
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 21 October 2022
Sec. Neuroendocrine Science
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.938659
This article is part of the Research TopicEarly Biomarkers in the Investigation of Peripheral and Central Diabetic NeuropathyView all 8 articles
 Weimiao Chen1*†
Weimiao Chen1*† Xiaohong Wu2†
Xiaohong Wu2† Shilin Li3†
Shilin Li3† Yan Zhang4†
Yan Zhang4† Yinqiong Huang2
Yinqiong Huang2 Yong Zhuang2
Yong Zhuang2 Xuefeng Bai2
Xuefeng Bai2 Xiaoyu Chen2
Xiaoyu Chen2 Xiahong Lin5,6*
Xiahong Lin5,6*Objective: To investigate the value of the retinal nerve fiber layer (RNFL) thickness in the optic disc and the cross-sectional area (CSA) of lower limb nerves in the diagnosis of diabetic peripheral neuropathy (DPN) separately and in combination.
Methods: A total of 140 patients with type 2 diabetes were enrolled, including 51 patients with DPN (DPN group) and 89 patients without DPN (NDPN group). Clinical data and biochemical parameters were collected. Electromyography/evoked potential instrument was performed for nerve conduction study. Optical coherence tomography was performed to measure the RNFL thickness of the optic disc. Color Doppler ultrasound was performed to measure CSA of lower limb nerves.
Results: The RNFL thickness was lower and the CSA of the tibial nerve (TN) in the DPN group was larger than that in the NDPN group. The album/urine creatinine ratio, diabetic retinopathy, and CSA of TN at 3 cm were positively correlated with DPN. The RNFL thickness in the superior quadrant of the optic disc was negatively correlated with DPN. For RNFL thickness to diagnose DPN, the area under the curve (AUC) of the superior quadrant was the largest, which was 0.723 (95% confidence interval [CI]: 0.645–0.805), and the best cutoff value was 127.5 μm (70.5% sensitivity, 72.1% specificity). For CSA of TN to diagnose DPN, the AUC of the distance of 5 cm was the largest, which was 0.660 (95% CI: 0.575–0.739), and the best cutoff value was 13.50 mm2 (82.0% sensitivity, 41.6% specificity). For the combined index, the AUC was greater than that of the above two indicators, which was 0.755 (95% CI: 0.664–0.846), and the best cutoff value was 0.376 (64.3% sensitivity, 83.0% specificity).
Conclusions: Patients with DPN have a reduction of the RNFL thickness and an increase in the CSA of TN, and these two changes are related to DPN. The RNFL thickness of the optic disc and the CSA of TN can be used as diagnostic indicators of DPN, and the combination of the two indicators has a higher diagnostic value.
The prevalence of diabetes is increasing year by year. According to the International Diabetes Federation, approximately 463 million adults (20–79 years old) are living with diabetes, and this will rise to 700 million by 2045 (1). Macrovascular and microvascular complications of diabetes are the main cause of morbidity and mortality of diabetes. Among them, diabetic peripheral neuropathy (DPN) is the most common diabetic microvascular complication. According to the statistics of the latest research, the prevalence of DPN for type 2 diabetes mellitus (T2DM) is 26.71% (2), and the prevalence of DPN for type 1 diabetes mellitus (T1DM) is 11% (3). The diagnosis of DPN is easily missed because of its hidden course and the characteristics of exclusive diagnosis. Approximately, 50% of DPN patients have no typical symptoms and need to be screened through physical and electrophysiological examinations. The consequences of DPN progression to late stage are severe, including pathological neuralgia, foot ulcers, Charcot’s joint, and even amputation (4, 5). DPN has a significant adverse impact on patients and society, but usually when DPN is diagnosed, its consequences are irreversible (6–8). In summary, the early screening and prevention of DPN are urgently needed.
The American Diabetes Association (ADA) (9) recommends that patients with T2DM and T1DM with a course of more than 5 years should be screened for complications every year. The screening for DPN includes symptom inquiry and five peripheral nerve tests (ankle reflex, vibration sensation, 10-g monofilament pressure sensation, superficial pain, and temperature sensation) (10). Nerve conduction study (NCS) is considered the most important DPN diagnosis method. Besides, quantitative assessment such as the Michigan neuropathy screening instrument is used for the screening of DPN. Corneal confocal microscopy, quantitative sensory testing, and skin biopsy technique are applied to diagnose small fibrous neuropathy. However, each of the above method has certain limitations. For example, some of them have poor repeatability and insufficient sensitivity and specificity, some are expensive and not accessible enough, and some are invasive methods that cannot easily be accepted by patients (11–15). Therefore, it is still necessary to explore convenient and efficient methods and strategies for screening and diagnosing DPN. From the perspective of anatomy, diabetes peripheral neuropathy can be divided into small fiber neuropathy, large fiber neuropathy, and mixed small and large fiber neuropathy (9). Retinal nerve fiber is a small unmyelinated nerve fiber, and the RNFL represents the axon of retinal ganglion cells. The tibial nerve (TN) is a large myelinated nerve fiber. Combining the assessment of large and small fiber neuropathy may improve the diagnostic efficiency for DPN. Previous studies of our team have shown that the use of optical coherence tomography (OCT) measuring the retinal nerve fiber layer (RNFL) thickness in the optic disc area has a value for clinical screening of DPN (16). Using OCT to measure the thickness of the RNFL is a method to assess small fiber neuropathy, and the detection method of large fiber neuropathy can be accomplished by color Doppler ultrasound (CDUS). In recent years, CDUS has been increasingly used in the evaluation of peripheral nerves. Many studies have proved that CDUS has broad prospects for application in the diagnosis of DPN (17). The above two examinations are both non-invasive, convenient, and inexpensive. In addition, they provided objective data indicators and relatively high repeatability. Combining the two examinations may improve the efficiency of early diagnosis of DPN. In this study, OCT was used to quantitatively measure the optic disc RNFL thickness, and CDUS was used to measure the cross-sectional area (CSA) of lower limb nerves. It is supposed to explore the diagnostic value of the combination of the two indicators and provide a new and efficient strategy for clinical screening and diagnosis of DPN.
Patients with T2DM who were hospitalized in the Department of Endocrinology of The Second Affiliated Hospital of Fujian Medical University (Quanzhou, China) from January 2018 to December 2020 were recruited. According to the inclusion and exclusion criteria, 140 patients were enrolled, including 51 patients with DPN (DPN group) and 89 patients without DPN (NDPN group). All the subjects were Chinese, with ages of 25–80 years. DPN was diagnosed according to the diagnostic criteria of the ADA position statement “confirmed DPN” (18). The inclusion criteria were as follows (1): patients with T2DM who met the diagnostic criteria of diabetes proposed by the World Health Organization in 1999 (2); corrected visual acuity of ≥4.6 (standard logarithmic visual acuity table); (3) diopter of ≤ ± 3.0 D; (4) binocular intraocular pressure range of ≤21 mm Hg (1 mm Hg = 0.133 kPa), no history of ocular hypertension; (5) no history of internal eye surgery, laser, or trauma; (6) no obvious abnormality in the anterior segment of the eye examined by a slit lamp; and (7) selection of the eye on the dominant hand side, and if it did not meet the standard, the other side was selected. The exclusion criteria were as follows: (1) fundus diseases caused by non-diabetes; (2) fundus examination with an obvious opacity of refractive media and diseases that could not be fixed; (3) acute or severe chronic complications of diabetes; (4) inflammatory or immune diseases, tumors, thyroid diseases, vitamin B deficiency, history of exposure to poison, or hereditary peripheral neuropathy; (5) neuropathy due to other causes like cerebral infarction, cerebral hemorrhage, Guillain–Barre syndrome cervical, lumbar lesions, and so on; (6) severe vascular diseases like lower limb arteriosclerosis obliterans, venous thrombosis in lower extremity, and so on; (7) history of severe trauma and surgery of the lower limbs; and (8) patients with severe cardiac, hepatic, and renal insufficiency. Flow chart of the study participants is shown in Figure S1 in Supplementary Materials.
Clinical data and biochemical parameters were collected. Glycosylated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (Model: D10, Bole Co., Ltd., USA). Urinary creatinine and urine microalbumin were detected by the automatic biochemistry analyzer (ARCHITECT c4000, Abbott Inc., USA). The estimated glomerular filtration rate and album/urine creatinine ratio (ACR) were calculated with Formula S1 and Formula S2 (Supplementary Materials).
A detailed inquiry and recording of clinical symptoms of DPN and five peripheral nerve tests (ankle reflex, vibration sensation, 10-g monofilament pressure sensation, superficial pain, and temperature sensation).
Nerve conduction velocity was detected by the same physician with an electromyography/evoked potential instrument (Keypoint 9033A07, Focus Company, Denmark), and the double-blind method was adopted. In a quiet room with a constant temperature of 25°C, the patient was in the supine position with limbs fully exposed and relaxed, and limb temperature was maintained at 32–36°C. It was performed according to the process recommended by the American Academy of Neurology and the American Association of Neuromuscular & Electrodiagnostic Medicine: (a) Measure the sensory conduction of the unilateral sural nerve (SN), median nerve, and ulnar nerve. (b) Measure the motor conduction and F wave of the unilateral common peroneal nerve, TN, median nerve, and ulnar nerve. Diagnostic criteria: It is judged to be DPN positive if there are abnormalities in two or more nerves, and one of which must be a lower limb nerve; otherwise, it is negative (19).
Best corrected visual acuity was measured with an autorefractor (Topcon KR 800, Topcon Medical Systems Inc., Japan) and corrected. Intraocular pressure was measured with a non-contact tonometer (Canon TX-20, Canon Inc., Japan).
A slit lamp (Topcon SL-3G, Topcon Medical Systems Inc., Japan) was used to observe the conjunctiva, cornea, anterior chamber, lens, and vitreous body in turn. The pupil was dilated with compound tropicamide eye drops, and then the fundus was examined with a slit lamp and Volk 90D front lens (Volk Inc., USA).
It was performed with a fundus camera (Canon CR-2, Canon Inc., Japan). The pupil was coaxial with the light, and the patients were told to move the eye in different directions. The physician then adjusted the focal length and definition of the image and selected the same ideal image as the object of study.
Spectral domain OCT (Spectralis®, Heidelberg Engineering Inc., Heidelberg, Germany) was used to scan the whole macular. The subject adopted a sitting position and placed their mandible on the jaw support at a suitable height. The subject was told to look at the green indicator light, and the lens was aimed at the pupil. The scanning centerline was adjusted to pass through the fovea center of the macular. The scanning mode was 768 × 496, and the scanning range was scanned with a macular fovea of 30°×25° volume. The scanning speed was 4,000 A/s, and the resolution was 5 μm longitudinally and 6 μm horizontally. A total of 61 B-scans were performed, and the mode of automatic real-time noise reduction was turned on to ensure that the quality of each scan was above 20 dB. For the RNFL measurement (Figure 1), we performed circular scanning of the optic disc with a diameter of 3.46 mm centered on the optic disc. The RNFL thickness was measured around the whole optic disc including four quadrants (superior, nasal, inferior, and temporal). Scanning was performed three times, and the clearest image with the strongest signal was selected and shown as a pattern diagram. Four-quadrant RNFL thicknesses and the overall average RNFL thickness were automatically analyzed using the analysis software supplied with the system. All of the above examinations were carried out by the same professionally trained ophthalmologist with the double-blind method.
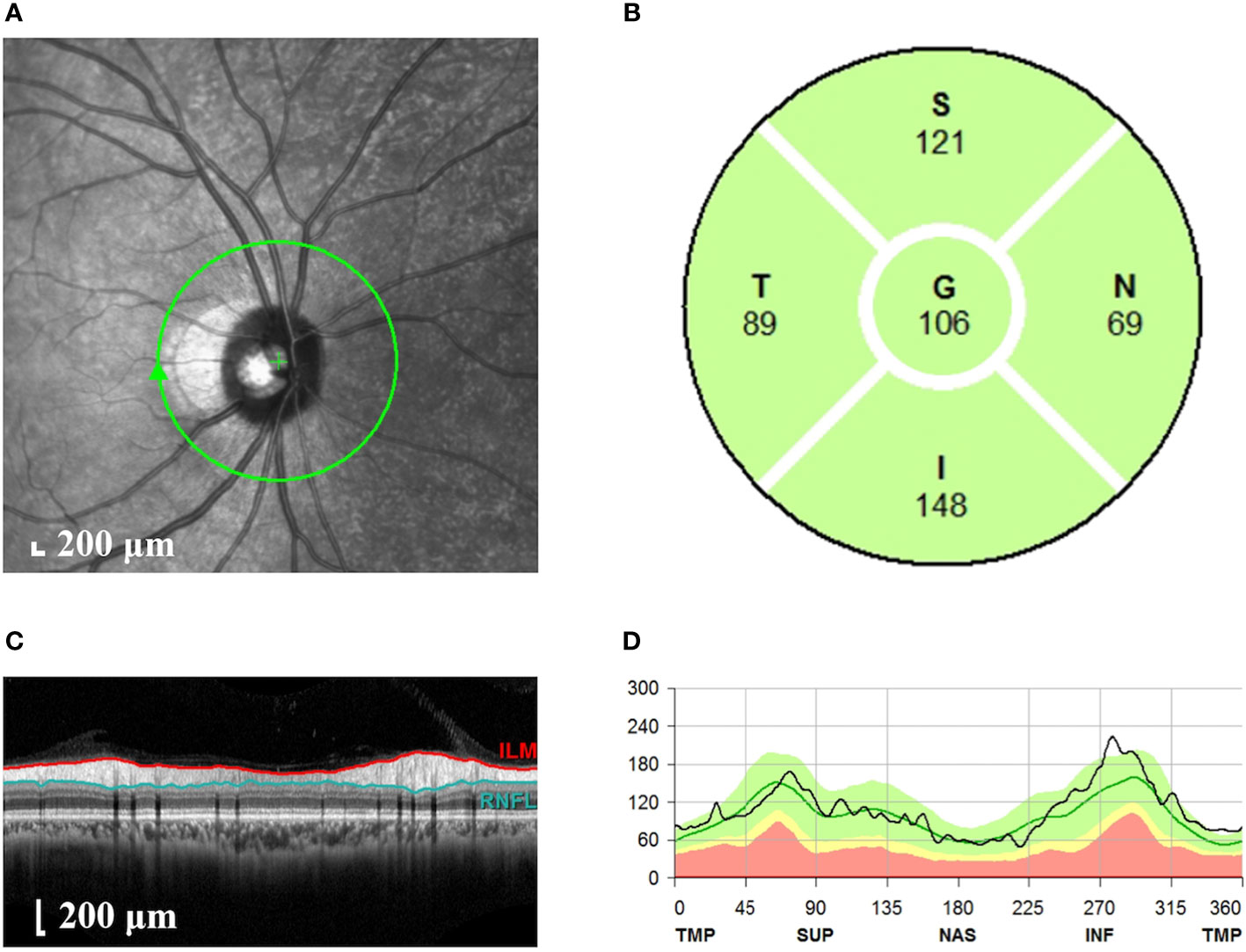
Figure 1 Measurement of the RNFL thickness of the optic disc. (A) Scanning image of the optic disc area. The green circle represents the scanning range. (B) Longitudinal section of the optic disc nerve fibers. The red line represents the internal limiting membrane; the blue line represents RNFL. (C) The thickness of RNFL in each quadrant of the optic disc. S, superior quadrant; N, nasal quadrant; I, inferior quadrant; T, temporal quadrant; G, overall average. (D) Pattern diagram of RNFL thickness. The abscissa indicates the quadrant, the ordinate indicates the thickness, the green line represents the normal value, and the black line represents the measured value. RNFL, retinal nerve fiber layer.
The examinations were performed by the same professionally trained sonographer with the double-blind method. CDUS (model: LOGIQ E9, GE Co., Ltd., USA) with linear array probe (model: ML6-15, GE Co., Ltd., USA) was used to detect nerves and measure the CSA of lower limb nerves. The probe frequency was 10–18 MHz, adjusted according to the depth of nerves. The subjects adopted a supine position in a quiet temperature-controlled room, with ankles positioned in slight plantar flexion and slightly rotated externally, cautioned not to move their feet or ankles during the CDUS examination. The sonographer detected the TN at the medial malleolus and 1, 3, and 5 cm to the medial malleolus and the SN at 10 and 20 cm to the heel. The nerve contour in the CDUS image based on the high echo of the nerve membrane was outlined by the sonographer, and the CSA of lower limb nerves were measured automatically (Figure 2).
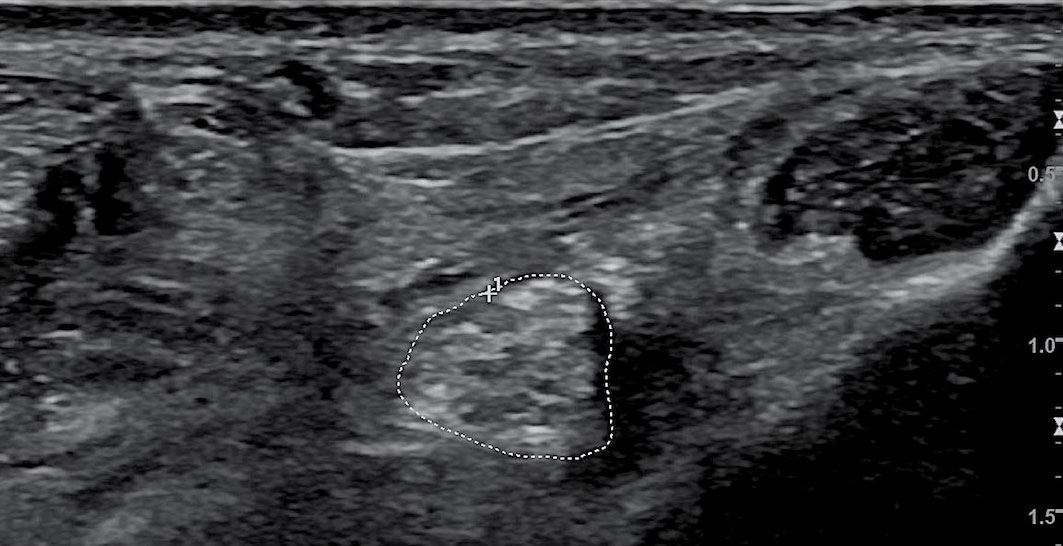
Figure 2 Color Doppler ultrasound image of the tibial nerve. The area circled by the dashed line is the CSA of the tibial nerve. CSA, cross-sectional area.
SPSS 26.0 statistical software was used. The data were tested for normality, and those with a normal distribution were expressed as mean ± standard deviation, whereas those with a non-normal distribution were expressed as median (quartile intervals). For quantitative data with a normal distribution, Student’s t-test was used to test the significance of differences between the two groups. To measure data with a non-normal distribution, the Mann–Whitney U non-parametric test was used. The qualitative data were tested by the chi-square test. Binary logistic regression (LR backward) was used to analyze the correlation of clinical parameters, CSA of nerves, RNFL thickness, and DPN. The receiver operator characteristic (ROC) curve was drawn using MedCalc software (version 20.0.15, MedCalc Software Ltd., Belgium) to calculate the area under the curve (AUC). The CSA of nerves, RNFL thicknesses, and the combined index of the CSA of nerves and RNFL thicknesses in the diagnosis of DPN were compared. P < 0.05 was considered to indicate a statistically significant difference.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China. Informed consent was obtained from all patients for being included in the study.
There were 51 cases in the DPN group, with an average age of 55.29 ± 12.44 years, and 89 cases in the NDPN group, with an average age of 51.12 ± 10.29 years. The duration of diabetes mellitus (DM) in the DPN group was longer than that in the NDPN group (9.57 [4.00, 13.00] vs. 5.64 [1.00, 8.50] years, p = 0.000). The systolic blood pressure (SBP) in the DPN group was higher than that in the NDPN group (130.80 ± 16.79 vs. 125.00 ± 11.79 mm Hg, p = 0.032). The blood urea nitrogen (BUN) in the DPN group was higher than that in the NDPN group (6.25 ± 3.74 vs. 5.01 ± 1.42 mmol/L, p = 0.033). The ACR in the DPN group was higher than that in the NDPN group (256.44 [10.08, 180.35] vs. 21.21 [6.10, 18.26] mg/g, p = 0.000). The diabetic retinopathy (DR) (%) in the DPN group was higher than that in the NDPN group (47.06% vs. 8.99%, p = 0.000) (Table 1).
There were significant differences in the overall average, superior quadrant, and inferior quadrant RNFL thicknesses. The RNFL thicknesses in the DPN group were lower than that in the NDPN group in the overall average (102.05 ± 12.91 vs. 106.94 ± 10.73 μm, p = 0.025), superior quadrant (119.28 ± 20.04 vs. 135.14 ± 17.72 μm, p = 0.000), and inferior quadrant (129.95 ± 18.12 vs. 140.98 ± 21.32 μm, p = 0.005). There were no significant differences in temporal quadrant or nasal quadrant RNFL thickness between the NDPN and DPN groups (Table 2).
There were significant differences in the CSA of TN at 1, 3, and 5 cm to the medial malleolus. The CSA of TN in the DPN group was larger than that in the NDPN group at the distances of 1 cm (19.44 ± 6.63 vs. 17.31 ± 4.72 mm², p = 0.048), 3 cm (18.07 ± 5.75 vs. 15.81 ± 3.95mm², p = 0.016), and 5 cm (17.62 ± 5.01 vs. 15.01 ± 4.59 mm², p = 0.002) to the medial malleolus. There were no significant differences in the CSA of TN at the medial malleolus and the SN at 10 and 20 cm to heel between the NDPN and DPN groups (Table 3).
Binary logistic regression (LR backward) was used to analyze the correlation of clinical parameters, RNFL thickness of the optic disc area, CSA of lower limb nerves, and DPN. There were significant statistical differences in the duration of DM, SBP, BUN, ACR, DR, RNFL thickness in overall average, superior and inferior quadrant of the optic disc, and the CSA of TN at 1, 3, and 5 cm to the medial malleolus between the DPN group and the NDPN group. The above variables were taken as independent variables, and DPN was taken as a dependent variable. ACR (B = 0.008, p = 0.021), DR (B = 1.235, p = 0.014), and the CSA of TN at 3 cm from the medial malleolus (B = 0.157, p = 0.008) were positively correlated with DPN.The RNFL thickness in the superior quadrant of the optic disc (B = −0.037, p = 0.010) was negatively correlated with DPN (Table 4).
The diagnostic values of the RNFL thickness in optic disc, the CSA of TN, and the combined index were evaluated by calculating the AUC of the ROC curve (Figure 3). A comparison of the diagnostic value of the indices is shown in Table 5, and the comparison of ROC curves is shown in Table S1. There was no significant difference in the AUC of the overall average, superior quadrant, or inferior quadrant RNFL thickness for DPN diagnosis. The AUC of the superior quadrant of RNFL thickness for diagnosing DPN was the largest, which was 0.723 (95% confidence interval [CI]: 0.645–0.805); sensitivity was 70.5%, and specificity was 72.1%; the best cutoff value was 127.50 μm. There was no significant difference in the AUC of the CSA of TN at 1, 3, and 5 cm from the medial malleolus for DPN diagnosis. The AUC of the CSA of TN at 5 cm for diagnosing DPN was the largest, which was 0.660 (95% CI: 0.575–0.739); sensitivity was 82.0%, and specificity was 41.6%; the best cutoff value was 13.50 mm2. The AUC of the combined index was greater than that of the above two indices, which was 0.755 (95% CI: 0.664–0.846); sensitivity was 64.3%, and specificity was 83.0%; the best cutoff value was 0.376.
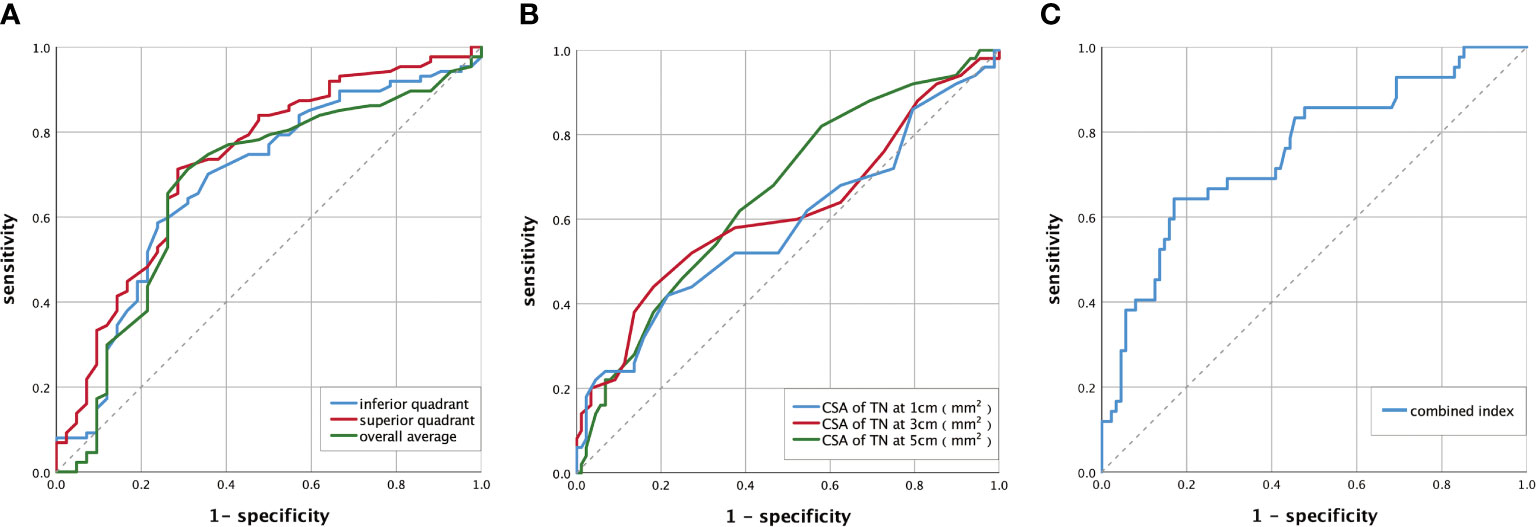
Figure 3 ROC curve of DPN diagnosed by RNFL thickness of the optic disc, the CSA of TN, and the combined index. (A) The ROC curve of DPN diagnosed by RNFL thickness of the optic disc. The AUC of the overall average was 0.675(95% CI: 0.587–0.755). The AUC of the superior quadrant was 0.723 (95% CI: 0.645–0.805). The AUC of the inferior quadrant was 0.686 (95% CI: 0.599–0.765). (B) The ROC curve of DPN diagnosed by CSA of TN. The AUC of the CSA of TN was 0.584 (95% CI: 0.497–0.667) at 1 cm, 0.617 (95% CI: 0.530–0.698) at 3 cm, and 0.660 (95% CI: 0.575–0.739) at 5 cm. (C) The ROC curve of DPN diagnosed by the combined index. The AUC of the combined index was 0.755 (95% CI: 0.664-0.846). AUC, area under the curve; ROC, receiver operator characteristic; DPN, diabetic peripheral neuropathy; CI, confidence interval; CSA, the cross-sectional area; TN, tibial nerve; RNFL, retinal nerve fiber layer. Combined index: the combined index of the CSA of TN at 3 cm and RNFL thickness in the superior quadrant. The calculation process is shown in Supplementary Materials and Formula S3 (Supplemental Data).
This study suggests that patients with DPN have a reduction of the RNFL thickness and an increase in the CSA of TN, and these two changes are related to DPN. The RNFL thickness of the optic disc and the CSA of TN can be used as diagnostic indicators of DPN, and the combination of the two indicators has a higher diagnostic value. The AUC of the combined index was 0.755 (95% CI: 0.664–0.846); sensitivity was 64.3%, and specificity was 83.0%; the best cutoff value was 0.376.
DPN has a huge impact on the life of patients and brings a heavy burden to society. Early identification and treatment of DPN can relieve symptoms, reduce sequelae, and improve the quality of patients’ life. The ADA (9) recommend that patients with T2DM and T1DM with a course of more than 5 years should be screened for complications every year, including DPN. However, the proportion of routine DPN screening in clinical practice is still low (20). DPN is an exclusive diagnosis with insidious onset, and about half of DPN patients have no typical symptoms. A definitive diagnosis of DPN requires a complete and detailed symptom inquiry, a neurological examination, and an electromyography examination. There are limitations in all the existing screening and diagnosis methods. For example, some examination methods, such as the five peripheral nerve tests for DPN, have low repeatability. Twelve physicians assessed 24 patients with DM with physical features and voice disguised. The result showed that diagnosis from signs and symptoms was excessively variable among physicians (15). It may be because of the differences in the operation skills of the physicians and the subjective factors of the patients. Therefore, it is important to seek new ones.
DPN can be divided into small fiber neuropathy, large fiber neuropathy, or a mixture of large and small fiber neuropathy according to the types of nerve fibers involved (21). Some previous studies have suggested that small nerve fibers are abnormal and large nerve fibers are complete in the early stage of DPN (22). Unmyelinated small fibers are first affected during the DPN course, probably because they lack the protection provided by the myelin sheath (23, 24). Ziegler’s studies have shown that patients with early DPN had homogenous damage to both large and small nerve fibers (25). Therefore, the combined screening of large and small fiber neuropathy has a good theoretical basis as a strategy for screening DPN. Some studies have confirmed the feasibility of the DPN screening strategy by combining large and small nerves. Tesfaye used DPNCheck to evaluate large nerve fibers and Sudoscan to evaluate small nerve fibers for screening DPN. The prevalence of DPN was diagnosed at 51.5% (sensitivity 0.84, specificity 0.68) by DPNCheck, 38.2% (sensitivity 0.77, specificity 0.68) by Sudoscan, and 61.9% (sensitivity 0.93, specificity 0.53) by the combination of DPNCheck and Sudoscan (26). It improved the screening sensitivity and verified the feasibility of this strategy. A cross-sectional study of 145 patients with T2DM has shown that the combination of large fiber neuropathy assessed by NCS and small fiber neuropathy assessed by the thermal threshold test can identify the vast majority of patients with DPN, subclinical DPN, and clinical diagnosis of DPN (27). This study also inspires us that using the strategy of combining large and small nerves to screen DPN may be an effective way for the early detection of DPN. It may help in achieving early intervention of DPN, slowing down the disease progression and minimizing the harm. However, Sudoscan equipment is expensive and not easily accessible. The determination of the thermal threshold depends on the subjective feelings of the patient, and there are many influencing factors. In this study, we tried to find out two methods that are more commonly used and more accessible in clinical practice to detect large and small nerves, respectively, and combine them to diagnose DPN.
In recent years, a few studies have suggested that CDUS can be used for the assessment of peripheral neuropathy. It has the advantages of affordability, convenience, and non-invasiveness. The data indicate that ultrasound machines allowed real-time and centralized imaging of nerves and surrounding structures with high fidelity, which are able to quickly identify peripheral neuropathy (28–30). Besides, the patient has no discomfort or radiation exposure during the examination period. Diagnosis of neuropathy by CDUS is mainly based on the reliability of high resolution for measurement of large nerve fiber CSA (31, 32). In some previous studies, CDUS was used to detect CSA of peripheral nerves in patients with diabetes. It has been found that the CSA of the median nerve and TN in DPN patients is significantly higher than that in the non-DPN group. The degree of CSA increase is related to the severity of DPN. Our study showed that the CSA of TN was significantly larger in the DPN group than in the NDPN group at the distances of 1, 3, and 5 cm to the medial malleolus. The increase in nerve CSA is a manifestation of nerve swelling, and its main reasons include the increase in water content during the reductase conversion of glucose to sorbitol (33), the accumulation of microfibrillar material in the vicinity of perineurial cells, the increased diameter of collagen fibers in the endoneurium (34), the thickening of the vasculature and the combination of basement membrane and Schwann cells, etc. Using the CSA of nerve fibers measured by CDUS to diagnose DPN, sensitivity and specificity can reach 69.0%–80% and 70%–77%, respectively (35, 36). In a cross-sectional study, Riazi and his teammates used nerve ultrasound examination to detect DPN in patients. The result showed that the CSA of the posterior TN in the DPN group at the distances of 1, 3, and 5 cm to the medial malleolus was significantly larger compared with the control group. The CSA measured at 3 cm to the medial malleolus had an optimal threshold value for identification of DPN in their study, which was 19.01 mm2 (sensitivity 0.690, specificity 0.770) (36). In this study, the diagnostic value of CSA at 5 cm to the medial malleolus for the diagnosis of DPN is the greatest, and the cutting value is 13.50 mm² (sensitivity 0.820, specificity 0.416). There is no significant difference in the ROC curve of CSA of TN in diagnosing DPN at 1, 3, and 5 cm to the medial malleolus, so the result of this research is consistent with that of previous research. Based on the above, using CDUS to measure the CSA of nerve fibers may be an accessible and effective way to evaluate large nerves.
Previous views indicated that the retinal nerve was an extension of the central nerve and was closely related to central neuropathy. For example, retinal neurodegeneration often occurs in Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease (37). In recent years, studies have found that retinal neuropathy is also closely related to peripheral neuropathy, and OCT has been introduced as a surrogate end point for evaluating retinal nerve fiber loss (38). Retinal nerve fiber is a small unmyelinated nerve fiber, and the RNFL represents the axon of retinal ganglion cells. It has been found that diabetic retinopathy is related to peripheral neuropathy; the RNFL thickness is related to the neuropathy disability score (NDS) of DPN patients. The RNFL thickness in the overall average, superior quadrant, and inferior quadrant of the optic disc in DPN patients with an NDS of ≥3 is significantly smaller than that in patients with diabetes but without DPN (39–41). These conclusions provide a reliable basis for RNFL thickness measured by OCT to diagnose DPN. Previous research by our team used OCT to measure the thickness of RNFL in the optic disc area and found that the thickness of RNFL was related to DPN and can be used as a diagnostic method for DPN. In this study, the result shows that RNFL thicknesses were significantly lower in the DPN group than that in the NDPN group in the overall average, superior quadrant, and inferior quadrant. However, there were no significant differences in temporal quadrant or nasal quadrant RNFL thickness between the NDPN and DPN groups. Studies have shown that there are significant differences in the shape of the four quadrants of the human posterior sclera sieve plate. The number and area of the ethmoidal foramen are significantly larger in the superior and inferior quadrants than in the nasal and temporal quadrants, so there are significantly more nerve fiber bundles in the superior or inferior quadrant than in the nasal or temporal quadrant (42). The decrease in nerve fiber bundle penetration indicates that the dynamic range of measurable RNFL thickness is very small, which may explain why there is no difference in RNFL thickness between the two groups in the temporal and nasal quadrants. The result of our previous study shows that the AUC of RNFL thickness in the inferior quadrant for diagnosing DPN was the largest, which was 0.755 (95% CI: 0.652–0.840, p < 0.001). The best cutoff value was 131 μm; sensitivity was 81.67%, and specificity was 68.97% (16). In this study, the AUC of the superior quadrant of RNFL thickness for diagnosing DPN is the largest, which is 0.723 (95% CI: 0.645–0.805). Sensitivity is 70.5%, and specificity is 72.1%; the best cutoff value is 127.5μm. There is no significant difference in the AUC of RNFL thickness in the overall average, superior quadrant, and inferior quadrant for the diagnosis of DPN. Therefore, the conclusion of this research is consistent with that of previous studies. Our study suggests that using OCT to measure RNFL thickness can be a feasible method to evaluate small fiber neuropathy. In addition, chronic diabetes complications must be regularly screened according to the global guidelines for the prevention and treatment of diabetes. Fundus examination of diabetes should include OCT to assess macular degeneration or edema. Early screening of DPN can be achieved while regularly evaluating fundus lesions in patients with diabetes. Therefore, it is a good choice to use OCT to evaluate small fiber neuropathy and screen DPN.
In this study, the RNFL thickness measured by OCT can reflect the lesions of small nerve fibers, whereas the CSA of the lower limb nerve measured by CDUS can reflect the lesions of large nerve fibers. Both examinations are non-invasive, are simple to operate, and have been widely used in clinical practice. Sensitivity/specificity of RNFL thickness, CSA, and the combined index to diagnose DPN was 70.5%/72.1%, 82.0%/41.6%, and 64.3%/83.0%, respectively. The AUC of the RNFL thickness, CSA, and the combined index were 0.723 (95% CI: 0.645–0.805), 0.660 (95% CI: 0.575–0.739), and 0.755 (95% CI: 0.664–0.846). The results show that the CSA of TN and RNFL thicknesses in the optic disc can be used as the diagnostic index of DPN, respectively. Furthermore, the DPN diagnostic value and specificity of the combination of CDUS and OCT is better than the CDUS or OCT alone. Therefore, the combination of neural CDUS and OCT in the diagnosis of DPN can be used as a new clinical strategy for the diagnosis of DPN. It is inspired by combining two methods detecting small and large fiber neuropathy could be a new strategy in DPN diagnosis. It may play an important role in clinical practice and benefit patients with DPN in the future. It is expected that DPN can be identified in an early stage and be intervened as soon as possible. Consequently, heavy stress caused by DPN on individuals and society could be released. In clinical practice, clinicians should make a reasonable choice of diagnostic methods according to the needs of the disease and the actual situation.
There are some limitations to this study. Because of the small sample size of this study, the subjects in the study were divided into the DPN group and the NDPN group, and the criteria for DPN was “confirmed DPN” according to ADA. In the consequent study, we will enroll more participants and divide the groups according to the definitions of minimal criteria for DPN (possible, probable, confirmed, and subclinical DPN) (18). Another limitation is that the RNFL thickness and the CSA of lower limb nerves are affected by many factors such as race, age, duration of diabetes, and the degree of retinal microangiopathy. Consequently, these findings must be verified by multicenter, large-sample, prospective observational studies.
Patients with DPN have a reduction of the RNFL thickness and an increase in the CSA of the TN, and these two changes are related to DPN. The RNFL thickness of the optic disc and the CSA of TN can be used as diagnostic indicators of DPN, and the combination of the two indicators has a higher diagnostic value.
The processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study. Requests to access the datasets should be directed to XL, MjQxMzM2ODc5MkBxcS5jb20=.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Fujian Medical University, Quanzhou, China. The patients/participants provided their written informed consent to participate in this study.
XL designed the study. YaZ and S L performed Optical Coherence Tomography and Color Doppler Ultrasound examinations, respectively. WC, XW, XB, YoZ and X C collected the data. WC, XW, and YH performed data processing and data analysis. WC and XW wrote and edited the manuscript. All authors edited and approved the final manuscript, and they have full access to all the data in the study and take responsibility for the integrity and security of the data.
This work was supported by the Fujian Provincial Health Technology Project, China (grant number 2020CXA044); the Natural science Foundation of Fujian Province, China (grant number 2020J01221); Key Young Talents Health Training Project of Fujian Province, China (grant number 2020GGA057).
Many thanks to colleagues at the Department of Endocrinology at The Second Affiliated Hospital of Fujian Medical University (China) for providing comprehensive help to carry out the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.938659/full#supplementary-material
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Lu Y, Xing P, Cai X, Luo D, Li R, Lloyd C, et al. Prevalence and risk factors for diabetic peripheral neuropathy in type 2 diabetic patients from 14 countries: Estimates of the interpret-dd study. Front Public Health (2020) 8:534372. doi: 10.3389/fpubh.2020.534372
3. Mizokami-Stout KR, Li Z, Foster NC, Shah V, Aleppo G, McGill JB, et al. The contemporary prevalence of diabetic neuropathy in type 1 diabetes: Findings from the T1d exchange. Diabetes Care (2020) 43(4):806–12. doi: 10.2337/dc19-1583
4. Zhang Y, Zhang M, Lu D-b. Analysis of the causes and characteristic of amputation in diabetic patients with peripheral neuropathy. Med J West China (2011) 7:1241–3. doi: 10.3969/.issn.1672-3511.2011.07.014
5. Gylfadottir SS, Christensen DH, Nicolaisen SK, Andersen H, Callaghan BC, Itani M, et al. Diabetic polyneuropathy and pain, prevalence, and patient characteristics: A cross-sectional questionnaire study of 5,514 patients with recently diagnosed type 2 diabetes. Pain (2020) 161(3):574. doi: 10.1097/j.pain.0000000000001744
6. Weisman A, Bril V, Ngo M, Lovblom LE, Halpern EM, Orszag A, et al. Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PloS One (2013) 8(3):e58783. doi: 10.1371/journal.pone.0058783
7. Selvarajah D, Kar D, Khunti K, Davies MJ, Scott AR, Walker J, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol (2019) 7(12):938–48. doi: 10.1016/S2213-8587(19)30081-6
8. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. Jama (2005) 293(2):217–28. doi: 10.1001/jama.293.2.217
9. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: A position statement by the American diabetes association. Diabetes Care (2017) 40(1):136–54. doi: 10.2337/dc16-2042
10. Association AD. Standards of medical care in diabetes–2013. Diabetes Care (2013) 36(Supplement 1):S11–66. doi: 10.2337/dc13-S011
11. Dyck PJB, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: New insights into pathophysiology and treatment. Muscle Nerve (2002) 25(4):477–91. doi: 10.1002/mus.10080
12. Alam U, Asghar O, Azmi S, Malik RA. General aspects of diabetes mellitus. Handb Clin Neurol. (2014) 126:211–22. doi: 10.1016/B978-0-444-53480-4.00015-1
13. Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care (2004) 27(8):1974–9. doi: 10.2337/diacare.27.8.1974
14. Massie R, Mauermann ML, Staff NP, Amrami KK, Mandrekar JN, Dyck PJ, et al. Diabetic cervical radiculoplexus neuropathy: A distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain (2012) 135(10):3074–88. doi: 10.1093/brain/aws244
15. Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJB, et al. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. Nphys Trial. Muscle Nerve (2010) 42(2):157–64. doi: 10.1002/mus.21661
16. Wu X-h, Fang J-w, Huang Y-q, Bai X-f, Zhuang Y, Chen X-y, et al. Diagnostic value of optic disc retinal nerve fiber layer thickness for diabetic peripheral neuropathy. J Zhejiang University-SCIENCE B (2020) 21(11):911–20. doi: 10.1631/jzus.B2000225
17. Gallardo E, Y-i N, Simon NG. Ultrasound in the diagnosis of peripheral neuropathy: Structure meets function in the neuromuscular clinic. J Neurology Neurosurg Psychiatry (2015) 86(10):1066–74. doi: 10.1136/jnnp-2014-309599
18. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care (2010) 33(10):2285–93. doi: 10.2337/dc10-1303
19. England J, Gronseth G, Franklin G, Miller R, Asbury A, Carter G, et al. Distal symmetric polyneuropathy: A definition for clinical research: Report of the American academy of neurology, the American association of electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology (2005) 64(2):199–207. doi: 10.1212/01.WNL.0000149522.32823.EA
20. Liu F, Bao Y, Hu R, Zhang X, Li H, Zhu D, et al. Screening and prevalence of peripheral neuropathy in type 2 diabetic outpatients: A randomized multicentre survey in 12 city hospitals of China. Diabetes/metabolism Res Rev (2010) 26(6):481–9. doi: 10.1002/dmrr.1107
21. Marathe PH, Gao HX, Close KL. American D iabetes a ssociation s tandards of m edical c are in d iabetes 2017. J Diabetes (2017) 9(4):320–4. doi: 10.1111/1753-0407.12524
22. Dyck PJ, O’Brien PC, Litchy WJ, Harper CM, Klein CJ, Dyck PJB. Monotonicity of nerve tests in diabetes: Subclinical nerve dysfunction precedes diagnosis of polyneuropathy. Diabetes Care (2005) 28(9):2192–200. doi: 10.2337/diacare.28.9.2192
23. Frank T, Nawroth P, Kuner R. Structure–function relationships in peripheral nerve contributions to diabetic peripheral neuropathy. Pain (2019) 160:S29–36. doi: 10.1097/j.pain.0000000000001530
24. Sumner C, Sheth S, Griffin J, Cornblath D, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology (2003) 60(1):108–11. doi: 10.1212/wnl.60.1.108
25. Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes (2014) 63(7):2454–63. doi: 10.2337/db13-1819
26. Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: An effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabetic Med (2018) 35(7):887–94. doi: 10.1111/dme.13630
27. Weng Y-C, Tsai S-S, Lyu R-K, Chu C-C, Ro L-S, Liao M-F, et al. Diabetic distal symmetrical polyneuropathy: Correlation of clinical, laboratory, and electrophysiologic studies in patients with type 2 diabetes mellitus. J Diabetes Res (2020) 2020:1–11. doi: 10.1155/2020/6356459
28. Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Handb Surg (2006) 31(5):726–32. doi: 10.1016/j.jhsa.2006.01.020
29. Hobson-Webb LD, Massey JM, Juel VC. Nerve ultrasound in diabetic polyneuropathy: Correlation with clinical characteristics and electrodiagnostic testing. Muscle Nerve (2013) 47(3):379–84. doi: 10.1002/mus.23625
30. Nakamichi KI, Tachibana S. Ultrasonographic measurement of median nerve cross-sectional area in idiopathic carpal tunnel syndrome: Diagnostic accuracy. Muscle Nerve: Off J Am Assoc Electrodiagnostic Med (2002) 26(6):798–803. doi: 10.1002/mus.10276
31. Alshami AM, Cairns CW, Wylie BK, Souvlis T, Coppieters MW. Reliability and size of the measurement error when determining the cross-sectional area of the tibial nerve at the tarsal tunnel with ultrasonography. Ultrasound Med Biol (2009) 35(7):1098–102. doi: 10.1016/j.ultrasmedbio.2009.01.011
32. Noto Y-i, Shiga K, Tsuji Y, Mizuta I, Higuchi Y, Hashiguchi A, et al. Nerve ultrasound depicts peripheral nerve enlargement in patients with genetically distinct charcot-Marie-Tooth disease. J Neurology Neurosurg Psychiatry (2015) 86(4):378–84. doi: 10.1136/jnnp-2014-308211
33. Lee D, Dauphineíe DM. Morphological and functional changes in the diabetic peripheral nerve: Using diagnostic ultrasound and neurosensory testing to select candidates for nerve decompression. J Am Podiatric Med Assoc (2005) 95(5):433–7. doi: 10.7547/0950433
34. Yagihashi S, Yamagishi S-I, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: Correlation with clinical signs and symptoms. Diabetes Res Clin Pract (2007) 77(3):S184–S9. doi: 10.1016/j.diabres.2007.01.054
35. Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, et al. Sonographic evaluation of the peripheral nerve in diabetic patients: The relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med (2010) 29(5):697–708. doi: 10.7863/jum.2010.29.5.697
36. Riazi S, Bril V, Perkins BA, Abbas S, Chan VW, Ngo M, et al. Can ultrasound of the tibial nerve detect diabetic peripheral neuropathy?: a cross-sectional study. Diabetes Care (2012) 35(12):2575–9. doi: 10.2337/dc12-0739
37. Yu J-g, Feng Y-f, Xiang Y, Huang J-h, Savini G, Parisi V, et al. Retinal nerve fiber layer thickness changes in Parkinson disease: A meta-analysis. PloS One (2014) 9(1):e85718. doi: 10.1371/journal.pone.0085718
38. Lamirel C, Newman NJ, Biousse V. Optical coherence tomography (Oct) in optic neuritis and multiple sclerosis. Rev neurologique (2010) 166(12):978–86. doi: 10.1016/j.neurol.2010.03.024
39. Srinivasan S, Pritchard N, Vagenas D, Edwards K, Sampson GP, Russell AW, et al. Retinal tissue thickness is reduced in diabetic peripheral neuropathy. Curr eye Res (2016) 41(10):1359–66. doi: 10.3109/02713683.2015.1119855
40. Shahidi AM, Sampson GP, Pritchard N, Edwards K, Vagenas D, Russell A, et al. Retinal nerve fibre layer thinning associated with diabetic peripheral neuropathy. Diabetic Med (2012) 29(7):e106–e11. doi: 10.1111/j.1464-5491.2012.03588.x
41. Srinivasan S, Pritchard N, Sampson GP, Edwards K, Vagenas D, Russell AW, et al. Diagnostic capability of retinal thickness measures in diabetic peripheral neuropathy. J optometry (2017) 10(4):215–25. doi: 10.1016/j.optom.2016.05.003
Keywords: diabetes mellitus, peripheral neuropathy, optical correlation tomography, color doppler ultrasound, diagnosis
Citation: Chen W, Wu X, Li S, Zhang Y, Huang Y, Zhuang Y, Bai X, Chen X and Lin X (2022) Optical coherence tomography of the retina combined with color Doppler ultrasound of the tibial nerve in the diagnosis of diabetic peripheral neuropathy. Front. Endocrinol. 13:938659. doi: 10.3389/fendo.2022.938659
Received: 07 May 2022; Accepted: 03 October 2022;
Published: 21 October 2022.
Edited by:
Bin Lu, Fudan University, ChinaReviewed by:
Xiaole Chen, Fujian Medical University, ChinaCopyright © 2022 Chen, Wu, Li, Zhang, Huang, Zhuang, Bai, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiahong Lin, MjQxMzM2ODc5MkBxcS5jb20=; bGlueGg2N0BtYWlsLnN5c3UuZWR1LmNu; Weimiao Chen, NjUxODYwMTgzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.