared to the gold standard hyperinsulinemic-euglycemic glucose clamp approach (15). High-sensitivity C-reactive protein (hs-CRP), also known as CRP assessed by high-sensitivity assays, is a typical protein produced in response to inflammation, infection, and tissue damage that has been linked to chronic disorders such as cardiovascular disease (CVD) and cancer (16, 17). Based on the evidence above, we assume that the close association of abdominal obesity rather than general obesity with CRC risk might be mediated by IR and inflammation, which, to our knowledge, has never been systematically explored in large-scale prospective studies.
The Kailuan study is an ongoing, prospective cohort study that includes biennial follow-ups for each participant. The anthropometric and laboratory parameter measurements offer us a valuable opportunity to investigate 1) the association of the TyG index and CRP with the risk of CRC incidence and 2) the mediation effects of the TyG index and CRP on the associations of obesity (general and abdominal) with CRC risk.
As previously stated (17), this study was based on the Kailuan Study, a community-based ongoing cohort study performed in Tangshan city. The current study investigated the risk factors for chronic diseases such as cancer. In short, a sum of 101,510 individuals including 81,110 males and 20,400 females underwent a standardized questionnaire, physical examination, and laboratory testing from June 2006 to October 2007 (baseline). Follow-up examinations were carried out biennially to keep participants up to update participant status on the parameters above.
In this study, individuals were excluded if they 1) were diagnosed with cancer previously(n=377); 2) had missing data on BMI, WC, CRP and the components of the TyG index, including fasting blood glucose (FBG, in mmol/L) and triglycerides (TG, in mmol/L) (n=1,342); and 3) lacked information about any potential confounders, including age, sex, social economic factors, laboratory tests and lifestyle behaviors (n=6,132). After factoring for the exclusion criteria, 93,659 individuals were enrolled, including 18,988 women and 74,671 men (Figure 1).
Patient’s venous blood was drawn into EDTA tubes after an overnight fast (8–12 h). All blood samples were analyzed in the Central Laboratory of Kailuan General Hospital using an autoanalyzer (Hitachi 747; Hitachi). The details regarding the assessment of plasma CRP, FBG, HDL-C, TG, and TC can be found elsewhere (18). The TyG index was estimated using the following calculations: ln [TG (mg/dL) × FBG (mg/dL)/2]. According to the Centers for Disease Control and Prevention and the American Heart Association guidelines, low-grade inflammation was defined as CRP ≥ 3 mg/L (19). The median of the TyG index (8.59) was used as the cutoff for the definition of IR.
For the duration of the follow-up, incident CRC cases were identified via: 1) tracking participants’ biennial health check-ups; 2) examining medical records linked with the Tangshan medical insurance system and the Kailuan Social Security Information System once a year, and 3) checking death certificates from the Provincial Vital Statistics Offices (PVSO) to further confirmed the outcome yearly. Clinical professionals assessed medical records and pathology reports to reconfirm the diagnosis of incident CRC, and CRC patients were categorized as C18-21 using the International Classification of Diseases, Tenth Revision (ICD-10).
Information on age, sex, socioeconomic factors, living habits, and personal and family members’ medical histories was collected via a standard questionnaire. BMI was calculated as body weight divided by the square of the body height and was grouped into the following three categories: normal (< 24.0 kg/m2), overweight (24.0-27.9 kg/m2), and general obesity (≥ 28.0 kg/m2). Abdominal obesity was defined as WC >90 cm in men and >85 cm in women. Drinking was defined as consuming ≥ 100 mL/day of alcoholic beverages for more than 6 months. Smoking status was defined as consuming ≥ 1 cigarette/day for more than 6 months. Physical activity was defined as having ≥ 3 times weekly with each time lasting ≥ 30 minutes based on the response to the question of frequency of Physical activity. Tea consumption was defined as ≥ 5 times weekly regardless of the tea types. High-fat diets were evaluated in the questionnaire as “Regularly” consumed.
The mean ± SD and T-test were used to describe and compare continuous variables in the normal distribution. The median (IQR) and nonparametric tests (Kruskal-Wallis test) were used to describe and compare the skewed distributed variables (e.g., CRP and TG). Absolute values with percentages and the chi-square test were utilized to represent and compare categorical variables. Person-years were computed from the date of the baseline examination to the date of CRC diagnosis, death, or December 31, 2019, whichever occurred first. The hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of incident CRC were calculated using Cox proportional hazards models. We firstly explored the association of general obesity (assessed by BMI) and central obesity (assessed by WC) with subsequent CRC risk among men and women, due to the distinct body compositions between men and women. Secondly, we investigated the effect of IR (assessed by TyG index) and inflammation (assessed by CRP) on the occurrence of CRC, multiplicative models were used to examine the interactions between CRP, TyG index, and CRC risks. Third, because there was an interaction between TyG index, CRP, and CRC risk, participants were further divided into four groups based on the presence/absence of the elevated TyG index (≥ 8.59) and CRP (>1 mg/L).
The CAUSALMED procedure was used to perform the mediation analyses based on the variance-covariance matrix and the maximum likelihood method. It calculated the total effect (the total of the direct and indirect effects), the direct effect (the effect without the mediator’s influence), and the indirect effect (the effect of the independent variable on the mediator multiplied the effect of the mediator on the outcome). All P values < 0.05 (two-sided) were judged statistically significant. Statistical analyses were carried out using SAS software (SAS Institute, Cary, NC, USA), version 9.4.
The baseline characteristics of the participants stratified by sex are listed in Table 1. The average age of the study population was 51.48 ± 12.44 years. Significant age differences, and levels of FBG, HDL-C, TC, TG, CRP, BMI, and WC, were found across the different sex groups. In addition, the percentages of the male sex, reported income, marital status, educational levels, physical exercise, tobacco, alcohol and tea consumption, sedentary lifestyle, high-fat diets, hypertension, diabetes mellitus, and family history of cancer differed considerably across the two groups.
During 13 years of follow-up, a total of 586 incident CRC was developed. Table 2 shows the association of general obesity or abdominal obesity with CRC risk. Among the male group, participants with general obesity and abdominal obesity had a 1.29-fold (HR [95%] CI: 1.29, 1.01-1.64) and a 1.28-fold (HR [95%] CI: 1.28, 1.07-1.52) increased risk of CRC. However, a significant association was only observed among female individuals with abdominal obesity (WC >85.0 vs. ≤85.0, HR [95%] CI: 1.22, 1.03-1.50).
The adjusted HRs (95% CI) for the association of the TyG index and CRP with the risk of CRC are shown in Table 3. TyG index (continuous) and elevated TyG index (≥ 8.59 vs. <8.59) were positively related to the risk of CRC incidence, with corresponding HRs (95% CI) of 1.21 (1.06-1.37) and 1.41 (1.17, 1.67), respectively. A significant interaction between the TyG index and inflammation (CRP> 3 mg/L) was found for the risk of CRC (P for interaction<0.05). There was a statistically significant trend of increasing relative risks of CRC across different CRP groups (CRP >3 vs. <1, HR [95%] CI: 1.29, 1.05-1.60; p for trend=0.042). Figure 2 illustrates the subgroup analyses stratified by sex, age, abdominal obesity, and diabetes. Significant associations of an elevated TyG index with CRC risk were found among participants who were male, young, middle-aged, elderly, and without abdominal obesity or diabetes. Age significantly modified the associations between CRP and CRC risk (P for interaction < 0.05). The associations were more pronounced among young participants than middle-aged and elderly adults. The positive results were also observed when participants were stratified by sex, abdominal obesity and diabetes.
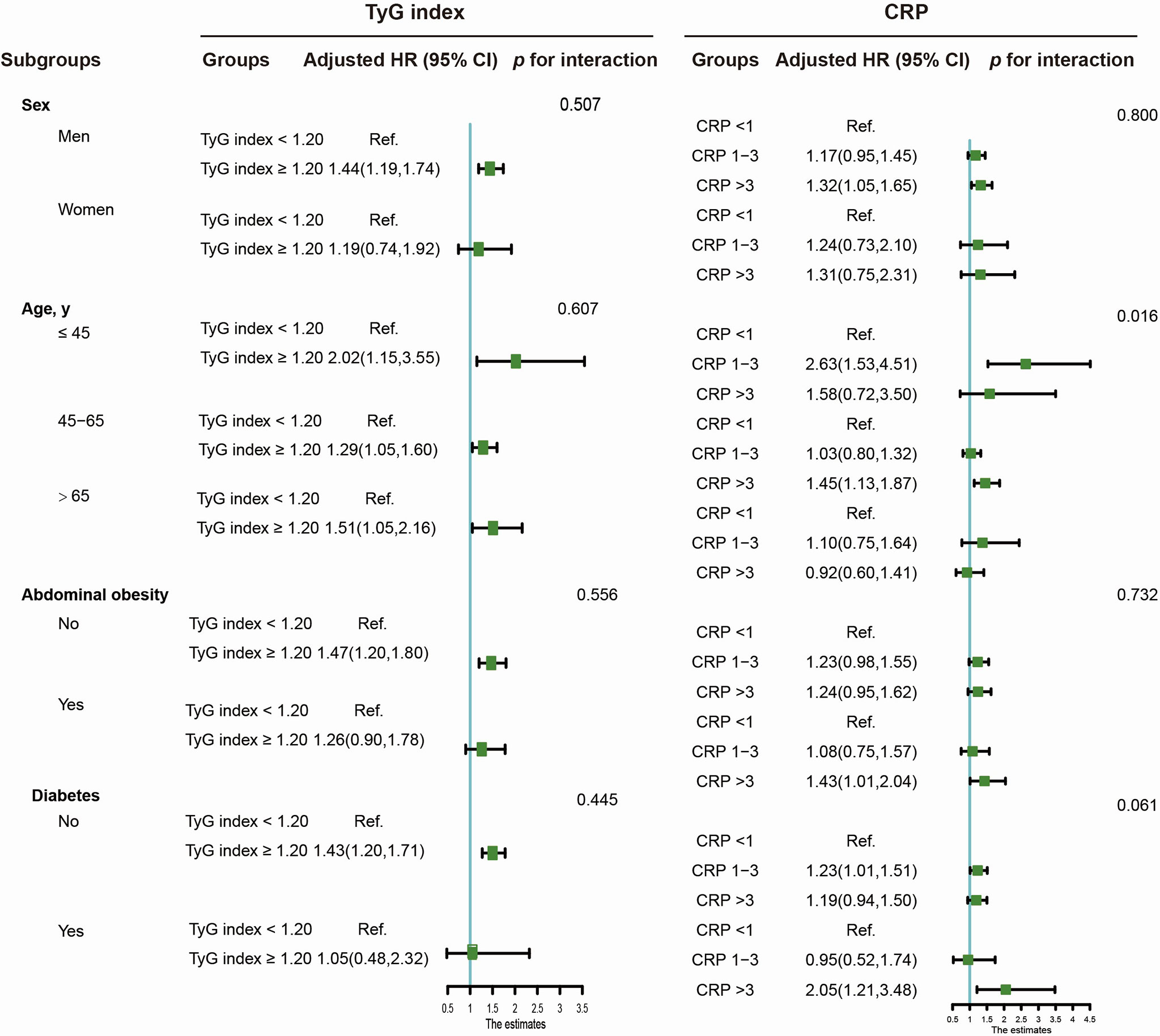
Figure 2 Subgroup analysis of the TyG index or CRP level association with CRC risk. Adjusted models were adjusted for age (every 10 years), sex, family income, educational background, marital status, WC, TC, smoking status, drinking status, physical activity, sedentary lifestyle, tea consumption, salt intake, high fat diet, hypertension, and family history of cancer.
The significant interaction between the TyG index and inflammation affects CRC development. We further divided participants into four groups based on the absence/presence of an elevated TyG index and CRP (Table 4). After adjustments were made for the potential confounders, compared with the low TyG index and CRP group, participants with only an elevated TyG index or with an elevated TyG index and CRP had a 1.41-fold (HR [95%] CI: 1.41, 1.16-1.72) and 1.74-fold (HR [95%] CI: 1.74, 1.31-2.28) elevated risk of CRC.
In the mediation effect analysis, both CRP and the TyG index significantly mediated the positive association between abdominal obesity (elevated WC) and CRC risk after adjustments were made for the confounding factors. However, null or weaker mediation effects of the TyG index and CRP were found to associate general obesity with the risk of CRC incidence (Figure 3).
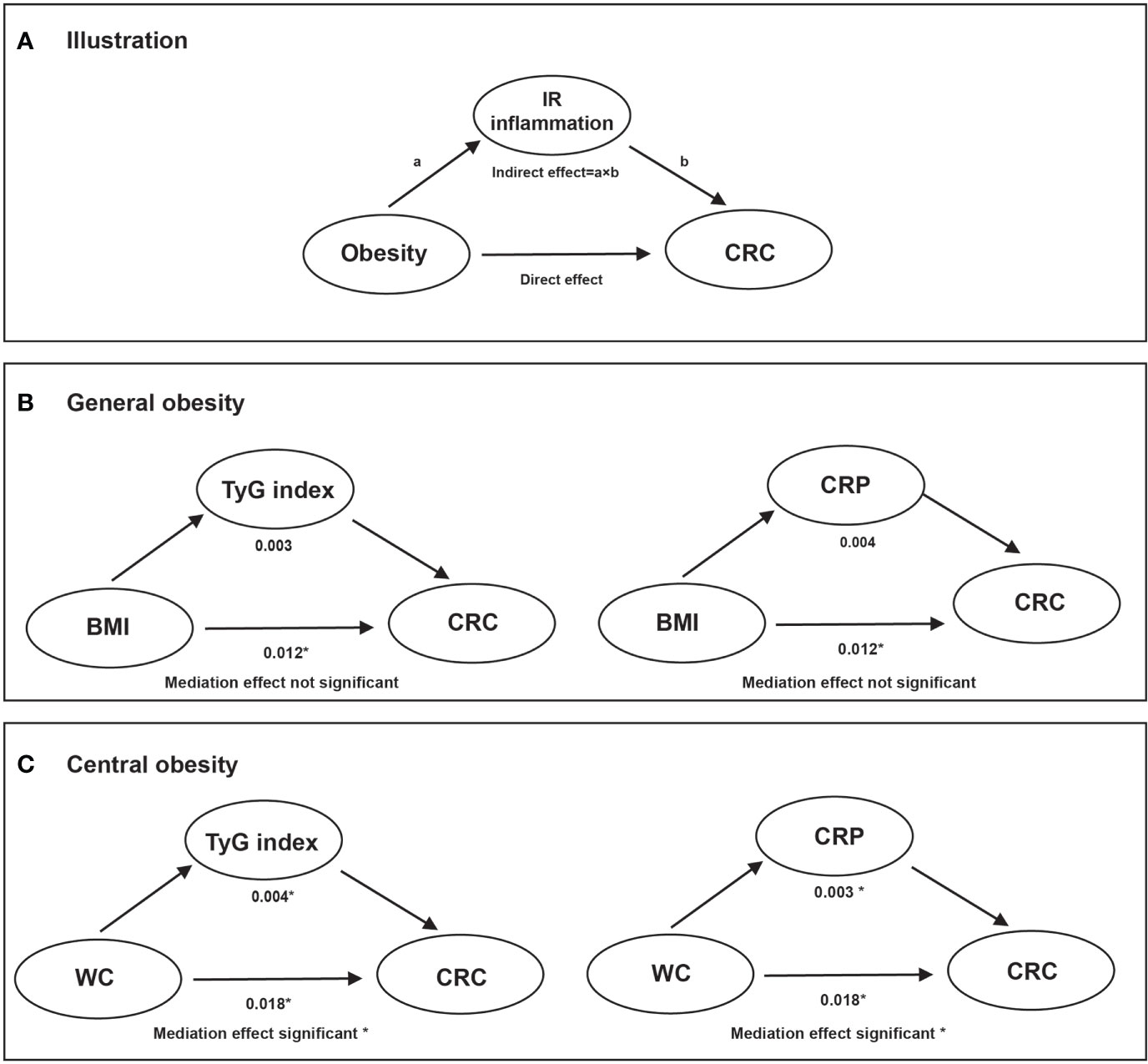
Figure 3 The mediation effect of TyG index and CRP on the association of obesity with CRC risk. Note: Adjusted models were adjusted for age (every 10 years), sex, family income, educational background, marital status, WC, TC, smoking status, drinking status, physical activity, sedentary lifestyle, tea consumption, salt intake, high fat diet, hypertension, and family history of cancer. (A): illustration; (B): overall obesity; (C): central obesity. * Values were statistically significant.
In this large-scale community-based cohort study, we found the following: I) abdominal obesity was associated with an elevated risk of CRC in both sexes, while general obesity was found to only increase the risk of CRC in men; II) TyG and CRP could raise the risk of CRC independently. In addition, IR along with inflammation may function synergistically to accelerate the initiation of CRC; III) CRP and TyG index only mediated the association between obesity assessed by WC and CRC risk, indicating the IR and inflammation hypotheses may help to explain the etiological importance of abdominal fat disposition, rather than overall adiposity.
We found that general obesity (BMI> 28 kg/m2) increased the risk of CRC among male participants, while abdominal obesity (WC>90 cm in men and >85 cm in women) was associated with an elevated risk of CRC incidence in both sexes. This finding is consistent with most previous epidemiological studies (2–6). The close associations between inflammation and CRC incidence have also been demonstrated in previous studies. A report from the general Danish population, which included 10,408 participants, found that elevated levels of CRP in cancer-free individuals were associated with an increased risk of cancer of any type and possibly CRC (20). A case-control study nested within the Japan Public Health Center-based prospective study found a 1.6-fold increased risk of subsequent colon cancer for the highest versus the lowest quartile of CRP (21). A systematic review including 5 nested case-control and 3 cohort studies identified a positive but weak association between pre-diagnostic circulating CRP concentrations and colorectal and colon cancer risk (12).
Little research has been designed to investigate the effect of the TyG index on the occurrence of CRC risk. In a retrospective population-based cohort study of 27,944 individuals, Takuro Okamura et al. found that the TyG index was a useful and accessible tool for predicting incident CRC. Recently, by analyzing 510,471 individuals from six European cohorts, Josef Fritz et al. found that the TyG index was associated with an increased risk of cancers of the digestive system, including colon, rectal, liver, and pancreatic cancer (22). Similarly, several epidemiological studies reported a close association between IR assessed by the homeostasis model assessment method (HOMA-IR) and the risk of colorectal cancer (23, 24), as did experimental studies (25, 26). A study speculated sulphonylureas may play a role in CRC carcinogenesis impairing the physiological insulin secretion among diabetes participants (27).
Epidemiological studies have found that WC is more significantly associated with CRC risk than BMI (28, 29), emphasizing the etiological importance of abdominal fat disposition rather than total adiposity. However, further direct evidence is needed to confirm this association. We found that CRP and the TyG index mediated the association of obesity, assessed by WC rather than BMI, with CRC risk. This finding partly explains the strong association between central obesity and CRC risk, that abdominal obesity-induced carcinogenesis may be through inflammatory and IR pathways. By using UK Biobank data, Dashti SG et al. exanimated the role of obesity-related factors including CRP, hemoglobin-A1c (HbA1c), sex hormone-binding globulin (SHBG), and testosterone in the association of adiposity and CRC risk (30). They found pathways influenced by CRP explained a small proportion of the adiposity-CRC association in both men and women. A prospective cohort study found that substantial proportions of the effect of BMI were mediated by the TyG index for cancers of the pancreas, rectum, colon, kidney, and liver. However, there were two limitations to the previous study. First, it did not explore the significance of those mediation effects. Second, WC was not assessed as an indicator of obesity. Abdominal obesity is a condition marked by low-grade chronic inflammation and IR. Adipose tissue functions as an endocrine organ, secreting a variety of proteins that regulate metabolism, energy intake, and fat storage, including leptin, adiponectin, interleukin- (IL-) 6, and tumor necrosis factor-alpha (TNF-α) (31).
The underlying mechanism by which inflammation and IR increase subsequent CRC risk includes two aspects. First, long-term, low-grade inflammation, which causes protein and DNA damage, can increase tumor growth and progression. Critical pathways that maintain normal cellular homeostasis can be altered by genetic and epigenetic variations in response to inflammatory mediators such as cytokines, free radicals, prostaglandins, and growth factors. Point mutations in tumor suppressor genes, DNA methylation, and posttranslational modifications are examples of these alterations, all of which can contribute to the existence and development of cancer (32). Second, insulin promotes colon cancer progression by increasing the expression of acyl-coenzyme A: cholesterol acyltransferase-1 (33), increasing the expression of vascular cell adhesion molecule-1 in intestinal tumor endothelial cells and causing a proinflammatory state (34), and elevating the levels of IGF-1, which promotes cell proliferation, survival, and angiogenesis by stimulating the synthesis of vascular endothelial growth factor (35).
The main strength of the current study is that it provides a unique perspective on the possible mediation effects of inflammation and IR on the association of obesity with future CRC risk based on a population-based cohort study. Additionally, this study fully considered the influence of potential confounders, such as lifestyle habits and a history of cancer-related illnesses. Additionally, the strengths of this study include the prospective study design, large sample size, and long-term follow-up. Sensitivity analysis and subgroup analyses were conducted to infer the robustness of our conclusion.
There are certain limitations in this study that should be mentioned. First, colon and rectal cancer could not be analyzed separately due to the scarcity of data. Inflammation and IR may have distinct carcinogenic impacts on the development of colon and rectal cancers. Second, although we controlled for most potential confounders, we could not exclude the possibility of residual cancer-related causal variables, such as the consumption of cereal, vegetable, and high-fiber foods, owing to a lack of information on how these products are consumed. On the other hand, dietary factors are substantially associated with BMI, TC, and TG. Because those factors were adjusted in the multivariate analysis, they may only have had a modest influence on the findings. Third, all participants were from the Kailuan community, with a higher proportion of men than women. As a result, this group could not be considered typical of the Chinese population. The findings could not be immediately extrapolated to other communities with various cultures and socioeconomic backgrounds. Fourth, instead of the gold standard, HOMA-IR, the TyG index was used as an indicator of insulin resistance, which may have resulted in misclassification and underestimation of the potential effect of IR.
The results of this prospective cohort study showed that elevated CRP and TyG index increased the risk of CRC independently and synergistically. Mediation effects of CRP and the TyG index were found for the association between abdominal obesity and CRC risk, which may help to elucidate the etiological importance of abdominal fat disposition rather than overall adiposity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Aerospace Center Hospital and Kailuan General Hospital. The patients/participants provided their written informed consent to participate in this study.
All authors have read and approved the manuscript. WQL: Methodology, Software, Writing-Original draft preparation. TL: Writing-Reviewing and Editing. LQ: Writing-Reviewing and Editing. YMW: Supervision, Validation. XMM: Software. LYC: Resources. QSZ: Conceptualization, Supervision. JQ: Conceptualization, Supervision, Validation, Resources. All authors contributed to the article and approved the submitted version.
We thank all the staff and participants of the Kailuan study for their important contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BMI, body mass index; CIs, confidence intervals; CRC, colorectal cancer; CRP, C-reactive protein; CVD, cardiovascular disease; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, hyperinsulinemic-euglycemic glucose clamp; HRs: hazard ratios; TC, total cholesterol; TG, triglyceride; TyG, fasting triglyceride-glucose; WC, waist circumference.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Terry P, Giovannucci E, Bergkvist L, Holmberg L, Wolk A. Body weight and colorectal cancer risk in a cohort of Swedish women: Relation varies by age and cancer site. Br J Cancer (2001) 85(3):346–9. doi: 10.1054/bjoc.2001.1894
3. Ku MS, Chiu SY, Chien KL, Lee YC, Chen SL, Chen CD. Gender difference in metabolic syndrome and incident colorectal adenoma: A prospective observational study (KCIS No.42). Medicine (2021) 100(22):e26121. doi: 10.1097/MD.0000000000026121
4. Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in framingham study adults. Int J Obes Relat Metab Disord (2004) 28(4):559–67. doi: 10.1038/sj.ijo.0802606
5. MacInnis RJ, English DR, Hopper JL, Haydon AM, Gertig DM, Giles GG. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev (2004) 13(4):553–9. doi: 10.1158/1055-9965.553.13.4
6. Lin J, Zhang SM, Cook NR, Rexrode KM, Lee IM, Buring JE. Body mass index and risk of colorectal cancer in women (United states). Cancer Causes Control (2004) 15(6):581–9. doi: 10.1023/B:CACO.0000036168.23351.f1
7. Lönnqvist F, Thörne A, Large V, Arner P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arterioscler Thromb Vasc Biol (1997) 17(7):1472–80. doi: 10.1161/01.ATV.17.7.1472
8. Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. importance of regional adipose tissue distribution. J Clin Invest (1983) 72(3):1150–62. doi: 10.1172/JCI111040
9. Guglielmo D, Hootman JM, Murphy LB, Boring MA, Theis KA, Belay B, et al. Health care provider counseling for weight loss among adults with arthritis and overweight or obesity - united states, 2002-2014. MMWR Morb Mortal Wkly Rep (2018) 67(17):485–90. doi: 10.15585/mmwr.mm6717a2
10. Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res (2002) 10 Suppl 2:97s–104s. doi: 10.1038/oby.2002.202
11. Palau-Rodriguez M, Marco-Ramell A, Casas-Agustench P, Tulipani S, Miñarro A, Sanchez-Pla A, et al. Visceral adipose tissue phospholipid signature of insulin sensitivity and obesity. J Proteome Res (2021) 20(5):2410–9. doi: 10.1021/acs.jproteome.0c00918
12. Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: A systematic review of prospective studies. Int J Cancer (2008) 123(5):1133–40. doi: 10.1002/ijc.23606
13. Cirillo F, Catellani C, Sartori C, Lazzeroni P, Amarri S, Street ME. Obesity, insulin resistance, and colorectal cancer: Could miRNA dysregulation play a role? Int J Mol Sci (2019) 20(12):2922. doi: 10.3390/ijms20122922
14. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab (2010) 95(7):3347–51. doi: 10.1210/jc.2010-0288
15. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol (1979) 237(3):E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
16. Allin KH, Nordestgaard BG. Elevated c-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci (2011) 48(4):155–70. doi: 10.3109/10408363.2011.599831
17. Liu T, Zhang Q, Song C, Siyin ST, Chen S, Zhang Q, et al. C-reactive protein trajectories and the risk of all cancer types: A prospective cohort study. Int J Cancer (2022) 151(2):297–307. doi: 10.1002/ijc.34012
18. Liu T, Zhang Q, Xiao X, Wang Y, Ma X, Song M, et al. High salt intake combined with hypertension elevated the risk of primary liver cancer: a prospective cohort study. Front Oncol (2022) 12:916583. doi: 10.3389/fonc.2022.916583
19. Wu Z, Huang Z, Jin W, Rimm EB, Lichtenstein AH, Kris-Etherton PM, et al. Peripheral inflammatory biomarkers for myocardial infarction risk: A prospective community-based study. Clin Chem (2017) 63(3):663–72. doi: 10.1373/clinchem.2016.260828
20. Allin KH, Bojesen SE, Nordestgaard BG. Baseline c-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol (2009) 27(13):2217–24. doi: 10.1200/JCO.2008.19.8440
21. Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma c-reactive protein and risk of colorectal cancer in a nested case-control study: Japan public health center-based prospective study. Cancer Epidemiol Biomarkers Prev (2006) 15(4):690–5. doi: 10.1158/1055-9965.EPI-05-0708
22. Fritz J, Bjørge T, Nagel G, Manjer J, Engeland A, Häggström C, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol (2020) 49(1):193–204. doi: 10.1093/ije/dyz053
23. Yamamoto S, Nakagawa T, Matsushita Y, Kusano S, Hayashi T, Irokawa M, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care (2010) 33(1):184–9. doi: 10.2337/dc09-1197
24. Limburg PJ, Stolzenberg-Solomon RZ, Vierkant RA, Roberts K, Sellers TA, Taylor PR, et al. Insulin, glucose, insulin resistance, and incident colorectal cancer in male smokers. Clin Gastroenterol Hepatol (2006) 4(12):1514–21. doi: 10.1016/j.cgh.2006.09.014
25. Koohestani N, Tran TT, Lee W, Wolever TM, Bruce WR. Insulin resistance and promotion of aberrant crypt foci in the colons of rats on a high-fat diet. Nutr Cancer (1997) 29(1):69–76. doi: 10.1080/01635589709514604
26. Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr Cancer (2000) 37(1):19–26. doi: 10.1207/S15327914NC3701_2
27. Sinagra E, Guarnotta V, Raimondo D, Mocciaro F, Dolcimascolo S, Rizzolo CA, et al. Colorectal cancer in patients with type 2 diabetes mellitus: A single-center experience. J Biol Regul Homeost Agents (2017) 31(4):1101–7.
28. Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst (1999) 91(13):1147–54. doi: 10.1093/jnci/91.13.1147
29. Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, et al. Body size and risk of colon and rectal cancer in the European prospective investigation into cancer and nutrition (EPIC). J Natl Cancer Inst (2006) 98(13):920–31. doi: 10.1093/jnci/djj246
30. Dashti SG, Viallon V, Simpson JA, Karahalios A, Moreno-Betancur M, English DR, et al. Explaining the link between adiposity and colorectal cancer risk in men and postmenopausal women in the UK biobank: A sequential causal mediation analysis. Int J Cancer (2020) 147(7):1881–94. doi: 10.1002/ijc.32980
31. Stolarczyk E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr Opin Pharmacol (2017) 37:35–40. doi: 10.1016/j.coph.2017.08.006
32. Hussain SP, Harris CC. Inflammation and cancer: An ancient link with novel potentials. Int J Cancer (2007) 121(11):2373–80. doi: 10.1002/ijc.23173
33. Chen X, Liang H, Song Q, Xu X, Cao D. Insulin promotes progression of colon cancer by upregulation of ACAT1. Lipids Health Dis (2018) 17(1):122. doi: 10.1186/s12944-018-0773-x
34. Wang X, Häring MF, Rathjen T, Lockhart SM, Sørensen D, Ussar S, et al. Insulin resistance in vascular endothelial cells promotes intestinal tumour formation. Oncogene (2017) 36(35):4987–96. doi: 10.1038/onc.2017.107
Keywords: insulin resistance, inflammation, colorectal cancer, obesity, mediation
Citation: Li W, Liu T, Qian L, Wang Y, Ma X, Cao L, Zhang Q and Qu J (2022) Insulin resistance and inflammation mediate the association of abdominal obesity with colorectal cancer risk. Front. Endocrinol. 13:983160. doi: 10.3389/fendo.2022.983160
Received: 30 June 2022; Accepted: 24 October 2022;
Published: 03 November 2022.
Edited by:
Valentina Guarnotta, University of Palermo, ItalyReviewed by:
Mengmeng Song, Beijing Shijitan Hospital, Capital Medical University, ChinaCopyright © 2022 Li, Liu, Qian, Wang, Ma, Cao, Zhang and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Qu, cXVqdW5jaGllZkAxNjMuY29t; Qingsong Zhang, a2x5eTg4ODg4OEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.