- 1Division of Pediatric Endocrinology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
- 2Neuroendocrine Unit, Center for Endocrinology and Diabetes of Bahia State, Salvador, Brazil
- 3Neuroendocrine Unit, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
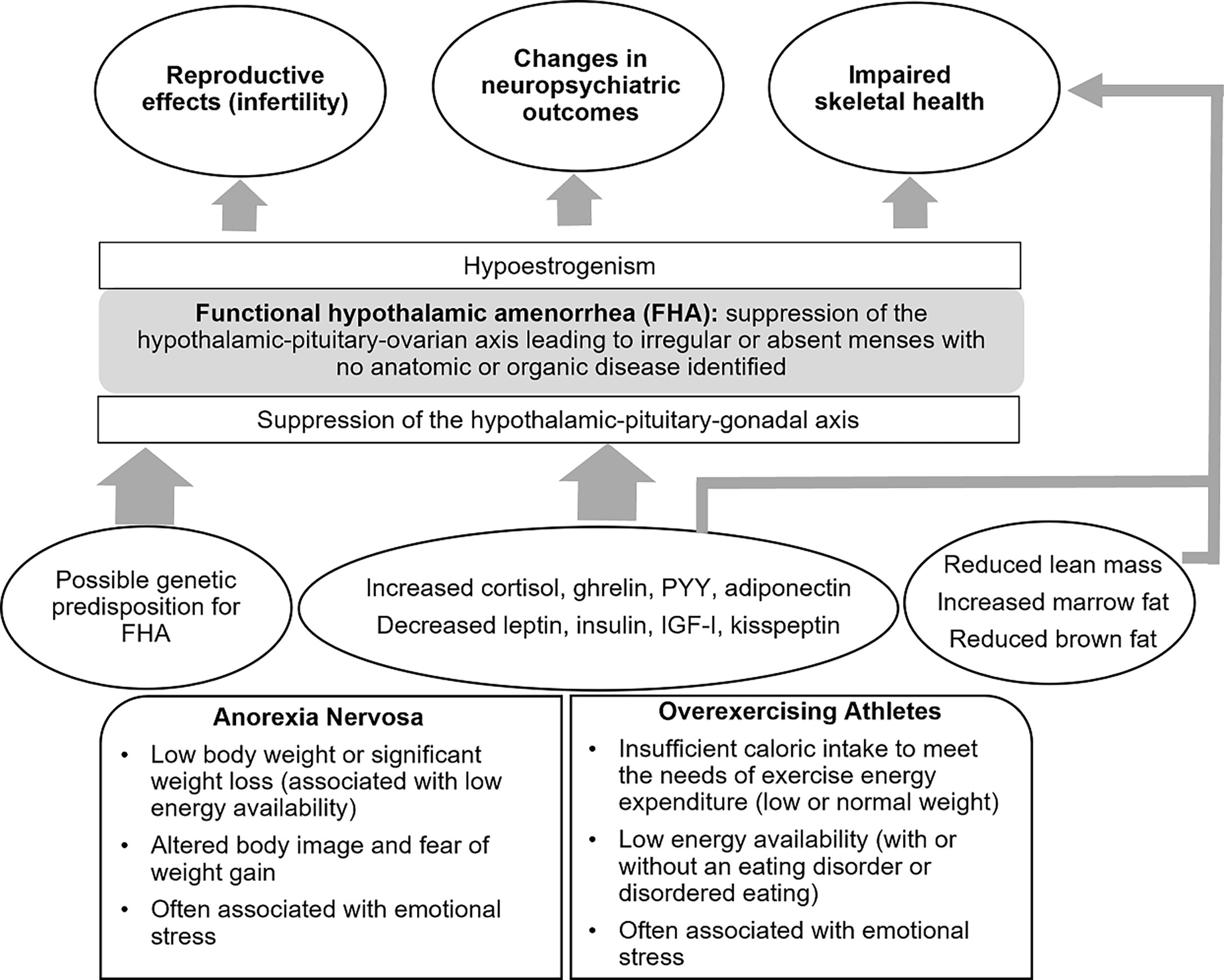
Functional hypothalamic amenorrhea is a state of reversible hypogonadism common in adolescents and young women that can be triggered by energy deficit or emotional stress or a combination of these factors. Energy deficit may be a consequence of (i) reduced caloric intake, as seen in patients with eating disorders, such as anorexia nervosa, or (ii) excessive exercise, when caloric intake is insufficient to meet the needs of energy expenditure. In these conditions of energy deficit, suppression of the hypothalamic secretion of gonadotrophin-releasing hormone (with resulting hypoestrogenism) as well as other changes in hypothalamic-pituitary function may occur as an adaptive response to limited energy availability. Many of these adaptive changes, however, are deleterious to reproductive, skeletal, and neuropsychiatric health. Particularly, normoestrogenemia is critical for normal bone accrual during adolescence, and hypoestrogenemia during this time may lead to deficits in peak bone mass acquisition with longstanding effects on skeletal health. The adolescent years are also a time of neurological changes that impact cognitive function, and anxiety and depression present more frequently during this time. Normal estrogen status is essential for optimal cognitive function (particularly verbal memory and executive function) and may impact emotion and mood. Early recognition of women at high risk of developing hypothalamic amenorrhea and its timely management with a multidisciplinary team are crucial to prevent the severe and long-term effects of this condition.
Introduction
Functional hypothalamic amenorrhea (FHA) is a condition characterized by irregular or absent menses due to suppression of the hypothalamic–pituitary–ovarian (HPO) axis, and the condition is termed ‘functional’ because no anatomical or organic disease is identified (1). Abnormalities in gonadotropin-releasing hormone (GnRH) secretion (2, 3) result in hypogonadotropic hypogonadism with impaired luteinizing hormone (LH) pulsatile secretion (4–8), and insufficient LH and follicle-stimulating hormone (FSH) concentrations (5, 9) to maintain full folliculogenesis and therefore ovulatory function, and the condition is also referred to as functional hypogonadotropic hypogonadism. FHA is a common cause of secondary amenorrhea in young premenopausal women and results in severe hypoestrogenism. According to the American Society of Reproductive Medicine, FHA is responsible for 20–35% of secondary amenorrhea (10). It typically occurs in the setting of low body weight, such as anorexia nervosa (AN), excessive exercise (exercise induced or athletic amenorrhea), stress, or a combination of these factors (5). AN is a chronic, relapsing disease defined in the Diagnostic and Statistical Manual -5 (DSM-5) as a state of low body weight in the setting of altered body image and fear of weight gain (11). The Female Athlete Triad (TRIAD) refers to the triad of low energy availability, menstrual dysfunction, and low bone mineral density (BMD) (12–14). Energy deficiency, from either a frank deficit in caloric intake or relative to excessive exercise, leads to hormonal adaptations that aim to optimize energy availability and prioritize this for body functions essential for survival.
Adolescents and young women with FHA typically present with amenorrhea of 6 months’ duration or longer (15). However, menstrual status can vary ranging from subclinical menstrual dysfunction (including a shortened luteal phase or anovulatory cycles) to frank oligo-amenorrhea. In adolescents, this condition may be difficult to distinguish from immaturity of the hypothalamic–pituitary–ovarian axis during the early postmenarchal years. However, several reports now indicate that even during the initial postmenarchal years menstrual cycles in adolescents typically are no longer than 45 days, thus irregular or absent menses are concerning (16–18).
The process by which GnRH is suppressed in FHA is multifactorial, as there are many inhibitory and stimulatory neuromodulatory signals that impair GnRH pulsatility. Kisspeptin plays a fundamental role in regulating reproductive function. In the human brain, kisspeptin neurons are found in the hypothalamus, basal ganglia, and periventricular region (19, 20). Kisspeptin has been implicated as the common intermediate signaling factor modulating GnRH activity, acting downstream of leptin and other neuromodulatory systems (21). Studies have demonstrated reduced kisspeptin secretion in conditions of energy deficit in rodents (22). This may be mediated by reductions in levels of hormones such as leptin, insulin, and insulin-like growth factor-1 (IGF-1), and increases in hormones such as ghrelin, cortisol, and adiponectin (23–26).
There may also be a genetic predisposition for the development of FHA. One small study identified heterozygous mutations in the fibroblast growth factor receptor 1 gene FGFR, the prokineticin receptor 2 gene PROKR2, the hypothalamic gonadotrophin-releasing hormone receptor gene GNRHR, and the Kallmann syndrome 1 sequence gene KAL1 (27) in patients with FHA, suggesting an increased vulnerability to develop hypothalamic amenorrhea. These mutations were not found in healthy controls. However, these findings have not been replicated and more data are necessary to determine whether these findings hold in a larger sample.
The prolonged hypoestrogenemia in FHA has profound effects on many body systems including metabolic, skeletal, neuropsychiatric, and reproductive systems. Estrogen plays an important role in bone health and therefore estrogen deficiency can impact bone mass deleteriously (4, 28). This is particularly important during adolescence, a critical period for bone mass accrual, and lack of estrogen during this time can lead to decreased BMD and increased fracture risk, both immediate and in the long-term. Another area of concern is the impact of prolonged hypogonadism and changes in hormones such as cortisol, leptin, and peptide YY (PYY) on neurocognitive status, emotion, and mood (29–32), thus posing additional challenges at an age when emotional lability is already common. The adolescent years are very important for optimal development of neuropsychiatric function and normoestrogenemia may be essential in this context.
Many young women are not aware of these long-term effects of FHA; thus, it is important to identify these women early, and address with them the importance of adequate energy availability and resumption of menses. Treatment includes correction of the energy deficit state to improve GnRH pulsatility and restore normal functioning of the HPO axis.
Impact of conditions associated with functional hypothalamic hypogonadism on bone
In situations of prolonged hypoestrogenism, as seen in states of functional hypothalamic (or hypogonadotropic) hypogonadism, changes are noted in areal BMD (aBMD), bone microarchitecture and strength estimates, associated with an increased risk of fractures in these individuals.
Anorexia nervosa and bone
Bone health has been extensively reviewed among adolescents with AN. Compared to controls, adolescent and young adult women with AN have low aBMD, which is driven independently by both low body weight (percent expected body weight for height, EBW-Ht, ≤ 80 or 90%) and amenorrhea (33), and alterations in hormones such as IGF-1, the gonadal steroids, cortisol, leptin, insulin, adiponectin, PYY and oxytocin. Older studies indicate that as many as 67% of these individuals may have an aBMD Z-score < -2 (34) while more recent findings (given earlier diagnosis of AN) report that ~ 52% have an aBMD Z-score < -1 at one or more sites, with trabecular bone (spine) being commonly affected (35). Of particular concern, in AN, bone accrual is stalled during adolescence, a vital period that determines long term bone health and fracture risk (36, 37). BMD improves with weight gain and menstrual recovery. However, it is uncertain whether full catch-up occurs and whether this is sufficient to ensure optimal long-term bone health (37).
Furthermore, studies using high-resolution peripheral quantitative computed tomography (HRpQCT) and microfinite element analysis (µFEA) (Figure 1) have demonstrated changes in cortical and trabecular volumetric BMD (vBMD), bone geometry and microarchitecture, and bone strength estimates in AN. At the distal radius (a non-weight bearing site), compared to controls, adolescents and young women with AN have lower total and trabecular vBMD, increased cortical porosity, lower cortical area and thickness, increased trabecular area and separation, and lower strength estimates (stiffness and failure load) (38). At the distal tibia (a weight-bearing site) adolescents and young adult women with AN, compared with controls, have lower total and cortical vBMD, increased cortical porosity, lower cortical area and thickness, lower trabecular number and lower stiffness and failure load (39). Another study showed, lower trabecular bone volume fraction (BV/TV) and trabecular thickness and higher trabecular separation in girls with AN using flat panel volume computed tomography (CT) when compared with normal-weight controls (40). IGF-1, leptin and androgen levels predict bone microarchitecture in adult women with AN, with lower levels appearing to have deleterious effects (41). Marrow adipose tissue (MAT) measured by 1H-magnetic resonance spectroscopy (1H-MRS) is higher in AN than controls, indicating increased differentiation of the common mesenchymal progenitor stem cell in marrow along the adipocyte rather than the osteoblast lineage, and higher marrow adipose tissue is related to lower bone strength estimates in young women with this condition (39).
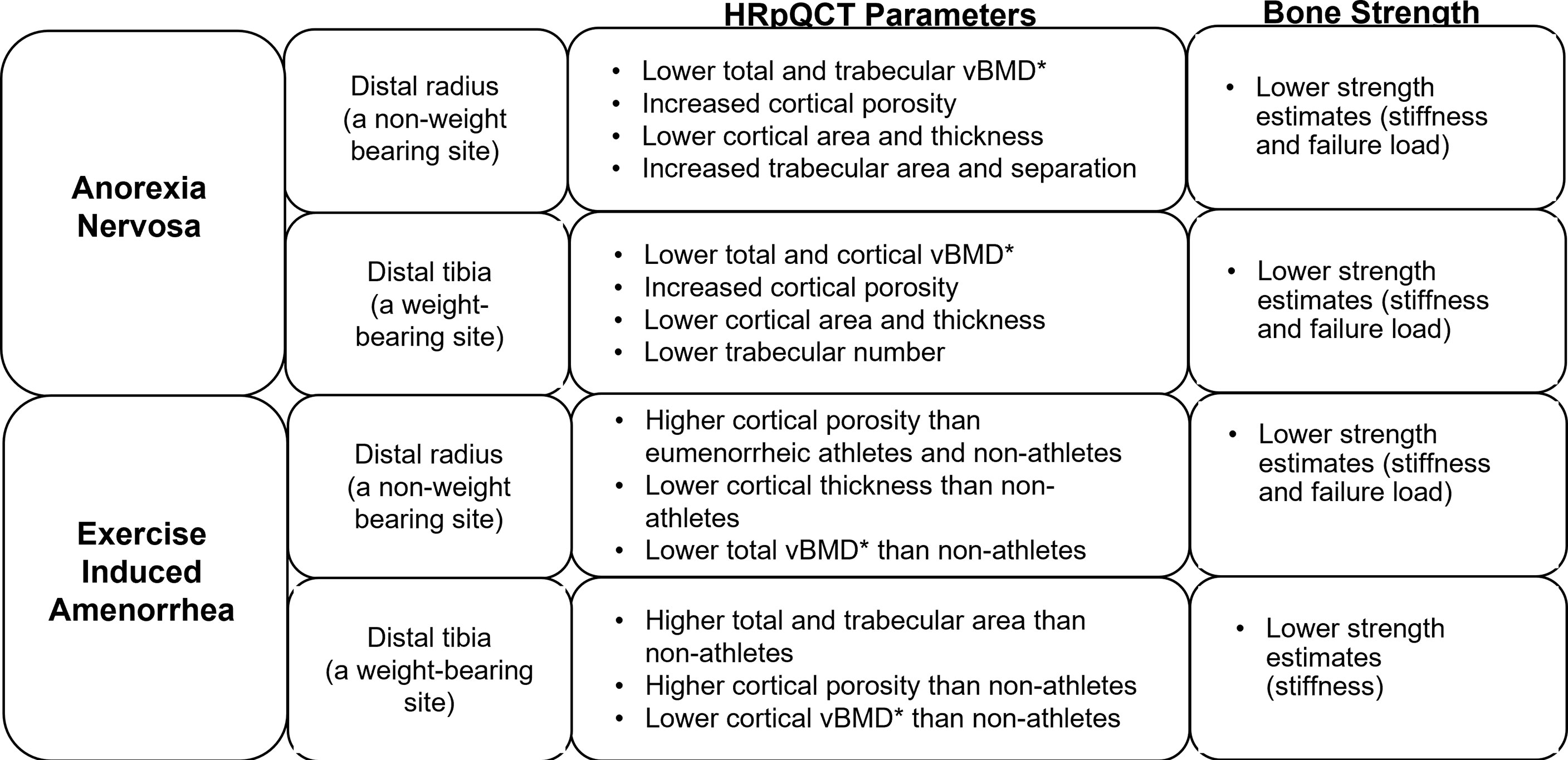
Figure 1 Impact of functional hypothalamic amenorrhea on bone parameters as assessed by high-resolution peripheral quantitative computed tomography (HRpQCT) and microfinite element analysis (μFEA). HRpQCT was used to assess volumetric bone mineral density (vBMD)*, bone geometry and structure, and μFEA to assess bone strength estimates at the distal radius and tibia.
These bone changes translate to higher fracture risk in adolescents and adult women with AN compared with controls that persists over time (42–44). One study reported no difference across groups in the site of fracture (upper extremity, lower extremity or non-extremity); the AN group just had many more fractures across all sites than the control group (44).
Exercise induced amenorrhea and bone
Similarly, in oligomenorrheic athletes, bone density, bone microarchitecture and strength are altered, a consequence of both low energy availability (from insufficient caloric intake and increased metabolic demands) and hypoestrogenism, as well as alterations in hormones such as IGF-1, other gonadal steroids, cortisol, and other metabolically regulated hormones. Oligoamenorrheic athletes have lower spine, hip and whole body aBMD than eumenorrheic athletes (45, 46). As in AN, there is concern that catch-up may be insufficient even after menses resume given the narrow window for optimizing bone accrual during and after puberty (47, 48).
However, using only BMD to assess bone health may be insufficient (14). In oligomenorrheic athletes, HRpQCT has been used to study changes in bone geometry and microarchitecture, and findings differ at non-weight bearing compared with weight bearing sites (Figure 1). At non-weight bearing sites such the distal radius, oligomenorrheic athletes have higher cortical porosity and lower strength estimates than eumenorrheic athletes, and higher cortical porosity with lower cortical thickness and total vBMD than non-athletes (46). At weight bearing sites such as the tibia, oligomenorrheic athletes have higher total and trabecular area, higher cortical porosity and lower cortical vBMD than non-athletes, and lower strength estimates than both eumenorrheic athletes and non-athletes (46, 49). Further, in one study, trabecular number was lower and separation higher in oligomenorrheic athletes compared to eumenorrheic athletes and non-athletes at the tibia (50). More recently, a study assessed the effects of energy deficiency (defined as energy intake below 45 kcal/kg fat-free mass/day) in long-distance triathletes without hypoestrogenism compared to non-athletes and found that several bone parameters (total and trabecular area, trabecular vBMD and trabecular microstructure) were better in athletes than non-athletes (consistent with the adaptive effects of bone loading), but inferior in athletes with low energy availability compared to those with adequate energy availability (51).
Fractures, particularly stress fractures, are more prevalent among oligo-amenorrheic athletes than eumenorrheic athletes and non-athletes (46). A history of two or more fractures in oligo-amenorrheic athletes has been associated with lower spine and whole-body BMD Z-scores, lower radial cross-sectional area, trabecular vBMD and strength estimates, and lower tibial strength estimates compared to oligoamenorrheic athletes with less than 2 fractures (46). Thus, the protective mechanism bestowed by mechanical loading on athletes is deficient in a state of hypoestrogenism.
Anorexia nervosa versus female athlete triad
Bone health and fracture risk are affected differently in adolescents and young adult women with AN and oligo-amenorrheic athletes (52). In AN, whole body less head (WBLH) and hip aBMD Z-scores, and several measures at the weight-bearing tibia (total vBMD, cortical area and thickness, trabecular number, and estimated strength) were lower than in oligo-amenorrheic athletes and controls in one study (52). In contrast, both AN and oligo-amenorrheic athletes had lower spine aBMD Z-scores, lower radius total, cortical and trabecular vBMD, cortical area, cortical thickness, and estimated strength, and lower tibial cortical vBMD than controls (52). Although fracture risk was higher in women with AN and oligo-amenorrheic athletes, the fracture type varied with nonstress fractures being more common in AN and stress fractures being more common in oligo-amenorrheic athletes.
Determinants of bone health in functional hypothalamic hypogonadism
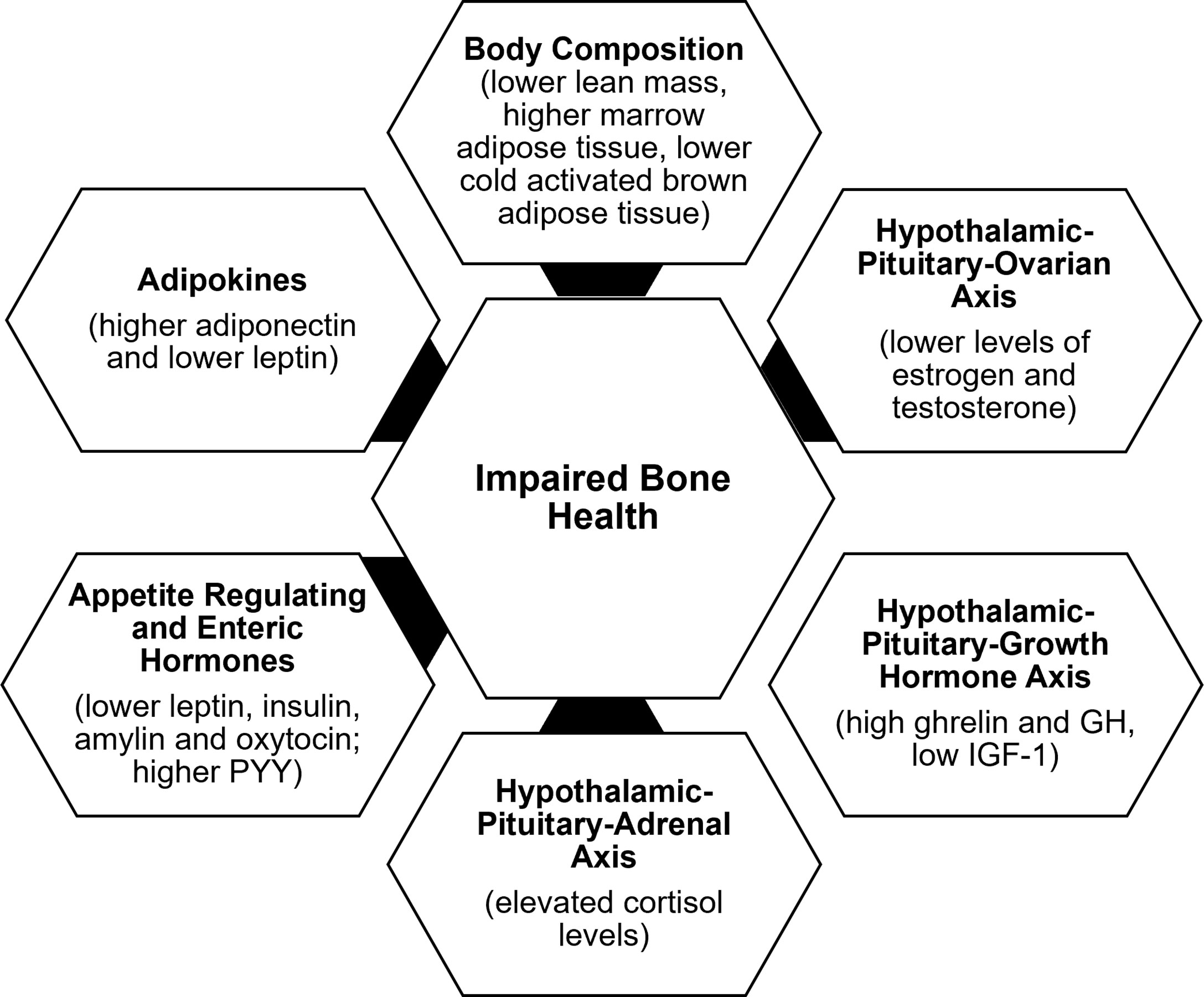
Determinants of bone outcome in these patients include changes in body composition, alterations in the HPO axis, growth homone-IGF-1 axis, hypothalamic-pituitary-adrenal axis, and appetite regulating and other hormones, consequent to the low energy availability state (Figure 2).
Body composition
In addition to the lower body mass index (BMI), changes in body composition in the setting of energy deficiency impact bone health. Overall, restrictive caloric intake in AN leads to lower fat and lean mass and lower resting energy expenditure (53). A compensatory increase in cortisol concentrations occurs in energy deficient states such as AN and exercise induced amenorrhea, and in AN, higher cortisol concentrations are associated with lower extremity lean mass (54). Lower lean mass is an independent predictor of BMD at almost every site, consistent with the pull of muscle on bone being osteogenic (37). With weight regain, increases in lean mass are associated with increases in BMD (36). Further, marrow adipose tissue (known to reduce biomechanical strength) is higher in AN than controls and in oligo-amenorrheic athletes than in eumenorrheic athletes and is associated with lower BMD and lower strength estimates (55, 56). In contrast, cold-activated brown adipose tissue is lower in AN. Some believe that brown adipose tissue plays a role in the differentiation of a common marrow progenitor mesenchymal stem cell preferentially into osteoblasts instead of adipocytes (57). This would explain a direct association of lower brown adipose tissue with lower BMD and an inverse relationship with levels of pre-adipocyte factor-1 (pref-1), a hormone that inhibits differentiation of this mesenchymal stem cell along the osteoblast pathway (57).
Hypothalamic-pituitary-ovarian axis
The gonadal steroids, including estrogen, testosterone and dehydroepiandrosterone (DHEA) (an ovarian and adrenal androgen and estrogen precursor), have important effects on bone (28).The estrogens (estradiol and estrone) inhibit osteoclastic bone resorption by increasing osteoprotegerin and decreasing receptor activator of nuclear factor kappa-B ligand (RANKL) secretion by osteoblasts (28). They may also increase bone formation by inhibiting secretion of sclerostin and pref-1, both of which otherwise inhibit osteoblast differentiation. Effects of testosterone on bone are mediated via its aromatization to estrogen; however, it also has direct osteoanabolic and anti-resorptive effects. DHEA is weakly bone anabolic, and also anti-resorptive through its aromatization to estrogen. Levels of estrogen and testosterone are lower in AN and in oligo-amenorrheic athletes compared with controls (36, 50). The duration of amenorrhea, consistent with the duration of hypogonadism, and menarchal age predict the extent of bone health impairment.
Hypothalamic-pituitary-growth hormone axis
Energy deficiency results in a state of growth hormone (GH) resistance with elevated GH concentrations and low IGF-1 levels in AN vs. controls (58), and in amenorrheic athletes vs. non-athletes (45), consistent with a hepatic resistance to GH, likely mediated by a downregulation of the GH receptor in end organs (as indicated by lower levels of GH binding protein) (59) and elevated fibroblast growth factor (FGF)-21 concentrations (60). This state of GH resistance is commonly seen in conditions of undernutrition. In athletes, lower IGF-1 levels have been associated with higher intensity of training (61, 62). GH stimulates osteoblast precursors and mature osteoblasts both directly and indirectly through the action of IGF-1 (63). While higher GH concentrations are associated with higher levels of bone turnover markers in healthy normal-weight controls, this association is not evident in AN (despite higher GH concentrations), indicative of a resistance to GH at the level of bone (in addition to the liver) (58). A study using supraphysiologic doses of recombinant human GH demonstrated a decrease in fat mass in women with AN (consistent with its IGF-1 independent lipolytic effects) without a corresponding increase in IGF-1 concentrations or concentrations of P1NP (a bone formation marker), further corroborating a hepatic and skeletal resistance to GH in AN (64). Further, low IGF-1 concentrations are associated with low bone density in conditions of functional hypothalamic hypogonadism (36, 45), and administration of replacement doses of recombinant human IGF-1 (rhIGF-1) has been associated with an increase in levels of bone formation markers in adolescents and adults with AN (65, 66).
Hypothalamic- pituitary-adrenal axis
One of the neuroendocrine adaptations of FHA includes overactivity of the hypothalamic–pituitary–adrenal axis (HPA), with increased secretion of corticotropin-releasing hormone (CRH), adrenocorticotropin hormone (ACTH), cortisol, and endogenous opioids (67–71). Higher cortisol levels have also been found in the cerebrospinal fluid of women with FHA compared to eumenorrheic women (7, 72). There is a tight link between activation of the HPA axis and reduction in GnRH drive in those with FHA (5, 70, 73, 74), such that an increase in CRH suppresses GnRH pulsatility (7). Further, cortisol inhibits kisspeptin release (75, 76), and its elevation contributes to the cascade of impairment in GnRH release, with higher cortisol concentrations being associated with lower secretion of LH (70). One study has shown that in AN, adolescents with the lowest BMI, fat mass, fasting glucose and insulin levels (thus with the lowest energy availability) have the highest cortisol concentrations, suggesting that the increase in cortisol levels is an adaptative mechanism, likely to maintain euglycemia in a state of low availability given its gluconeogenic effects (71).
These relatively high cortisol levels may have immediate and long-term effects on bone health in patients with FHA. The deleterious effects of hypercortisolemia on bone are mediated by many different mechanisms including reduced osteoblastic activity, increased osteoclastic activity, inhibition of intestinal calcium absorption, impaired renal handling of calcium, and reduced secretion of GH and IGF-1 (77). Higher cortisol levels predict lower percent extremity lean mass (54) and lower bone density in FHA (30, 71). As previously discussed, lean body mass is an important determinant of bone density (35, 78). High cortisol levels in women with AN are inversely correlated with markers of bone turnover and may contribute to low BMD through suppression of bone formation (71, 79).
Insulin, enteric peptides and adipokines
Appetite regulating hormones (such as leptin, insulin, PYY, and oxytocin, which are anorexigenic, and ghrelin, which is orexigenic) as well as adipokines, such as adiponectin, are modulators of energy availability and play a critical role in the regulation of hypothalamic dysfunction and in bone metabolism in FHA (80, 81). Leptin and insulin stimulate kisspeptin release, while ghrelin and adiponectin inhibit its release (25, 75, 76, 82, 83). Similarly, PYY can modulate reproductive function (84, 85). Women with FHA have lower leptin (6, 8, 9, 86–90), insulin (9, 86, 87, 91), and oxytocin levels (92), and higher ghrelin (8, 90, 93–95), PYY (94, 96, 97), and adiponectin (91, 98) levels than controls. In FHA, many of these hormonal alterations have been associated with suppression of the HPO axis (25, 80, 81, 99, 100).
All these hormonal alterations contribute to hypogonadism in FHA and consequently to low bone mass. In addition, these hormones have direct effects on bone. Leptin is osteoanabolic and antiresorptive (101–103), and insulin, amylin and ghrelin also have bone anabolic effects. Lower levels of leptin, insulin and amylin correlate with lower BMD and impaired bone microstructure in those with AN (41, 91, 104). Ghrelin levels correlate with bone endpoints in healthy normal-weight controls, but not in girls with AN, consistent with a ghrelin resistant state (105). PYY inhibits osteoblastic activity (106), and in AN, high PYY levels are associated with lower BMD in adults, and with lower levels of bone turnover markers in adolescents (96, 107). Similarly, higher PYY levels are associated with lower levels of bone formation markers and lower BMD in adolescent athletes and non-athletes (97). Oxytocin is now known to be bone anabolic, and lower oxytocin concentrations in AN have been associated with lower bone density (108). Adiponectin receptors are expressed on osteoblasts and osteoclasts (109, 110), and high levels of adiponectin are deleterious to bone. High adiponectin levels are associated with low BMD in healthy adults (111, 112) and in girls with AN (91).
Treatment strategies
The first line of management of FHA is lifestyle intervention, aimed at normalization of HPO axis function and resumption of menses. The approach should be multidisciplinary and include involvement of a physician to coordinate care (preferably an eating disorder specialist), a dietician, and a psychologist or psychiatrist (particularly when there is a co-existing eating disorder), with engagement of the parents or other family members and the athletic trainer or coach (for hyperexercisers). The condition is generally reversible and resolves after restoration of energy balance and resolution of underlying emotional stress. While targeting the triggers of FHA, such as disordered eating, excessive exercise or emotional stress is the first approach, convincing patients to change long-standing behavior can be challenging.
A dietary evaluation and consequent counseling are important to optimize caloric intake (including healthy fat), and micronutrients such as calcium and vitamin D. Energy availability should meet established weight goals and other clinical criteria for athletes to continue exercising, and these athletes may need to modify their training and competition regimen if such goals are not met (46, 113). Consistent with this, sports consensus groups, including the Female Athlete Triad Coalition and International Olympic Committee, recommend that athletes with FHA undergo screening for various components of the Triad and meet certain energy availability requirements to be permitted to continue to exercise (12, 13). In a small study, three out of four amenorrheic athletes resumed menses after a 20-week program that included nutritional supplementation and one rest day per week (114).
Similarly, psychological support for treating stress and enhancing behavioral change is critical (14). Behavioral modifications can result in a reduction in cortisol levels (115) and resumption of ovarian function in some women with FHA (116). One study showed that 71% of patients recovered over a period of 7-9 years and predictive factors of recovery included lower serum cortisol concentrations and higher basal BMI (117).
There has been some debate over whether a critical increase in weight is required to resume menstrual cycles. Based on one study, a recommendation is that goal weight should be at least 2 kg higher than the weight at which point menses were lost (118). This longitudinal study involving adolescents with AN also showed that menstruation resumed at a mean body weight that was 91.6 ± 9.1% of ideal body weight and that it could take 6-12 months or longer of being at a ‘healthy’ weight before menses resumed (118). Another study suggested that about 50% of women are expected to resume their menstrual cycle when they are at or above a BMI of 19 kg/m2 with ≥ 23% body fat (119). Overall, the recommendations are to regain any recent weight loss, to aim for a body weight that is at least 2 kg greater than at which menses were lost and a body weight that corresponds to greater than 90% of median BMI for age (or greater than 18.5 kg/m2 for adults). However, there is significant variability in the set point for menstrual recovery from one individual to another, thus establishing goal weight can be challenging. Further, if a woman continues to be amenorrheic despite being at a healthy weight for a prolonged period, it is important to consider other causes of amenorrhea such as persistent emotional stress or conditions such as polycystic ovarian syndrome.
In women, particularly athletes, who are at a ‘healthy’ weight and yet have FHA, low caloric intake may still be a contributor to the amenorrheic state, these women often have lower fat mass than eumenorrheic women, consistent with a state of energy deficit. In such women, careful assessment of caloric intake and expenditure may be necessary to demonstrate the state of low energy availability. Individualized meal plans are often helpful in such instances, and it is important to emphasize optimizing caloric intake before and after periods of intense exercise. Recommendations from the Female Athlete Triad Coalition include increasing calories through intake of food such as nuts, dried fruit, energy bars and drinks, avocado and fatty fish (12).
Management of low bone density
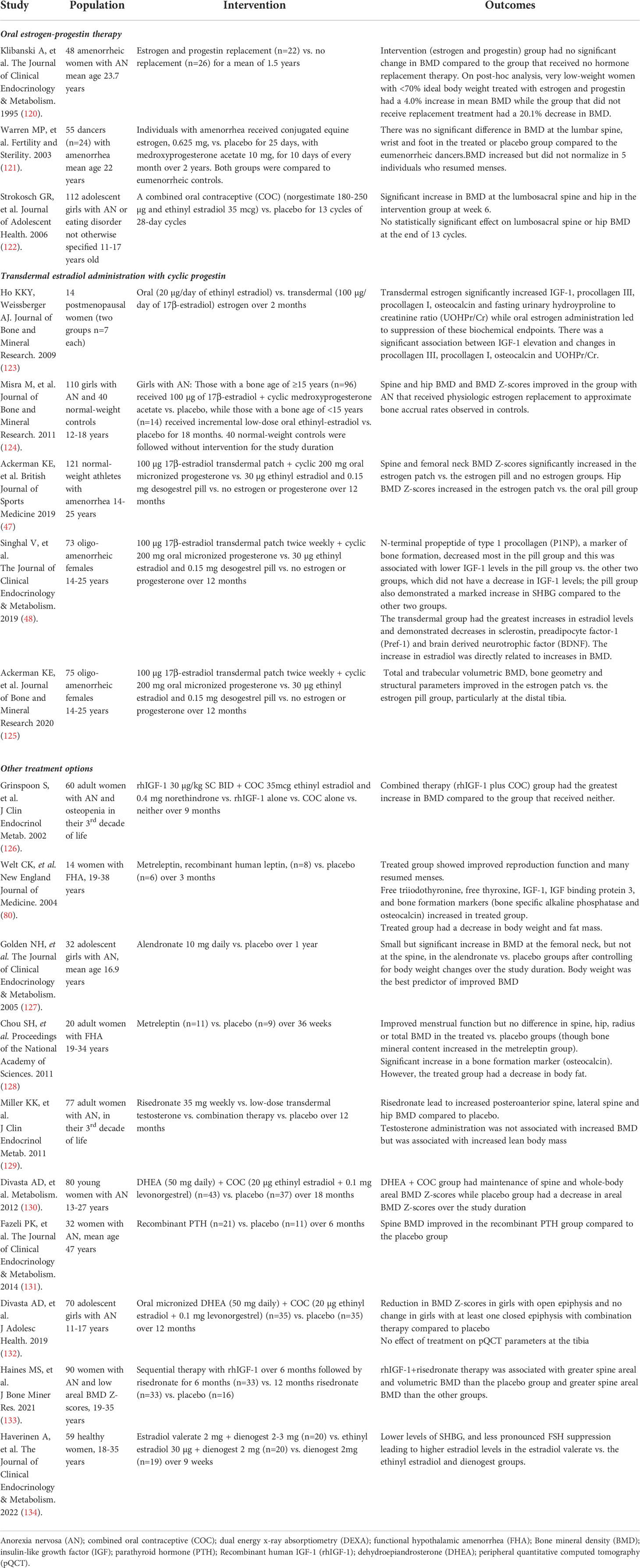
The management of low bone density in FHA is summarized in Figure 3. Table 1 summarizes interventional studies addressing bone outcomes in FHA.
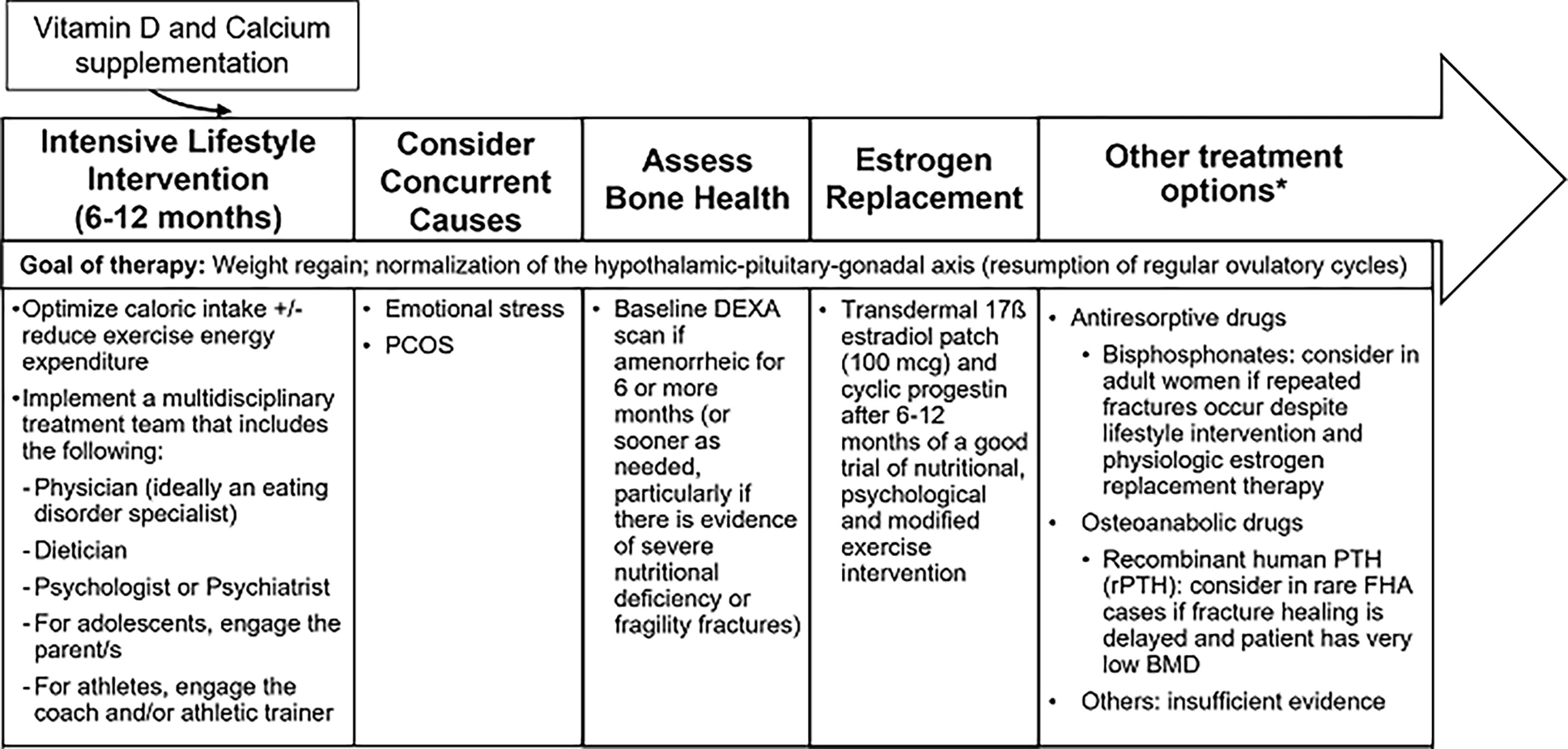
Figure 3 Management of Functional Hypothalamic Amenorrhea. HPO, Hypothalamic-pituitary-ovarian axis; PCOS, Polycystic ovarian syndrome; DEXA, Dual-energy X-ray absorptiometry; rPTH, Recombinant parathyroid hormone. *Treatment is similar in both adult and adolescent women except for "other treatment options" which at this time, only apply to adults.
The most important strategy to improve bone density in adolescent and adult women with FHA is normalization of menstrual function and recovery of weight (for those who are undernourished, underweight or have had recent weight loss). It is important to supplement vitamin D to maintain 25(OH) vitamin D levels above 30 ng/ml (135) and to recommend adequate calcium intake (1000-1500 mg daily). Clinicians should obtain a baseline BMD measurement by dual-energy X-ray absorptiometry (DXA) for any adolescent or woman with 6 or more months of amenorrhea, and even earlier in patients with a history of severe nutritional deficiency, other energy deficit state, and/or skeletal fragility (136).
Although estrogen deficiency is an important determinant of low BMD in FHA, many studies have shown lack of a protective effect of combined oral contraceptives (COCs) on bone (120–122, 137). A possible reason for the lack of efficacy of oral estrogen in increasing BMD is the suppression of IGF-1, a key osteoanabolic hormone (particularly during the adolescent years), by COCs because of hepatic first pass metabolism (123, 138). In contrast, transdermal 17-β estradiol, administered in replacement doses, is not IGF-1 suppressive (138, 139), and randomized clinical trials (47, 124) over 12 or 18 months have demonstrated that transdermal 17-β estradiol replacement is effective in increasing spine and hip BMD Z-scores in oligo-amenorrheic athletes and adolescents with AN, although catch-up is incomplete. This lack of complete catch-up is likely due to residual alterations that persist in other hormones that may impact bone that are not fixed by estrogen replacement. Importantly, the impact of estrogen replacement on fracture risk in women with FHA remains unclear. The Endocrine Society guidelines suggest short-term use of transdermal 17-β estradiol with cyclic oral progestin in adolescents and women with FHA who do not resume menses after a reasonable trial of nutritional, psychological, and/or modified exercise intervention (136).
The route of estrogen administration may have an impact on bone that extends beyond effects on IGF-1. A study that examined the impact of route of estrogen administration in oligo-amenorrheic athletes showed that transdermal estradiol replacement was associated with an increase in estradiol levels (associated with increases in bone density), and a decrease in factors that inhibit osteoblastic activity such as sclerostin, Pref-1, and brain-derived neurotrophic factor (BDNF). Further, while COCs led to a significant increase in sex hormone binding globulin levels with a decrease in levels of bioavailable gonadal steroids, this effect was not observed in the transdermal estrogen group (139). All these mechanisms may contribute to the efficacy of transdermal estrogen (but not COCs) in improving bone outcomes. A study examining effects of estradiol valerate versus ethinyl estradiol in oral contraceptive pills with the same progestin found a less pronounced FSH suppression in the estradiol valerate group, leading to higher estradiol levels and suggesting more positive effects of natural estradiol on bone mass (134).
Adolescent girls and adult women with AN and amenorrheic athletes have lower levels of testosterone than control groups. However, one study of transdermal testosterone given in replacement doses vs. placebo in adult women with AN was not associated with increases in BMD, despite an initial increase in bone formation markers (129).
Few studies have evaluated the use of anti-resorptive medications such as bisphosphonates and denosumab in FHA. One randomized controlled study of risedronate vs. placebo in adult women with AN reported small but significant increases in BMD (2-3%) at the spine and hip (129), while another study of alendronate vs. placebo in adolescents with AN reported a small increase at the femoral neck (but not at the spine) (127). When considering these drugs as a therapeutic strategy (particularly in women who have repeated fractures despite lifestyle intervention, optimization of calcium and vitamin D status, and estrogen replacement), caution needs to be exercised during the reproductive years given concerns regarding their long half-life. Data for denosumab are not available at this time in women with FHA.
Similar to the anti-resorptives, few studies have examined the impact of osteoanabolic drugs [such as teriparatide, recombinant PTH (rPTH), abaloparatide, romosozumab, recombinant leptin (metreleptin), rhIGF-1, and DHEA] on bone outcomes in FHA. A 6-month study of teriparatide vs. placebo in older pre-menopausal women with AN reported improvements in spine BMD (131); however, studies over a longer duration are currently lacking. There are also no studies that have reported on the impact of abaloparatide or romosozumab on bone outcomes in FHA.
Although a small 3-month study of metreleptin vs. placebo in adult women with FHA demonstrated improvement in menstrual function and increases in levels of IGF-1 and markers of bone formation with metreleptin, the medication led to subjective reductions in appetite and a significant decrease in body weight and fat mass (80). A subsequent small 9-month study similarly showed improved menstrual function and an increase in bone mineral content at the lumbar spine following metreleptin treatment. However, the group that received this drug had a significant decrease in body fat despite careful dose titration to prevent weight loss, an undesirable side effect in individuals with FHA (128).
Recombinant human IGF-1 given with a COC was demonstrated to increase spine and hip BMD in adult women with AN in a 9-month RCT in which the women were randomized to receive the combination regimen, rhIGF-1 alone, COC alone or neither (126), suggesting that administering rhIGF-1 in replacement doses may mitigate the IGF-1 suppressive effects of a COC. However, in a 12-month randomized controlled trial in adolescents with AN in which all received transdermal 17-β estradiol (given in replacement doses with cyclic oral progestin) with half being randomized to receive replacement doses of rhIGF-1 and half randomized to placebo, adding rhIGF-1 to transdermal 17-β estradiol did not lead to a further improvement in bone outcomes (140). In contrast, in a study in adults with AN, sequential therapy with rhIGF-1 for 6 months followed by risedronate for 6 months (vs. 12 months of risedronate or double placebo) led to greater increases in spine aBMD and vBMD than in the double placebo group, and greater increases in lateral spine aBMD than in both other groups (133).
Finally, one 18-month study demonstrated that a combination regimen of DHEA (50 mg daily) with a COC (vs. double placebo) led to a maintenance of BMD Z-scores at multiple sites compared to a decrease in these measures in the placebo group in young women with AN 13-27 years old (130). However, a subsequent study of this combination regimen vs. placebo in younger girls with AN 11-17 years old reported a reduction in spine and whole-body BMD Z-scores with the combination regimen in the younger girls with open epiphyses, and no change in these BMD measures in older girls with open epiphyses (132).
Based on these and other studies, current guidelines caution against using denosumab, metreleptin and androgens to improve bone outcomes in FHA. In rare adult FHA cases, the guidelines suggest short-term use of teriparatide as an option in patients with delayed fracture healing and very low BMD (136). Given that recent studies have demonstrated that bisphosphonates improve bone outcomes in adults with AN (133) this may be a consideration in those women who continue to have fractures despite attention to caloric intake, a reduction in exercise activity, and optimization of vitamin D, calcium, and estrogen status.
Neuropsychiatric outcomes
Adequate estrogen status is essential for optimal cognitive function and may also impact mood and emotion (141, 142).
Cognitive Function: Hypoestrogenism has deleterious effects on verbal memory and executive function (specifically cognitive flexibility) in oligo-amenorrheic athletes compared to eumenorrheic ones and/or non-athletes (143). Other studies have also demonstrated cognitive dysfunction in adolescent and adult women with FHA (144, 145), with an improvement in these measures with menstrual resumption or estrogen administration (144). Importantly, estrogen replacement as the transdermal 17-β estradiol patch with cyclic oral progesterone given for a 6-month period improved both verbal memory and cognitive flexibility in oligo-amenorrheic athletes compared to a no-estrogen group (with a COC group demonstrating intermediate effects) (143).
Emotion and Mood: Women with FHA have significantly higher depression and anxiety scores compared to healthy controls (146). Studies reported that depression and anxiety are common in women with FHA, suggesting a role for estrogen in mediating these effects (146–149). Another study reported that administration of transdermal estradiol reduced trait anxiety in girls with AN and prevented the increase in state anxiety observed with weight gain over time than in those who received placebo (150).
Eating Behaviors and Attitudes: Women with FHA exhibit more dysfunctional attitudes such as perfectionistic behavior and extra attention to peoples’ judgments and have great difficulty coping with daily stress in comparison with eumenorrheic women (151, 152). Additionally, women with FHA report greater internal feelings of insecurity, inadequacy, and lack of control over their lives (146). One study in athletes and non-athletes reported greater cognitive restraint, drive for thinness, feelings of ineffectiveness and greater interoceptive awareness in oligo-amenorrheic athletes compared to eumenorrheic athletes and non-athletes (153). A subsequent study showed a significant improvement in drive for thinness and body dissatisfaction scores, and a reduction in uncontrolled eating after 12 months of treatment with transdermal estradiol with cyclic progesterone (154) (not observed in those who received COCs). Further, transdermal estradiol replacement in adolescent girls with AN has been demonstrated to prevent the increase in body dissatisfaction that occurs with weight gain over time in those who remain hypoestrogenic (150).
Hormonal Correlates of Neuropsychiatric Outcomes: Estrogen has an influence on many areas of the brain including to the hypothalamus, cerebellum, nigrostriatal and mesolimbic system, amygdala, hippocampus, cerebral cortex, and brainstem (155). Estrogen also modulates many neurotransmitters including serotonin, acetylcholine, dopamine, and norepinephrine (156). Although hypoestrogenemia plays a major role in the neurocognitive impairment in FHA, hypercortisolemia due to HPA dysregulation and fluctuations in neuropeptides and neurotransmitters can work synergistically to promote the neuropsychiatric disturbances in this condition. One study showed that amenorrheic women present greater increases in heart rate, systolic and diastolic blood pressure, and serum cortisol levels in response to neuropsychological stress exposure than eumenorrheic women (151). In another study of 21 healthy controls, 18 amenorrheic women with AN, and 13 normal-weight women with FHA, cortisol levels showed a strong correlation with anxiety and depressive symptoms (30). Lower levels of gonadal hormones, oxytocin, and leptin, and higher levels of cortisol and PYY have been implicated in eating disorder psychopathology and symptoms of anxiety and depression in AN (31, 32, 108, 157).
The relationship between psychological stress and FHA is bidirectional, as stress can trigger the suppression of the HPO axis and, conversely, low levels of estrogen greatly impact the neuropsychological status, thus creating a vicious cycle. Therefore, psychological support is essential to break the cycle. The Endocrine Society Clinical Practice Guidelines (136) suggest psychological treatment such as cognitive behavioral therapy (CBT) to improve the ability to cope with psychological stressors. In a study, eight women with FHA were randomized to CBT and eight to observation for 20 weeks. Among women who received CBT, most (six of eight) achieved ovulatory recovery compared to only one of eight in the observation group (116). In another study, CBT lowered cortisol levels, and increased leptin and TSH levels in women with FHA (115). The long-term impact of CBT in amenorrheic women needs to be studied.
Conclusion
FHA from AN, low energy availability in athletes or chronic stress is a frequent cause of oligo-amenorrhea in young women and can go undiagnosed for long periods of time. FHA results from disruption of the HPO axis consequent to other endocrine changes and possibly a genetic predisposition, with an impact on reproductive, neuropsychiatric and skeletal health (summarized in Figure 4). Early recognition of patients at risk of developing FHA is very important due to the long-term consequences of low energy availability on the reproductive system, and the impact of low energy availability and hypoestrogenism on bone and neurocognitive outcomes, particularly during the critical adolescent and young adult years when skeletal and neurological systems are maturing. Treatment aims to optimize energy availability with restoration of gonadal function and generally requires a multidisciplinary team. Transdermal estrogen therapy is a proven useful tool in those women who do not respond to nutritional, psychological, and/or modified exercise intervention, and has beneficial effects on bone accrual, as well as neuropsychiatric outcomes.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gordon CM. Functional hypothalamic amenorrhea. N Engl J Med (2010) 363(4):365–71. doi: 10.1056/NEJMcp0912024
2. Yen SSC, Rebar R, Vandenberg G, Judd H. Hypothalamic amenorrhea and hypogonadotropinism: Responses to synthetic LRF. J Clin Endocrinol Metab (1973) 36(5):811–6. doi: 10.1210/jcem-36-5-811
3. Laughlin GA, Dominguez CE, Yen SSC. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab (1998) 83(1):25–32. doi: 10.1210/jc.83.1.25
4. Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: Spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab (1999) 84(6):1905–11. doi: 10.1210/jc.84.6.1905
5. Berga SL, Mortola JF, Girton L, Suh B, Laughlin G, Pham P, et al. Neuroendocrine aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab (1989) 68(2):301–8. doi: 10.1210/jcem-68-2-301
6. Couzinet B, Young J, Brailly S, le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (1999) 50(2):229–35. doi: 10.1046/j.1365-2265.1999.00649.x
7. Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J Clin Endocrinol Metab (1988) 66(4):733–9. doi: 10.1210/jcem-66-4-733
8. Ackerman KE, Slusarz K, Guereca G, Pierce L, Slattery M, Mendes N, et al. Higher ghrelin and lower leptin secretion are associated with lower LH secretion in young amenorrheic athletes compared with eumenorrheic athletes and controls. Am J Physiol Endocrinol Metab (2012) 302(7):E800–6. doi: 10.1152/ajpendo.00598.2011
9. Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, et al. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril (2007) 87(4):876–85. doi: 10.1016/j.fertnstert.2006.09.011
10. Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril (2004) 82(Suppl 1):33–9. doi: 10.1016/j.fertnstert.2004.07.001
11. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). Codas (2013) 25(2):190–1. doi: 10.1590/S2317-17822013000200017
12. de Souza MJ, Nattiv A, Joy E, Misra M, Williams NI, Mallinson RJ, et al. Female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, may 2012 and 2nd international conference held in Indianapolis, Indiana, may 2013. Br J Sports Med (2014) 48(4):289. doi: 10.1136/bjsports-2013-093218
13. Mountjoy M, Sundgot-Borgen JK, Burke LM, Ackerman KE, Blauwet C, Constantini N, et al. IOC consensus statement on relative energy deficiency in sport (RED-s): 2018 update. Br J Sports Med (2018) 52(11):687–97. doi: 10.1136/bjsports-2018-099193
14. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. The female athlete triad. Med Sci Sports Exerc (2007) 39(10):1867–82. doi: 10.1249/mss.0b013e318149f111
15. Liu JH, Bill AH. Stress-associated or functional hypothalamic amenorrhea in the adolescent. Ann N Y Acad Sci (2008) 1135(1):179–84. doi: 10.1196/annals.1429.027
16. Flug D, Largo RH, Prader A. Menstrual patterns in adolescent Swiss girls: a longitudinal study. Ann Hum Bio (1984) 11(6):495–508. doi: 10.1080/03014468400007411
17. Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab (2000) 85(3):1021–5. doi: 10.1210/jc.85.3.1021
18. Campbell H, Edstrom K, Engstrom L, Kodagoda N, Lunenfeld B, Romer M, et al. World health organization multicenter study on menstrual and ovulatory patterns in adolescent girls. II. longitudinal study of menstrual patterns in the early postmenarcheal period, duration of bleeding episodes and menstrual cycles. world health organization task force on adolescent reproductive health. J Adolesc Health Care (1986) 7(4):236–44.
19. Hrabovszky E. Neuroanatomy of the human hypothalamic kisspeptin system. Neuroendocrinology (2014) 99(1):33–48. doi: 10.1159/000356903
20. Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, et al. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin b neurons. Eur J Neurosci (2010) 31(11):1984–98. doi: 10.1111/j.1460-9568.2010.07239.x
21. McCarthy MM. A piece in the puzzle of puberty. Nat Neurosci (2013) 16(3):251–3. doi: 10.1038/nn.3339
22. Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology (2005) 146(9):3917–25. doi: 10.1210/en.2005-0337
23. Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology (2021) 162(2):1–18. doi: 10.1210/endocr/bqaa204
24. Dees WL, Hiney JK, Srivastava VK. IGF-1 influences gonadotropin-releasing hormone regulation of puberty. Neuroendocrinology (2021) 111(12):1151–63. doi: 10.1159/000514217
25. Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett (2009) 460(2):143–7. doi: 10.1016/j.neulet.2009.05.060
26. Morrison AE, Fleming S, Levy MJ. A review of the pathophysiology of functional hypothalamic amenorrhoea in women subject to psychological stress, disordered eating, excessive exercise or a combination of these factors. Clin Endocrinol (2021) 95(2):229–38. doi: 10.1111/cen.14399
27. Caronia LM, Martin C, Welt CK, Sykiotis GP, Quinton R, Thambundit A, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med (2011) 364(3):215–25. doi: 10.1056/NEJMoa0911064
28. Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev (2002) 23(3):279–302. doi: 10.1210/edrv.23.3.0465
29. Miller KK, Wexler TL, Zha AM, Lawson EA, Meenaghan EM, Misra M, et al. Androgen deficiency: Association with increased anxiety and depression symptom severity in anorexia nervosa. J Clin Psychiatry (2007) 68(06):959–65. doi: 10.4088/JCP.v68n0621
30. Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab (2009) 94(12):4710–6. doi: 10.1210/jc.2009-1046
31. Lawson EA, Eddy KT, Donoho D, Misra M, Miller KK, Meenaghan E, et al. Appetite-regulating hormones cortisol and peptide YY are associated with disordered eating psychopathology, independent of body mass index. Eur J Endocrinol (2011) 164(2):253–61. doi: 10.1530/EJE-10-0523
32. Lawson EA, Miller KK, Blum JI, Meenaghan E, Misra M, Eddy KT, et al. Leptin levels are associated with decreased depressive symptoms in women across the weight spectrum, independent of body fat. Clin Endocrinol (2012) 76(4):520–5. doi: 10.1111/j.1365-2265.2011.04182.x
33. Kandemir N, Becker K, Slattery M, Tulsiani S, Singhal V, Thomas JJ, et al. Impact of low-weight severity and menstrual status on bone in adolescent girls with anorexia nervosa. Int J Eat Disord (2017) 50(4):359–69. doi: 10.1002/eat.22681
34. Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics (1990) 86(3):440–7. doi: 10.1542/peds.86.3.440
35. Misra M, Aggarwal A, Miller KK, Almazan C, Worley M, Soyka LA, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics (2004) 114(6):1574–83. doi: 10.1542/peds.2004-0540
36. Soyka LA, Misra M, Frenchman A, Miller KK, Grinspoon S, Schoenfeld DA, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab (2002) 87(9):4177–85. doi: 10.1210/jc.2001-011889
37. Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab (2008) 93(4):1231–7. doi: 10.1210/jc.2007-1434
38. Faje AT, Karim L, Taylor A, Lee H, Miller KK, Mendes N, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab (2013) 98(5):1923–9. doi: 10.1210/jc.2012-4153
39. Singhal V, Tulsiani S, Campoverde KJ, Mitchell DM, Slattery M, Schorr M, et al. Impaired bone strength estimates at the distal tibia and its determinants in adolescents with anorexia nervosa. Bone (2018) 106:61–8. doi: 10.1016/j.bone.2017.07.009
40. Bredella MA, Misra M, Miller KK, Madisch I, Sarwar A, Cheung A, et al. Distal radius in adolescent girls with anorexia nervosa: Trabecular structure analysis with high-resolution flat-panel volume CT. Radiology (2008) 249(3):938–46. doi: 10.1148/radiol.2492080173
41. Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, et al. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone (2010) 46(2):458–63. doi: 10.1016/j.bone.2009.09.005
42. Vestergaard P, Emborg C, Støving RK, Hagen C, Mosekilde L, Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders-a nationwide register study. Int J Eat Disord (2002) 32(3):301–8. doi: 10.1002/eat.10101
43. Lucas AR, Melton LJ, Crowson CS, O’Fallon WM. Long-term fracture risk among women with anorexia nervosa: A population-based cohort study. Mayo Clin Proc (1999) 74(10):972–7. doi: 10.1016/S0025-6196(11)63994-3
44. Faje AT, Fazeli PK, Miller KK, Katzman DK, Ebrahimi S, Lee H, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord (2014) 47(5):458–66. doi: 10.1002/eat.22248
45. Christo K, Prabhakaran R, Lamparello B, Cord J, Miller KK, Goldstein MA, et al. Bone metabolism in adolescent athletes with amenorrhea, athletes with eumenorrhea, and control subjects. Pediatrics (2008) 121(6):1127–36. doi: 10.1542/peds.2007-2392
46. Ackerman KE, Cano Sokoloff N, de Nardo Maffazioli G, Clarke HM, Lee H, Misra M. Fractures in relation to menstrual status and bone parameters in young athletes. Med Sci Sports Exerc (2015) 47(8):1577–86. doi: 10.1249/MSS.0000000000000574
47. Ackerman KE, Singhal V, Baskaran C, Slattery M, Campoverde Reyes KJ, Toth A, et al. Oestrogen replacement improves bone mineral density in oligo-amenorrhoeic athletes: a randomised clinical trial. Br J Sports Med (2019) 53(4):229–36. doi: 10.1136/bjsports-2018-099723
48. Singhal V, Reyes KC, Pfister B, Ackerman K, Slattery M, Cooper K, et al. Bone accrual in oligo-amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone (2019) 120:305–13. doi: 10.1016/j.bone.2018.05.010
49. Ackerman KE, Putman M, Guereca G, Taylor AP, Pierce L, Herzog DB, et al. Cortical microstructure and estimated bone strength in young amenorrheic athletes, eumenorrheic athletes and non-athletes. Bone (2012) 51(4):680–7. doi: 10.1016/j.bone.2012.07.019
50. Ackerman KE, Nazem T, Chapko D, Russell M, Mendes N, Taylor AP, et al. Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J Clin Endocrinol Metab (2011) 96(10):3123–33. doi: 10.1210/jc.2011-1614
51. Gama E, Kasuki L, Paranhos-Neto F, Madeira M, Mendonça L, Schtscherbyna A, et al. Low energy availability interferes with exercise-associated bone effects in female long-distance triathletes as detected by HR-pQCT. J Clin Densitom (2022) 25((2):160–7. doi: 10.1016/j.jocd.2021.01.013
52. Kandemir N, Slattery M, Ackerman KE, Tulsiani S, Bose A, Singhal V, et al. Bone parameters in anorexia nervosa and athletic amenorrhea: Comparison of two hypothalamic amenorrhea states. J Clin Endocrinol Metab (2018) 103(6):2392–402. doi: 10.1210/jc.2018-00338
53. Misra M, Tsai P, Anderson EJ, Hubbard JL, Gallagher K, Soyka LA, et al. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr (2006) 84(4):698–706. doi: 10.1093/ajcn/84.4.698
54. Misra M, Miller KK, Almazan C, Worley M, Herzog DB, Klibanski A. Hormonal determinants of regional body composition in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab (2005) 90(5):2580–7. doi: 10.1210/jc.2004-2041
55. Singhal V, Torre Flores LP, Stanford FC, Toth AT, Carmine B, Misra M, et al. Differential associations between appendicular and axial marrow adipose tissue with bone microarchitecture in adolescents and young adults with obesity. Bone (2018) 116:203–6. doi: 10.1016/j.bone.2018.08.009
56. Singhal V, Maffazioli GDN, Cano Sokoloff N, Ackerman KE, Lee H, Gupta N, et al. Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone (2015) 77:83–90. doi: 10.1016/j.bone.2015.04.005
57. Bredella MA, Fazeli PK, Freedman LM, Calder G, Lee H, Rosen CJ, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower pref-1 than women without brown adipose tissue: A study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab (2012) 97(4):E584–90. doi: 10.1210/jc.2011-2246
58. Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab (2003) 88(12):5615–23. doi: 10.1210/jc.2003-030532
59. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab (1992) 75(3):762–7. doi: 10.1210/jcem.75.3.1381372
60. Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J Clin Endocrinol Metab (2010) 95(1):369–74. doi: 10.1210/jc.2009-1730
61. Elloumi M. IGFBP-3, a sensitive marker of physical training and overtraining. Br J Sports Med (2005) 39(9):604–10. doi: 10.1136/bjsm.2004.014183
62. Zietz B, Schnabl S, Nerlich M, Schoelmerich J, Schaeffler A. Nutritional composition in different training stages in young female athletes (Swimming) and association with leptin, IGF-1 and estradiol. Exp Clin Endocrinol Diabetes (2008) 117(06):283–8. doi: 10.1055/s-0028-1085996
63. Ohlsson C, Bengtsson BÅ, Isaksson OGP, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev (1998) 19(1):55–79. doi: 10.1210/edrv.19.1.0324
64. Fazeli PK, Lawson EA, Prabhakaran R, Miller KK, Donoho DA, Clemmons DR, et al. Effects of recombinant human growth hormone in anorexia nervosa: A randomized, placebo-controlled study. J Clin Endocrinol Metab (2010) 95(11):4889–97. doi: 10.1210/jc.2010-0493
65. Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. J Clin Investig (1995) 96(2):900–6. doi: 10.1172/JCI118137
66. Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, et al. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone (2009) 45(3):493–8. doi: 10.1016/j.bone.2009.06.002
67. Gambacciani M, Yen SSC, Rasmussen DD. GnRH release from the mediobasal hypothalamus. Neuroendocrinology (1986) 42(2):181–3. doi: 10.1159/000124271
68. Petraglia F, Sutton S, Vale W, Plotsky P. Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology (1987) 120(3):1083–8. doi: 10.1210/endo-120-3-1083
69. Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzani AR. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynaecol Endocrinol (2008) 24(1):4–11. doi: 10.1080/09513590701807381
70. Ackerman KE, Patel KT, Guereca G, Pierce L, Herzog DB, Misra M. Cortisol secretory parameters in young exercisers in relation to LH secretion and bone parameters. Clin Endocrinol (2013) 78(1):114–9. doi: 10.1111/j.1365-2265.2012.04458.x
71. Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab (2004) 89(10):4972–80. doi: 10.1210/jc.2004-0723
72. Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab (2006) 91(4):1561–5. doi: 10.1210/jc.2005-2422
73. Villanueva AL, Schlosser C, Hopper B, Liu JH, Hoffman DI, Rebar RW. Increased cortisol production in women runners. J Clin Endocrinol Metab (1986) 63(1):133–6. doi: 10.1210/jcem-63-1-133
74. Biller BMK, Federoff HJ, Koenig JI, Klibanski A. Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab (1990) 70(2):311–7. doi: 10.1210/jcem-70-2-311
75. Cravo RM, Frazao R, Perello M, Osborne-Lawrence S, Williams KW, Zigman JM, et al. Leptin signaling in Kiss1 neurons arises after pubertal development. PloS One (2013) 8(3):e58698. doi: 10.1371/journal.pone.0058698
76. Talbi R, Navarro VM. Novel insights into the metabolic action of Kiss1 neurons. Endocr Connect (2020) 9(5):R124–33. doi: 10.1530/EC-20-0068
77. Hahn TJ, Halstead LR, Teitelbaum SL, Hahn BH. Altered mineral metabolism in glucocorticoid-induced osteopenia. effect of 25-hydroxyvitamin d administration. J Clin Investig (1979) 64(2):655–65. doi: 10.1172/JCI109506
78. Grinspoon S. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med (2000) 133(10):790–4. doi: 10.7326/0003-4819-133-10-200011210-00011
79. Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, et al. Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic Amenorrhea1. J Clin Endocrinol Metab (1999) 84(6):2049–55. doi: 10.1210/jcem.84.6.5792
80. Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med (2004) 351(10):987–97. doi: 10.1056/NEJMoa040388
81. Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab (2003) 88(1):109–16. doi: 10.1210/jc.2002-020645
82. Wen JP, Liu C, Bi WK, Hu YT, Chen Q, Huang H, et al. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J Endocrinol (2012) 214(2):177–89. doi: 10.1530/JOE-12-0054
83. Sliwowska JH, Fergani C, Gawałek M, Skowronska B, Fichna P, Lehman MN. Insulin: Its role in the central control of reproduction. Physiol Behav (2014) 133:197–206. doi: 10.1016/j.physbeh.2014.05.021
84. Fernández-Fernández R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett (2004) 362(2):103–7. doi: 10.1016/j.neulet.2004.03.003
85. Fernandez-Fernandez R, Aguilar E, Tena-Sempere M, Pinilla L. Effects of polypeptide YY 3–36 upon luteinizing hormone-releasing hormone and gonadotropin secretion in prepubertal rats: In vivo and in vitro studies. Endocrinology (2005) 146(3):1403–10. doi: 10.1210/en.2004-0858
86. Andrico S. Leptin in functional hypothalamic amenorrhoea. Hum Reprod (2002) 17(8):2043–8. doi: 10.1093/humrep/17.8.2043
87. Miller KK, Parulekar MS, Schoenfeld E, Anderson E, Hubbard J, Klibanski A, et al. Decreased leptin levels in normal weight women with hypothalamic amenorrhea: The effects of body composition and nutritional Intake1. J Clin Endocrinol Metab (1998) 83(7):2309–12. doi: 10.1210/jcem.83.7.4975
88. Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab (1999) 84(3):873–7. doi: 10.1210/jcem.84.3.5551
89. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab (2005) 289(3):E373–81. doi: 10.1152/ajpendo.00041.2005
90. Christo K, Cord J, Mendes N, Miller KK, Goldstein MA, Klibanski A, et al. Acylated ghrelin and leptin in adolescent athletes with amenorrhea, eumenorrheic athletes and controls: a cross-sectional study. Clin Endocrinol (2008) 69(4):628–33. doi: 10.1111/j.1365-2265.2008.03237.x
91. Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab (2007) 92(6):2046–52. doi: 10.1210/jc.2006-2855
92. Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, et al. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol (2013) 168(3):457–64. doi: 10.1530/EJE-12-0869
93. Schneider LF, Warren MP. Functional hypothalamic amenorrhea is associated with elevated ghrelin and disordered eating. Fertil Steril (2006) 86(6):1744–9. doi: 10.1016/j.fertnstert.2006.05.051
94. Scheid JL, Williams NI, West SL, VanHeest JL, de Souza MJ. Elevated PYY is associated with energy deficiency and indices of subclinical disordered eating in exercising women with hypothalamic amenorrhea. Appetite (2009) 52(1):184–92. doi: 10.1016/j.appet.2008.09.016
95. Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. Am J Physiol Endocrinol Metab (2005) 289(2):E347–56. doi: 10.1152/ajpendo.00615.2004
96. Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab (2006) 91(3):1027–33. doi: 10.1210/jc.2005-1878
97. Russell M, Stark J, Nayak S, Miller KK, Herzog DB, Klibanski A, et al. Peptide YY in adolescent athletes with amenorrhea, eumenorrheic athletes and non-athletic controls. Bone (2009) 45(1):104–9. doi: 10.1016/j.bone.2009.03.668
98. Russell M, Misra M. Influence of ghrelin and adipocytokines on bone mineral density in adolescent female athletes with amenorrhea and eumenorrheic athletes. Med Sport Sci Basel: KARGER (2010) 55:103–13. doi: 10.1159/000321975
99. Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology (2007) 148(1):401–10. doi: 10.1210/en.2006-1019
100. Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJG. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LβT2 gonadotropes. Mol Endocrinol (2008) 22(3):760–71. doi: 10.1210/me.2007-0330
101. Martin A, de Vittoris R, David V, Moraes R, Bégeot M, Lafage-Proust MH, et al. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology (2005) 146(8):3652–9. doi: 10.1210/en.2004-1509
102. Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res (2005) 20(6):994–1001. doi: 10.1359/JBMR.050103
103. Cornish J, Callon K, Bava U, Lin C, Naot D, Hill B, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol (2002) 175(2):405–15. doi: 10.1677/joe.0.1750405
104. Wojcik MH, Meenaghan E, Lawson EA, Misra M, Klibanski A, Miller KK. Reduced amylin levels are associated with low bone mineral density in women with anorexia nervosa. Bone (2010) 46(3):796–800. doi: 10.1016/j.bone.2009.11.014
105. Misra M, Miller KK, Stewart V, Hunter E, Kuo K, Herzog DB, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. J Clin Endocrinol Metab (2005) 90(9):5082–7. doi: 10.1210/jc.2005-0512
106. Wong IPL, Driessler F, Khor EC, Shi YC, Hörmer B, Nguyen AD, et al. Peptide YY regulates bone remodeling in mice: A link between gut and skeletal biology. PloS One (2012) 7(7):e40038. doi: 10.1371/journal.pone.0040038
107. Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, et al. (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone (2008) 43(1):135–9. doi: 10.1016/j.bone.2008.03.007
108. Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry (2011) 72(11):1546–51. doi: 10.4088/JCP.10m06617
109. Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone (2004) 35(4):842–9. doi: 10.1016/j.bone.2004.06.008
110. Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun (2005) 331(2):520–6. doi: 10.1016/j.bbrc.2005.03.210
111. Jürimäe J, Rembel K, Jürimäe T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res (2005) 37(5):297–302. doi: 10.1055/s-2005-861483
112. Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone (2003) 33(4):646–51. doi: 10.1016/S8756-3282(03)00237-0
113. Barrack MT, Gibbs JC, de Souza MJ, Williams NI, Nichols JF, Rauh MJ, et al. Higher incidence of bone stress injuries with increasing female athlete triad–related risk factors. Am J Sports Med (2014) 42(4):949–58. doi: 10.1177/0363546513520295
114. Kopp-Woodroffe SA, Manore MM, Dueck CA, Skinner JS, Matt KS. Energy and nutrient status of amenorrheic athletes participating in a diet and exercise training intervention program. Int J Sport Nutr (1999) 9(1):70–88. doi: 10.1123/ijsn.9.1.70
115. Michopoulos V, Mancini F, Loucks TL, Berga SL. Neuroendocrine recovery initiated by cognitive behavioral therapy in women with functional hypothalamic amenorrhea: a randomized, controlled trial. Fertil Steril (2013) 99(7):2084–91.e1. doi: 10.1016/j.fertnstert.2013.02.036
116. Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril (2003) 80(4):976–81. doi: 10.1016/S0015-0282(03)01124-5
117. Falsetti L, Gambera A, Barbetti L, Specchia C. Long-term follow-up of functional hypothalamic amenorrhea and prognostic factors. J Clin Endocrinol Metab (2002) 87(2):500–5. doi: 10.1210/jcem.87.2.8195
118. Golden NH. Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med (1997) 151(1):16–21. doi: 10.1001/archpedi.1997.02170380020003
119. Tinahones FJ, Martínez-Alfaro B, Gonzalo-Marín M, García-Almeida JM, Garrido-Sánchez L, Cardona F. Recovery of menstrual cycle after therapy for anorexia nervosa. Eating anEat Weight Disord (2005) 10(3):e52–5. doi: 10.1007/BF03327550
120. Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab (1995) 80(3):898–904. doi: 10.1210/jcem.80.3.7883849
121. Warren MP, Brooks-Gunn J, Fox RP, Holderness CC, Hyle EP, Hamilton WG, et al. Persistent osteopenia in ballet dancers with amenorrhea and delayed menarche despite hormone therapy: a longitudinal study. Fertil Steril (2003) 80(2):398–404. doi: 10.1016/S0015-0282(03)00660-5
122. Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (Norgestimate/Ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: A double-blind, placebo-controlled study. J Adolesc Health (2006) 39(6):819–27. doi: 10.1016/j.jadohealth.2006.09.010
123. Ho KKY, Weissberger AJ. Impact of short-term estrogen administration on growth hormone secretion and action: Distinct route-dependent effects on connective and bone tissue metabolism. J Bone Miner Res (2009) 7(7):821–7. doi: 10.1002/jbmr.5650070711
124. Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res (2011) 26(10):2430–8. doi: 10.1002/jbmr.447
125. Ackerman KE, Singhal V, Slattery M, Eddy KT, Bouxsein ML, Lee H, et al. Effects of estrogen replacement on bone geometry and microarchitecture in adolescent and young adult oligoamenorrheic athletes: a randomized trial. J Bone Miner Res (2020) 35(2):248–60. doi: 10.1002/jbmr.3887
126. Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. J Clin Endocrinol Metab (2002) 87(6):2883–91. doi: 10.1210/jcem.87.6.8574
127. Golden NH, Iglesias EA, Jacobson MS, Carey D, Meyer W, Schebendach J, et al. Alendronate for the treatment of osteopenia in anorexia nervosa: A randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab (2005) 90(6):3179–85. doi: 10.1210/jc.2004-1659
128. Chou SH, Chamberland JP, Liu X, Matarese G, Gao C, Stefanakis R, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci (2011) 108(16):6585–90. doi: 10.1073/pnas.1015674108
129. Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. J Clin Endocrinol Metab (2011) 96(7):2081–8. doi: 10.1210/jc.2011-0380
130. Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism (2012) 61(7):1010–20. doi: 10.1016/j.metabol.2011.11.016
131. Fazeli PK, Wang IS, Miller KK, Herzog DB, Misra M, Lee H, et al. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J Clin Endocrinol Metab (2014) 99(4):1322–9. doi: 10.1210/jc.2013-4105
132. Divasta AD, Feldman HA, O’Donnell JM, Long J, Leonard MB, Gordon CM. Impact of adrenal hormone supplementation on bone geometry in growing teens with anorexia nervosa. J Adolesc Health (2019) 65(4):462–8. doi: 10.1016/j.jadohealth.2019.04.003
133. Haines MS, Kimball A, Meenaghan E, Bachmann KN, Santoso K, Eddy KT, et al. Sequential therapy with recombinant human IGF-1 followed by risedronate increases spine bone mineral density in women with anorexia nervosa: A randomized, placebo-controlled trial. J Bone Miner Res (2021) 36(11):2116–26. doi: 10.1002/jbmr.4420
134. Haverinen A, Luiro K, Kangasniemi MH, Piltonen TT, Hustad S, Heikinheimo O, et al. Estradiol valerate vs ethinylestradiol in combined oral contraceptives: Effects on the pituitary-ovarian axis. J Clin Endocrinol Metab (2022) 107(7):e3008–e3017. doi: 10.1210/clinem/dgac150
135. Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol (2014) 2(7):581–92. doi: 10.1016/S2213-8587(13)70180-3
136. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2017) 102(5):1413–39. doi: 10.1210/jc.2017-00131
137. Bulik CM, Sullivan PF, Fear JL, Pickering A, Dawn A, McCullin M. Fertility and reproduction in women with anorexia nervosa. J Clin Psychiatry (1999) 60(2):130–5. doi: 10.4088/JCP.v60n0212
138. Weissberger AJ, Ho KKY, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women. J Clin Endocrinol Metab (1991) 72(2):374–81. doi: 10.1210/jcem-72-2-374
139. Singhal V, Ackerman KE, Bose A, Flores LPT, Lee H, Misra M. Impact of route of estrogen administration on bone turnover markers in oligoamenorrheic athletes and its mediators. J Clin Endocrinol Metab (2019) 104(5):1449–58. doi: 10.1210/jc.2018-02143
140. Singhal V, Bose A, Slattery M, Haines MS, Goldstein MA, Gupta N, et al. Effect of transdermal estradiol and insulin-like growth factor-1 on bone endpoints of young women with anorexia nervosa. J Clin Endocrinol Metab (2021) 106(7):2021–35. doi: 10.1210/clinem/dgab145
141. Lund TD, Rovis T, Chung WCJ, Handa RJ. Novel actions of estrogen receptor-β on anxiety-related behaviors. Endocrinology (2005) 146(2):797–807. doi: 10.1210/en.2004-1158
142. Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology (1996) 21(7):609–20. doi: 10.1016/S0306-4530(96)00015-7
143. Baskaran C, Cunningham B, Plessow F, Singhal V, Woolley R, Ackerman KE, et al. Estrogen replacement improves verbal memory and executive control in Oligomenorrheic/Amenorrheic athletes in a randomized controlled trial. J Clin Psychiatry (2017) 78(5):e490–7. doi: 10.4088/JCP.15m10544
144. Chui HT, Christensen BK, Zipursky RB, Richards BA, Hanratty MK, Kabani NJ, et al. Cognitive function and brain structure in females with a history of adolescent-onset anorexia nervosa. Pediatrics (2008) 122(2):e426–37. doi: 10.1542/peds.2008-0170
145. Baskaran C, Plessow F, Ackerman KE, Singhal V, Eddy KT, Misra M. A cross-sectional analysis of verbal memory and executive control across athletes with varying menstrual status and non-athletes. Psychiatry Res (2017) 258:605–6. doi: 10.1016/j.psychres.2016.12.054
146. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril (2001) 76(2):310–6. doi: 10.1016/S0015-0282(01)01921-5
147. Braun DL, Sunday SR, Halmi KA. Psychiatric comorbidity in patients with eating disorders. Psychol Med (1994) 24(4):859–67. doi: 10.1017/S0033291700028956
148. Herzog DB, Keller MB, Sacks NR, Yeh CJ, Lavori PW. Psychiatric comorbidity in treatment-seeking anorexics and bulimics. J Am Acad Child Adolesc Psychiatry (1992) 31(5):810–8. doi: 10.1097/00004583-199209000-00006
149. Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry (2004) 161(12):2215–21. doi: 10.1176/appi.ajp.161.12.2215
150. Misra M, Katzman DK, Estella NM, Eddy KT, Weigel T, Goldstein MA, et al. Impact of physiologic estrogen replacement on anxiety symptoms, body shape perception, and eating attitudes in adolescent girls with anorexia nervosa. J Clin Psychiatry (2013) 74(08):e765–71. doi: 10.4088/JCP.13m08365
151. Gallinelli A, Matteo ML, Volpe A, Facchinetti F. Autonomic and neuroendocrine responses to stress in patients with functional hypothalamic secondary amenorrhea. Fertil Steril (2000) 73(4):812–6. doi: 10.1016/S0015-0282(99)00601-9
152. Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril (1993) 60(3):486–92. doi: 10.1016/S0015-0282(16)56165-2
153. Cano Sokoloff N, Eguiguren ML, Wargo K, Ackerman KE, Baskaran C, Singhal V, et al. Bone parameters in relation to attitudes and feelings associated with disordered eating in oligo-amenorrheic athletes, eumenorrheic athletes, and nonathletes. Int J Eat Disord (2015) 48(5):522–6. doi: 10.1002/eat.22405
154. Plessow F, Singhal V, Toth AT, Micali N, Eddy KT, Misra M. Estrogen administration improves the trajectory of eating disorder pathology in oligo-amenorrheic athletes: A randomized controlled trial. Psychoneuroendocrinology (2019) 102:273–80. doi: 10.1016/j.psyneuen.2018.11.013
155. McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav Neurosci (2012) 126(1):4–16. doi: 10.1037/a0026708
156. Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cognit Neurosci Rev (2005) 4(1):43–58. doi: 10.1177/1534582305277152
Keywords: functional hypothalamic amenorrhea, estrogen deficiency, bone health, anxiety, depression, adolescent
Citation: Pedreira CC, Maya J and Misra M (2022) Functional hypothalamic amenorrhea: Impact on bone and neuropsychiatric outcomes. Front. Endocrinol. 13:953180. doi: 10.3389/fendo.2022.953180
Received: 25 May 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Huseyin Demirbilek, Hacettepe University, TurkeyReviewed by:
Ayhan Abacı, Dokuz Eylül University, TurkeyVed Bhushan Arya, King’s College Hospital NHS Foundation Trust, United Kingdom
Serap Demircioglu Turan, Marmara University, Turkey
Copyright © 2022 Pedreira, Maya and Misra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clarissa Carvalho Pedreira, Y3BlZHJlaXJhQG1naC5oYXJ2YXJkLmVkdQ==
 Clarissa Carvalho Pedreira
Clarissa Carvalho Pedreira Jacqueline Maya
Jacqueline Maya Madhusmita Misra
Madhusmita Misra