
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Endocrinol. , 11 August 2022
Sec. Neuroendocrine Science
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.955687
This article is part of the Research Topic Rare Neuroendocrine Neoplasms View all 29 articles
Doege–Potter syndrome is a rare paraneoplastic syndrome characterized by non-islet cell tumor hypoglycemia secondary to a solitary fibrous tumor. Doege–Potter syndrome always presents with recurrent fasting hypoglycemia, which can occasionally be life-threatening. The best choice of treatment for Doege–Potter syndrome and solitary fibrous tumor is complete resection. However, when it is unfeasible, local-regional treatment can be used as a palliative therapy. Herein, we report a case of a 46-year-old man with Doege–Potter syndrome that occurred secondary to the liver and pancreatic metastatic solitary fibrous tumors. After he received six rounds of targeting-intratumoral-lactic-acidosis transcatheter-arterial-chemoembolization (TILA-TACE) treatment in our hospital, his hypoglycemia was clinically cured, and the liver metastatic tumor was well controlled. We suggest that TILA-TACE can be considered when curative resection is unfeasible for metastatic liver solitary fibrous tumors to help a patient obtain further surgery opportunities.
Solitary fibrous tumors (SFTs) are rare neoplasms that originate from mesenchymal spindle cells. They were first reported by Wagner in 1870, and their histopathological report was subsequently described by Klemperer and Rabin in 1931 (1). In 2013, based on the World Health Organization (WHO) classification of tumors of soft tissue and bone, SFTs were considered benign tumors with potential for malignant transformation (2). Most SFTs are inert, without local or distant recurrence. However, the 10-year disease-specific survival rate for pleural and extra-pleural SFT is about 73%–100%, and the 10-year recurrence rate is 10%–25% (3–8). Meningeal hemangiopericytoma in the central nervous system and SFT were classified as the same tumor by WHO. On the other hand, hemangiopericytoma/SFT is more aggressive; local recurrence is frequent and rapid, with the possibility of meningeal spread and early distant metastasis to the bone. According to the WHO classification, though most of SFTs are considered benign, some SFTs can be considered malignant with the following histopathologic characteristics: cytological atypia, hypercellularity, tumor necrosis, high mitotic rate (>4 per high-power field (HPF)), and/or infiltrative margins (9). At present, it is not recommended to use “benign” or “malignant” to evaluate the prognosis of patients; it is more applicable to apply the risk of recurrence/metastasis for SFTs. Patients with the following conditions are more likely to have tumor recurrence/metastasis: incomplete surgical resection, metastatic disease at the time of visiting, tumor greater than 10 cm, high mitosis rate (>4 mitotic images/10 HPF), and tumor necrosis (4, 10–14). Ki-67 greater than 5% is also considered a marker (15). The clinical manifestations of SFTs are usually nonspecific. Hypoglycemia occurs in approximately 5% of SFT cases, in a rare and challenging paraneoplastic syndrome known as the Doege–Potter syndrome (DPS), characterized as non-islet cell tumor hypoglycemia (NICTH) (16, 17). DPS usually manifests as a rare, refractory, and severe hypoglycemia.
For both SFTs and DPS, the best treatment is complete resection of the tumor. However, when curative resection is not feasible, short-term therapy, including continuous intravenous infusion of glucose and medical therapy, is beneficial. Other treatments include adjuvant chemotherapy and radiotherapy (18, 19). It is reported that pazopanib can effectively control the progression of the tumor and can be considered a first-line treatment for some advanced typical SFTs (20–23). Other drugs, like temozolomide and bevacizumab, could also play a role. However, more study is needed to verify the effects of these drugs (24). For primary and metastatic liver SFTs, local-regional treatments such as transcatheter arterial chemoembolization (TACE) have been reported (19, 25). Zhong et al. have recently reported a case of hepatic SFT in a patient who was treated with curative ex situ hepatectomy and liver autotransplantation (26). All of these approaches can play a part in treating hepatic SFTs.
Herein, we report the case of a patient with DPS caused by multiple metastatic SFTs in the liver and pancreas. Due to multiple liver metastases and serious hyperglycemia, he was not a suitable candidate for direct surgery. He had previously been treated with diet therapy, continuous infusion of glucose, low-dose glucocorticoid treatment, and sorafenib tosylate, as well as four cycles of conventional TACE (cTACE) in other hospitals, yet experienced little significant improvement. Fortunately, in our hospital, his hypoglycemia was clinically controlled after six cycles of targeting-intratumoral-lactic-acidosis (TILA)-TACE, and his metastatic liver tumors became almost necrotic after eight cycles of TILA-TACE treatment, which was first reported by our team (27). The patient then underwent a successful combined operation of the pancreatic body and tail resection, splenectomy, and left lateral lobe hepatectomy after his hypoglycemia was clinically cured and the further progression of SFTs was controlled.
In this case, our patient benefited from TILA-TACE rather than the original cTACE treatment. Hence, we suggest that for patients with primary and metastatic liver SFTs and who are not eligible for curative surgery, TILA-TACE may be a successful therapeutic alternative.
A 46-year-old Chinese man visited our hospital for recurrent paroxysmal unconsciousness occurring for 10 months (from January 2017). At first, his hypoglycemia usually occurred in the morning, and later it was irregular, accompanied by sweating, weakness, and occasional urinary incontinence, and these symptoms disappeared after eating. He had no history of diabetes but had a history of drinking for 20 years and had been sober for 1 year before the onset of hypoglycemia. The results of laboratory test were as follows: serum blood glucose at 1.26 mmol/L (normal range: 3.89–6.11 mmol/L), serum insulin level at <3.48 pmol/L (normal range: 17.8–173.0 pmol/L), C-peptide at 0.03 nmol/L (normal range: 0.27–1.28 nmol/L), and insulin-like growth factors IGF-1 at < 25.0 ng/ml (normal range: 94.0–252 ng/ml). Glutamate acid decarboxylase antibodies, anti-insulin autoantibodies, and anti-islet cell antibodies were negative. Positron emission tomography showed scattered low-density lesions in the liver and an enlarged lymph node behind the pancreas with increased glucose uptake. Liver contrast-enhanced magnetic resonance imaging (MRI) was performed in May 2017 and revealed pancreatic and multiple liver masses, showing at least six large masses (diameter >5 cm) and multiple small metastases in the liver, the largest one of which was approximately 14 cm in diameter. All of the masses demonstrated a slightly high mixed signal on T2-weighted imaging and diffusion-weighted imaging and a slightly low mixed signal on T1-weighted imaging. In contrast, all these masses showed obvious enhancement. In addition, a mass was found located in the tail of the pancreas that measured 3.6 cm in diameter with short T1 and long T2 signals on MRI, and the mass showed evident enhancement after contrast. Neuroendocrine tumors were first considered (Figure 1). Later, liver mass biopsy and immunohistochemistry results showed positive staining with antibodies against STAT6 and CD34. Thus, the patient was diagnosed with SFT and DPS, and initially received four cycles of cTACE to reduce the side effects as direct surgery might be life-threatening. However, no improvement was observed, and the patient still had severe hypoglycemia. Previous medical history showed that he had resection of a right fossa pterygopalatine tumor in 2010, and reoperation in 2012 as the tumor recurred; a further pathology report showed a spindle cell tumor diagnosed as a hemangiopericytoma.
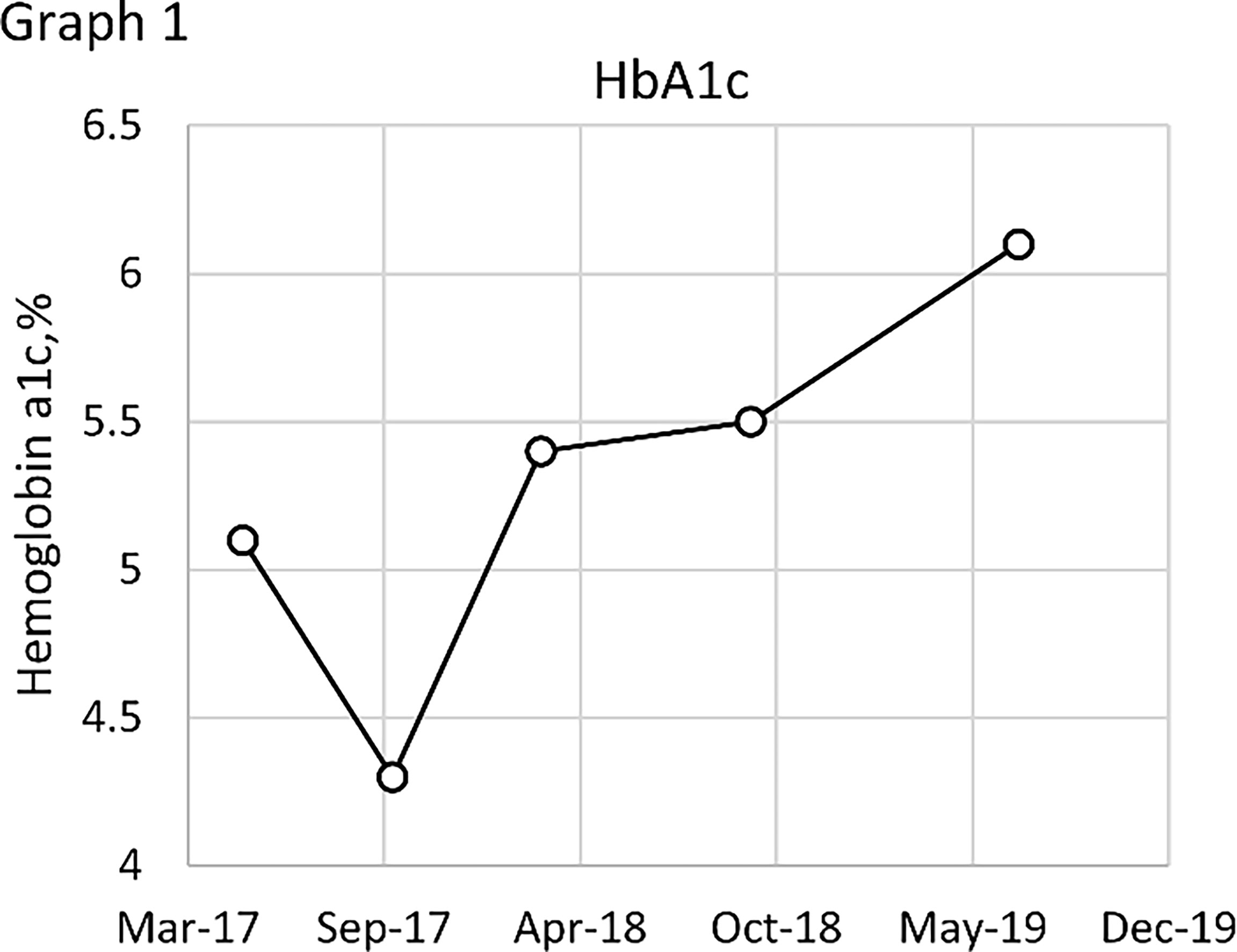
Figure 1 Contrast-enhanced magnetic resonance imaging (MRI) of the liver before TILA-TACE. Contrast-enhanced MRI of the liver showed at least six large masses in the liver, the largest one of which was approximately 14 cm in diameter. All of the masses demonstrated a slightly low mixed signal on T1WI (A) and a slightly high mixed signal on T2WI (B); after contrast, all these masses were clearly enhanced (C, D). In addition, a mass located in the tail of the pancreas that measured 3.6 cm in diameter was observed (arrow), with short T1 (E) and long T2 (F) signals on MRI, and the mass was evidently enhanced after contrast (G, H).
On physical examination, he was an overweight man with a body mass index of 29.01 kg/m2. Facial changes included acrochordons and rhinophyma (Figure 2). Abdominal bulging was observed, and abdominal palpation revealed an enlarged liver. No other physical abnormalities were found.
Laboratory data showed fasting hypoglycemia, and the results were as follows: serum blood glucose at 1.25 mmol/L (normal range: 3.89–6.11 mmol/L), serum insulin level at <3.48 pmol/L (normal range: 17.8–173.0 pmol/L), C-peptide at 0.03 nmol/L (normal range: 0.27–1.28 nmol/L), IGF-2 at 1,964.33 ng/ml (normal range: 400–736 ng/ml), IGF-1 at <25.0 ng/ml (normal range: 94.0–252 ng/ml), pro-IGF-2 at 28.79 ng/ml, and IGF-2/IGF-1 >10. Tumor markers such as alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) were negative. Routine biochemical indicators such as transaminase were normal in laboratory tests. Thyroid dysfunction, functional islet cell tumors, and adrenal dysfunction were excluded. Head MRI showed no obvious abnormalities except the VR space of the right cerebral peduncle and the lacunar foci in the right semioval area. Enhanced MRI of the nasopharyngeal area showed postoperative changes in the right pterygopalatine fossa tumor. There were multiple nodular abnormal signals in the right temporal fossa that may be a local recurrence. A contrast-enhanced computed tomography (CT) scan of the abdomen showed multiple nodules and masses of varying sizes in the liver, with unclear borders, which were enlarged compared to May 2017. The largest one was located in the eighth segment of the liver, with a diameter of 15.9 cm. The masses were unevenly enhanced in the arterial phase; in the portal phase and the delayed phase, the enhancement of the mass was weakened but still higher than the surrounding liver tissue. A mass in the posterior and lower parts of the pancreas was noted that was unevenly enhanced with a blurred border.
Based on the aforementioned evidence, the patient was diagnosed with metastatic liver and pancreatic SFT, along with the manifestation of DPS. We speculated that the right fossa pterygopalatine tumor was the primary SFT based on the WHO classification of hemangiopericytoma/SFT. Intravenous boluses of 10% dextrose were administered to correct his hypoglycemia, and his blood glucose was monitored through capillary tests and a blood glucose instantaneous sensor. There was a decrease in the frequency of the episodes of unconsciousness, but they occurred irregularly at least once a day.
The effects of diet and drugs were poor, cTACE did not work, and surgery was unavailable; thus, after a multidisciplinary discussion, he was treated with TILA-TACE therapy. Laboratory tests after the first TILA-TACE showed that big-IGF-2 and IGF-2 had obviously decreased, 18.05 and 922.33 ng/ml, respectively, and the serum blood glucose level had also significantly improved. However, hypoglycemia recurred after 3 days at a frequency and severity similar to those before TILA-TACE. One month later, after the second TILA-TACE cycle, the recurrence-free time had extended to 1 week. When the third TILA-TACE cycle was completed, the symptoms and the blood glucose level significantly improved, and no drug treatment was required. Hemoglobin A1c (HbA1c) during hospitalization before and after TILA-TACE treatment is shown in Graph 1. Insulin, C-peptide, IGF-1, HbA1c, and glucose levels during treatment are shown in Table 1. Contrast-enhanced MRI of the liver performed after the third cycle of TILA-TACE showed that almost all of the large liver lesions had complete necrosis (Figure 3). After six cycles of TILA-TACE, the patient’s blood glucose was basically back to normal; no extra meals were required.
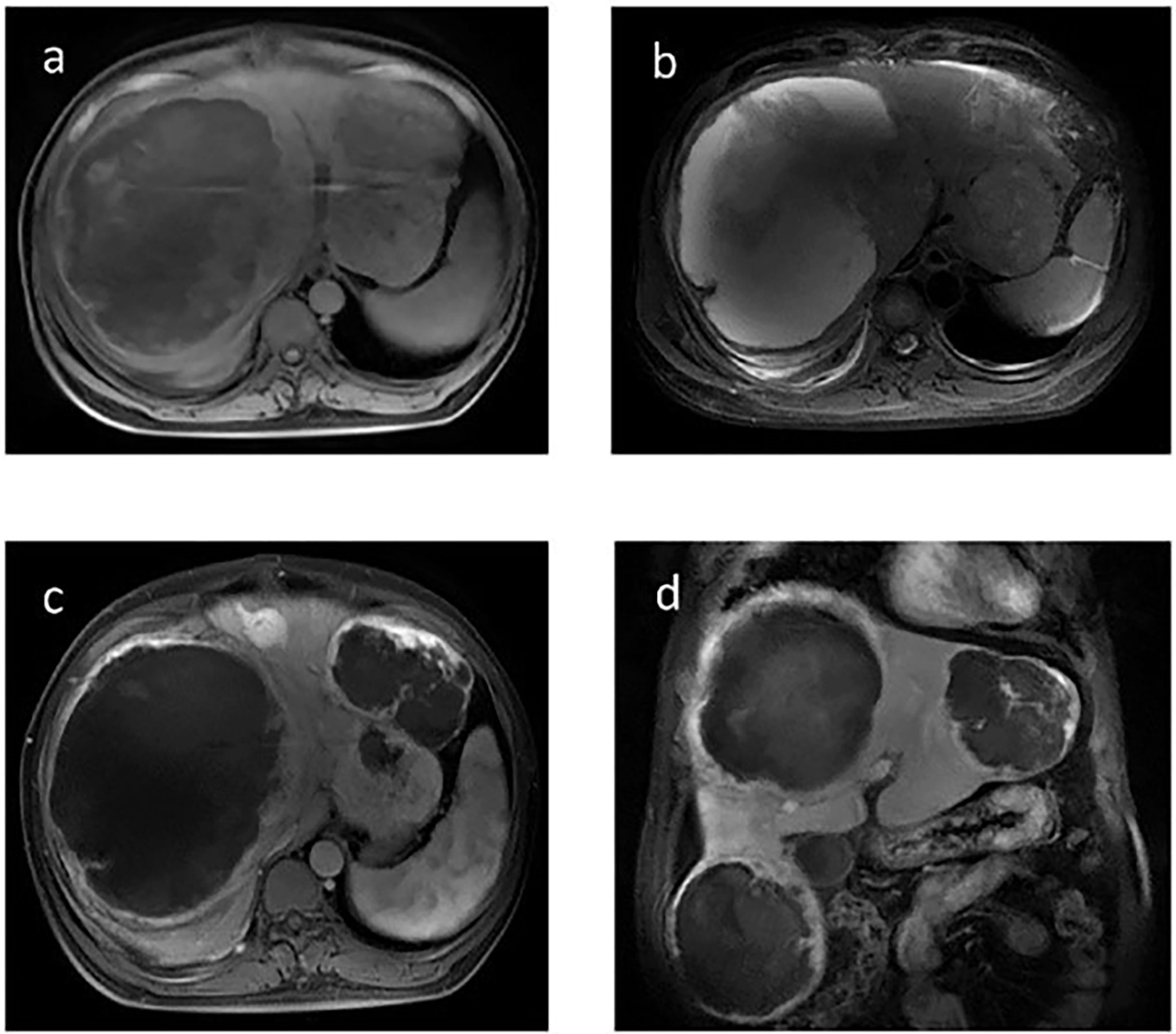
Figure 3 Contrast-enhanced MRI of the liver after the third TILA-TACE cycle showed that all of the masses demonstrated slightly low mixed signal on T1WI (A) and high mixed signal on T2WI (B). After contrast, most of the masses were almost necrosed (C, D).
After the glucose level had stabilized, the patient underwent a combined operation of the pancreatic body and tail resection, splenectomy, and left lateral lobe hepatectomy after six cycles of TILA-TACE treatment. Due to multiple liver and pancreatic metastases, the surgeon did not suggest complete resection of the tumor. A pathological examination of the pancreas revealed a spindle cell tumor with an abundance of heterotypic cells. Microscopic examination with hematoxylin–eosin staining showed that the tumor had a high proliferation rate of four to five mitotic figures per 10 HPF, and the margin was negative. Immunohistochemistry findings revealed positive staining with antibodies against CD34, Bcl-2, STAT6, and CD31, and Ki-67 was 10% (Figure 4). After surgery, his blood glucose was basically back to normal (Table 1), and no extra meals were required. Unfortunately, we determined that the residual tumors had progressed, so two more cycles of TILA-TACE were performed after surgery.
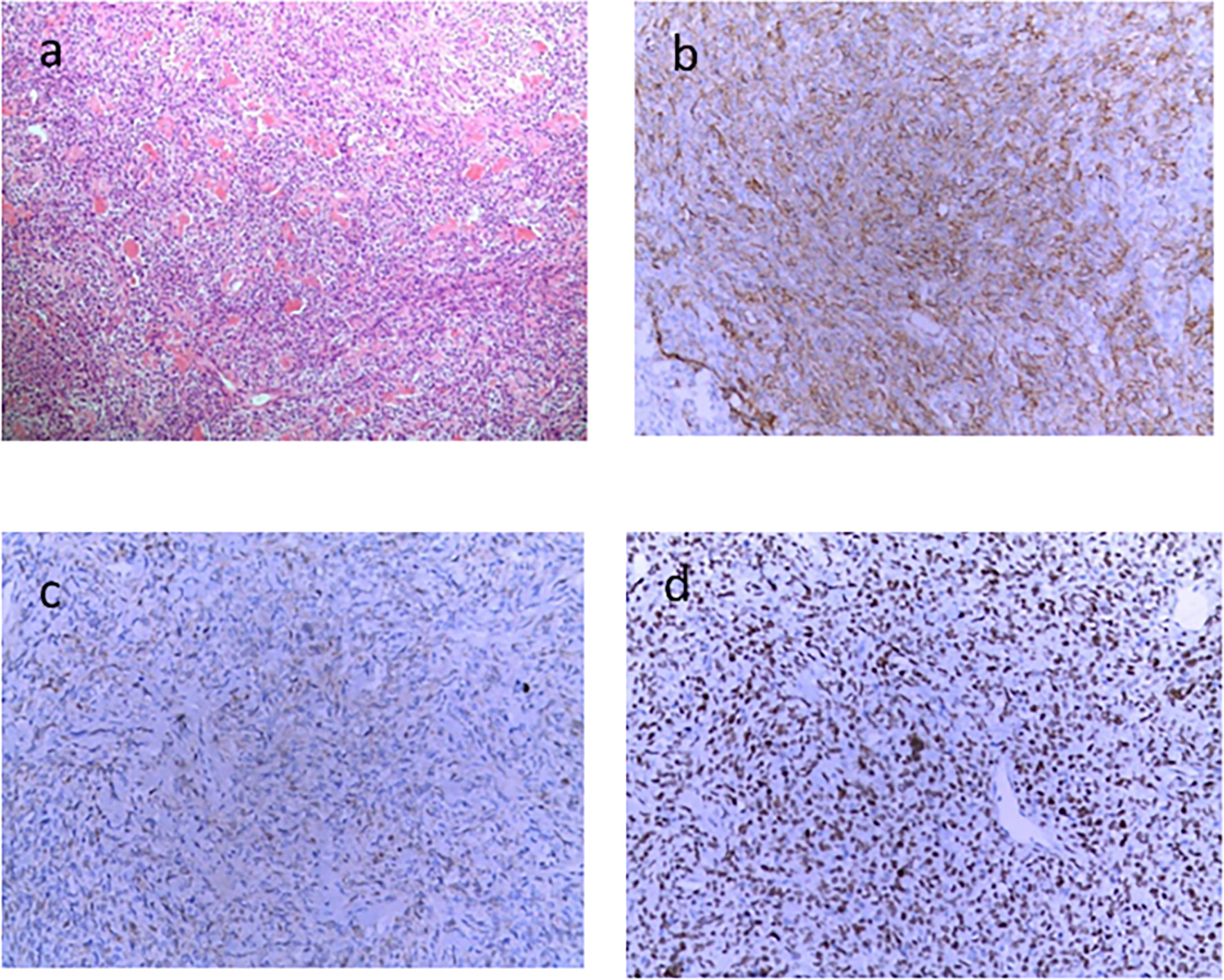
Figure 4 Microscopic examination of the pancreatic lesion (6.5 × 5 cm in size) demonstrated the presence of spindle-shaped cells, abundant tumor cells with atypia, approximately four to five mitotic figures/10 HPFs, and local infarction, in accordance with the World Health Organization grade III. (A) HE staining (×100). (B) CD34 immunohistochemical stain (×200). (C) BCL2 immunohistochemical stain (×200). (D) STAT-6 immunohistochemical stain (×200).
Eight months after surgery, the patient was readmitted to our hospital because of instability while walking and left lower limb weakness. Contrast-enhanced MRI of the thoracic spine confirmed thoracic spine metastasis, and one metastatic nodule had projected into the spinal canal, compressing the spinal cord (Figure 5). A review of his previous imaging revealed that a metastatic thoracic spine tumor had been present in November 2017. After an uneventful thoracic spine tumor resection, he recovered well with no paraplegic symptoms. Histopathology also showed a spindle cell tumor (Figure 6).
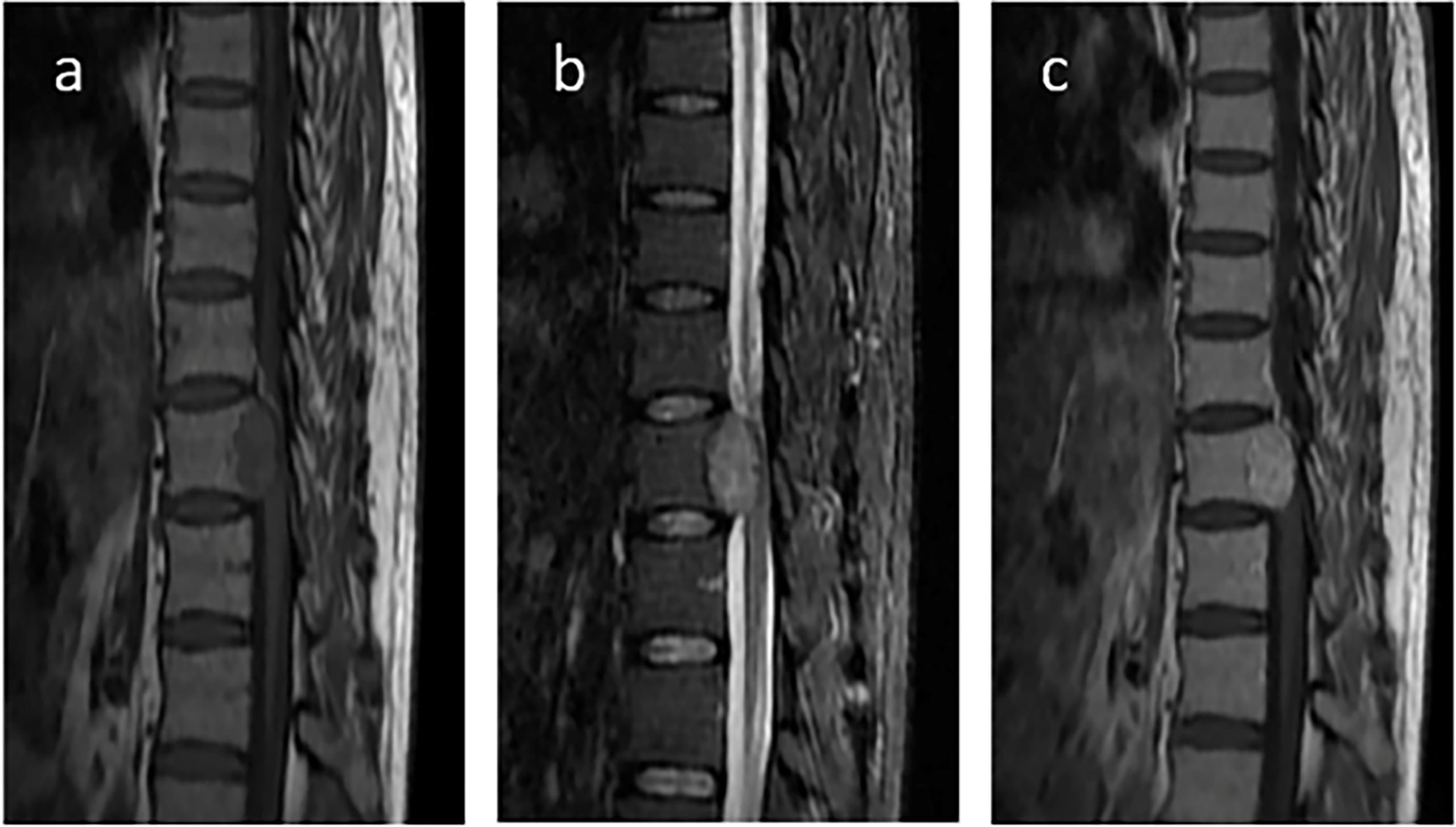
Figure 5 Contrast-enhanced magnetic resonance imaging (MRI) of the thoracic spine. Contrast-enhanced MRI of the thoracic spine confirmed thoracic spine metastasis and one metastatic nodule projected into the spinal canal and compressed the spinal cord. (A) T1WI; (B) T2WI; (C) T1WI postcontrast.
Since 2017, the patient has received a total of eight cycles of TILA-TACE and has undergone a combined operation of the pancreatic body and tail resection, splenectomy, and left extrahepatic tumor resection and thoracic spine tumor resection in our hospital. He last visited our hospital for an evaluation in May 2020, and, according to his examination results, the disease was thought to be temporarily stable and his hypoglycemia clinically cured. There is no doubt that TILA-TACE is effective for metastatic liver SFTs and DPS. However, the subsequent treatment of the patient remains an open question because of multiple metastases. Due to economic and personal reasons, the patient did not want to receive further drug or surgical treatment for SFTs after March 2020. The patient died following tumor progression 7 months ago, before the submission of this paper. He had maintained stable blood glucose before his death, with no obvious hypoglycemia events occurring according to his family’s description.
DPS is mediated by several mechanisms. The first mechanism is the consumption of glucose by a large tumor (28). However, some studies have reported that hypoglycemia did not occur again when the tumor recurred and regrew to its former size, indicating that glucose consumption by the tumor may not be the main cause of hypoglycemia (28). Second, abnormalities in the EGR-IGF system have been reported to lead to hypoglycemia (28). It is generally believed that the enhanced insulin-like effect is caused by oversecretion of big IGF-2 (29). Moreover, IGF2 is an EGR target gene and is regulated by the chimeric transcription factor NAB2-STAT6, leading to abnormalities. NAB2-STAT6 gene fusion induced proliferation in cultured cells and activated the expression of EGR-responsive genes (30). The fusion of NAB2 and STAT6 produced the NAB2-STAT6 chimeric transcription factor, which is located in the nucleus, where it is currently believed to drive tumorigenesis by constitutively activating NAB2 target genes (30). The NAB2-STAT6 fusion gene, as a unique molecular feature of SFT, appears in up to 100% of cases and has not yet been detected in other tumors (31). RT-PCR detection can be used to identify the NAB2-STAT6 fusion gene, but due to the diversity of fusion types, its sensitivity is much lower than that of STAT6. Therefore, STAT6 detection is more widely used in clinical settings (32–34).
The IGF system consists of two ligands, IGF-1 and IGF-2, as well as their two receptors. Normally, approximately 70%–80% of IGFs bind to insulin-like growth factor binding protein (IGFBP)-3 in serum, whereas residual IGFs bind to other IGFBPs, leaving less than 1% of IGFs free (28). This mechanism effectively protects IGFs from degradation and avoids hypoglycemia by limiting their binding to receptors (28, 35). Bertherat et al. studied the specific expression of the IGF-2 gene in NICTH/DPS caused by pleural fibrosarcoma (36). They observed a loss of imprinting of parent alleles, which resulted in an excess expression of the IGF-2 gene and an increase in incompletely processed IGF-2, termed big-IGF-2 or pro-IGF-2. Moreover, tumor cells did not seem to have enough enzymes to act on pro-IGF-2; thus, the excess pro-IGF-2 competes with IGF-1 and IGF-2 in binding to IGFBP to form a 40–50–kDa binary complex. In that case, the process results in an excess of free IGF-2 (37) and IGF-1 in the plasma (38, 39). The increased IGF-1 causes a negative feedback, leading to a decrease in the upstream GH secretion, followed by a decline in IGF-1 and IGFBP-3 levels. IGF-2, on the other hand, will not be reduced since its secretion is not regulated by feedback because it is automatic and paracrine (20). This leads to an increased ratio of serum IGF-2/IGF-1 and an inversely proportional relationship between IGFBP-3 and IGF-2. Meanwhile, the binary complex and free IGF-2 pass through capillary membranes relatively easily and bind to insulin receptors, subsequently causing hypoglycemia (35). In DPS, as pro-IGF-2 tests have not been commercialized, the IGF-2:IGF-1 ratio is considered to be a surrogate marker for pro-IGF-2. DPS is diagnosed when the IGF-2:IGF-1 ratio is greater than 10 (40).
The best treatment for SFTs is complete resection of the tumor. Nevertheless, when curative resection is not available, metastasectomy, chemotherapy, and radiotherapy may be beneficial (18, 19). A retrospective work (n = 64) suggested that surgical resection of localized SFT can result in a 10-year overall survival (OS) of 58% when compared to that of metastatic SFT, which is 11% (21). Although there are various chemotherapies, no standard regimen has been recommended for metastatic SFTs. Several case reports and small sample studies have reported that pazopanib effectively controlled the progression of the tumor in metastatic SFTs, and the best median progression-free survival rate was 6.2 months (22). When it comes to metastatic liver SFTs, TACE may be a good alternative therapy. Velayati et al. summarized the safety and efficacy of the treatment of TACE (19). However, TACE may not always be effective. El-Khouli et al. reported the first use of cTACE: after three cycles of TACE treatments, no significant reduction in tumor size was observed (41). This is a condition similar to our patient. The reason for the low response of cTACE in metastatic liver SFTs needs further studies.
With regard to DPS, the therapies include the following: correct hypoglycemia immediately and treat the potential tumor or prevent recurrent episodes of hypoglycemia when the tumor cannot be controlled. There are several ways to correct hypoglycemia, but the best treatment is still surgery. Administrating quick-acting carbohydrates (such as glucose tablets, sugared fruit juices, or hard candy), intravenous glucose infusion, or injection of glucagon are good methods to correct hypoglycemia immediately (42). When the tumor secretes IGF, complete removal of the tumor can cure hypoglycemia (43). If surgery is not available, the methods mentioned above and glucocorticoid therapy can be considered alternatives. In spite of that, the efficacy is clear but not entirely curative. Several case reports have shown that TACE is a good choice for patients with liver SFT and DPS (25, 44). However, in these cases, there were no multiple metastases and some patients need multimodal treatment to control hypoglycemia after cTACE treatment (43). We speculated that cTACE treatment still has some limitations on both SFTs and DPS.
TILA-TACE is a powerful method to treat both SFTs and DPS. It can effectively induce tumor cell necrosis, which is more effective than that induced by cTACE. The mechanism of cTACE is by embolizing blood vessels and then creating a microenvironment with a low concentration of glucose, which affects energy metabolism and causes tumor cell death. However, in the condition of our patient, he had no improvement in blood glucose and tumor prognosis after four cycles of cTACE. The possible reasons were as follows: firstly, we know cTACE causes hypoxia and glucose deprivation, but hypoxia can simultaneously induce transcriptional activation of the IGF-2 gene, leading to increased production of pro-IGF-2, which compromises the effect of tumor cell death (28); secondly, tumor cells can survive with the help of proton and lactate, which may lead to treatment failure (27). cTACE blocks tumor-feeding arteries; the amount of glucose in the embolized tumor is limited, and the low oxygen level speeds up glycolysis and glutaminolysis. As a result, lactate and proton accumulation create a chemical environment called lactic acidosis (high lactate concentration with acidic pH). When glucose is used up, lactate and protons accumulated together can rescue cancer cells from glucose deprivation-induced death. TILA-TACE, on the other hand, using bicarbonate to neutralize tumor bed, can convert intratumoral lactic acidosis to lactosis, which can effectively prevent tumor cells from using glucose and accelerate cell necrosis. This demonstrates a superior activity in the local control of large tumors (45). In our previous nonrandomized study, the 1-, 2-, and 3-year survival rates in patients with large hepatocarcinoma treated with cTACE were lower than those treated with TILA-TACE. The median survival of the former was 14 months, and the median survival time was 41 months for TILA-TACE (p < 0.05) (27). The hypothetical therapeutic mechanism of the novel treatment TILA-TACE is presented as a flowchart (Figure 7).
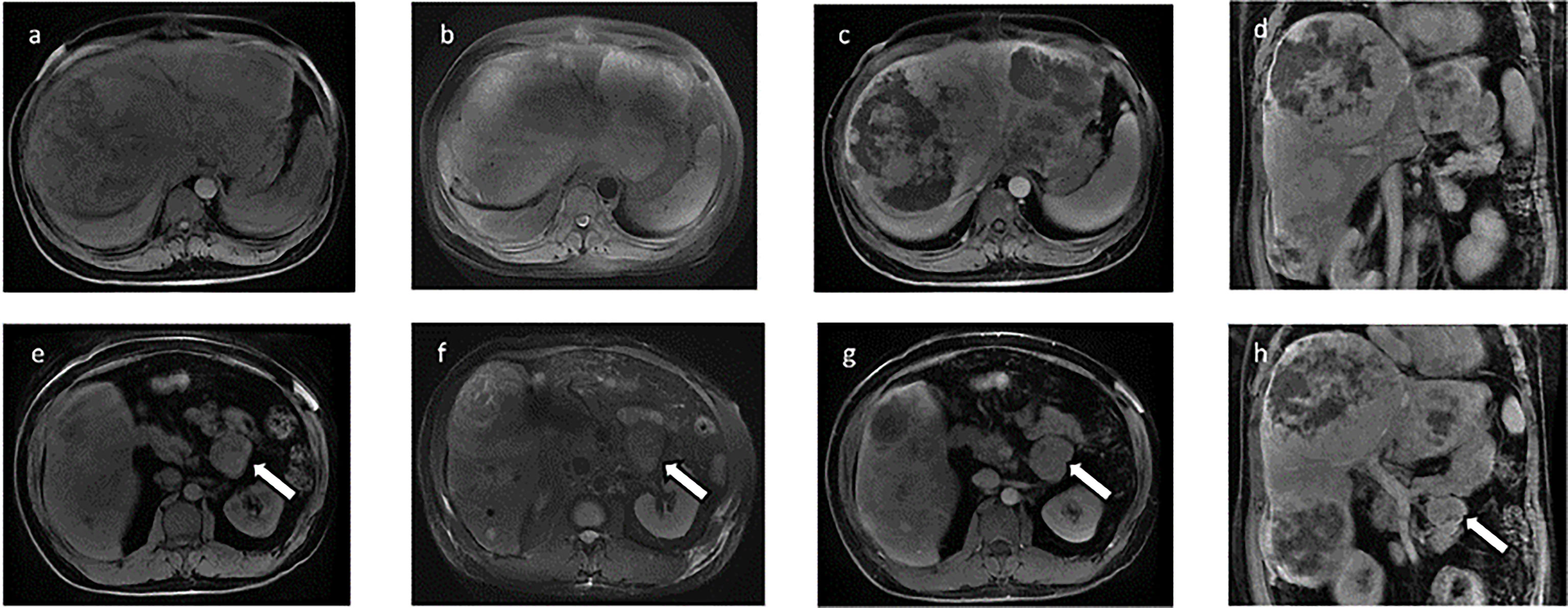
Figure 7 The hypothetical approach of TILA-TACE to treating large liver metastatic solitary fibrous tumors by targeting intratumoral lactic acidosis. cTACE embolizes tumor-feeding arteries that block glucose supply but also create a hypoxia condition and trap lactic acidosis. Intratumoral lactic acidosis rescues cancer cells from glucose deprivation. Hypoxia-enhanced angiogenesis could also significantly contribute to tumor survival. On the contrary, TILA-TACE is designed for neutralizing lactic acidosis by bicarbonate, which rapidly kills cancer cells before revascularization and thus significantly improves the therapeutic efficiency.
Considering the case of our patient, the primary lesion is likely to be intracranial SFT. The patient’s overall survival was about 10 years, with about 40 months after the development of multiple metastatic SFTs. He had severe hypoglycemia caused by metastatic SFTs in the liver and pancreas. Laboratory examinations revealed that fasting hypoglycemia, GH, and IGF-1 levels were lower than the measurable values, along with a low fasting C-peptide level. Additionally, levels of IGF-2, pro-IGF-2, and the molar ratio of IGF-2 to IGF-1 were increased. After cTACE treatment, the symptoms and laboratory tests showed no improvement, and abdominal CT even indicated an enlarged liver tumor. However, after TILA-TACE treatment, most of the metastatic liver SFTs had completely undergone necrosis and the serum levels of pro-IGF-2 and IGF-2 had decreased. Thereafter, his hypoglycemia was significantly improved, and, after tumor partial resection, his blood glucose had improved to normal levels. No other extra meals were required. Overall, TILA-TACE played a vital role in the treatment of our patient: it cured hypoglycemia and provided our patient with surgical opportunities for SFT metastases.
Therefore, it is reasonable to believe that the main cause of DPS in our patient was the excess of IGF-2 and pro-IGF-2. TILA-TACE is not only an effective measure for the treatment of metastatic SFTs but is also applicable to patients with DPS. TALI-TACE can effectively reduce tumor size, control tumor progression, provide patients with surgery opportunities and prolong survival in metastatic SFTs. Meanwhile, based on the good effect of TALI-TACE on the rapidly growing giant liver tumor, we also speculate that TALI-TACE is suitable for patients with primary SFTs in the liver. Overall, the effect of intratumoral lactic acidosis on tumor cells in a combination of hypoxia-enhanced revascularization significantly contributes to the cTACE therapeutic bottleneck (46). Whereas, destroying intratumoral lactic acidosis by TILA-TACE will be a potential protocol for hypervascular primary tumor in the liver and neuroendocrine tumor liver metastasis. Further prospective clinical trials are needed to verify the effectiveness of TILA-TACE.
In summary, DPS is a rare paraneoplastic syndrome associated with SFTs, characterized by NICTH. We report the first case of metastatic SFTs in the liver, pancreas, and thoracic spine with DPS, which was successfully treated with TILA-TACE. This led to a clinical cure of DPS in a 3-year follow-up and subsequently earned the opportunity for tumor resection. Therefore, we suggest that TILA-TACE be recommended not only for patients with SFTs and DPS but also for patients with hypervascular primary or metastatic liver tumors when curative surgery is not applicable.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine and was performed in accordance with the Declaration of Helsinki of 2013 for Human Research. Written informed consent for publication of the clinical details and images was obtained from the patient.
XS and MC conceptualized the study. KJ analyzed and interpreted all data and wrote the manuscript. SZ and LL collected and assembled data and wrote the manuscript. JW and YW analyzed the data. LL and WC interpreted study data and revised the manuscript. WG revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grant number 81300083 to XS), Zhejiang Provincial Medical and Health Technology Project (grant numbers 2020380946 and 2022502078 to XS), and Major Project of Zhejiang Provincial Medical and Health (2018c03009 to MC). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Professor Xun Hu (Cancer Institute, The Second Affiliated Hospital, Zhejiang University School of Medicine) for critical reading of this manuscript and constructive comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
WHO, World Health Organization; SFTs, solitary fibrous tumors; DPS, Doege–Potter syndrome; NTCTH, non-islet cell tumor hypoglycemia; TACE, transcatheter arterial chemoembolization; cTACE, conventional transcatheter arterial chemoembolization; TILA-TACE, targeting-intratumoral-lactic-acidosis transcatheter-arterial-chemoembolization; IGF, insulin-like growth factors; IGFBP, insulin-like growth factor binding protein; HPF, high-power field; MRI, magnetic resonance imaging; CT, computed tomography; HbA1c, hemoglobin A1c; AFP, alpha-fetoprotein; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen.
1. Klemperer P, Coleman BR. Primary neoplasms of the pleura. a report of five cases. Am J Ind Med (1992) 22(1):1–31. doi: 10.1002/ajim.4700220103
2. Rosenberg AE. WHO classification of soft tissue and bone, fourth edition: summary and commentary. Curr Opin Oncol (2013) 25(5):571–3. doi: 10.1097/01.cco.0000432522.16734.2d
3. Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer (2002) 94(4):1057–68. doi: 10.1002/cncr.10328
4. Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol (2012) 25(9):1298–306. doi: 10.1038/modpathol.2012.83
5. Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol (1999) 30(12):1464–73. doi: 10.1016/S0046-8177(99)90169-7
6. Cranshaw IM, Gikas PD, Fisher C, Thway K, Thomas JM, Hayes AJ. Clinical outcomes of extra-thoracic solitary fibrous tumours. Eur J Surg Oncol (2009) 35(9):994–8. doi: 10.1016/j.ejso.2009.02.015
7. Vallat-Decouvelaere A, Dry S, Fletcher C. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol (1998) 22(12):1501–11. doi: 10.1097/00000478-199812000-00007
8. Gronchi A, Miceli R, Allard M, Callegaro D, Le Péchoux C, Fiore M, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann Surg Oncol (2015) 22(5):1447–54. doi: 10.1245/s10434-014-4130-7
9. Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology (2014) 64(1):2–11. doi: 10.1111/his.12267
10. van Houdt W, Westerveld C, Vrijenhoek J, van Gorp J, van Coevorden F, Verhoef C, et al. Prognosis of solitary fibrous tumors: a multicenter study. Ann Surg Oncol (2013) 20(13):4090–5. doi: 10.1245/s10434-013-3242-9
11. Tapias L, Mercier O, Ghigna M, Lahon B, Lee H, Mathisen D, et al. Validation of a scoring system to predict recurrence of resected solitary fibrous tumors of the pleura. Chest (2015) 147(1):216–23. doi: 10.1378/chest.14-1180
12. Nawojowska A, Rodrigues C, Mendes S, Cabral D, Antunes M, Torres C, et al. Solitary fibrous tumors of the pleura - 20-year experience. Rev Portuguesa Cirurgia Cardio Toracica e Vascular: Orgao Oficial da Sociedade Portuguesa Cirurgia Cardio Toracica e Vasc (2020) 27(4):273. doi: 10.1378/chest.14-1180
13. Chang Y, Lee Y, Wu C. Thoracic solitary fibrous tumor: clinical and pathological diversity. Lung Cancer (Amsterdam Netherlands) (1999) 23(1):53–60. doi: 10.1016/S0169-5002(98)00096-8
14. Brozzetti S, D’Andrea N, Limiti M, Pisanelli M, De Angelis R, Cavallaro A. Clinical behavior of solitary fibrous tumors of the pleura. an immunohistochemical study. Anticancer Res (2000) 20:4701–6. doi: 10.1016/s0169-5002(98)00096-8
15. Beltrán MA. Solitary fibrous tumor of the liver: a review of the current knowledge and report of a new case. J Gastrointest Cancer (2015) 46(4):333–42. doi: 10.1007/s12029-015-9769-1
16. Doege KW. FIBRO-SARCOMA OF THE MEDIASTINUM. Ann Surg (1930) 92(5):955–60. doi: 10.1007/s12029-015-9769-1
17. Kiely NP, Sinha R, Tang K, Wan KM. Doege-potter syndrome: a systematic review of the literature and case presentation of a rare pelvic malignant solitary fibrous tumour. BMJ Case Rep (2021) 14(8):955–60. doi: 10.1136/bcr-2021-242447
18. Haas RL, Walraven I, Lecointe-Artzner E, Scholten AN, van Houdt WJ, Griffin AM, et al. Radiation therapy as sole management for solitary fibrous tumors (SFT): A retrospective study from the global SFT initiative in collaboration with the sarcoma patients EuroNet. Int J Radiat Oncol Biol Phys (2018) 101(5):1226–33. doi: 10.1016/j.ijrobp.2018.04.024
19. Velayati S, Erinjeri JP, Brody LA, Ziv E, Boas FE, Brown KT, et al. Safety and efficacy of hepatic artery embolization in treating solitary fibrous tumor metastatic to the liver. Sarcoma (2019) 2019:3060658. doi: 10.1155/2019/3060658
20. Martin-Broto J, Cruz J, Penel N, Le Cesne A, Hindi N, Luna P, et al. Pazopanib for treatment of typical solitary fibrous tumours: a multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(3):456–66. doi: 10.1016/S1470-2045(19)30826-5
21. Friis R, Safwat A, Baad-Hansen T, Aggerholm-Pedersen N. Solitary fibrous tumour: A single institution retrospective study and further validation of a prognostic risk assessment system. Clin Oncol (Royal Coll Radiologists (Great Britain)) (2018) 30(12):798–804. doi: 10.1016/j.clon.2018.08.015
22. Ebata T, Shimoi T, Bun S, Miyake M, Yoshida A, Shimomura A, et al. Efficacy and safety of pazopanib for recurrent or metastatic solitary fibrous tumor. Oncology (2018) 94(6):340–4. doi: 10.1159/000486623
23. Lee S, Kim S, Park S, Choi Y, Park J, Kim S, et al. Successful use of pazopanib for treatment of refractory metastatic hemangiopericytoma. Clin Sarcoma Res (2014) 4:13. doi: 10.1186/2045-3329-4-13
24. Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer (2011) 117(21):4939–47. doi: 10.1002/cncr.26098
25. Mačák J, Buzrla P, Dvořáčková J, Prokop P, Jalůvka F. [Hypoglycemia in a solitary fibrous tumor of the liver]. Cesk Patol (2016) 52(1):41–4. doi: 10.1002/cncr.26098
26. Sun Z, Ding Y, Jiang Y, Zhang Q, Li Z, Xiang J, et al. Ex situ hepatectomy and liver autotransplantation for a treating giant solitary fibrous tumor: A case report. Oncol Lett (2019) 17(1):1042–52. doi: 10.3892/ol.2018.9693
27. Chao M, Wu H, Jin K, Li B, Wu J, Zhang G, et al. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. Elife (2016) 5:1042–52. doi: 10.7554/eLife.15691
28. de Groot JW, Rikhof B, van Doorn J, Bilo HJ, Alleman MA, Honkoop AH, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer (2007) 14(4):979–93. doi: 10.1677/ERC-07-0161
29. Daughaday WH, Emanuele MA, Brooks MH, Barbato AL, Kapadia M, Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. New Engl J Med (1988) 319(22):1434–40. doi: 10.1056/NEJM198812013192202
30. Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, et al. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet (2013) 45(2):180–5. doi: 10.1038/ng.2509
31. Mohammed T, Ozcan G, Siddique AS, Araneta Iii RN, Slater DE, Khan A. Doege-potter syndrome with a benign solitary fibrous tumor: A case report and literature review. Case Rep Oncol (2021) 14(1):470–6. doi: 10.1159/000512823
32. Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet (2013) 45(2):131–2. doi: 10.1038/ng.2522
33. Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, von Steyern FV, et al. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer (2013) 52(10):873–86. doi: 10.1002/gcc.22083
34. Barthelmeß S, Geddert H, Boltze C, Moskalev E, Bieg M, Sirbu H, et al. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol (2014) 184(4):1209–18. doi: 10.1016/j.ajpath.2013.12.016
35. Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev (2013) 34(6):798–826. doi: 10.1210/er.2012-1033
36. Bertherat J, Logié A, Gicquel C, Mourriéras F, Luton JP, Le Bouc Y. Alterations of the 11p15 imprinted region and the IGFs system in a case of recurrent non-islet-cell tumour hypoglycaemia (NICTH). Clin Endocrinol (2000) 53(2):213–20. doi: 10.1046/j.1365-2265.2000.01064.x
37. Izutsu T, Ito H, Fukuda I, Tamura H, Matsumoto S, Antoku S, et al. Early improvement of non-islet cell tumor hypoglycemia by chemotherapy using lenvatinib in a case with type 2 diabetes and hepatocellular carcinoma producing big IGF-II. Internal Med (2021) 60(9):1427–32. doi: 10.2169/internalmedicine.5328-20
38. Nauck MA, Reinecke M, Perren A, Frystyk J, Berishvili G, Zwimpfer C, et al. Hypoglycemia due to paraneoplastic secretion of insulin-like growth factor-I in a patient with metastasizing large-cell carcinoma of the lung. J Clin Endocrinol Metab (2007) 92(5):1600–5. doi: 10.1210/jc.2006-2573
39. Frystyk J, Skjaerbaek C, Zapf J, Orskov H. Increased levels of circulating free insulin-like growth factors in patients with non-islet cell tumour hypoglycaemia. Diabetologia (1998) 41(5):589–94. doi: 10.1007/s001250050951
40. Teale JD, Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) (1990) 33(1):87–98. doi: 10.1111/j.1365-2265.1990.tb00469.x
41. El-Khouli RH, Geschwind JF, Bluemke DA, Kamel IR. Solitary fibrous tumor of the liver: magnetic resonance imaging evaluation and treatment with transarterial chemoembolization. J Comput Assisted Tomography (2008) 32(5):769–71. doi: 10.1097/RCT.0b013e3181557453
42. Beato-Vibora PI, Arroyo-Diez FJ. New uses and formulations of glucagon for hypoglycaemia. Drugs Context (2019) 8:212599. doi: 10.7573/dic.212599
43. Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab (2014) 99(3):713–22. doi: 10.1210/jc.2013-3382
44. De Los Santos-Aguilar RG, Chávez-Villa M, Contreras AG, García-Herrera JS, Gamboa-Domínguez A, Vargas-Sánchez J, et al. Successful multimodal treatment of an IGF2-producing solitary fibrous tumor with acromegaloid changes and hypoglycemia. J Endocr Soc (2019) 3(3):537–43. doi: 10.1210/js.2018-00281
45. Chao M, Wu H, Jin K, Hu X. TILA-TACE–an approach for effective local control of hepatocellular carcinoma. J Intervent Med (2018) 000(001):58–63. doi: 10.1210/jc.2013-3382
Keywords: TILA-TACE, solitary fibrous tumor, Doege–Potter syndrome, non-islet cell tumor, hypoglycemia
Citation: Jin K, Zhong S, Lin L, Wu J, Wang Y, Cui W, Gu W, Chao M and Song X (2022) Targeting-intratumoral-lactic-acidosis transcatheter-arterial-chemoembolization for non-islet cell tumor hypoglycemia secondary to a liver metastatic solitary fibrous tumor: A case report and literature review. Front. Endocrinol. 13:955687. doi: 10.3389/fendo.2022.955687
Received: 29 May 2022; Accepted: 18 July 2022;
Published: 11 August 2022.
Edited by:
Barbara Altieri, University Hospital of Wuerzburg, GermanyReviewed by:
Vivek Pant, Federal University of Rio de Janeiro, BrazilCopyright © 2022 Jin, Zhong, Lin, Wu, Wang, Cui, Gu, Chao and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxiao Song, eHNvbmcxMDNAemp1LmVkdS5jbg==; Ming Chao, Y2hhb21pbmdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.