
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 28 July 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.955241
This article is part of the Research Topic Association of Novel Anthropometric Indexes with Metabolic Syndrome and Beyond, volume II View all 16 articles
 Yue Zhang1,2
Yue Zhang1,2 Wenxing Gao1
Wenxing Gao1 Binqi Li3,2
Binqi Li3,2 Yang Liu2
Yang Liu2 Kang Chen2
Kang Chen2 Anping Wang2
Anping Wang2 Xulei Tang4
Xulei Tang4 Li Yan5
Li Yan5 Zuojie Luo6
Zuojie Luo6 Guijun Qin7
Guijun Qin7 Lulu Chen8
Lulu Chen8 Qin Wan9
Qin Wan9 Zhengnan Gao10
Zhengnan Gao10 Weiqing Wang11
Weiqing Wang11 Guang Ning11
Guang Ning11 Yiming Mu2*
Yiming Mu2*Background: Obesity, especially visceral obesity, seems to be one of the most decisive risk factors for chronic kidney disease. A Body Shape Index (ABSI) is an emerging body size measurement marker of visceral obesity. This study aimed to explore whether ABSI is associated with albuminuria in Chinese community adults.
Methods: This cross-sectional study enrolled 40,726 participants aged 40 or older from seven provinces across China through a cluster random sampling method. ABSI was calculated by body mass index, waist circumference, and height. Increased albuminuria was defined as urinary albumin–creatinine ratio (UACR) ≥ 30 mg/g, indicating kidney injury. For ABSI, we divided it by quartile cutoff points and tried to determine the association between ABSI levels and UACR by multiple regression analysis. DAG (Directed Acyclic Graph) was plotted using literature and expert consensus to identify potential confounding factors.
Results: The average age of subjects with elevated UACR was 61.43 ± 10.07, and 26% were men. The average age of subjects with normal UACR was 57.70 ± 9.02, and 30.5% were men. Multiple logistic regression analysis was conducted and demonstrated that the ABSI quartiles were related to elevated UACR positively (OR [95% CI] Q2 vs. Q1: 1.094 [1.004, 1.197]; OR [95% CI] Q3 vs. Q1: 1.126 [1.030, 1.231]; OR [95% CI] Q4 vs. Q1: 1.183 [1.080, 1.295], p for trend < 0.001) after adjustments for confounding factors. The stratified analysis further showed that with the mounting for ABSI levels, elevated UACR more easily occurred in the people characterized by the elderly, men, and hypertension.
Conclusions: In Chinese community adults, people with higher ABSI levels can be deemed as high-risk individuals with UACR elevation, and it will be beneficial for them to lose weight and significantly reduce visceral fat.
Nowadays, chronic kidney disease (CKD) has been changed into a global public health threat. The estimated prevalence of CKD was 9.1% worldwide in 2017, ranking as the 12th leading cause of death (1). The onset of CKD is insidious, and it was easily advanced to end-stage renal disease (ESRD), which has a poor prognosis and high mortality, posing a heavy burden on public health and the economy (2). UACR, as a sensitive marker of early kidney injury, is currently used in clinical screening for CKD to identify high-risk populations (3).
There is a remarkable phenomenon that the prevalence of obesity in patients with CKD is high, mounting from 38.1% in 1999–2002 to 44.1% in 2011–2014 in the United States (4). A large population of European survey demonstrated that among the risk factors of new CKD, obesity is one of the strongest one (5). Body mass index (BMI), used for obesity measurement most commonly (6), remains limited by its inability to provide information on fat distribution and distinguish fat accumulation from muscle (7). Krakauer invented ABSI (8), which consists of waist circumference (WC), height, and BMI. High ABSI values correspond to high visceral fat, which not only predicts the risk of premature death independent of BMI, but also is a marker of abdominal obesity and insulin resistance in men (9).
pt?>A cohort study of 5,438 urban residents in Japan found that ABSI can predict subjects at risk of renal function decline more effectively than WC (10). A cross-sectional survey of 7,053 older people in South Korea showed that the ABSI had a better capacity to discriminate the CKD stage than BMI (11). However, to our knowledge, evidence of the relationship between ABSI and UACR in the large-sample population is lacking. Therefore, in this study, we collected data from 40,726 Chinese adults to explore the relationship between ABSI and albuminuria and identify high-risk individuals as early as possible to provide evidence for CKD prevention.
Data from the cross-sectional study came from the REACTION (China’s Risk Evaluation of cAncers in Chinese diabeTic Individuals, a lONgitudinal study), which recruited 47,808 individuals over the age of 40 from May to December 2011 in seven geographically diverse regional centers in China (Zhengzhou, Dalian, Luzhou, Shanghai, Wuhan, Guangzhou, and Lanzhou) (12). Participants with a diagnosis of primary kidney disease, a history of malignancy, prior use of antihypertensive medications (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers), or lack of significant data were excluded. Finally, we enrolled 33,303 participants (Figure 1). The Clinical Research Ethics Committee approved this study of Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine (2014-25). This study was performed according to the Declaration of Helsinki. All participants, before participation, provided informed consent.
Trained investigators collected basic information about participants through standardized questionnaires, including age, gender, history of underlying diseases, medication history, lifestyle, smoking habits, and drinking habits. Anthropometric measurements include weight, height, WC, diastolic blood pressure (DBP), and systolic blood pressure (SBP). Participants removed their clothes and shoes before the measurement. Blood pressure was measured three times with a mercury sphygmomanometer and averaged. All subjects had to sit still for at least 5 min before the measurement. The definition of WC is the abdominal circumference connecting the lower margin of the thorax to the midpoint of the iliac crest, the hip circumference (HC) was defined as the length of the hip joint protrusion horizontally. Fasting blood samples and morning urine were collected after 10 h of fasting. Biochemical parameters included aspartate transferase (AST), alanine transferase (ALT), glutamyltransferase (GGT), fasting blood glucose (FBG), 2 h postprandial blood glucose (PBG), rapid insulin determination (0 min, 120 min), glycosylated hemoglobin A1c (HbA1c), triglyceride (TG), total cholesterol (TC), serum creatinine (Scr), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
BMI is the weight (kg) divided by height squared (m2). ABSI was calculated by WC (m)/[BMI2/3 (kg/m2) × height1/2 (m)]. Waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) are WC divided by HC and WC divided by height, respectively. Smoking habits were defined as non-smoking, occasional smoking (less than one cigarette per day or less than seven cigarettes per week), and regular smoking (at least one cigarette per day). Normal blood pressure is defined as SBP of less than 120 and DBP of less than 80; hypertension was defined as SBP greater than 140 or DBP greater than 90; between the two categories is pre-hypertension. UACR was calculated as urinary albumin (mg)/urinary creatinine (g). According to the KDIGOCKD guidelines (13), UACR ≥ 30 mg/g was the definition of increased proteinuria, suggesting kidney damage. The UACR group was divided into two groups: normal proteinuria group: UACR < 30 mg/g and increased proteinuria: UACR ≥ 30 mg/g. For ABSI, we divide it by quartile cutoff points. eGFR was estimated from a simplified equation developed from data from the Modification of Diet in Renal Disease (MDRD) study as follows (14): eGFR (ml/min/1.73 m2) = 186 × [SCr (mg/dl)/88.4]-1.154 × (age)-0.203 × (0.742 if women) × 1.233.
We performed the Kolmogorov–Smirnov test to explore whether the continuous variables were normally distributed. Continuous variables were presented as mean ± SD or median (IQR) for skewed variables; the classification variables were represented by percentage (%). The Mann–Whitney U test was used to compare the difference between continuous variables, and the Chi-square test was used to compare the categorical variables. Logistic regression analysis was conducted to estimate odds ratios (ORs) and 95% confidence intervals (Cis) to determine the association between the ABSI quartile and increased proteinuria, with the lowest quartile as the reference group. We identify conf variables for the relationship between ABSI and albuminuria by reviewing the literature and drawing the DAG. After the univariate analysis between the confounders and UACR, the confounders with a p-value less than 0.2 were included in the final model, and the multiple logistic regression model of ABSI and all potential confounders was established, and the optimal model was fitted by stepwise backward regression method. To further investigate the association between ABSI quartile and increased risk of proteinuria, the relationship between gender, age (<60/≥60 years), and eGFR (<90/≥90 ml/min/1.73 m2) was stratified. The software used for data analysis was SPSS Version 25.0 (IBM, Chicago, IL, USA). The results were considered statistically significant if the bilateral p-value < 0.05.
A total of 40,726 participants were recruited for the study; 29.9% were men, and 70.1% were women. The average age of participants was 58.3 ± 9.26. Table 1 shows the clinical and biochemical demographics of the subjects, which are divided into two groups based on whether their UACR is elevated or not. Compared with the normal UACR group, the UACR ≥ 30 mg/g group was older, had higher rates of DM and CHD, and had higher LDL-C, WC, GGT, AST, BMI, HbA1c, FBG, PBG, SBP, DBP, and lower HDL-C, education level, and eGFR values (all p< 0.001)
By plotting DAG (Figure 2), we found that age, BMI, education level, smoking habits, sex, eGFR, blood pressure level, sedentary time, diabetes, LDL-C, physical activity, TG, FBG, diabetes, CHD, and smoking habits were confounders of the relationship between ABSI and albuminuria. Through single-factor analysis, statistically significant age, sex, smoking habits, CHD history, DM history, education status, blood pressure level, FBG, TG, LDL, and eGFR were selected as confounding factors for multivariate logistic regression analysis to investigate the relationship between ABSI quartiles and UACR elevation (Table 2). The correlation of model was significant (OR [95% CI] Q2 vs. Q1: 1.094 [1.001, 1.197]; OR [95% CI] Q3 vs. Q1: 1.126 [1.030, 1.231]; OR [95% CI] Q4 vs. Q1: 1.183 [1.080, 1.295], p trend < 0.001).
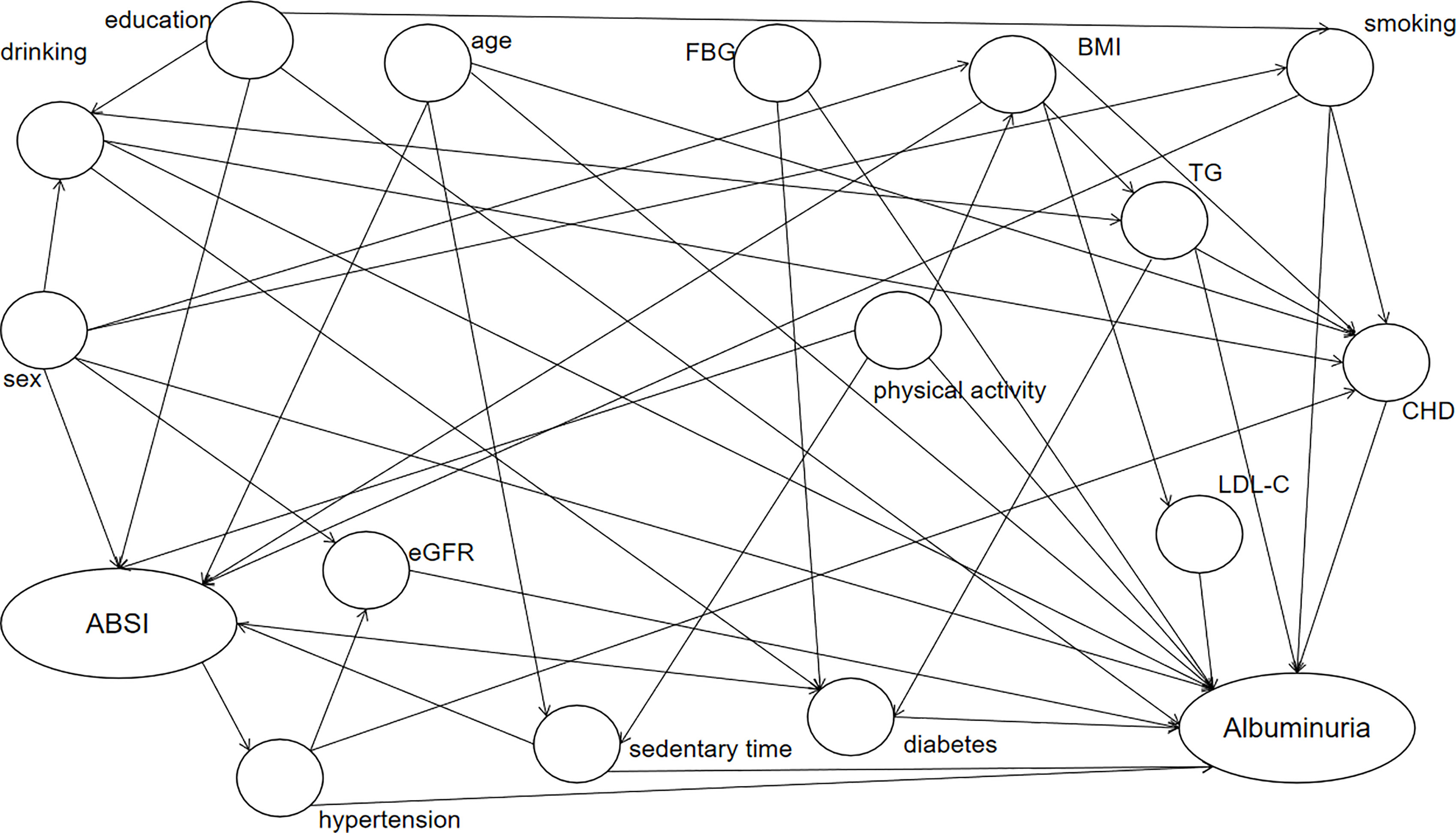
Figure 2 DAG diagram of the ABSI and albuminuria association study. Elliptic nodes represent independent variables ABSI and dependent variables albuminuria, circular nodes indicate possible confounders, and arrows indicate causality. ABSI, A Body Shape Index; BMI, body mass index; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate.
Stratified analysis was adopted to further verify the stability of the correlation between ABSI and UACR in different populations after comprehensive adjustment of age, sex, smoking habits, CHD history, DM history, education status, blood pressure level, FBG, TG, LDL, and eGFR (Table 3). Stratified by sex (p-interaction = 0.026), ABSI in the fourth quartile was associated with increased UACR in women (OR [95% CI] Q4 vs. Q1: 1.144 [1.031, 1.268]); however, increased UACR has a significantly association with ABSI in the third and fourth quartile in men(OR [95% CI] Q3 vs. Q1: 1.245 [1.028, 1.507]; OR [95% CI] Q4 vs. Q1: 1.314 [1.080, 1.599]). When subjects have a normal blood pressure (SBP < 120 and DBP < 80) according to Stratification (p-interaction < 0.001), the probability of UACR increased gradually from the lowest quartile of ABSI to the highest quartile (OR [95% CI] Q2 vs. Q1: 1.228 [1.038, 1.452]; OR [95% CI] Q3 vs. Q1: 1.200 [1.008, 1.429]; OR [95% CI] Q4 vs. Q1: 1.235 [1.027, 1.485], p trend = 0.048). The same trend was observed at the pre-hypertension group (120 ≤ SBP < 140 and/or 80 ≤ DBP < 90) (OR [95% CI] Q3 vs. Q1: 1.229 [1.057, 1.428]; OR [95% CI] Q4 vs. Q1: 1.404 [1.225, 1.610], p trend < 0.001). However, the OR was highest in the hypertensive group (SBP ≥ 140 or DBP ≥ 90) (OR [95% CI] Q3 vs. Q1: 1.239 [1.075, 1.429]; OR [95% CI] Q4 vs. Q1: 1.449 [1.249, 1.682], p trend < 0.001). Stratified by age (p-interaction < 0.001), the elderly (age ≥ 60 years) in Q3 and Q4 were more likely to increase UACR (OR [95% CI] Q3 vs. Q1: 1.183 [1.025, 1.365]; OR [95% CI] Q4 vs. Q1: 1.372 [1.198, 1.572], p trend < 0.001). In the younger participants (age < 60 years), ABSI was also significantly associated with increased UACR (OR [95% CI] Q2 vs. Q1: 1.119 [1.001, 1.250]; OR [95% CI] Q3 vs. Q1: 1.142 [1.017, 1.283]; OR [95% CI] Q4 vs. Q1: 1.159 [1.113, 1.185], p trend = 0.027).
In this study, we found that the ABSI levels are associated with increased UACR significantly, and correlation is abated after adjustment of smoking habits, FBG, diabetes history, LDL-C, and CHD history, indicating that history of smoking, blood glucose or lipid metabolic disorders, and history of CHD increase the risk of increased proteinuria in Chinese adults. Furthermore, stratified analysis showed that individuals with higher ABSI levels were more likely to have elevated UACR than those with lower ABSI levels, especially those in the elderly, men, and with hypertension. This study is the first multicenter, large-sample clinical to investigate the relationship between ABSI and UACR in Chinese adults. Early prevention and intervention of proteinuria are crucial; early detection and decreasing abnormal fat distribution may be helpful in preventing adverse outcomes such as CHD, obesity, and DM for patients.
With rapid economic development and lifestyle changes, the incidence of overweight and obesity has increased significantly worldwide. Given this growing trend, it is expected that as many as 57.8% of the population will be overweight or obese by 2030 (15). Obesity is divided into central obesity and peripheral obesity. An increase in visceral fat characterizes central obesity. Excess visceral fat can cause diabetes, hypertension, heart diseases, non-alcoholic fatty liver diseases, kidney disorders, cancer, and other health problems. Traditional anthropometric indicators include WC, BMI, WHtR, and WHR. BMI cannot distinguish fat accumulation from muscle, and WC cannot distinguish visceral fat from subcutaneous fat (16); magnetic resonance imaging (MRI) and computed tomography (CT) are considered to be the gold standard for the distribution of visceral obesity. However, they cannot be routinely used in epidemiological investigations due to the risk of radiation exposure, which is time-consuming and expensive. According to the study (17), ABSI is apparently associated with central obesity and has a better ability to predict type 2 diabetes mellitus (T2DM) than BMI. ABSI has been proved to be associated with all-cause mortality (8), metabolic syndrome (18), DM (19), and hypertension (20). Therefore, ABSI can better measure body size and is expected to become a new standard for health assessment.
The Dutch Prevention of Renal and Vascular End-Stage Disease (PREVEND) study, published in 2003, reported a prevalence of microalbuminuria of 21% or 13%, depending on central or peripheral obesity patterns (21). In a cross-sectional study of adults with T2DM, visceral obesity was significantly associated with UACR (22). Similarly, a follow-up study of 2,393 participants over 4 years observed that participants who had increased visceral fat mass had higher albuminuria (23). Notably, few studies have investigated the relationship between ABSI and proteinuria in individuals who are most likely to develop CKD and have underlying cardiovascular risk factors. Munkhaugen conducted a 20-year cohort study in Norway that assessed 75,000 volunteers and found a strong correlation between BMI and CKD risk, with obese people more likely to develop kidney disease (24). In another large population-based case–control study reported by Ejerblad, patients with a BMI of 25 kg/m2 at age 20 had a threefold increased risk of new kidney disease, even after adjusting for hypertension and DM (25). In our study, which included 40,726 Chinese adults, we found that higher visceral obesity as assessed by ABSI was independently associated with an increased risk of proteinuria. These results are consistent with previous studies.
Multiple biological mechanisms may mediate the association between obesity and proteinuria. Recent studies have shown that adipose tissue can secrete adipose tissue-derived adipokines (26) and cytokines (27), such as leptin, which has local effects on mesangial cells, podocytes, and renal tubules, promoting glomerular hyperfiltration (28), which is an independent predictor of proteinuria (29). Participate in the pathogenesis of CKD. In addition, mechanisms such as insulin resistance (30), oxidative stress (31), systemic chronic low-level inflammation (32), and inappropriate activation of the renin–angiotensin–aldosterone system (33) are also involved in developing proteinuria. Prevention of REnal and Vascular ENd-stage Disease study data showed that men have a higher urinary albumin excretion, a known factor for progression of CKD, at any given age, plasma glucose, and BMI than women (34). Our data also showed a gender difference, and we speculated that this might be due to potential anti-fibrotic and anti-apoptotic effects of estrogen or deleterious pro-inflammatory effects of testosterone, as evidenced in animal studies (35). Moreover, age is a well-known independent risk factor for renal impairment, and albuminuria is more likely to occur in the elderly than in middle-aged adults (36). Chronic hypertension can lead to gradual thickening of the glomerular arteries, which can lead to atherosclerotic changes, decreased renal blood flow, decreased kidney function, and decreased filtration of the kidneys, thus leading to increased albuminuria.
Our study provides additional evidence to confirm the association between visceral fat and proteinuria as assessed by ABSI and demonstrates the value of ABSI as a simple, reliable, and effective screening tool for kidney disease risk. In clinical practice, improvements in the distribution and deposition of visceral fat, rather than just weight loss, should be proposed to reduce the associated risk of kidney disease and cardiovascular disease. We believe that ABSI should be used as part of a management strategy to reduce the risk of kidney disease in further clinical practice.
As we know, this study is the first cross-sectional study to explore the relationship between ABSI and elevated UACR with a large sample. However, some limitations need to be considered: First, it cannot clarify the causal relationship between ABSI and elevated UACR as it is a retrospective cross-sectional study, so further prospective studies are necessary. Secondly, the participants were all enrolled from China and were older than 40 years old, so our conclusions may not be applied to other regions and people. Finally, because the application of MRI or CT in such a large population is expensive and inconvenient, our study did not accurately assess visceral adipose tissue. However, previous studies have demonstrated the apparent association between ABSI and visceral adipose tissue, and we suggested that ABSI has the potential to be a reliable and simple tool in proteinuria screening for high-risk people.
This study demonstrated that increased ABSI levels positively correlated with elevated UACR among adults in the Chinese community. Albuminuria was increased in men, the elderly, and hypertension. Therefore, ABSI can be used as a clinical tool to identify the high-risk population of CKD in Chinese adults. Considering the significant association between visceral fat, proteinuria, and CKD, we should pay more attention to obese individuals and guide them to change their lifestyle and regular exercise, and weight loss is not the only significant benefit of reducing visceral fat deposition.
All data used to support the conclusions of the study are not freely available in the view of the privacy principle of Chinese PLA General Hospital, and of protecting the privacy of participants.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YZ analyzed the data and wrote the manuscript. WG, BL, and YL provided great help in the operation and application of SPSS. KC, AW, XT, LY, ZL, GQ, LC, QW, ZG, WW, and GN offered advice and assistance. YM contributed by revising the article. All authors contributed to the article and approved the submitted version.
The authors would like to thank the participants in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD Chronic Kidney Disease Collaboratin. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet (2020) 395(10225):709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Adair KE, von Waaden N, Rafalski M, Hess BW, Weaver SP, Bowden RG. Metabolic phenotypes and chronic kidney disease: A cross-sectional assessment of patients from a Large federally qualified health center. Life (Basel) (2021) 11(2):175. doi: 10.3390/life11020175
3. Smith ER, Cai MM, McMahon LP, Wright DA, Holt SG. The value of simultaneous measurements of urinary albumin and total protein in proteinuric patients. Nephrol Dial Transplant (2012) 27(4):1534–41. doi: 10.1093/ndt/gfr708
4. Martin WP, Bauer J, Coleman J, Dellatorre-Teixeira L, Reeve JLV, Twomey PJ, et al. Obesity is common in chronic kidney disease and associates with greater antihypertensive usage and proteinuria: evidence from a cross-sectional study in a tertiary nephrology centre. Clin Obes (2020) 10(6):e12402. doi: 10.1111/cob.12402
5. Obermayr RP, Temml C, Knechtelsdorfer M, Gutjahr G, Kletzmayr J, Heiss S, et al. Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant (2008) 23(4):1265–73. doi: 10.1093/ndt/gfm790
6. Giudici KV, Martini LA. Comparison between body mass index and a body shape index with adiponectin/leptin ratio and markers of glucose metabolism among adolescents. Ann Hum Biol (2017) 44(6):489–94. doi: 10.1080/03014460.2017.1327617
7. Gomez-Ambrosi J, Silva C, Galofre J, Escalada JC, Santos S, Millán D, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) (2012) 36(2):286–94. doi: 10.1038/ijo.2011.100
8. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One (2012) 7(7):e39504. doi: 10.1371/journal.pone.0039504
9. Biolo G, Di Girolamo FG, Breglia A, Chiuc M, Baglio V, Vinci P, et al. Inverse relationship between "a body shape index" (ABSI) and fat-free mass in women and men: Insights into mechanisms of sarcopenic obesity. Clin Nutr (2015) 34(2):323–7. doi: 10.1016/j.clnu.2014.03.015
10. Nagayama D, Fujishiro K, Tsuda S, Watanabe Y, Yamaguchi T, Suzuki K, Saiki A, et al. Enhanced prediction of renal function decline by replacing waist circumference with "A body shape index (ABSI)" in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes (Lond) (2022) 46(3):564–73. doi: 10.1038/s41366-021-01026-7
11. Kim B, Kim G, Kim E, Park J, Isobe T, Sakae T, et al. The a body shape index might be a stronger predictor of chronic kidney disease than BMI in a senior population. Int J Environ Res Public Health (2021) 18(24):12874. doi: 10.3390/ijerph182412874
12. Ning G, Reaction Study G. Risk evaluation of cAncers in Chinese diabeTic individuals: a lONgitudinal (REACTION) study. J Diabetes (2012) 4(2):172–3. doi: 10.1111/j.1753-0407.2012.00182.x
13. Stevens PE, Levin A, Members. KDIGOCKDGDWG. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med (2013) 158(11):825–30. doi: 10.7326/0003-4819-158-11-201306040-00007
14. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol (2006) 17(10):2937–44. doi: 10.1681/ASN.2006040368
15. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) (2008) 32(9):1431–7. doi: 10.1038/ijo.2008.102
16. Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Despres JP. Visceral obesity and the heart. Int J Biochem Cell Biol (2008) 40(5):821–36. doi: 10.1016/j.biocel.2007.12.001
17. Bawadi H, Abouwatfa M, Alsaeed S, Kerkadi A, Shi Z. Body shape index is a stronger predictor of diabetes. Nutrients (2019) 11(5):1018. doi: 10.3390/nu11051018
18. Ji M, Zhang S, An R. Effectiveness of a body shape index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev (2018) 19(5):737–59. doi: 10.1111/obr.12666
19. Krakauer NY, Krakauer JC. Dynamic association of mortality hazard with body shape. PLoS One (2014) 9(2):e88793. doi: 10.1371/journal.pone.0088793
20. Rico-Martin S, Calderon-Garcia JF, Sanchez-Rey P, Franco-Antonio C, Martinez Alvarez M, Sanchez Munoz-Torrero JF. Effectiveness of body roundness index in predicting metabolic syndrome: A systematic review and meta-analysis. Obes Rev (2020) 21(7):e13023. doi: 10.1111/obr.13023
21. Pinto-Sietsma S-J, Navis G, Janssen WMT. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Diseases (2003) 41(4):733–41. doi: 10.1016/S0272-6386(03)00020-9
22. Hanai K, Toya K, Suzuki K, Babazono T, Nyumura I. Involvement of visceral fat in the pathogenesis of albuminuria in patients with type 2 diabetes with early stage of nephropathy. Clin Exp Nephrol (2010) 14:132–6. doi: 10.1007/s10157-009-0245-8
23. Kim J-K, Kwon Y-J, Young Rim S, Kim Y-S. Four-year changes in visceral fat mass and the risk of developing proteinuria in the general population. PloS One (2015) 10(6):e0131119. doi: 10.1371/journal.pone.0131119
24. Munkhaugen J, Lydersen S, Widerøe T-E, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis (2009) 54(4):638–46. doi: 10.1053/j.ajkd.2009.03.023
25. Ejerblad E, Fored M, Lindblad P, Fryzek J, McLaughlin JK, O Nyre´n. Obesity and risk for chronic renal failure. Am Soc Nephrology (2006) 17:1695–702. doi: 10.1681/ASN.2005060638
26. Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes (2014) 7:347–55. doi: 10.2147/DMSO.S46674
27. Hunley TE, Ma LJ, Kon V. Scope and mechanisms of obesity-related renal disease. Curr Opin Nephrol Hypertens (2010) 19(3):227–34. doi: 10.1097/MNH.0b013e3283374c09
28. Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol (2012) 8(5):293–300. doi: 10.1038/nrneph.2012.19
29. Kramer H, Reboussin D, Bertoni AG, Marcovina S, Lipkin E, Greenway FL 3rd, et al. Obesity and albuminuria among adults with type 2 diabetes: the look AHEAD (Action for health in diabetes) study. Diabetes Care (2009) 32(5):851–3. doi: 10.2337/dc08-2059
30. Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol (2006) 26(3):232–44. doi: 10.1159/000093632
31. Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol (2008) 19(3):593–9. doi: 10.1681/ASN.2007030355
32. Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol (2007) 2(3):550–62. doi: 10.2215/CJN.04071206
33. Ruster C, Wolf G. The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol (2013) 33(1):44–53. doi: 10.1016/j.semnephrol.2012.12.002
34. Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int (2008) 74(4):505–12. doi: 10.1038/ki.2008.200
35. Brar A, Markell M. Impact of gender and gender disparities in patients with kidney disease. Curr Opin Nephrol Hypertens (2019) 28(2):178–82. doi: 10.1097/MNH.0000000000000482
Keywords: a body shape index, albuminuria, visceral obesity, chronic kidney disease, body mass index
Citation: Zhang Y, Gao W, Li B, Liu Y, Chen K, Wang A, Tang X, Yan L, Luo Z, Qin G, Chen L, Wan Q, Gao Z, Wang W, Ning G and Mu Y (2022) The association between a body shape index and elevated urinary albumin–creatinine ratio in Chinese community adults. Front. Endocrinol. 13:955241. doi: 10.3389/fendo.2022.955241
Received: 28 May 2022; Accepted: 27 June 2022;
Published: 28 July 2022.
Edited by:
Mostafa Qorbani, Alborz University of Medical Sciences, IranReviewed by:
Shirin Djalalinia, Ministry of Health and Medical Education, IranCopyright © 2022 Zhang, Gao, Li, Liu, Chen, Wang, Tang, Yan, Luo, Qin, Chen, Wan, Gao, Wang, Ning and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Mu, bXV5aW1pbmdAMzAxaG9zcGl0YWwuY29tLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.