- Department of Thyroid Surgery, The First Hospital of China Medical University, Shenyang, China
Objective: Sex-specific thyroid cancer risk exists in patients diagnosed with diabetes mellitus (DM). However, thyroid cancer risk in different types of DM is still unclear. This meta-analysis aims to identify the real correlation between different types of DM and thyroid cancer risk in both sexes.
Methods: Studies were identified by an electronic search of PubMed, EMBASE, and Cochrane Library on 16 January 2022. A random-effects model was used to estimate the relative risks (RRs). The Cochran’s Q and I2 statistics were computed to detect heterogeneity between studies.
Results: In comparison with non-DM counterparts, patients with DM had a 1.32-fold higher risk of thyroid cancer (95% CI, 1.22–1.44) with 1.26-fold (95% CI, 1.12–1.41) in men and 1.36-fold (95% CI, 1.22–1.52) in women, respectively. Subgroup analysis by the type of DM showed that the RR of thyroid cancer in patients with type 2 diabetes was 1.34 (95% CI, 1.17–1.53) in the study population with 1.32 (95% CI, 1.12–1.54) in men and 1.37 (95% CI, 1.12–1.68) in women, respectively; the RR of thyroid cancer was 1.30 (95% CI, 1.17–1.43) in patients with gestational diabetes; the risk of thyroid cancer in patients with type 1 diabetes was 1.51-fold in women but not in men. Although there were some heterogeneities, it did not affect the above results of this study.
Conclusion: This study indicates that, compared with non-DM individuals, patients with any type of DM have an elevated thyroid cancer risk. This positive correlation between type 2 diabetes and thyroid cancer risk exists in both men and women.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, CRD42022304028.
Introduction
The incidence of thyroid cancer has increased greatly in the past 3 decades (1).Thyroid cancer is the most popular malignancy in endocrine system, accounting for an estimated 43,800 new cases with threefold higher overall incidence rates in American women in 2022 (2). On the basis of the latest Chinese cancer statistics, thyroid cancer is the fourth most frequent cancer in women with its incidence rising by 12.4% every year (3, 4). The rapidly increased incidence of thyroid cancer is considered to be predominantly owing to overdiagnosis. However, it is not yet clear whether the epidemic of thyroid cancer is also caused by exposure to certain risk factors (5).
Risk factors of thyroid cancer have not yet been established. Ionizing radiation and family history of thyroid cancer are the accepted risk factors of thyroid cancer. Additional potential risk factors include obesity and reproductive and environmental factors (6–8). The potential impact of other risk factors for thyroid carcinogenesis warrants further exploration. Here, we explore the possible impact of diabetes mellitus (DM) on subsequent thyroid cancer.
DM represents one of the most rapidly increasing global public health problems. The worldwide prevalence of DM is estimated to grow from 2.8% to 4.4% between 2000 and 2030 and projected to be one of the five leading contributors to disease burden by 2030 (9). China has the largest number of DM people in the world with the rise in prevalence from 0.9% in 1980 to 10.9% in 2013 and more than 90% of whom have type 2 diabetes (T2D) (10, 11). DM has been positively associated with the risk of cancer at different sites, including prostate, oral, breast, and pancreas (12–15). The prevalence of both DM and thyroid cancer has increased all over the world, providing theoretic support that DM may be a driver for thyroid cancer, although early studies did not find an association between them (16). The insufficient cancer cases among the exposed group might account for the lack of association. Furthermore, all of these studies were launched in the USA, possibly leading to the insufficiency of the heterogeneity of exposure and the power of statistics. Two recent meta-analyses have illustrated that the risk of thyroid cancer in female patients with DM has significantly increased in comparison with their non-diabetic counterparts (17, 18). However, there are still some shortcomings in these studies. First, the type of DM was not differentiated and thyroid cancer risk caused by various kinds of DM could not be highlighted. Second, the previous meta-analysis included patients with metabolic syndrome, which may lead to confounding bias. Furthermore, the correlation between DM and thyroid cancer in male patients is not clear, owing to the small sample size. Thus, this meta-analysis was designed to determine the association between various kinds of DM and the risk of thyroid cancer and whether the discrepancy exists in sex based on the available cohort studies.
Methods
This study was designed and carried out according to the guidelines for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) (19). The protocol has been registered in the International Prospective Register of Systematic Reviews platform. The registration number of this meta-analysis is CRD42022304028.
Search strategy
A literature search-up was conducted using Cochrane Library, EMBASE, and PubMed and without language restrictions from databases on 16 January 2022. Medical Subject Heading (MESH) terms and keywords were (“Diabetes Mellitus” OR “Diabetes”) AND (“Thyroid Tumor” OR “Thyroid Neoplasm*” OR “Thyroid Adenoma*” OR “Thyroid Cancer*” OR “Thyroid Carcinoma*”). Furthermore, a manual screening of the reference lists of selected studies was also conducted to find extra potentially relevant studies. The integrated search strategy is shown in the Appendix (Table 1–3).
Eligibility criteria
Studies were included if the following criteria were met (1): cohort studies, whether prospective or retrospective (2); the exposed group could be patients with any type of DM, the most common being T2D, T1D, or gestational diabetes (GD), and the control group consisted of non-DM individuals (3); thyroid cancer risk as the outcome that expressed as a hazard ratio (HR), adjusted odds ratio (OR), relative risk (RR), or standardized incidence ratio (SIR); (4) thyroid cancer should be a risk occurring naturally under observation, and drug intervention to reduce thyroid cancer risk was not considered.
Exclusion criteria
The exclusion criteria were (1) conference abstracts or erratum, (2) duplicate published studies based on the same observation population, and (3) incomplete data or no interested outcome.
Research selection
The eligible studies were independently screened by two investigators (Wenwu Dong and Dalin Zhang). First, they excluded duplicate and irrelevant literatures by title and abstract. Then, each of them independently read the full text of each potentially eligible article and finally identified all studies. Any disagreement was resolved by discussion with a third investigator (Hao Zhang) until consensus was achieved.
Data extraction
Two investigators (Wenwu Dong and Dalin Zhang) independently extracted data. The following pertinent information was included: name of first author, country, publication year, sample size, DM type, cases of thyroid cancer, mean age at baseline, follow-up years, diagnosis criteria of thyroid cancer, and adjusted confounders (20).
Risk-of-bias assessment
The quality of the selected studies was assessed on the basis of the Newcastle-Ottawa Quality Assessment Scale (NOS). The range of scores was from 0 to 9, and the higher the score, the higher the study quality. NOS scores ≥ 7, 4–6, and 0–3 mean high, medium, and low quality, respectively.
Statistical analysis
SIR, RR, HR, or adjusted OR and 95% confidence intervals (CI) were extracted to estimate thyroid cancer risk in different types of DM. Because of the low attack rate of thyroid cancer, RR is approximately equal to OR, HR, and SIR, so a pooled analysis can be performed. Heterogeneity was assessed using the chi-square test and I2 value. P-value < 0.1 or I2 > 50% was considered to indicate significant heterogeneity, and random-effects model was adopted. Otherwise, a fixed-effects model was employed. The sensitivity analysis was conducted to examine the robustness of our results. Subgroup analysis was conducted for different types of DM in different sex. Finally, funnel plots and Egger’s regression test were conducted to assess publication bias. A two-sided P-value less than 0.05 was considered significant. Statistical analyses were performed using the Stata software (version 15.1).
Results
Literature search
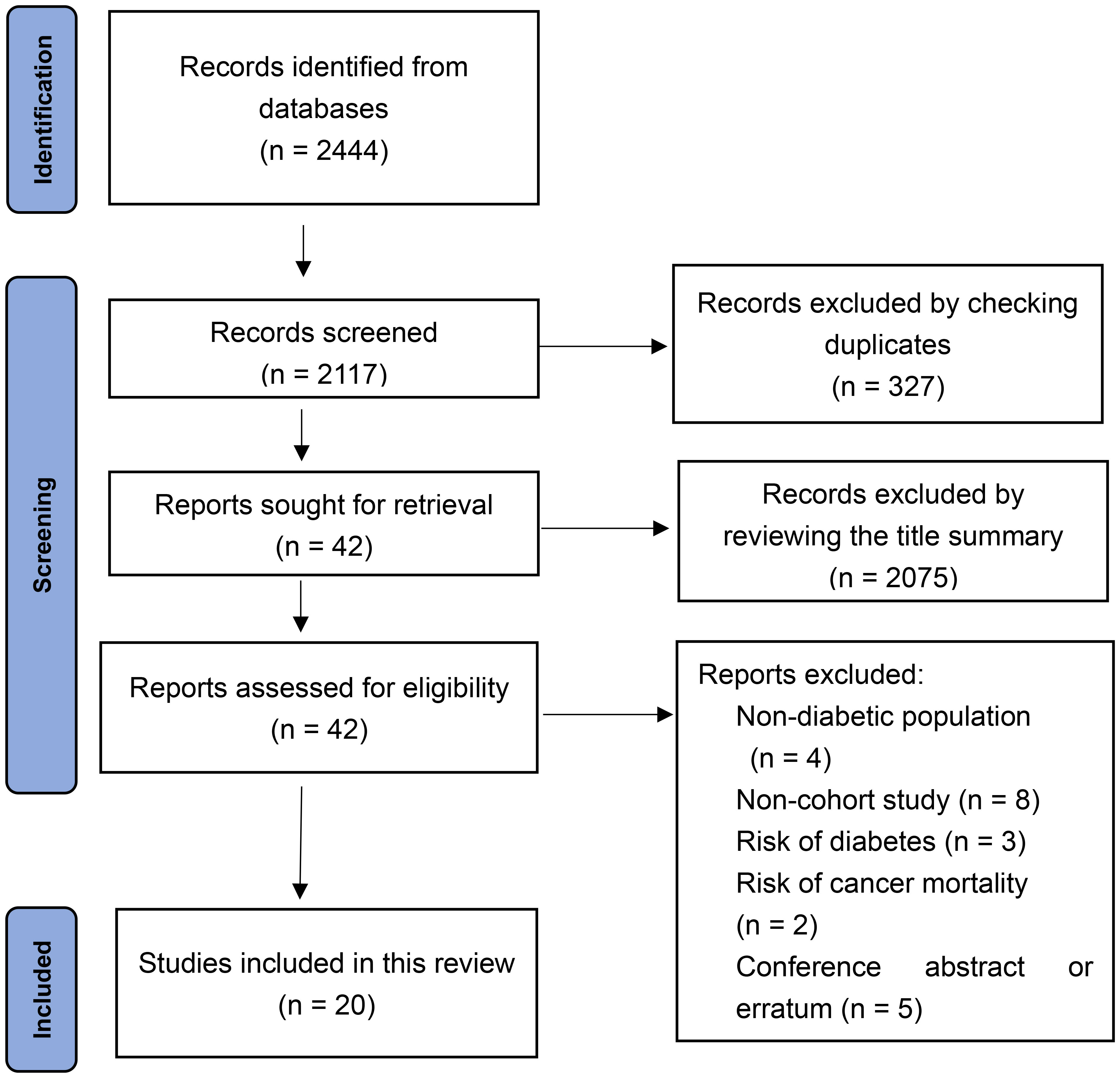
From the electronic search, a total of 2,444 records were identified. A total of 327 articles were excluded by checking duplicates. Another 2,075 articles were excluded after reviewing the title summary. The 42 remaining articles were examined in full text. Finally, 20 cohort studies were included according to the inclusion criteria (16, 21–39). The literature search algorithm is shown in Figure 1.
Study characteristics
The included cohort studies (16, 21–39) were published between 1991 and 2019 from nine countries and regions, including Europe, Asia, and America, with the largest number of studies from Asia. Types of DM varied in these cohort studies. T2D (21, 24–26, 36–38) was the most frequently studied, followed by GD (22, 23, 27, 28). Three studies (34, 35, 39) did not identify the type of DM, and only one (29) reported thyroid cancer risk in patients with T1D. The sample size of the included studies was greater than 300,000, and a total of 11,091 cases of thyroid cancer occurred during follow-up. The mean follow-up time ranged from 3.0 to 20.8 years. Most studies on the diagnosis criteria of thyroid cancer were in accordance with International Classification of Diseases (ICD), and the confounding factors (e.g., sex and body mass index) were well controlled. The baseline characteristics of the included studies are presented in Table 1.
Quality assessment
Specific assessments with the NOS scores are shown in Table 1. Six studies with a score of 6 were deemed of moderate quality, and 14 studies with a score of ≥ 7 were classified as high quality. The mean score was seven points, suggesting a high overall quality.
Sex-specific risk of thyroid cancer in any type of DM
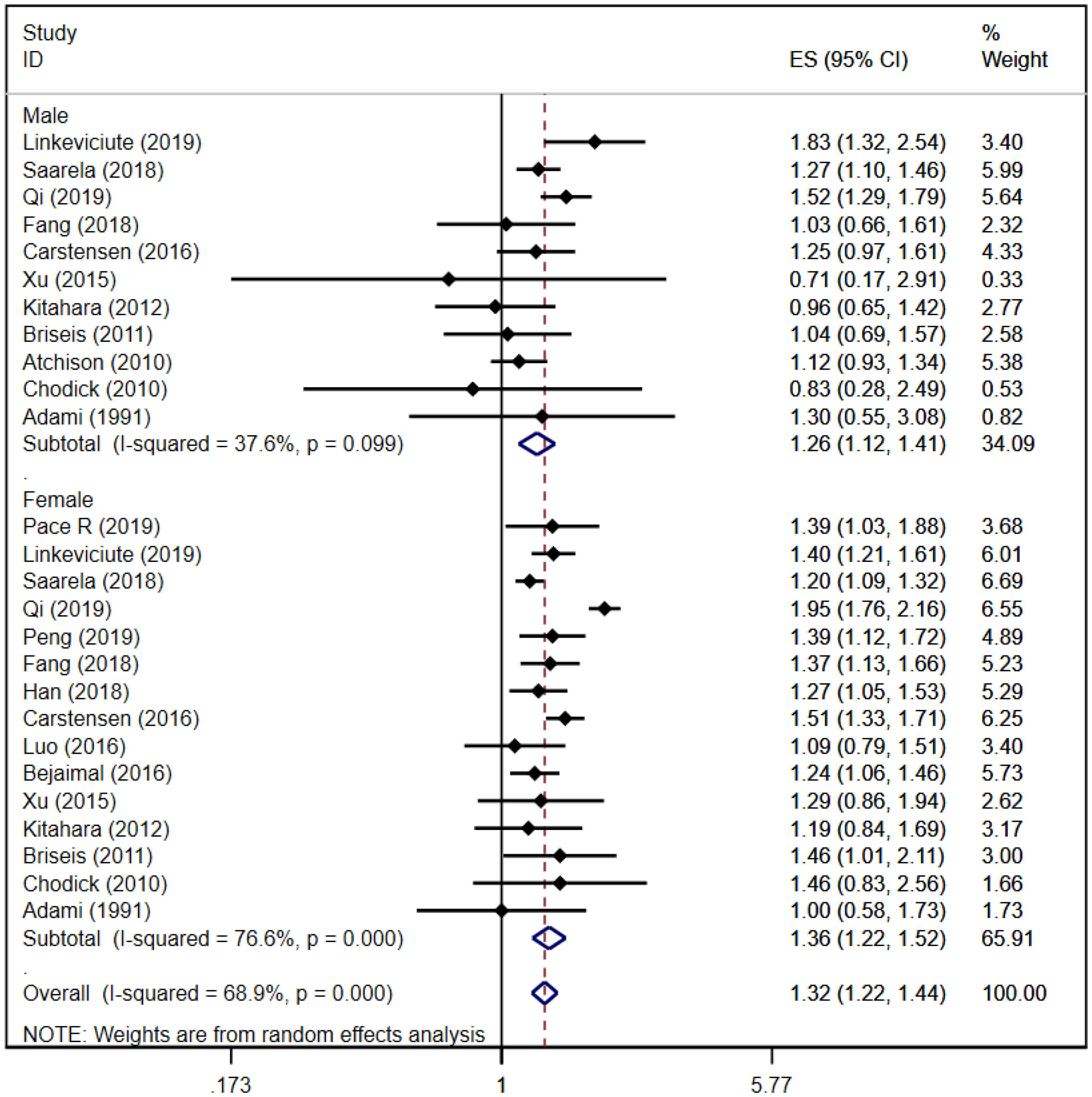
A total of 11 (16, 21, 24–26, 29, 32, 34, 35, 37, 39) and 15 (16, 21–29, 32, 34, 35, 37, 39) cohort studies reported that thyroid cancer risk in male and female patients with DM, respectively. The pooled analysis showed that thyroid cancer risk in male patients was [RR = 1.26, 95% CI (1.12, 1.41), I2 = 36.7%, P = 0.000]; thyroid cancer risk in female patients was [RR = 1.36, 95% CI (1.22, 1.52), I2 = 76.6%, P = 0.000]; and the total thyroid cancer risk in the overall study populations was [RR = 1.32, 95% CI (1.22, 1.44), I2 = 68.9%, P = 0.000]. The risk of thyroid cancer in female patients was slightly higher than that in male patients. Forest plots of sex-specific thyroid cancer risk in any type of DM is shown in Figure 2. Because of some heterogeneity, sensitivity analyses were performed by sequentially excluding each study in the pooled analysis. The results did not affect the overall conclusions, suggesting that the conclusions of this study are reliable. Sensitivity analysis plot is in Appendix Figure 1.
Risk of thyroid cancer in T2D
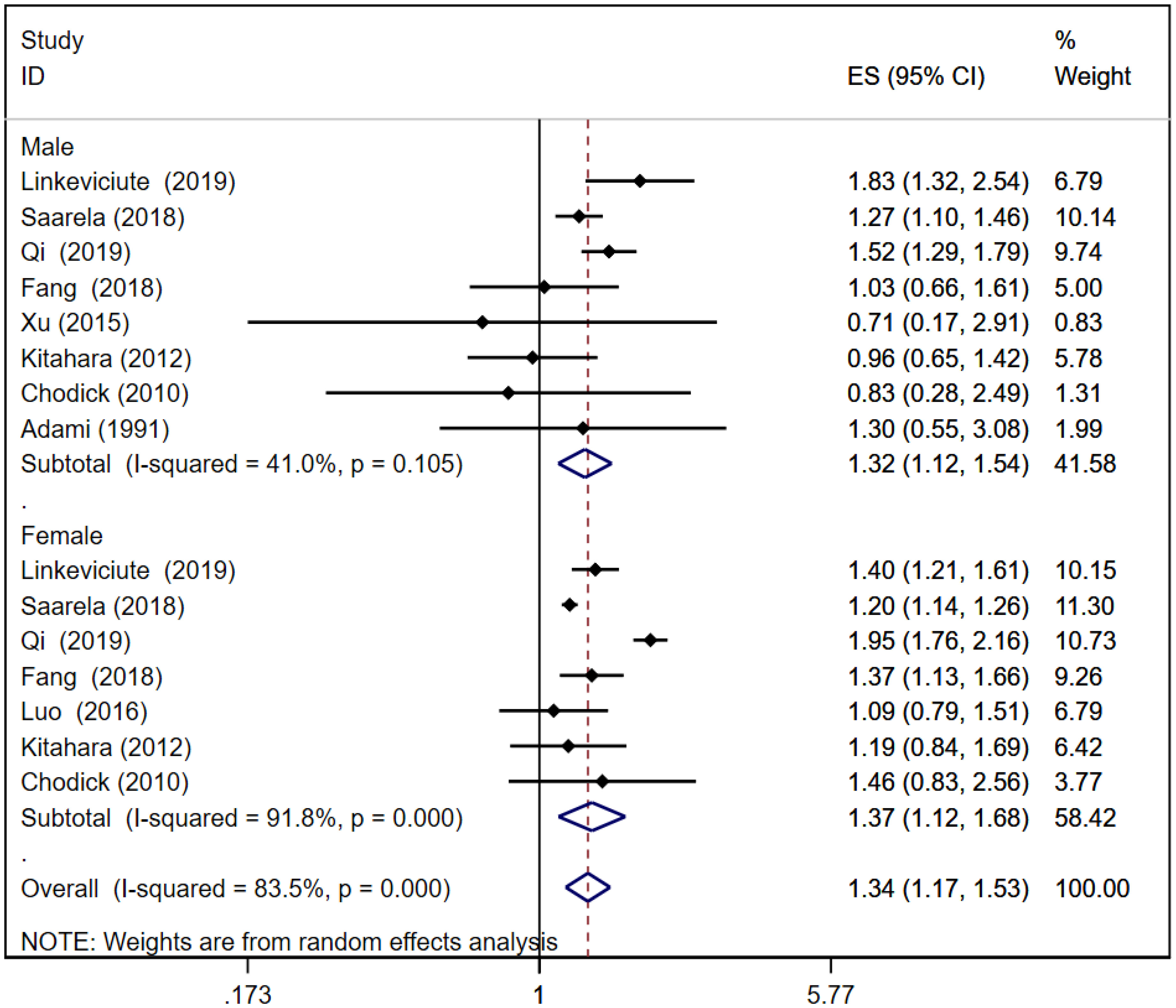
A total of eight (16, 21, 24–26, 32, 37, 39) and seven (16, 21, 24–26, 31, 37) cohort studies reported thyroid cancer risk in male and female patients with T2D, respectively. The pooled analysis showed that thyroid cancer risk in male patients was [RR = 1.32, 95% CI (1.12, 1.54), I2 = 41.0%, P = 0.001]; thyroid cancer risk in female patients was [RR = 1.37, 95% CI (1.12, 1.68), I2 = 91.8%, P = 0.002]; and the total thyroid cancer risk in T2D patients was [RR = 1.34, 95% CI (1.17, 1.53), I2 = 83.5%, P = 0.000]. In T2D, thyroid cancer risk in female patients was slightly higher than that in male patients. Forest plots of thyroid cancer risk in T2D is shown in Figure 3. Because of large heterogeneity, sensitivity analyses were performed by excluding each study sequentially from the pooled analysis, but the overall conclusion was not affected, suggesting that the results of our meta-analysis are robust. Sensitivity analysis plot is presented in Appendix Figure 2.
Risk of thyroid cancer in GD
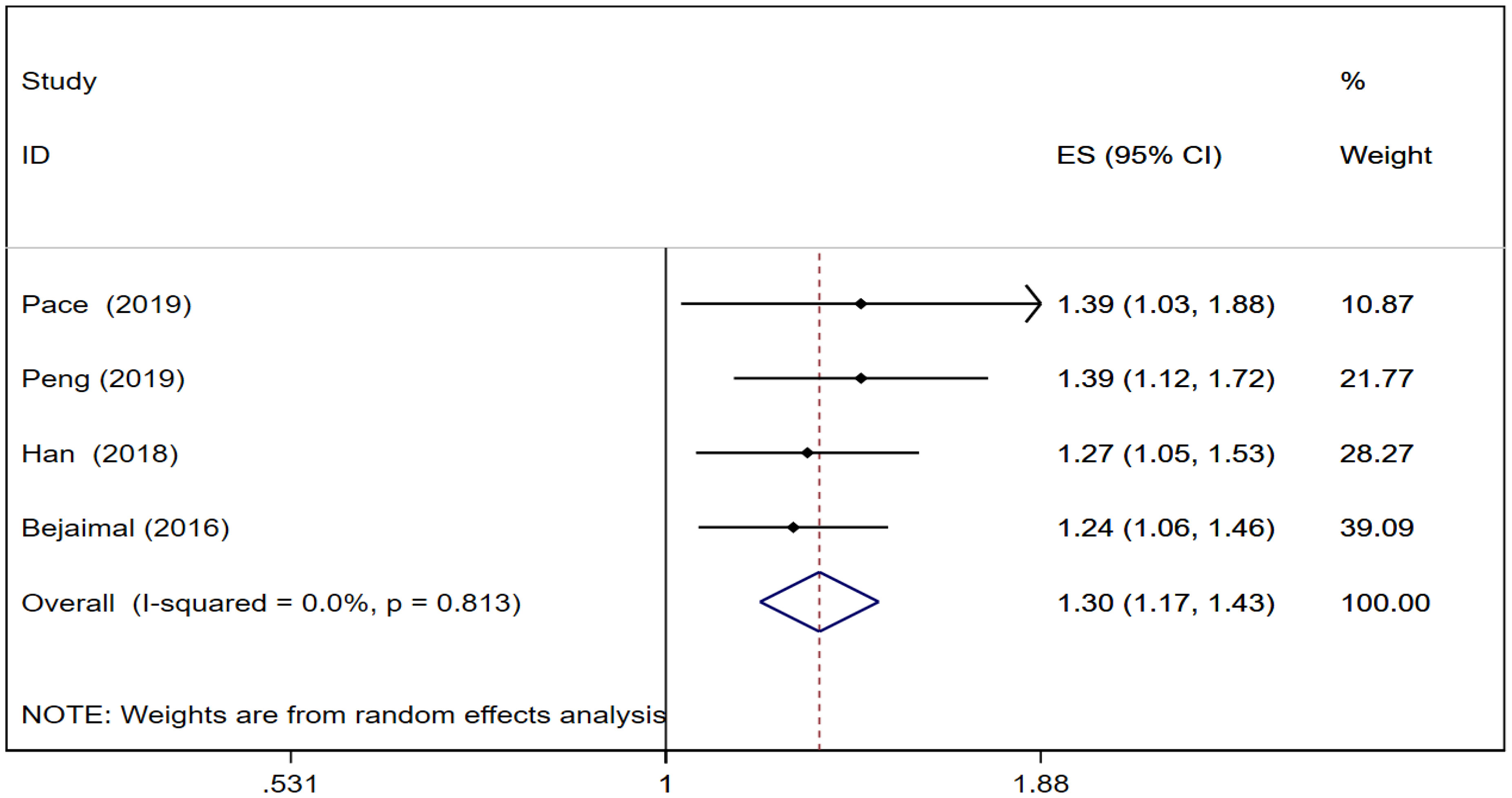
Four cohort studies (22, 23, 27, 28) reported thyroid cancer risk in GD. The pooled analysis showed that thyroid cancer risk was [RR = 1.30, 95% CI (1.17, 1.43), I2 = 0.0%, P = 0.000]. Forest plots of thyroid cancer risk in GD is shown in Figure 4.
Risk of thyroid cancer in T1D
Only one cohort study (29) reported that the risk of thyroid cancer increased in patients with T1D. Among them, no association was found between male patients and the risk of thyroid cancer. The risk of thyroid cancer in female patients was 1.51-fold.
Publication bias
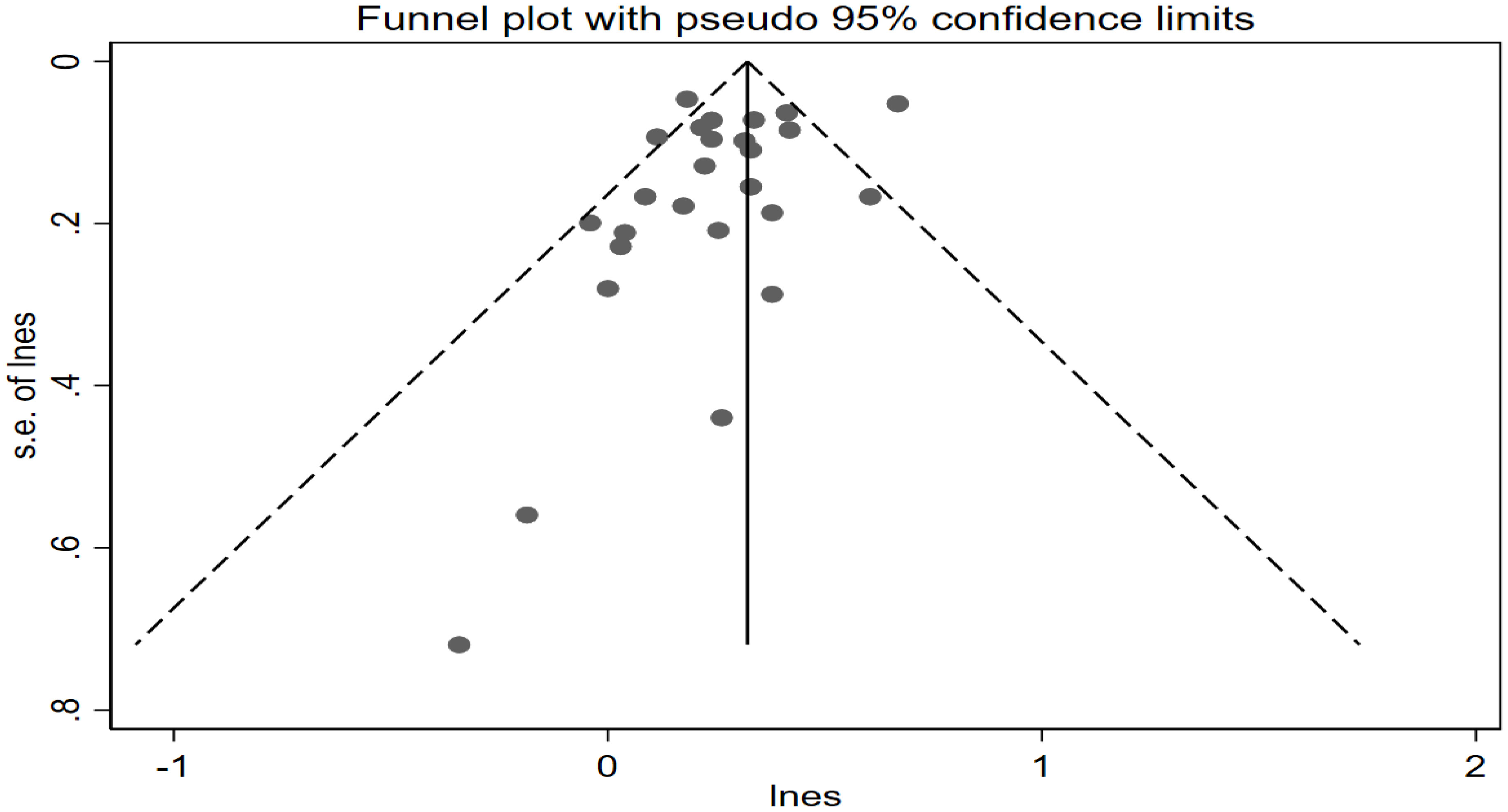
No evidence of publication bias was observed in the visual distribution of the funnel plot (Figure 5). The result of Egger’s regression tests is P = 0.108 ≥ 0.05, indicating no publication bias in this meta-analysis.
Discussion
This meta-analysis of 20 cohort studies included more than 300,000 individuals, providing evidence that thyroid cancer risk increased approximately 30% in DM for the entire study populations, with a 36% increase among female patients and a 26% increase among male patients. The increased thyroid cancer risk varies among patients with different kinds of DM with 34%, 30%, and 51% in patients with T1D, GD, and T2D, respectively. Unexpectedly, the correlation between T2D and thyroid cancer risk existed not only in female patients but also in male patients. This contradicts previous reports that speculated that this correlation between DM and thyroid cancer was prominent in female patients but not in male patients (17, 18). Our positive findings in men were mainly due to the more accurate patient enrolment, the subgroup analysis of different types of DM and the enlargement of sample size.
The global prevalence of DM in 2017 was 8.8% with a further increase expected to 9.9% by the year 2045, nearly 90% of which was T2D (40). Our findings indicated that T2D was a risk factor for thyroid cancer in both male and female patients, and therefore, male sex was not a protective factor for thyroid cancer in patients with T2D. However, the association between T1D and the risk of thyroid cancer was evident in female patients but not in male patients reported by only one cohort study (29). Thus, more research needs to be undertaken to confirm the findings and to explore the underlying molecular mechanisms. GD was also a highly prevalent condition affecting 9.3%–25.5% of pregnant women (41). Thus, pregnant women were potential susceptible population of thyroid cancer. Understanding potential sex discrepancy in risk of thyroid cancer is critical from both a population health and clinical perspective. From the perspective of public health, assessment of sex differences sends messages to targeted public health and is conducive to draw projections of the future disease burden of thyroid cancer and estimating relevant public health costs. Clinically, knowledge of sex differences may contribute to patient selection for thyroid cancer screening.
Some molecular biological mechanisms may explain this correlation. First, chronic elevated insulin levels observed in patients with DM may influence thyroid cancer risk, which was mediated by insulin receptors overexpressed in cancer cells and tissues (42). Insulin may activate insulin and insulin-like growth factor-1 (IGF-1) pathway to inhibit cell apoptosis and promote proliferation. Insulin may also activate mitogen-activated protein kinase (MAPK) and the phosphatidylinositide 3-kinase pathways by mimicking IGF-1 and binding to IGF-1 receptor to promote thyroid carcinogenesis (43). This also reflects that the presence of T2D is associated with insulin resistance, leptin resistance, increased oxidized low-density lipoprotein cholesterol, and obesity, which are present, but to a lesser extent, in T1D. Furthermore, the majority of tumors occurred in patients with T2D, postulating that cancer mainly exists in the older people, in which T2D is more frequent (44). Therefore, an increase in thyroid cancer incidence in T1D may be an artefact of diagnosis because clinicians may pay more medical attention to these patients than non-DM individuals (29). In addition, exogenous insulin is an absolute demand for patients with T1D because they lack endogenous insulin. Moreover, unlike patients with T2D, patients with T1D lack a long prediabetes and diabetes history with compensatory endogenous hyperinsulinemia. As hyperinsulinemia decreases IGF-binding protein–1 (IGFBP-1) and IGFBP-2 levels, insulin may reduce certain IGFBP levels and increase bioavailable IGF levels to indirectly enhance the IGF–IGF-1R signaling pathway. In the situation of diabetes, hyperinsulinemia may therefore directly enhance tumor growth and progression or indirectly promote malignant transformation through IGF-1 signaling (45). Second, the increase of TSH levels is three times more common in patients with D2M than in those without DM (46). Chronic higher level of serum TSH was related to an elevated likelihood of differentiated thyroid cancer and more aggressive tumor stage (47). Interestingly, patients with malignancy may have normal TSH levels, whereas patients with benign tumors often have low TSH levels caused by toxic nodules, goiter, and Graves’ disease. Thyroid hormones have been implicated in promoting thyroid inflammation and thyroid cancer through genomic and non-genomic (membrane integrin receptor) actions. Genomic action of thyroid hormones promotes thyroid carcinogenesis by binding to specific nuclear receptors, but accumulated evidence suggests that the activation of the MAPK signaling pathway is the pathophysiological mechanism, which has been noted in the pathogenesis of papillary thyroid carcinoma. Recently, a novel pathway mediated by a membrane receptor located in integrin αVβ3 has been revealed. The proliferative and angiogenic effects of THs have been postulated through this mechanism. It is not yet clear whether the tumorigenic action noted in other neoplasms may play a role on DTC (48). Third, increased oxidative stress caused by hyperglycemia influences tumor cell growth and proliferation (17). Patients with DM suffered from increased permanent pro-inflammatory and oxidative stress caused by metabolic abnormalities. The intracellular anti-oxidant capacity reduced by prolonged inflammatory responses may increase the risk of carcinogenesis of susceptible cells (49). Increased oxidative stress determines thyroid cell inflammatory effects through Toll-like receptor (TLR) activation. Increased TLR expression, activation, and signaling adaptor molecules were found in peripheral blood mononuclear cells from patients with autoimmune thyroid disease (AITD), and TLRs may participate in the pathogenesis of AITD. A significant elevation in TLR endogenous ligands was also observed in the serum of AITD group (50). Moreover, accumulating evidence indicates the association between overactivation of TLR signaling and thyroid cancer progression (51, 52). This reveals the molecular mechanism of thyroid carcinogenesis caused by hyperglycemia to a certain extent. Finally, 70% of patients with DM have vitamin D deficiency (53). Inactivation of deiodinase II (DIO2) enzyme by a Vitamin D deficient environment in patients with DM leads to decreased glucose transporter 4 (GLUT4) transcription by skeletal muscle and adipose tissue, thus resulting in insulin resistance and thyroid carcinogenesis (34, 53).
The strengths of this study should be highlighted as follows: the analyses were limited to cohort studies, which could minimize the influence of biases such as recall and selection biases; the large sample size provided powerful statistical power for quantitative analysis of this correlation between DM and thyroid cancer risk, obtaining more robust results than any single study; subgroup analyses by type of DM and sex were performed to explore the associations between various kinds of DM and thyroid cancer risk and further investigate sex-specific thyroid cancer risk in any type of DM.
This meta-analysis also had some potential limitations. Because DM is shown in the vast majority of studies as a no or yes variable, it is not possible to assess the severity of DM and further explore its association with thyroid cancer risk. Moreover, we could not distinguish controlled vs. uncontrolled DM, because information on DM treatment was not available. In some studies, potential confounding factors were not adjusted such as obesity and age. Although they were adjusted, the adjustment models varied across the selected studies, which might affect the validity of the results. TSH levels and/or TNM classification or staging of thyroid cancer were not monitored in the original literatures included in this study, we cannot assess whether the difference of TSH levels exists between patients with DM and non-DM counterparts and also cannot explore the correlation between DM and progression of thyroid cancer. We expect this problem to be solved in the prospective studies in the future. Diabetes status was ascertained by a self-report in some previous studies. Thus, prediabetes and T2D can be misleading and undiagnosed glucose disorders account for 45.8% on a global scale. However, because of wide area coverage (nine countries), the large sample size (greater than 300,000, and a total of 11,091 cases of thyroid cancer), and the recent literatures accounting for the majority, the impact of this problem is minimized. We believe that our conclusion is still valid.
Conclusion
Our results provided evidence that T2D could increase thyroid cancer risk in both sexes, underlining the demand for developing management and prevention strategies to mitigate thyroid cancer risk in any individual with T2D. Patients with GD are also at high risk for thyroid cancer. Further study is warranted to explore the association between T1D and thyroid cancer risk. Thus, given the increased thyroid cancer risk in patients with DM, DM prevention should be encouraged in all individuals, especially in the high-risk group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HZ and W-WD conceptualized the research. W-WD and D-LZ conducted statistical analysis. Z-HW, C-ZL, and PZ contributed to data interpretation. W-WD wrote the manuscript draft. All authors contributed to the draft revision and approved the final draft of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.971213/full#supplementary-material
References
1. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol (2021) 9(4):225–34. doi: 10.1016/s2213-8587(21)00027-9
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
3. Wang J, Yu F, Shang Y, Ping Z, Liu L. Thyroid cancer: incidence and mortality trends in China, 2005-2015. Endocrine (2020) 68(1):163–73. doi: 10.1007/s12020-020-02207-6
4. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Center (2021) 1(1):2–11. doi: 10.1016/j.jncc.2020.12.001
5. Li M, Brito JP, Vaccarella S. Long-term declines of thyroid cancer mortality: An international age-Period-Cohort analysis. Thyroid (2020) 30(6):838–46. doi: 10.1089/thy.2019.0684
6. Haymart MR. Progress and challenges in thyroid cancer management. Endocr Pract (2021) 27(12):1260–3. doi: 10.1016/j.eprac.2021.09.006
7. Fiore M, Oliveri Conti G, Caltabiano R, Buffone A, Zuccarello P, Cormaci L, et al. Role of emerging environmental risk factors in thyroid cancer: A brief review. Int J Environ Res Public Health 16(7) (2019) 16(7):1185. doi: 10.3390/ijerph16071185
8. Mannathazhathu AS, George PS, Sudhakaran S, Vasudevan D, Krishna Km J, Booth C, et al. Reproductive factors and thyroid cancer risk: Meta-analysis. Head Neck (2019) 41(12):4199–208. doi: 10.1002/hed.25945
9. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care (2004) 27(5):1047–53. doi: 10.2337/diacare.27.5.1047
10. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev (2019) 35(6):e3158. doi: 10.1002/dmrr.3158
11. Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia (2018) 61(6):1249–60. doi: 10.1007/s00125-018-4557-7
12. Jian Gang P, Mo L, Lu Y, Runqi L, Xing Z. Diabetes mellitus and the risk of prostate cancer: an update and cumulative meta-analysis. Endocr Res (2015) 40(1):54–61. doi: 10.3109/07435800.2014.934961
13. Gong Y, Wei B, Yu L, Pan W. Type 2 diabetes mellitus and risk of oral cancer and precancerous lesions: a meta-analysis of observational studies. Oral Oncol (2015) 51(4):332–40. doi: 10.1016/j.oraloncology.2015.01.003
14. Zhou Y, Zhang X, Gu C, Xia J. Diabetes mellitus is associated with breast cancer: systematic review, meta-analysis, and in silico reproduction. Panminerva Med (2015) 57(3):101–8.
15. Song S, Wang B, Zhang X, Hao L, Hu X, Li Z, et al. Long-term diabetes mellitus is associated with an increased risk of pancreatic cancer: A meta-analysis. PloS One (2015) 10(7):e0134321. doi: 10.1371/journal.pone.0134321
16. Kitahara CM, Platz EA, Beane Freeman LE, Black A, Hsing AW, Linet MS, et al. Physical activity, diabetes, and thyroid cancer risk: a pooled analysis of five prospective studies. Cancer Causes Control (2012) 23(3):463–71. doi: 10.1007/s10552-012-9896-y
17. Yeo Y, Ma SH, Hwang Y, Horn-Ross PL, Hsing A, Lee KE, et al. Diabetes mellitus and risk of thyroid cancer: a meta-analysis. PloS One (2014) 9(6):e98135. doi: 10.1371/journal.pone.0098135
18. Li H, Qian J. Association of diabetes mellitus with thyroid cancer risk: A meta-analysis of cohort studies. Med (Baltimore) (2017) 96(47):e8230. doi: 10.1097/md.0000000000008230
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372 n71 (2021) 372:n71. doi: 10.1136/bmj.n71
20. Taylor KS, Mahtani KR, Aronson JK. Summarising good practice guidelines for data extraction for systematic reviews and meta-analysis. BMJ Evid Based Med (2021) 26(3):88–90. doi: 10.1136/bmjebm-2020-111651
21. Linkeviciute-Ulinskiene D, Patasius A, Zabuliene L, Stukas R, Smailyte G. Increased risk of site-specific cancer in people with type 2 diabetes: A national cohort study. Int J Environ Res Public Health (2019) 17(1):246. doi: 10.3390/ijerph17010246
22. Pace R, Rahme E, Dasgupta K. Gestational diabetes mellitus and risk of incident primary cancer: A population-based retrospective cohort study. J Diabetes (2020) 12(1):87–90. doi: 10.1111/1753-0407.12988
23. Peng YS, Lin JR, Cheng BH, Ho C, Lin YH, Shen CH, et al. Incidence and relative risk for developing cancers in women with gestational diabetes mellitus: a nationwide cohort study in Taiwan. BMJ Open (2019) 9(2):e024583. doi: 10.1136/bmjopen-2018-024583
24. Qi J, He P, Yao H, Song R, Ma C, Cao M, et al. Cancer risk among patients with type 2 diabetes: A real-world study in shanghai, China. J Diabetes (2019) 11(11):878–83. doi: 10.1111/1753-0407.12926
25. Saarela K, Tuomilehto J, Sund R, Keskimäki I, Hartikainen S, Pukkala E. Cancer incidence among Finnish people with type 2 diabetes during 1989-2014. Eur J Epidemiol (2019) 34(3):259–65. doi: 10.1007/s10654-018-0438-0
26. Fang Y, Zhang X, Xu H, Smith-Warner SA, Xu D, Fang H, et al. Cancer risk in Chinese diabetes patients: a retrospective cohort study based on management data. Endocr Connect (2018) 7(12):1415–23. doi: 10.1530/ec-18-0381
27. Han KT, Cho GJ, Kim EH. Evaluation of the association between gestational diabetes mellitus at first pregnancy and cancer within 10 years postpartum using national health insurance data in south Korea. Int J Environ Res Public Health 15(12) (2018) 15(12):2646. doi: 10.3390/ijerph15122646
28. Bejaimal SA, Wu CF, Lowe J, Feig DS, Shah BR, Lipscombe LL. Short-term risk of cancer among women with previous gestational diabetes: a population-based study. Diabetes Med (2016) 33(1):39–46. doi: 10.1111/dme.12796
29. Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson AM, et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia (2016) 59(5):980–8. doi: 10.1007/s00125-016-3884-9
30. Dankner R, Boffetta P, Balicer RD, Boker LK, Sadeh M, Berlin A, et al. Time-dependent risk of cancer after a diabetes diagnosis in a cohort of 2. 3 Million Adults. Am J Epidemiol (2016) 183(12):1098–106. doi: 10.1093/aje/kwv290
31. Luo J, Phillips L, Liu S, Wactawski-Wende J, Margolis KL. Diabetes, diabetes treatment, and risk of thyroid cancer. J Clin Endocrinol Metab (2016) 101(3):1243–8. doi: 10.1210/jc.2015-3901
32. Xu HL, Fang H, Xu WH, Qin GY, Yan YJ, Yao BD, et al. Cancer incidence in patients with type 2 diabetes mellitus: a population-based cohort study in shanghai. BMC Cancer (2015) 15:852. doi: 10.1186/s12885-015-1887-4
33. Lo SF, Chang SN, Muo CH, Chen SY, Liao FY, Dee SW, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. Int J Cancer (2013) 132(1):182–8. doi: 10.1002/ijc.27597
34. Aschebrook-Kilfoy B, Sabra MM, Brenner A, Moore SC, Ron E, Schatzkin A, et al. Diabetes and thyroid cancer risk in the national institutes of health-AARP diet and health study. Thyroid (2011) 21(9):957–63. doi: 10.1089/thy.2010.0396
35. Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U. S. veterans diabetes. Int J Cancer (2011) 128(3):635–43. doi: 10.1002/ijc.25362
36. Johnson JA, Bowker SL, Richardson K, Marra CA. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia (2011) 54(9):2263–71. doi: 10.1007/s00125-011-2242-1
37. Chodick G, Heymann AD, Rosenmann L, Green MS, Flash S, Porath A, et al. Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control (2010) 21(6):879–87. doi: 10.1007/s10552-010-9515-8
38. Hemminki K, Li X, Sundquist J, Sundquist K. Risk of cancer following hospitalization for type 2 diabetes. Oncologist (2010) 15(6):548–55. doi: 10.1634/theoncologist.2009-0300
39. Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control (1991) 2(5):307–14. doi: 10.1007/bf00051670
40. Standl E, Khunti K, Hansen TB, Schnell O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur J Prev Cardiol (2019) 26(2_suppl):7–14. doi: 10.1177/2047487319881021
41. Popova PV, Klyushina AA, Vasilyeva LB, Tkachuk AS, Vasukova EA, Anopova AD, et al. Association of common genetic risk variants with gestational diabetes mellitus and their role in GDM prediction. Front Endocrinol (Lausanne) (2021) 12:628582. doi: 10.3389/fendo.2021.628582
42. Hard GC. Recent developments in the investigation of thyroid regulation and thyroid carcinogenesis. Environ Health Perspect (1998) 106(8):427–36. doi: 10.1289/ehp.106-1533202
43. Oberman B, Khaku A, Camacho F, Goldenberg D. Relationship between obesity, diabetes and the risk of thyroid cancer. Am J Otolaryngol (2015) 36(4):535–41. doi: 10.1016/j.amjoto.2015.02.015
44. Gristina V, Cupri MG, Torchio M, Mezzogori C, Cacciabue L, Danova M. Diabetes and cancer: A critical appraisal of the pathogenetic and therapeutic links. BioMed Rep (2015) 3(2):131–6. doi: 10.3892/br.2014.399
45. Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia (2013) 18(3-4):277–89. doi: 10.1007/s10911-013-9303-7
46. Tamez-Pérez HE, Martínez E, Quintanilla-Flores DL, Tamez-Peña AL, Gutiérrez-Hermosillo H, Díaz de León-González E. The rate of primary hypothyroidism in diabetic patients is greater than in the non-diabetic population. an observational study. Med Clin (Barc) (2012) 138(11):475–7. doi: 10.1016/j.medcli.2011.08.009
47. Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab (2008) 93(3):809–14. doi: 10.1210/jc.2007-2215
48. Zafón C. TSH-suppressive treatment in differentiated thyroid cancer. A dogma under review. Endocrinol Nutr (2012) 59(2):125–30. doi: 10.1016/j.endonu.2011.10.002
49. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer (2009) 16(4):1103–23. doi: 10.1677/erc-09-0087
50. Peng S, Li C, Wang X. Et al: Increased toll-like receptors activity and TLR ligands in patients with autoimmune thyroid diseases. Front Immunol 7:578 (2016) 7:578. doi: 10.3389/fimmu.2016.00578
51. McCall KD, Harii N, Lewis CJ. Et al: High basal levels of functional toll-like receptor 3 (TLR3) and noncanonical Wnt5a are expressed in papillary thyroid cancer and are coordinately decreased by phenylmethimazole together with cell proliferation and migration. Endocrinology (2007) 148(9):4226–37. doi: 10.1210/en.2007-0459
52. Peyret V, Nazar M, Martín M. Et al: Functional toll-like receptor 4 overexpression in papillary thyroid cancer by MAPK/ERK-induced ETS1 transcriptional activity. Mol Cancer Res (2018) 16(5):833–45. doi: 10.1158/1541-7786.MCR-17-0433
Keywords: diabetes mellitus, thyroid cancer, meta-analysis, cohort study, risk
Citation: Dong W-w, Zhang D-L, Wang Z-H, Lv C-Z, Zhang P and Zhang H (2022) Different types of diabetes mellitus and risk of thyroid cancer: A meta-analysis of cohort studies. Front. Endocrinol. 13:971213. doi: 10.3389/fendo.2022.971213
Received: 16 June 2022; Accepted: 05 September 2022;
Published: 23 September 2022.
Edited by:
Janete Maria Cerutti, Federal University of São Paulo, BrazilReviewed by:
Rosario Le Moli, University of Catania, ItalyTereza Grimmichová, Institute of Endocrinology (Prague), Czechia
Copyright © 2022 Dong, Zhang, Wang, Lv, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhang, aGFvemhhbmdAY211LmVkdS5jbg==
 Wen-wu Dong
Wen-wu Dong Da-Lin Zhang
Da-Lin Zhang Zhi-Hong Wang
Zhi-Hong Wang Hao Zhang
Hao Zhang