
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 19 August 2022
Sec. Pituitary Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.947085
This article is part of the Research TopicInsights in Cushing’s Syndrome and DiseaseView all 10 articles
Purpose: We aimed to perform a retrospective analysis of a rare subtype of corticotroph adenoma, Crooke’s cell adenoma, to better understand its clinical features.
Methods: We collected T-PIT-positive pituitary adenomas and screened Crooke’s cell adenomas from January 2020 to December 2021 in our center. Case reports of such tumors were also collected through a literature search. Clinical data such as biochemical tests, imaging examinations, and pathological data of the above cases were analyzed.
Results: A total of 101 T-PIT-positive patients were treated in our center in the last 2 years, and 4 were finally pathologically diagnosed with Crooke’s cell adenomas. All of these patients were male with elevated adrenocorticotropic hormone levels, and 50.0% presented with hypercortisolemia, Cushing’s syndrome, visual impairment, and headache. The tumor diameter was significantly larger in these 4 patients (37.0 mm) than in the other patients (26.0 mm), and their tumor invasive behavior was more pronounced. Cases reported in the literature were mainly female (72.8%), and the clinical presentation was also dominated by Cushing’s syndrome (65.1%) and hormonal dysfunction. Tumors were more common as macroadenomas (33.2 mm) and suprasellar growths (63.8%). The tumor recurrence rate was as high as 55.6%, with 6 cases progressing to pituitary carcinomas and 7.7% of tumor-related deaths. Our further integrated analysis of our center and reported cases revealed that gender, Cushing’s syndrome, visual dysfunction, hormonal disorders, and tumor growth characteristics were statistically different in different tumor categories.
Conclusion: Crooke’s cell adenoma is a tumor subtype with obvious clinical aggressive behavior, and an in-depth analysis of its clinical characteristics may assist in developing a comprehensive treatment plan.
Pituitary Crooke’s cell adenomas (CCAs) are a rare subtype of corticotroph adenomas typically associated with Cushing’s disease, accounting for less than 1% of pituitary adenomas (1). These tumors, first described by Arthur Carleton Crooke in 1935 (2), represent a distinct clinicopathological subtype of pituitary adenomas. They were remarkably aggressive and showed the characteristics of Crooke’s cell - cytokeratin (CK) filaments accumulating heavily around the nucleus, making them appear distinctly hyaline in hematoxylin and eosin (HE) staining. The neoplastic Crooke’s cells are strongly immunoreactive T-PIT and CK8/18 and exhibit variable adrenocorticotropic hormone (ACTH) immunoreactivity. It has been suggested that CCA should meet the diagnostic criteria of at least 50% of neoplastic Crooke’s cells in corticotroph adenomas. The presence of hyaline change is reported to be the result of respondence of excess glucocorticoids, but the mechanisms have not been well understood. CCAs usually take the form of invasive macroadenomas with a high rate of recurrence after standard resection. We reviewed 4 cases of CCA, with clinical, radiological, and histopathological features. We are seeking a better understanding of their clinical-pathological characteristics, as well as assessing their immunophenotype and prognosis.
We performed a retrospective analysis of patients with pituitary adenoma who underwent surgery at The First Affiliated Hospital of Sun Yat-sen University from January 2020 to December 2021. All masses were removed by the same surgical team, and their pathological findings supported the diagnosis of T-PIT-positive pituitary adenoma. We identified CCAs with a multidisciplinary synergistic diagnosis of neurosurgery, pathology, endocrinology, etc.
During the perioperative period, hormonal and imaging studies are performed in our center on patients with pituitary adenomas. The reference ranges for hormones are as follows: 8 AM Cortisol, Urinary cortisol, 24h urinary cortisol, ACTH, PRL, GH, TSH, LH, and FSH. Above or below these ranges are considered abnormal hormone secretion. We used 3.0T Magnetic Resonance Imaging (MRI) for routine tumor screening. For the pituitary gland, thin cuts in coronal and sagittal positions are used to facilitate visualization of the cavernous sinus and optic chiasma. For microadenomas, dynamic MRI scans have been used to increase the sensitivity; while for macroadenomas, conventional coronal and sagittal pituitary MRI with contrast is generally adequate for treatment planning. The direction of tumor growth was classified as supra-sella, para-sella (cavernous sinus), clivus and infra-sella (sphenoidal sinus).
We used HE staining and reticulin fiber staining for the initial diagnosis of tumorigenic lesions in the sella area masses, based on which T-PIT (AM0486), PIT-1 (AM0451), and SF-1 (AM0443) immunostaining was used to distinguish the tumor cell spectrum origin. The transcription factor antibodies are all purchased from Xiamen Talent Biomedical Technology Co., Ltd. (Fujian Province, China). ACTH, PRL, GH, TSH, LH, FSH, and CK8/18 immunostaining was used for further classification of tumor categories. The Ki-67 index was used to determine the proliferative activity of the tumor. The final pathology report was issued by two experienced pathologists after discussion.
We searched the PubMed database using the terms “Crooke’s cell”, “Crooke’s cell hyaline deposition”, and “Crooke’s cell adenoma”, etc. We reviewed all relevant English-language literature published before December 2021 and performed a summary analysis of the case reports.
Statistical analysis was performed using SPSS software (version 19.0, IBM Corp.). Continuous variables with normal distribution were presented as mean ± standard deviation. Comparing two sets of quantitative data following a normal distribution using the Student’s t test. The frequencies of categorical variables were compared using a chi-square test. A value of p<0.05 was considered statistically significant.
Over the past 2 years, we treated a total of 418 patients with pituitary-related disorders, of whom 391 completed pathological testing for transcription factors and hormones, while a total of 101 patients (25.8%) were positive for T-PIT. 4 patients eventually received a pathological diagnosis of CCA, the prevalence of which was approximately 1.0%.
Of the 391 patients, there were 244 clinically silent adenomas, of which 66 (27.0%) were T-PIT positive cases. As shown in Table 1, of the 97 non-CCA cases recorded in our center, their average age was 45.5 ± 14.0 years, and 17.5% (17/97) were male; whereas no female patients were found in CCA cases and the mean age was 45.0 ± 8.0 years. Clinically, a total of 18 (18.6%) non-CCA patients with significant clinical symptoms associated with Cushing’s Syndrome, and 50.0% (2/4) CCA patients exhibited similar symptoms. Visual dysfunction was observed in 50.5% (49/97) of non-CCA patients and 50.0% (2/4) of CCA patients. 25.8% (25/97) of non-CCA patients and 50.0% (2/4) of CCA patients presented with headache. For hormone secretion, 8.3% (8/97) of non-CCA patients had hypercortisolism, while half of the CCA patients had significantly elevated cortisol levels. All CCA cases had abnormal secretion of ACTH, while non-CCA patients presented more often with non-functioning adenomas (NFPAs), with only 18.6% (18/97) having this hematological feature. The overlap with clinical and hormone immunohistochemistry in 101 cases was shown in Supplemental Table 1. The mean tumor diameter was 26.0 mm in non-CCA patients and 37.0 mm in CCA patients, the latter being significantly larger than the former. Tumors in CCA patients showed significant invasive behavior to surrounding structures such as the cavernous sinus (100.0%, 4/4), sphenoidal sinus (100.0%, 4/4), suprasellar region (75.0%, 3/4), and posterior cranial fossa (75.0%, 3/4). Non-CCA tumor invaded mainly towards the suprasellar region (59.8%, 58/97) and the cavernous sinus (50.5%, 49/97). The recurrence rate of CCA (75.0%, 3/4) is significantly higher than that of non-CCA (39.2%, 38/97). One CCA patient (25.0%, 1/4) died due to tumor complications.
A patient aged 32 suddenly developed symptoms such as right eyelid ptosis and visual impairment in 2016, and cranial MRI suggested a 18×38×30 mm (Knosp grading 4) lesion in the sella area. Transsphenoidal resection was performed in November of the same year, and postoperative pathology confirmed the ACTH adenoma. Clinical symptoms related to the occupying effect of the tumor were alleviated, but the MRI suggested tumor recurrence in the second month after the operation. Transsphenoidal surgery was performed again, followed by gamma knife radiotherapy while the MRI showed a residual tumor in the left cavernous sinus area. In February 2018, the patient presented with weakness, memory loss, abdominal purple striae, and central obesity. Cranial MRI suggested a 18×19×9 mm lesion in the sella area. In September 2018, after intolerable side effects from mifepristone, he underwent bilateral adrenalectomy in our hospital and gradually developed postoperative hyperpigmentation of the face and limbs. In December, nasal bleeding was appeared and endoscopy revealed a neoplasm at the olfactory fissure protruding into the common nasal tract with a maximum diameter of about 34 mm, and tumor recurrence is considered. In January 2019, a surgical resection was performed and the pathology reported an aggressive ACTH adenoma with Ki-67 of about 20%. A residual lesion was found and radiotherapy was re-performed postoperatively. In July 2019, symptoms of nasal bleeding re-emerged and ACTH was significantly elevated (Table 2). A nasal endoscopic biopsy was performed and the pathology suggested CCA (Figure 1). In April 2020, the pituitary MRI showed a recurrent mass of 37×43×34 mm in the operated area encapsulating the left internal carotid artery (Figure 2). In August 2020, the mass had invaded into the cerebellopontine angle region, and the headache was relieved after surgery, but vision loss and left-sided facial hypoesthesia occurred. In November, MRI scanning showed that the mass size increased to 53×39×26 mm, compressing the left temporal lobe and brainstem. After considering the risk of surgery, the patient and his family decided to take “Temozolomide 100 mg/day combined with Anlotinib 8 mg/day”. At the same time, symptomatic treatments such as hormone replacement, intracranial pressure reduction, and maintenance of water-electrolyte balance were administered. The patient was discharged from the hospital with occasional symptoms such as choking on water, difficulty urinating, chest tightness, dizziness and headache, which were treated symptomatically. MRI was repeated in December 2020, and the lesion further expanded to 65×47×50 mm, involving the temporal lobe and brainstem, with supratentorial hydrocephalus. Unfortunately, the patient died in 2021 due to severe complications.
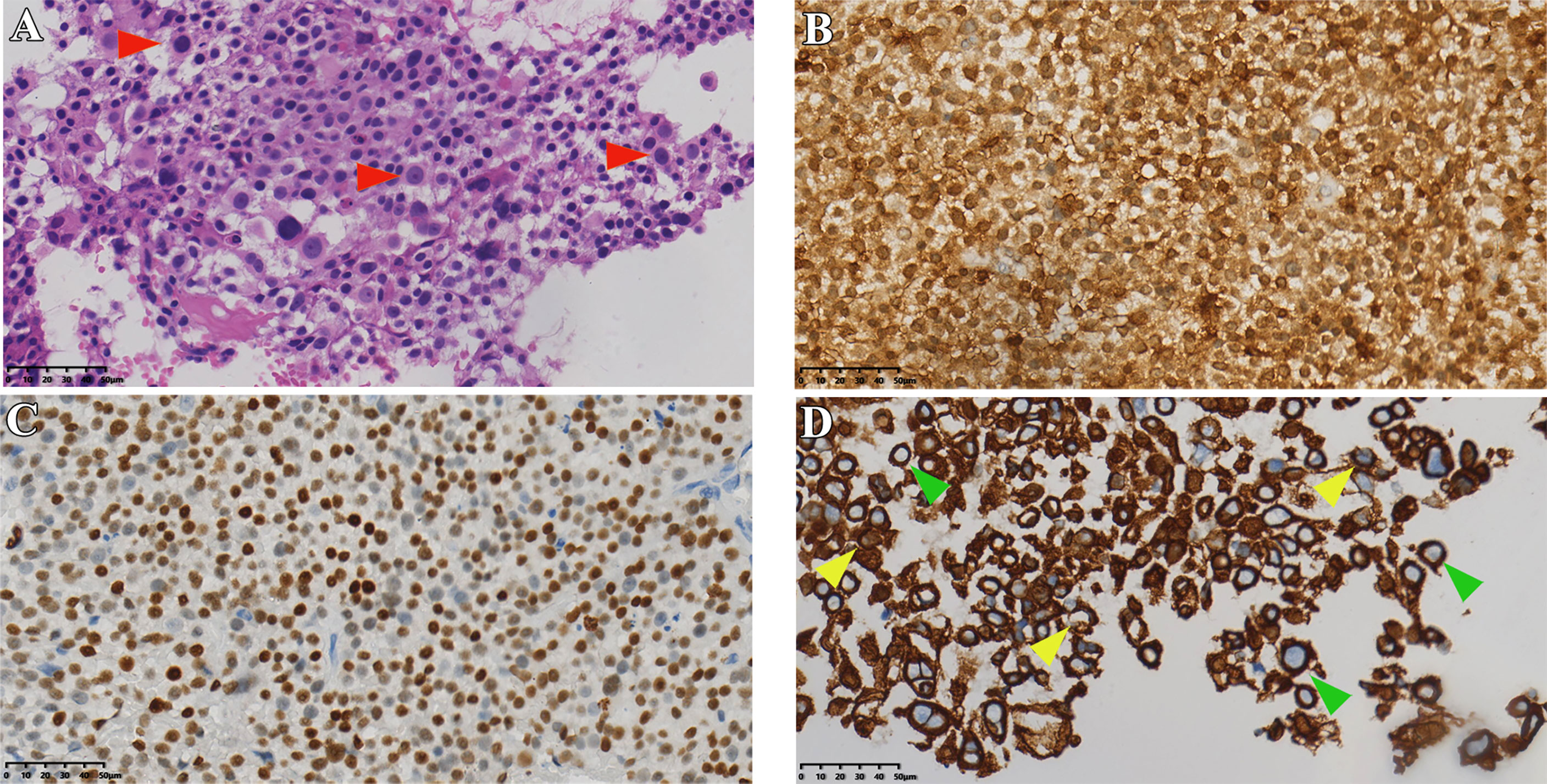
Figure 1 Histopathological data of case 1. (A) HE staining of tumor tissue (red arrows indicate typical Crooke’s hyaline change). (B, C) Immunohistochemical staining for ACTH and T-PIT. (D) Immunohistochemical staining of cells keratin showing Crooke hyaline changes (green and yellow arrows show a round and semi-circular immunostaining of CK8/18, respectively). Original magnification, 40×.
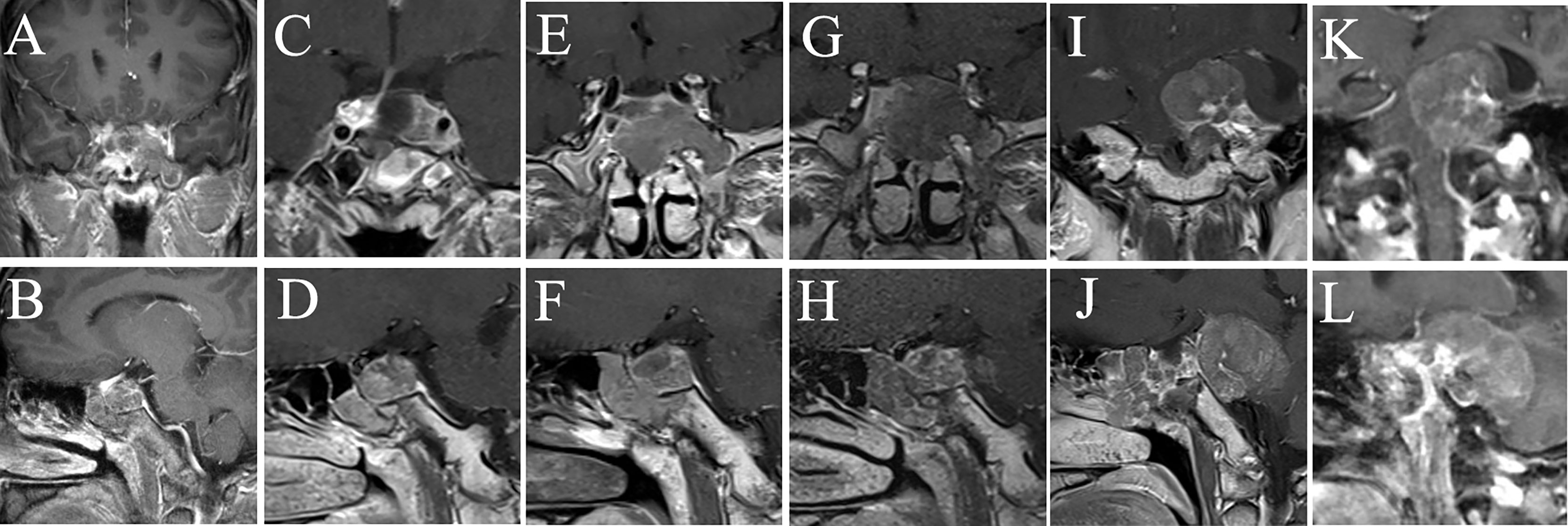
Figure 2 MRI examination of case 1. The images show the patient’s coronal and sagittal MRI of the head before (A, B), one week after (C, D), six months after (E, F), eight months after (G, H), sixteen months after (I, J), and seventeen months after (K, L) the fourth surgery, respectively.
A 54 years old male patient gradually developed symptoms such as hyperphagia, full moon face and buffalo back 7 years ago, without accompanying purple striae, subcutaneous bleeding spots or petechiae. Starting in 2017, he developed generalized skin pigmentation, which was evident in the axillae, buttocks, neck, and joints of the extremities, accompanied by swelling of both lower extremities. In June 2020, the patient was found to have elevated blood pressure, significantly elevated blood cortisol and ACTH. MRI of the pituitary gland suggested an occupying lesion of about 25×16×24 mm (Knosp grading 2) in the sella area, with compression of structures like optical chiasma and cavernous sinus. Initial consideration was given to pituitary macroadenoma. Abdominal CT showed multiple nodular hypodense shadows in the left adrenal gland. After admission to our hospital, ACTH, cortisol and 24-hour urinary cortisol were found to be elevated (Table 2). The patient underwent transsphenoidal surgery in July 2020, and the postoperative pathology suggested a CCA with CK8/18 > 50% (Figure 3), T-PIT (+), and Ki-67 about 3%. No significant postoperative urinary cortisol relief was seen, and no evident residual mass was found in the second day postoperative follow-up CT. However, MRI after 4 months suggested a mass in the operative area, measuring approximately 15×18×14 mm (Figure 4). Cortisol concentration was still high at 34 ug/dL at 8 months after the intervention. Twenty-one months after surgery, blood cortisol was 32.1 ug/dL, ACTH was 59.79 pmol/L, and MRI showed a 22*29*23 mm mass in the sellar area. The patient underwent a second surgery in our hospital in April 2022. Intraoperatively, the tumor was seen to be separated by the pseudocapsule, with a tough texture, and pathological staining showed Ki-67 8%. Cortisol and ACTH did not return to normal on repeat examination, and CT suggested residual tumor.
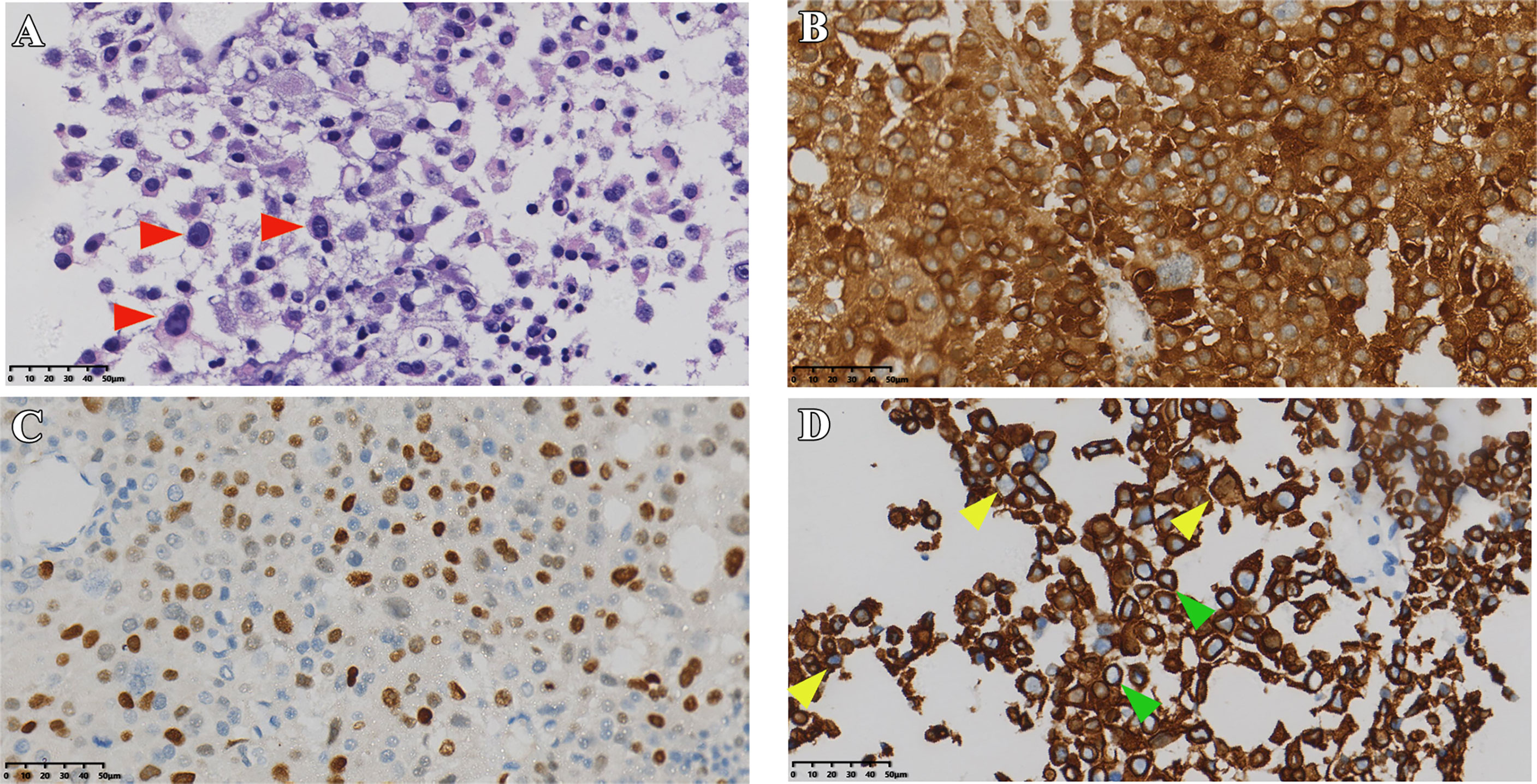
Figure 3 Pathological sections of case 2. (A) HE staining of tumor (red arrows indicate typical Crooke’s hyaline change). (B, C) Immunostaining of ACTH, T-PIT, and CK8/18 (D) green and yellow arrows show a round and semi-circular immunostaining of keratin, respectively). Original magnification, 40×.
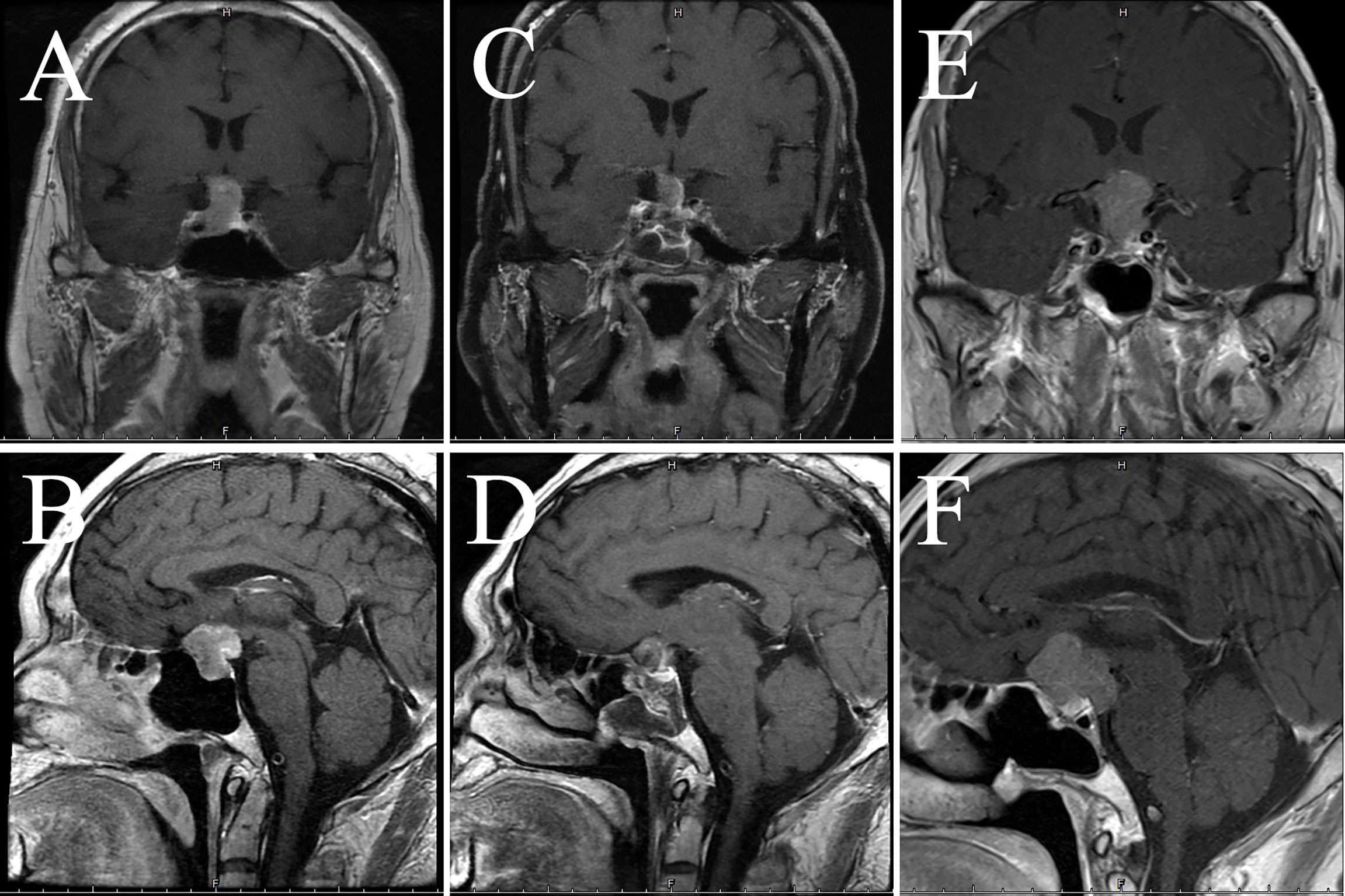
Figure 4 Imaging data of case 2. The images show coronal and sagittal MRI of the patient’s head preoperatively (A, B), three months postoperatively (C, D), and twenty-one months postoperatively (E, F), respectively.
One man presented with dizziness, occasional nausea and vomiting at the age of 42 in 2018. Starting the following year, the above symptoms got worse, so he went to our outpatient clinic in 2021. Pituitary MRI suggested an enlarged pituitary fossa and an abnormal signal occupying the lesion in the sella, the size was about 24×20×17 mm (Knosp grading 3), the initial consideration was a pituitary macroadenoma. 1 month later, the head CT revealed that the mass diameters were about 25mm, 23mm, and 18mm. The tumor was aggressive and the bone of the sella base was damaged, and the cavernous sinus was also compressed. The patient underwent microsurgery in October 2021, following which the elevated ACTH was relieved (Table 2), and the pathology suggested cyclic CK8/18 staining of about 80% (Figure 5), Ki-67 of about 10%, and infiltration of Crooke cells were observed in bone trabeculae. Hematology and MRI in January 2022 showed no evidence of recurrence (Figure 6).
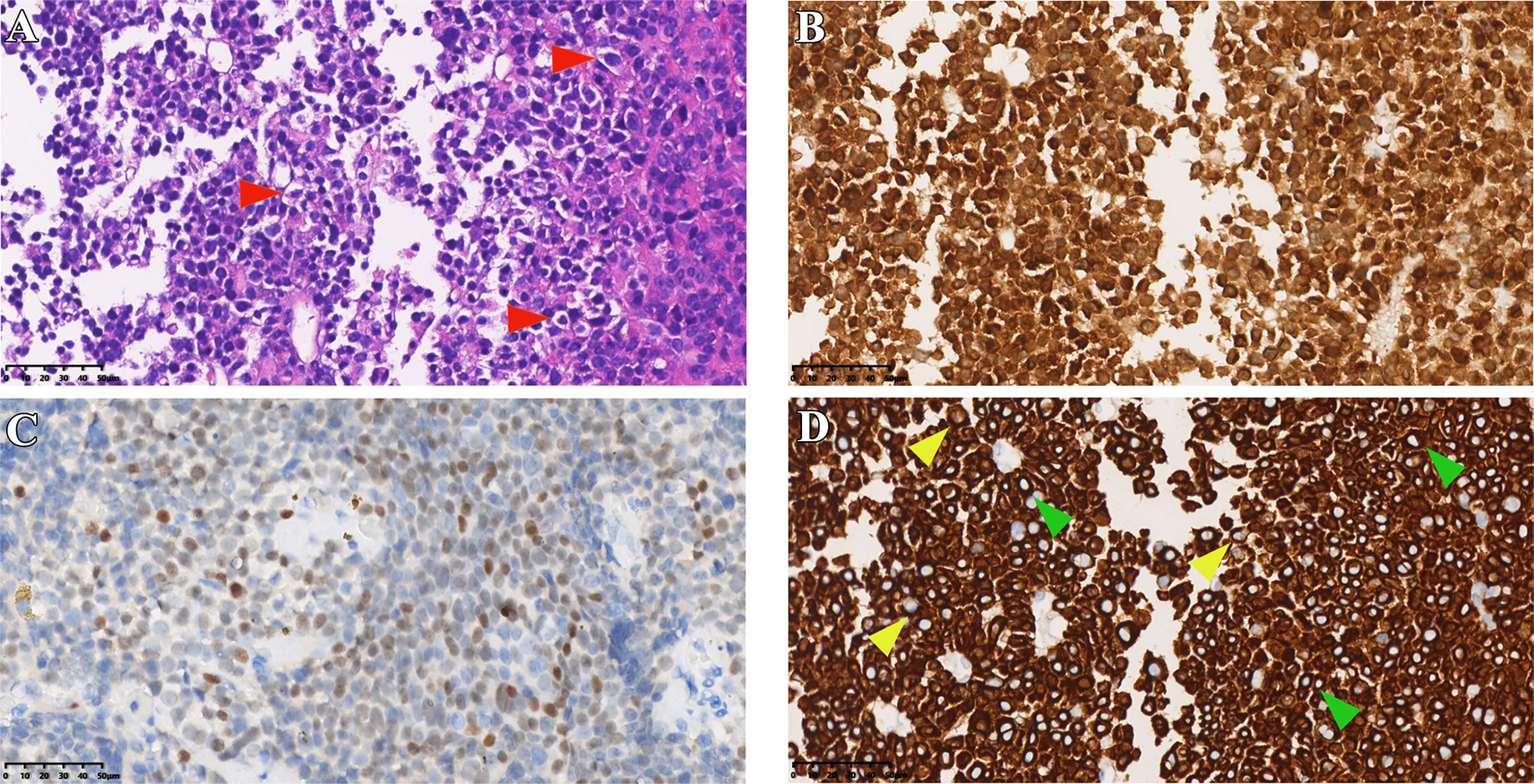
Figure 5 Immunostaining results of case 3. (A) HE staining of tumor tissue (red arrows indicate typical Crooke’s hyaline change). (B, C) Immunohistochemical staining for ACTH and T-PIT. (D) Immunohistochemical staining of cells keratin (green and yellow arrows show a round and semi-circular immunostaining of CK8/18, respectively). Original magnification, 40×.
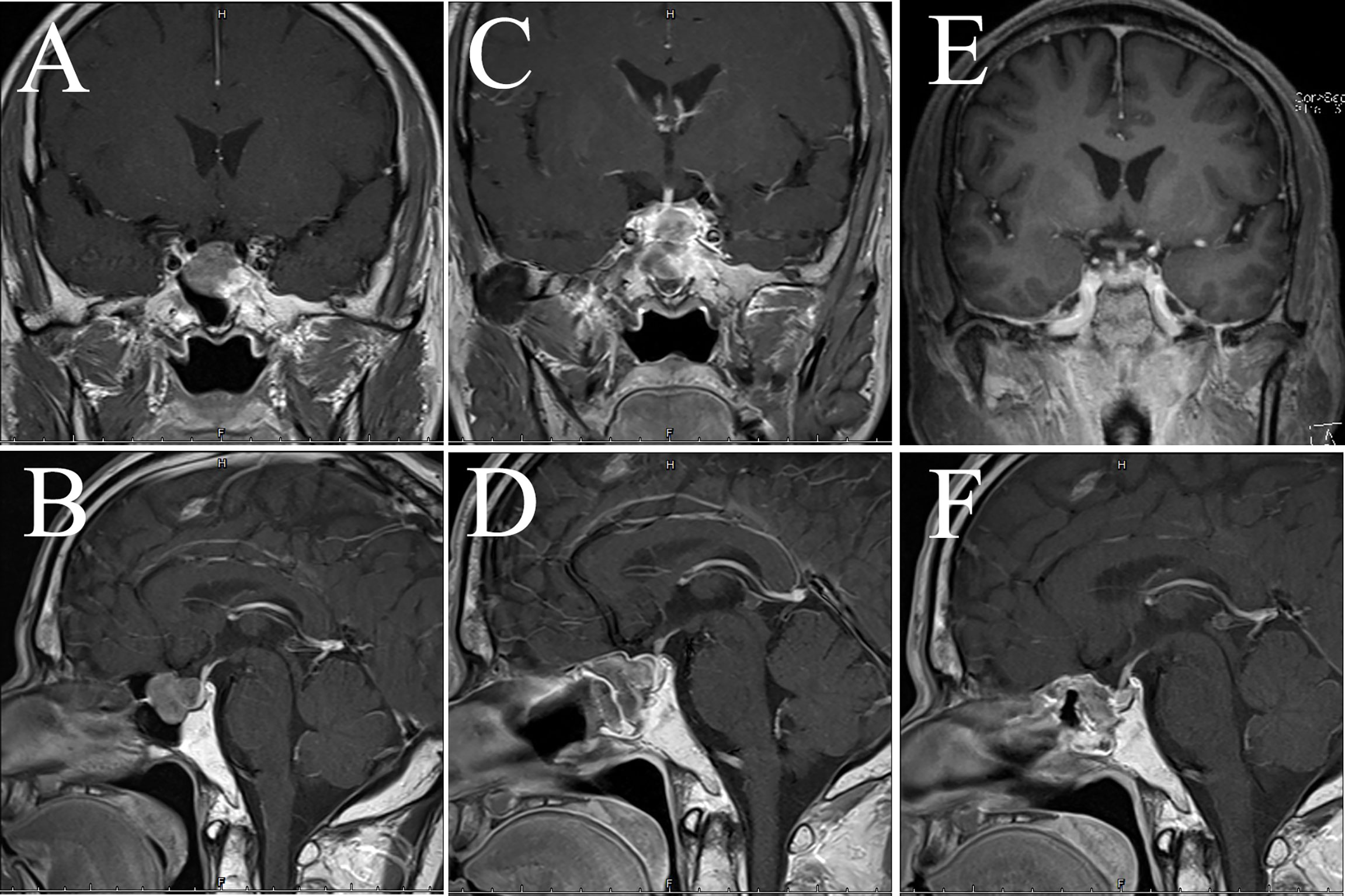
Figure 6 MRI examination of case 3. The images show coronal and sagittal MRI of the patient’s head preoperatively (A, B), one week postoperatively (C, D), and three months postoperatively (E, F), respectively.
Case 4 is a 50-year-old man with a headache following a “resection of left vocal cord squamous cell carcinoma” in December 2021. The headache was mainly in the right temporal region, with paroxysmal traction pain, with double vision, blurred vision and drooping right eyelid, and elevated ACTH on blood examination (Table 2). The cranial MRI showed an occupancy in the sella area with a size of about 21×17 mm (Knosp grading 3), and a pituitary macroadenoma was considered. The head CT showed an irregular mass in the sella and supra-sella area, about 33×22 mm in size, with uniform density. The mass wrapped around the siphon segment of the right internal carotid artery and part of the left posterior communicating artery. Also, the neoplasm was close to the left internal carotid artery, and invaded the dorsum of the sella and part of the slope bone downward. The magnetic resonance DTI sequence showed that part of the right optic nerve fiber bundle was interrupted and significantly reduced compared to the other side. Transsphenoidal surgery was performed, and the pathology suggested that the tumor tissue was T-PIT (+), ACTH positive >95%, Crooke cells accounted for about 80% (Figure 7), and Ki-67 about 1% (+). Normalized ACTH was found in a postoperative reexamination. However, recent imaging data suggested a suspicious residual tumor (Figure 8).

Figure 7 Histopathological staining of case 4. (A) HE staining of tumor tissue (red arrows indicate typical Crooke’s hyaline change). (B, C) Immunohistochemical staining for ACTH and T-PIT. (D) Immunostaining of CK8/18 (green and yellow arrows show a round and semi-circular pattern, respectively). Original magnification, 40×.
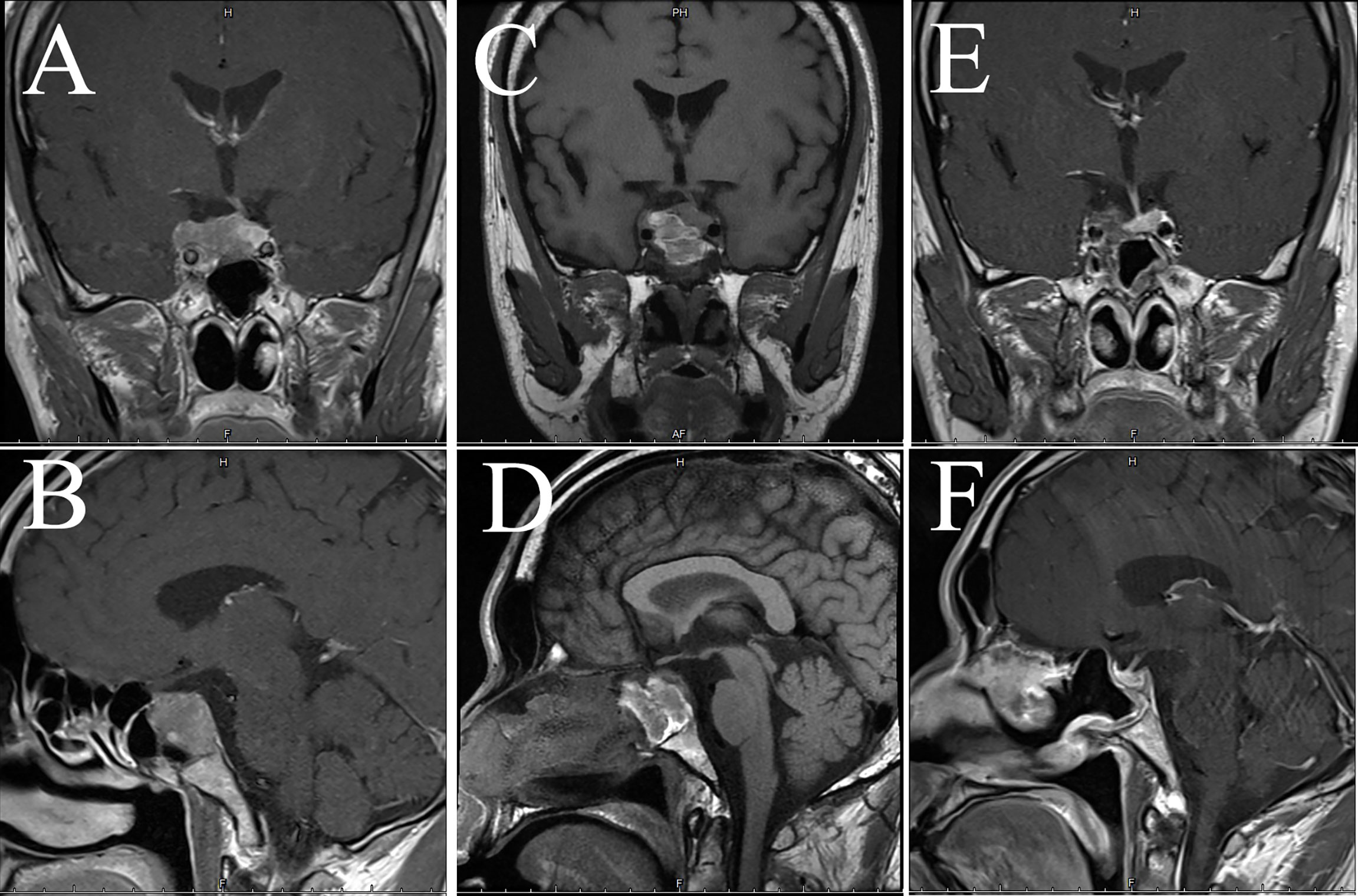
Figure 8 Imaging examination of case 4. The images show coronal and sagittal MRI of the patient’s head preoperatively (A, B), one week postoperatively (C, D), and three months postoperatively (E, F), respectively.
To date, over 100 CCA cases have been published (Table 3) (3–35). Since the hyalinization of corticotropin cells was proposed by Crooke in 1935 (2), Felix et al. (3) first reported 3 cases of adrenocorticotropin adenoma with a large amount of Crook’s hyaline deposition in 1981. Since then, cases of a variety of sizes have come forward. In 2003, George et al. reported the largest number of 36 cases to date (13). The authors described their clinical manifestations, pathological manifestations, and therapeutic strategies, and underlined their invasive clinical features. Early symptoms in some cases were consistent with asymptomatic NFPAs. With the development of the disease, they are transformed into functional tumors of hypercortisolism, and finally into pituitary cancer. CCA is mainly macroadenoma with obvious clinical invasiveness. In comparison to microadenomas, the macroadenomas that cause Cushing’s disease have different biochemical characteristics, invasive nature, initial remission rate, and post-operative recurrence rate.
We made a retrospective summary of the published cases (Table 4) to better understand the clinical features of CCA. Overall, their mean age was 47.8 ± 13.4 years. There were more female patients (72.8%, 59/81) than men (27.2%, 22/81) in CCA patients. The major symptoms in most patients were Cushing’s syndrome (65.1%, 56/86), optic nerve disorder (33.7%, 29/86), and headache (29.1%, 25/86). Notably, up to 71.4% (45/63) of patients were accompanied by an increase in ACTH, while more than half had hypercortisolism. The average diameter of the tumor was 33.2 ± 12.8 mm, and we found that the tumor mainly grew toward the suprasellar (63.8%, 44/69), which coincided with the symptoms of visual impairment in most patients. Additionally, the number of patients with tumor recurrence reached 55.6% (35/63), 6 patients with progression to pituitary carcinoma were reported (9.7%, 6/62), and tumor-related deaths accounted for 7.7% (5/65).
To better understand the clinical characteristics of CCA, we integrated our cases with the CCA cases previously reported for analysis. As illustrated in Table 5, the age composition of the two groups was similar and the data were relatively comparable. Regarding gender, CCA was clearly dominated by female patients. Although this is contrary to the characteristics of our center, it is similar to the overall reporting. In addition, CCA was predominantly a functional tumor with a significantly higher proportion of concomitant Cushing’s syndrome, hypercortisolism and elevated ACTH than non-CCA, supporting the notion that cortisol disorders promote the formation of CCA. The relatively moderate hormone secretion in the non-CCA group resulted in a higher proportion of visual dysfunction than in the CCA group. No significant difference was observed between the two groups with respect to headache. The tumor diameter was significantly larger in CCA than in the control group, but the tendency for tumor invasion into the sphenoid sinus was lower than in non-CCA. In terms of tumor recurrence, the therapeutic effect of CCA was much worse than that of the control group.
Pituitary adenomas are the most common masses of the sellar region arising from adenohypophyseal cells. The pituitary-specific transcription factors, including PIT-1, T-PIT, SF-1, and GATA-2, involved in adenohypophysial cell differentiation and maturation are now regarded as key diagnostic tools for the further characterization of pituitary adenomas. Based on the specific transcription factor tested by IHC, the new WHO classification of pituitary tumors has provided an integrated approach for the diagnosis and classification of pituitary adenomas (36). T-PIT-driving corticotroph adenomas represent 10 to 15 percent of all pituitary adenomas and are divided into three recognized variants: densely granulated adenomas, sparsely granulated adenomas, and Crooke’s cell adenomas. Crooke’s cell adenoma is an uncommon variant of ACTH-immunoreactive adenoma in which the cells recapitulate Crooke’s hyaline change observed in the non-neoplastic pituitary gland under the influence of elevated cortisol levels. In 1935, Crooke et al. first reported the hyaline change of basophils in the anterior pituitary (2). Subsequent studies found intermediate filament-rich Crooke’s cells, with ring-like cytoplasmic filaments accumulating, causing dispersal of secretory granules (and both PAS and ACTH reactivity) to the peripheral submembrane region or displaced internally next to the nucleus. Porcile and Racodot (37) confirmed that the core material of the hyalinization was made up of 7nm filaments arranged in parallel circles surrounding the nucleus by the ultrastructural method. The remaining secretory granules and other organelles were divided into perinuclear and perimembrane groups. Subsequently, Newman et al. (38) revealed that Crook’s hyaline deposition was immunohistochemically composed of intermediate filament keratin. Yet, until now, there is no clear mechanism to explain the phenomenon of increased keratin. Some studies suggest that Crooke-like hyalinization is a cellular inhibitory response to cortisol stimulation, such as the fact that Crooke’s cells are commonly accompanied by hypercortisolemia, and the phenomenon that organelles like the Golgi apparatus are squeezed around the cell membrane is considered a manifestation of inhibition of cell function (5, 9). Our results also confirm the above point by analyzing about 200 cases (Table 5). Irregular processing of precorticotropin (POMC), a precursor of corticotropin, is thought to be the cause of clinical silence in these tumors (27). Crooke-like change is generally a change in the response of normal corticotropin cells, which is not often involved in ACTH tumor cells. This is due to the expression of the glucocorticoid receptors in normal corticotropin cells, while the low or no expression of the glucocorticoid receptors in tumor cells leads to their tolerance to hypercortisolemia (12). Studies have confirmed that the keratin filaments accumulated in Crooke-like ACTH cells are CK8 and CK18 (39). Some studies have shown that CK20 is also involved in the formation of Crooke-like deposition. According to 2022 WHO classification guidelines (36) and previous case reports, CK8/18 immunostaining is described as a “perinuclear and ring-shaped” change. In 2003, George et al. (13) published a study with the largest number of cases to date. They took the positive rate of CK8/18 circular immunostaining > 50% as a diagnostic criterion for CCAs, which is still in use today.
Pathological diagnosis of CCA depends on the results of typical histology and immunostaining. In a typical Crooke’s cell adenoma, more than 50% of tumor cells exhibit obvious intracytoplasmic circular hyalines stained by HE and CK8/18 immunostaining, lack of intact reticulin scaffold, and positive T-PIT immunostaining (13). In addition, there is an eccentric rhabdoid keratin staining with neoplastic Crooke’s cells, although circular enhancement is the earliest and most characteristic change (21). Our medical records also show that semi-circular and strip-shaped keratin staining co-exists with circular enhancement in variable proportions. We note that the circular characteristic of CK8/18 staining is not the only way of expression, and the types of semi-cyclic and strip staining should attract the attention of researchers as well. It is not an unusual phenomenon that our cases contain various semi-ringlike CAM 5.2 positive cells (Figures 1D, 3D, 5D, 7D). The Ki-67 is even as high as 40% in case1, reminding us that despite the existence of atypical Crooke-like cells, the invasiveness of the tumor still needs the close attention of researchers. In addition, the proportion of Crooke-like positive cells is worthy of further study. Conversion of adrenocorticotropic hormone cells to Crooke’s cells is reported to account for up to 25% of 52% of ACTH adenomas (40). Another study found that in 177 patients with neuroendocrine tumors with positive corticotropin staining confirmed by histology and 213 patients with Cushing’s syndrome diagnosed by pituitary surgery, about 74% of the tumor samples experienced Crooke-like changes (41). Also, it has been suggested that as long as the anterior lobe biopsy measures more than 25% of Crook’s hyaline deposition, it may indicate that the functional recovery of the HPA axis is slower after the operation (25, 42). A recent study pointed out that due to local infiltration and growth, non-tumor tissue is very common in ACTH tumor specimens, and how to identify normal tissue in pathological diagnosis is very important (39). It is not known whether different percentages of Crooke’s cells indirectly indicate the degree of tumor invasion, but it can be predicted that once Crooke-like hyaline change occurs in tumor tissues, its invasive behavior is also stronger than that of general ACTH adenomas (9, 43), which deserves the continuous attention of clinicians. Combined with previous literature reports, considering the formation of Crooke’s cells is a long-term outcome of hypercortisolemia, which makes us wonder: is “50%” in the previous diagnostic criteria appropriate? After all, Crooke’s cell tumor is a highly invasive tumor, and its early diagnosis has a positive sign for the clinical prognosis of the disease.
Treatment of CCAs remains challenging due to its aggressive nature and high recurrence rate. According to the case summaries in our center and the literatures, the diameter of the CCAs is significantly larger than that of non-CCAs’ (Tables 1 and 5). Unlike the proportion reported, all CCAs from our center were male (Table 1). In general, the surgical treatment goal of CCAs is still gross-total resection. However, despite the effectiveness of surgical resection, its recurrence rate remains as high as 56.7% (38/67) and the success rate of reoperation is low: case 1 died of severe complications despite multiple treatments, while global tumor-related mortality reached 8.7% (6/69). Radiotherapy may be considered for patients with postoperative recurrence, postoperative residues, or strong invasiveness. A recent study (44) reported that 40% of the patients who received radiotherapy had a 30% reduction in tumor volume, and there was no tumor recurrence or growth during the 12-month follow-up, no complications of radiotherapy, and no patients experienced a second dose of radiotherapy. In this study, radiotherapy is used as an adjuvant for surgery rather than as an independent treatment, since all patients have received at least one surgical treatment. Temozolomide (TMZ), a first-line drug against glioma, is also effective in treating refractory pituitary adenomas. One study revealed that the overall effective rate of TMZ in the treatment of refractory pituitary adenomas was 45%, and 27% of people were in stable condition (26). ACTH adenomas, particularly invasive tumors, have a low level of MGMT (18), which is an indication for TMZ treatment. However, some studies have shown that as the malignant degree of CCA increases, its MGMT level increases, and the effectiveness of TMZ treatment decreases. The effectiveness of TMZ has received positive signals in clinical practice. At least 18-33 months (22, 24) after discontinuation of TMZ treatment, the disease is still in remission, and these data demonstrate the safety and effectiveness of discontinuing temozolomide treatment. A recent study confirmed that no tumor recurrence was found in the seven years after TMZ treatment (30). In addition to using the level of MGMT as a reference for tumor treatment, genetic mutations and small molecule RNA also provide potential entry points for tumor treatment. Kyohei Hayashi et al. (45) reviewed 60 cases of ACTH adenoma (including 15 cases of Crooke’s cell adenoma). They found that the USP8 mutation rate was high and the downstream POMC content was also increased in microadenomas, while macroadenomas such as CCAs had a low USP8 mutation rate accompanied by decreased expression of POMC and MGMT, suggesting the suitability of CCAs for TMZ therapy. Garbicz et al. (46) found that miR-106b-25 and its host gene MCM7 are potential novel biomarkers of invasive corticotropin immunopositive pituitary adenomas. We have a number of T-PIT cases in our NFPAs, and the previously reported anti-oncogene MEG3 (47) may also serve as a breakthrough in treatment. The percentage of T-PIT-positive in NFPAs reported in our center is close to the results of a Japanese study (26.9%) (48), but higher than the prevalence reported in previous literature (5.0-19.0%) (49). The reasons for this result were considered to be caution in diagnoses making and a decrease in the number of asymptomatic patients seeking medical care.
CCA is a rare pituitary adenoma that has received significant attention because of its aggressive nature and high recurrence rate. It should be assisted by a multidisciplinary consultation to deal with this particular type of tumor from the initial stages rather than once the recurrence has already occurred. However, the percentage of tumors immunopositive for keratin and their presentation status are confronted with clinical diversity, and very few studies involving the exploration of their pathogenesis have been performed. Therefore, more cases need to be investigated to further reveal the clinical features of CCA and its underlying mechanisms.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
DZ: literature review and draft writing; ZW: clinical data analysis; TT and XW: clinical data collection; DH and YZ: manuscript revision; DL and HW: pathological sections analysis and research design. All authors contributed to the article and approved the submitted version.
Our research was supported by the Guangzhou Science and Technology Project (grant no. 202102021116) and Sun Yat-sen University Clinical Research 5010 Program (grant no. 2016008).
Thank the members of Prof. Yonghong Zhu’s team for their help during the implementation of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.947085/full#supplementary-material
1. Di Ieva A, Davidson JM, Syro LV, Rotondo F, Montoya JF, Horvath E, et al. Crooke's cell tumors of the pituitary. Neurosurgery (2015) 76(5):616–22. doi: 10.1227/NEU.0000000000000657
2. Crooke AC. Change in the basophil cells of the pituitary gland common to conditions which exhibit the syndrome attributed to basophil adenoma. J Pathol Bacteriology (1935) 41(2):339–49. doi: 10.1002/path.1700410215
3. Felix IA, Horvath E, Kovacs K. Massive crooke's hyalinization in corticotroph cell adenomas of the human pituitary. a histological, immunocytological, and electron microscopic study of three cases. Acta Neurochir (Wien) (1981) 58(3-4):235–43. doi: 10.1007/bf01407130
4. Martin R, Cetin Y, Fehm HL, Fahlbusch R, Voigt KH. Multiple cellular forms of corticotrophs in surgically removed pituitary adenomas and periadenomatous tissue in cushing's disease. Am J Pathol (1982) 106(3):332–41.
5. Horvath E, Kovacs K, Josse R. Pituitary corticotroph cell adenoma with marked abundance of microfilaments. Ultrastruct Pathol (1983) 5(2-3):249–55. doi: 10.3109/01913128309141842
7. Franscella S, Favrod-Coune CA, Pizzolato G, Asa SL, Gaillard R, Berney J, et al. Pituitary corticotroph adenoma with crooke's hyalinization. Endocr Pathol (1991) 2(2):111–6. doi: 10.1007/bf02915332
8. Kamijo K, Sato M, Saito T, Yachi A, Minase T, Noro H, et al. An acth and fsh producing invasive pituitary adenoma with crooke's hyalinization. Pathol Res Pract (1991) 187(5):637–41. doi: 10.1016/s0344-0338(11)80162-7
9. Ikeda H, Yoshimoto T, Ogawa Y, Mizoi K, Murakami O. Clinico-pathological study of cushing's disease with Large pituitary adenoma. Clin Endocrinol (Oxf) (1997) 46(6):669–79. doi: 10.1046/j.1365-2265.1997.1741013.x
10. Coire CI, Horvath E, Kovacs K, Smyth HS, Ezzat S. Cushing's syndrome from an ectopic pituitary adenoma with peliosis: A histological, immunohistochemical, and ultrastructural study and review of the literature. Endocr Pathol (1997) 8(1):65–74. doi: 10.1007/bf02739709
11. Holthouse DJ, Robbins PD, Kahler R, Knuckey N, Pullan P. Corticotroph pituitary carcinoma: Case report and literature review. Endocr Pathol (2001) 12(3):329–41. doi: 10.1385/ep:12:3:329
12. Roncaroli F, Faustini-Fustini M, Mauri F, Asioli S, Frank G. Crooke's hyalinization in silent corticotroph adenoma: Report of two cases. Endocr Pathol (2002) 13(3):245–9. doi: 10.1385/ep:13:3:245
13. George DH, Scheithauer BW, Kovacs K, Horvath E, Young WJ, Lloyd RV, et al. Crooke's cell adenoma of the pituitary: An aggressive variant of corticotroph adenoma. Am J Surg Pathol (2003) 27(10):1330–6. doi: 10.1097/00000478-200310000-00005
14. Lopez JA, Kleinschmidt-Demasters BB, Sze CI, Woodmansee WW, Lillehei KO. Silent corticotroph adenomas: Further clinical and pathological observations. Hum Pathol (2004) 35(9):1137–47. doi: 10.1016/j.humpath.2004.04.016
15. Kovacs K, Diep CC, Horvath E, Cusimano M, Smyth H, Lombardero CC, et al. Prognostic indicators in an aggressive pituitary crooke's cell adenoma. Can J Neurol Sci (2005) 32(4):540–5. doi: 10.1017/s0317167100004583
16. Sahli R, Christ ER, Seiler R, Kappeler A, Vajtai I. Clinicopathologic correlations of silent corticotroph adenomas of the pituitary: Report of four cases and literature review. Pathol Res Pract (2006) 202(6):457–64. doi: 10.1016/j.prp.2006.01.007
17. Mohammed S, Kovacs K, Mason W, Smyth H, Cusimano MD. Use of temozolomide in aggressive pituitary tumors: Case report. Neurosurgery (2009) 64(4):E773–4. doi: 10.1227/01.Neu.0000339115.12803.4e
18. Takeshita A, Inoshita N, Taguchi M, Okuda C, Fukuhara N, Oyama K, et al. High incidence of low O(6)-methylguanine DNA methyltransferase expression in invasive macroadenomas of cushing's disease. Eur J Endocrinol (2009) 161(4):553–9. doi: 10.1530/eje-09-0414
19. Rotondo F, Cusimano M, Scheithauer BW, Coire C, Horvath E, Kovacs K. Atypical, invasive, recurring crooke cell adenoma of the pituitary. Hormones (Athens) (2012) 11(1):94–100. doi: 10.1007/bf03401542
20. Atkinson DM, Hamilton RL. A 52-Year-Old Male with visual changes. Brain Pathol (2012) 22(4):575–8. doi: 10.1111/j.1750-3639.2012.00607.x
21. Kovacs GL, Goth M, Rotondo F, Scheithauer BW, Carlsen E, Saadia A, et al. Acth-secreting crooke cell carcinoma of the pituitary. Eur J Clin Invest (2013) 43(1):20–6. doi: 10.1111/eci.12010
22. Asimakopoulou A, Tzanela M, Koletti A, Kontogeorgos G, Tsagarakis S. Long-term remission in an aggressive crooke cell adenoma of the pituitary, 18 months after discontinuation of treatment with temozolomide. Clin Case Rep (2014) 2(1):1–3. doi: 10.1002/ccr3.39
23. Sathiyabama D, Asha U, Shwetha SD, Thakkar R, Dil JS, Santosh V. Crooke's cell adenoma of the pituitary: A histological, immunocytochemical, and electron microscopic study of a rare case. Neurol India (2014) 62(2):216–7. doi: 10.4103/0028-3886.132435
24. Kurowska M, Tarach JS, Malicka J, Zielinski G, Maksymowicz M, Denew P. Long-term complete remission of crooke's corticotropinoma after temozolomide treatment. Endokrynol Pol (2016) 67(5):526–33. doi: 10.5603/ep.2016.0060
25. Giri D, Roncaroli F, Sinha A, Didi M, Senniappan S. Silent crooke's cell corticotroph adenoma of the pituitary gland presenting as delayed puberty. Endocrinol Diabetes Metab Case Rep (2017) 2017:16-0153. doi: 10.1530/edm-16-0153
26. Gilis-Januszewska A, Wilusz M, Pantoflinski J, Turek-Jabrocka R, Sokolowski G, Sowa-Staszczak A, et al. Temozolomide therapy for aggressive pituitary crooke's cell corticotropinoma causing cushing's disease - a case report with literature review. Endokrynol Pol (2018) 69(3):306–12. doi: 10.5603/EP.a2018.0011
27. Todnem N, Ward A, Segar S, Rojiani AM, Rahimi SY. Clinically silent adrenocorticotropic hormone-positive crooke cell adenoma: Case report and review of literature. World Neurosurg (2018) 119:197–200. doi: 10.1016/j.wneu.2018.07
28. Khatri KJ, Javanmard P, Pawha PS, Miller JD. Clinically silent adrenocorticotropic hormone-secreting crooke cell adenoma presenting as unilateral ear pain. Aace Clin Case Rep (2019) 5(2):e150–e3. doi: 10.4158/ACCR-2018-0347
29. Krug RN, Chang AY, Raghunathan A, Van Gompel JJ. Apoplectic silent crooke cell adenoma with adjacent pseudoaneurysms: Causation or bystander? World Neurosurg (2019) 122:480–4. doi: 10.1016/j.wneu.2018.10.232
30. Tanaka S, Yamamoto M, Morita M, Takeno A, Kanazawa I, Yamaguchi T, et al. Successful reduction of acth secretion in a case of intractable cushing's disease with pituitary crooke's cell adenoma by combined modality therapy including temozolomide. Endocr J (2019) 66(8):701–8. doi: 10.1507/endocrj.EJ18-0547
31. Dogansen SC, Bilgic B, Yalin GY, Tanrikulu S, Yarman S. Clinical significance of granulation pattern in corticotroph pituitary adenomas. Turk Patoloji Derg (2019) 35(1):9–14. doi: 10.5146/tjpath.2018.01434
32. Schwann K, Bogiatzi C, Algird A, Lu JQ. Invasive crooke cell adenoma in a patient with diffuse Large b-cell lymphoma. J Clin Neurosci (2020) 73:318–21. doi: 10.1016/j.jocn.2020.01.014
33. Cortez GM, Monteiro A, Agnoletto G, Bit-Ivan EN, Sauvageau E, Hanel RA. Aggressive pituitary tumor with crooke's cells and invasion of the posterior fossa. World Neurosurg (2020) 138:530–4.e1. doi: 10.1016/j.wneu.2020.02.137
34. Sood R, Das L, Gupta K, Kumar A, Tripathi M, Ahuja CK, et al. Crooke cell adenoma: Uncommon and aggressive variant of corticotroph adenoma. Clin Neuropathol (2021) 40(5):295-8. doi: 10.5414/np301360
35. Giraldi EA, Neill SG, Mendoza P, Saindane A, Oyesiku NM, Ioachimescu AG. Functioning crooke cell adenomas: Case series and literature review. World Neurosurg (2021) 158:e754-65. doi: 10.1016/j.wneu.2021.11.049
36. Asa SL, Mete O, Perry A, Osamura RY. Overview of the 2022 who classification of pituitary tumors. Endocr Pathol (2022) 33(1):6–26. doi: 10.1007/s12022-022-09703-7
37. Porcile E, Racadot J, Kovacs GL, Goth M, Rotondo F, Scheithauer BW, et al. [Ultrastructure of crooke cells seen in the human hypophysis during cushing's disease]. C R Acad Hebd Seances Acad Sci DActh-Secreting Crooke Cell Carcinoma Pituitary (1966) 263(14):948–51. doi: 10.1111/eci.12010
38. Neumann PE, Horoupian DS, Goldman JE, Hess MA. Cytoplasmic filaments of crooke's hyaline change belong to the cytokeratin class. an immunocytochemical and ultrastructural study. Am J Pathol (1984) 116(2):214–22.
39. Asa SL, Mete O. Cytokeratin profiles in pituitary neuroendocrine tumors. Hum Pathol (2021) 107:87–95. doi: 10.1016/j.humpath.2020.10.004
40. Saeger W, Geisler F, Ludecke DK. Pituitary pathology in cushing's disease. Pathol Res Pract (1988) 183(5):592–5. doi: 10.1016/s0344-0338(88)80018-9
41. Oldfield EH, Vance ML, Louis RG, Pledger CL, Jane JJ, Lopes MB. Crooke's changes in cushing's syndrome depends on degree of hypercortisolism and individual susceptibility. J Clin Endocrinol Metab (2015) 100(8):3165–71. doi: 10.1210/jc.2015-2493
42. Flitsch J, Ludecke DK, Knappe UJ, Saeger W. Correlates of long-term hypocortisolism after transsphenoidal microsurgery for cushing's disease. Exp Clin Endocrinol Diabetes (1999) 107(3):183–9. doi: 10.1055/s-0029-1212095
43. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C. How to classify the pituitary neuroendocrine tumors (Pitnet)S in 2020. Cancers (Basel) (2020) 12(2):514. doi: 10.3390/cancers12020514
44. Snyder MH, Shabo L, Lopes MB, Xu Z, Schlesinger D, Sheehan JP. Gamma knife radiosurgery in patients with crooke cell adenoma. World Neurosurg (2020) 138:e898–904. doi: 10.1016/j.wneu.2020.03.152
45. Hayashi K, Inoshita N, Kawaguchi K, Ibrahim AA, Suzuki H, Fukuhara N, et al. The Usp8 mutational status may predict drug susceptibility in corticotroph adenomas of cushing's disease. Eur J Endocrinol (2016) 174(2):213–26. doi: 10.1530/EJE-15-0689
46. Garbicz F, Mehlich D, Rak B, Sajjad E, Maksymowicz M, Paskal W, et al. Increased expression of the microrna 106b~25 cluster and its host gene Mcm7 in corticotroph pituitary adenomas is associated with tumor invasion and crooke's cell morphology. Pituitary (2017) 20(4):450–63. doi: 10.1007/s11102-017-0805-y
47. Zhu D, Xiao Z, Wang Z, Hu B, Duan C, Zhu Z, et al. Meg3/Mir-376b-3p/Hmga2 axis is involved in pituitary tumor invasiveness. J Neurosurg (2020), 1–13. doi: 10.3171/2019.10.JNS191959
48. Nishioka H, Inoshita N, Mete O, Asa SL, Hayashi K, Takeshita A, et al. The complementary role of transcription factors in the accurate diagnosis of clinically nonfunctioning pituitary adenomas. Endocr Pathol (2015) 26(4):349–55. doi: 10.1007/s12022-015-9398-z
Keywords: Crooke’s cell, pituitary adenoma, ACTH, Cushing disease, hyalinization
Citation: Zhu D, Wang Z, Tian T, Wu X, He D, Zhu Y, Liu D and Wang H (2022) Prevalence and clinical characteristics of Crooke’s cell adenomas in 101 patients with T-PIT-positive pituitary adenomas: Case series and literature review. Front. Endocrinol. 13:947085. doi: 10.3389/fendo.2022.947085
Received: 18 May 2022; Accepted: 03 August 2022;
Published: 19 August 2022.
Edited by:
Fabienne Langlois, Centre Hospitalier Universitaire de Sherbrooke, CanadaReviewed by:
Prashant Chittiboina, National Institute of Neurological Disorders and Stroke (NIH), United StatesCopyright © 2022 Zhu, Wang, Tian, Wu, He, Zhu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dawei Liu, bGl1ZHdlaUBtYWlsLnN5c3UuZWR1LmNu; Haijun Wang, d2FuZ2hhaWpAbWFpbC5zeXN1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.