
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 09 August 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.945159
This article is part of the Research TopicNew Progress in Understanding and Treatment of OsteoporosisView all 28 articles
Background: Osteoporosis (OP) and osteopenia are common bone disorders in old age, and lots of patients suffering from OP or osteopenia need to take antiplatelet agents to treat basic diseases. However, clinical data on the link between osteopenia or OP and antiplatelet agents are limited.
Methods: Data in this study were collected and screened from the NHANES from 2013 to 2014 and 2017 to 2018. The variables were extracted from interviews and compared between OP or osteopenia participants and normal. The relationship between OP or osteopenia and taking antiplatelet drugs was analyzed by weighted multivariate logistic regression
Results: After excluding individuals who were not eligible and had invalid data, we finally identified 894 participants for inclusion in the study. We found a negative association between OP or osteopenia and taking antiplatelet agents (OR = 0.53; 95% CI, 0.33–0.84; p < 0.05). These results did not change on multiple imputations (OR = 0.32, 95% CI, 0.19–0.56; p <0.01). In the subgroup analyses, the associations were more significant in women (OR = 0.18, 95% CI, 0.05–0.62; p <0.05).
Conclusion: This study demonstrated that the association between OP or osteopenia and taking antiplatelet agents was significant. Therefore, it is necessary to confirm the result by extending further research.
Osteoporosis (OP) and osteopenia are systemic skeletal diseases that lead to increased fracture risk. Hip and spine fractures are the most common damage types among them (1–3). Venous thromboembolism (VTE) is a kind of common complication for clinical patients (4). Guidelines recommend an antiplatelet agent: low molecular weight heparin is an optimal treatment for VTE in patients undergoing hip fracture and joint replacement surgery (5). Patients with the above types of surgery tend to be older, and most of them need to take antiplatelet agents (6–8). Some common diseases in endocrinology can also be affected by antiplatelet drugs (9–11).
Bone mineral density (BMD) is the preferred method to diagnose OP and osteopenia. BMD should be measured at the hip and its subregions as well as the lumbar spine using dual-energy X-ray absorptiometry (DXA) (1). Normal BMD is defined as BMD within 1 SD of white women aged 20–29 in the National Health and Nutrition Examination Survey (NHANES) III, osteopenia is defined as 1.0–2.5 SDs below that of a young white woman, and OP is defined as 2.5 and more SDs inferior to that of a young white woman (12). According to new research, ticagrelor can inhibit osteoclast differentiation in vitro while promoting bone regeneration in mice with calvarial defects (13). A trend toward higher lumbar BMD in users of acetylsalicylic acid compared to nonexposed (14) was observed.
However, clinical data on the link between osteopenia or OP and antiplatelet agents are limited. In this research, we aim to demonstrate the relationship between osteopenia or OP and antiplatelet agents in a national sample of Americans. The results of our study will lead to further treatment and prevention of OP and osteopenia.
Data were collected and screened from the NHANES. The NHANES is a representative multilevel and multidimensional project, its continuity is due to the organization’s annual survey of a sample of the American people (15, 16). Participants completed the NHANES and signed an informed consent.
We combined data from the 2013 to 2014 and 2017 to 2018 waves of NHANES. These two waves were chosen because data on femoral neck BMD were not available in the 2015–2016 wave. The study population was restricted to participants who had undergone femoral neck and total lumbar spine BMD. Since participants in the 2013–2014 wave were 40–80 years old and in the 2017–2018 wave were 50–80 years old, participants aged 50–80 years old were selected as research objects. Considering that the data came from different waves, we performed statistical analysis on the data of different years before the combined analysis, and the results showed that the difference between the data of these two waves in each variable was not statistically significant (the detailed analysis results can be found in Supplementary Table S1). Thus, data can be combined for analysis. Among the 5,413 eligible participants, we excluded missing data, participants younger than 50 years of age, and those who had taken other drugs. Ultimately, 894 participants were included in the analysis.
The outcome variable of this study was whether the participants were diagnosed with OP or osteopenia. The diagnoses of OP and osteopenia were based on T-scores calculated from the BMD of the neck of the femur and lumbar vertebra with DXA. The femur and lumbar spine scans were analyzed with the APEX software (17). BMD was calculated as T-scores via a method. T-scores were divided into ≥ −1, −1 to −2.5, and ≤ −2.5, representing normal, osteopenia, and OP (1, 18).
As collected data from the NHANES, we additionally calculated the proportion of participants who were taking antiplatelet agents (clopidogrel, aspirin, cilostazol, prasugrel, dipyridamole) under the diagnostic subgroup.
Concomitant variables of this research included sex, age, race, education, federal poverty level (FPL), body mass index (BMI), smoke, drink, calcium daily intake (g/day), and vitamin D daily intake (µg/day). Age was classified as 50–60, 60–70, and 70–80. Covariates about sex, race, education, age, FPL, smoke, and drink were obtained from structured questionnaires. Calcium and vitamin D daily dietary intake was collected from 24-h dietary recalls. The race was classified as White race, Black race, Mexican, and Others. Education was categorized into high school and below, college (and equivalent educational attainment), and above college. FPL was classified as <200% FPL and ≥200% FPL. If BMI is defined as the underweight range when the index is less than 18.5. Healthy weight is in the range of 18.5 to 25, while the overweight range is 25–30. It is in the obesity range if BMI is ≥30. Obesity is further divided into classes 1–3. BMI was 30–35, 35–40, and ≥40, respectively (19). Smoke was divided into former, never, and now. Smoking less than 100 cigarettes in life was described as never. Smoking more than 100 cigarettes in life and smoke not at all now were defined as former; the definition of now was smoking more than 100 cigarettes in life and smoking some days or every day (from the questionnaire in the database: smoked at least 100 cigarettes in life). The drink was divided into no, mild, moderate, and heavy as follows: heavy alcohol user: ≥3 drink times/day for women or ≥4 drink times/day for men or binge drinking on ≥5 days/month; moderate alcohol user: ≥2 drink times/day for women or ≥3 drink times/day for men or binge drinking ≥2 days/month; mild alcohol user: none of the above but drinking currently; and no was defined as never drinking (20).
All data were weighted to produce estimates for the US population, and designed layering and clustering were used in the analysis. Continuous variables are expressed as mean (95% CI), while categorical variables are expressed as count (percentage). The relationship between taking antiplatelet agents and OP or osteopenia was analyzed via weighted logistical regression. The confounding factors were added to the multivariate logistic model to adjust the relationship analysis. Statistical analysis of all data was performed by R Studio. A two-tailed p < 0.05 was regarded as significant.
We fully enrolled 894 individuals after excluding participants who did not complete BMD testing, used other drugs, and had incomplete information. Among 4,519 excluded participants, 11.77% were Mexican, 21.09% were Black, 41.93% were White, and 25.20% were Other; 50.81% were women and 49.19% were men. Individuals <50, 50–60, 60–70, and 70–80 were 19.85%, 24.43%, 29.14%, and 26.58%, respectively. Of the 894 included participants, 448 were diagnosed with OP or osteopenia, while 446 were considered normal. The general characteristics are shown in Table 1. Overall, OP and osteopenia individuals were older (p < 0.01) and thinner (p < 0.05), were less likely to be Black (p < 0.001), had a higher proportion of women (p < 0.001), and were less likely to take antiplatelet agents (p < 0.05).
We compared race- and sex-adjusted T-scores across the age groups by using linear regression. With the increase of age, the T-score of all race groups decreased, among which the most significant decrease was in women who had just menopause (50–60 years) and 70–80 years (Table 2).
A logistic regression model was established to investigate the relationship between the use of antiplatelet agents and OP or osteopenia, as shown in Table 3. Model 1 was a univariate logistic regression model that showed a negative association between OP or osteopenia and taking antiplatelet agents (OR = 0.53; 95% CI, 0.33–0.84; p < 0.05). This association was not altered after adjusting for age, sex, race, education, BMI range, and FPL in model 2 (OR = 0.37; 95% CI, 0.22–0.63; p < 0.01). In model 3, which was further adjusted for model 2 plus smoke, drink, calcium, and vitamin D, this association was not altered (OR = 0.32, 95% CI, 0.19–0.56; p < 0.01). We further explored the association of taking antiplatelet agents with OP or osteopenia stratified by sex groups (Table 4). The association between taking antiplatelet agents and OP or osteopenia was more significant in the female participants in model 2 (OR = 0.21, 95% CI, 0.06–0.70; p < 0.05) and model 3 (OR = 0.18, 95% CI, 0.05–0.62; p <0.05).
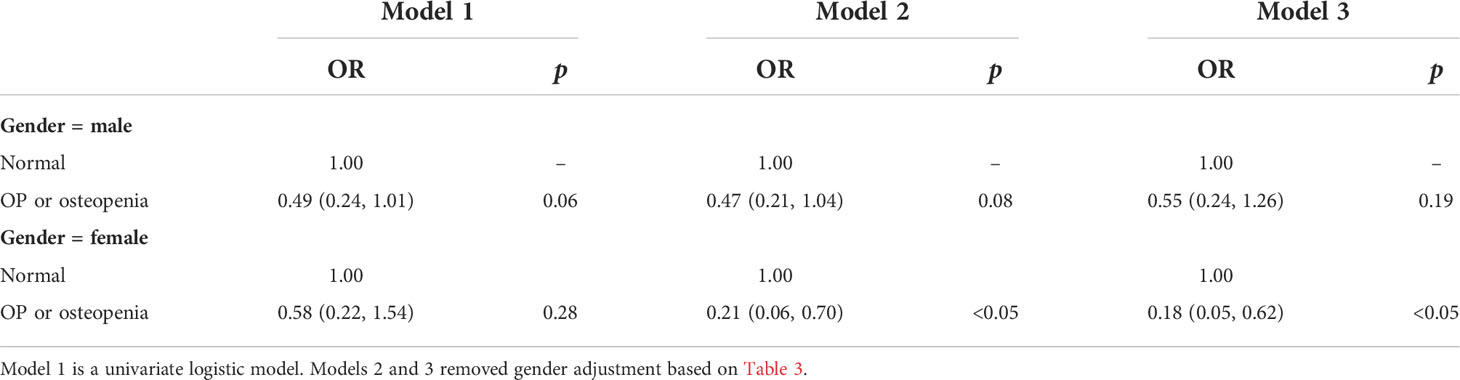
Table 4 Association between taking antiplatelet agents and OP or osteopenia (subgroup analysis stratified by gender).
Our study concluded that OP and osteopenia were associated with taking antiplatelet agents. The relationship remained the same even after other factors were added (demographic factors and smoking, drinking, calcium, and vitamin D).
It is widely believed that menopause and aging lead to bone loss (21–23). The white race is considered an established risk factor for OP (24, 25). Calcium and vitamin D are two trace elements that affect bone metabolism. Calcium deficiency may be associated with reduced bone mass and OP, while chronic deficiency of vitamin D will cause osteomalacia (26). Calcium and vitamin D deficiency aggravate bone loss in osteoporotic mice (27). Studies have found a relationship between serum vitamin D status and resistance to clopidogrel (28, 29). Obesity increases the fracture risk, and the higher BMI, the less protective the bones are (30).
A few studies had confirmed that antiplatelet agents can treat OP and OP-related diseases or stop the disease from getting worse. A study in the population of the 70–79 age group demonstrated higher BMD in aspirin users compared with those without using aspirin (31). Drugs modified with aspirin can accelerate the repair of osteoporotic bone defects (32). Aspirin inhibits osteoclast differentiation and promotes osteogenic differentiation (33, 34). Aspirin inhibits osteoclast formation through related signaling pathways such as NF-κB signaling pathway (35, 36). Continued perioperative use of clopidogrel does not have the predicted negative effects but promotes fracture healing (37). Even after adjusting for covariables, the results demonstrated a negative association between antiplatelet agents and OP or osteopenia. The association between taking antiplatelet agents and OP or osteopenia was more significant in women. A previous study found that using aspirin regularly might benefit BMD in postmenopausal women (38). However, studies have shown that long-term use of antiplatelet agents can negatively affect bones (39). Contrary research conclusions are due to differences in research methods, drug use methods, drug dosage, drug dosage form, course of treatment, and other aspects.
The acquisition and screening of NHANES data adopt a standardized, unified scheme, so the accuracy and consistency of data included in the study and the reliability of results can be guaranteed. A large number of community samples and a weighted analysis of data ensured the reliability of the results. It is more intuitive and comprehensive to include some important confounding factors in regression analysis compared with mechanism research. Nevertheless, this research still has some limitations. Firstly, since this study is a cross-sectional survey based on NHANES, the causal relationship between dependent variables, independent variables, and covariables cannot be deduced. The analysis of the relationship between specific drugs and OP or osteopenia could not be completed due to insufficient sample size and uneven distribution of the antiplatelet agent-taking population. Due to the lack of records of the dosage and course of treatment of medication, it is impossible to verify the relationship between long-term, high-volume intake of antiplatelet drugs with OP with osteopenia. To further strengthen and verify our results, a large-scale cohort study is required to complete the validation. Second, NHANES data sources are measured or collected only once, which increases the possibility of data bias. Therefore, it is suggested that the database can be repeated multiple times in the following study.
In conclusion, for adults, taking antiplatelet agents is associated with OP and osteopenia in women but not in men. This suggests that taking antiplatelet agents is likely to impact BMD for women but is likely to have no impact on men.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Protocol #2018-01 (Effective beginning October 26, 2017) Continuation of Protocol #2011-17 (Effective through October 26, 2017). The patients/participants provided their written informed consent to participate in this study.
HL designed the study, analyzed the data, and wrote the manuscript. YZ, JW, ZH, ZW, and MQ analyzed the data. TJ revised the manuscript. HL is the first author. All authors read and approved the final manuscript.
This work was supported by the Nature Science Foundation of Anhui University of Chinese Medicine (2020yfyzc27), the Key Research and Development Projects in Anhui Province (202104j07020010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.945159/full#supplementary-material
1. Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med (2017) 167(3):ITC17–32. doi: 10.7326/AITC201708010
2. Karaguzel G, Holick MF. Diagnosis and treatment of osteopenia. Rev Endocr Metab Disord (2010) 11(4):237–51. doi: 10.1007/s11154-010-9154-0
3. Khosla S, Melton LJ 3rd. Clinical practice. Osteopenia. N Engl J Med (2007) 356(22):2293–300. doi: 10.1056/NEJMcp070341.
4. Flevas DA, Megaloikonomos PD, Dimopoulos L, Mitsiokapa E, Koulouvaris P, Mavrogenis AF. Thromboembolism prophylaxis in orthopaedics: An update. EFORT Open Rev (2018) 3(4):136–48. doi: 10.1302/2058-5241.3.170018
5. Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, et al. Prevention of vte in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th Ed: American college of chest physicians evidence-based clinical practice guidelines. Chest (2012) 141(2):e278S. doi: 10.1378/chest.11-2404
6. Yang Z, Ni J, Long Z, Kuang L, Gao Y, Tao S. Is hip fracture surgery safe for patients on antiplatelet drugs and is it necessary to delay surgery? A systematic review and meta-analysis. J Orthop Surg Res (2020) 15(1):105. doi: 10.1186/s13018-020-01624-7
7. Pailleret CA-O, Ait Hamou Z, Rosencher N, Samama CM, Eyraud V, Chilot F, et al. A retrospective comparison between delayed and early hip fracture surgery in patients taking clopidogrel: Same total bleeding but different timing of blood transfusion. Int Orthop (2017) 41(9):1839–44. doi: 10.1007/s00264-017-3571-6
8. Matharu GS, Kunutsor SK, Judge A, Blom AW, Whitehouse MR. Clinical effectiveness and safety of aspirin for venous thromboembolism prophylaxis after total hip and knee replacement: A systematic review and meta-analysis of randomized clinical trials. AMA Intern Med (2020) 180(3):376–84. doi: 10.1001/jamainternmed.2019.6108
9. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med (2018) 379(16):1529–39. doi: 10.1056/NEJMoa1804988
10. Leiter LA, Bhatt DL, McGuire DK, Teoh H, Fox K, Simon T, et al. Diabetes-related factors and the effects of ticagrelor plus aspirin in the themis and themis-pci trials. J Am Coll Cardiol (2021) 77(19):2366–77. doi: 10.1016/j.jacc.2021.03.298
11. Mansour A, Samadi M, Sanginabadi M, Gerami H, Karimi S, Hosseini S, et al. Effect of resveratrol on menstrual cyclicity, hyperandrogenism and metabolic profile in women with PCOS. Clin Nutr (2021) 40(6):4106–12. doi: 10.1016/j.clnu.2021.02.004
12. Klibanski A, Adams-Campbell L, Bassford T, Blair N, Russell W. Nih consensus development panel on osteoporosis prevention, diagnosis, and therapy, march 7-29, 2000: Highlights of the conference. South Med J (2001) 94(6):569–73. doi: 10.1097/00007611-200194060-00004
13. Mediero A, Wilder T, Reddy VS, Cheng Q, Tovar N, Coelho PG, et al. Ticagrelor regulates osteoblast and osteoclast function and promotes bone formation in vivo via an adenosine-dependent mechanism. FASEB J (2016) 30(11):3887–900. doi: 10.1096/fj.201600616R
14. Vestergaard P, Hermann P, Jensen JEB, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: Results of the Danish osteoporosis prevention study (Dops). Osteoporos Int (2012) 23(4):1255–65. doi: 10.1007/s00198-011-1692-0
15. Seligman HK, Laraia BA, Kushel MB. Food insecurity is associated with chronic disease among low-income nhanes participants. J Nutr (2010) 140(2):304–10. doi: 10.3945/jn.109.112573
16. Kan B, Guo D, Yuan B, Vuong AM, Jiang D, Zhang M, et al. Dietary carotenoid intake and osteoporosis: The national health and nutrition examination survey, 2005-2018. Arch Osteoporos (2021) 17(1):2. doi: 10.1007/s11657-021-01047-9
17. Wahner HW, Looker A, Dunn WL, Walters LC, Hauser MF, Novak C. Quality control of bone densitometry in a national health survey (Nhanes iii) using three mobile examination centers. J Bone Miner Res (1994) 9(6):951–60. doi: 10.1002/jbmr.5650090621
18. Looker AC, Wahner Hw, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of us adults. Osteoporos Int (1998) 8(5):468–89. doi: 10.1007/s001980050093
19. Organization WH. Obesity and overweight. In: Fact sheet N°311 (January 2015). doi: 10.1007/978-1-4419-1695-2_447
20. Rattan PA-OX, Penrice DA-O, Ahn JA-O, Ferrer A, Patnaik M, Shah VA-OX, et al. Inverse association of telomere length with liver disease and mortality in the us population. Hepatol Commun (2022) 6(2):399–410. doi: 10.1002/hep4.1803
21. Prestwood KM, Pilbeam Cc Fau - Raisz LG, Raisz LG. Treatment of osteoporosis. Annu Rev Med (1995) 46:249–56. doi: 10.1146/annurev.med
22. Khosla S, Hofbauer LC. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol (2017) 5(11):898–907. doi: 10.1016/S2213-8587(17)30188-2
23. Mundy GR. Osteoporosis and inflammation. Nutr Rev (2007) 65(12 Pt 2):147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x
24. Kelsey JL. Risk factors for osteoporosis and associated fractures. Public Health Rep (1989) 104(Suppl):14–20. doi: 10.2307/4628728
25. Noel SA-O, Santos MP, Wright NA-O. Racial and ethnic disparities in bone health and outcomes in the united states. J Bone Miner Res (2021) 36(10):1881–905. doi: 10.1002/jbmr.4417
26. Gennari C. Calcium and vitamin d nutrition and bone disease of the elderly. Public Health Nutr (2001) 4(2B):547–59. doi: 10.1079/PHN2001140
27. Fischer VA-O, Haffner-Luntzer MA-O, Prystaz K, Vom Scheidt A, Busse BA-O, Schinke T, et al. Calcium and vitamin-d deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep (2017) 7(1):7223. doi: 10.1038/s41598-017-07511-2
28. Lu BC, Shi XJ, Liang L, Dong N, Liu ZZ. Platelet surface Cd62p and serum vitamin d levels are associated with clopidogrel resistance in Chinese patients with ischemic stroke. J Stroke Cerebrovasc Dis (2019) 28(5):1323–8. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.031
29. Verdoia M, Pergolini P, Rolla R, Sartori C, Nardin M, Schaffer A, et al. Vitamin d levels and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. Platelets (2016) 27(6):576–82. doi: 10.3109/09537104.2016.1149159
30. Palermo A, Tuccinardi D, Defeudis G, Watanabe M, D’Onofrio L, Lauria Pantano A, et al. Bmi and bmd: The potential interplay between obesity and bone fragility. Int J Environ Res Public Health (2016) 13(6):544. doi: 10.3390/Ijerph13060544
31. Carbone LD, Cauley JA, Harris TB, Lang TF, Bauer DC, Barrow KD, et al. Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: Impact of cyclooxygenase selectivity. J Bone Miner Res (2003) 18(10):1795–802. doi: 10.1359/jbmr.2003.18.10.1795
32. Tao ZS, Zhou WS, Xu HG, Yang M. Aspirin modified strontium-doped b-tricalcium phosphate can accelerate the healing of femoral metaphyseal defects in ovariectomized rats. Biomed Pharmacother (2020) 132:110911. doi: 10.1016/j.biopha.2020.110911
33. Shi S, Yamaza T Fau - Akiyama K, Akiyama K. Is aspirin treatment an appropriate intervention to osteoporosis? Future Rheumatol (2008) 3(6):499–502. doi: 10.2217/17460816.3.6.499
34. Lussana F, Squizzato A, Permunian ET, Cattaneo M. A systematic review on the effect of aspirin in the prevention of post-operative arterial thrombosis in patients undergoing total hip and total knee arthroplasty. Thromb Res (2014) 134(3):599–603. doi: 10.1016/j.thromres.2014.06.027
35. Zeng YP, Yang C, Li Y, Fan Y, Yang HJ, Liu B, et al. Aspirin inhibits osteoclastogenesis by suppressing the activation of nf-kb and mapks in rankl-induced Raw264. 7 Cells. Mol Med Rep (2016) 14(3):1957–62. doi: 10.3892/mmr.2016.5456
36. Wu L, Luo Z, Liu Y, Jia L, Jiang Y, Du J, et al. Aspirin inhibits rankl-induced osteoclast differentiation in dendritic cells by suppressing nf-kb and Nfatc1 activation. Stem Cell Res Ther (2019) 10(1):375–. doi: 10.1186/s13287-019-1500-x
37. Lillis T, Veis A, Sakellaridis N, Tsirlis A, Dailiana Z. Effect of clopidogrel in bone healing-experimental study in rabbits. World J Orthop (2019) 10(12):434–45. doi: 10.5312/wjo.v10.i12.434
38. Bauer DC, Orwoll Es, Fox KM, Vogt TM, Lane NE, Hochberg MC, et al. Aspirin and nsaid use in older women: Effect on bone mineral density and fracture risk. Study Osteoporotic Fractures Res Group. J Bone Miner Res 1996 (1996) 11(1):29–35. doi: 10.1002/jbmr.5650110106
Keywords: osteoporosis, osteopenia, antiplatelet agents, NHANES, cross-sectional survey
Citation: Lv H, Wang J, Zhu Y, Hu Z, Wang Z, Qiao M and Jiang T (2022) Association between osteoporosis or osteopenia and taking antiplatelet agents in general US population of NHANES. Front. Endocrinol. 13:945159. doi: 10.3389/fendo.2022.945159
Received: 16 May 2022; Accepted: 14 July 2022;
Published: 09 August 2022.
Edited by:
Xiang Hang Luo, Xiangya Hospital, Central South University, ChinaReviewed by:
Rupesh K. Srivastava, All India Institute of Medical Sciences, IndiaCopyright © 2022 Lv, Wang, Zhu, Hu, Wang, Qiao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Jiang, amlhbmd0aW5nNzBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.