
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 10 August 2022
Sec. Neuroendocrine Science
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.957114
This article is part of the Research TopicThe neuroendocrine female brain: from normal reproductive function to diseaseView all 9 articles
Classically the neurobiology of aggression has been studied exclusively in males. Thus, females have been considered mildly aggressive except during lactation. Interestingly, recent studies in rodents and humans have revealed that non-lactating females can show exacerbated and pathological aggression similarly to males. This review provides an overview of recent findings on the neuroendocrine mechanisms regulating aggressive behavior in females. In particular, the focus will be on novel rodent models of exaggerated aggression established in non-lactating females. Among the neuromodulatory systems influencing female aggression, special attention has been given to sex-steroids and sex-steroid-sensitive neuronal populations (i.e., the core nuclei of the neural pathway of aggression) as well as to the neuropeptides oxytocin and vasopressin which are major players in the regulation of social behaviors.
Typically aggressive behavior is defined as an innate social behavior expressed whenever animals face conflicts over essential resources for their survival such as mates, food, water, and/or territory (1, 2). The fact that in nature aggression is displayed in those territorial settings has led scientists to assume that females are only, if so, mildly aggressive (1–4) except during the physiologically unique period of lactation where females will exacerbate their aggressive behavior to defend their offspring (5). These assumptions come from the fact that males and females differ significantly in terms of body size and weaponry to aggress (claws, canines, and horns) as well as in selective pressure, regarding their reproductive fitness (4, 6, 7). In general, males tend to adopt a polygynous strategy to guarantee their reproductive success, meaning that males will try to mate with as many females as possible. To accomplish that, they will need to gain access to more resources (food, water, and/or territory) to find their mates and to have the physical strength necessary to protect those mates against possible competitors (1, 8).
By contrast, according to this theory, female reproductive fitness is not based on a polygynous strategy as females are generally fertilized by one male (in most vertebrate species at least) (9). Thus, in theory, females do not need to compete for those resources. In addition, aggression might be a risk for females as the possibility of severe injury could strongly decrease their reproductive success (8). However, once pregnancy and lactation are achieved, this picture shifts as females invest a lot of their energy in pregnancy, lactation, and maternal care. Therefore, exacerbated maternal aggression becomes an adaptative response to protect their offspring and consequently achieve reproductive success (4–6, 8).
Although this evolutionary theory of sexual dimorphism on aggression appears quite appealing at first sight, it is necessary to point out that females might also compete for crucial resources for their survival especially when those resources are limited. For example, females need to get access to nutrition and territory as those are necessary for fitness. Thus, one could hypothesize that females compete to gain access to the best options available (7, 10). When it comes to sexual courtship and mate choice, females for may also compete for the “fittest” mates, with perhaps the best examples the lekking topi antelopes and female prairie voles which actively compete for sexual partners (10). Female voles develop wander behavior with polyandrous (mating with several males) strategy similarly to wander males (polygynous) (11), which is also accompanied by increased aggression and decreased affiliative behaviors towards other females (12). Additionally, one could predict that females need to be able to reject unsuitable mating partners and defend themselves in case of retaliation. Altogether, those scenarios illustrate some situations, in which non-lactating females should display some levels of aggression in order to either protect themselves from threats or to guarantee their survival and/or fitness.
Accordingly, aggression in territorial/”rivalry” related conditions have been reported in females of several species from invertebrates, such as fruit flies (13, 14) and octopus (15) to vertebrates, such as fish (7), birds (16, 17), rodents (mice (18–22), rats (23–28), hamsters (29–35), mole rats (6), voles (12), lemurs, marmoset, hyenas (6), antelopes (10), and humans (8, 36–38). Despite this compelling evidence of the occurrence of female aggression in nature, the neural substrates and neuroendocrine factors underlying this behavior have only recently gained scientific attention. In fact, the neurobiological underpinnings of non-maternal female aggression are still relatively unknown in comparison to intermale aggression.
Here it is important to highlight that besides showing adaptative aggression, females might also develop pathological and disruptive aggression. For instance, recent reports in humans have demonstrated that girls and women develop aggression disorders such as conduct and antisocial personality disorder similarly to boys and men, respectively (8, 36–39). Thus, the study of the neurobiological mechanisms underlying female aggression is not only relevant for basic but also for translational neuroscience research in order to find suitable treatment options for aggression disorders in both sexes.
In this review, we will describe the recent discoveries on the neuroendocrine mechanisms regulating female aggression especially focusing on non-lactating animal models of female aggression. Additionally, we will particularly emphasize the role of neurosteroid-responsive neurons, core regulators of the neural pathway of aggression (1), as well as the neurohypophyseal hormones oxytocin and vasopressin, major modulators of social and aggressive behaviors (40).
In most rodent species, females will typically exhibit a dramatic increase in their aggressive behavior during the first week of lactation, the so-called maternal aggression. Maternal aggression wanes over the last half of lactation, disappearing around weaning or when pups are culled (3, 5, 18). Conventionally, maternal aggression is considered an important part of the repertory of behaviors displayed by lactating rodents as the survival of pups depends on a combination of maternal care and maternal aggression (5). In fact, several authors do not consider maternal aggression as a typical antisocial behavior, but rather a prosocial type of aggression as it is displayed not to protect oneself, but rather to protect others (5, 41, 42). Despite this hypothesis, maternal aggression is a highly adaptative behavior that assures fitness similarly to territorial aggression (1, 4, 5).
Interestingly, although the pure levels of maternal aggression do not seem to differ from the levels of aggression displayed by males in the resident intruder test (RIT) (18, 43), qualitatively, lactating females seem to show severer attacks than males, as they attack vulnerable targets such as the head and genitals (43). From a neurobiological point of view, various neuropeptitidergic, as well as hormonal systems, are known to be up-regulated during lactation and involved in the expression of maternal aggression (4, 5). Of note, it is important to mention the participation of oxytocin (OXT) (5), vasopressin (AVP) (5), corticotropin-releasing hormone (CRH) (44), gonadotropin-releasing hormone (GnRH) (45), prolactin (46), estrogen receptor α (Erα)- (47), and aromatase-expressing neurons (43).
Importantly, when pertinent, we will make a parallel between the systems involved in maternal and non-maternal female aggression.
Although females are assumed to be less aggressive than males, in some rodent species, virgin females are known to display high levels of aggression, for a review please see (6, 35). Among those species, we will particularly focus on hamsters and California mice due to the important behavioral neuroendocrinology research conducted over the last decades on those animals.
Female Syrian hamsters (Mesocricetus auratus) are highly territorial and show aggression towards male and female intruders. In fact, female hamsters will only reduce their aggression towards males when they are sexually receptive (32–35, 48). Additionally, external factors such as social isolation (48) and successive aggressive interactions (32, 34) escalate the already high levels of aggression displayed by those animals.
Virgin female Peromyscus californicus are known to display high levels of aggression towards female intruders. Aggression was found to be especially dependent on photoperiod and experience, with higher scores on short days and after multiple testing (19). Notably, aggression in this species seems to be independent of the estrous cycle (22).
Although anecdotal data has described aggression among wild female mice, most of the studies using laboratory mouse strains have reported mild or no aggression in female mice (3, 4). However, recent findings have shown striking strain differences in naturally occurring aggression in female mice. Swiss but not C57BL/6N females display aggression towards male juveniles (in 80% of the encounters) and adult female intruders (in about 60% of the encounters). In those animals, social isolation was also reported to enhance aggression. Additionally, the estrous cycle of the resident female was found to not influence aggressive behavior (47).
Co-habitating a male resident with a stimulus female is a well-established method to induce reliable levels of aggression in male mice (3) and rats (49, 50). However, until recently this protocol was never used in females. Newman et al. (18) have shown that cohabitation with a male significantly increases the levels of aggression exhibited by Swiss female mice, which could be reduced by either social isolation or ovariectomy. Additionally, after pup culling, postpartum mice recovered their levels of aggression after being cohoused with gonadectomized males. Accordingly, nulliparous females cohoused with gonadectomized males displayed high levels of aggression (65% of the females presented more than 15 bites within the first 2 minutes of interaction), which were similar to the levels normally displayed by male mice, with females showing more rapid bite bouts and pursuit bites than males. Also in this model, no apparent effect of the estrous cycle on female aggression was found (18).
Recently, a new study has refined these findings by showing that co-housed CFW mice display similar levels of aggression independent of sex. Interestingly, although mice did not differ in attack latency and duration, they exhibited striking differences in the composition of their aggressive behavior with males displaying more wrestling and lung (threat-like behavior) compared to females. Females on the other hand showed a higher number of attack bites. Sex differences also emerged when the Hidden Markov Model was applied to characterize the behavioral strategies used by both sexes. Briefly, males engaged in persistent attack bouts (characterized by the occurrence of only aggressive behavior) whereas females displayed intermittent attacks which occurred in association with investigative social behaviors. This suggests that males show a more marked transition between investigative social interactions and aggressive behavior, whereas females appear to display mixed interactions (20). Furthermore, together with the fact that females display less wrestling and lung behavior, this indicates that aggression in female mice has a reactive and/or defensive phenotype, suggesting that they do not seek aggressive interactions. In fact, in the same study, aggressive interactions were not rewarding and/or reinforcing (20) in females in the aggression-conditioned place preference test (51) and aggression operant self-administration procedure (52).
Similar results have been reported in an early study in female Wistar rats (24) where females were co-housed with a sterilized (ligation and incision of the vasa deferentia) male to induce pseudopregnancy. After pseudopregnancy vanished, those animals were found to display aggression towards an ovariectomized intruder. Differently from mice, in rats, aggression was found to be dependent on the estrous cycle as females in estrus were less aggressive than females in metestrus and diestrus (24).
In female prairie voles, cohabitation with a male is also known to enhance aggression and diminish affiliative behaviors (12). Likewise, cohabitation and the development of a pair bond have been suggested to increase aggression towards same-sex intruders in female California mice (21, 22).
Wistar rats typically display mild levels of aggression, regardless of sex (24, 28, 49, 50, 53). Nevertheless, male Wistar rats have been consistently used to study aggression in combination with the most diverse paradigms to induce high levels of aggression, for a review please see (49, 54, 55). Females, on the other hand, have been poorly studied. Recent findings using the female intruder test (FIT), a direct analog to the RIT, where an intact normally cycling female is confronted with a smaller (10-20% lighter) same-sex intruder for 10 min, showed that females display “rivalry” aggression towards conspecifics (25). Interestingly, group-housed male and female Wistar rats did not differ significantly in their levels of aggression (25, 28). Additionally, neither intruder nor the resident’s estrous cycle had an apparent effect on aggression (25).
Using the same paradigm, Oliveira et al. (23) found that a combination of social isolation (9 days) accompanied by successive aggressive interactions, i.e. daily 10 min exposition to a FIT in three consecutive days, strongly exacerbated the mild levels of aggression displayed by virgin female Wistar rats. Both socially isolated (IS) and isolated and trained (IST) females displayed higher levels of aggression (>20% on average) than non-trained group-housed (GH) females (<10% on average). Interestingly, isolation-induced aggression was affected by the estrous cycle as no increase in aggression was found in IS females in proestrus or estrus. The effects of the estrus cycle completely vanished in IST females (23).
Behavioral neurobiologists have established rodent models of excessive and pathological aggression using male Wistar rats as model organisms to understand which are the mechanisms underlying disruptive aggression in humans. Those models are known to exhibit the so-called abnormal aggression which is characterized by: i) mismatch between provocation and response (increased number of attacks or attack in neutral arenas); ii) disregard for species-specific rules (attacking juveniles, females, or vulnerable targets, such as gonads, paws, head or belly) and iii) insensitivity towards social signs of the intruder (attacking anesthetized and/or submissive intruders), for a review please see (55, 56).
Male rats selectively bred for low (LAB) and high (HAB) anxiety-related behavior display high and abnormal levels of aggression (57, 58). Particularly, LABs are known to be extremely aggressive towards females, anesthetized intruders, and to attack vulnerable targets (57). By contrast, maternal (5) and non-maternal (25) female aggression were found to be higher in HAB rather than in LAB rats, implying a sex-specific effect of the trait of anxiety on aggressive behavior. In fact, in virgin females, time spend in the open arm of the elevated plus-maze negatively correlated with aggression, meaning that a highly anxious female also showed high levels of aggression (25). Yet, it remains to be assessed whether HAB and LAB females show abnormal aggression similarly to males.
Briefly, exposition to a combination of fear-inducing stressors such as an open field, an elevated platform in bright-light conditions, and the synthetic fox odor trimethylthizoline (TMT), is shown to induce excessive and abnormal aggression in male Wistar rats (i.e., attack to vulnerable targets, smaller and anesthetized intruders) (56, 59). Importantly, in this protocol, animals are exposed (for 25 min) to those stressors in unpredictable and semi-random order with one or two stressors a day, during the peripubertal period, from postnatal day 28 until 42 (p28 to p42, a total of 7 days) (56, 59).
In females, peripubertal stress was found to increase aggression towards an ovariectomized intruder in the RIT, independently of the phase of the estrous cycle. Those animals were also reported to exhibit aggression towards males during the cohabitation period prior to parturition and to develop higher levels of maternal aggression (shorter attack latency), compared to non-stressed controls (26). It remains to be verified whether PB also induces other signs of abnormal aggression in females (such as attacks towards vulnerable targets or juveniles). Nevertheless, this model confirms the deleterious effects of stress during early life on aggressive behavior, independent of sex.
Social deprivation after weaning (p21) was described to robustly enhance the levels of aggression displayed by male Wistar rats and mice (56, 60, 61). Specifically, in male rats, PWSI is known to induce exaggerated as well as abnormal aggression (consisting of hard bites, attacks towards vulnerable targets, and non-signaled attacks, i.e. without threat or from defense) (61).
Similar results have been found in females as PWSI induced excessive and abnormal aggressive behavior in virgin female Wistar rats. Although isolated males and females did not differ in total levels of aggression, the number of attacks, and attacks towards vulnerable body parts, abnormal aggression was displayed in a sex-specific manner, with females showing higher levels of aggression towards juveniles and males tending to show a higher number of attacks and attacks towards vulnerable targets (28). Similar to PB, these data depict PWSI as a robust and reliable model to induce excessive and pathological aggression in both sexes.
Although the neurocircuitry of maternal aggression has been extensively characterized over the last decades (62, 63), the neural substrates of aggression in virgin/non-lactating females are largely unknown. A couple of studies using either the expression of immediate early genes and/or longitudinal in vivo calcium recordings (fiber photometry) have revealed some brain regions which might be implicated in the display of aggression by non-lactating females, for an overview please see Table 1.
Concerning hypothalamic areas, aggression was shown to activate the medial portion of the ventrolateral part of the ventromedial nucleus of the hypothalamus (VMHvl) (47, 64) as well as the hypothalamic attack area [HAA, mediobasal hypothalamus (55)] (25) in virgin female mice and rats, respectively. Additionally, in female Wistar rats, aggression was found to reduce the number of phosphorylated ERK (pERK) positive cells in the paraventricular nucleus of the hypothalamus (PVN) (25). Regarding limbic regions, the bed nucleus of stria terminalis (BNST) and the medial amygdala (MeA) show increased pERK after aggression (19) in California mice. In highly aggressive IST female rats, GABAergic neurons within the ventral lateral septum (vLS) (23), as well as the central amygdala (CeA) (27), were found to be activated after FIT exposure. By contrast, in the dorsal lateral septum (dLS), GABAergic neurons showed reduced pERK after the FIT. Additionally, activity (pERK/VGAT colocalization) negatively correlated with aggression in those animals (23). The participation of those subregions of the LS on female aggression was further confirmed using pharmacology: muscimol infusion into the dLS enhanced whereas, in the vLS, it decreased female aggression in Wistar rats (23). Regarding the brainstem, aggression was found to activate the lateral periaqueductal gray matter in female mice (47). Although those studies indicate the participation of crucial hubs regulating intermale aggression such as the LS (65) and mediobasal hypothalamus (66) also in female aggression, future studies should focus on dissecting those pathways and better characterizing the connections among those regions.
Sex steroids (estradiol, progesterone, and testosterone) are mainly produced in the gonads under the influence of the hypothalamus-pituitary-gonad (HPG) axis. Briefly, gonadotropin-releasing hormone (GnRH) will be released from the hypothalamus into the median eminence acting in gonadotropes at the anterior pituitary and triggering the release of luteinizing (LH) and follicle-stimulating hormones (FSH), which will then act at the gonads stimulating the production and release of sex-steroids (67, 68). In turn, sex steroids will travel back to the brain and act on neurons generating a negative feedback loop (68) but also modulating behavior (69–71).
Social behaviors such as social investigation, sexual and aggressive behavior, are influenced by sex steroids (69–71). Particularly estrogen receptor alpha (Erα) is expressed in the main nuclei embedded in the neural pathway of aggression such as the MeA, the lateral septum (LS), the BNST, the medial pre-optic area (MPOA), and VMHvl (1, 4, 72, 73). This pattern of expression together with the fact that this receptor exerts a crucial role in intermale aggression (1, 4, 71), has raised the hypothesis that sex differences in aggressive behavior are mainly driven by masculinization of the neural network of aggression by aromatized estradiol (4). Accordingly, aromatase knockout (ArKO) males show feminized brain responses (74) and reduced aggressive behavior towards a male but increased aggressive behavior towards an estrous female (75). Additionally, masculinization of the female brain, i.e. by exposing females to testosterone or estrogen during perinatal development, has been shown to exacerbate aggression in females as well as to make females more sensitive to the pro-aggressive activational effects of testosterone in adulthood, by increasing female attacks towards male intruders (76).
Although the data described above suggest an interaction between the organizational (70) and activational (70) effects of estrogens on the neural pathway of aggression to orchestrate the sex-specific behavioral patterns seen in adults, more research is needed. It is unlikely that femininization of the brain hubs controlling aggression completely abolishes aggressive behavior in females since females exhibit extreme levels of maternal aggression during lactation (5, 18). Thus, one could hypothesize that sex hormones would rather modulate or temporarily suppress female aggression.
Conversely, female aggression in several rodent species was found to be independent of the estrous cycle (18, 19, 22, 23, 25, 26, 28, 47), indicating that subtle alterations in plasma concentrations of sex steroids are unlikely to modulate aggression in females. Nevertheless, a minimum concentration of circulating sex hormones seems to be necessary for the display of aggression in females as ovariectomy completely suppressed aggression in female mice and rats, respectively (18, 24). Also, hormonal alterations and treatments were associated with aggression in female rodents. For instance, a reduced progesterone/testosterone ratio was found in the plasma of California mice after a confrontation with a same-sex intruder (77). Accordingly, in highly aggressive hamsters, progesterone infusion in estrogen-primed females abolished aggression (33). In another study, dihydrotestosterone infusion alone or combined with estradiol increased aggression in female rats (78). Of note, in those females, estradiol alone did not affect aggressive behavior. Again, those early studies (33, 78) somehow challenge the hypothesis that the neural circuitry of aggression is completely feminized in early development in females and as a result, it becomes irresponsive to sex steroids such as testosterone (4, 76).
Complementary evidence of the participation of sex hormones in non-lactating female aggression comes from studies using chemo- and optogenetics to manipulate the activity of ERα-positive neurons and knocking down the estrogen receptors in specific brain regions. ER-α knockout female mice (ERKO) were reported to display high levels of aggression towards gonadectomized and/or estrogen-primed female intruders (79). Ovariectomy only affected aggressive behavior in those animals when performed before aggression testing, meaning that once those animals displayed aggression towards an intruder for the first time, ovariectomy did no longer affect their aggressive behavior (79). This suggests that aggressive experience overrules circulating levels of sex steroids, in line with that aggression training is reported to overshadow the effect of the estrous cycle on aggressive behavior displayed by isolated rats (23). Later studies refined this approach demonstrating that specific knockdown of ER-α within the VMH increased aggressive behavior towards juveniles in female Wistar rats (80).
Recent studies have further dissected the participation of progesterone receptor (PR) and ER-α+ neurons (co-expressed in a ratio of 1:1 in the VMHvl) within the VMHvl on female aggression. Briefly, ER-α-PR+ neurons in the medial part of the VMHvl were found to be specifically activated during aggression in virgin and lactating Swiss mice. Additionally, inhibition and stimulation of those neurons reduced and enhanced aggression in Swiss mice, respectively, regardless of the reproductive state (47). Interestingly, stimulation of Erα-PR+ neurons did not induce aggression but only mount behavior in C57 virgin females. However, stimulation and inhibition of those neurons in lactating C57 females resembled the effects seen in lactating Swiss females (47). Altogether, those results indicate that i) Erα+ neurons within the VMHvl are important for the display of aggression in females independent of the reproductive state and ii) strain differences might impact aggression in female mice.
Furthermore, another study has refined these previous findings by showing that among the ER-α+ neurons in the VMHvl, particularly neuropeptide Y receptor 2 (Npy2r) expressing neurons are crucial for the display of aggression in females, regardless of the reproductive state (virgin x lactating). Strikingly, stimulation of those neurons was able to induce aggression in C57 virgin females (towards adult male and female intruders) even during ongoing sexual behavior. In fact, VMHvl ERα-Npy2r+ (also called β cells) were suggested to suppress sexual behavior by inhibiting ER-α-Npy2r- (α cells) (64). Thus, those findings i) confirm the existence of subpopulations of neurons within the VMHvl-ERα population controlling aggressive or sexual behaviors in female mice, ii) suggest that the neural circuitry of aggression is normally suppressed rather than absent in females and iii) suggest that the internal states such as lactation might inhibit sexual behavior by disinhibiting/activating aggression promoting neurons. Yet it remains to be addressed i) which are the endogenous factors leading to the shift between β and α cells activity, ii) the mechanisms by which these cells might inhibit each other (as the VMHvl ERα neurons are glutamatergic) and iii) whether the release/neurotransmission of the endogenous neuromodulators, i.e. NPY or estrogens, binding to its respective receptors resemble the effects of chemo- and optogenetic manipulations on behavior.
Finally, apart from ERα+ neurons, aromatase-positive neurons within the MeA amygdala were found to be necessary for maternal and intermale aggression (43). However, whether those neurons and aromatase activity also play a role in non-lactating female aggression still needs to be investigated. Of note, the expression of aromatase in fibers surrounding the VMHvl is sexually dimorphic and sensitive to the organizational effects of estrogen (76). Thus, aromatized estradiol may constitute the endogenous signal activating ERα+ neurons and subsequently triggering aggression in both sexes (47, 81). Additionally, the fact that aromatase fibers in the VMHvl are less abundant in females than in males (76) might explain the differences in the levels of aggression seen in mice.
Oxytocin is produced in the paraventricular (PVN) and supra-optic nucleus (SON) of the hypothalamus. Magnocellular OXT neurons release OXT into the bloodstream from the neurohypophysis during suckling and birth (82). However, within the brain, OXT release and signaling are known to be essential for the display of various social behaviors including aggression, for a review, please see (40, 82, 83). Typically in male mice and rats, OXT is reported to have anti-aggressive effects (40, 84–88). Consistent with the fact that during lactation the OXT system is up-regulated with elevated peptide content, OXT release, and OXT receptor (OXTR) binding (5, 82), maternal aggression was found to trigger OXT release in several regions within the aggression network, namely the PVN (89), CeA (89), BNST and LS (5).
In virgins, the role of the endogenous OXT system on aggressive behavior has been less studied. In the highly aggressive rodent species such as California mice, elevated levels of plasma OXT were found after an aggressive encounter, when housed in long days. This was accompanied by a higher number of OXT and pERK neurons in the PVN of long-day versus short-day housed females, independently of being exposed to aggression (90). Accordingly, high- and abnormally aggressive PWSI female Wistar Rats exhibit elevated levels of OXT mRNA in the PVN (28). Similar findings were obtained in highly aggressive IST rats with IST females showing elevated OXT release in their cerebrospinal fluid (CSF) and in the LS after and during the FIT, respectively. Importantly, those animals exhibited higher OXT release (CSF and locally in the LS) when compared to low-aggressive GH controls. Furthermore, OXT content positively correlated with aggression (23). Additionally, activation of OXT neurons in the PVN and SON via chemogenetics as well as stimulation of OXT axons in the vLS, by optogenetics, enhanced the mild levels of aggression displayed by GH females (23). Likewise, infusion of synthetic OXT i.c.v. or locally in the CeA exacerbated aggression in GH and IST rats, respectively (23, 27). Altogether, these data strongly indicate that OXT release promotes aggression in non-lactating females. This was further confirmed by the blockade of OXTRs either intracerebroventricularly (i.c.v.) or locally within the vLS in IST rats, OXTR antagonist (OXTR-A) was found to reduce the high levels of aggressive behavior displayed by IST females, independent of the site of infusion (23). These data strongly suggest a pro-aggressive effect of OXT in virgin females.
However, contrasting data have also been reported in rats and hamsters. In group-housed mildly aggressive female Wistar rats, exposure to the FIT decreased the activation of OXT neurons (measured by pERK) in the PVN (25). Although this might seem contradictory at first glance, one could hypothesize that this lower activity of the OXT neurons reflects the elevated release during the aggressive encounter. Therefore further studies should address how OXT neuronal activity relates to OXT release during aggression. Furthermore, synthetic infusion with OXT was found to decrease aggression in virgin LAB and NAB (non-selected for anxiety-related behavior, i.c.v.) (25) and hamsters (into the MPOA-AH) (91). Those pharmacological results should be interpreted carefully as cross-reactivity of synthetic OXT binding to vasopressin 1a receptor (V1aR) has been reported in the context of aggression (87, 92, 93). In fact, i.c.v. OXT infusion was found to decrease aggression in IST female rats, however, those effects were mediated via V1aRs (23).
Further evidence of the pro-aggressive role of OXT comes from the fact that OXTR binding is upregulated in lactating females in areas such as the LS, BNST, and MPOA (94). Interestingly, in highly aggressive virgin rats, OXTR binding was found to be reduced in the vLS (23) and nucleus accumbens (NAc) (28) but increased in the CeA (27). Those results although contradictory might only reflect differences in local OXT release as both increased and decreased OXTR binding have been associated with enhanced OXT release, for a review please see (82, 83). The decreased OXTR binding in the NAc is particularly interesting as escalated female aggression was shown to induce plasticity changes in this region in hamsters (32, 34).
Taken together, these data support a pro-aggressive role of endogenous OXT independently of the reproductive state. From an evolutionary point of view, adopting the same system to regulate lactational and non-lactational female aggression is to be expected. Especially, knowing that during lactation, both aggressive behavior (4, 5) and OXT system activity (increased synthesis, release, and receptor binding) are elevated (5, 82). It remains to be addressed, however, whether OXT acts on the same neuronal pathways/populations to trigger aggression in both states (virgin x lactating) and whether differences in the severity of aggressive behavior in lactating females are also reflected by changes in the OXT system (receptor binding, release, and signaling).
Vasopressin is the sister peptide of OXT. It is mainly produced in the PVN and SON, but parvocellular vasopressin expressing neurons are also found in the BNST, MeA, suprachiasmatic nucleus of the hypothalamus, and olfactory bulb (95, 96). Of note AVP positive cell bodies and axon terminal density, differ significantly across sexes, with males typically exhibiting more cell bodies in the BNST and MeA and higher fiber density in various hypothalamic regions and in the LS (95). Therefore, AVP has been suggested to drive male-typical social behaviors such as aggression (40, 83).
Accordingly, local AVP release was reported after aggression in male Wistar rats within the LS (40, 97). However, the link between AVP release/signaling and intermale aggression appears to be more complex depending on several factors such as levels of aggression (97), brain region (97), traits of anxiety (98), the display of abnormal aggression (98, 99) and dominance (100), for a review please see (40, 101).
Similar to males, the role of AVP on aggression displayed by lactating females seems to be intricate as well. Although AVP release within the CeA has been described during the display of maternal aggression in HAB rats (102), i.c.v. infusion of synthetic AVP was shown to decrease aggression in stressed lactating rats (103). Additionally, blockade of V1aRs was found to increase maternal aggression in Sprague-Dawley rats (104, 105), suggesting an anti-aggressive role of endogenous AVP. Yet, early life experiences also seem to affect the link between maternal aggression and AVP, as plasma AVP was found to be positively and negatively correlated with maternal aggression in PB stressed and control female Wistar rats, respectively (26).
In non-lactating females, compelling evidence has suggested an anti-aggressive role of the AVP system on aggression. In Syrian hamsters, infusion of synthetic AVP into the AH was shown to decrease female aggression (30). Additionally, AVP neurons in the PVN were not activated after aggression exposure in dominant female hamsters (30). In female Wistar rats, decreased or blunted AVP release centrally (CSF) or locally within the LS was reported after or during the FIT, respectively (23). Interestingly, highly aggressive IST females showed reduced AVP release in the dLS when compared to low-aggressive GH females. Accordingly, infusion of synthetic AVP either i.c.v. or within the dLS was shown to reduce female aggression in IST rats. Further evidence of the anti-aggressive effects of the endogenous AVP system on female aggression, comes from the fact that blockade of V1aRs in the dLS (region rich in V1aR but not OXTR binding) increases aggression in female Wistar rats independently of the level of aggression or housing condition of those animals (GH x IST) (23). Contrasting results have been obtained for the CeA where AVP was described to instigate aggression in highly aggressive IST females (27). Interestingly, blockade of V1aRs within the CeA did not affect aggression, indicating that the endogenous AVP release locally is not necessary for the display of female aggression (27).
Regarding V1aR densities, decreased V1aR binding in the anterior BNST, lateral hypothalamus, and dentate gyrus was associated with increased aggression in abnormally aggressive PWSI female Wistar rats. Importantly, V1aRs in the anterior BNST were differently affected by PWSI in males and females, i.e. PWSI females showed a decrease whereas males an increase in receptor binding after PWSI (28). Furthermore, reduced V1aR binding was reported in the dLS of highly aggressive IST females (23). Accordingly, knocking out V1aRs (globally) in female hamsters strongly enhanced aggression (106). Taken together, these data imply a blunted AVP signaling in various limbic and hypothalamic regions to be associated with exacerbated aggression in virgin females.
Apart from V1aRs, AVP binds also to vasopressin 1 b receptors (V1bRs) which are widespread in the rodent brain (107, 108). Expression of V1bRs (109, 110), as well as stimulation of V1bR-positive terminals projecting from the hippocampal CA2 to the dLS (111), increase aggression in male mice. Those effects seem to be sexually dimorphic as activation of V1b receptors either centrally (i.c.v.) or within the MPOA or BNST did not affect maternal aggression (112–114). To the best of our knowledge, the participation of V1bRs in non-maternal female aggression has not been investigated.
Although the anti-aggressive role of AVP seems to be evident in virgin females, the AVP neuronal population (s) contributing to those anti-aggressive effects still needs to be identified. This is especially important in the context of the sex-specific modulation of aggression by septal AVP release (23, 97). Additionally, the contribution of sex-specific distribution of AVP fibers (higher in males) and V1aRs (higher in females) to those sex-dependent effects still needs to be addressed. Future studies should dissect how endogenous AVP signaling differently contributes to instigating aggression in both sexes.
Other neuroendocrine systems have been associated with maternal aggression in rats and mice. Of note, blockade of GnRH receptors i.c.v (45). as well as activation of prolactin and CRH 2 receptors (44) were found to reduce maternal aggression. However, the role of those peptides in virgin female aggression remains unknown. Thus we will focus on two other systems, i.e., the hypothalamic-pituitary-adrenal (HPA) axis and serotonin.
The HPA axis, as well as corticosterone (CORT), are known to strongly modulate aggression in male rodents (56, 101). Typically both high and low plasma CORT levels, as well as increased and decreased HPA axis activity, have been associated with aggressive behavior in males (101). Additionally, early life stress which is known to hyperstimulate (activate) the HPA axis is frequently described as a consistent model for inducing exaggerated and abnormal aggression in males [for a review please see (56, 101, 115)].
In females, the link between stress, HPA axis activity, CORT, and aggression is less clear. Nevertheless, all classical early life stress protocols used in male rodents to induce pathological aggression (56, 115), i.e. maternal separation, post-weaning social isolation, and peripubertal stress are known to increase aggression in females as well. In contrast to males, maternal separation was reported to enhance maternal aggression in mice (116). In female Wistar rats, both PB and PWSI exacerbate aggression in virgin females (26, 28), for further details please see section 3.
Notably, the role of the HPA axis and CORT in virgin female aggression is largely understudied. Confrontation with an intruder in the RIT leads to increased plasma CORT in naturally aggressive female California mice (90), suggesting that aggression triggers CORT release in females. Interestingly, aggression was found to negatively correlate with plasma CORT in female Wistar rats after the FIT (23), consistent with previous data in humans and animals associating hypo- arousal and CORT with exaggerated and abnormal aggression (56, 101, 117). Similarly, in PB female rats, plasma CORT was found to negatively correlate with non-maternal aggression, those animals also showed a reduced CORT response to an elevated platform when compared to controls (26). Intriguingly, those effects seem to be reproductive state-dependent as exposition to a male intruder and consequently display of maternal aggression increases maternal anxiety and decreases maternal care (both indicators of stress) in dams (118). However, whether those behavioral differences found in mothers are reflected by alterations in the HPA axis activity is unclear.
Altogether, those findings indicate that i) stress (especially during early life) strongly impacts aggression in females, ii) as in males, aggression appears to lead to increased CORT release in females, and iii) female aggression in non-lactating rats seems to negatively correlate with plasma CORT. Future studies should address the time course between CORT secretion and aggression display in females as well as acutely manipulate CORT levels and evaluate its effects on aggressive behavior. These studies should also address the potential reproductive state-dependent effects (virgin x lactation) on the HPA axis activity and subsequent aggressive behavior.
The serotoninergic system is among one of the most studied neurotransmitter systems in the context of aggression. Typically, serotonin (or agonism of its receptors) is known to be anti-aggressive in males (1, 119–121). Additionally, the elevation of 5-HT levels via selective serotonin reuptake inhibitors (SSRIs) has been consistently used to treat pathological aggression in humans and rodent models (122).
In females, the link between serotonin and aggression is less clear. In naturally aggressive female but not male hamsters, the acquisition of dominance and display of aggression was linked to increased activation of serotoninergic neurons within the anterior and posterior dorsal Raphe nucleus. Additionally, treatment with either fluoxetine (SSRI) or OH-DPAT (selective 5-HT1a receptor agonist) exacerbated aggression in female hamsters (30). Conversely in non-lactating female Wistar rats, SSRIs (fluoxetine and escitalopram) were found to decrease aggression (23, 24). Studies in female mice support a similar picture as tryptophan hydroxylase 2 (Tph2) knockout (Tph2KO) mice showed escalated female aggression (123).
Taken together, these data suggest the participation of the serotoninergic system in non-lactating female aggression. Yet, it remains to be determined i) which are the serotoninergic neuronal populations relevant for this behavior in females, ii) the role of different serotonin receptors on female aggression and iii) how changes in serotonin neurotransmission relate to aggressive behavior in females.
We summarized here recent findings on the neurobiological mechanisms regulating female aggression. Importantly, in the last years, several rodent models of territorial/”rivalry”related aggression have been established in female rodents modeling different aspects of this behavior. Those protocols offer to behavioral neurobiologists a wide range of possibilities to focus either on ethological aspects (naturally aggressive species), instigations protocols (co-housing, social isolation, and/or aggression training), or on abnormal and pathological aggression (MS, PWSI, and PB). Thus, the development and use of those behavioral paradigms and/or models might allow scientists to shed light on the neural underpinnings regulating aggression in both sexes.
Regarding the neuroendocrine mechanisms underlying aggressive behavior in females, we highlighted the role of ER-α+ neurons within the VMHvl and of the peptides, OXT, and AVP. Interestingly, these systems were shown to regulate maternal and non-maternal female aggression similarly. As mentioned above, activation of ER-α+ neurons in the VMHvl, as well as OXT release, seems to promote aggression independently of the reproductive state. Additionally, AVP seems to be anti-aggressive in both lactating and virgin females. Again, co-opting the same neuroendocrine systems to regulate aggression in virgins and dams makes sense from an evolutionary point of view. However, it remains to be addressed whether i) the same neural circuits are regulating aggression in both states and ii) whether the high levels of aggression (severer attacks, for instance) in lactating females are also associated with an up-regulation of those systems, i.e. release, synthesis, receptor densities and neuronal activity (Figure 1).
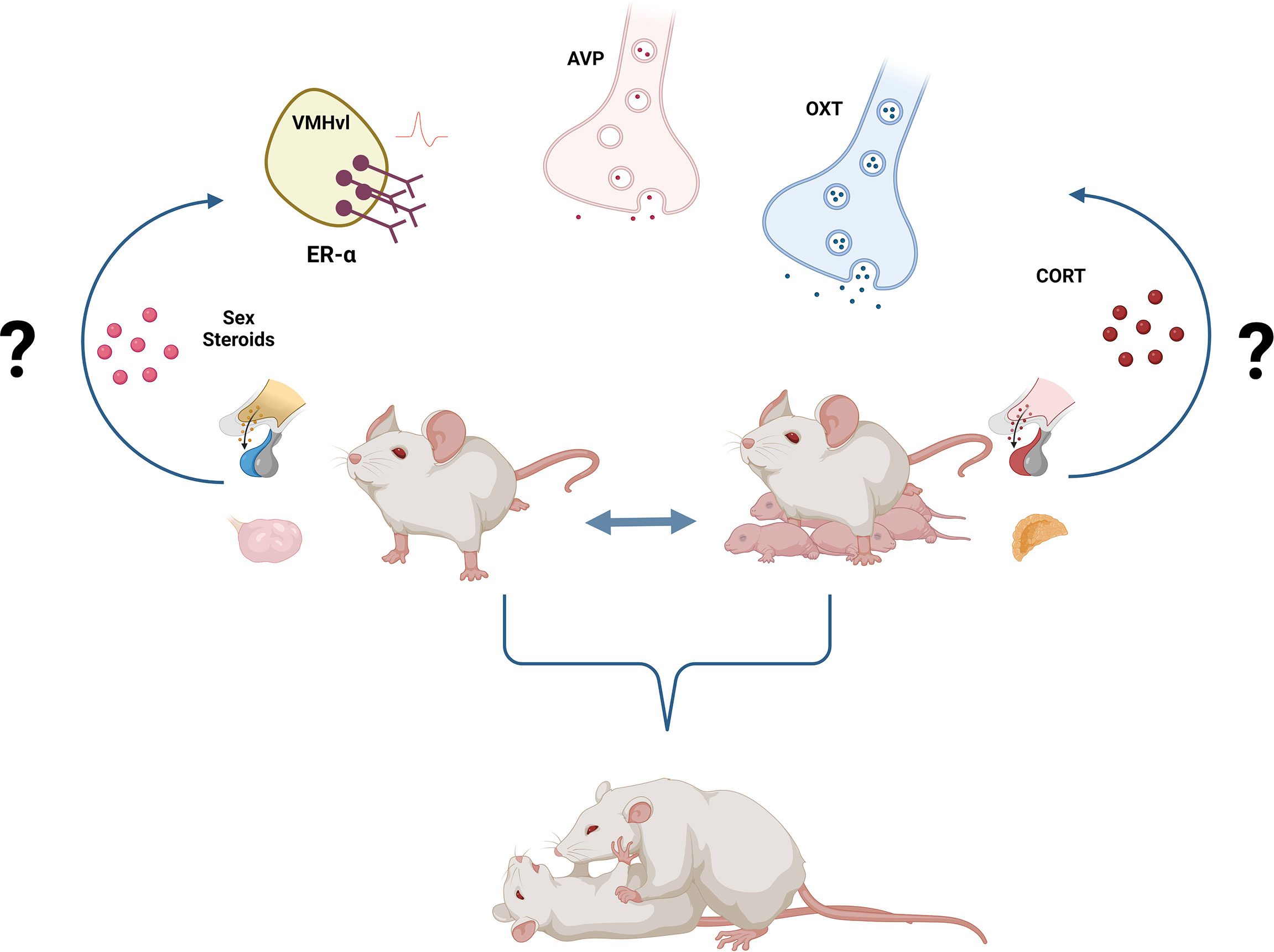
Figure 1 Neuroendocrine regulation of female aggression. Activation of ER-α+ neurons in the VMHvl as well as increased OXT and decreased AVP signaling underly female aggression, independently of the reproductive state. Yet, the activational, as well as the organizational role of sex steroids either activating ER-α+ neurons or masculinizing/feminizing the neural circuitry of female aggression remains poorly understood. Similarly, the well-established link between stress, hypothalamic-pituitary-adrenal (HPA) axis, corticosterone, and aggressive behavior needs to be further dissected in females. Created with BioRender.com. .
Finally, there are still several unanswered questions regarding the neurobiological underpinnings of female aggression such as i) how does brain masculinization/feminization impacts the neural circuits regulating aggression in females (are those effects reproductive state-dependent?); ii) how does estrogen signaling affects aggression in females (are neuroestrogens necessary for female aggression?); iii) which AVP neuronal population (s) is/are relevant for female aggression?, how does AVP signaling differently impact aggression in males and females? and iv) are other neurobiological systems implicated in intermale aggression also important for female aggression?, i.e. serotonin, HPA axis, dopamine, and the reward system.
VO: wrote the manuscript and drafted the figure and table with the input of JB. JB: revised the manuscript. All authors contributed to the article and approved the submitted version.
This review was supported by the Fonds De La Recherche Scientifique - FNRS (PDR T.0015.20 to JB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci (2007) 8:536–46. doi: 10.1038/nrn2174
2. Lischinsky JE, Lin D. Neural mechanisms of aggression across species. Nat Neurosci (2020) 23:1317–28. doi: 10.1038/s41593-020-00715-2
3. Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res (2001) 125:167–81. doi: 10.1016/S0166-4328(01)00298-4
4. Hashikawa K, Hashikawa Y, Lischinsky J, Lin D. The neural mechanisms of sexually dimorphic aggressive behaviors. Trends Genet (2018) 10:755–76. doi: 10.1016/j.tig.2018.07.001
5. Bosch OJ. Maternal aggression in rodents: Brain oxytocin and vasopressin mediate pup defence. Philos Trans R Soc B: Biol Sci (2013) 368:1–11. doi: 10.1098/rstb.2013.0085
6. French JA, Mustoe AC, Cavanaugh J, Birnie AK. The influence of androgenic steroid hormones on female aggression in “atypical” mammals. Philos Trans R Soc B: Biol Sci (2013) 368:1–12. doi: 10.1098/rstb.2013.0084
7. Pandolfi M, Scaia MF, Fernandez MP. Sexual dimorphism in aggression: Sex-specific fighting strategies across species. Front Behav Neurosci (2021) 15:659615. doi: 10.3389/fnbeh.2021.659615
8. Campbell A. Staying alive: Evolution, culture, and women’s intrasexual aggression. Behav Brain Sci (1999) 22:203–52. doi: 10.1017/S0140525X99001818
9. Lenschow C, Lima SQ. In the mood for sex: neural circuits for reproduction. Curr Opin Neurobiol (2020) 60:155–68. doi: 10.1016/j.conb.2019.12.001
10. Rosenthal GG, Ryan MJ. Sexual selection and the ascent of women: Mate choice research since Darwin. Sci (1979) (2022) 375:1–28. doi: 10.1126/science.abi6308
11. Shuster SM, Willen RM, Keane B, Solomon NG. Alternative mating tactics in socially monogamous prairie voles, microtus ochrogaster. Front Ecol Evol (2019) 7:7. doi: 10.3389/fevo.2019.00007
12. Bowler CM, Cushing BS, Carter CS. Social factors regulate female-female aggression and affiliation in prairie voles. Physiol Behav (2002) 76:559–66. doi: 10.1016/S0031-9384(02)00755-2
13. Bath E, Edmunds D, Norman J, Atkins C, Harper L, Rostant WG, et al. Sex ratio and the evolution of aggression in fruit flies. Proc R Soc B: Biol Sci (2021) 288:1–6. doi: 10.1098/rspb.2020.3053
14. Schretter CE, Aso Y, Robie AA, Dreher M, Dolan MJ, Chen N, et al. Cell types and neuronal circuitry underlying female aggression in drosophila. Elife (2020) 9:1–82. doi: 10.7554/eLife.58942
15. Huffard CL, Caldwell RL, Boneka F. Male-Male and male-female aggression may influence mating associations in wild octopuses (abdopus aculeatus). J Comp Psychol (2010) 124:38–46. doi: 10.1037/a0017230
16. Holtmann B, Buskas J, Steele M, Solokovskis K, Wolf JBW. Dominance relationships and coalitionary aggression against conspecifics in female carrion crows. Sci Rep (2019) 9:1–8. doi: 10.1038/s41598-019-52177-7
17. Lipshutz SE, Rosvall KA. Nesting strategy shapes territorial aggression but not testosterone: A comparative approach in female and male birds. Hormones Behav (2021) 133:104995. doi: 10.1016/j.yhbeh.2021.104995
18. Newman EL, Covington HE III, Suh J, Bicakci MB, Ressler KJ, Debold JF, et al. Fighting females : Neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry (2019) 86:657–68. doi: 10.1016/j.biopsych.2019.05.005
19. Silva AL, Fry WHD, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res (2010) 208:528–34. doi: 10.1016/j.bbr.2009.12.038
20. Aubry A, Burnett CJ, Goodwin NL, Li L, Navarrete J, Zhang Y, et al. Sex differences in appetitive and reactive aggression. Neuropsychopharmacol (2022). doi: 10.1101/2022.02.22.481480
21. Gubernick DJ, Alberts JR. The biparental care system of the California mouse, peromyscus californicus. J Comp Psychol (1987) 101:169–77. doi: 10.1037/0735-7036.101.2.169
22. Karelina K, Walton JC, Weil ZM, Norman GJ, Nelson RJ, DeVries AC. Estrous phase alters social behavior in a polygynous but not a monogamous peromyscus species. Hormones Behav (2010) 58:193–9. doi: 10.1016/j.yhbeh.2010.03.022
23. Oliveira VEM, Lukas M, Wolf HN, Durante E, Lorenz A, Mayer A-L, et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat Commun (2021) 12:1–15. doi: 10.1038/s41467-021-23064-5
24. Ho HP, Olsson M, Westberg L, Melke J, Eriksson E. The serotonin reuptake inhibitor fluoxetine reduces sex steroid-related aggression in female rats: An animal model of premenstrual irritability? Neuropsychopharmacology (2001) 24:502–10. doi: 10.1016/S0893-133X(00)00219-0
25. de Jong TR, Beiderbeck DI, Neumann ID. Measuring virgin female aggression in the female intruder test (FIT): Effects of oxytocin, estrous cycle, and anxiety. PloS One (2014) 9:e91701. doi: 10.1371/journal.pone.0091701
26. Cordero MI, Sandi C. Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology (2013) 201:2758–69. doi: 10.1016/j.psyneuen.2013.07.005
27. Oliveira VEM, de Jong TR, Neumann ID. Synthetic oxytocin and vasopressin act within the central amygdala to exacerbate aggression in female wistar rats. Front Neurosci (2022) 16:906617. doi: 10.3389/fnins.2022.906617
28. Oliveira VEM, Neumann ID, de Jong TR. Post-weaning social isolation exacerbates aggression in both sexes and affects the vasopressin and oxytocin system in a sex-specific manner. Neuropharmacology (2019) 156:107504. doi: 10.1016/j.neuropharm.2019.01.019
29. Borland JM, Walton JC, Norvelle A, Grantham KN, Aiani LM, Larkin TE, et al. Social experience and sex-dependent regulation of aggression in the lateral septum by extrasynaptic δGABAA receptors. Psychopharmacol (Berl) (2019) 237:329–44. doi: 10.1007/s00213-019-05368-z
30. Terranova JI, Song Z, Larkin TE, Hardcastle N, Norvelle A, Riaz A, et al. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U.S.A. (2016) 113:13233–8. doi: 10.1073/pnas.1610446113
31. Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus). Eur J Neurosci (2010) 31:1655–63. doi: 10.1111/j.1460-9568.2010.07190.x
32. Staffend NA, Meisel RL. Aggressive experience increases dendritic spine density within the nucleus accumbens core in female syrian hamsters. Neuroscience (2012) 227:163–9. doi: 10.1016/j.neuroscience.2012.09.064
33. Meisel RL, Sterner MR, Diekman MA. Differential hormonal control of aggression and sexual behavior in female Syrian hamsters. Hormones Behav (1988) 22:453–66. doi: 10.1016/0018-506X(88)90050-5
34. Been LE, Moore KM, Kennedy BC, Meisel RL. Metabotropic glutamate receptor and fragile x signaling in a female model of escalated aggression. Biol Psychiatry (2016) 79:685–92. doi: 10.1016/j.biopsych.2015.07.021
35. Been LE, Gibbons AB, Meisel RL. Towards a neurobiology of female aggression. Neuropharmacology (2019) 156:1–44. doi: 10.1016/j.neuropharm.2018.11.039
36. Freitag CM, Konrad K, Stadler C, De Brito SA, Popma A, Herpertz SC, et al. Conduct disorder in adolescent females: current state of research and study design of the FemNAT-CD consortium. Eur Child Adolesc Psychiatry (2018) 9:1077–93. doi: 10.1007/s00787-018-1172-6
37. Ackermann K, Kirchner M, Bernhard A, Martinelli A, Anomitri C, Baker R, et al. Relational aggression in adolescents with conduct disorder: sex differences and behavioral correlates. J Abnormal Child Psychol (2019) 47:1625–37. doi: 10.1007/s10802-019-00541-6
38. Denson TF, O’Dean SM, Blake KR, Beames JR. Aggression in women: behavior, brain and hormones. Front Behav Neurosci (2018) 12:81. doi: 10.3389/fnbeh.2018.00081
39. Mancke F, Bertsch K, Herpertz SC. Gender differences in aggression of borderline personality disorder. Borderline Pers Disord Emotion Dysregulation (2015) 2:7. doi: 10.1186/s40479-015-0028-7
40. Lukas M, de Jong TR. Conspecific Interactions in Adult Laboratory Rodents: Friends or Foes? Curr Top Behav Neurosci (2017) 30:3–24. doi: 10.1007/7854_2015_428
41. Shamay-tsoory SG, Abu-akel A. Review the social salience hypothesis of oxytocin. Biol Psychiatry (2015) 79:1–9. doi: 10.1016/j.biopsych.2015.07.020
42. Campbell A. Attachment, aggression and affiliation: The role of oxytocin in female social behavior. Biol Psychol (2008) 77:1–10. doi: 10.1016/j.biopsycho.2007.09.001
43. Unger EK, Burke KJ, Fuller PM, Unger EK, Burke KJ, Yang CF, et al. Medial amygdalar aromatase neurons regulate aggression in both sexes report medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep (2015) 10:453–62. doi: 10.1016/j.celrep.2014.12.040
44. Klampfl SM, Brunton PJ, Bayerl DS, Bosch OJ. Hypoactivation of CRF receptors, predominantly type 2, in the medial-posterior BNST is vital for adequate maternal behavior in lactating rats. J Neurosci (2014) 34:9665–76. doi: 10.1523/JNEUROSCI.4220-13.2014
45. Bayerl DS, Klampfl SM, Bosch OJ. More than reproduction: Central gonadotropin-releasing hormone antagonism decreases maternal aggression in lactating rats. J Neuroendocrinol (2019) 31:1–8. doi: 10.1111/jne.12709
46. Georgescu T. Prolactin-mediated restraint of maternal aggression in lactation.J PNAS (2022) 119:1–10. doi: 10.1073/pnas.2116972119/-/DCSupplemental.Published
47. Hashikawa K, Hashikawa Y, Tremblay R, Zhang J, Feng JE, Sabol A, et al. Esr1+cells in the ventromedial hypothalamus control female aggression. Nat Neurosci (2017) 20:1580–90. doi: 10.1038/nn.4644
48. Brain PF. Effects of isolation/grouping on endocrine function and fighting behavior in male and female golden hamsters (Mesocricetus auratus waterhouse). Behav Biol (1972) 7:349–57. doi: 10.1016/S0091-6773(72)80106-8
49. Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. The resident-intruder paradigm: A standardized test for aggression, violence and social stress. J Visualized Experiments (2013) 77:4367. doi: 10.3791/4367
50. Koolhaas JM, Schuurman T, Wiepkema PR. The organization of intraspecific agonistic behaviour in the rat. Prog Neurobiol (1980) 15:247–68. doi: 10.1016/0301-0082(80)90024-6
51. Aleyasin XH, Flanigan XME, Golden XSA, Takahashi XA, Menard XC, Pfau ML, et al. Cell-type-specific role of ⌬; fosb in nucleus accumbens in modulating intermale aggression. J Neurosci (2018) 38:5913–24. doi: 10.1523/JNEUROSCI.0296-18.2018
52. Golden SA, Heins C, Venniro M, Caprioli D, Zhang M, Epstein DH, et al. Compulsive addiction-like aggressive behavior in mice. Biol Psychiatry (2017) 82:239–48. doi: 10.1016/j.biopsych.2017.03.004
53. de Jong TR, Neumann ID. Moderate role of oxytocin in the pro-ejaculatory effect of the 5-ht1areceptor agonist 8-oh-dpat. J Sexual Med (2015) 12:17–28. doi: 10.1111/jsm.12742
54. Haller J. Preclinical models of conduct disorder – principles and pharmacologic perspectives. Neurosci Biobehav Rev (2018) 91:112–20. doi: 10.1016/j.neubiorev.2016.05.032
55. Haller J. The neurobiology of abnormal manifestations of aggression-a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res Bull (2013) 93:97–109. doi: 10.1016/j.brainresbull.2012.10.003
56. Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci (2015) 16:290–304. doi: 10.1038/nrn3918
57. Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety — involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology (2012) 37:1969–80. doi: 10.1016/j.psyneuen.2012.04.011
58. Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci (2010) 4:1–12. doi: 10.3389/fnbeh.2010.00012
59. Marquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, et al. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry (2013) 3:e216. doi: 10.1038/tp.2012.144
60. Chang CH, Gean PW. The ventral hippocampus controls stress-provoked impulsive aggression through the ventromedial hypothalamus in post-weaning social isolation mice. Cell Rep (2019) 28:1195–1205.e3. doi: 10.1016/j.celrep.2019.07.005
61. Toth M, Mikics E, Tulogdi A, Aliczki M, Haller J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Hormones Behav (2011) 60:28–36. doi: 10.1016/j.yhbeh.2011.02.003
62. Hasen NS, Gammie SC. Differential fos activation in virgin and lactating mice in response to an intruder. Physiol Behav (2005) 84:681–95. doi: 10.1016/j.physbeh.2005.02.010
63. Hasen NS, Gammie SC. Maternal aggression: New insights from egr-1. Brain Res (2006) 1108:147–56. doi: 10.1016/j.brainres.2006.06.007
64. Liu M, Kim D-W, Zeng H, Anderson DJ. Make war not love: The neural substrate underlying a state-dependent switch in female social behavior. Neuron (2022) 110. doi: 10.1016/j.neuron.2021.12.002
65. Menon R, Süß T, Oliveira VE de M, Neumann ID, Bludau A. Neurobiology of the lateral septum: regulation of social behavior. Trends Neurosci (2021) 45. doi: 10.1016/j.tins.2021.10.010
66. Toth M, Tulogdi A, Biro L, Soros P, Mikics E, Haller J. The neural background of hyper-emotional aggression induced by post-weaning social isolation. Behav Brain Res (2012) 233:120–9. doi: 10.1016/j.bbr.2012.04.025
67. Spergel DJ. Neuropeptidergic modulation of GnRH neuronal activity and GnRH secretion controlling reproduction: insights from recent mouse studies. Cell Tissue Res (2019) 375:179–91. doi: 10.1007/s00441-018-2893-z
68. Goodman HM. Basic medical endocrinology. Elsevier science and technology books: AP academic press (2009) pp. 1–309 p.
69. Bhargava A, Arnold AP, Bangasser DA, Denton KM, Gupta A, Hilliard Krause LM, et al. Considering sex as a biological variable in basic and clinical studies: An endocrine society scientific statement. Endocrine Rev (2021) 42:219–58. doi: 10.1210/endrev/bnaa034
70. Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: Dogma and beyond. Front Neurosci (2012) 6:26. doi: 10.3389/fnins.2012.00026
71. Trainor BC, Kyomen HH, Marler CA. Estrogenic encounters: How interactions between aromatase and the environment modulate aggression. Front Neuroendocrinol (2006) 27:170–9. doi: 10.1016/j.yfrne.2005.11.001
72. Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor α in select brain nuclei are associated with individual differences in aggression. Hormones Behav (2006) 50:338–45. doi: 10.1016/j.yhbeh.2006.04.002
73. Ruscio MG, King SB, Kinley-Cooper SK, McKendrick G. Social environment affects central distribution of estrogen receptor-α in peromyscus californicus. Gen Comp Endocrinol (2018) 269:81–7. doi: 10.1016/j.ygcen.2018.08.018
74. Szymanski L, Bakker J. Aromatase knockout mice show normal steroid-induced activation of gonadotrophin-releasing hormone neurones and luteinising hormone surges with a reduced population of kisspeptin neurones in the rostral hypothalamus. J Neuroendocrinol (2012) 24:1222–33. doi: 10.1111/j.1365-2826.2012.02334.x
75. Matsumoto T, Honda SI, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology (2003) 77:416–24. doi: 10.1159/000071313
76. Wu M v., Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda SI, et al. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell (2009) 139:61–72. doi: 10.1016/j.cell.2009.07.036
77. Davis ES, Marler CA. The progesterone challenge: Steroid hormone changes following a simulated territorial intrusion in female peromyscus californicus. Hormones Behavior Acad Press Inc (2003) 44:185–98. doi: 10.1016/S0018-506X(03)00128-4
78. van de Poll NE, van Zanten S, de Jonge FH. Effects of testosterone, estrogen, and dihydrotestosterone upon aggressive and sexual behavior of female rats. Hormones Behav (1986) 20:418–31. doi: 10.1016/0018-506X(86)90004-8
79. Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-α gene expression in reproduction-related behaviors in female mice. Endocrinology (1998) 139:5070–81. doi: 10.1210/endo.139.12.6357
80. Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A. The role of the estrogen receptor α in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res (2010) 210:211–20. doi: 10.1016/j.bbr.2010.02.033
81. Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature (2014) 509:627–32. doi: 10.1038/nature13169
82. Jurek B, Neumann ID. The oxytocin receptor: From intracellular signaling to behavior. Physiol Rev (2018) 98:1805–908. doi: 10.1152/physrev.00031.2017
83. Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci (2012) 35:649–59. doi: 10.1016/j.tins.2012.08.004
84. Anpilov S, Shemesh Y, Eren N, Wagner S, Yizhar O, Chen A, et al. Wireless optogenetic stimulation of oxytocin neurons in a semi-natural setup dynamically elevates both pro-social and agonistic behaviors. Neuron (2020) 107:1–12. doi: 10.1016/j.neuron.2020.05.028
85. Calcagnoli F, De Boer SF, Althaus M, Den Boer JA, Koolhaas JM. Antiaggressive activity of central oxytocin in male rats. Psychopharmacol (Berl) (2013) 229:639–51. doi: 10.1007/s00213-013-3124-7
86. de Jong TR, Neumann ID. Oxytocin and aggression. Curr Topics Behav Neurosci (2017) 35:1–18. doi: 10.1007/7854_2017_13
87. Tan O, Musullulu H, Raymond JS, Wilson B, Langguth M, Bowen MT. Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice. Neuropharmacology (2019) 156:107573. doi: 10.1016/j.neuropharm.2019.03.016
88. Oliveira VEM, Jong de, Trynke R, Neumann ID. Modelling sexual violence in male rats : the sexual aggression test (SxAT). Transl Psychiatry (2022) 12:1–12. doi: 10.1038/s41398-022-01973-3
89. Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal Aggression : Link to anxiety. J Neurosci (2005) 25:6807–15. doi: 10.1523/JNEUROSCI.1342-05.2005
90. Trainor BC, Takahashi EY, Silva AL, Crean KK, Hostetler C. Sex differences in hormonal responses to social conflict in the monogamous California mouse. Hormones Behav (2010) 58:506–12. doi: 10.1016/j.yhbeh.2010.04.008
91. Harmon AC, Huhman KL, Moore TO, Albers HE. Oxytocin inhibits aggression in female Syrian hamsters. J Neuroendocrinol (2002) 14:963–9. doi: 10.1046/j.1365-2826.2002.00863.x
92. Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology (2014) 50:14–9. doi: 10.1016/j.psyneuen.2014.08.005
93. Jiang Y, Platt ML. Oxytocin and vasopressin flatten dominance hierarchy and enhance behavioral synchrony in part via anterior cingulate cortex. Sci Rep (2018) 8:1–14. doi: 10.1038/s41598-018-25607-1
94. Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, et al. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J Neuroendocrinol (2011) 23:1113–24. doi: 10.1111/j.1365-2826.2011.02224.x
95. DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol (2017) 525:2549–70. doi: 10.1002/cne.24216
96. Lukas M, Suyama H, Egger V. Vasopressin cells in the rodent olfactory bulb resemble non-bursting superficial tufted cells and are primarily inhibited upon olfactory nerve stimulation. eNeuro (2019) 6:1–19. doi: 10.1523/ENEURO.0431-18.2019
97. Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Hormones Behav (2010) 58:273–81. doi: 10.1016/j.yhbeh.2010.03.006
98. Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci (2007) 26:3597–605. doi: 10.1111/j.1460-9568.2007.05974.x
99. Compaam JC, Buijs RM, Pool CW, de Ruiter AJH, Koolhaas JM. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull (1993) 30:1–6. doi: 10.1016/0361-9230(93)90032-7
100. Lee W, Hiura LC, Eilene Y, Broerkman KA, Ophir AG, Curley JP. Social status in mouse social hierarchies is associated with variation in oxytocin and vasopressin 1a receptor densities. Hormones Behav (2019) 114:104551. doi: 10.1016/j.yhbeh.2019.06.015
101. Masis-Calvo M, Schmidtner AK, de Moura Oliveira VE, Grossmann CP, de Jong TR, Neumann ID. Animal models of social stress: the dark side of social interactions. Stress (2018) 5:41–432. doi: 10.1080/10253890.2018.1462327
102. Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci (2010) 31:883–91. doi: 10.1111/j.1460-9568.2010.07115.x
103. Coverdill AJ, Mccarthy M, Bridges RS, Nephew BC. Effects of chronic central arginine vasopressin (AVP) on maternal behavior in chronically stressed rat dams. Brain Sci (2012) 2:589–604. doi: 10.3390/brainsci2040589
104. Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav (2008) 91:77–83. doi: 10.1016/j.pbb.2008.06.013
105. Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology (2010) 58:102–6. doi: 10.1016/j.neuropharm.2009.06.032
106. Taylor JH, Walton JC, Mccann KE, Norvelle A, Liu Q, Vander JW. CRISPR-Cas9 editing of the arginine – vasopressin V1a receptor produces paradoxical changes in social behavior in Syrian hamsters. PNAS (2022) 119:1–8. doi: 10.1073/pnas.2121037119/-/DCSupplemental.Published
107. Koshimizu Ta, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol Rev (2012) 92:1813–64. doi: 10.1152/physrev.00035.2011
108. Hernando F, Schoots O, Lolait SJ, Peter J, Burbach H. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: Anatomical support for its involvement in the central effects of vasopressin* (2001). Available at: https://academic.oup.com/endo/article-abstract/142/4/1659/2989089.
109. Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry (2002) 7:975–84. doi: 10.1038/sj.mp.4001195
110. Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, Dudek SM, et al. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry (2015) 20:490–9. doi: 10.1038/mp.2014.47
111. Leroy F, Park J, Asok A, Brann DH, Meira T, Boyle LM, et al. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature (2018) 564:213–8. doi: 10.1038/s41586-018-0772-0
112. Bayerl DS, Klampfl SM, Bosch OJ. Central V1b receptor antagonism in lactating rats: Impairment of maternal care but not of maternal aggression. J Neuroendocrinol (2014) 26:918–26. doi: 10.1111/jne.12226
113. Bayerl DS, Kaczmarek V, Jurek B, van den Burg EH, Neumann ID, Gaßner BM, et al. Hormones and behavior antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats. Hormones and Behavior (2016) 79:18–27. doi: 10.1016/j.yhbeh.2015.12.003
114. Bayerl DS, Bosch OJ. Brain vasopressin signaling modulates aspects of maternal behavior in lactating rats. Genes, Brain and Behavior (2019) 18:1–13. doi: 10.1111/gbb.12517
115. Haller J, Harold G, Sandi C, Neumann ID. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J Neuroendocrinol (2014) 26:724–38. doi: 10.1111/jne.12182
116. Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology (2007) 32:437–50. doi: 10.1016/j.psyneuen.2007.02.008
117. Haller J, Halász J, Mikics É, Kruk MR. Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. J Neuroendocrinol (2004) 16:550–7. doi: 10.1111/j.1365-2826.2004.01201.x
118. Rosinger ZJ, Mayer HS, Geyfen JI, Orser MK, Stolzenberg DS. Ethologically relevant repeated acute social stress induces maternal neglect in the lactating female mouse. Dev Psychobiol (2021) 63:1–15. doi: 10.1002/dev.22173
119. De Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: Dopamine, serotonin and GABA. Eur J Pharmacol (2005) 526:51–64. doi: 10.1016/j.ejphar.2005.10.004
120. De Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: A pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol (2005) 526:125–39. doi: 10.1016/j.ejphar.2005.09.065
121. Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci (2001) 24:713–9. doi: 10.1016/S0166-2236(00)01996-2
122. Carrillo M, Ricci LA, Cappersmith GA, Melloni RH Jr. The effect of increased serotonergic neurotransmission on aggression : A critical meta-analytical review of preclinical studies. Psychopharmacol (Berl) (2009) 205:349–68. doi: 10.1007/s00213-009-1543-2
Keywords: aggressive behavior, social behavior, estrogen receptors, oxytocin (OXT), vasopressin (AVP), corticosterone, sex differences, aromatase
Citation: Oliveira VEdM and Bakker J (2022) Neuroendocrine regulation of female aggression. Front. Endocrinol. 13:957114. doi: 10.3389/fendo.2022.957114
Received: 30 May 2022; Accepted: 19 July 2022;
Published: 10 August 2022.
Edited by:
Mauro S. B. Silva, Brigham and Women’s Hospital and Harvard Medical School, United StatesReviewed by:
Brian Trainor, University of California, Davis, United StatesCopyright © 2022 Oliveira and Bakker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julie Bakker, amJha2tlckB1bGllZ2UuYmU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.