
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 24 August 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.973820
This article is part of the Research Topic Research Advances in Gestational Diabetes Mellitus, Neonatal Diabetes Mellitus, and Metabolic Disorders Volume II View all 8 articles
 Kaat Beunen1*†
Kaat Beunen1*† Lies Vercauter2
Lies Vercauter2 Paul Van Crombrugge3
Paul Van Crombrugge3 Carolien Moyson1
Carolien Moyson1 Johan Verhaeghe4
Johan Verhaeghe4 Sofie Vandeginste5
Sofie Vandeginste5 Hilde Verlaenen5
Hilde Verlaenen5 Chris Vercammen6
Chris Vercammen6 Toon Maes6
Toon Maes6 Els Dufraimont7
Els Dufraimont7 Nele Roggen7
Nele Roggen7 Christophe De Block8
Christophe De Block8 Yves Jacquemyn9
Yves Jacquemyn9 Farah Mekahli10
Farah Mekahli10 Katrien De Clippel11
Katrien De Clippel11 Annick Van Den Bruel12
Annick Van Den Bruel12 Anne Loccufier13
Anne Loccufier13 Annouschka Laenen14
Annouschka Laenen14 Roland Devlieger4
Roland Devlieger4 Chantal Mathieu1
Chantal Mathieu1 Katrien Benhalima1†
Katrien Benhalima1†Aims: To characterize women with gestational diabetes mellitus (GDM) positive for type 1 diabetes-related autoimmune antibodies (T1D-related autoantibodies) in pregnancy and to evaluate their risk for long-term glucose intolerance.
Methods: In a multi-centric prospective cohort study with 1843 women receiving universal screening for GDM with a 75 g oral glucose tolerance test (OGTT), autoantibodies were measured in women with GDM: insulin autoantibodies (IAA), islet cell antibodies (ICA), insulinoma-associated protein-2 antibodies (IA-2A) and glutamic acid decarboxylase antibodies (GADA). Long-term follow-up ( ± 4.6 years after delivery) with a 75 g OGTT and re-measurement of autoantibodies was done in women with a history of GDM and autoantibody positivity in pregnancy.
Results: Of all women with GDM (231), 80.5% (186) received autoantibody measurement at a mean of 26.2 weeks in pregnancy, of which 8.1% (15) had one positive antibody (seven with IAA, two with ICA, four with IA-2A and two with GADA). Characteristics in pregnancy were similar but compared to women without autoantibodies, women with autoantibodies had more often gestational hypertension [33.3% (5) vs. 1.7% (3), p<0.001] and more often neonatal hypoglycemia [40.0% (6) vs. 12.5% (19), p=0.012]. Among 14 of the 15 autoantibody positive women with an early postpartum OGTT, two had impaired fasting glucose (IFG). Of the 12 women with long-term follow-up data, four tested again positive for T1D-related autoantibodies (three positive for IA-2A and one positive for ICA and IAA). Five women were glucose intolerant at the long-term follow-up of which two had IA-2A (one had IFG and one had T1D) and three without autoantibodies. There were no significant differences in long-term characteristics between women with and without autoantibodies postpartum.
Conclusions: Systematic screening for T1D-related autoantibodies in GDM does not seem warranted since the low positivity rate for autoantibodies in pregnancy and postpartum. At 4.6 years postpartum, five out of 12 women were glucose intolerant but only two still had autoantibodies. In women with clinically significant increased autoantibody levels during pregnancy, postpartum autoantibody re-measurement seems useful since the high risk for further increase of autoantibody levels.
Gestational diabetes mellitus (GDM) is a common medical condition during pregnancy. It is defined as glucose intolerance diagnosed in the second or third trimester that was not clearly overt diabetes in early pregnancy (1). GDM raises the risk of pregnancy complications such as gestational hypertension, preeclampsia, preterm delivery, and large for gestational age (LGA) infants (2–5). Pregnancy outcomes can be improved by GDM screening and treatment between 24-28 weeks of pregnancy (4, 5). A universal one-step screening approach with 2-h 75 g oral glucose tolerance test (OGTT) between 24-28 weeks and using stringent diagnostic criteria is currently recommended by the ‘International Association of Diabetes and Pregnancy Study Groups’ (IADPSG) to diagnose GDM (3, 6). Generally, glucose levels are restored to normal shortly after delivery. However, women with a history of GDM are at increased risk of developing future type 2 diabetes (T2D), cardiovascular disorders, and metabolic syndrome compared to normal glucose tolerant (NGT) women (7–10).
Not all gestational hyperglycemia has the same etiology. Gestational hyperglycemia develops when the β-cell insulin response, normally adapting to increased physiological needs and functional demands of pregnancy, is inadequate (11). GDM screening strategies mainly focus on evaluating glucose homeostasis based on diagnostic criteria rather than reflecting the underlying pathophysiology. However, the underlying pathophysiology might contribute to adverse pregnancy outcomes (12).
Sometimes, GDM masquerades undetected autoimmune type 1 diabetes mellitus (T1D) (13). In a small percentage of women with GDM, usually <10%, GDM diagnosis is associated with autoimmunity against pancreatic β-cells (i.e. autoimmune destruction of β-cells), following expression of T1D-related autoimmune antibodies (autoantibodies) such as insulin autoantibodies (IAA), islet cell antibodies (ICA), insulinoma-associated protein-2 antibodies (IA-2A), glutamic acid decarboxylase antibodies (GADA), and zinc transporter 8 antibodies (ZnT8A) (13–15). Data on the exact prevalence and levels of individual autoantibodies in GDM women remain inconclusive. Some studies showed no differences in pregnancy outcomes between GDM women with and without autoantibodies (16–18). This may imply that maternal hyperglycemia, regardless of the cause, is the main determinant of adverse pregnancy outcomes (13). Nevertheless, women with a history of GDM and autoantibody positivity in pregnancy have a higher risk to develop future impaired glucose regulation, T1D or Latent Autoimmune Diabetes of Adulthood (LADA) (13, 15, 19–22). Identification of T1D-related autoantibodies in GDM women might therefore facilitate better understanding of the pathophysiology underlying gestational hyperglycemia and contribute to more accurate classification of GDM (15, 16). Moreover, identification of these women might optimize antenatal management strategies to avoid adverse pregnancy outcomes related to T1D, or acute onset of diabetes with diabetic ketoacidosis (15, 16). So far, there are no clear recommendations in which women with GDM it would be clinically relevant to screen for autoantibodies. In addition, data on the long-term prevalence of autoantibodies after GDM and the risk to develop glucose intolerance postpartum are limited (13, 14). We aimed therefore to characterize women with GDM and T1D-related autoantibodies in pregnancy and to evaluate their long-term risk for glucose intolerance.
This is a sub-analysis of the ‘Belgian Diabetes in Pregnancy’ (BEDIP-N) study, a multi-centric prospective cohort study previously described in detail (23–26). The study was registered in ClinicalTrials.gov (NCT02036619). The study protocol received approval by Institutional Review Boards of all participating centers and all investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008. Between April 2014 and March 2017, women between 18–45 years who presented for prenatal care between 6–14 weeks of pregnancy in two university and four non-university hospitals in Belgium, were invited to participate in the study. Before inclusion, participants provided informed consent. In the first trimester, women were screened for overt diabetes and impaired fasting glycemia (IFG) in early pregnancy, as defined by the American Diabetes Association (ADA), using fasting plasma glucose (FPG) (27). Participants with FPG <100 mg/dL were universally screened for GDM between 24-28 weeks, using both a non-fasting 50 g glucose-challenge test (GCT) and 2-h 75 g OGTT. Participants and health care providers were blinded for the GCT result, so all women received an OGTT irrespective of the GCT result (24, 25). GDM diagnosis was based on IADPSG criteria. Women with GDM were treated according to ADA recommended glycemic targets (27, 28). If targets were not reached within two weeks after start of lifestyle measures, insulin treatment was started. GDM women were invited 6–16 weeks postpartum to receive a 2-h 75 g OGTT. Glucose intolerance postpartum [diabetes, IFG and/or impaired glucose tolerance (IGT)] was defined using ADA criteria (24, 27).
At the first antenatal visit (6-14 weeks), baseline characteristics and obstetrical history were collected (24). Minority ethnic background was defined as having at least one parent from non-Caucasian origin. At first visit and at the OGTT, anthropometric measurements were collected and self-administered questionnaires were completed (24). Blood pressure (BP) was measured twice at 5 min intervals with an automatic BP monitor (24). Hypertension was defined as systolic BP (SBP) ≥140 mmHg and/or diastolic BP (DBP) ≥90 mmHg. Overweight was defined as BMI ≥25 kg/m2 and obesity as BMI ≥30 kg/m2 based on body mass index (BMI) at first visit. FPG, insulin, lipid profile [total cholesterol, high-density (HDL), and low-density lipoprotein (LDL) cholesterol and triglycerides (TG)], and hemoglobin A1c (HbA1c) were measured fasting between 6-14 weeks (24). At the GCT, non-fasting glycemia was evaluated, followed by consumption of a 50 g glucose load to evaluate 1-h plasma glucose. At the OGTT, fasting lipid profile and HbA1c were determined. Glucose and insulin levels were measured fasting, at 30, 60, and 120 min (24). Analyses of FPG at first visit and glucose measurements of the OGTT were performed locally at each center, while analyses of GCT samples, insulin, lipids, and HbA1c were performed centrally at the UZ Leuven laboratory. Extra serum samples were collected in GDM women to detect autoantibodies according to routine guidelines of the ‘Belgian Diabetes Registry’ (BDR; new diagnosis of diabetes or GDM in women <40 years). Autoantibodies were analyzed by the laboratory of UZ Brussel using liquid-phase radiobinding assay for IAA, IA-2A, and GADA detection and indirect immunofluorescence for ICA detection, as described previously (29–33). Cut-off values for antibody-positivity were determined as the 99th percentile of antibody levels in 761 non-diabetic controls, after removing outliers. The upper normal limit was <0.6% binding for IAA, <12 Juvenile Diabetes Foundation units (JDF U) for ICA, <1.4 WHO U/mL for IA-2A, and <23 WHO U/mL for GADA. Internal quality controls (negative, positive low and/or high) are applied on the detection methods for all autoantibodies at least once per run. Once per two year, an external quality control named Islet Autoantibody Standardization Program (IASP) is applied on the detection method for GADA and IA-2A. At the early postpartum OGTT, anthropometric measurements were performed and self-administered questionnaires were completed (24). Continuation of breastfeeding during the OGTT was recorded. Glucose and insulin levels from the OGTTs were used to calculate different indices of insulin sensitivity [Matsuda index and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)] and β-cell function [Homeostatic Model Assessment for β-cell function (HOMA-B), insulinogenic index divided by HOMA-IR and insulin secretion-sensitivity index-2 (ISSI-2)] (24, 34–38). These measures have been validated for use in women with GDM and have been used in the BEDIP-N study (24, 34–38).
We collected pregnancy outcome data such as gestational age, preeclampsia (de novo BP ≥140/90 mmHg >20 weeks with proteinuria or signs of end-organ dysfunction), gestational hypertension (de novo BP ≥140/90 mmHg >20 weeks), type of labor and delivery, birth weight, macrosomia (>4 kg), birth weight ≥4.5 kg, LGA and small for gestational age (SGA) defined as birth weight >P90 and <P10 according to standardized Flemish birth charts adjusted for the baby’s sex and parity, respectively (39), preterm delivery (<37 weeks), neonatal hypoglycemia (<40 mg/dL), and neonatal intensive care unit (NICU) admission (24). NICU admission was decided in line with normal routine care by the local neonatologist. Gestational weight gain in early pregnancy was calculated as the difference in weight between the first antenatal visit and the OGTT, and total gestational weight gain as the difference in weight between the first antenatal visit and delivery. Excessive total gestational weight gain was defined according to 2009 Institute of Medicine guidelines (40).
Long-term follow-up in women with GDM and autoantibodies in pregnancy was standardized across all centers by performing a 2-h 75 g OGTT and re-measurement of autoantibodies. The following data were collected: results of the glucose and insulin levels at the OGTT, HbA1c, fasting C-peptide, lipid profile (total cholesterol, HDL, LDL, and TG), levels of autoantibodies measured by the BDR [IAA, ICA, IA-2A, and GADA detection as described above, and ZnT8A detection using liquid-phase radiobinding assay with upper normal limit <1.02% binding and internal and external (IASP) quality control], weight, BMI, waist circumference, BP, different indices of insulin sensitivity [Matsuda index, HOMA-IR, and reciprocal HOMA-IR (1/HOMA-IR)], and β-cell function (HOMA-B, the insulinogenic index/HOMA-IR, ISSI-2, and Stumvoll index) (24, 34–38, 41, 42).
Descriptive statistics were presented as frequencies and percentages for categorical variables and means with standard deviations or medians with interquartile range for continuous variables. Categorical variables were analyzed using the Chi-square test or Fisher exact test in case of low (<5) cell frequencies, whereas continuous variables were analyzed using the Kruskal-Wallis test for not normally distributed variables or One-way ANOVA test for normally distributed variables. A p-value <0.05 was considered significant. Analyzes were performed by statistician A. Laenen using SAS version 9.4.
In total, 1843 women received GDM screening between 26-28 weeks using an OGTT (Figure 1). Of all women with GDM (231), 80.5% (186) were screened for T1D-related autoantibodies at a mean gestational age of 26.2 weeks, of which 8.1% (15) had one positive autoantibody [seven with IAA, two with ICA, four with IA-2A, and two with GADA] (Table 1). Of these 15 women, ten had borderline increased autoantibodies (at or just above the upper limit of normal). Of the 12 women with long-term follow-up data ±4.6 years after delivery, four tested again positive for autoantibodies: three were positive for IA-2A and one was positive for both ICA and IAA (Table 1). Of all women with borderline increased autoantibodies in pregnancy and follow-up data, none had clinically significant increased autoantibodies at long-term follow-up (Table 1). In contrast, of the five women with clinically significant increased autoantibodies in pregnancy [two with IAA, two with IA-2A, and one with GADA], four had higher autoantibodies levels at long-term of which two developed glucose intolerance (one with IFG and one with T1D).
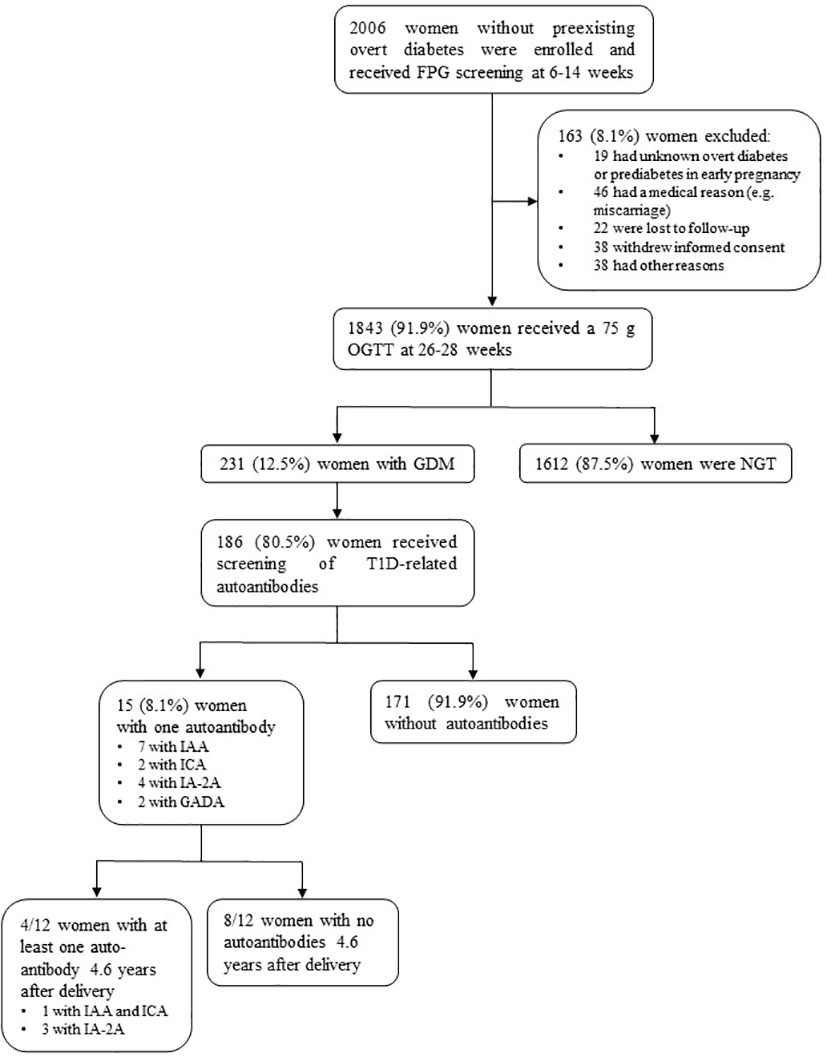
Figure 1 Flow diagram of the BEDIP-N study cohort. FPG, fasting plasma glucose; OGTT, oral glucose tolerance test; GDM, gestational diabetes mellitus; NGT, normal glucose tolerant; T1D, type 1 diabetes mellitus; IAA, insulin autoantibodies; ICA, islet cell antibodies; IA-2A, insulinoma-associated protein-2 antibodies; GADA, glutamic acid decarboxylase antibodies.
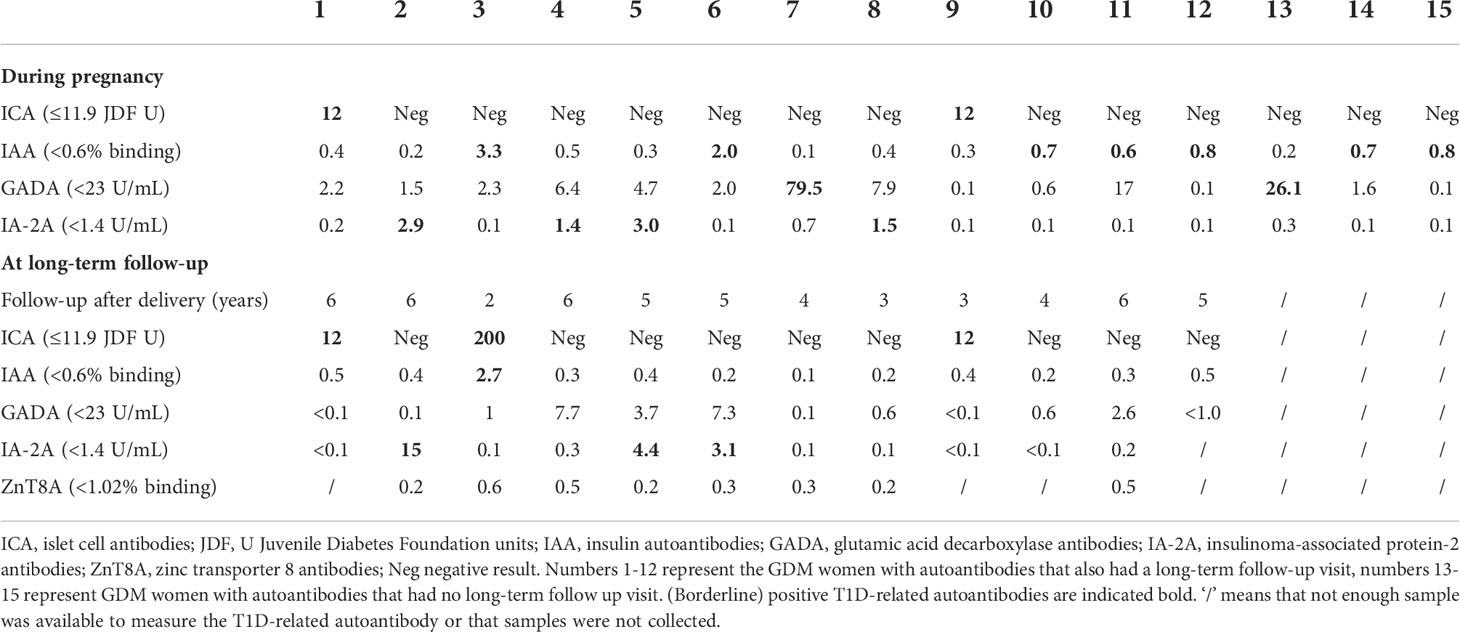
Table 1 Levels of T1D-related autoantibodies in women with GDM in pregnancy and at long-term follow-up.
At baseline and at 26-28 weeks in pregnancy, characteristics were similar between GDM women with autoantibodies (15) and GDM women without autoantibodies (171) (Table 2, and Appendix 1). Compared to GDM without autoantibodies, GDM with autoantibodies had more often gestational hypertension [33.3% (5) vs. 1.7% (3), p<0.001] and more often neonatal hypoglycemia [40.0% (6) vs. 12.5% (19), p=0.012] (Table 2). The rate of glucose intolerance at ±12.9 weeks postpartum (early postpartum) was not significantly different between GDM women with and without autoantibodies: 14.3% (2) of GDM women with autoantibodies (one with IA-2A and one with IAA) were diagnosed with IFG, while 18.3% (28) of all GDM women without autoantibodies had glucose intolerance (Table 3). Besides a lower fasting LDL-cholesterol in GDM women with autoantibodies, there were no significant differences in early postpartum characteristics between both groups (Table 3, Appendix 2, 3). Long-term follow-up data were available for 12 of the 15 women with a history of GDM and autoantibodies in pregnancy. One woman did not attend both the early postpartum and long-term follow-up OGTT, and two women who were NGT at early postpartum did not attend the long-term follow-up OGTT. At long-term follow-up, five women were glucose intolerant of which two with IA-2A (one had IFG and one had T1D) and three without autoantibodies (one had IGT, one had both IFG and IGT, and one had T2D) (Table 1 and Appendix 3). Indices of insulin resistance, beta-cell function and fasting C-peptide were similar between women with and without autoantibodies at long-term (Table 4). Other long-term characteristics were also not significantly different between both groups (Table 4 and Appendix 3).
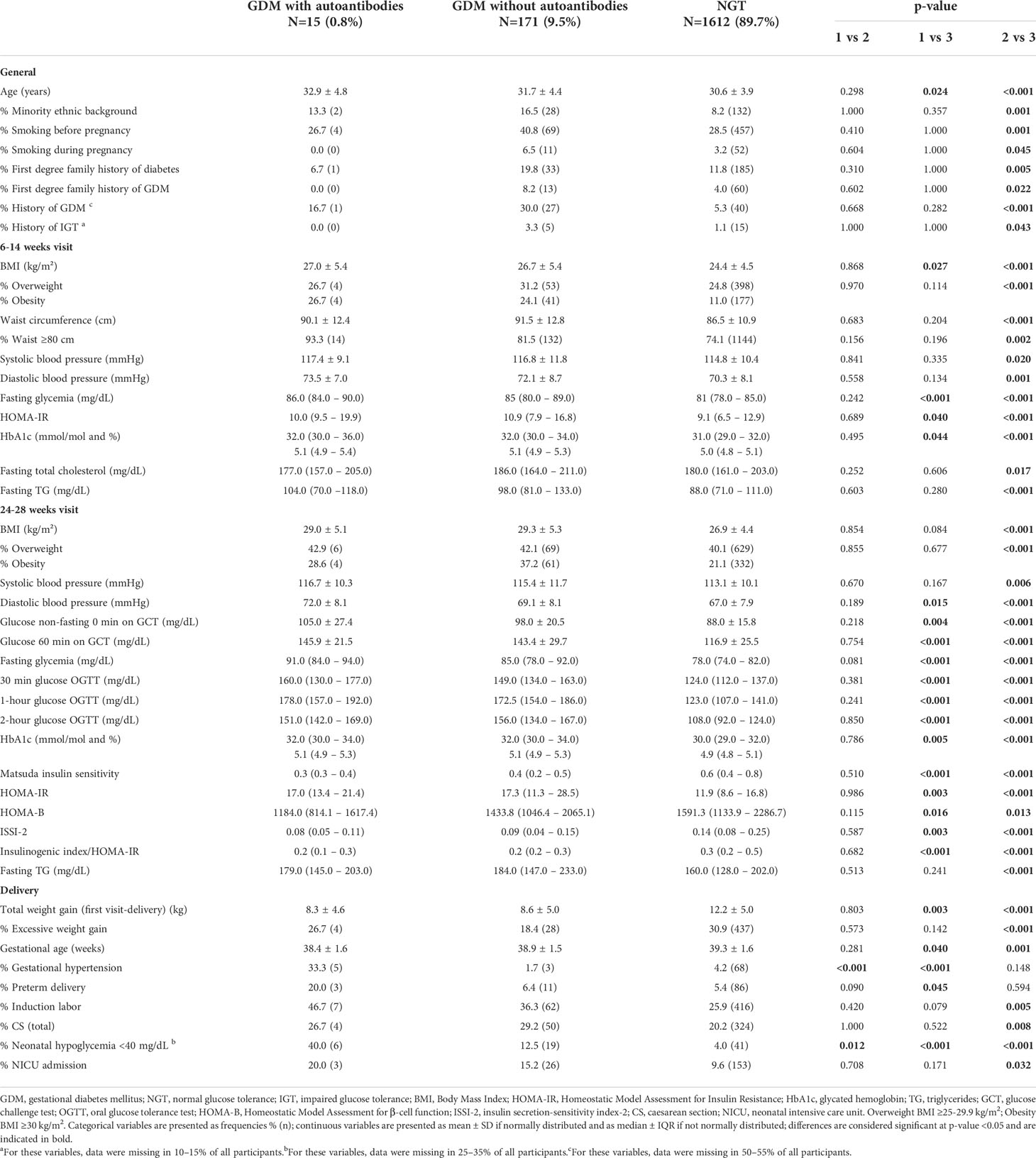
Table 2 Comparison of characteristics and pregnancy outcomes between GDM with autoantibodies (group 1), GDM without autoantibodies (group 2) and NGT women (group 3).
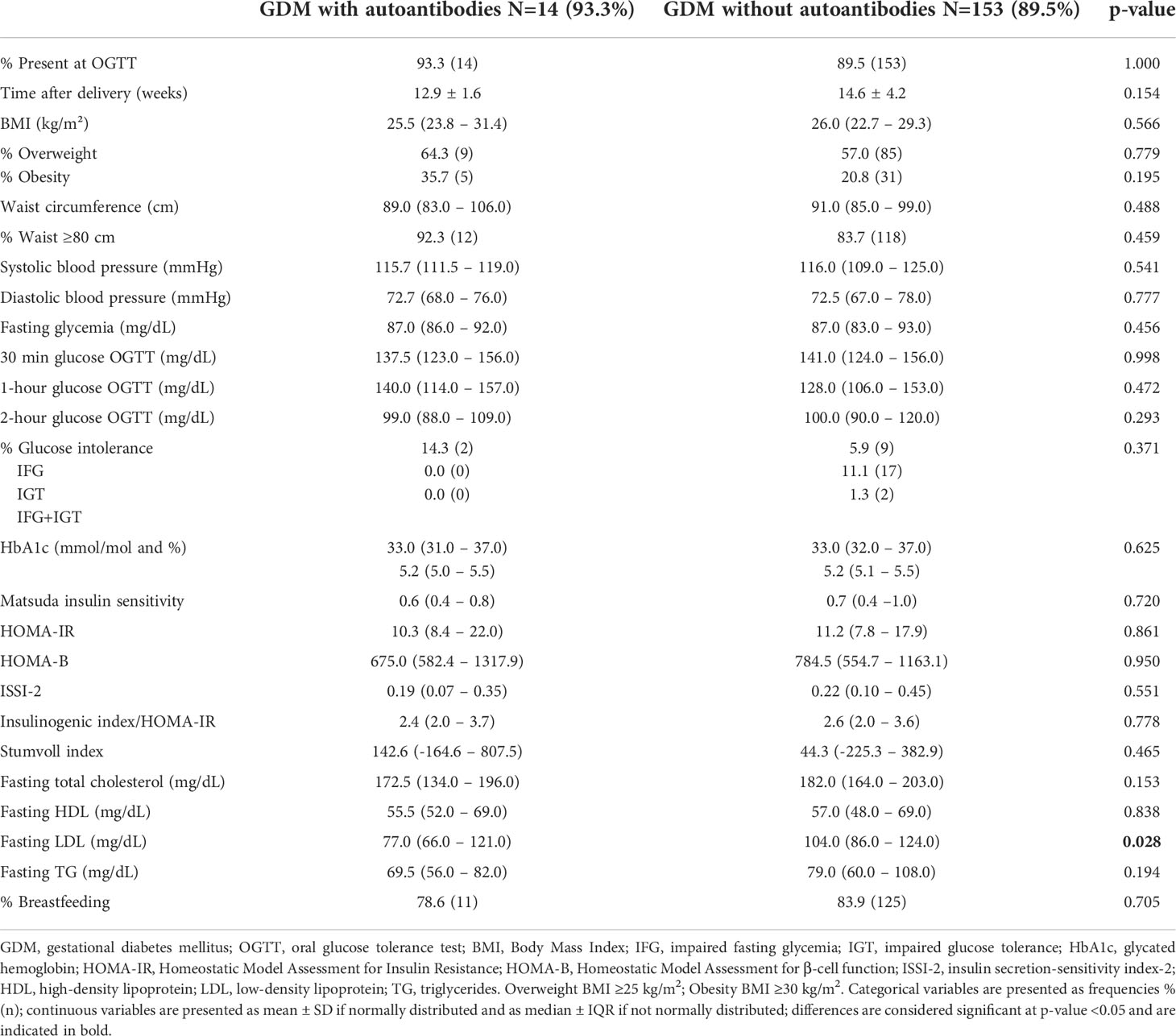
Table 3 Comparison of characteristics between GDM with autoantibodies and GDM without autoantibodies at the early postpartum OGTT.
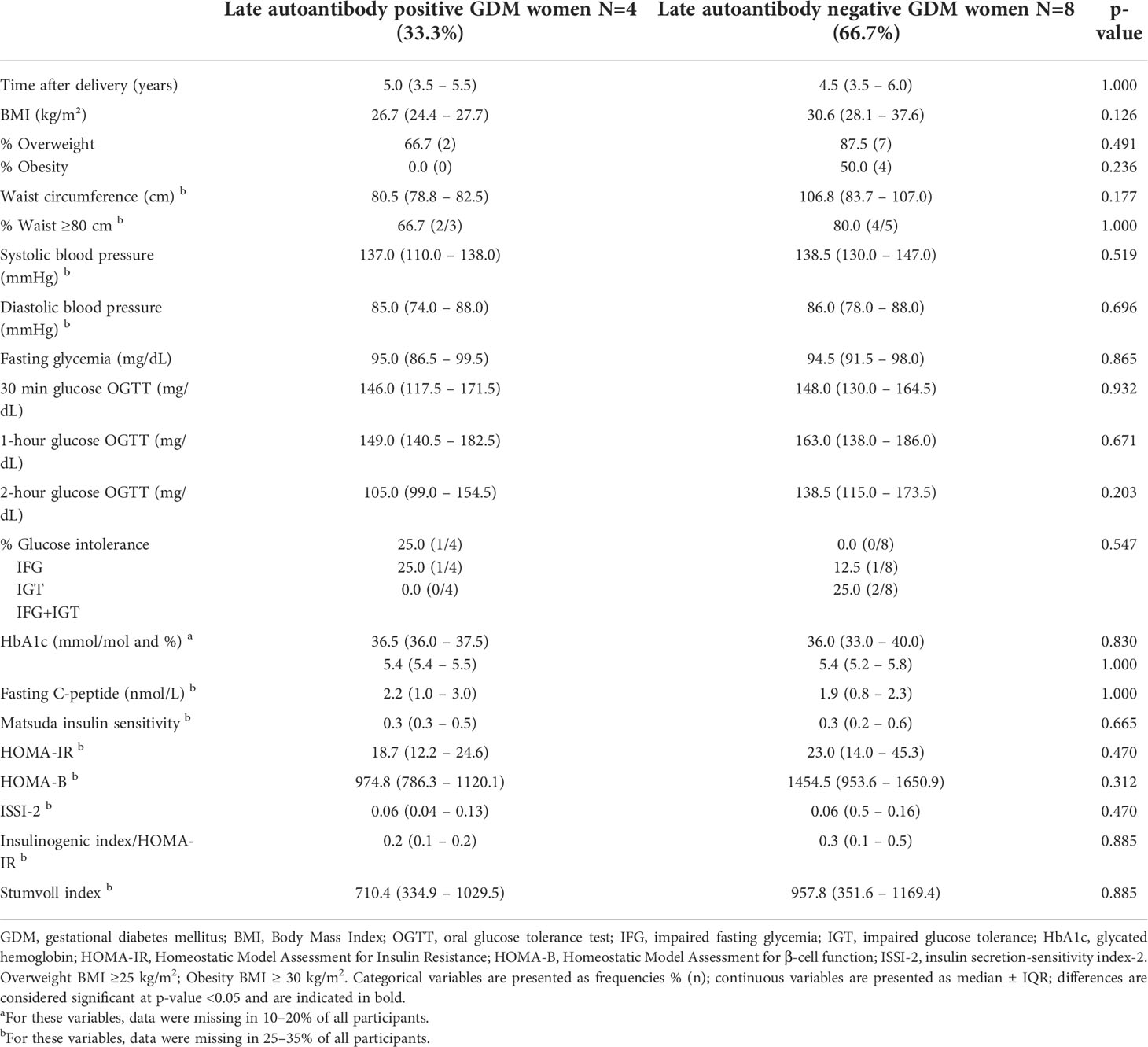
Table 4 Comparison of long-term follow-up data among women with a history of GDM and T1D-related autoantibodies in pregnancy (n=12).
Controversy still exists regarding the clinical relevance of screening for T1D-related autoantibodies during GDM pregnancy. In this large Belgian cohort, overall prevalence of autoantibodies in GDM women was 8.1%, confirming previous findings. Surprisingly, IAA was the most frequent positive autoantibody (3.8%), which is in contrast with previous observations, rarely reporting IAA in this population (14, 43). GADA positivity (1.1%) and IA-2A frequency (2.1%) in our cohort was in accordance with previous reports (0-10.8% and 0-6.2%, respectively) (13, 44). The rate of ICA positivity was rather low (1.1%). However, ICA frequency varies considerably between studies (1-35%) due to use of a not-standardized assay and consequently yielding numerous false positive results (13, 15, 16). Variations in autoantibody positivity rate in GDM women can be explained by the type of autoantibodies measured, the GDM diagnostic criteria, the ethnicity, and the T1D risk of the background population.
Antibody levels are generally lower in GDM compared to newly diagnosed T1D cases (13–15). Gestational immunomodulation may alter presence and levels of autoantibodies, possibly resulting in false negative results in pregnancy (13, 15, 21). Consequently, postpartum autoantibody re-evaluation is suggested (13, 14). We show that postpartum autoantibody re-measurement seems unwarranted in GDM women with borderline increased autoantibodies during pregnancy as these autoantibodies were negative or again only borderline increased at follow-up. In contrast, in GDM women with clinically significant increased autoantibodies during pregnancy, postpartum re-evaluation seems useful since autoantibodies further increased at follow-up. One woman, with clinical characteristics of the metabolic syndrome and no other autoimmune disorders, probably had false positive GADA during pregnancy (79.5 U/mL) as she developed glucose intolerance postpartum but without increased autoantibodies.
Overall, our data suggest that systematic screening for T1D-related autoantibodies in GDM women does not seem warranted since the low positivity rate for autoantibodies in pregnancy and postpartum. So far, there are no clear recommendations in which women with GDM it would be clinically relevant to screen for autoantibodies (13, 14). In our cohort, clinical and biochemical characteristics including insulin sensitivity and beta-cell function in pregnancy and postpartum were similar between GDM women with and without autoantibodies. We could therefore not uncover specific clinical and biochemical risk factors suggestive for autoimmune GDM. This is in line with most other studies, reporting no important dissimilarities in characteristics between both groups (14, 19, 45, 46). Only few studies could detect some characteristics associated with autoimmunity in GDM like younger age, lower BMI, lower fasting insulin level, and more frequent need for insulin therapy in pregnancy (16, 22, 47–49). These studies had several limitations, as in one study, all women with gestational hyperglycemia were considered, including women without GDM (16), while in two other studies, autoantibodies were measured at delivery (48, 49). In contrast with previous studies, we observed that GDM women with autoantibodies had more often gestational hypertension and more often neonatal hypoglycemia. This might suggest that besides hyperglycemia, autoimmunity might also affect the risk for adverse pregnancy outcomes. However, caution is warranted for interpretation of these study results due to the small sample size.
Previous studies have shown that the first two years postpartum seem to be the most critical for development of T1D (21, 49). This is in contrast with our findings, showing a very low risk for progression to T1D within 4 years postpartum (only one T1D diagnosis). We observed no differences in early postpartum rate of glucose intolerance between GDM women with and without autoantibodies. Moreover, we demonstrate that at long-term follow-up, presence of autoantibodies remained limited and only two women with glucose intolerance still showed autoantibody positivity while the majority with glucose intolerance was no longer autoantibody positive. However, rates can change rapidly and therefore an additional follow-up visit around 8-10 years postpartum might be recommended, especially in women with clinically significant increased autoantibodies in pregnancy and early postpartum.
The highest accuracy in predicting autoimmune diabetes seems to be achieved by screening with GADA (63% sensitivity) compared to ICA (48%) and IA-2A (34%), but single GADA appeared to have limited predictive power (21, 49). In our study, none showed GADA positivity at follow-up. Three women were positive for IA-2A (one with prediabetes at early postpartum and one with T1D). This confirms that IA-2A is associated with rapid β-cell dysfunction, indicating a higher risk of developing clinical signs within a shorter term (49). According to some studies, an increasing number of positive autoantibodies is highly predictive for progression towards T1D (13, 49). However, in our cohort, the only woman positive for two autoantibodies was still NGT at follow-up. In line with other studies, ZnT8A did not show additional predictive power for postpartum autoimmune diabetes in our study (13, 14, 17, 50).
A strength of this study is the availability of a large prospective cohort in pregnancy with long-term follow-up data, which allowed to evaluate various characteristics and biomarkers over time. Long-term follow-up data were available for most women with a history of GDM and autoantibodies in pregnancy from our cohort. Nevertheless, this involves a small group of GDM women with autoantibodies, though consistent with previous studies (14, 18). In future studies, estimation of optimal power for reliable statistical comparison between the groups is recommended. Another limitation is the lack of autoantibody re-evaluation in all women complicated with GDM at the early postpartum OGTT. Moreover, ZnT8A positivity was evaluated at follow-up but not during pregnancy as evidence about an association with T1D was limited at start of the study (51). Autoantibodies were not measured in women without GDM. However, in an Italian population, no significant difference in autoantibody positivity was found between women with and without GDM (5.6% vs. 8.3%, p=0.47) (14). In addition, no data were available on stimulated C-peptide. An association between autoimmunity against β-cells and other autoimmune disorders could not be assessed in our cohort due to limited data on other autoimmune diseases. Furthermore, our population was mainly Caucasian, indicating that our results might not be applicable to different ethnic populations.
Systematic screening for T1D-related autoantibodies in GDM does not seem warranted since the low positivity rate for autoantibodies in pregnancy and postpartum. At 4.6 years postpartum, five out of 12 women were glucose intolerant but only two still had autoantibodies. The clinical characteristics, including insulin resistance and β-cell function were similar, independent of presence of autoantibodies. However, in women with clinically significant increased autoantibody levels during pregnancy, postpartum autoantibody re-measurement seems useful since the high risk for further increase of autoantibody levels.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee Research UZ/KU Leuven (EC Research). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
KBen, PVC and CMa conceived the project. KBeu and CMo prepared the data and ALa did the statistical analysis. KBeu did the literature review. KBeu and KBen wrote the first draft of the manuscript. All authors contributed to the study design, including data collection, data interpretation and manuscript revision, and gave final approval to submit for publication.
This investigator-initiated study was funded by the Belgian National Lottery, the Fund of the Academic studies of UZ Leuven, and the Fund Yvonne and Jacques François-de Meurs of the King Baudouin Foundation.
KBen and RD are the recipient of a ‘Fundamenteel Klinisch Navorserschap FWO Vlaanderen’.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.973820/full#supplementary-material
1. American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes. Diabetes Care (2022). 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
2. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, et al. 2 Vol. 45. . Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care (2022) p. S17–38.
3. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med (2008) 358(19):1991–2002. doi: 10.1056/NEJMoa0707943
4. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med (2005) 352(24):2477–86. doi: 10.1056/NEJMoa042973
5. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med (2009) 361(14):1339–48. doi: 10.1056/NEJMoa0902430
6. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care (2010) 33(3):676–82. doi: 10.2337/dc10-0719
7. Benhalima K, Lens K, Bosteels J, Chantal M. The risk for glucose intolerance after gestational diabetes mellitus since the introduction of the IADPSG criteria: A systematic review and meta-analysis. J Clin Med (2019) 8(9):1431. doi: 10.3390/jcm8091431
8. Song C, Lyu Y, Li C, Liu P, Li J, Ma RC, et al. Long-term risk of diabetes in women at varying durations after gestational diabetes: A systematic review and meta-analysis with more than 2 million women. Obes Rev (2018) 19(3):421–9. doi: 10.1111/obr.12645
9. Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X. Metabolic syndrome risk after gestational diabetes: A systematic review and meta-analysis. PloS One (2014) 9(1):e87863. doi: 10.1371/journal.pone.0087863
10. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia. (2019) 62(6):905–14. doi: 10.1007/s00125-019-4840-2
11. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis primers (2019) 5(1):47. doi: 10.1038/s41572-019-0098-8
12. Saravanan P, Diabetes in Pregnancy Working Group, Maternal Medicine Clinical Study Group, Royal College of Obstetricians and Gynaecologists, UK. Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol (2020) 8(9):793–800. doi: 10.1016/S2213-8587(20)30161-3
13. Incani M, Baroni MG, Cossu E. Testing for type 1 diabetes autoantibodies in gestational diabetes mellitus (GDM): Is it clinically useful? BMC endocrine Disord (2019) 19(1):44. doi: 10.1186/s12902-019-0373-4
14. Cossu E, Incani M, Pani MG, Gattu G, Serafini C, Strazzera A, et al. Presence of diabetes-specific autoimmunity in women with gestational diabetes mellitus (GDM) predicts impaired glucose regulation at follow-up. J endocrinol Invest (2018) 41(9):1061–8. doi: 10.1007/s40618-018-0830-3
15. Lapolla A, Dalfrà MG, Fedele D. Diabetes related autoimmunity in gestational diabetes mellitus: Is it important? Nutr Metab Cardiovasc Dis (2009) 19(9):674–82. doi: 10.1016/j.numecd.2009.04.004
16. Bo S, Menato G, Pinach S, Signorile A, Bardelli C, Lezo A, et al. Clinical characteristics and outcome of pregnancy in women with gestational hyperglycaemia with and without antibodies to beta-cell antigens. Diabetes Med (2003) 20(1):64–8. doi: 10.1046/j.1464-5491.2003.00721.x
17. Rudland VL, Pech C, Harding AJ, Tan K, Lee K, Molyneaux L, et al. Zinc transporter 8 autoantibodies: What is their clinical relevance in gestational diabetes? Diabet Med (2015) 32(3):359–66. doi: 10.1111/dme.12629
18. Yu SH, Park S, Kim HS, Park SY, Yim CH, Han KO, et al. The prevalence of GAD antibodies in Korean women with gestational diabetes mellitus and their clinical characteristics during and after pregnancy. Diabetes/metabol Res Rev (2009) 25(4):329–34. doi: 10.1002/dmrr.963
19. Mauricio D, Balsells M, Morales J, Corcoy R, Puig-Domingo M, de Leiva A. Islet cell autoimmunity in women with gestational diabetes and risk of progression to insulin-dependent diabetes mellitus. Diabetes Metab Rev (1996) 12(4):275–85. doi: 10.1002/(SICI)1099-0895(199612)12:4<275::AID-DMR170>3.0.CO;2-W
20. Jarvela IY, Juutinen J, Koskela P, Hartikainen AL, Kulmala P, Knip M, et al. Gestational diabetes identifies women at risk for permanent type 1 and type 2 diabetes in fertile age: Predictive role of autoantibodies. Diabetes Care (2006) 29(3):607–12. doi: 10.2337/diacare.29.03.06.dc05-1118
21. Nilsson C, Ursing D, Törn C, Aberg A, Landin-Olsson M. Presence of GAD antibodies during gestational diabetes mellitus predicts type 1 diabetes. Diabetes Care (2007) 30(8):1968–71. doi: 10.2337/dc07-0157
22. Wucher H, Lepercq J, Timsit J. Onset of autoimmune type 1 diabetes during pregnancy: Prevalence and outcomes. Best Pract Res Clin Endocrinol Metab (2010) 24(4):617–24. doi: 10.1016/j.beem.2010.06.002
23. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. A modified two-step screening strategy for gestational diabetes mellitus based on the 2013 WHO criteria by combining the glucose challenge test and clinical risk factors. J Clin Med (2018) 7(10):351. doi: 10.3390/jcm7100351
24. Benhalima K, Van Crombrugge P, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, et al. The Belgian diabetes in pregnancy study (BEDIP-n), a multi-centric prospective cohort study on screening for diabetes in pregnancy and gestational diabetes: Methodology and design. BMC Pregnancy Childbirth (2014) 14:226. doi: 10.1186/1471-2393-14-226
25. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. The sensitivity and specificity of the glucose challenge test in a universal two-step screening strategy for gestational diabetes mellitus using the 2013 world health organization criteria. Diabetes Care (2018) 41(7):e111–e2. doi: 10.2337/dc18-0556
26. Benhalima K, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia. (2019) 62(11):2118–28. doi: 10.1007/s00125-019-4961-7
27. American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care (2013) 36 Suppl 1:S11–66. doi: 10.2337/dc13-S011
28. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A world health organization guideline. Diabetes Res Clin Pract (2014) 103(3):341–63. doi: 10.1016/j.diabres.2013.10.012
29. Vermeulen I, Weets I, Costa O, Asanghanwa M, Verhaeghen K, Decochez K, et al. An important minority of prediabetic first-degree relatives of type 1 diabetic patients derives from seroconversion to persistent autoantibody positivity after 10 years of age. Diabetologia. (2012) 55(2):413–20. doi: 10.1007/s00125-011-2376-1
30. Vandewalle CL, Falorni A, Svanholm S, Lernmark A, Pipeleers DG, Gorus FK, et al. High diagnostic sensitivity of glutamate decarboxylase autoantibodies in insulin-dependent diabetes mellitus with clinical onset between age 20 and 40 years. J Clin Endocrinol Metab (1995) 80(3):846–51. doi: 10.1210/jcem.80.3.7883841
31. Vandewalle CL, Decraene T, Schuit FC, De Leeuw IH, Pipeleers DG, Gorus FK, et al. Insulin autoantibodies and high titre islet cell antibodies are preferentially associated with the HLA DQA1*0301-DQB1*0302 haplotype at clinical type 1 (insulin-dependent) diabetes mellitus before age 10 years, but not at onset between age 10 and 40 years. Diabetologia. (1993) 36(11):1155–62. doi: 10.1007/BF00401060
32. Gorus FK, Goubert P, Semakula C, Vandewalle CL, De Schepper J, Scheen A, et al. IA-2-autoantibodies complement GAD65-autoantibodies in new-onset IDDM patients and help predict impending diabetes in their siblings. Diabetologia. (1997) 40(1):95–9. doi: 10.1007/s001250050648
33. De Grijse J, Asanghanwa M, Nouthe B, Albrecher N, Goubert P, Vermeulen I, et al. Predictive power of screening for antibodies against insulinoma-associated protein 2 beta (IA-2beta) and zinc transporter-8 to select first-degree relatives of type 1 diabetic patients with risk of rapid progression to clinical onset of the disease: implications for prevention trials. Diabetologia. (2010) 53(3):517–24. doi: 10.1007/s00125-009-1618-y
34. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883
35. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care (1999) 22(9):1462–70. doi: 10.2337/diacare.22.9.1462
36. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. (2003) 46(1):3–19. doi: 10.1007/s00125-002-1009-0
37. Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: Validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care (2001) 24(9):1602–7. doi: 10.2337/diacare.24.9.1602
38. Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabetes Med (2009) 26(12):1198–203. doi: 10.1111/j.1464-5491.2009.02841.x
39. Devlieger H, Martens G, Bekaert A, Eeckels R. Standaarden van geboortegewicht-voor-zwangerschapsduur voor de vlaamse boreling. Tijdschrift voor geneeskunde (2000) 56:1–14. doi: 10.2143/TVG.56.1.5000625
40. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US) (2009).
41. Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care (2001) 24(4):796–7. doi: 10.2337/diacare.24.4.796
42. Vermeulen I, Weets I, Asanghanwa M, Ruige J, Van Gaal L, Mathieu C, et al. Contribution of antibodies against IA-2β and zinc transporter 8 to classification of diabetes diagnosed under 40 years of age. Diabetes Care (2011) 34(8):1760–5. doi: 10.2337/dc10-2268
43. Vandewalle CL, Falorni A, Lernmark A, Goubert P, Dorchy H, Coucke W, et al. Associations of GAD65- and IA-2- autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes Care (1997) 20(10):1547–52. doi: 10.2337/diacare.20.10.1547
44. de Leiva A, Mauricio D, Corcoy R. Diabetes-related autoantibodies and gestational diabetes. Diabetes Care (2007) 30 Suppl 2:S127–33. doi: 10.2337/dc07-s204
45. Dozio N, Beretta A, Belloni C, Castiglioni M, Rosa S, Bosi E, et al. Low prevalence of islet autoantibodies in patients with gestational diabetes mellitus. Diabetes Care (1997) 20(1):81–3. doi: 10.2337/diacare.20.1.81
46. Fallucca F, Di Mario U, Gargiulo P, Iavicoli M, Galfo C, Contreas G, et al. Humoral immunity in diabetic pregnancy: Interrelationships with maternal/neonatal complications and maternal metabolic control. Diabete Metab (1985) 11(6):387–95.
47. Murgia C, Orrù M, Portoghese E, Garau N, Zedda P, Berria R, et al. Autoimmunity in gestational diabetes mellitus in Sardinia: A preliminary case-control report. Reprod Biol Endocrinol (2008) 6:24. doi: 10.1186/1477-7827-6-24
48. Lobner K, Knopff A, Baumgarten A, Mollenhauer U, Marienfeld S, Garrido-Franco M, et al. Predictors of postpartum diabetes in women with gestational diabetes mellitus. Diabetes. (2006) 55(3):792–7. doi: 10.2337/diabetes.55.03.06.db05-0746
49. Füchtenbusch M, Ferber K, Standl E, Ziegler AG. Prediction of type 1 diabetes postpartum in patients with gestational diabetes mellitus by combined islet cell autoantibody screening: A prospective multicenter study. Diabetes. (1997) 46(9):1459–67. doi: 10.2337/diab.46.9.1459
50. Dereke J, Palmqvist S, Nilsson C, Landin-Olsson M, Hillman M. The prevalence and predictive value of the SLC30A8 R325W polymorphism and zinc transporter 8 autoantibodies in the development of GDM and postpartum type 1 diabetes. Endocrine. (2016) 53(3):740–6. doi: 10.1007/s12020-016-0932-7
Keywords: gestational diabetes mellitus, autoimmune antibodies, type 1 diabetes mellitus, pregnancy, follow-up, long-term risk, glucose intolerance
Citation: Beunen K, Vercauter L, Van Crombrugge P, Moyson C, Verhaeghe J, Vandeginste S, Verlaenen H, Vercammen C, Maes T, Dufraimont E, Roggen N, De Block C, Jacquemyn Y, Mekahli F, De Clippel K, Van Den Bruel A, Loccufier A, Laenen A, Devlieger R, Mathieu C and Benhalima K (2022) Type 1 diabetes-related autoimmune antibodies in women with gestational diabetes mellitus and the long-term risk for glucose intolerance. Front. Endocrinol. 13:973820. doi: 10.3389/fendo.2022.973820
Received: 20 June 2022; Accepted: 04 August 2022;
Published: 24 August 2022.
Edited by:
Ihtisham Bukhari, Henan Provincial People’s Hospital, ChinaReviewed by:
Shuoming Luo, Second Xiangya Hospital, Central South University, ChinaCopyright © 2022 Beunen, Vercauter, Van Crombrugge, Moyson, Verhaeghe, Vandeginste, Verlaenen, Vercammen, Maes, Dufraimont, Roggen, De Block, Jacquemyn, Mekahli, De Clippel, Van Den Bruel, Loccufier, Laenen, Devlieger, Mathieu and Benhalima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaat Beunen, a2FhdC5iZXVuZW5Aa3VsZXV2ZW4uYmU=
†ORCID ID: Kaat Beunen, orcid.org/0000-0003-3557-0022
Katrien Benhalima, orcid.org/0000-0002-3325-0263
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.