
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 22 July 2022
Sec. Developmental Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.945543
This article is part of the Research TopicMaternal-Fetal Interface: New Insight in Placenta ResearchView all 18 articles
 Laurel Moar1†
Laurel Moar1† Chloe Simela1†
Chloe Simela1† Surabhi Nanda1,2
Surabhi Nanda1,2 Andreas Marnerides3
Andreas Marnerides3 Mudher Al-Adnani3
Mudher Al-Adnani3 Catherine Nelson-Piercy1,2
Catherine Nelson-Piercy1,2 Kypros H. Nicolaides1,4
Kypros H. Nicolaides1,4 Panicos Shangaris1,2,5*
Panicos Shangaris1,2,5*Background: Chronic histiocytic intervillositis (CHI) is a rare placental lesion with a high recurrence rate and poor perinatal outcomes. There are currently limited guidelines regarding the diagnosis of this condition in the index pregnancy and treatment where recurrence is suspected.
Objective: The primary objective of this systematic review and meta-analysis was to determine the perinatal outcomes of pregnancies affected by chronic histiocytic intervillositis and to what extent they can be improved with treatment. The secondary objective was to assess the relationship between CHI lesion severity and pregnancy loss.
Methods: A systematic search of Ovid Embase, Web of Science, Science Direct, PubMed, Ovid Medline, Google Scholar and CINAHL was carried out. Case reports, cohort, case-control and randomised controlled trials (RCT) detailing the perinatal outcomes of CHI pregnancies, both treated and untreated, were included.
Results: No RCTs were identified. However, in a review population of 659 pregnancies, with additional 7 in case reports, CHI treatments included aspirin, prednisone, prednisolone, low molecular weight heparin (LMWH), hydroxychloroquine and adalimumab. A descriptive synthesis of data found mixed results for treatments in relation to live birth, miscarriage and fetal growth restriction outcomes. Furthermore, quantitative synthesis of 38 pregnancies revealed a non-significant improvement in live birth rate with CHI targeted treatment (OR 1.79 [95% CI 0.33-9.61] (p=0.50), while meta-analysis of CHI severity in line with pregnancy loss, in a sample of 231 pregnancies, revealed lower odds of pregnancy loss with less severe lesions (OR: 0.17 [0.03-0.80], p=0.03).
Conclusions: This systematic review and meta-analysis reinforce notions surrounding the insufficient evidence for CHI treatment. It also strengthens previous hypotheses detailing the positive association between CHI lesion severity and odds of pregnancy loss. Aspirin, LMWH, prednisolone, hydroxychloroquine and adalimumab are candidates with varying levels of weak to moderate evidence supporting their use. Further prospective research is required to obtain robust evidence pertaining to treatment safety and efficacy and optimal drug regimes.
Systematic Review Registration: [website], identifier CRD42021237604
Chronic histiocytic intervillositis (CHI) (also referred as CHIV) is a rare but severe placental condition characterised by the presence of an inflammatory mononuclear infiltrate in the intervillous space. CHI has been estimated to affect 6 in every 10 000 pregnancies that reach 12 weeks gestation (1, 2) and the term was first introduced by Labarrere and Mullen in 1987 as massive chronic intervillositis (3). This pathology has since been referred to by several names across the literature, including chronic intervillositis of unknown aetiology (CIUE), massive chronic intervillositis, massive perivillous histiocytosis and shortened forms - chronic intervillositis or intervillitis. Diagnostic understanding has also grown in differentiating CHI from other placental lesions through its non-infectious origin and minimal involvement of the villi or basal plate (1). Although the lesion is well described in the placental pathology literature, there is no agreed standardised diagnostic criteria or grading system for the severity of this lesion (4–7). However, the paucity of knowledge surrounding CHI biomarkers only permits retrospective diagnosis following examination of the placenta postpartum.
Furthermore, while the aetiology of CHI remains undetermined, the pathology’s high recurrence rate, reported to lie between 70 and 100% (8), highlights the need for effective treatment in future pregnancies. Some evidence of pathogenic immune mechanisms has been proposed, including an absence of the usual Th1 to Th2 immune response shift observed in normal pregnancy (1, 9–13). This raises the question of possible therapeutic benefits for immunologically based CHI treatments, with steroids and other immunosuppressants having been used in some cases.
This systematic review summarises the available research on treatment approaches for gravid women diagnosed with CHI in a previous pregnancy, including antithrombotic and immunosuppressive drug combinations. Primary objectives include quantifying the effectiveness of these therapies in improving perinatal outcomes compared to baseline data without treatment. Moreover, further analysis was undertaken to determine how CHI severity impacts perinatal outcomes.
PROSPERO registration for the review was fulfilled following the completion of the protocol form (CRD42021237604).
The literature search was carried out by the two reviewers, CS and LM, across seven databases: PubMed, OVID MEDLINE, Google Scholar, OVID EMBASE, Web of Science, Science Direct and CENTRAL (Cochrane Central Register of Controlled Trials) between 15th February and 16th February 2021. Each reviewer used the search strategy (1) comprising all known terms for CHI and a period spanning from Jan 1990 to December 2020, in three databases. CENTRAL was searched by both reviewers. Formatting adjustments were made to the search terms to optimise the strategy for each database, and email alerts were put in place to notify the reviewers of new results after the search period (Table 1). The two reviewers screened the search results independently, and discussions were held at both screening stages to resolve any disagreements before the final papers were decided.
Selection criteria were based on the following predetermined characteristics from the protocol, starting with the population in question being identified as pregnant women of any age diagnosed with CHI in a previous pregnancy. CHI was defined as an idiopathic inflammatory lesion in the intervillous space in line with the criteria devised by Bos and colleagues (4). All other similar but distinct placental lesions were excluded, including villitis, even if in co-occurrence with CHI. Interventions described included varying doses of drug-based mono or combination therapy for CHI. However, studies without interventions were also included for comparison. Outcomes assessing the efficacy of treatment and impact of disease severity included, but were not limited to, quantitative measures such as live birth rate and birth weight. Only randomised controlled trials, cohort, case-control studies and case reports were included, with abstracts excluded. While there were no geographical limits, time constraints enforced excluding texts not in English.
A modified Cochrane Public Health Group (CPHG) data extraction form was used to collect population, intervention and outcome data from the chosen papers independently by both reviewers (C.S and L.M). Additionally, each structure underwent a data checking process against the original article to detect and minimise human error. The specific information collected for each study included authors, publication year; study design, location; the number of women, number of CHI pregnancies, and number undergoing treatment. Authors were contacted to confirm information regarding population data where clarification was needed (14, 15).
Outcome measures reported in the systematic review included growth restriction and preterm birth, while those of interest in the meta-analysis were live birth rate and pregnancy loss. The preterm birth rate was defined as a live birth before 37 + 0 weeks gestation. Fetal growth restriction (FGR) or intrauterine growth restriction (IUGR) was defined as a birth weight below the 10th percentile (16).
Several terms were used when determining rates of pregnancy loss in CHI. In this paper, pregnancy loss data were divided into early miscarriage (before 14 + 0 weeks gestation), late miscarriages (14 + 1 to 24 + 0 weeks gestation) and stillbirths (intrauterine death after 24 + 0 weeks gestation). Some studies also included neonatal deaths as an outcome, where this term described the loss of an infant within seven days of birth (17).
In some cases (such as with FGR), there was variation in how outcomes were classified (e.g., birth weight below 10th percentile versus 3rd percentile, stillbirth including pregnancy loss as early as 20 weeks versus after 24 weeks) in different studies. Such instances of outcome measure variation are indicated in the review.
The extent of intervillous space involvement defined CHI lesion severity as a quantifiable and standard measure. In this case, low to moderate grading referred to infiltrate occupying less than 50% of the intervillous space, and severe grading infiltrate more than 50% of the intervillous space [Parant (18), Simula (6), Sauvestre (15) but not Marchaudon (19)].
The risk of bias and quality of included studies was assessed independently by both reviewers using adapted versions of the Newcastle-Ottawa scale (20) and Critical Appraisal Skills Programme (CASP) checklist (21).
The 7-point system of the Newcastle Ottawa scale contained domains of selection, comparability, and outcome, while the CASP checklist aimed to detect instances of selection, measurement, classification or reporting bias. Additional consideration was given to the lack of accountability for confounding factors.
The Cohen kappa coefficient was used to assess agreement between the two reviewers at the full-text eligibility stage before generating a narrative synthesis of the findings. A high ratio indicated a reasonable level of agreement between the reviewers.
Inclusion in the meta-analysis component depended on cohort studies with a suitable differentiation of outcomes according to the presence of treatment or severity of lesions. The chosen effect measures for the meta-analyses were relative risk (RR) of pregnancy loss in moderate vs severe CHI and odds ratios (OR) for live births in treated vs untreated pregnancies, each with a 95% confidence interval and calculated based on the number of pregnancies and events. All analyses herein were carried out using RevMan (Review Manager (RevMan) Version 5.4.1, The Cochrane Collaboration 2020). Heterogeneity was measured using the I2 statistic, with an I2 >50% indicating significant heterogeneity, not due to chance. As recommended, random-effects models were used to determine the summary effect estimate where considerable heterogeneity was detected (I2>30%) (22).
Due to the small meta-analysis population, no additional sensitivity, subgroup, or meta-regression analyses were undertaken.
Figure 1 summarises (23) the study selection process and its outcomes. In total, 805 papers were found, and of these, 384 remained for the title and abstract screening once duplicates were removed. Titles and abstracts were excluded for review format, language limitation and lack of relevance leading to 66 full texts, which were narrowed down to the final 20 for systematic review inclusion. Reasons for exclusion included irrelevance, language limitation, conference abstract, poster or thesis format, full-text unavailability, and data insufficiency.
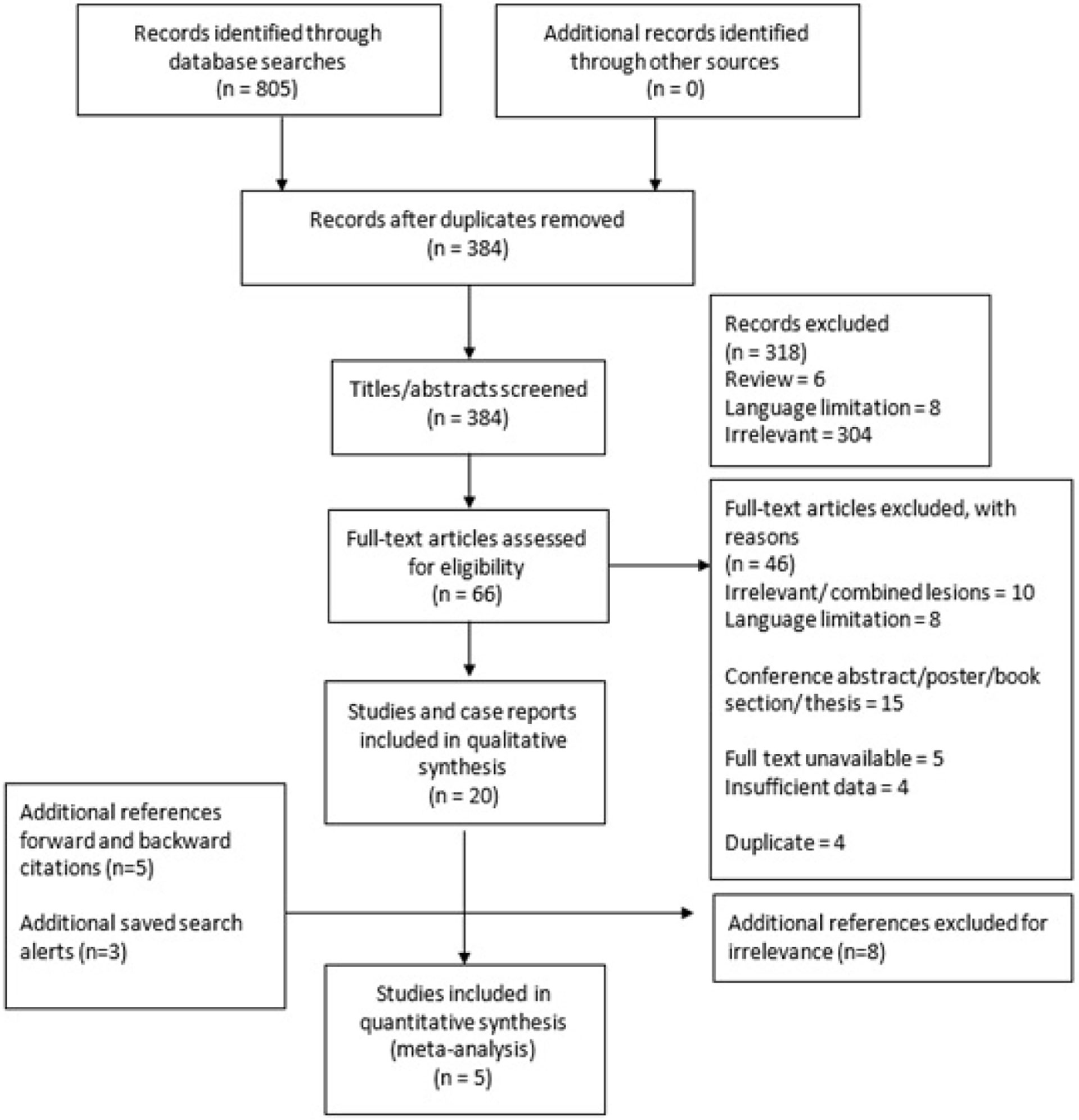
Figure 1 Study selection process of the systematic review and meta-analysis into outcomes of pregnancies affected by CHI.
The Cohen kappa coefficient for full-text screening between the two reviewers was 0.81, indicating almost perfect agreement at this stage of study selection.
The final twenty papers comprised 12 cohort studies and 8 case reports as no RCTs were identified. The characteristics of these mainly retrospective studies and case reports are summarised in Table 2. Five of the twelve cohort studies were selected for inclusion in either meta-analysis. The limited number of studies selected for meta-analysis was due to treated vs untreated outcomes being unavailable for pooling in the other seven studies.
Excluding case reports and series, the review population included the outcomes of 527 pregnancies affected by CHI in 439 women with a mean age of approximately 31.9 years. Fifty-eight of these cohort study pregnancies were treated, and sample size ranged from 6 to 122 pregnancies. A further 6 instances of treatment were reported in case reports and series. In total, 64 pregnancies were treated out of 554 across cohort studies, case reports and case series.
Email correspondence with authors confirmed a shared cohort between two papers, and this is highlighted in all subsequent results tables to avoid duplication and reporting errors. For review population totals, these cohorts have been counted as one (14, 15).
The risk of bias scores for each case-control and cohort study can be seen in Table 3. Following a judgement by both reviewers, the risk of bias was considered generally low, with an average bias score of 1.83 out of 4 across the twelve studies.
Selection bias was reported in four of the twelve studies due to how participants were selected from a pathology database (15, 18, 37, 39). Furthermore, in other cases, selection bias was detected because of limited search terms comprising only of ‘CIUE and chronic intervillositis’ in one study and ‘intervillositis’ in another (15, 39).
Seven studies indicated measurement/classification bias in diagnosis and grading of CHI, whereby pathologists were aware of and not blinded to the previous CHI diagnosis.
Reporting bias was undetected. However, ten out of twelve studies did not state accountability for confounding variables.
Study quality assessment outcomes are shown in Table 4. High scores were obtained in the selection domain, with participants seen as broadly representative of the population in question. The area in which numerous studies were deficient was comparability and outcome measures. Lack of differentiation between early and late miscarriage outcomes and methods that did not describe the assessment of lesion severity as a possible mediator of perinatal outcome was also suboptimal. Furthermore, lack of information on population comorbidities or medication also led to lower quality scores in the outcome domain.
None of the cohorts included in the systematic review was fully treated. The proportion of the four cohorts receiving targeted treatment for CHI ranged from 18-88%, and the other eight cohorts were labelled as untreated. Table 5 summarises the treatment combination details in the treated pregnancies.
There was a variation of gestational ranges when the treatment was commenced. Those containing aspirin were the most common of the treatment regimes, followed by low molecular weight heparin (LMWH) and finally corticosteroid regimes (prednisone/corticoid), either alone or in combination.
Table 6 summarises the perinatal outcomes of these studies. Continuous variables are reported as means followed by the standard deviation and range in brackets if available.
The live birth rate varied considerably between studies, ranging from 30.4% to 100%. Interestingly, neither of the cohorts displaying results at these extremes were indicated as receiving target CHI treatment (19, 40). There was also variance within similar populations, as several of the papers were based within the same countries yet yielded different live birth rates for example the study by Bos et al., 2020 reported a live birth rate more than twice as high as that reported by Reus et al., 2013 (11, 37). Comparable live birth rates of 67% and 70% were observed in two studies with a similar cohort size despite 88% of one cohort receiving targeted treatment (25) versus none of the other (27). Overall, the average live birth rate for cohorts in which no treatment was stated was 58.3% versus 40.9% in cohorts with a partial treatment.
Data on average live birth weight were available in seven studies, three of which had a proportion receiving treatment. In most cases, this outcome rarely reached 2500g however, the highest mean birth weight of 2493g was achieved in one study in which 88% of the cohort received treatment (25). Furthermore, a low average birth weight of 995g was reported by Traeder and colleagues (40) across a case series of four untreated pregnancies. However, this positive treatment effect was not always reflected, with higher birth weight observed in untreated cohorts (19, 27, 28) compared to partially treated cohorts (18, 38).
In five out of six studies, a low average birth weight was accompanied by an average gestational age at delivery <37+0 weeks. The general trend towards high preterm birth in both untreated and treated CHI pregnancies is also evident in a preterm birth rate of 40.2% across the twelve studies. Interestingly, the study by Mekinian and colleagues in which the largest proportion of the cohort was treated (88%) had a lower preterm birth rate of 31.25% (25). However, this was not the case for all partially treated cohorts, which, for the most part, had a lower mean gestational age at delivery (38) and higher preterm live birth rate than the untreated cohort (19, 27, 28).
Many infants were small for gestational age. Studies reported fetal growth restriction of below 10th centile (with some even below 3rd centile) in both live and stillborn or neonatal death groups.
Similar prevalence of FGR < 10th centiles was reported in two studies (66.7% and 69.7%, respectively), both of which had entirely untreated cohorts (27, 31). In treated cohorts, this same variable ranged from 12.5% (25) to 72.7% (18)19 with treated proportions being 88% and 42.8%, respectively.
Severe FGR, <3rd centile, was only reported as an outcome in 5 studies (19, 31, 37, 40). Of these, one study had an 18.9% treated cohort with a severe FGR prevalence of 55.9% (38). Four untreated study cohorts have reported an occurrence rate of severe FGR as 69.7%, 42.1%, 34.8% and 50% (19, 31, 37, 40) respectively.
Overall, miscarriage rates ranged between 2.63% and 20% in untreated cohorts (11, 37). The miscarriage rate was much wider in treated cohorts (range between 5.4% and 75%). In some cases, miscarriage timing was before 14 weeks rather than after 14 weeks, but this was only in five out of the twelve cohorts (11, 15, 18, 28, 39).
Stillbirth rates varied between 10.7% and 19.8% in treated pregnancies (38, 39). Like miscarriage rates, this was comparatively higher than the untreated cohort range of 7.9% to 13.1% (15, 22). Interestingly, there were also instances of no reported stillbirths in untreated populations with small sample sizes (28, 40).
Neonatal death was not widely reported as an outcome and ranged from 1.11% up to 23.3% across four studies (11, 14, 15, 38). There are no reported cases in both partially treated and untreated cohorts (18, 27).
As shown in Table 7, individual patient outcomes were available from seven case reports, one case series, and two retrospective cohort studies that reported individual patient details. 21 of the 31 case pregnancies were treated, and 9 of these resulted in a live birth, of which 4 were at term. This is compared to 6 out of 10 untreated pregnancies resulting in live births and 1 of these 6 occurring at term.
Meta-analysis of live birth outcomes in two studies in which treated and untreated data was available revealed a non-significant improvement in live birth rates with treatment (Odds Ratio: 1.79 [0.33-9.61], p=0.50) (Figure 2). The pooled population included 27 treated and 11 untreated pregnancies. Heterogeneity (I2) was estimated to be 6%, using fixed effect model.
Meta-analysis of pregnancy loss in relation to CHI severity across four studies with a pooled population of 174 low to moderate severity cases and 84 severe cases indicated significantly lower odds of pregnancy loss in cases with less severe lesions vs those with increased severity (Odds Ratio: 0.17 [0.03-0.80], p=0.03) (Figure 3). Largely homogenous grading criteria in all four studies permitted low to moderate lesions to be defined as <50% intervillous infiltrate involvement and >50% leading to a severe classification (supplementary data file).
The primary objective of this systematic review and meta-analysis was to identify and quantify the effectiveness of current treatment regimens for pregnant women diagnosed with CHI following a previous pregnancy. Effectiveness was quantified by comparing perinatal outcomes, including live birth, miscarriage, stillbirth rates and neonatal death in treated and untreated pregnancies, birth weight (normal, IUGR), as well as preterm birth rate, with a positive effect in such measures indicating effective treatment.
The commonly used treatment regimens for CHI, either as standalone or as combination therapy include aspirin, prednisone, low molecular weight heparin, biological agents most commonly adalimumab and hydroxychloroquine (Table 8). The partially treated cohorts often performed equally or even worse in the outcome domains of live birth rates, preterm birth, fetal growth restriction, miscarriage and stillbirth rates. Indeed, the pooled treatment effect for live birth rates in treated vs untreated pregnancies produced an odds ratio of 1.79 [95% CI 0.33-9.61] (p=0.50). This converts to a risk ratio of 1.4 [95% CI 0.48-4.05] (p=0.54), which reveals the likelihood of live births in the treated group was 1.4 times that in the untreated, although this was not statistically significant. Taken collectively, this suggests that antithrombotic and immunosuppressive treatment for CHI cannot be significantly effective in improving perinatal outcomes in affected pregnancies. However, classifying these results as clinically insignificant due to exceeding the arbitrary cut off p=0.05 would be misleading as such a small, pooled population of 27 treated and 11 untreated pregnancies suggests a degree of imprecision. Consequently, we can conclude that these findings are inconclusive, and there is insufficient evidence of a treatment effect, as opposed to proof of no treatment effect (34).
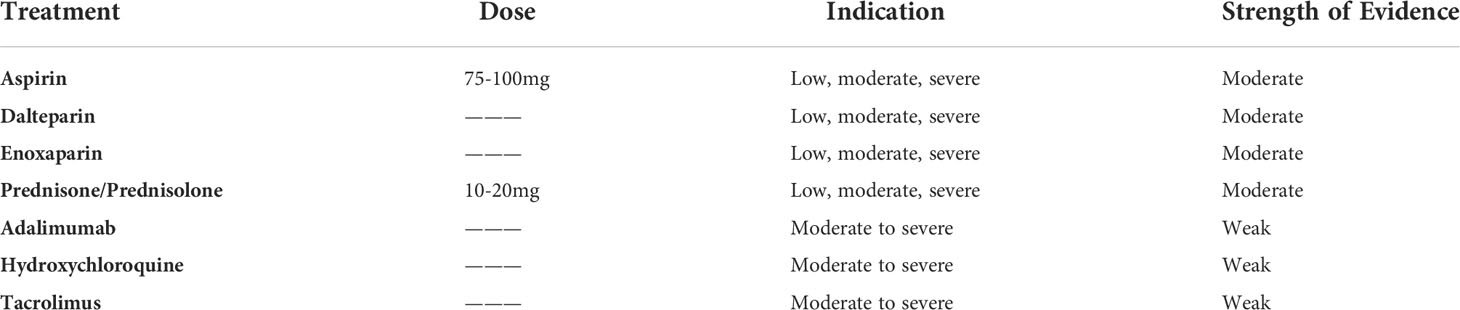
Table 8 Summary of CHI treatments, their indication in mild, moderate, or severe cases and the strength of evidence surrounding each.
The secondary objective of this review was to determine the extent to which CHI severity – measured as a percentage of intervillous space occupied by infiltrate- impacts perinatal outcomes. It was anticipated that establishing a relationship between progression of placental pathology, in subsequent pregnancies, and extent of adverse outcomes would improve understanding of treatment potential as seen by the effect of treatment (monotherapy or combination therapy) on the placental tissue. This in turn would help to explore if improvements in lesions (with or without complete remission) positively correlate with more favourable perinatal outcomes. Results demonstrate a small severity effect in relation to pregnancy loss (Odds Ratio: 0.17 [0.03-0.80], (p=0.03). The larger pooled population of 174 low or moderately affected pregnancies and 84 pregnancies affected with severe CHI infers a more reliable evidence that any improvement in the severity of lesions correlates to a reduction (albeit small) in the odds of miscarriage or stillbirth.
The proposed hypothesis around the effect of treatment regimens is the reduction in the severity of lesions through targeted anti-inflammatory, immunosuppressive and anti-thrombotic mechanisms, which in turn would help improve the perinatal outcome.
The discrepancies in severity classification systems between papers cannot be ignored. It is known that CHI can exist with or without fibrin deposition and so while it is not necessary for diagnosis, the role of fibrin in severity classification systems is interesting. While in some, fibrin was acknowledged, this was not the case in studies with methods that did not call for fibrin to be noted (19, 39). Table 9 illustrates the varying inclusion and exclusion criteria for CHI classification in all the studies included.
In retrospective studies, it was not possible for all placentas in the database to be examined for CHI. Therefore, many relied on the assumption that CHI placentas had accurately been documented as such and the search terms used to recover them were adequate (38).
Furthermore, there was an invariable absence of adjustment for confounders known to increase risk of adverse perinatal outcomes such as maternal BMI and smoking which at times, were not measured during the process of data gathering in some cases or noted in many of the case reports.
Overall, the findings of this review were consistent with those previously available evidence. For instance, the strong association between FGR, miscarriage, and stillbirth reflected in Table 6 (2). FGR frequency <10th percentile, was estimated at 61% across nine studies in our review population compared to 48% in a previous meta-analysis (35). The total population in our review did not confirm a higher prevalence of early miscarriage compared to late, as was seen by Rota and colleagues (5), however it was found that there was a higher rate of intrauterine deaths in the remit of early miscarriage rather than stillbirth or late miscarriage. Nevertheless, the relatively high incidence of either early or late pregnancy loss emphasises the impact of placental insufficiency without maternal vascular malperfusion on increased adverse pregnancy outcomes.
Interestingly, our conclusion that increased CHI lesion severity correlates with increased risk of pregnancy loss disagrees with the findings of the most prominent prospective study in the area (25) which states that maintenance of visible CHI pathology is not always indicative of adverse outcomes. However, this study did find that the absence of these lesions was linked to more positive outcomes. Further investigation may be required to find a more definitive answer. It is worth acknowledging here that there has previously been a lack of international consensus on indications for sending placental pathology (and for reporting by perinatal pathologists), following an adverse pregnancy outcome, and therefore CHI itself may be under-reported. However, there has been a reform in this area more recently due to the creation of the international Amsterdam consensus criteria in 2016 (36).
The lack of significant treatment effects in CHI, though cautiously interpreted by our review, was also highlighted by Contro et al (41). This 2010 review on CHI concluded that treatments investigated at the time had no significant, and even a detrimental, effect on perinatal outcome. Our review provides evidence supporting this conclusion, despite numerous novel studies conducted in the 10-years since the last systematic review (41). However, one of the explanations for this may be a lack of consensus about the case selection for treatment, gestation of commencing treatment (and stopping), and treatment regimens (monotherapy or combination therapy). Further explanation for individual worse outcomes in combination therapy pregnancies has been highlighted by Mekinian et al., pertaining to confounding by indication and severity – whereby pregnancies with worse prognoses (maternal history of previous IUD) are targeted with combination treatment, while those with prognostically better outcomes are not (25).
Historically, there has been a lack of evidence around the generic use of immunosuppressive therapies in the management of recurrent pregnancy loss (42). A previous study, albeit with suboptimal case selection and design, highlighted a possible association between first-trimester prednisone exposure and cleft lips and palates in infants (43). This has since been disputed (44), and there is considerable experience in the use of oral steroids for many a condition in pregnancy, including lupus, transplants, severe asthma, etc. Nevertheless, long term use of steroids has been linked with increased preterm delivery rates (secondary to preterm prelabour rupture of membranes), neonatal intensive care admission and low birth weights in the neonates, and increased chance of maternal dependence on steroids and steroid induced diabetes (and its associated complications) (45–47). While it is not clear whether any of these adverse outcomes were documented as a result of oral steroids like prednisone in our review studies, its use in the management of pregnancies previously affected by CHI as a part of off-label treatment regimes in the UK, in order to optimise pregnancy outcome (improve live birth rate, and prolong gestation at delivery), is still surrounded by controversy (48).
Antithrombotic therapy is relatively effective in treating pregnancy loss, particularly in those with associated antiphospholipid syndrome (42, 46). A recent Cochrane review concluded that combined treatment of aspirin with heparin is more likely to lead to higher birth rates in women with recurrent pregnancy loss associated with antiphospholipid antibodies, compared to aspirin alone (49). Though still yet to be confirmed, this offers a potential means of treating the proposed antibody mediated and pro-inflammatory pathophysiology of CHI. A recent review drew upon the application of immunomodulatory drugs in autoimmune conditions during pregnancy to emphasise the safety and merit of hydroxychloroquine in CHI (50).
Additionally, CHI has been associated with maternal hypertensive disorders in some studies but evaluating this was outside the remit of this paper (8). Antithrombotic therapy such as aspirin is also cited by The American College of Obstetricians and Gynaecologists as appropriate in the prevention of fetal growth restriction, which in some cases is due to placental insufficiency (16, 51). Hence, despite further research being warranted, the practice of the use of aspirin and heparin in the management of pregnancies affected by CHI, is not discordant with current practice. Anti-thrombotic drugs such as unfractionated and low molecular weight heparin, adjunctive low dose aspirin (with or without other immunosuppressive agents such as hydroxychloroquine, azathioprine, adalimumab, tacrolimus) currently form the basis of off-label CHI treatment in the UK (48). A 2021 study by Brady and colleagues has however offered some promising results in the application of hydroxychloroquine and prednisolone (in conjunction with mainstay aspirin and heparin) in improving CHI lesion severity and bringing about a 62.3% reduction in subsequent pregnancy loss (52). The study reported lower FGR, preterm, stillbirth and neonatal death rates in treated pregnancies although statistical significance was restricted by the small sample size (52).
Co-occurrence of placental lesions was encountered as frequently as 30% in one study and 25% in another (5, 18). While these cases were beyond the scope of the review, the reality of coexisting pathology is worth recognising (50). In one study, combined lesions formed a considerable proportion of placental samples (35%). Although these were excluded from our data, the debate on viewing combined lesions as separate entities continues (27). The application of aspirin and corticosteroids in a pregnancy affected by both villitis and CHI was documented as having a positive outcome in one case report (53). It is questionable whether the same treatment effect would be observed in pregnancies affected by other placental lesions adjunct to CHI, and further research into this should be encouraged. We stress the value of a multidisciplinary discussion involving perinatal pathologists in such cases, where there are such services available, to have a case-based consensus of treatment regimes (36).
This is a rare condition. As discussed above, there is a lack of consensus and guidance on indications for placental pathology following pregnancy loss or adverse pregnancy outcomes. Not all placental pathology is assessed by perinatal pathologists. Therefore, it can be acknowledged that the actual incidence of CHI is underreported. There is only a small amount of literature on CHI, and it is unlikely that any relevant studies were omitted through the search strategy. It has been noted that observational study titles can be misleading and require full-text screening for relevance (22). The manageable number of search results enabled manual screening and several checking processes to be carried out to ensure that no relevant studies were missed. Saved search alerts permitted new papers released after the search period to be identified and screened equally.
However, it must be acknowledged that there was an enforcement of an English-language criterion due to time constraints, and it is possible that this introduced a level of selection bias to this systematic review and meta-analysis.
Lastly, not all relevant data, i.e., treated vs untreated outcomes, appeared to be included in studies, and although raw data were requested through email correspondence, this was not available. As a result, only 38 untreated and treated cases across two studies could be included in the meta-analysis for treatment effect concerning live birth rates specifically, and while data from individual studies appeared to favour untreated cohorts, pooled data in the meta-analysis revealed a non-significant improvement in treated outcomes may suggest publication bias. This is likely due to the exclusion of multiple studies from the meta-analysis. There was a lack of data distinguishing treated vs untreated outcomes in all these other partially treated cohorts (18, 25).
Associated maternal outcomes like hypertensive disorders in pregnancy and treatment-related adverse effects like steroid-induced diabetes have been noted in case reports and from experience with our group. However, evaluating this was outside the remit of this review (54).
This review examined the outcomes of 554 pregnancies, 64 of which were treated by either aspirin, prednisolone or LMWH alone or in conjunction. Additional therapies included hydroxychloroquine and adalimumab. Based on efficacy being defined as significantly reducing the prevalence of adverse perinatal outcomes in affected pregnancies, the findings have further strengthened the available evidence that there is no known effective treatment for CHI in pregnancy. The paucity of research using comparison groups in a case-control design has led to challenging analysis and equivocal results. Gaining a deeper insight into novel, effective therapeutics will require international collaboration due to the rare nature of this pathology.
Current therapies for the general treatment of recurrent pregnancy loss form a basis for building these next steps for CHI treatment. Existing recommendations support the use of antithrombotic therapies more than immunosuppressants. The likely need for combination therapy in the event of previous IUFD has been highlighted (25), and this review showed the general value of combination therapy in current practice. Novel therapies not previously reviewed, such as adalimumab and hydroxychloroquine, warrant further research as uncertainty surrounding their efficacy and safety has led to their application only informally recommended in moderate to severe cases.
Compared to other lesions, the high recurrence rate and more severe perinatal outcomes associated with CHI underscore the value of a precise differential diagnosis. Further research may permit a stepwise approach utilising aspirin, heparin, and hydroxychloroquine to form the basis of individualised treatment plans with cost/benefit analysis in line with the previous pregnancy outcomes and multidisciplinary treatment counselling.
Fundamentally, research needs to address the aetiology of CHI to gain a complete understanding of possible ways to treat it. Once this is achieved, observational studies are of value, and even if still retrospective, augmentation of database results with additional hospital data will help account for confounding. Our review only identified one complete and one ongoing prospective multi-centre study. A prospective registry of women with pregnancies previously affected by CHI, involving non-profit organisations like CHI support (48), who may provide ongoing support to these families, may help with a holistic multidisciplinary approach to the management of these pregnancies and improve our understanding of this rare pathology. Equally, a prospective multi-centre design should be the objective model of future studies with additional outcome measures such as Apgar Scores and NICU admissions and later achievement of neurodevelopmental milestones.
Interventions should include, but not be limited to, aspirin, prednisone, and heparin, with or without other immunosuppressive agents such as hydroxychloroquine, azathioprine, adalimumab, tacrolimus). Researchers should be attentive to side effects caused by therapies and their comparative effectiveness in combination or alone. This will help develop future guidelines for CHI treatment, and hopefully, long-term follow-up data regarding childhood outcomes in untreated versus treated CHI pregnancies will also become available.
The best effort to improve pregnancy outcomes in pregnancies associated with CHI is surveillance and identification of screening tools in the index pregnancy, where CHI is suspected. Equally, where CHI has been previously identified, screening tools may help increase surveillance and tailor treatment modalities and regimens. Use of first-trimester markers (55, 56) like placental growth factor or second-trimester markers like alkaline phosphatase, ultrasound markers like increased uterine artery pulsatility index and reduction in amniotic fluid in the third trimester, and use of fetal placental MRI using diffusion imaging have all been reported either alone or in conjunction with each other. Although a single sufficiently specific biomarker is yet to be identified (31), further research into these potential prognostic biomarkers would be invaluable (54) in reducing the prevalence of adverse perinatal outcomes in this pathology by permitting earlier intervention and better treatment.
PS, LM and CS conceptualized the topic and structure of the systematic review. LM and CS drafted and revised the manuscript. SN, AM, MA-A, CN-P, KN and PS provided expert opinion, edited, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
PS is funded by an NIHR Clinical Lectureship (CL-2018-17-002). This study was funded by the Fetal Medicine Foundation (KHN) (registered charity 1037116), National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St Thomas’ National Health Service Foundation Trust and King’s College London (IS-BRC-1215–20006). The views expressed in this Article are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.945543/full#supplementary-material
1. Boyd TK, Redline RW. Chronic histiocytic intervillositis: A placental lesion associated with recurrent reproductive loss. Hum Pathol (2000) 31:1389–96. doi: 10.1016/S0046-8177(00)80009-X
2. Brady CA, Williams C, Sharps MC, Shelleh A, Batra G, Heazell AEPP, et al. Chronic histiocytic intervillositis: A breakdown in immune tolerance comparable to allograft rejection? Am J Reprod Immunol (2021) 85:e13373. doi: 10.1111/aji.13373
3. Labarrere C, Mullen E. Fibrinoid and trophoblastic necrosis with massive chronic intervillositis: An extreme variant of villitis of unknown etiology. Am J Reprod Immunol Microbiol (1987) 15:85–91. doi: 10.1111/j.1600-0897.1987.tb00162.x
4. Bos M, Nikkels PGJ, Cohen D, Schoones JW, Bloemenkamp KWM, Bruijn JA, et al. Towards standardized criteria for diagnosing chronic intervillositis of unknown etiology: A systematic review. Placenta (2018) 61:80–8. doi: 10.1016/j.placenta.2017.11.012
5. Rota C, Carles D, Schaeffer V, Guyon F, Saura R, Horovitz J. Pronostic périnatal des grossesses compliquées d’intervillites chroniques placentaires. J Gynécologie Obs Biol la Reprod (2006) 35:711–9. doi: 10.1016/S0368-2315(06)76468-7
6. Simula NK, Terry J, Kent NE, Robertson J, Purkiss S, Bedaiwy MA. Chronic intervillositis of unknown etiology (CIUE): a cause for reproductive failure. Fertil Steril (2019) 112:e402. doi: 10.1016/j.fertnstert.2019.07.1144
7. Ongaro D, Terry J. Reproducibility of grading in chronic intervillositis of unknown etiology. Pediatr Dev Pathol (2020) 23:210–4. doi: 10.1177/1093526619882522
8. Lee AX, Tan BRY, Kho CL, Tan KT. Chronic histiocytic intervillositis (CHI): an under-recognised condition with potential serious sequelae in pregnancy. BMJ Case Rep (2021) 14:e241637. doi: 10.1136/bcr-2021-241637
9. Doss BJ, Greene MF, Hill J, Heffner LJ, Bieber FR, Genest DR. Massive chronic intervillositis associated with recurrent abortions. Hum Pathol (1995) 26:1245–51. doi: 10.1016/0046-8177(95)90201-5
10. Salafia C, Cowchock F. Placental pathology and antiphospholipid antibodies: A descriptive study. Am J Perinatol (1997) 14:435–41. doi: 10.1055/s-2007-994176
11. Reus AD, van Besouw NM, Molenaar NM, Steegers EAP, Visser W, de Kuiper RP, et al. An immunological basis for chronic histiocytic intervillositis in recurrent fetal loss. Am J Reprod Immunol (2013) 70:230–7. doi: 10.1111/aji.12125
12. Labarrere CA, Bammerlin E, Hardin JW, DiCarlo HL. Intercellular adhesion molecule-1 expression in massive chronic intervillositis: Implications for the invasion of maternal cells into fetal tissues. Placenta (2014) 35:311–7. doi: 10.1016/j.placenta.2014.02.006
13. Bendon RW, Coventry S, Thompson M, Rudzinski ER, Williams EM, Oron AP. Significance of C4d immunostaining in placental chronic intervillositis. Pediatr Dev Pathol (2015) 18:362–8. doi: 10.2350/14-12-1582-OA.1
14. Mattuizzi A, Sauvestre F, André G, Poingt M, Camberlein C, Carles D, et al. Adverse perinatal outcomes of chronic intervillositis of unknown etiology: an observational retrospective study of 122 cases. Sci Rep (2020) 10:12611. doi: 10.1038/s41598-020-69191-9
15. Sauvestre F, Mattuizzi A, Sentilhes L, Poingt M, Blanco P, Houssin C, et al. Chronic intervillositis of unknown etiology. Am J Surg Pathol (2020) 44:1367–73. doi: 10.1097/PAS.0000000000001549
16. Suhag A, Berghella V. Intrauterine growth restriction (IUGR): Etiology and diagnosis. Curr Obstet Gynecol Rep (2013) 2:102–11. doi: 10.1007/s13669-013-0041-z
17. MacGregor B, Shakespeare J, Kotnis R, Knight M, Hillman S. MBRRACE 2021: preventing maternal deaths — we are all part of the solution. Br J Gen Pract (2022) 72:148–9. doi: 10.3399/bjgp22X718829
18. Parant O, Capdet J, Kessler S, Aziza J, Berrebi A. Chronic intervillositis of unknown etiology (CIUE): Relation between placental lesions and perinatal outcome. Eur J Obstet Gynecol Reprod Biol (2009) 143:9–13. doi: 10.1016/j.ejogrb.2008.06.012
19. Marchaudon V, Devisme L, Petit S, Ansart-Franquet H, Vaast P, Subtil D. Chronic histiocytic intervillositis of unknown etiology: Clinical features in a consecutive series of 69 cases. Placenta (2011) 32:140–5. doi: 10.1016/j.placenta.2010.11.021
20. Luchini C, Stubbs B, Solmi M, Veronese N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa scale. World J Meta Analysis (2017) 5:80. doi: 10.13105/wjma.v5.i4.80
21. Long HA, French DP, Brooks JM. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res Methods Med Heal Sci (2020) 1:31–42. doi: 10.1177/2632084320947559
22. Lefebvre C, Glanville J BS, et al. Cochrane handbook for systematic reviews of interventions version 6.2. cochrane; 2021 (2021). Available at: https://training.cochrane.org/handbook.
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
24. Ramya T, Chaitra V UG. Chronic histiocytic intervillositis- a rare placental cause of poor obstetric outcome: a clinicopathological study and literature review. Int J Reprod Contraception Obstet Gynecol (2014) 3:1146. doi: 10.5455/2320-1770.ijrcog20141258
25. Mekinian A, Costedoat-Chalumeau N, Masseau A, Botta A, Chudzinski A, Theulin A, et al. Chronic histiocytic intervillositis: Outcome, associated diseases and treatment in a multicenter prospective study. Autoimmunity (2015) 48:40–5. doi: 10.3109/08916934.2014.939267
26. Crawford A, Moore L, Bennett G, Savarirayan R, Manton N, Khong Y, et al. Recurrent chronic histiocytic intervillositis with intrauterine growth restriction, osteopenia, and fractures. Am J Med Genet Part A (2016) 170:2960–4. doi: 10.1002/ajmg.a.37856
27. Nowak C, Joubert M, Jossic F, Masseau A, Hamidou M, Philippe H-JJ, et al. Perinatal prognosis of pregnancies complicated by placental chronic villitis or intervillositis of unknown etiology and combined lesions: About a series of 178 cases. Placenta (2016) 44:104–8. doi: 10.1016/j.placenta.2016.04.017
28. Sabra S, Zurriaga CR, Saborit A, Gómez Roig MD. A series of rare chronic histiocytic intervillositis cases and its association with fetal growth restriction. Gynecol Obstet Res - Open J (2016) 3:26–31. doi: 10.17140/GOROJ-3-133
29. Ozawa N, Yamaguchi K, Shibata M, Sugibayashi R, Yagi H, Sago H, et al. Chronic histiocytic intervillositis in three consecutive pregnancies in a single patient: Differing clinical results and pathology according to treatment used. J Obstet Gynaecol Res (2017) 43:1504–8. doi: 10.1111/jog.13404
30. Vardi L, Paterson H, Hung NA. Successful pregnancy following treatment of recurrent chronic histiocytic intervillositis. BMJ Case Rep (2017) 2017:bcr2016217886. doi: 10.1136/bcr-2016-217886
31. Koby L, Keating S, Malinowski AK, D’Souza R. Chronic histiocytic intervillositis - clinical, biochemical and radiological findings: An observational study. Placenta (2018) 64:1–6. doi: 10.1016/j.placenta.2018.02.002
32. Mekinian A, Houfflin-Debarge V, Kolanska K, Cohen J, Abisror N, Bornes M, et al. Antagonists of TNFalpha for recurrent miscarriages: 2 illustrative cases. Eur J Obstet Gynecol Reprod Biol (2019) 236:263–4. doi: 10.1016/j.ejogrb.2019.02.036
33. Nohr EW, Wright JRJ EWN, Wright JR. Diffuse subamniotic calcification: A novel pattern of placental calcification. Pediatr Dev Pathol (2020) 23:438–42. doi: 10.1177/1093526620957843
34. Alderson P. Research pointers: Survey of claims of no effect in abstracts of cochrane reviews. BMJ (2003) 326:475–5. doi: 10.1136/bmj.326.7387.475
35. Mekinian A, Costedoat-Chalumeau N, Carbillon L, Coulomb-L’Hermine A, Le Guern V, Masseau A, et al. Intervillites chroniques histiocytaires : bilan et prise en charge. La Rev Médecine Interne (2018) 39:117–21. doi: 10.1016/j.revmed.2017.10.422
36. Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler M-A, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med (2016) 140:698–713. doi: 10.5858/arpa.2015-0225-CC
37. Bos M, Harris-Mostert ETMSTMS, van der Meeren LEE, Baelde JJJ, Williams DJJ, Nikkels PGJGJ, et al. Clinical outcomes in chronic intervillositis of unknown etiology. Placenta (2020) 91:19–23. doi: 10.1016/j.placenta.2020.01.001
38. Homatter C, Stichelbout M, Devisme L, Chudzinski A, Debarge V, Garabedian C, et al. Is chronic histiocytic intervillositis a severe placental disease? a case-control study. Placenta (2020) 91:31–6. doi: 10.1016/j.placenta.2019.12.020
39. Simula NK, Terry J, Kent NE, Robertson J, Purkiss S, Bloomenthal D, et al. Chronic intervillositis of unknown etiology (CIUE): Prevalence, patterns and reproductive outcomes at a tertiary referral institution. Placenta (2020) 100:60–5. doi: 10.1016/j.placenta.2020.07.032
40. Traeder J, Jonigk D, Feist H, Bröcker V, Länger F, Kreipe H, et al. Pathological characteristics of a series of rare chronic histiocytic intervillositis of the placenta. Placenta (2010) 31:1116–9. doi: 10.1016/j.placenta.2010.09.012
41. Contro E, DeSouza R, Bhide A. Chronic intervillositis of the placenta: A systematic review. Placenta (2010) 31:1106–10. doi: 10.1016/j.placenta.2010.10.005
42. Gynaecologists RC of O. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. In: RCOG green-top guidel no 17. London: Royal College of Obstetricians and Gynaecologists (2011). p. 1–18.
43. Carmichael SL, Shaw GM, Ma C, Werler MM, Rasmussen SA, Lammer EJ. Maternal corticosteroid use and orofacial clefts. Am J Obstet Gynecol (2007) 197:585.e1–7. doi: 10.1016/j.ajog.2007.05.046
44. Skuladottir H, Wilcox AJ, Ma C, Lammer EJ, Rasmussen SA, Werler MM, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res Part A Clin Mol Teratol (2014) 100:499–506. doi: 10.1002/bdra.23248
45. Empson MB, Lassere M, Craig JC, Scott JR. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev (2005) 2012:CD002859. doi: 10.1002/14651858.CD002859.pub2
46. Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open (2018) 2018:1–12. doi: 10.1093/hropen/hoy004
47. Laskin CA, Bombardier C, Hannah ME, Mandel FP, Ritchie K, Farewell V, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med (1997) 337:148–54. doi: 10.1056/nejm199707173370302
48. Belardo CAA. Summary of suggested treatments for chronic histiocytic intervillositis (CHI) (2019). Available at: https://chisupport.org/wp-content/uploads/2019/01/Summary-of-suggested-treatments-for-Chronic-Histiocytic-Intervillositis.docx-Google-Docs.pdf.
49. Hamulyák EN, Scheres LJJJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database Syst Rev (2020) 2020:CD012852. doi: 10.1002/14651858.CD012852.pub2
50. Bouariu A, Gică N, Ciobanu AM, Scutelnicu AM, Popescu MR, Panaitescu AM. The potential benefit of hydroxychloroquine in chronic placental inflammation of unknown etiology associated with adverse pregnancy outcomes. Healthcare (2022) 10:168. doi: 10.3390/healthcare10010168
51. Berghella V. Prevention of recurrent fetal growth restriction. Obstet Gynecol (2007) 110:904–12. doi: 10.1097/01.AOG.0000267203.55718.aa
52. Brady CA, Williams C, Batra G, Church E, Tower CL, Crocker IP, et al. Immunomodulatory therapy reduces the severity of placental lesions in chronic histiocytic intervillositis. Front Med (2021) 8:753220. doi: 10.3389/fmed.2021.753220
53. Boog G, Le Vaillant C, Alnoukari F, Jossic F, Barrier J, Muller J-YY, et al. Combining corticosteroid and aspirin for the prevention of recurrent villitis or intervillositis of unknown etiology. J Gynecol Obstet Biol la Reprod (2006) 35:396–404. doi: 10.1016/s0368-2315%2806%2976411-0
54. Cornish EF, McDonnell T, Williams DJ. Chronic inflammatory placental disorders associated with recurrent adverse pregnancy outcome. Front Immunol (2022) 13:825075. doi: 10.3389/fimmu.2022.825075
55. Tan MY, Syngelaki A, Poon LC, Rolnik DL, O’Gorman N, Delgado JL, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol (2018) 52:186–95. doi: 10.1002/uog.19112
Keywords: CHI, recurrent miscarriage, intervillositis, stillbirth, small gestation age (SGA)
Citation: Moar L, Simela C, Nanda S, Marnerides A, Al-Adnani M, Nelson-Piercy C, Nicolaides KH and Shangaris P (2022) Chronic histiocytic intervillositis (CHI): current treatments and perinatal outcomes, a systematic review and a meta-analysis. Front. Endocrinol. 13:945543. doi: 10.3389/fendo.2022.945543
Received: 16 May 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Reinaldo Marín, Instituto Venezolano de Investigaciones Científicas (IVIC), VenezuelaReviewed by:
Chloe A. Brady, The University of Manchester, United KingdomCopyright © 2022 Moar, Simela, Nanda, Marnerides, Al-Adnani, Nelson-Piercy, Nicolaides and Shangaris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Panicos Shangaris, cGFuaWNvcy5zaGFuZ2FyaXNAa2NsLmFjLnVr
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.