
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 16 August 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.969149
Objectives: Diabetic peripheral neuropathic pain (DPNP) is a prevalent chronic complication in patients with diabetes. Using a questionnaire is helpful for DPNP screening in outpatients. In this retrospective cohort, we aimed to examine whether DPNP diagnosed based on scoring questionnaires could predict long-term mortality in outpatients with type 2 diabetes.
Methods: We enrolled 2318 patients who had joined the diabetes pay-for-performance program and completed the annual assessments, including both the identification pain questionnaire (ID pain) and Douleur Neuropathique en 4 questionnaire (DN4), between January 2013 and October 2013. Information on registered deaths was collected up to August 2019.
Results: There was high consistency in the scores between the ID pain and DN4 (r = 0.935, P < 0.001). During the median follow-up of 6.2 years (interquartile range: 5.9−6.4 years), 312 patients deceased. Patients with an ID pain score of ≥ 2 had a higher mortality risk than those with a score of < 2 (hazard ratio [HR] = 1.394, 95%CI: 1.090−1.782), and patients with a DN4 score of ≥ 4 had a higher mortality risk than those with a score of < 4 (HR = 1.668, 95% confidence interval [CI]: 1.211−2.297). Patients consistently diagnosed with DPNP by the ID pain and DN4 had a significantly higher mortality risk (HR = 1.713, 95% CI: 1.223−2.398, P = 0.002), but not those discrepantly diagnosed with DPNP (P = 0.107), as compared with those without DPNP.
Conclusions: Both the ID pain and DN4 for DPNP screening were predictive of long-term mortality in patients with type 2 diabetes. However, a discrepancy in the diagnosis of DPNP weakened the power of mortality prediction.
Diabetic peripheral neuropathy (DPN) is the presence of peripheral nerve dysfunction in patients with diabetes after the exclusion of other causes (1, 2). DPN is prevalent in patients with type 2 diabetes (3, 4). The prevalence of DPN was approximately 26.7% and showed high variations in ethnicity based on interviews with patients with type 2 diabetes across 14 countries in the International Prevalence and Treatment of Diabetes and Depression study (5). In Taiwan, the prevalence of DPN was 26.8% according to the Neurological Symptom Score questionnaire (6), and was 34.5% by physical examination according to the Michigan Neuropathy Screening Instrument (MNSI) criteria (7). DPN has become a heavy burden on health and the economy due to subsequent foot complications or pain relief treatments (8, 9). An investigation determined that most patients with diabetes did not undergo foot examination in the rural community (7). Non-invasive questionnaires would be helpful for early screening of DPN in clinical practice (6).
DPN is a heterogeneous group of disorders with diverse clinical manifestations (1). More than 40% of patients with DPN suffer from peripheral neuropathic pain (10). Diabetic peripheral neuropathic pain (DPNP) is a common problem and patients with DPNP should be treated for symptom relief (10, 11). The presence of pain is associated with several comorbidities, including mental disorders, and has been reported to increase health-care costs (9–12). Since objective measurements are not sensitive for evaluating painful symptoms, the diagnosis of DPNP is challenging. To facilitate awareness of neuropathic pain, several questionnaires have been developed. The identification pain questionnaire (ID pain) is a self-administered questionnaire with the six items (13). The Douleur Neuropathique en 4 questionnaire (DN4) is a clinician-administered questionnaire with ten items (14). The DN4 has been validated in the assessment of DPNP with a high diagnostic accuracy (15), and there is good consistency between DN4 and ID pain in the diagnosis of neuropathic pain (16).
It was reported that DPN diagnosed based on low signal amplitude and slow conduction velocity in a nerve conduction study is associated with total mortality in patients with type 2 diabetes (17). DPN diagnosed based on the MNSI could predict a high incidence of cardiovascular (CV) disease; however, it was not significantly associated with total mortality in patients with type 2 diabetes (18). Notably, DPNP based on electronic health records might be associated with a significantly higher mortality risk than DPN without pain in patients with type 2 diabetes (19). We hypothesized that pain symptoms are predictive of mortality in patients with type 2 diabetes. Therefore, we aimed to examine the association between mortality and neuropathic pain screened using the ID pain and DN4 in patients with type 2 diabetes.
This retrospective cohort study was conducted at Taichung Veterans General Hospital. The inclusion criteria were [1] adult patients who had joined the diabetes pay-for-performance program and [2] patients who had completed both the ID pain and DN4 for DPNP screening between January 2013 and October 2013. The exclusion criteria were [1] end-stage renal disease; [2] an open wound or amputation of the lower extremities; [3] diabetes other than type 2; [4] malignancy, psychiatric disorders, current use of any pain medication involving the central nervous system, other severe systemic diseases, or pregnancy; and [5] other conditions unsuitable for the study.
The diabetes pay-for-performance program is an important health policy for clinical diabetic care (20). Patients who had been diagnosed with diabetes and repeatedly visited our hospital within ninety days were encouraged to participate in the program (21). The completion of a comprehensive survey was requested in this program, including diabetic neuropathy screening every year. The questionnaires for scoring DPNP were administrated by a well-trained educator at our Diabetes Care Center. We only collected the last assessment records for the participants who had undergone repeated annual comprehensive assessments in the enrollment period.
The six items of ID pain were reported by patients themselves: each item was scored 0 when a participant answered “no”. Items 1−5 received a score of 1 when a participant answered “yes”, and Item 6 received a score of -1 when a participant answered “yes” (13). The total ID pain score ranges from -1 to 5, and a score ≥ 2 indicates that a diagnosis of peripheral neuropathic pain is likely (22). The Mandarin Chinese version of the ID pain has been validated in previous studies (22–24). The ten items of DN4 were assessed by a trained educator certified by the Taiwanese Association of Diabetes Educators: each item was assigned a score of 0 when a participant answered “no” and a score of 1 when a participant answered “yes”. The total DN4 score ranges from 0 to 10, and a score ≥ 4 indicates a diagnosis of peripheral neuropathic pain (14, 15). The Mandarin Chinese version of the DN4 has been validated previously (25).
Furthermore, laboratory data, including plasma glucose, glycated hemoglobin (HbA1c), serum levels of total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and creatinine, and urinary levels of albumin and creatinine, were collected during the annual comprehensive survey. Information on the use of antidiabetic drugs, antihypertensive drugs, antiplatelet drugs, and statins was also collected.
Plasma glucose levels were determined using the oxidase-peroxidase method (Wako Diagnostics, Tokyo, Japan). HbA1c levels were determined by cation-exchange high performance liquid chromatography (National Glycohemoglobin Standardization Program certified; G8, TOSOH, Tokyo, Japan). Serum concentrations of total cholesterol, HDL cholesterol, and triglycerides were determined using enzymatic methods (Advia 1800, Siemens, New York, USA). Creatinine levels were determined using the Jaffé method (Advia 1800, Siemens, New York, USA). Urinary albumin was determined using the polyethylene glycol enhanced immunoturbidimetric method (Advia 1800, Siemens, New York, USA). The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation as follows: 141 × (serum creatinine [mg/dL]/0.9)-0.411 × 0.993age (year) in men with serum creatinine ≤ 0.9 mg/dL; 141 × (serum creatinine [mg/dL]/0.9)-1.209 × 0.993age (year) in men with serum creatinine > 0.9 mg/dL; 144 × (serum creatinine [mg/dL]/0.7)-0.329 × 0.993age (year) in women with serum creatinine ≤ 0.7 mg/dL; or 144 × (serum creatinine [mg/dL]/0.9)-1.209 × 0.993age (year) in women with serum creatinine > 0.7 mg/dL (26, 27). CKD was defined as an eGFR < 60 mL/min/1.73 m2 (2, 28). The urinary albumin-to-creatinine ratio (UACR) was calculated as follows: albumin (mg)/creatinine (g), and albuminuria was defined as a UACR ≥ 300 mg/g (2, 28). Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, a diastolic blood pressure ≥ 90 mmHg, or current use of an antihypertensive drug. Hypertriglyceridemia was defined as serum triglycerides ≥ 150 mg/dL (1.7 mmol/L), and low HDL cholesterol was defined as a serum HDL cholesterol < 40 mg/dL (1.0 mmol/L) in men or < 50 mg/dL (1.3 mmol/L) in women (29).
We present the mean ± standard deviation (SD) for continuous variables and numbers with percentages (%) for categorical data. The clinical variables were tested for statistically significant differences using Student’s t test for continuous variables between two groups, and the χ2 test for categorical variables. The relationship between the scores of the questionnaires was determined by Pearson’s correlation.
The reproducibility of the ID pain and DN4 was examined in a group of 51 subjects by repeated assessments on different days. The median period between repeated measurements was 161 days (interquartile range [IQR] between 112 and 252 days). Highly positive correlations of ID pain score (correlation coefficient [r] = 0.870, P < 0.001) and DN4 score (r = 0.789, P < 0.001) were observed between the first and second measurements. The 95% confidence intervals (CIs) were 0.078 ± 0.158 for the bias of the ID pain and 0.020 ± 0.221 for the DN4 between repeated measurements based on Bland-Altman plots (30).
The primary endpoint was the occurrence of all-cause mortality. Information on deaths registered through August 31, 2019, was obtained from the Ministry of Health and Welfare, Executive Yuan, Taiwan. The univariate cumulative risk for all-cause mortality was assessed by Kaplan-Meier analysis, and significance was tested by the log-rank test. The receiver operating characteristic (ROC) curve was used to determine the prediction for mortality in patients with DPNP according to different criteria. Multivariate Cox proportional hazards regression analyses were used to determine the primary endpoint according to the diagnosis of DPNP. The CV mortality was defined based on the International Classification of Diseases 10th Revision, and the codes included those for heart diseases (I01−I25, I27, and I30−I52), stroke (I60−I69), and peripheral artery disease (I70.2−I75). A two-tailed P value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 22.0 (IBM, Armonk, NY, USA).
Of a total of 2318 patients, 505 (21.8%) and 179 (7.7%) were diagnosed with DPNP based on ID pain scores ≥ 2 and DN4 score ≥ 4, respectively. The baseline characteristics of the patients grouped by DPNP are shown in Table 1. The patients with DPNP were significantly older than those without DPNP (P < 0.001 based on either the ID pain or DN4). The proportions of males were significantly lower among patients with DPNP than among those without DPNP (P < 0.001 based on the ID pain and P = 0.004 based on the DN4, respectively). The patients with DPNP had a significantly longer diabetes duration than those without DPNP (P < 0.001 based on either the ID pain or DN4). There were significantly higher proportions of previous coronary artery disease (CAD) history and hypertension in the patients with DPNP than in those without DPNP (P < 0.05 based on either the ID pain or DN4). The patients with DPNP had significantly higher HbA1c levels than those without DPNP (P = 0.013 based on the ID pain and P = 0.007 based on the DN4, respectively). The patients with DPNP had significantly lower eGFR and higher UACR than those without DPNP (P < 0.001 based on either the ID pain or DN4). There were significantly higher proportions of antiplatelet agent, antihypertensive drug, and insulin injection therapy use in the patients with DPNP than in those without DPNP (P < 0.01 based on either the ID pain or DN4). Among the oral antihyperglycemic drugs, only the proportions of metformin use were significantly different between the patients with and without DPNP (P = 0.009 based on the ID pain and P = 0.041 based on the DN4, respectively).
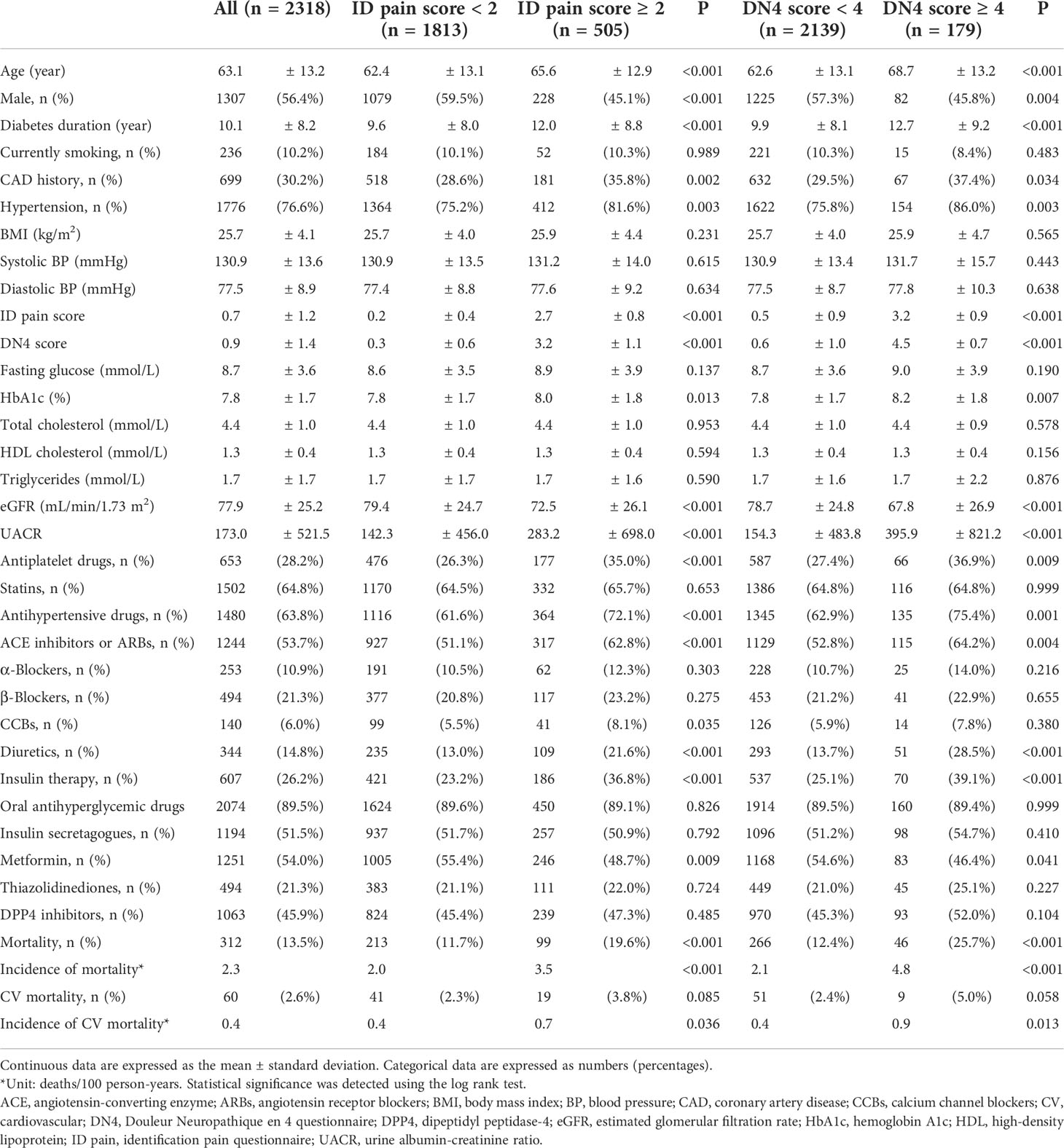
Table 1 The baseline characteristics of all patients, categorized by peripheral neuropathic pain scores.
During a median follow-up of 6.2 years (IQR: 5.9−6.4 years), 312 patients died (Figure 1). The incidences of mortality were 3.5 deaths/100 person-years in patients with ID pain scores ≥ 2 and 4.8 deaths/100 person-years in patients with DN4 scores ≥ 4; and 2.0 deaths/100 person-years in patients with ID pain scores < 2 and 2.1 deaths/100 person-years in patients with DN4 scores < 4. Notably, 60 of 312 deaths (19.2%) caused by CV diseases (Table 1). The CV−total mortality ratios were not significantly different between patients with ID pain scores ≥ 2 and < 2 (19.2% vs. 19.2%, P = 0.999). Similarly, the CV−total mortality ratios were not significantly different between patients with DN4 scores ≥ 4 and < 4 (19.6% vs. 19.2%, P = 0.999).
The Kaplan-Meier analysis showed that the survival rates were significantly different between patients with and without DPNP (log-rank test P < 0.001 based on the ID pain, Figure 2; or based on the DN4, Figure 3). According to each item in the ID pain, all positive symptoms were significantly predictive of mortality, except the Item 6, which was designed to exclude DPNP; according to each item in the DN4, all positive symptoms were significantly predictive of mortality, except the Items 2 and 4, as few patients experienced the symptoms (Table 2). Using multivariable Cox proportional hazard regression models, the ID pain score was a significant predictor for total mortality with an HR of 1.394 (95% CI: 1.090−1.782, P = 0.008; Table 3), and the DN4 was also a significant predictor for total mortality with an HR of 1.668 (95% CI: 1.211−2.297, P = 0.002; Table 3) after adjusting for age, sex, hypertension, CAD history, diabetes duration, HbA1c level, HDL cholesterol level, CKD, the UACR, and the use of antiplatelet drugs, antihypertensive drugs, insulin, and metformin.
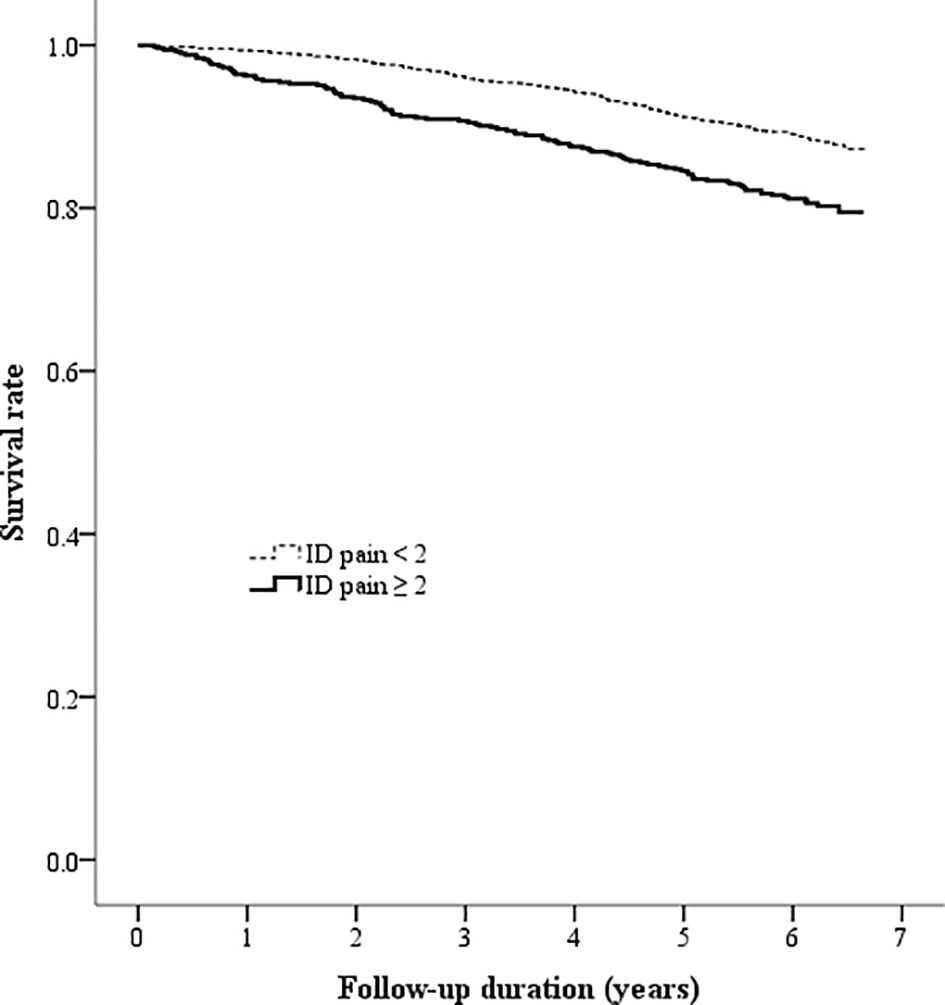
Figure 2 Kaplan-Meier curves showing the survival rate between the two patient groups categorized by the baseline ID pain score (ID pain = identification pain questionnaire).
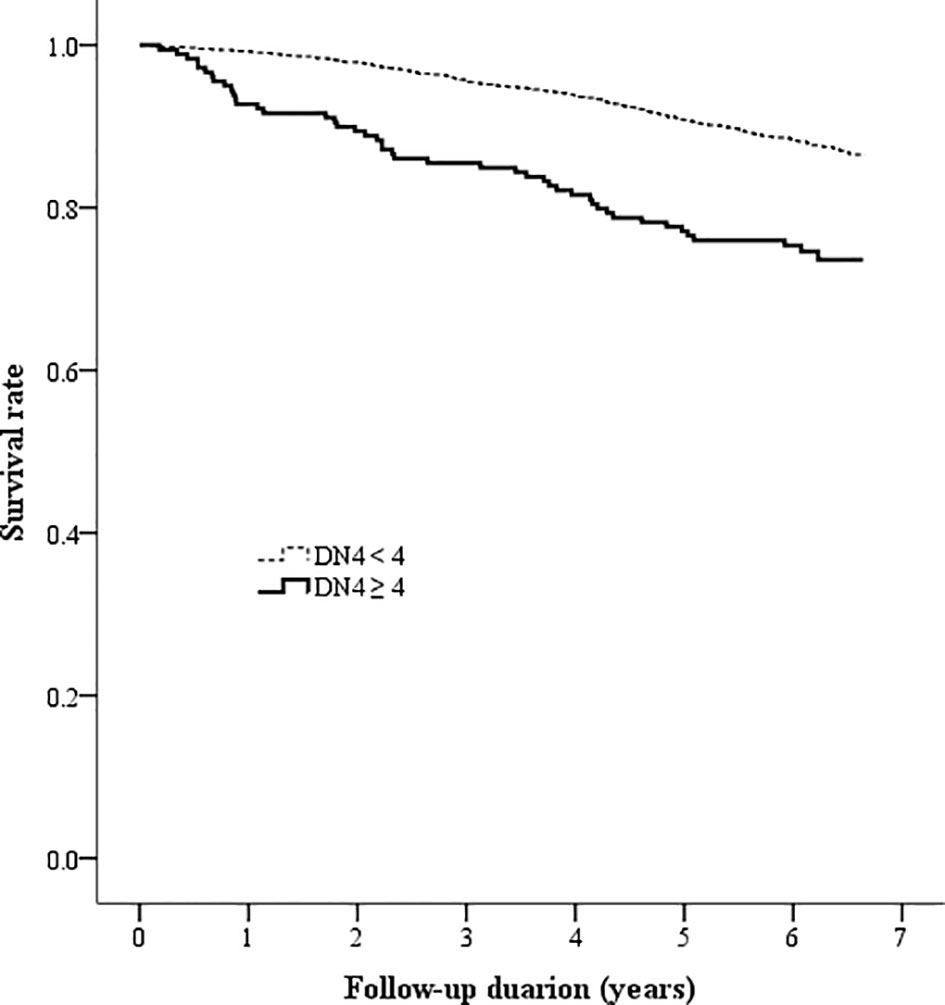
Figure 3 Kaplan-Meier curves showing the survival rate between the two patient groups categorized by the baseline DN4 score (DN4 = Douleur Neuropathique en 4 questionnaire).
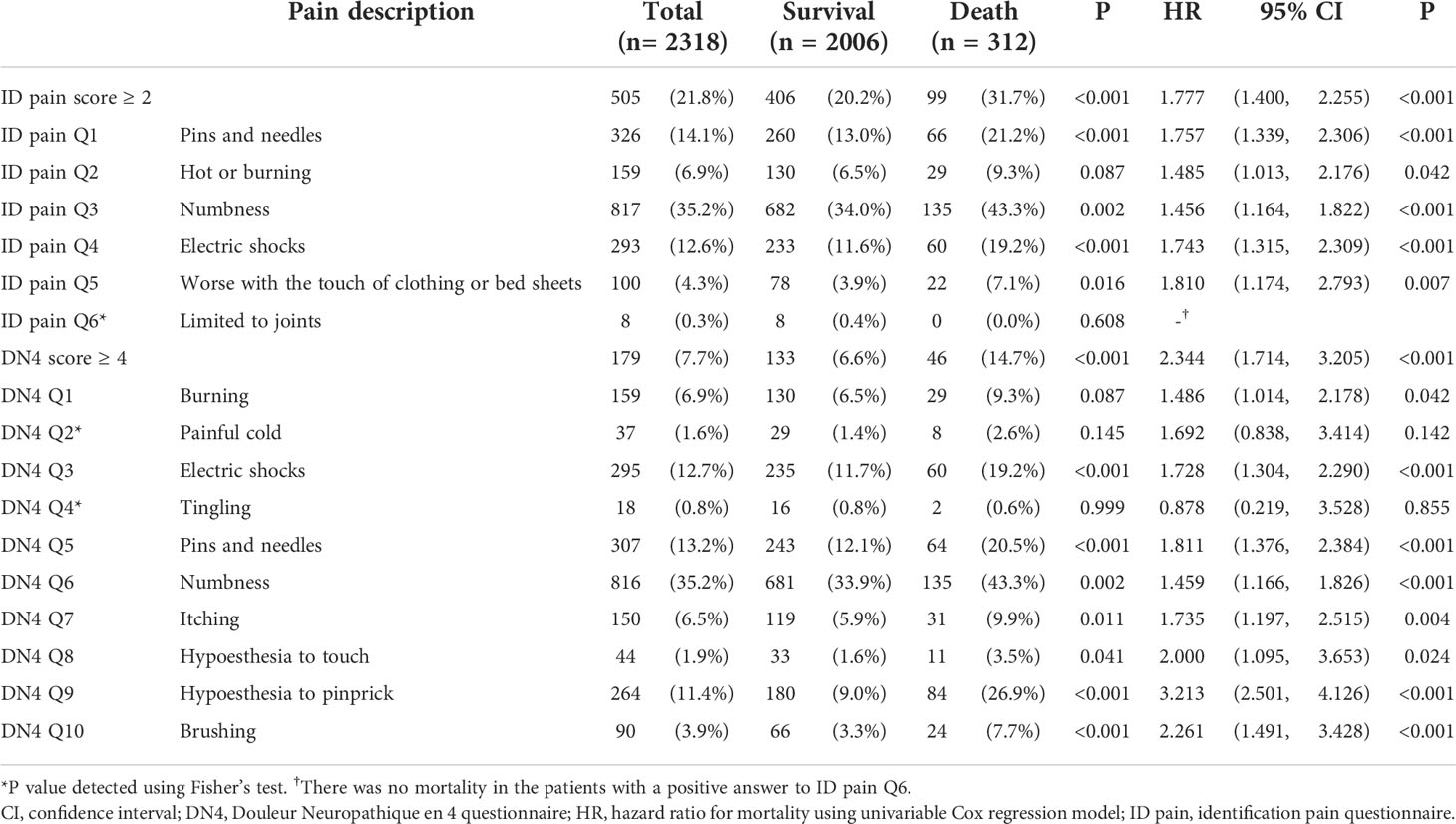
Table 2 The number (percentage) and mortality HR (95% CI) of patients with positive symptoms for each question item.
There was a high correlation between the ID pain and DN4 scores (r = 0.935, P < 0.001). According to the criteria of peripheral neuropathic pain based on these two scoring questionnaires, 1805 (77.9%) patients were consistently grouped as no DPNP, and 171 (7.4%) patients were consistently grouped as DPNP. However, 8 (0.3%) patients were grouped as no DPNP based on the ID pain score but were discrepantly grouped as DPNP based on the DN4 score; 334 (14.4%) patients were grouped as DPNP based on the ID pain score but were discrepantly as no DPNP based on the DN4 score. The consistent group of diagnosed DPNP showed a sensitivity of 17.0%, a specificity of 92.6%, a positive predictive value (PPV) of 25.1%, and a negative predictive value (NPV) of 88.4% to predict mortality; the discrepant group of DPNP diagnoses showed a sensitivity of 21.9%, a specificity of 84.9%, a PPV of 17.3%, and a NPV of 88.4% to predict mortality during follow-up (Table 4). The consistent group of diagnosed DPNP based on the ID pain and DN4 had the highest mortality risk (Figure 4), and was significantly predictive of mortality compared to the consistent group of no DPNP (HR = 1.713, 95% CI: 1.223−2.398, P =0.002; Table 3). However, the discrepant group of DPNP diagnoses weakened the mortality prediction toward a null hypothesis (HR = 1.273, 95% CI: 0.949−1.709; P = 0.107) after adjusting for the associated factors.
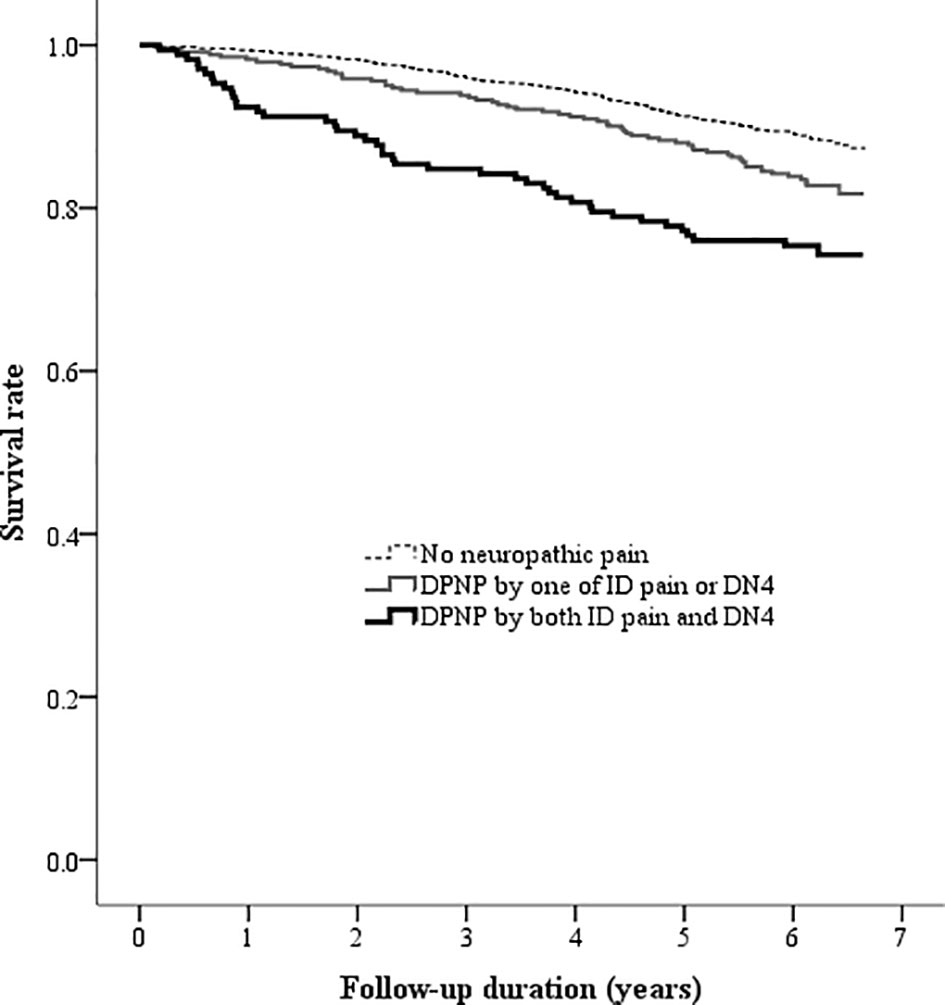
Figure 4 Kaplan-Meier curves showing the survival rate among the three groups categorized by the DPNP diagnosis. (DN4, Douleur Neuropathique en 4 questionnaire; DPNP, diabetic peripheral neuropathic pain; ID pain, identification pain questionnaire).
The main finding of our study revealed that the scores of peripheral neuropathic pain using the ID pain and DN4 were predictive of total mortality in patients with type 2 diabetes, with a median follow-up duration of 6.2 years. There was a high consistency in the diagnosis of DPNP between the ID pain and DN4 scores. Notably, a discrepancy in the diagnosis of DPNP attenuated the prediction of long-term mortality. The ID pain and DN4 are widely used tools for screening neuropathic pain, and Padua et al. (16) reported a low discrepancy rate of 16% between the ID pain and DN4 in diagnosing neuropathic pain in Italians with established peripheral nerve diseases. A strength of the present study is that use of these two questionnaires to actively screen peripheral neuropathic pain could predict total mortality risk in patients with type 2 diabetes. In line with our findings, Lapin et al. (19) reported that peripheral neuropathic pain defined by medical records or medication usage predicted CV disease and total mortality in patients with type 2 diabetes. Since chronic pain has been reported as a CV risk factor (31), the early detection of peripheral neuropathic pain is important to evaluate the associated comorbidities and to categorize the long-term mortality risk for patients with type 2 diabetes (32).
The mechanisms of pain are complex in diabetes. Generally, peripheral neuropathic pain might result from the facilitated excitation of pain signals and reduced inhibition of the central nervous system (33). Small-fiber neuropathy can generate action potentials even without an external stimulus in patients with diabetes (34). Several CV risk factors, including metabolic disorders and vasculopathy, are associated with neural injury (35). In the present study, higher pain scores were associated with age, diabetes duration, hypertension, CAD, hyperglycemia, CKD, and albuminuria. DPNP was an independent predictor of mortality after adjusting for all assessed potential risk factors; however, several nontraditional CV risks associated with DPN were not assessed in the present study (36). Variability of fasting glucose was reported to be associated with DPNP, peripheral artery disease, and mortality (37–39). Retinopathy was reported to be associated with DPN, CKD, and mortality (40, 41). Endothelial dysfunction, a well-known CV risk factor, was also reported to be associated with DPN (42).
In addition to comorbidities between DPNP and CV disease, imbalanced glycolytic metabolites and decreased neuroprotective factors may be involved in the mechanism between DPNP and mortality. Methylglyoxal, which might be generated during glycolysis in cells, is associated with the hyperexcitability of neurons (43). In patients with type 2 diabetes, the plasma methylglyoxal level was higher in individuals with DPNP than in those without pain (44). Hannssen et al. (45) reported that a high plasma methylglyoxal level was associated with an increased risk of diabetic nephropathy, CV events, and all-cause mortality. Alterations in the function and structure of the brain were observed in patients with DPN. N-acetyl aspartate was decreased in the thalamus of patients with DPNP (46), and reduced inhibition of central nervous system might be associated with psychological comorbidities (47–49). A decrease in serum brain-derived neurotrophic factor (BDNF), a neurotrophin family member, is associated with peripheral neuropathy in patients with type 2 diabetes (50). Previously, we reported that a low serum BDNF level was also associated with depressive symptoms and predictive of long-term mortality (51, 52).
The prevalence of peripheral neuropathic pain was approximately 13% in patients with diabetes (53, 54), and was higher in patients with type 2 diabetes than in those with type 1 diabetes (54, 55). According to electronic health records, approximately 20.7% of the patients with type 2 diabetes had experienced any peripheral neuropathic pain (19). An optimal cutoff ID pain score of ≥ 2 with a sensitivity of 77% is suggested for the diagnosis of peripheral neuropathic pain in the Taiwanese population (24). In the present study, the prevalence of DPNP was 21.8% based on the cutoff ID pain score of ≥ 2. Notably, a low prevalence (7.7%) of DPNP was found using the cutoff DN4 score of ≥ 4 in the present study. It has been reported that an optimal cutoff DN4 score of ≥ 3 with a similar sensitivity of 77% is suggested for the diagnosis of peripheral neuropathic pain in the Taiwanese population (25). The prevalence of DPNP was 15.7% when using the criterion of a cutoff DN4 score ≥ 3 (data not shown) in the present study, and was similar to the prevalence of 17.9% in outpatients with type 2 diabetes reported in Belgium (55). However, in the present study, we still used the criterion of a cutoff DN4 score ≥ 4, which has been validated for diagnosing DPNP in most countries (25).
Insulin was shown to possibly augment the mechanical responsiveness to mechanical stimuli and induce excitation in peripheral nerves in an in vitro study (56). In line with our findings, Huang et al. (40) reported that the use of insulin is an independent risk factor for DPN in patients with type 2 diabetes. On the other hand, metformin possibly attenuated neuropathic pain in a diabetic rat model (57). According to UK biobank data, the use of metformin had low odds of causing musculoskeletal pain in patients with type 2 diabetes (58). In the present study, the use of metformin was associated with a low pain score. Notably, long-term metformin use might cause vitamin B12 deficiency, and American Diabetes Association recommended monitoring vitamin B12 levels in patients with DPN (59).
Unlike prospective studies, we did not estimate case numbers before enrolling this retrospective cohort. Previous studies reported that DPNP prevalence was approximately 16.7% in type 2 diabetes (54, 55), and the cumulative mortality rate might be 11.5% in patients with DPNP and 8.5% in patients without DPNP during a median 3.1-year interval (19). The patient number of 2310 yielded statistical power > 0.85 for a median 6.2-year follow-up. However, CV diseases caused only 19.2% of total mortality in the present study, and the proportion of CV mortality was similar to the 19.6% in 2014 based on the Taiwan National Health Insurance Research Database (60). Despite the close relationship between neuropathic pain and CV risk, we only collected mortality data during follow-up, and could not assess CV event incidence. Based on the CV−total mortality ratio in the present study, the increased mortality risk in the patients with DPNP might not be driven only by CV diseases. Moreover, there are several limitations in the present study that should be acknowledged. First, we did not directly investigate the underlying mechanism between neuropathic pain and total mortality in patients with type 2 diabetes. Second, we did not collect all clinical information on DPNP, such as the duration of pain. Third, we did not investigate the effects of specific interventions for DPNP to improve prognosis in the observation study. Fourth, we aimed to use the convenient DPNP symptom scores to predict long-term mortality. A two-stage screening model was recommended for diagnosing DPN (6). However, we only collected questionnaire data but did not further diagnose DPN using electrodiagnostic tests. Finally, the ID pain and DN4 questionnaires are screening tools for diagnosing DPNP instead of estimating DPNP severity. Therefore, we could not speculate that a high score was correlated with high severity in DPNP.
In the present study, we found that diagnosis of DPNP using the ID pain and DN4 were predictive of mortality. Furthermore, there was high consistency between the application of the ID pain and DN4 scores when assessing neuropathic pain among outpatients with type 2 diabetes. However, the discrepancy in the diagnosis of DPNP weakened the prediction of mortality. Screening for DPNP by the ID pain and DN4 questionnaires is helpful for categorizing the long-term risk of mortality in patients with type 2 diabetes. Further larger-scale studies are required to fully investigate the potential mechanisms between neuropathic pain and mortality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Institutional Review Board of Taichung Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YL and IL conceived and designed the study. YL and IL organized all data. YL, SL, and IL, analyzed and visualized the results. YL and SL wrote the manuscript. IL reviewed and edited the manuscript. IL is the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This research was funded by the Taichung Veterans General Hospital, Taiwan (grant number TCVGH-1113501C), the National Health Research Institute (grant number NHRI-EX111-10927HT), and the Ministry of Science and Technology, Taiwan (grant number MOST 110-2314-B-075A-004 -MY3). The funders had no role in the decision to publish the results.
We thank the Diabetes Care Center of Taichung Veterans General Hospital for their support. Statistical analysis was performed by the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: A position statement by the American diabetes association. Diabetes Care (2017) 40(1):136–54. doi: 10.2337/dc16-2042
2. American Diabetes Association. 11. microvascular complications and foot care: Standards of medical care in diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S151–S67. doi: 10.2337/dc21-S011
3. Lee CC, Perkins BA, Kayaniyil S, Harris SB, Retnakaran R, Gerstein HC, et al. Peripheral neuropathy and nerve dysfunction in individuals at high risk for type 2 diabetes: The PROMISE cohort. Diabetes Care (2015) 38(5):793–800. doi: 10.2337/dc14-2585
4. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers (2019) 5(1):41. doi: 10.1038/s41572-019-0092-1
5. Lu Y, Xing P, Cai X, Luo D, Li R, Lloyd C, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: Estimates of the INTERPRET-DD study. Front Public Health (2020) 8:534372. doi: 10.3389/fpubh.2020.534372
6. Hsu WC, Chiu YH, Chiu HC, Liou HH, Jeng YC, Chen TH. Two-stage community-based screening model for estimating prevalence of diabetic polyneuropathy (KCIS no. 6) Neuroepidemiology (2005) 25(1):1–7. doi: 10.1159/000085306
7. Lee CM, Chang CC, Pan MY, Chang CF, Chen MY. Insufficient early detection of peripheral neurovasculopathy and associated factors in rural diabetes residents of Taiwan: a cross-sectional study. BMC Endocr Disord (2014) 14:89. doi: 10.1186/1472-6823-14-89
8. Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care (2003) 26(6):1790–5. doi: 10.2337/diacare.26.6.1790
9. Mehra M, Merchant S, Gupta S, Potluri RC. Diabetic peripheral neuropathy: Resource utilization and burden of illness. J Med Econ (2014) 17(9):637–45. doi: 10.3111/13696998.2014.928639
10. Gylfadottir SS, Christensen DH, Nicolaisen SK, Andersen H, Callaghan BC, Itani M, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: A cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain (2020) 161(3):574–83. doi: 10.1097/j.pain.0000000000001744
11. Gylfadottir SS, Weeracharoenkul D, Andersen ST, Niruthisard S, Suwanwalaikorn S, Jensen TS. Painful and non-painful diabetic polyneuropathy: Clinical characteristics and diagnostic issues. J Diabetes Investig (2019) 10(5):1148–57. doi: 10.1111/jdi.13105
12. Naranjo C, Ortega-Jimenez P, Del Reguero L, Moratalla G, Failde I. Relationship between diabetic neuropathic pain and comorbidity. Their impact on pain intensity, diabetes complications and quality of life in patients with type-2 diabetes mellitus. Diabetes Res Clin Pract (2020) 165:108236. doi: 10.1016/j.diabres.2020.108236
13. Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID pain. Curr Med Res Opin (2006) 22(8):1555–65. doi: 10.1185/030079906X115702
14. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain (2005) 114(1-2):29–36. doi: 10.1016/j.pain.2004.12.010
15. Spallone V, Morganti R, D'Amato C, Greco C, Cacciotti L, Marfia GA. Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabetes Med (2012) 29(5):578–85. doi: 10.1111/j.1464-5491.2011.03500.x
16. Padua L, Briani C, Truini A, Aprile I, Bouhassira D, Cruccu G, et al. Consistence and discrepancy of neuropathic pain screening tools DN4 and ID-pain. Neurol Sci (2013) 34(3):373–7. doi: 10.1007/s10072-012-1011-3
17. Hsu WC, Chiu SY, Yen AM, Chen LS, Fann CY, Liao CS, et al. Somatic neuropathy is an independent predictor of all- and diabetes-related mortality in type 2 diabetic patients: a population-based 5-year follow-up study (KCIS no. 29). Eur J Neurol (2012) 19(9):1192–8. doi: 10.1111/j.1468-1331.2011.03659.x
18. Bjerg L, Nicolaisen SK, Christensen DH, Nielsen JS, Andersen ST, Jorgensen ME, et al. Diabetic polyneuropathy early in type 2 diabetes is associated with higher incidence rate of cardiovascular disease: Results from two Danish cohort studies. Diabetes Care (2021) 44(7):1714–21. doi: 10.2337/dc21-0010
19. Lapin BR, Pantalone KM, Milinovich A, Morrison S, Schuster A, Boulos F, et al. Pain in patients with type 2 diabetes-related polyneuropathy is associated with vascular events and mortality. J Clin Endocrinol Metab (2020) 105(9):dgaa394. doi: 10.1210/clinem/dgaa394
20. Lee IT, Hsu CC, Sheu WH, Su SL, Wu YL, Lin SY. Pay-for-performance for shared care of diabetes in Taiwan. J Formos Med Assoc (2019) 118 Suppl 2:S122–S9. doi: 10.1016/j.jfma.2019.08.011
21. Chen YC, Lee CT, Lin BJ, Chang YY, Shi HY. Impact of pay-for-performance on mortality in diabetes patients in Taiwan: A population-based study. Med (Baltimore) (2016) 95(27):e4197. doi: 10.1097/MD.0000000000004197
22. Li J, Feng Y, Han J, Fan B, Wu D, Zhang D, et al. Linguistic adaptation, validation and comparison of 3 routinely used neuropathic pain questionnaires. Pain Physician (2012) 15(2):179–86.
23. Chan A, Wong S, Chen PP, Tsoi TH, Lam J, Ip WY, et al. Validation study of the Chinese identification pain questionnaire for neuropathic pain. Hong Kong Med J (2011) 17(4):297–300.
24. Yang CC, Ro LS, Tsai YC, Lin KP, Sun WZ, Fang WT, et al. Development and validation of a Taiwan version of the ID pain questionnaire (ID pain-T). J Chin Med Assoc (2018) 81(1):12–7. doi: 10.1016/j.jcma.2017.06.019
25. Wang YF, Yang CC, Ro LS, Tsai YC, Lin KP, Sun WZ, et al. Development and validation of a Taiwan version of the DN4-T questionnaire. J Chin Med Assoc (2019) 82(8):623–7. doi: 10.1097/JCMA.0000000000000129
26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI 3rd, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
27. Targher G, Zoppini G, Mantovani W, Chonchol M, Negri C, Stoico V, et al. Comparison of two creatinine-based estimating equations in predicting all-cause and cardiovascular mortality in patients with type 2 diabetes. Diabetes Care (2012) 35(11):2347–53. doi: 10.2337/dc12-0259
28. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis (2014) 63(5):713–35. doi: 10.1053/j.ajkd.2014.01.416
29. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American heart Association/National heart, lung, and blood institute scientific statement. Circulation (2005) 112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
30. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (1986) 1(8476):307–10. doi: 10.1016/S0140-6736(86)90837-8
31. Fayaz A, Ayis S, Panesar SS, Langford RM, Donaldson LJ. Assessing the relationship between chronic pain and cardiovascular disease: A systematic review and meta-analysis. Scand J Pain (2016) 13:76–90. doi: 10.1016/j.sjpain.2016.06.005
32. Ziegler D, Tesfaye S, Spallone V, Gurieva I, Al Kaabi J, Mankovsky B, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: International expert consensus recommendations. Diabetes Res Clin Pract (2022) 186:109063. doi: 10.1016/j.diabres.2021.109063
33. Sloan G, Selvarajah D, Tesfaye S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol (2021) 17(7):400–20. doi: 10.1038/s41574-021-00496-z
34. Orstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, et al. Abnormal function of c-fibers in patients with diabetic neuropathy. J Neurosci (2006) 26(44):11287–94. doi: 10.1523/JNEUROSCI.2659-06.2006
35. Tesfaye S, Kempler P. Painful diabetic neuropathy. Diabetologia (2005) 48(5):805–7. doi: 10.1007/s00125-005-1721-7
36. Calcutt NA. Diabetic neuropathy and neuropathic pain: a (con)fusion of pathogenic mechanisms? Pain (2020) 161(Suppl 1):S65–86. doi: 10.1097/j.pain.0000000000001922
37. Pai YW, Lin CH, Lee IT, Chang MH. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab (2018) 44(2):129–34. doi: 10.1016/j.diabet.2018.01.015
38. Chang YS, Lee LY, Lee IT. Variability in annual fasting glucose and the risk of peripheral artery disease in patients with diabetes mellitus. Diabetes Metab Syndr Obes (2021) 14:4109–19. doi: 10.2147/DMSO.S330606
39. Xu D, Fang H, Xu W, Yan Y, Liu Y, Yao B. Fasting plasma glucose variability and all-cause mortality among type 2 diabetes patients: A dynamic cohort study in shanghai, China. Sci Rep (2016) 6:39633. doi: 10.1038/srep39633
40. Huang L, Shen X, Huang L, Yan S, Wu P. Identification of independent risk factors for diabetic neuropathy progression in patients with type 2 diabetes mellitus. J Int Med Res (2021) 49(9):3000605211044366. doi: 10.1177/03000605211044366
41. Li YH, Sheu WH, Lee IT. Effects of retinopathy and chronic kidney disease on long-term mortality in type 2 diabetic inpatients with normal urinary albumin or protein: a retrospective cohort study. BMJ Open (2018) 8(7):e021655. doi: 10.1136/bmjopen-2018-021655
42. Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J Clin Endocrinol Metab (2016) 101(9):3401–8. doi: 10.1210/jc.2016-2030
43. Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, et al. Methylglyoxal evokes pain by stimulating TRPA1. PloS One (2013) 8(10):e77986. doi: 10.1371/journal.pone.0077986
44. Bierhaus A, Fleming T, Stoyanov S, Leffler A, Babes A, Neacsu C, et al. Methylglyoxal modification of Nav1.8 facilitates nociceptive neuron firing and causes hyperalgesia in diabetic neuropathy. Nat Med (2012) 18(6):926–33. doi: 10.1038/nm.2750
45. Hanssen NMJ, Westerink J, Scheijen J, van der Graaf Y, Stehouwer CDA, Schalkwijk CG, et al. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease and mortality in individuals with type 2 diabetes. Diabetes Care (2018) 41(8):1689–95. doi: 10.2337/dc18-0159
46. Sorensen L, Siddall PJ, Trenell MI, Yue DK. Differences in metabolites in pain-processing brain regions in patients with diabetes and painful neuropathy. Diabetes Care (2008) 31(5):980–1. doi: 10.2337/dc07-2088
47. Sloan G, Shillo P, Selvarajah D, Wu J, Wilkinson ID, Tracey I, et al. A new look at painful diabetic neuropathy. Diabetes Res Clin Pract (2018) 144:177–91. doi: 10.1016/j.diabres.2018.08.020
48. Jung YH, Kim H, Jeon SY, Kwon JM, Lee WJ, Jang JH, et al. Peripheral and central metabolites affecting depression, anxiety, suicidal ideation, and anger in complex regional pain syndrome patients using a magnetic resonance spectroscopy: A pilot study. Psychiatry Investig (2018) 15(9):891–9. doi: 10.30773/pi.2018.06.17
49. Jollant F, Near J, Turecki G, Richard-Devantoy S. Spectroscopy markers of suicidal risk and mental pain in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry (2016), S0278–5846(16)30167-1. doi: 10.1016/j.pnpbp.2016.10.005
50. Azoulay D, Abed S, Sfadi A, Sheleg O, Shaoul E, Shehadeh M, et al. Low brain-derived neurotrophic factor protein levels and single-nucleotide polymorphism Val66Met are associated with peripheral neuropathy in type II diabetic patients. Acta Diabetol (2020) 57(7):891–8. doi: 10.1007/s00592-020-01508-6
51. Lee IT, Fu CP, Lee WJ, Liang KW, Lin SY, Wan CJ, et al. Brain-derived neurotrophic factor, but not body weight, correlated with a reduction in depression scale scores in men with metabolic syndrome: a prospective weight-reduction study. Diabetol Metab Syndr (2014) 6(1):18. doi: 10.1186/1758-5996-6-18
52. Lee IT, Li YH, Sheu WH. Brain-derived neurotrophic factor during oral glucose tolerance test predicts cardiovascular outcomes. Int J Mol Sci (2020) 21(14):5008. doi: 10.3390/ijms21145008
53. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA augsburg surveys S2 and S3. Pain Med (2009) 10(2):393–400. doi: 10.1111/j.1526-4637.2008.00555.x
54. Truini A, Spallone V, Morganti R, Tamburin S, Zanette G, Schenone A, et al. A cross-sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain (2018) 159(12):2658–66. doi: 10.1097/j.pain.0000000000001378
55. Van Acker K, Bouhassira D, De Bacquer D, Weiss S, Matthys K, Raemen H, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab (2009) 35(3):206–13. doi: 10.1016/j.diabet.2008.11.004
56. Hotta N, Katanosaka K, Mizumura K, Iwamoto GA, Ishizawa R, Kim HK, et al. Insulin potentiates the response to mechanical stimuli in small dorsal root ganglion neurons and thin fibre muscle afferents in vitro. J Physiol (2019) 597(20):5049–62. doi: 10.1113/JP278527
57. Cao XJ, Wu R, Qian HY, Chen X, Zhu HY, Xu GY, et al. Metformin attenuates diabetic neuropathic pain via AMPK/NF-kappaB signaling pathway in dorsal root ganglion of diabetic rats. Brain Res (2021) 1772:147663. doi: 10.1016/j.brainres.2021.147663
58. Carvalho ESAP, Harmer AR, Ferreira ML, Ferreira PH. The effect of the anti-diabetic drug metformin on musculoskeletal pain: A cross-sectional study with 21,889 individuals from the UK biobank. Eur J Pain (2021) 25(6):1264–73. doi: 10.1002/ejp.1747
59. American Diabetes Association. 3. prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S34–9. doi: 10.2337/dc21-S003
Keywords: diabetes, DN4, ID pain, mortality, neuropathy, painful
Citation: Liau YJ, Lin SF and Lee IT (2022) Scores of peripheral neuropathic pain predicting long-term mortality in patients with type 2 diabetes: A retrospective cohort study. Front. Endocrinol. 13:969149. doi: 10.3389/fendo.2022.969149
Received: 14 June 2022; Accepted: 27 July 2022;
Published: 16 August 2022.
Edited by:
Rayaz A. Malik, Weill Cornell Medicine- Qatar, QatarReviewed by:
Shazli Azmi, Manchester University NHS Foundation Trust (MFT), United KingdomCopyright © 2022 Liau, Lin and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I-Te Lee, aXRsZWVAdmdodGMuZ292LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.