- 1Society of Meta-research and Biomedical Innovation, London, United Kingdom
- 2Department of Nutrition and Dietetics, Homerton University Hospital Foundation Trust, London, United Kingdom
- 3Department of Life Sciences, Faculty of Natural Sciences, Imperial College London, London, United Kingdom
- 4Department of Endocrinology and Diabetes, Guy’s and St Thomas’ National Health Service (NHS) Foundation Trust, London, United Kingdom
- 5Department of Metabolism, Digestion and Reproduction, Faculty of Medicine, Imperial College London, London, United Kingdom
The newly developed COVID-19 vaccines have established a safe profile, yet some individuals experience a wide range of adverse events. Recently, thyroid dysfunction, including Graves’ disease, has been observed after administration of different COVID-19 vaccines, although causality remains a matter of debate. The aim of this systematic review was to examine the available literature and provide an overview of reported cases of Graves’ disease following COVID-19 vaccination. We identified 21 eligible articles which included 57 patients with Graves’ disease following COVID-19 vaccination. Fourteen participants were males (25%, 14/57) and 43 (75%, 44/57) were females with a mean age of 44.3 years. The most common presenting symptom was palpitations (63%, 27/43) followed by weight loss (35%, 15/43). The majority of patients received thionamides (47%, 25/53). The clinical status after treatment was provided for 37 patients and it was improved in the majority of them (84%, 31/37). Graves’ disease is possibly a condition clinicians may expect to encounter in patients receiving COVID-19 vaccines. While the above adverse event is rare, considering the scarcity of available data in scientific literature, and causality is not yet confirmed, the increased awareness of clinicians and the early recognition of the disorder are important for the optimal management of these patients.
Introduction
An outbreak of an atypical viral pneumonia initially reported at the end of 2019, was later declared a public health emergency of international concern in March 2020 (1, 2). The aetiology was a novel coronavirus strain called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), which has now disseminated across the globe with hundreds of millions affected (3, 4).
Different vaccines have been used widely against COVID-19 including: COMIRNATY (the COVID-19 mRNA vaccine BNT162b2 by BioNTech–Pfizer); COVID-19 Vaccine Moderna (mRNA-1273 by Moderna); VAXZEVRIA (ChAdOx1-nCoV19 by AstraZeneca-Oxford University); COVID-19 Vaccine Janssen (Ad26.COV2.S by Janssen); and CoronaVac COVID19 vaccine (Vero cell by Sinovac Biotech) (5, 6). Almost two thirds of the world population has now received at least one dose of a COVID-19 vaccine with 12 billion doses already administered worldwide (7).
Time has proven the aforementioned vaccines both safe and effective, with serious adverse events being rare, while providing 70-95% protection against severe disease (8–11). However, adverse reactions following vaccination remain inevitable, considering the extent and scale required to control seasonal outbreaks of COVID-19 infection (12–14). At present, patients experience numerous commonly reported adverse symptoms following COVID-19 vaccination, including muscle pain, fever, headache, nausea and vomiting. Beyond the most commonly presenting adverse effects post-COVID-19 vaccination, a diverse range of complaints and symptoms have been reported by patients, including also cases of immune-mediated adverse events (12–17). More recently though, there is an increasing number of reports pertained to thyroid disorders described in patients after the first or second doses of COVID-19 vaccination; however, they are not yet fully clarified.
Recent evidence suggests that viral effects of COVID-19 infection might be associated with thyroid function, possibly by contributing to the onset of thyroid disease or to the exacerbation of a pre-existing one (18–20). To date, COVID-19 vaccine administration has not been considered as a precipitating factor of thyroid dysfunction. In this study, we comprehensively examined the currently available literature to provide an overview of the reported cases of Graves’ disease following vaccination against SARS-CoV-2.
Methods
This review was reported based on the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines.
Literature search
Two reviewers (KKT, PG) searched PubMed and Scopus library databases from inception until May 2022 independently. The search included the following terms: “(COVID 19 vaccin* OR SARS-COV2 vaccin*) AND (Graves’ disease OR Basedow Disease OR Exophthalmic Goiter OR Thyroiditis)”. No restrictions regarding study design, geographic region or language were applied. A manual search of references cited in the selected articles and published reviews were also ensued for undetected studies. Discrepancies in the literature search process were resolved by a third investigator (KSK).
Eligibility criteria
We included studies that provided data for new onset or exacerbation of Graves’ disease following COVID-19 vaccination with at least one dose. All study designs were considered eligible for inclusion. Review articles, abstracts submitted in conferences and non-peer reviewed sources were not eligible for inclusion. Studies on in vitro and animal models were excluded.
Data extraction and handling
In all studies, patient data was retrieved and handled by two authors (KKT, PG) who conducted the data extraction independently. We collected the following information: sex, age, comorbidities, type of vaccine, number of doses received, presenting symptoms after vaccination, history of COVID-19 infection, laboratory measurements, primary diagnosis, imaging findings, treatment, clinical outcome. Any disagreements were discussed and resolved by a third author (KSK).
Quality assessment
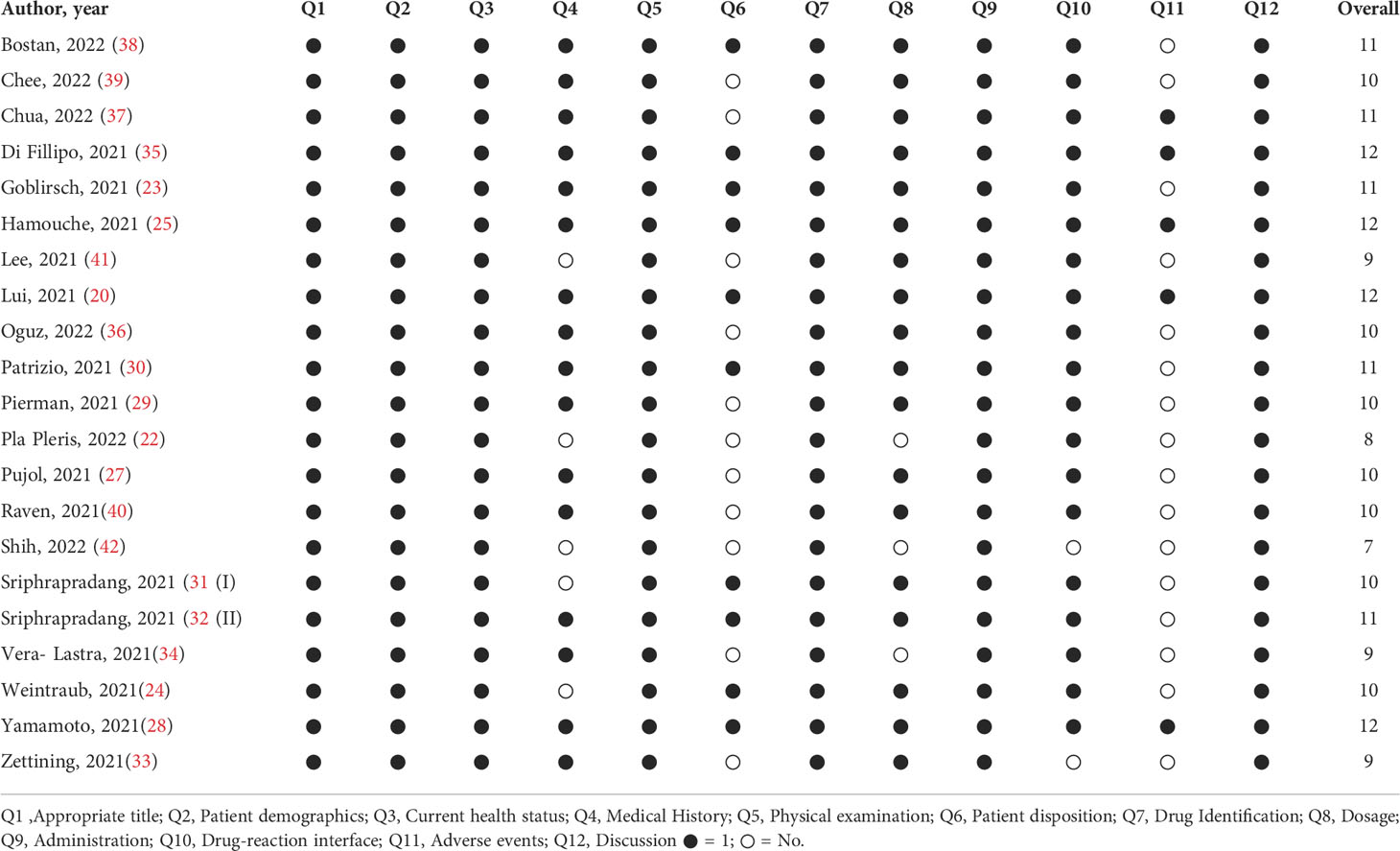
The studies were evaluated using the criteria established by the Task Force for Reporting Adverse Events of the International Society for Pharmacoepidemiology (ISPE) and the International Society of Pharmacovigilance (ISoP) (21). The assessment was based on the adequate reporting of 12 different elements namely: title, patient demographics, current health status, medical history, physical examination, patient disposition, drug identification, dosage, administration/drug reaction interface, concomitant therapies, adverse events, and discussion. The studies scored either 0 (absence of information) or 1 (containing the information) for every element.
Results
Study characteristics
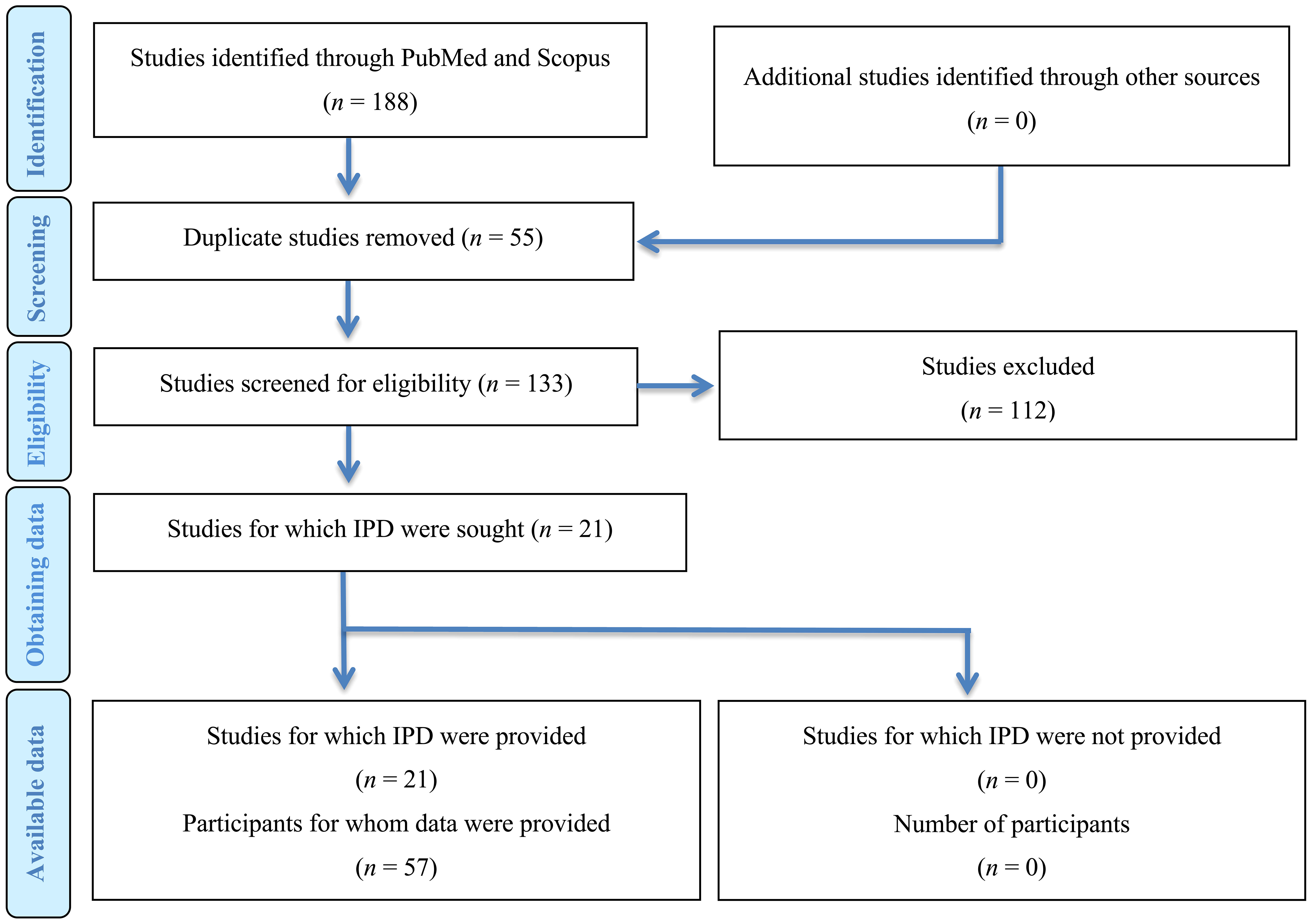
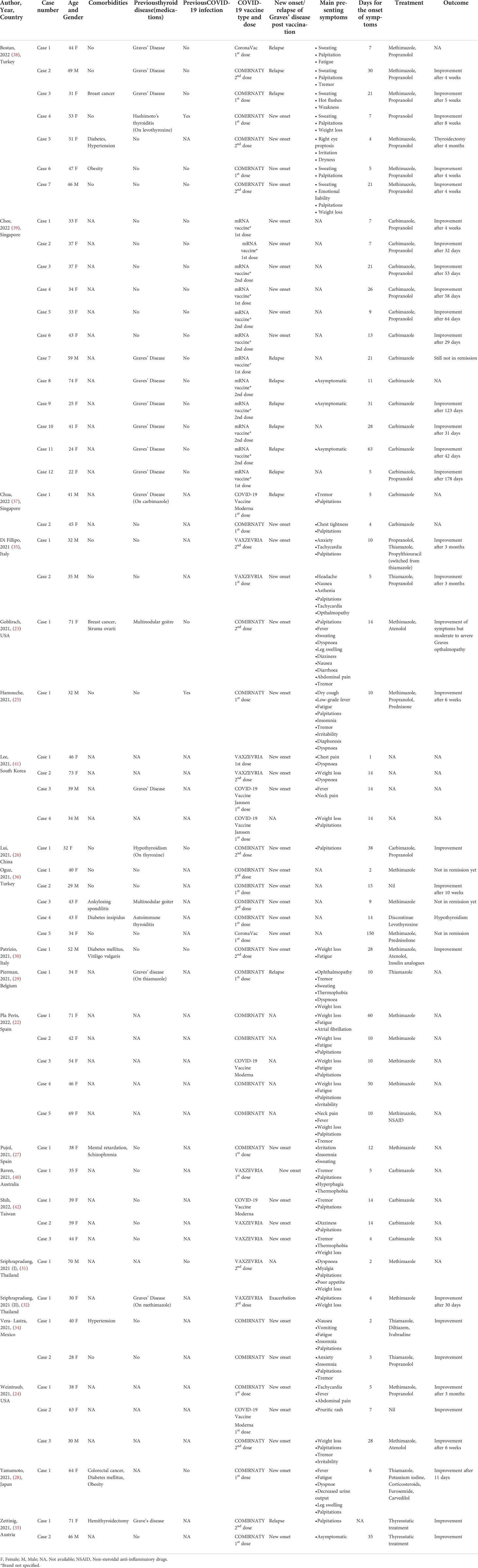
The initial literature search yielded 188 publications. In the first screening 165 studies were excluded as irrelevant. After the exclusion phase, 21 studies (22–42) were eligible for the systematic review (Figure 1). Ten of the studies were conducted in Asia, 6 in Europe, 4 in Americas, and 1 in Australia. In terms of design, 12 studies were case series and 9 were case reports (Table 1).
We identified a total of 57 cases of Graves’ disease following COVID-19 vaccination. Fourteen participants were males (25%, 14/57) and 43 (75%, 43/57) were females with a mean age of 44.3 years (median: 41.5, interquartile range: 34-51.5). Data regarding medical history was provided for 30 cases and half of them had no past medical history (50%, 15/30) with two patients having hypothyroidism before vaccination (66%, 2/30). From the included patients 37 (74%, 37/50) were characterised as new-onset, 12 (26%, 12/50) as relapse and one (2%, 2/50) as exacerbation. The mean age of individuals with Graves’ disease relapse was 42.9 (median: 41, interquartile range: 28-59) with the majority of them receiving mRNA vaccines (92%, 11/12).
For most of the patients (58%, 33/57) data regarding COVID-19 infection before or at the time of Graves’ diagnosis was not provided. Among the remaining patients only 2 were previously infected with SARS-CoV-2. In 12 patients, vaccine brand was not mentioned (21%, 12/57). The majority of the patients received COMIRNATY (64%, 29/45), followed by VAXZEVRIA (18%, 8/45), while a fraction of participants received COVID-19 Vaccine Moderna (9%, 4/45), COVID-19 Vaccine Janssen (4%, 2/45) and CoronaVac (4%, 2/45).
Data regarding the day of the onset of symptoms was provided for 56 cases. On average, the symptoms developed 14.8 days (median: 10, interquartile range: 5-21) after the administration of the vaccine irrespective of the dose. A significant proportion of patients developed symptoms after the 1st dose (55%, 26/47), followed by the 2nd dose (38%, 18/47). Only 3 cases (6%, 3/47) developed symptoms after the 3rd dose.
Data regarding symptomatology was provided for 43 cases. The most common symptom was palpitations (63%, 27/43) followed by weight loss (35%, 15/43). Other common symptoms included tremor (25%, 11/43) and fatigue/weakness (23%, 10/43). Almost all patients had positive thyrotropin receptor antibody (TRAb) or Thyroid stimulating immunoglobin (TSI) (96%, 55/57) except for two people who had imaging findings consistent with Graves’ disease (3%, 2/57). Thyroid stimulating hormone (TSH) levels were provided for 54 patients and they were decreased in all of them (100%, 54/54).
Thyroid ultrasound data was provided for 36 patients. Twenty-four of them had increased vascularity (67%, 24/36) (Table 2). Data regarding thyroid scintigraphy was provided for only 12 cases, with the majority having findings of increased diffuse uptake consistent with Graves’ disease (75%, 9/12). Data regarding treatment was available for 53 cases. Most of them received thionamides (47%, 25/53). The clinical status after treatment was provided for 37 patients and it was improved in the majority of them (84%, 31/37).
Quality of the studies
The mean quality score indicated that the studies reported on average 10 of the recommended 12 elements, defined by the guidelines. Only 3 studies had a perfect score of 12 while the second most common score was 11. The most frequently missing information was the following: adverse events after vaccine administration (76%, 16/21) (Table 3).
Discussion
COVID-19 vaccine administration has not been considered a triggering factor for thyroid autoimmune disorders. However, emerging evidence, mainly from case reports and case series, suggests a potential association between COVID-19 vaccination and the development or recurrence of thyroid dysfunction including Graves’ disease. In our systematic review, we comprehensively examined the currently available literature to provide an overview of the reported cases of Graves’ disease following vaccination against SARS-CoV-2. Our study included 21 reports, which comprised 57 patients, in which Graves’ disease was reported after the administration of different COVID-19 vaccines. The onset of the symptoms started after administration of the first dose in most cases and clinical improvement was reported for the majority of patients.
Results in the context of the literature
Graves’ disease is an autoimmune disorder most commonly presenting with hyperthyroidism and seropositivity for autoantibodies against the thyrotropin receptor (43–45). TRAb production is secondary to a Th1 immune response in which T cells react with peptides derived from thyroid autoantigens leading to increased secretion of autoantibodies from B cells. TRAb stimulates thyroid hormone synthesis, which leads to thyroid growth and diffuse goiter. Multiple precipitating factors have been proposed including female gender, genetic predisposition, stress, smoking, medication, iodine, pregnancy and infection. Several cases of Graves’ disease have been reported following COVID-19 infection with the T cell sensitization to the TSH receptor antigen being proposed as the driving mechanism in people with genetic predisposition (45). Specifically, in a systematic review, Tutal et al. reported 14 cases of Graves’ disease post COVID-19 infection (45).
Apart from COVID-19 infection, our study showed that COVID-19 vaccination may potentially be associated with Graves’ disease although evidence is still inconclusive. Following the sex distribution reported in the literature (46), Graves’ disease post vaccination presented most commonly in females (75%) with palpitations and weight loss. Overall, 19 people had a pre-existing thyroid disorder such as multinodular goiter, Graves’ disease, autoimmune thyroiditis or subclinical hypothyroidism. Interestingly, most patients with background thyroid dysfunction had received an mRNA vaccine. Regrettably, the impact of previous COVID-19 infection could not be assessed considering the lack of data in the majority of cases but remains a possibility. Based on the short interval between vaccination and initiation of symptoms, Graves’ disease might have preceded vaccination on certain occasions. As expected, most cases were treated with thionamides and beta blockers. Steroids were used only in three cases for the amelioration of symptoms by reducing the conversion of T4 to T3. Although steroids consist one of the main therapeutic approaches in people with subacute thyroiditis, more concrete instructions on their use in Graves’ disease are needed considering their potential impact on the immune response triggered by vaccination.
Two reviews have attempted to present the evidence on thyroid dysfunction and COVID-19 vaccination so far. Caironi et al. and Jafarzadeh et al. included 29 and 21 number of patients with Graves’ disease respectively (47, 48). Our study focused solely on Graves’ disease including 57 patients. Overall our findings were in agreement regarding presenting symptoms, onset of symptoms post-vaccination and management. Distribution on different vaccine types was also similar.
Although the exact mechanism behind the potential association between COVID-19 vaccination and Graves’ disease remains to be elucidated, several theories have been suggested. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) is the most frequently cited theory (49). Adjuvants are used to increase immune response to the active substance and although essential for adequate immune system stimulation, they have been considered the etiological factor of ASIA following Hepatitis B and HPV immunization in the past most likely due to an intense immune response or genetic predisposition (50). This results from the formation of autoantibodies or systemic/localised inflammation, it rarely involves autoimmune thyroid disease and it’s most commonly reported within the first 3 weeks post vaccination (51). Although, mRNA vaccines do not use of adjuvants, they contain lipid nanoparticles which facilitate mRNA transport into cells and could potentially induce immune response in predisposed people (52). Additionally, the presence of the ACE-2 receptor in the thyroid gland could offer another explanation for the endocrine effects reported in individuals following the SARS-CoV-2 infection or vaccination since it constitutes the entry point of the virus into host cells (53). Cellular entry could lead to a direct inflammatory or immune mediated injury on thyroid cells with subsequent clinical manifestations (54). It is worth noting that the mRNA of ACE-2 receptor is also expressed in thyroid cells as confirmed by studies in thyroid tissue specimens and cultures, making them a potential target for viral entry (55, 56).
Another theory includes the possible effect of molecular mimicry in the development of autoimmune thyroid disorders (29). Thyroid peroxidase peptide sequences in thyroid tissue share similarities with the SARS-CoV-2 proteins, such as the spike protein that comprise a major target of the mRNA vaccines (57). It has been speculated that this could lead to cross-recognition between the modified SARS-CoV-2 spike protein encoded in the mRNA vaccine and the thyroid target proteins resulting in autoimmunity and it has been demonstrated that spike protein, nucleoprotein and membrane protein all cross-react with thyroid peroxidase (57). Additionally, cytokines such as Interferon gamma have been identified in both Graves’ disease and the SARS-CoV-2 infection (58). Results from a phase I/II vaccine candidate mRNA BNT162b1 suggest a Th1 type immune response involving interferon gamma, which could imply a modification of the cytokine environment that could favor the Th1 population and subsequently the production of autoantibodies (59).
Strengths and limitations
Our study is the first to systematically review the association between COVID-19 vaccination and onset or exacerbation of Graves’ disease. Our findings present a comprehensive review of the currently available literature and highlight published data with rigorous quality assessment of included studies.
However, some limitations still persist. A broader drawback underlies the low-quality nature of case reports and case series included in our review, which affects the validity and scope of conclusions that can be reached. Specifically, the potential risk of bias of these studies is inevitable, as these are exposed to the risk of overinterpretation and selection bias. In this way, their reported data although interesting may be far from the truth without reflecting a valid description. Thus, causality cannot be inferred and requires insight from mechanistic studies.
Conclusion
Although the currently available COVID-19 vaccines have established a safe profile and the benefits of vaccination outweigh the possible adverse events, patients can potentially experience mild to moderate side effects including thyroid related complications. Graves’ disease is possibly a condition physicians and other healthcare professionals may expect to see in patients receiving COVID-19 vaccines. While the above adverse event is rare, considering the scarcity of available data in scientific literature, and causality is not yet confirmed, the increased awareness of clinicians and the early recognition of the disorder is important for the optimal management of these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, KKT, KSK; Methodology, KKT, KSK; Validation, KKT, DS, KSK; Investigation, KKT, KSK; Resources, KKT, DS, KSK; Writing—Original Draft Preparation, KKT, PG, DS, KSK; Writing—Review & Editing, KKT, DS, KSK; Visualization, KKT, DS, KSK; Supervision, DS, KSK; Project Administration, PG, DS, KSK. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in wuhan, China: The mystery and the miracle. J Med Virol (2020) 92(4):401. doi: 10.1002/jmv.25678
2. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med (2020) 8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5
3. Kechagias K, Giannos P, Katsikas Triantafyllidis K, Falagas ME. Spotlight on early COVID-19 research productivity: A 1-year bibliometric analysis. Front Public Health (2022) 10:811885. doi: 10.3389/fpubh.2022.811885
4. Patel N, Nicolae R, Geropoulos G, Mandal P, Christou C, Gavala M, et al. Pneumomediastinum in the COVID-19 era: To drain or not to drain? Monaldi Arch Chest Dis (2022). doi: 10.4081/monaldi.2022.2338
5. Loubet P, Wittkop L, Tartour E, Parfait B, Barrou B, Blay J, et al. A French cohort for assessing COVID-19 vaccine responses in specific populations. Nat Med (2021) p:1–3. doi: 10.1038/s41591-021-01435-1
6. Tanriover MD, Doganay HL, Akova M, Guner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet (2021) 398(10296):213–22. doi: 10.1016/S0140-6736(21)01429-X
7. Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav (2021) 5(7):947–53. doi: 10.1038/s41562-021-01122-8
8. Livingston EH, Malani PN, Creech CB. The Johnson & Johnson vaccine for COVID-19. Jama (2021) 325(15):1575–5. doi: 10.1001/jama.2021.2927
9. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature (2020) 586(7830):589–93. doi: 10.1038/s41586-020-2639-4
10. Barrett JR, Belij-Rammerstorfer S, Dold C, Ewer KJ, Folegatti PM, Gilbride C, et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med (2021) 27(2):279–88. doi: 10.1038/s41591-020-01179-4
11. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. New Engl J Med (2020) 383(25):2427–38. doi: 10.1056/NEJMoa2028436
12. Katsikas Triantafyllidis K, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID-19 vaccination: A systematic review of case reports. Vaccines (2021) 9(9):1013. doi: 10.3390/vaccines9091013
13. Giannos P, Katsikas Triantafyllidis K, Geropoulos G, Kechagias KS. Persistent hiccups as an atypical presentation of SARS-CoV-2 infection: A systematic review of case reports. Front Neurol (2022) 13. doi: 10.3389/fneur.2022.819624
14. Giannos P, Prokopidis K. Gut dysbiosis and long COVID-19: Feeling gutted. J Med Virol (2022) 94(7):2917–8. doi: 10.1002/jmv.27684
15. Dias L, Soares-Dos-Reis R, Meira J, Ferrao D, Soares PR, Pastor A, et al. Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2 vaccine. J Stroke Cerebrovascular Dis (2021) 30(8):105906. doi: 10.1016/j.jstrokecerebrovasdis.2021.105906
16. Bril F, Diffalha SA, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol (2021) 75(1):222–4. doi: 10.1016/j.jhep.2021.04.003
17. Vuille-Lessard É́, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun (2021) 123:102710. doi: 10.1016/j.jaut.2021.102710
18. Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev endocrine Metab Disord (2021) 22(4):803–15. doi: 10.1007/s11154-020-09615-z
19. Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of graves’ disease after COVID-19. J endocrinological Invest (2020) 43(10):1527–8. doi: 10.1007/s40618-020-01366-7
20. Lui DTW, Lee CH, Chow WS, Lee ACH, Tam AR, Fong CHY, et al. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J Clin Endocrinol Metab (2021) 106(2):e926–35. doi: 10.1210/clinem/dgaa813
21. Kelly WN, Arellano FM, Barnes J, Bergman U, Edwards RI, Fernandez AM, et al. Guidelines for submitting adverse event reports for publication. Pharmacoepidemiology Drug Saf (2007) 16(5):581–7. doi: 10.1002/pds.1399
22. Pla Peris B, Alfaro AAM, Royo FJM, Galiana PA, Naranjo SP, Boillos MG, et al. Thyrotoxicosis following SARS-COV-2 vaccination: A case series and discussion. J Endocrinological Invest (2022) 45(5):1071–7. doi: 10.1007/s40618-022-01739-0
23. Goblirsch TJ, Paulson AE, Tashko G, Mekonned AJ. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep CP (2021) 14(12):e246432. doi: 10.1136/bcr-2021-246432
24. Weintraub MA, Ameer B, Sinha Gregory N. Graves disease following the SARS-CoV-2 vaccine: Case series. J Invest Med High Impact Case Rep (2021) 9:23247096211063356. doi: 10.1177/23247096211063356
25. Hamouche W, El Soufi Y, Okafor BV, Zhang F, Paras C. A case report of new onset graves’ disease induced by SARS-CoV-2 infection or vaccine? J Clin Trans Endocrinology: Case Rep (2022) 23:100104. doi: 10.1016/j.jecr.2021.100104
26. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of graves' disease after SARS-CoV-2 mRNA vaccination: A case report and literature review. Front Public Health (2021) 9. doi: 10.3389/fpubh.2021.778964
27. Pujol A, Gomez LA, Gallegos C, Nicolau J, Sanchis P, Gonzalez-Freire M, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV2 vaccination: From graves’ disease to silent thyroiditis. J Endocrinological Invest (2022) 45(4):875–82. doi: 10.1007/s40618-021-01707-0
28. Yamamoto K, Mashiba T, Takano K, Suzuki T, Kami M, Takita M, et al. A case of exacerbation of subclinical hyperthyroidism after first administration of BNT162b2 mRNA COVID-19 vaccine. Vaccines (2021) 9(10):1108. doi: 10.3390/vaccines9101108
29. Pierman G, Delgrange E, Jonas C. Recurrence of graves’ disease (a Th1-type cytokine disease) following SARS-CoV-2 mRNA vaccine administration: A simple coincidence? Eur J Case Rep Internal Med (2021) 8(9):2807. doi: 10.12890/2021_002807
30. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of graves' disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun (2021) 125:102738. doi: 10.1016/j.jaut.2021.102738
31. Sriphrapradang C, Shantavasinkul PC. Graves’ disease following SARS-CoV-2 vaccination. Endocrine (2021) 74(3):473–4. doi: 10.1007/s12020-021-02902-y
32. Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with graves’ disease. Endocrine (2021) 74(2):226–7. doi: 10.1007/s12020-021-02879-8
33. Zettinig G, Krebs M. Two further cases of graves’ disease following SARS-Cov-2 vaccination. J Endocrinological Invest (2022) 45(1):227–8. doi: 10.1007/s40618-021-01650-0
34. Vera-Lastra O, Navarro AO, Domiguez MPC, Medina G, Valadez TIS, Jara LJ. Two cases of graves' disease following SARS-CoV-2 vaccination: An autoimmune/inflammatory syndrome induced by adjuvants. Thyroid (2021) 31(9):1436–9. doi: 10.1089/thy.2021.0142
35. di Filippo L, Castellino L, Giustina A. Occurrence and response to treatment of graves’ disease after COVID vaccination in two male patients. Endocrine (2022) 75(1):19–21. doi: 10.1007/s12020-021-02919-3
36. Oğuz SH, Sendur SN, Iremli BG, Gurlek A, Erbas T, Unluturk U. SARS-CoV-2 vaccine–induced thyroiditis: Safety of revaccinations and clinical follow-up. J Clin Endocrinol Metab (2022) 107(5):e1823–34. doi: 10.1210/clinem/dgac049
37. Chua MWJ. Graves' disease after COVID-19 vaccination. Ann Acad Medicine Singapore (2022) 51(2):127–8. doi: 10.47102/annals-acadmedsg.2021398
38. Bostan H, Ucan B, Kizilgul M, Calapkulu M, Hepsen S, Gul U, et al. Relapsed and newly diagnosed graves’ disease due to immunization against COVID-19: A case series and review of the literature. J Autoimmun (2022) 128:102809. doi: 10.1016/j.jaut.2022.102809
39. Chee YJ, Liew H, Hoi WH, Lee Y, Lim B, Chin HX, et al. SARS-CoV-2 mRNA vaccination and graves’ disease: A report of 12 cases and review of the literature. J Clin Endocrinol Metab (2022) 107(6):e2324–30. doi: 10.1210/clinem/dgac119
40. Raven LM, McCormack AI, Greenfield JR. Letter to the Editor from raven et al:”Three cases of subacute thyroiditis following SARS-CoV-2 vaccine”. J Clin Endocrinol Metab (2022) 107(4):e1767–8. doi: 10.1210/clinem/dgab822
41. Lee K, Kim YJ, Jin HY. Thyrotoxicosis after COVID-19 vaccination: seven case reports and a literature review. Endocrine (2021) 74(3):470–2. doi: 10.1007/s12020-021-02898-5
42. Shih S-R, Wang C-Y. SARS-CoV-2 vaccination related hyperthyroidism of graves’ disease. J Formosan Med Assoc (2022) doi: 10.1016/j.jfma.2022.02.010.
43. Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol (2004) 4(11):841–55. doi: 10.1038/nri1485
44. Benvenga S, Guarneri F. Molecular mimicry and autoimmune thyroid disease. Rev Endocrine Metab Disord (2016) 17(4):485–98. doi: 10.1007/s11154-016-9363-2
45. Tutal E, Ozaras R, Leblebicioglu H. Systematic review of COVID-19 and autoimmune thyroiditis. Travel Med Infect Dis (2022) p:102314. doi: 10.1016/j.tmaid.2022.102314
46. Manji N, Carr-Smith JD, Boelaert K, Allahabadia A, Armitage M, Chatterjee VK, et al. Influences of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab (2006) 91(12):4873–80. doi: 10.1210/jc.2006-1402
47. Caironi V, Pitoia F, Trimboli P. Thyroid inconveniences with vaccination against SARS-CoV-2: The size of the matter. a systematic review. Front Endocrinol (Lausanne) (2022) 13:900964. doi: 10.3389/fendo.2022.900964
48. Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi SMJ. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J Endocrinol Invest (2022) p:1–29. doi: 10.1007/s40618-022-01786-7
49. Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine (2019) 37(24):3167–78. doi: 10.1016/j.vaccine.2019.04.055
50. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab (2020) 34(1):101412. doi: 10.1016/j.beem.2020.101412
51. Das L, Bhadada SK, Sood A. Post-COVID-vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest (2022) 45(2):465–7. doi: 10.1007/s40618-021-01681-7
52. Chen BM, Cheng TL, Roffler SR. Polyethylene glycol immunogenicity: Theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano (2021) 15(9):14022–48. doi: 10.1021/acsnano.1c05922
53. Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's reliance on ACE2. Endocrinology (2020) 161(9):108. doi: 10.1210/endocr/bqaa108
54. Soldevila B, Puig-Domingo M, Marazuela M. Basic mechanisms of SARS-CoV-2 infection. What endocrine systems could be implicated? Rev Endocr Metab Disord (2022) 23(2):137–50. doi: 10.1007/s11154-021-09678-6
55. Rotondi M, Coperchini F, Ricci G, Denegri M, Groce L, Ngnitejeu ST, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: A clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest (2021) 44(5):1085–90. doi: 10.1007/s40618-020-01436-w
56. İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: Postvaccination ASIA syndrome. J Clin Endocrinol Metab (2021) 106(9):2600–5. doi: 10.1210/clinem/dgab373
57. Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: Implications for autoimmune diseases. Front Immunol (2020) 11:617089. doi: 10.3389/fimmu.2020.617089
58. Croce L, Gangemi D, Ancona G, Liboa F, Bendotti G, Minelli L, et al. The cytokine storm and thyroid hormone changes in COVID-19. J Endocrinol Invest (2021) 44(5):891–904. doi: 10.1007/s40618-021-01506-7
Keywords: Graves’ disease, thyroiditis, COVID-19, SARS–CoV–2, vaccines
Citation: Triantafyllidis KK, Giannos P, Stathi D and Kechagias KS (2022) Graves‘ disease following vaccination against SARS-CoV-2: A systematic review of the reported cases. Front. Endocrinol. 13:938001. doi: 10.3389/fendo.2022.938001
Received: 06 May 2022; Accepted: 23 August 2022;
Published: 27 September 2022.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Mohammad Barary, Shahid Beheshti University of Medical Sciences, IranHayri Bostan, Dışkapı Yildirim Training and Research Hospital, Turkey
Cary Mariash, Purdue University Indianapolis, United States
Verdiana Caironi, Lugano Regional Hospital, Switzerland
Copyright © 2022 Triantafyllidis, Giannos, Stathi and Kechagias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos S. Kechagias, a29uc3RhbnRpbm9zLmtlY2hhZ2lhczE4QGltcGVyaWFsLmFjLnVr
†These authors have contributed equally to this work and share last authorship
 Konstantinos Katsikas Triantafyllidis
Konstantinos Katsikas Triantafyllidis Panagiotis Giannos
Panagiotis Giannos Dimitra Stathi
Dimitra Stathi Konstantinos S. Kechagias
Konstantinos S. Kechagias