- Endocrinology, Diabetes and Metabolism Department of Coimbra Hospital and University Center, Coimbra, Portugal
The thyroid-stimulating hormone receptor (TSH-R) is predominantly expressed in the basolateral membrane of thyrocytes, where it stimulates almost every aspect of their metabolism. Several extrathyroidal locations of the receptor have been found including: the pituitary, the hypothalamus, and other areas of the central nervous system; the periorbital tissue; the skin; the kidney; the adrenal; the liver; the immune system cells; blood cells and vascular tissues; the adipose tissue; the cardiac and skeletal muscles, and the bone. Although the functionality of the receptor has been demonstrated in most of these tissues, its physiological importance is still a matter of debate. A contribution to several pathological processes is evident in some cases, as is the case of Grave’s disease in its multiple presentations. Conversely, in the context of other thyroid abnormalities, the contribution of the TSH-R and its ligand is still a matter of debate. This article reviews the several different sites of expression of the TSH-R and its potential role in both physiological and pathological processes.
Introduction
The thyroid-stimulating hormone receptor (TSH-R), encoded by a gene located in 14q31 (1), belongs to the G protein-coupled receptor family. It has a large extracellular domain, seven transmembrane passages, and a small intracellular domain (2).
A single chained TSH-R with approximately 100 kDa has been described (3, 4), however, the holoreceptor is most frequently cleaved into two subunits, α and β, linked by disulfide bonds (5). Cleavage may be important in the induction of some of TSH-R signaling pathways (6). The membrane-spanning β subunit, with a molecular mass of ~30 kDa, is common to luteinizing hormone (LH), human chorionic gonadotropin (hCG) and follicle stimulating hormone (FSH). The α subunit, of ~50 kDa, and is TSH-specific, located in the extracellular region, and shed from the cell surface (2, 5).
The TSH-R couples to four subfamilies of G proteins (7): Gs, inducing adenyl-cyclase activity and cyclic AMP production [the most common pathway (8, 9)]; Gq/G11 activating phospholipase C; G13, inducing p44/42 mitogen-activated protein kinase (MAPK); Gi inhibiting adenyl-cyclase activity (7). The receptor is constitutionally active, however, TSH and TSHRAbs may enhance or, less frequently, block its signalling (5).
TSH Receptor Expression
In Thyroidal Tissue
The main site of expression of the TSH-R is the basolateral membrane of thyrocytes (10, 11). TSH-R activation stimulates iodine uptake, synthesis and secretion of thyroid hormones, and proliferation of thyroid follicular cells (9, 10, 12). In adult thyrocytes, TSH stimulation is paramount to maintaining follicular architecture and regulate the expression of thyroid-specific genes such as those coding thyroglobulin (Tg), thyroid peroxidase (TPO), and sodium/iodide symporter (NIS) (11).
TSH/TSH-R also seem to be relevant during the embryological development of the thyroid gland, as their expression was shown in embryonic stem cells (13). However, other factors are certainly involved at this stage (11).
In Extrathyroidal Tissue
In the last decades, TSH-R expression has been found in several extrathyroidal tissues. In this section, data on the extrathyroidal sites of TSH-R expression and suggested, albeit generally unproved, physiological roles are explored.
TSH-releasing hormone (TRH) is produced in the hypothalamus mainly in the paraventricular nucleus and upregulates TSH production (14). The production of both TRH and TSH is under strict negative feedback control by the thyroid hormones (15). Follicle-stellate cells constitute ~10% of the anterior pituitary cells and form a network with each other and with endocrine cells (16). TSH-R expression was found in these cells and it has been hypothesized to be responsible for fine-tuning of the TSH levels, through an ultra-short negative feedback mechanism in pituitary thyrotrophic cells (16, 17). In the hypothalamus, activation of the TSH-R may be relevant for regulation of food intake (18) and influence seasonal reproductive patterns in some animals (19). TSH-R has also been demonstrated in other areas of the human brain such as the cortex, amygdala, cerebellum, cingulate gyrus and frontal, occipital and temporal lobes (20).
The presence of a functional TSH-R has been widely documented in the periorbital tissue (10, 21, 22), where it may be important in regulating the differentiation of orbital fibroblasts (23).
TSH-R is also expressed in the epidermis and hair follicles (24, 25), and the skin has been found to synthesize TSH under the control of TRH and thyroid hormones (23, 26). Treatment of organic cultures with TSH resulted in altered gene expression in hair follicles and stimulation of epidermis differentiation (25).
Thyroid hormones have been proposed to modulate renal development, morphology, and function (27). TSH-R expression was also demonstrated in the kidney and adrenal (28) and TSH stimulation was shown to increase cAMP production in human kidney cells (29).
In the ovary, the TSH-R has been found in granulosa cells (30) where its expression is increased by gonadotropins and decreased by estrogen (23). In murine models, the presence of TSH-R has been demonstrated in the testis and TSH has been shown to inhibit steroidogenesis (31). A role of TSH/TSH-R in the seasonal effects on gonadal growth in some animals has also been suggested (23).
TSH/TSH-R have been proposed to influence immune regulation (32). TSH-R expression was found in bone marrow hematopoietic cells, where TSH may regulate TNFα production (33). In thymocytes, TSH acting on the TSH-R was proposed to constitute an important growth factor influencing the development of T-cells (34). In white blood cells, TSH-R may be involved in recruitment, development and immunoregulation (23). This receptor has been found to be expressed on monocytes, dendritic cells, natural killer cells, T and B cells (32). Stimulation with recombinant human TSH has been shown to promote proliferation of natural killer cells, T and B cells (35). In dendritic cells, TSH stimulation was shown to lead to the production of pro-inflammatory cytokines and increased phagocytic activity (36).
Expression of the TSH-R in eritrocytes has been documented to influence Na+/K+-ATPase conformation (37). In blood vessels, TSH-R appears to contribute to the stimulation of angiogenesis and vascular smooth muscle proliferation (38).
TSH/TSH-R have been suggested to have bone protective properties (39). In human bone marrow-derived mesenchymal stem cells, TSH-R seems to be important for self-renewal, maintenance and differentiation (40). TSH was suggested to stimulate osteoblastic differentiation and to inhibit osteoclastogenesis (39). Nevertheless, sufficient data on the physiological effects of TSH on bone is conflicting (41). On the one hand, in human osteoblasts, TSH-R seems to have low expression and functionality (42). Also no influence of genetic variants influencing TSH concentration or TSH-R expression was found by van Vliet et al. (43). On the other hand, findings from van der Deure et al. and Kim et al. support an independent effect of TSH levels in improving bone mineral density (44).
Functional TSH-R was found in white adipose tissue in preadipocytes and differentiated adipocytes. It may have a role in preadipocyte behaviour and contribute to regulation of lipolysis in adipocytes (45, 46). In brown adipose tissue, a role of the TSH-R in thermogenesis has been suggested through induction of uncoupling protein-1 and deiodinase 2 (47, 48).
Expression of TSH-R has also been documented in hepatocytes (49), where stimulation with TSH may up-regulate cholesterol synthesis (50) and hepatic glucose production (51).
In the skeletal muscle, TSH appears to improve insulin sensitivity and increase insulin substrate-1 receptor expression (52). In the cardiac muscle, expression of a functional TSH-R has also been demonstrated (53, 54) and it was shown to influence cardiac electric properties (55).
As pleiotropic expression and myriad effects of the TSH-R are identified, it is becoming increasingly clear that it may play a part in several human diseases, which will be explored in the following sections of this text.
TSH Receptor in Human Disease
TSH Receptor Relevance in Thyroid Gland Diseases
Graves’ disease (GD) is the most common form of hyperthyroidism in countries without iodine deficiency (56, 57). Its physiopathology involves an autoimmune response with infiltration of specific T cells against the TSH-R, more commonly its thyroid-specific α-subunit (58). The fact that the α-subunit is shed may be important for the development of antibodies, but it is not sufficient, as it seems to occur in all humans (59). TSHRAbs share many of the actions of TSH on the TSH-R, and mostly lead to thyroid hyperplasia with upregulated production and secretion of thyroid hormone (5). However, TSH induces a more regulated response of thyroid specific genes, whereas stimulatory TSHRAbs persistently upregulate those genes (60). Conversely, some TSHRAbs may decrease TSH effects (blocking antibodies) or have a neutral effect on TSH binding and cAMP production (5).
Chronic autoimmune thyroiditis is an even more common autoimmune thyroid disease (61). Antibodies against thyroid peroxidase (anti-TPO) and thyroglobulin (anti-Tg) are usually present. However, TSHRAbs can be identified in 6,3-12% of HT patients and in 12-59% of atrophic thyroiditis patients. As some TSHRAbs have a blocking effect, they may contribute to hypothyroidism (5), or be associated with a fluctuating course between hyper and hypothyroidism (62).
Several somatic and germline mutations in the TSH-R have been identified and are listed on the TSH-R database (https://www.tsh-receptor-mutation-database.org/) (63).
Activating mutations of the TSH-R have been implicated in thyroid autonomy and hyperthyroidism (64, 65), these are usually located in exons 9 and 10 that encode the transmembrane domain (8). Inherited germline mutations are implicated in Familial Non-Autoimmune Autosomal Dominant Hyperthyroidism (OMIM 609152) (66), a rare, autosomal dominant, disease that courses with hyperthyroidism of varying severity and age of onset, and goiter. De novo germline mutations cause Persistent Sporadic Congenital Non-Autoimmune Hyperthyroidism, which usually presents precociously with significant hyperthyroidism, but with no familial history (8, 67).
Somatic activating mutations of the TSH-R are far more common and are involved in the pathogenesis of a substantial proportion of autonomous nodules and toxic multinodular goiter, the remaining being usually caused by somatic mutations in GNAS (68–70).
At the opposite pole, there are inactivating mutations that may occur in different parts of the receptor structure and cause resistance to TSH action (71). The clinical phenotype varies from compensated TSH resistance to congenital hypothyroidism with severe thyroid hypoplasia (23, 64). In most cases there seems to be a genotype-phenotype correlation with the former being associated with residual function of at least one allele, whereas hypothyroidism arises in the context of two non-functioning alleles (72).
Mutations that extend receptor specificity have also been described. TSH, FSH, LH and hCG and their receptors have evolved from a common ancestor. In normal conditions hCG can weakly stimulate the TSH-R leading to to lower TSH values in the first trimester of pregnancy (73). It has been proposed that mutations that reverse evolution may be associated with hyperemesis gravidarum, making TSH-R more sensitive to hCG action (23).
Finally, an important role of TSH-R in differentiated thyroid cancer (DTC) has also been proposed. Most, but not all, data suggests that TSH-R expression is maintained in tumor cells (74). TSH-R activation may have a pro-oncogenic and growth-promoting role (10). In murine models its expression was shown to be necessary for the initiation of the neoplastic process. TSH, acting through its receptor, has the potential of stimulating the growth of DTC (64). TSH has also been shown to promote vascular endothelial growth factor production thus contributing to angiogenesis (75, 76) to induce genomic instability (77) and to contribute to invasion and immune evasion in thyroid tumors (78). Conversely, the TSH-R seems to have an important role in maintaining differentiation of thyroid cancer cells, and in advanced and dedifferentiated thyroid cancer, a decrease in its expression has commonly been reported (79).
TSH Receptor Relevance in Extrathyroidal Illness
Graves’ orbitopathy (GO) is present at GD presentation in ~26% of the cases, or, more rarely emerges during follow-up (80). It is an autoimmunity driven phenomena (81), causing orbital lymphocytic infiltration with a predominance of T lymphocytes, edema, and an increase in orbital connective tissue, adipose tissue and the extraocular muscles volume (82). The TSH-R is currently seen as the main autoantigen in ophthalmopathy and periorbital fibroblasts as the target of autoimmune attack (81). In patients with recent onset GO, TSHRAbs levels directly correlate with orbital disease activity (83) and may predict clinical course (84). Stimulation of TSH-R in orbital fibroblasts by TSHRAbs leads to activation of intracellular pathways, production of glycosaminoglycans and an increase in proliferation, adipogenesis and myofibrillogenesis (81). Indeed, enhanced TSH-R expression has been shown to be influenced by the autoimmune and inflammatory process (85) and to parallel with de novo adipogenesis (82). A role for the IGF-1 receptor has also been suggested and a crosstalk between IGF-1 receptor and the TSH-R is currently accepted as an important phenomenon in the pathophysiology of GO (86–90).
TSH-R has also been implicated in Graves’ dermatopathy. Similarly to what occurs with GO, this rare manifestation is associated with high titers of TSHRAbs, and characterized by a large amount of glycosaminoglycans dispersed in the reticular portion of the dermis. TSH-R immunoreactivity has been documented in the pretibium of patients with Graves’ dermatopathy (91).
The presence of TSH-R in thymocytes, may potentially explain the thymic hyperplasia seen in some patients with GD (34).
Hashimoto’s Encephalopathy, a rare aseptic form of encephalopathy, occurs in association with Hashimoto thyroiditis (92). Hypotheses proposed for its pathogenesis include: an immunopathological vasculitis; hormonal dysregulation; antibodies against antigens existent in the brain. The latter theory emphasizes the role of anti-thyroid antibodies such as TSHRAbs, anti-TPO and anti-Tg, since they are expressed in the brain (93). TSHRAbs might bind to TSH-R in cortical neurons and have a role in Hashimoto’s Encephalopathy (94). Homology between central nervous system antigens involved in Hashimoto’s Encephalopathy (such as alpha-enolase, dimethylargininase-I and aldehyde reductase-I) and thyroid antigens, including the TSH-R, has been found. This raises the possibility of cross-reactivity as an alternative pathophysiological mechanism (95).
Diffuse TSH-R expression in the brain may connect it with other neurological diseases. In the limbic system, abnormal interaction between anti-thyroid antibodies and the TSH-R may lead to neuronal inactivation/destruction and reduction of TSH-R expression, downregulating limbic-thyroid function, thus contributing to mood dysregulation and maniac-depressive disorders (20). Reduced TSH-R signaling may also be linked with declining cognitive function, as there is evidence suggesting an association between cognitive impairment and subclinical hyperthyroidism and in murine models, reduced TSH-R signaling was associated with impaired special learning and memory (96). TSHβ resistance has been associated with attention-deficit/hyperactivity disorder (ADHD) and TSH-R knockout in mice led to a ADHD phenotype (97). Conversely, both Alzheimer's disease and Down syndrome patients have greater expression of temporal and frontal lobe TSH-R, suggesting a potential role for TSH-R in neurogenerative disorders (20).
Thyroid disease is frequently accompanied by increased or decreased glomerular filtration rate or alterations in tubular transport (27), effects usually attributed to a direct action of thyroid hormones. As renal expression of TSH-R was documented, an influence of TSH itself has also been proposed (29). There are reports of nephritis due to thyroid antigen-antibody complexes in GD (98, 99). Despite these phenomena being generally attributed to circulating complexes, in light of the knowledge of TSH-R expression in the kidney, in situ antibody formation can also be considered (29).
Overt hypothyroidism has been associated with decreased fertility. For subclinical hypothyroidism this relationship is not clear, nevertheless TSH levels >4.0 mIU/L have been associated with adverse fertility outcomes (100). In a population with polycystic ovary syndrome undergoing in vitro fertilization, TSH levels in serum and in follicular fluid showed a negative correlation with oocyte maturation rate and fertilization (30). As such, one might wonder if TSH acting on the TSH-R in the granulosa cells, may contribute to the negative effects of hypothyroidism in fertility.
TSH acting on the testis may contribute to compromised secretion of androgens in hypothyroidism (31).
In murine models, absence of bone TSH-R expression was found to result in an osteoporotic phenotype. It is conceivable that the lack of TSH stimulation in thyrotoxicosis may contribute along with elevated thyroid hormones to increased bone loss in these patients (12, 101).
The presence of TSH-R on adipocytes has led some authors to question whether elevated TSH might contribute to the increased risk of obesity and cardiovascular disease associated with hypothyroidism (45). Overt hyperthyroidism has been associated with modest weight gain, however, for subclinical hypothyroidism a relationship with weight gain is not so clear. A positive correlation between TSH and body mass index has been found, although it’s been difficult to ascertain whether it is a contributing factor or a consequence of adiposity (102). There are some data to support a role of TSH-R signaling in the regulation of energy expenditure, thus contributing to weight variations associated with hyper and hypothyroidism (103). In murine models, a positive correlation between TSH-R expression and body mass index was found in diet-induced fat mice (104), and TSH-R knockout induced obesity resistance (105). It was proposed that TSH acting on the TSH-R on adipose tissue would promote triglyceride synthesis in adipocytes (105). A prior diagnosis of GD has been found to be a risk factor for a greater weight gain after treatment for hyperthyroidism (106) and it was hypothesized that TSHRAbs acting on adipose tissue might contribute to this effect (103).
Hypothyroidism is associated with abnormal cardiac repolarization and some data supports the possibility that increased stimulation of the TSH-R in cardiomyocytes may be a contributing factor (55, 107).
Since in hepatocytes TSH stimulation may increase both cholesterol and glucose synthesis, the presence of TSH-R in these cells may be one contributor to the worsening of cardiovascular risk factors associated with hypothyroidism (50, 51). Increased mitochondrial oxidative stress is associated with an incremental risk of conditions such as non-alcoholic hepatic liver disease. In murine models TSH signaling through its hepatic receptor has shown to upregulate oxidative stress (108).
Besides the above-mentioned role of the TSH-R in thyroid cancer, there are some data suggesting its expression in extrathyroidal malignancies such as melanoma (109), glioma/glioblastoma (110), lung (111), breast cancer (112), ovarian cancer (113) and hepatocellular carcinoma (114).
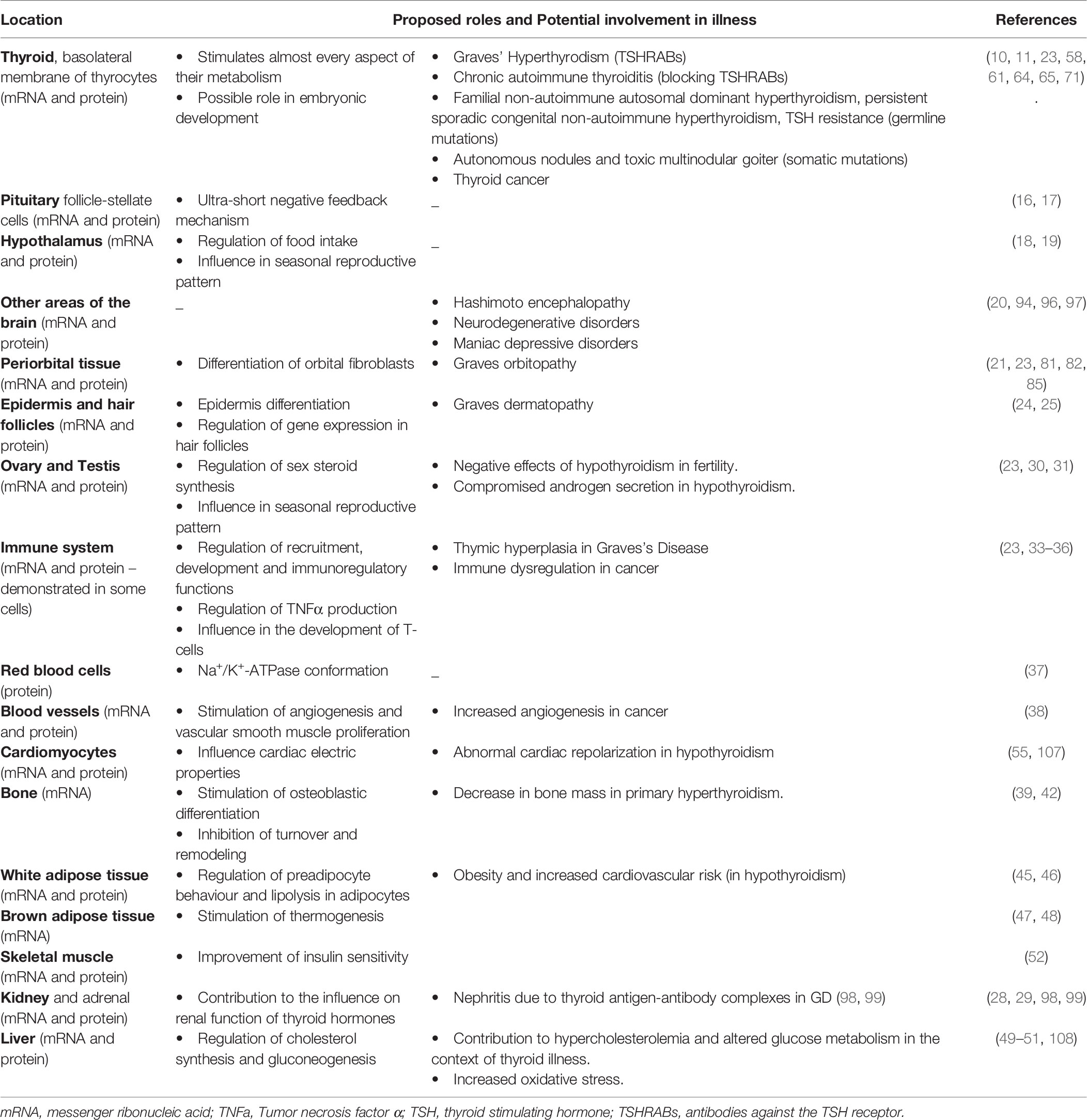
A summary of the locations of TSH-R and its potential influence on human disease is provided on Table 1.
Concluding Remarks
The discovery of TSH-R expression in several organs changes the perspective of TSH action from a simple stimulator of thyroid gland function to a hormone with pleiotropic actions that may have an influence on the clinical picture of thyroid gland dysfunction and in several human diseases.
However, the physiological and pathophysiological roles are difficult to establish given that: it requires the ability to distinguish the consequences of TSH deficiency from those of thyroid hormones’ excess (reciprocal relationship), the receptor is frequently expressed at low levels in peripheral tissues, and there is the potential for local TSH production (12).
It is possible that the fact that TSH-R has a constitutive activation and a biphasic controlled response to TSH, may contribute to less overt manifestations of subclinical thyroid disorders.
Detailed examination of extrathyroidal manifestations of patients with germline TSH-R mutations rendered euthyroid may shed a light. New molecules with the function of TSH.R agonists, antagonists or inverse agonists have recently emerged, and can also assist in increasing our understanding on the extrathyroidal roles of the TSH-R. Due to the pleiotropic expression of the TSH-R, the importance of such knowledge may be reflected on several human diseases and even contribute to the creation of a new theranostic tool.
Author Contributions
IV, DR, and IP were responsible for conceptualization and methodology; IV was responsible for literature review and preparation of the original draft; DR and IP supervised the manuscript creation and were responsible for its review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tomer Y, Barbesino G, Keddache M, Greenberg DA, Davies TF. Mapping of a Major Susceptibility Locus for Graves’ Disease (GD-1) to Chromosome 14q31. J Clin Endocrinol Metab (1997) 82(5):1645–8. doi: 10.1210/jcem.82.5.4064
2. Rapoport B, McLachlan SM. The Thyrotropin Receptor in Graves’ Disease. Thyroid Off J Am Thyroid Assoc (2007) 17(10):911–22. doi: 10.1089/thy.2007.0170
3. Russo D, Chazenbalk GD, Nagayama Y, Wadsworth HL, Seto P, Rapoport B. A New Structural Model for the Thyrotropin (TSH) Receptor, as Determined by Covalent Cross-Linking of TSH to the Recombinant Receptor in Intact Cells: Evidence for a Single Polypeptide Chain. Mol Endocrinol Baltim Md (1991) 5(11):1607–12. doi: 10.1210/mend-5-11-1607
4. Chen CR, Chazenbalk GD, Wawrowsky KA, McLachlan SM, Rapoport B. Evidence That Human Thyroid Cells Express Uncleaved, Single-Chain Thyrotropin Receptors on Their Surface. Endocrinology (2006) 147(6):3107–13. doi: 10.1210/en.2005-1514
5. Michalek K, Morshed SA, Latif R, Davies TF. TSH Receptor Autoantibodies. Autoimmun Rev (2009) 9(2):113–6. doi: 10.1016/j.autrev.2009.03.012
6. Kleinau G, Neumann S, Grüters A, Krude H, Biebermann H. Novel Insights on Thyroid-Stimulating Hormone Receptor Signal Transduction. Endocr Rev (2013) 34(5):691–724. doi: 10.1210/er.2012-1072
7. Boutin A, Krieger CC, Marcus-Samuels B, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC. TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes In Vitro. Front Endocrinol (2020) 11:276. doi: 10.3389/fendo.2020.00276
8. Ferraz C, Paschke R. Inheritable and Sporadic non-Autoimmune Hyperthyroidism. Best Pract Res Clin Endocrinol Metab (2017) 31(2):265–75. doi: 10.1016/j.beem.2017.04.005
9. Vassart G, Dumont JE. The Thyrotropin Receptor and the Regulation of Thyrocyte Function and Growth. Endocr Rev (1992) 13(3):596–611. doi: 10.1210/edrv-13-3-596
10. Chu Y-D, Yeh C-T. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells [Internet]. (2020) 9(7):1730. doi: 10.3390/cells9071730
11. Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, et al. Role of the Thyroid-Stimulating Hormone Receptor Signaling in Development and Differentiation of the Thyroid Gland. Proc Natl Acad Sci USA (2002) 99(24):15462–7. doi: 10.1073/pnas.242328999
12. Williams GR. Extrathyroidal Expression of TSH Receptor. Ann Endocrinol (2011) 72(2):68–73. doi: 10.1016/j.ando.2011.03.006
13. Ma R, Latif R, Davies TF. Thyrotropin-Independent Induction of Thyroid Endoderm From Embryonic Stem Cells by Activin a. Endocrinology (2009) 150(4):1970–5. doi: 10.1210/en.2008-1374
14. Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic Thyroid Hormone Feedback in Health and Disease. Prog Brain Res (2006) 153:189–207. doi: 10.1016/S0079-6123(06)53011-0
15. Chiamolera MI, Wondisford FE. Thyrotropin-Releasing Hormone and the Thyroid Hormone Feedback Mechanism. Endocrinology (2009) 150(3):1091–6. doi: 10.1210/en.2008-1795
16. Prummel MF, Brokken LJS, Wiersinga WM. Ultra Short-Loop Feedback Control of Thyrotropin Secretion. Thyroid Off J Am Thyroid Assoc (2004) 14(10):825–9. doi: 10.1089/thy.2004.14.825
17. Prummel MF, Brokken LJ, Meduri G, Misrahi M, Bakker O, Wiersinga WM. Expression of the Thyroid-Stimulating Hormone Receptor in the Folliculo-Stellate Cells of the Human Anterior Pituitary. J Clin Endocrinol Metab (2000) 85(11):4347–53. doi: 10.1210/jcem.85.11.6991
18. Burgos JR, Iresjö BM, Wärnåker S, Smedh U. Presence of TSH Receptors in Discrete Areas of the Hypothalamus and Caudal Brainstem With Relevance for Feeding Controls-Support for Functional Significance. Brain Res (2016) 1642:278–86. doi: 10.1016/j.brainres.2016.04.007
19. Yoshimura T. Neuroendocrine Mechanism of Seasonal Reproduction in Birds and Mammals. Anim Sci J Nihon Chikusan Gakkaiho (2010) 81(4):403–10. doi: 10.1111/j.1740-0929.2010.00777.x
20. Naicker M, Naidoo S. Cellular and Molecular Distribution of Thyroid-Specific Proteins, Thyroid-Stimulating Hormone Receptor (TSH-R) and Thyroglobulin (TG) in the Central Nervous System. Neurochem Int (2022) 155:105305. doi: 10.1016/j.neuint.2022.105305
21. Spitzweg C, Joba W, Hunt N, Heufelder AE. Analysis of Human Thyrotropin Receptor Gene Expression and Immunoreactivity in Human Orbital Tissue. Eur J Endocrinol (1997) 136(6):599–607. doi: 10.1530/eje.0.1360599
22. Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin Receptor Expression in Graves’ Orbital Adipose/Connective Tissues: Potential Autoantigen in Graves’ Ophthalmopathy. J Clin Endocrinol Metab (1998) 83(3):998–1002. doi: 10.1210/jcem.83.3.4676
23. Briet C, Suteau-Courant V, Munier M, Rodien P. Thyrotropin Receptor, Still Much to be Learned From the Patients. Best Pract Res Clin Endocrinol Metab (2018) 32(2):155–64. doi: 10.1016/j.beem.2018.03.002
24. Slominski A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Pisarchik A, et al. Expression of Hypothalamic–Pituitary–Thyroid Axis Related Genes in the Human Skin. J Invest Dermatol (2002) 119(6):1449–55. doi: 10.1046/j.1523-1747.2002.19617.x
25. Bodó E, Kromminga A, Bíró T, Borbíró I, Gáspár E, Zmijewski MA, et al. Human Female Hair Follicles Are a Direct, Nonclassical Target for Thyroid-Stimulating Hormone. J Invest Dermatol (2009) 129(5):1126–39. doi: 10.1038/jid.2008.361
26. Bodó E, Kany B, Gáspár E, Knüver J, Kromminga A, Ramot Y, et al. Thyroid-Stimulating Hormone, a Novel, Locally Produced Modulator of Human Epidermal Functions, Is Regulated by Thyrotropin-Releasing Hormone and Thyroid Hormones. Endocrinology (2010) 151(4):1633–42. doi: 10.1210/en.2009-0306
27. Katz AI, Emmanouel DS, Lindheimer MD. Thyroid Hormone and the Kidney. Nephron (1975) 15(3–5):223–49. doi: 10.1159/000180514
28. Dutton CM, Joba W, Spitzweg C, Heufelder AE, Bahn RS. Thyrotropin Receptor Expression in Adrenal, Kidney, and Thymus. Thyroid Off J Am Thyroid Assoc (1997) 7(6):879–84. doi: 10.1089/thy.1997.7.879
29. Sellitti DF, Akamizu T, Doi SQ, Kim GH, Kariyil JT, Kopchik JJ, et al. Renal Expression of Two “Thyroid-Specific” Genes: Thyrotropin Receptor and Thyroglobulin. Exp Nephrol (2000) 8(4–5):235–43. doi: 10.1159/000020674
30. Gao H, Lu X, Huang H, Ji H, Zhang L, Su Z. Thyroid-Stimulating Hormone Level Is Negatively Associated With Fertilization Rate in Patients With Polycystic Ovary Syndrome Undergoing In Vitro Fertilization. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet (2021) 155(1):138–45. doi: 10.1002/ijgo.13581
31. Dhole B, Gupta S, Shekhar S, Kumar A. A Novel Antigonadotropic Role of Thyroid Stimulating Hormone on Leydig Cell-Derived Mouse Leydig Tumor Cells-1 Line. Ann Natl Acad Med Sci India. (2020) 56(1):30–7. doi: 10.1055/s-0040-1709091
32. Wenzek C, Boelen A, Westendorf AM, Engel DR, Moeller LC, Führer D. The Interplay of Thyroid Hormones and the Immune System - Where We Stand and Why We Need to Know About It. Eur J Endocrinol (2022) 186(5):R65–77. doi: 10.1530/EJE-21-1171
33. Wang HC, Dragoo J, Zhou Q, Klein JR. An Intrinsic Thyrotropin-Mediated Pathway of TNF-Alpha Production by Bone Marrow Cells. Blood (2003) 101(1):119–23. doi: 10.1182/blood-2002-02-0544
34. van der Weerd K, van Hagen PM, Schrijver B, Heuvelmans SJWM, Hofland LJ, Swagemakers SMA, et al. Thyrotropin Acts as a T-Cell Developmental Factor in Mice and Humans. Thyroid Off J Am Thyroid Assoc (2014) 24(6):1051–61. doi: 10.1089/thy.2013.0396
35. Adamczewski Z, Stasiołek M, Zygmunt A, Śliwka PW, Wieczorek-Szukała K, Lewiński A. Recombinant Human Thyroid-Stimulating Hormone Increases the Percentages of Natural Killer T Cells and B Lymphocytes in Human Peripheral Blood In Vivo. Front Endocrinol (2020) 11:543845. doi: 10.3389/fendo.2020.543845
36. Bağriaçik EU, Klein JR. The Thyrotropin (Thyroid-Stimulating Hormone) Receptor Is Expressed on Murine Dendritic Cells and on a Subset of CD45RBhigh Lymph Node T Cells: Functional Role for Thyroid-Stimulating Hormone During Immune Activation. J Immunol Baltim Md (1950) 164(12):6158–65. doi: 10.4049/jimmunol.164.12.6158
37. Balzan S, Nicolini G, Forini F, Boni G, Del Carratore R, Nicolini A, et al. Presence of a Functional TSH Receptor on Human Erythrocytes. BioMed Pharmacother Biomed Pharmacother (2007) 61(8):463–7. doi: 10.1016/j.biopha.2007.04.009
38. Balzan S, Del Carratore R, Nicolini G, Beffy P, Lubrano V, Forini F, et al. Proangiogenic Effect of TSH in Human Microvascular Endothelial Cells Through its Membrane Receptor. J Clin Endocrinol Metab (2012) 97(5):1763–70. doi: 10.1210/jc.2011-2146
39. Baliram R, Latif R, Zaidi M, Davies TF. Expanding the Role of Thyroid-Stimulating Hormone in Skeletal Physiology. Front Endocrinol (2017) 8:252. doi: 10.3389/fendo.2017.00252
40. Bagriacik EU, Yaman M, Haznedar R, Sucak G, Delibasi T. TSH-Induced Gene Expression Involves Regulation of Self-Renewal and Differentiation-Related Genes in Human Bone Marrow-Derived Mesenchymal Stem Cells. J Endocrinol (2012) 212(2):169–78. doi: 10.1530/JOE-11-0404
41. Brancatella A, Marcocci C. TSH Suppressive Therapy and Bone. Endocr Connect (2020) 9(7):R158–72. doi: 10.1530/EC-20-0167
42. Tsai JA, Janson A, Bucht E, Kindmark H, Marcus C, Stark A, et al. Weak Evidence of Thyrotropin Receptors in Primary Cultures of Human Osteoblast-Like Cells. Calcif Tissue Int (2004) 74(5):486–91. doi: 10.1007/s00223-003-0108-3
43. van Vliet NA, Noordam R, van Klinken JB, Westendorp RG, Bassett JD, Williams GR, et al. Thyroid Stimulating Hormone and Bone Mineral Density: Evidence From a Two-Sample Mendelian Randomization Study and a Candidate Gene Association Study. J Bone Miner Res Off J Am Soc Bone Miner Res (2018) 33(7):1318–25. doi: 10.1002/jbmr.3426
44. van der Deure WM, Uitterlinden AG, Hofman A, Rivadeneira F, Pols HAP, Peeters RP, et al. Effects of Serum TSH and FT4 Levels and the TSHR-Asp727Glu Polymorphism on Bone: The Rotterdam Study. Clin Endocrinol (Oxf) (2008) 68(2):175–81. doi: 10.1111/j.1365-2265.2007.03016.x
45. Sorisky A, Bell A, Gagnon A. TSH Receptor in Adipose Cells. Horm Metab Res Horm Stoffwechselforschung Horm Metab (2000) 32(11–12):468–74. doi: 10.1055/s-2007-978672
46. Endo T, Kobayashi T. Expression of Functional TSH Receptor in White Adipose Tissues of Hyt/Hyt Mice Induces Lipolysis In Vivo. Am J Physiol Endocrinol Metab (2012) 302(12):E1569–1575. doi: 10.1152/ajpendo.00572.2011
47. Murakami M, Kamiya Y, Morimura T, Araki O, Imamura M, Ogiwara T, et al. Thyrotropin Receptors in Brown Adipose Tissue: Thyrotropin Stimulates Type II Iodothyronine Deiodinase and Uncoupling Protein-1 in Brown Adipocytes. Endocrinology (2001) 142(3):1195–201. doi: 10.1210/endo.142.3.8012
48. Martinez-deMena R, Anedda A, Cadenas S, Obregon MJ. TSH Effects on Thermogenesis in Rat Brown Adipocytes. Mol Cell Endocrinol (2015) 404:151–8. doi: 10.1016/j.mce.2015.01.028
49. Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, et al. Presence of Thyrotropin Receptor in Hepatocytes: Not a Case of Illegitimate Transcription. J Cell Mol Med (2009) 13(11–12):4636–42. doi: 10.1111/j.1582-4934.2008.00670.x
50. Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, et al. A Novel Role for Thyroid-Stimulating Hormone: Up-Regulation of Hepatic 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase Expression Through the Cyclic Adenosine Monophosphate/Protein Kinase A/cyclic Adenosine Monophosphate-Responsive Element Binding Protein Pathway. Hepatol Baltim Md (2010) 52(4):1401–9. doi: 10.1002/hep.23800
51. Wang T, Xu J, Bo T, Zhou X, Jiang X, Gao L, et al. Decreased Fasting Blood Glucose Is Associated With Impaired Hepatic Glucose Production in Thyroid-Stimulating Hormone Receptor Knockout Mice. Endocr J (2013) 60(8):941–50. doi: 10.1507/endocrj.EJ12-0462
52. Moon MK, Kang GH, Kim HH, Han SK, Koo YD, Cho SW, et al. Thyroid-Stimulating Hormone Improves Insulin Sensitivity in Skeletal Muscle Cells via cAMP/PKA/CREB Pathway-Dependent Upregulation of Insulin Receptor Substrate-1 Expression. Mol Cell Endocrinol (2016) 436:50–8. doi: 10.1016/j.mce.2016.07.018
53. Drvota V, Janson A, Norman C, Sylvén C, Häggblad J, Brönnegård M, et al. Evidence for the Presence of Functional Thyrotropin Receptor in Cardiac Muscle. Biochem Biophys Res Commun (1995) 211(2):426–31. doi: 10.1006/bbrc.1995.1831
54. Dong J, Gao C, Liu J, Cao Y, Tian L. TSH Inhibits SERCA2a and the PKA/PLN Pathway in Rat Cardiomyocytes. Oncotarget (2016) 7(26):39207–15. doi: 10.18632/oncotarget.9393
55. Alonso H, Fernández-Ruocco J, Gallego M, Malagueta-Vieira LL, Rodríguez-de-Yurre A, Medei E, et al. Thyroid Stimulating Hormone Directly Modulates Cardiac Electrical Activity. J Mol Cell Cardiol (2015) 89(Pt B):280–6. doi: 10.1016/j.yjmcc.2015.10.019
56. Smith TJ, Hegedüs L. Graves’ Disease. N Engl J Med (2016) 375(16):1552–65. doi: 10.1056/NEJMra1510030
57. Ehlers M, Schott M, Allelein S. Graves’ Disease in Clinical Perspective. Front Biosci Landmark Ed (2019) 24(1):35–47. doi: 10.2741/4708
58. Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association Guideline for the Management of Graves’ Hyperthyroidism. Eur Thyroid J. (2022) 7(4):167–86. doi: 10.1159/000490384
59. Rapoport B, McLachlan SM. TSH Receptor Cleavage Into Subunits and Shedding of the A-Subunit; A Molecular and Clinical Perspective. Endocr Rev (2016) 37(2):114–34. doi: 10.1210/er.2015-1098
60. Jang D, Morgan SJ, Klubo-Gwiezdzinska J, Banga JP, Neumann S, Gershengorn MC. Thyrotropin, But Not Thyroid-Stimulating Antibodies, Induces Biphasic Regulation of Gene Expression in Human Thyrocytes. Thyroid Off J Am Thyroid Assoc (2020) 30(2):270–6. doi: 10.1089/thy.2019.0418
61. McGrogan A, Seaman HE, Wright JW, de Vries CS. The Incidence of Autoimmune Thyroid Disease: A Systematic Review of the Literature. Clin Endocrinol (Oxf) (2008) 69(5):687–96. doi: 10.1111/j.1365-2265.2008.03338.x
62. Napolitano G, Bucci I, Di Dalmazi G, Giuliani C. Non-Conventional Clinical Uses of TSH Receptor Antibodies: The Case of Chronic Autoimmune Thyroiditis. Front Endocrinol (2021) 12:769084. doi: 10.3389/fendo.2021.769084
63. Stephenson A, Lau L, Eszlinger M, Paschke R. The Thyrotropin Receptor Mutation Database Update. Thyroid (2020) 30(6):931–5. doi: 10.1089/thy.2019.0807
64. Gershengorn MC, Neumann S. Update in TSH Receptor Agonists and Antagonists. J Clin Endocrinol Metab (2012) 97(12):4287–92. doi: 10.1210/jc.2012-3080
65. Führer D. Constitutive TSH Receptor Activation as a Hallmark of Thyroid Autonomy. Endocrine (2020) 68(2):274–8. doi: 10.1007/s12020-020-02270-z
66. Duprez L, Parma J, Van Sande J, Allgeier A, Leclère J, Schvartz C, et al. Germline Mutations in the Thyrotropin Receptor Gene Cause non-Autoimmune Autosomal Dominant Hyperthyroidism. Nat Genet (1994) 7(3):396–401. doi: 10.1038/ng0794-396
67. Paschke R, Niedziela M, Vaidya B, Persani L, Rapoport B, Leclere J. 2012 European Thyroid Association Guidelines for the Management of Familial and Persistent Sporadic Non-Autoimmune Hyperthyroidism Caused by Thyroid-Stimulating Hormone Receptor Germline Mutations. Eur Thyroid J (2012) 1(3):142–7. doi: 10.1159/000342982
68. Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, et al. Somatic Mutations in the Thyrotropin Receptor Gene Cause Hyperfunctioning Thyroid Adenomas. Nature (1993) 365(6447):649–51. doi: 10.1038/365649a0
69. Tonacchera M, Agretti P, Chiovato L, Rosellini V, Ceccarini G, Perri A, et al. Activating Thyrotropin Receptor Mutations Are Present in Nonadenomatous Hyperfunctioning Nodules of Toxic or Autonomous Multinodular Goiter. J Clin Endocrinol Metab (2000) 85(6):2270–4. doi: 10.1210/jcem.85.6.6634
70. Stephenson A, Eszlinger M, Stewardson P, McIntyre JB, Boesenberg E, Bircan R, et al. Sensitive Sequencing Analysis Suggests Thyrotropin Receptor and Guanine Nucleotide-Binding Protein G Subunit Alpha as Sole Driver Mutations in Hot Thyroid Nodules. Thyroid Off J Am Thyroid Assoc (2020) 30(10):1482–9. doi: 10.1089/thy.2019.0648
71. Grasberger H, Refetoff S. Resistance to Thyrotropin. Best Pract Res Clin Endocrinol Metab (2017) 31(2):183–94. doi: 10.1016/j.beem.2017.03.004
72. Narumi S, Hasegawa T. TSH Resistance Revisited. Endocr J (2015) 62(5):393–8. doi: 10.1507/endocrj.EJ15-0131
73. Kobaly K, Mandel SJ. Hyperthyroidism and Pregnancy. Endocrinol Metab Clin North Am (2019) 48(3):533–45. doi: 10.1016/j.ecl.2019.05.002
74. D’Agostino M, Sponziello M, Puppin C, Celano M, Maggisano V, Baldan F, et al. Different Expression of TSH Receptor and NIS Genes in Thyroid Cancer: Role of Epigenetics. J Mol Endocrinol (2014) 52(2):121–31. doi: 10.1530/JME-13-0160
75. Soh EY, Sobhi SA, Wong MG, Meng YG, Siperstein AE, Clark OH, et al. Thyroid-Stimulating Hormone Promotes the Secretion of Vascular Endothelial Growth Factor in Thyroid Cancer Cell Lines. Surgery (1996) 120(6):944–7. doi: 10.1016/S0039-6060(96)80038-9
76. Hoffmann S, Hofbauer LC, Scharrenbach V, Wunderlich A, Hassan I, Lingelbach S, et al. Thyrotropin (TSH)-Induced Production of Vascular Endothelial Growth Factor in Thyroid Cancer Cells In Vitro: Evaluation of TSH Signal Transduction and of Angiogenesis-Stimulating Growth Factors. J Clin Endocrinol Metab (2004) 89(12):6139–45. doi: 10.1210/jc.2004-1260
77. Orim F, Bychkov A, Shimamura M, Nakashima M, Ito M, Matsuse M, et al. Thyrotropin Signaling Confers More Aggressive Features With Higher Genomic Instability on BRAF(V600E)-Induced Thyroid Tumors in a Mouse Model. Thyroid Off J Am Thyroid Assoc (2014) 24(3):502–10. doi: 10.1089/thy.2013.0038
78. Wu Z, Xi Z, Xiao Y, Zhao X, Li J, Feng N, et al. TSH-TSHR Axis Promotes Tumor Immune Evasion. J Immunother Cancer (2022) 10(1):e004049. doi: 10.1136/jitc-2021-004049
79. Feng F, Han H, Wu S, Wang H. Crosstalk Between Abnormal TSHR Signaling Activation and PTEN/PI3K in the Dedifferentiation of Thyroid Cancer Cells. Front Oncol (2021) 11:718578. doi: 10.3389/fonc.2021.718578
80. Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and Natural History of Graves’ Orbitopathy in a Large Series of Patients With Newly Diagnosed Graves’ Hyperthyroidism Seen at a Single Center. J Clin Endocrinol Metab (2013) 98(4):1443–9. doi: 10.1210/jc.2012-3873
81. Łacheta D, Miśkiewicz P, Głuszko A, Nowicka G, Struga M, Kantor I, et al. Immunological Aspects of Graves’ Ophthalmopathy. BioMed Res Int (2019) 2019:7453260. doi: 10.1155/2019/7453260
82. Kumar S, Coenen MJ, Scherer PE, Bahn RS. Evidence for Enhanced Adipogenesis in the Orbits of Patients With Graves’ Ophthalmopathy. J Clin Endocrinol Metab (2004) 89(2):930–5. doi: 10.1210/jc.2003-031427
83. Nicolì F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, et al. Correlation Between Serum Anti-TSH Receptor Autoantibodies (TRAbs) and the Clinical Feature of Graves’ Orbitopathy. J Endocrinol Invest (2021) 44(3):581–5. doi: 10.1007/s40618-020-01353-y
84. Stöhr M, Oeverhaus M, Lytton SD, Horstmann M, Zwanziger D, Möller L, et al. Predicting the Course of Graves’ Orbitopathy Using Serially Measured TSH-Receptor Autoantibodies by Automated Binding Immunoassays and the Functional Bioassay. Horm Metab Res Horm Stoffwechselforschung Horm Metab (2021) 53(7):435–43. doi: 10.1055/a-1525-2070
85. Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose Thyrotrophin Receptor Expression Is Elevated in Graves’ and Thyroid Eye Diseases Ex Vivo and Indicates Adipogenesis in Progress In Vivo. J Mol Endocrinol (2003) 30(3):369–80. doi: 10.1677/jme.0.0300369
86. Smith TJ, Tsai CC, Shih MJ, Tsui S, Chen B, Han R, et al. Unique Attributes of Orbital Fibroblasts and Global Alterations in IGF-1 Receptor Signaling Could Explain Thyroid-Associated Ophthalmopathy. Thyroid Off J Am Thyroid Assoc (2008) 18(9):983–8. doi: 10.1089/thy.2007.0404
87. Krieger CC, Sui X, Kahaly GJ, Neumann S, Gershengorn MC. Inhibition of TSH/IGF-1 Receptor Crosstalk by Teprotumumab as a Treatment Modality of Thyroid Eye Disease. J Clin Endocrinol Metab (2022) 107(4):e1653–60. doi: 10.1210/clinem/dgab824
88. Girnita L, Smith TJ, Janssen JAMJL. It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease. J Clin Endocrinol Metab (2022) XX:1–12. doi: 10.1210/clinem/dgac045
89. Smith TJ. Recognizing the Putative Role for TSH Receptor Expressing Fibrocytes in Thyroid-Associated Ophthalmopathy may Solve Several Mysteries. Nat Rev Endocrinol (2015) 11(3):171–81. doi: 10.1038/nrendo.2014.226
90. Paik JS, Kim SE, Kim JH, Lee JY, Yang SW, Lee SB. Insulin-Like Growth Factor-1 Enhances the Expression of Functional TSH Receptor in Orbital Fibroblasts From Thyroid-Associated Ophthalmopathy. Immunobiology (2020) 225(2):151902. doi: 10.1016/j.imbio.2019.151902
91. Daumerie C, Ludgate M, Costagliola S, Many MC. Evidence for Thyrotropin Receptor Immunoreactivity in Pretibial Connective Tissue From Patients With Thyroid-Associated Dermopathy. Eur J Endocrinol (2002) 146(1):35–8. doi: 10.1530/eje.0.1460035
92. Zhou JY, Xu B, Lopes J, Blamoun J, Li L. Hashimoto Encephalopathy: Literature Review. Acta Neurol Scand. (2017) 135(3):285–90. doi: 10.1111/ane.12618
93. Churilov LP, Sobolevskaia PA, Stroev YI. Thyroid Gland and Brain: Enigma of Hashimoto’s Encephalopathy. Best Pract Res Clin Endocrinol Metab (2019) 33(6):101364. doi: 10.1016/j.beem.2019.101364
94. Moodley K, Botha J, Raidoo DM, Naidoo S. Immuno-Localisation of Anti-Thyroid Antibodies in Adult Human Cerebral Cortex. J Neurol Sci (2011) 302(1–2):114–7. doi: 10.1016/j.jns.2010.11.027
95. Benvenga S, Guarneri F. Homology Between TSH-R/Tg/TPO and Hashimoto’s Encephalopathy Autoantigens. Front Biosci Landmark Ed (2020) 25(2):229–41. doi: 10.2741/4804
96. Luan S, Bi W, Shi S, Peng L, Li Z, Jiang J, et al. Thyrotropin Receptor Signaling Deficiency Impairs Spatial Learning and Memory in Mice. J Endocrinol (2020) 246(1):41–55. doi: 10.1530/JOE-20-0026
97. Mouri A, Hoshino Y, Narusawa S, Ikegami K, Mizoguchi H, Murata Y, et al. Thyrotoropin Receptor Knockout Changes Monoaminergic Neuronal System and Produces Methylphenidate-Sensitive Emotional and Cognitive Dysfunction. Psychoneuroendocrinology (2014) 48:147–61. doi: 10.1016/j.psyneuen.2014.05.021
98. Becker BA, Fenves AZ, Breslau NA. Membranous Glomerulonephritis Associated With Graves’ Disease. Am J Kidney Dis Off J Natl Kidney Found (1999) 33(2):369–73. doi: 10.1016/S0272-6386(99)70314-8
99. Sasaki K, Yasuda K, Nakanishi K, Rakugi H, Isaka Y, Yamato M. Membranous Nephropathy Secondary to Graves’ Disease With Deposits of Thyroid Peroxidase in an Adult. CEN Case Rep (2014) 3(1):90–3. doi: 10.1007/s13730-013-0093-y
100. Poppe K, Bisschop P, Fugazzola L, Minziori G, Unuane D, Weghofer A. European Thyroid Association Guideline on Thyroid Disorders Prior to and During Assisted Reproduction. Eur Thyroid J (2021) 9(6):281–95. doi: 10.1159/000512790
101. Karga H, Papaioannou G, Polymeris A, Papamichael K, Karpouza A, Samouilidou E, et al. The Effects of Recombinant Human TSH on Bone Turnover in Patients After Thyroidectomy. J Bone Miner Metab (2010) 28(1):35–41. doi: 10.1007/s00774-009-0098-y
102. Sanyal D, Raychaudhuri M. Hypothyroidism and Obesity: An Intriguing Link. Indian J Endocrinol Metab (2016) 20(4):554–7. doi: 10.4103/2230-8210.183454
103. Draman MS, Stechman M, Scott-Coombes D, Dayan CM, Rees DA, Ludgate M, et al. The Role of Thyrotropin Receptor Activation in Adipogenesis and Modulation of Fat Phenotype. Front Endocrinol (2017) 8. doi: 10.3389/fendo.2017.00083
104. Lu S, Guan Q, Liu Y, Wang H, Xu W, Li X, et al. Role of Extrathyroidal TSHR Expression in Adipocyte Differentiation and its Association With Obesity. Lipids Health Dis (2012) 11:17. doi: 10.1186/1476-511X-11-17
105. Ma S, Jing F, Xu C, Zhou L, Song Y, Yu C, et al. Thyrotropin and Obesity: Increased Adipose Triglyceride Content Through Glycerol-3-Phosphate Acyltransferase 3. Sci Rep (2015) 5:7633. doi: 10.1038/srep07633
106. Dale J, Daykin J, Holder R, Sheppard MC, Franklyn JA. Weight Gain Following Treatment of Hyperthyroidism. Clin Endocrinol (Oxf) (2001) 55(2):233–9. doi: 10.1046/j.1365-2265.2001.01329.x
107. Fernandez-Ruocco J, Gallego M, Rodriguez-de-Yurre A, Zayas-Arrabal J, Echeazarra L, Alquiza A, et al. High Thyrotropin Is Critical for Cardiac Electrical Remodeling and Arrhythmia Vulnerability in Hypothyroidism. Thyroid Off J Am Thyroid Assoc (2019) 29(7):934–45. doi: 10.1089/thy.2018.0709
108. Wang X, Mao J, Zhou X, Li Q, Gao L, Zhao J. Thyroid Stimulating Hormone Triggers Hepatic Mitochondrial Stress Through Cyclophilin D Acetylation. Oxid Med Cell Longev (2020) 2020:1249630. doi: 10.1155/2020/1249630
109. Ellerhorst JA, Sendi-Naderi A, Johnson MK, Cooke CP, Dang SM, Diwan AH. Human Melanoma Cells Express Functional Receptors for Thyroid-Stimulating Hormone. Endocr Relat Cancer (2006) 13(4):1269–77. doi: 10.1677/erc.1.01239
110. Vastrad B, Vastrad C, Godavarthi A, Chandrashekar R. Molecular Mechanisms Underlying Gliomas and Glioblastoma Pathogenesis Revealed by Bioinformatics Analysis of Microarray Data. Med Oncol Northwood Lond Engl (2017) 34(11):182. doi: 10.1007/s12032-017-1043-x
111. Kim JWS, Lee S, Lui N, Choi H, Mulvihill M, Fang LT, et al. A Somatic TSHR Mutation in a Patient With Lung Adenocarcinoma With Bronchioloalveolar Carcinoma, Coronary Artery Disease and Severe Chronic Obstructive Pulmonary Disease. Oncol Rep (2012) 28(4):1225–30. doi: 10.3892/or.2012.1938
112. Govindaraj V, Yaduvanshi NS, Krishnamachar H, Rao AJ. Expression of Thyroid-Stimulating Hormone Receptor, Octamer-Binding Transcription Factor 4, and Intracisternal A Particle-Promoted Polypeptide in Human Breast Cancer Tissues. Horm Mol Biol Clin Investig (2012) 9(3):173–8. doi: 10.1515/hmbci-2011-0130
113. Huang WL, Li Z, Lin TY, Wang SW, Wu FJ, Luo CW. Thyrostimulin-TSHR Signaling Promotes the Proliferation of NIH:OVCAR-3 Ovarian Cancer Cells via Trans-Regulation of the EGFR Pathway. Sci Rep (2016) 6:27471. doi: 10.1038/srep27471
Keywords: thyroid, thyroid stimulating hormone, TSH receptor autoantibodies, thyroid diseases, receptors, G-protein coupled receptors
Citation: Vieira IH, Rodrigues D and Paiva I (2022) The Mysterious Universe of the TSH Receptor. Front. Endocrinol. 13:944715. doi: 10.3389/fendo.2022.944715
Received: 15 May 2022; Accepted: 10 June 2022;
Published: 12 July 2022.
Edited by:
Yuji Nagayama, Nagasaki University, JapanReviewed by:
Rauf Latif, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2022 Vieira, Rodrigues and Paiva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inês Henriques Vieira, bmFmbmljaUBnbWFpbC5jb20=
 Inês Henriques Vieira
Inês Henriques Vieira Dírcea Rodrigues
Dírcea Rodrigues