
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 26 July 2022
Sec. Endocrinology of Aging
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.934020
This article is part of the Research TopicWomen in Aging Endocrinology 2022View all 6 articles
Background: In this study, we investigated the epidemiological characteristics and predictors of diabetic peripheral neuropathy (DPN) in adult patients with type 2 diabetes mellitus (DM).
Methods: The study was designed as a retrospective cohort trial at the First Affiliated Hospital of Wenzhou Medical University. From January 2017 to December 2020, a total of 1,262 patients with DM were enrolled to assess the risk factors for DPN. The patients were divided into two groups (DPN group and non-DPN group). The Mann–Whitney U test or t-test, receiver operating characteristic (ROC) analyses, univariate chi-square analyses, and multiple logistic regression analyses were used to analyze the adjusted predictors of DPN.
Results: The overall prevalence of DPN in DM patients was 72.7% (n = 793/1,091). Multivariate analysis revealed that age > 66 years (odds ratio [OR], 2.647; 95% confidence interval [CI] 1.469–4.770; p = 0.002), history of hypertension (OR, 1.829; 95% CI 1.146–2.920; p = 0.011), neutrophil (NE) levels exceeding 4.0 × 109/L (OR 0.256; 95% CI 0.162–0.405; p = 0.001), lymphocyte (LY) levels over 3.0 × 109/L (OR 7.173; 95% CI 4.258–12.086; p = 0.000), HbA1c > 7.7% (OR 3.151; 95% CI 1.959–5.068; p = 0.000), and FT3 > 4.4 pmol/L (OR 0.417; 95% CI 0.263–0.662; p = 0.000) were six significant predictive factors for the prevalence of DPN.
Conclusions: High levels of LY, HbA1c, history of hypertension, and > 66 years of age increase the risk of DPN in adult patients with DM, while high levels of NE and FT3 were protective factors of DPN. Thus, the prediction of DPN can significantly be improved by identifying older patients over the age of 66 and history of hypertension, as well as establishing the biochemical cutoff values of NE, LY, HbA1c, and FT3.
Diabetes mellitus (DM) is a chronic metabolic disease associated with hyperglycemia owing to impaired insulin secretion, insulin activity, or both (1). High levels of blood glucose can cause damage to all organs of the body, including the cardiovascular system, eyes, kidneys, as well as the nervous system (2). The pathogenesis of diabetes is very complex, and a large number of studies are under way. Some studies have found that anemia, which could lead to tissue hypoxia, interferes with the diagnosis and management of diabetes because it affects pathological examination and drugs (3). In 2019, 488 million adults aged 20–99 years old (9.5%) live with diabetes, worldwide. The high cost of the treatment of diabetes and the loss of the ability to work normally as a result of diabetes are a burden on society (4).
Diabetic peripheral neuropathy (DPN) is the most widely recognized complication of DM (5). At present, DPN is divided into two clinical types: typical DPN (DSPN) and atypical DPNs. Symptoms or signs of DSPN may include the following: decreased sensation; positive neurosensory symptoms (such as “sleep numbness”, tingling, burning, or pain), mainly in the toes, feet, or legs; reduced distal sensory symmetry; and absent ankle reflexes. In the exploration of DPN, there are many previous studies suggesting that diabetes is closely related to other complications. Cardiovascular autonomic neuropathy is positively correlated with declined urinary albumin excretion rate and cardiac function in patients with diabetes (6). In patients with atypical DNPs, pain and autonomic and neuromorphologic abnormalities may be present (7). It mainly manifests as peripheral body pain (8), cardiovascular system damage (9), sudomotor dysfunction (10), bladder dysfunction, and erectile dysfunction (11). In a prospective study with long-term follow-up, the prevalence of DPN increased from 7.5% at the beginning of DM to 45% after 25 years with DM (12). The high prevalence of DPN is a major cause of death and disability resulting from diabetes, including recurrent limb infections, ulcers, and even progression to amputation. One study reported an 11% cumulative risk of lower limb amputation 25 years after the diagnosis of diabetes (13).
The pathogenesis of DPN has not been clearly elucidated. Some scholars have suggested that DPN can be attributed to chronic exposure to hyperglycemia and cardiovascular risk covariates (7). The existing examination and diagnosis methods, such as nerve biopsy, skin biopsy, and flare reaction, are difficult to operate and are not acceptable to patients. Due to the lack of treatments that target the underlying nerve damage, screening for laboratory indicators for DPN is critical in clinical practice, as it may detect the earliest stages of neuropathy, enabling early intervention (14). The objective of this retrospective study was to identify independent risk factors for DPN in DM patients and to provide insights for clinical diagnosis and management.
Information on diabetic patients who underwent treatment for diabetes at our hospital from January 2017 to December 2020 was extracted from a retrospective database. A total of 1,262 cases were reviewed, and 1,091 were analyzed (Figure 1). The exclusion criteria were as follows: (1) type 1 diabetes, gestational DM, and any other type of diabetes; (2) neuropathy due to other reasons, such as cervical and lumbar spondylopathy, Guillain–Barré syndrome, epilepsy, and severe arteriovenous vascular disease; (3) other serious diseases, such as progressive malignancy, acute infection, severe renal insufficiency, and heart failure; (4) several medications (thyroid hormones preparations, methimazole, propylthiouracil, amiodarone, lithium carbonate, corticosteroids, biotin, etc.) that are able to affect thyroid function or to influence thyroid hormone levels; (5) incomplete data; and (6) several medications (thyroid hormones preparations, methimazole, propylthiouracil, amiodarone, lithium carbonate, corticosteroids, biotin, etc.) that are able to affect thyroid function or to influence thyroid hormone levels were excluded.. The patients in our study were divided into 2 groups: DM patients who developed DPN and DM patients without DPN. The study was approved by the institutional ethics committee of the First Affiliated Hospital of Wenzhou Medical University, and all procedures were performed in accordance with the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all patients.
DPN is defined as clinical and(or) electrophysiological evidence of the definite presence of peripheral neuropathy in patients with diabetes. Other causes of peripheral neuropathy should also be ruled out. All T2DM patients underwent a complete neurological examination by experienced neurologists. Symptoms were assessed using the neuropathy disability score based on the examination of ankle reflexes, vibration, pinprick sensation, and temperature sensation. Nerve conduction studys (NCS) were performed on each patient by trained physicians. During the test, patients remained calm and relaxed, and local skin temperature was kept constant.
The diagnosis met the following criteria: (1) a clear history of diabetes or at least evidence of abnormal glucose metabolism; (2) neuropathy that occurs at or after the diagnosis of diabetes; (3) the clinical symptoms and signs were consistent with those of DPN; (4) if there are 2 or more abnormalities in the following 5 tests: (a) temperature sensing anomaly, (b) Semmes–Weinstein monofilament trial: decline or absence of sensation in the foot, (c) abnormal vibration sensation, (d) absence of ankle reflex, and (e) nerve conduction velocity decreased by 2 or more factors; (5) other neuropathy, such as cervical and lumbar lesions, was excluded.
The data came from six aspects: demographics, blood indicators, lipid metabolism, glucose metabolism, renal function, and thyroid hormones. The demographic data included age, gender, residence, body mass index (BMI), cigarette smoking, and alcohol consumption. The blood indicators included WBC, RBC, and platelet counts; neutrophil count; mononuclear cell count; and lymphocyte count. The lipid metabolism data included total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL). The glucose metabolism included HbA1c and fasting blood glucose (FBG). The renal function included creatinine, urea, and serum uric acid. The thyroid hormones included thyroid-stimulating hormone (TSH), free triiodothyronine 3 (FT3), and free thyroxine 4 (FT4). Associated laboratory variables were obtained within 24 h of admission. FT3, FT4, and TSH were tested by Beckman DXI800 (Beckman Coulter, Indiana, USA).
Statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as median, mean ± standard deviation (SD). Student’s t-test and Mann–Whitney U test were performed to compare continuous variables between DPN and non-DPN groups according to the homogeneity of variance test and normality test. For the continuous variables with statistical significance, receiver operating characteristic (ROC) analyses were performed to detect the optimum cutoff value, which was calculated by maximizing the sum of sensitivity and specificity in the ROC curve. Based on the cutoff value, continuous variables were converted into categorical variables before being subjected to logistic regression. Pearson chi-square test was used to determine the correlation between each classification variable and DPN. Predictors found to be significant in the single-factor analysis were subjected to stepwise multiple logistic regression analyses (backward LR) to screen for the adjusted factors. The odds ratio (OR) and 95% confidence interval (CI) were determined to evaluate the correlation magnitude between factors and DPN. p < 0.05 was considered to be statistically significant.
It is a retrospective single-center study involving a total of 1,262 patients with DM. Figure 1 shows the flowchart that represents the procedures applying for screening the study participants. A total of 1,262 diabetic patients were admitted to our institute. Among them, 12 patients suffered from type 1 diabetes, gestational DM, and any other type of diabetes; 15 patients had neuropathy due to other reasons, such as cervical and lumbar spondylopathy, Guillain–Barré syndrome, epilepsy, and severe arteriovenous vascular disease; 21 patients had other serious diseases, such as progressive malignancy, acute infection, severe renal insufficiency, and heart failure; 123 patients had incomplete data before admission. A total of 171 patients were excluded due to incomplete data. A total of 1,091 patients were eventually analyzed in this study; 678 (62%) were men and 413 (38%) were women. Their average age and BMI were 54.79 ± 12.32 years old vs. 60.40 ± 11.53 years old and 24.56 ± 3.76 kg/m2 vs. 24.10 ± 3.30 kg/m2, respectively. The prevalence of diabetic patients with DPN is 72.7%.
As shown in Table 1, thirteen continuous variables were compared between the DPN group and the non-DPN group. Five continuous variables including age, NE, LY, HbA1c, and FT3 were significantly different between the two groups. The optimum cutoff values for quantitative data related to DNP were determined by ROC curve analysis. Table 2 shows the area under the curve and the optimum cutoff values for the five continuous variables. The optimum cutoff values for age, NE, LY, HbA1c, and FT3 were 66.0 years, 4.0 × 109/L, 3.0 × 109/L, 7.7%, and 4.4 pmol/L, respectively.

Table 1 Comparison of general information and biochemical indicators between non-DPN group and DPN group.
The demographic and laboratory indicators were evaluated by univariate analysis in Table 3. A total of eight factors (gender, age, smoking, hypertension, NE, LY, HbA1c, and FT3) were correlated with DPN. Fifty-six percent of patients were male in the DPN group, and 64.4% of patients were male in the non-DPN group. The proportion of patients >66 years old was higher in DM patients with DPN than in those without DPN (32.3% vs. 13.1%) (p < 0.001). More patients with DPN had histories of hypertension (p < 0.001) and smoking (p < 0.020), but not hyperlipidemia (p = 0.835). Other characteristics, including NE > 4.0 × 109/L (p < 0.001), LY > 3.0 × 109/L (p < 0.001), HbA1c > 7.7% (p < 0.001), and FT3 > 4.4 pmol/L (p < 0.001), were statistically significantly different between the two groups. Therefore, these eight factors were subjected to the multiple logistic regression analysis.
The final variables of the multiple logistic regression analysis are shown in Table 4. There were six independent risk factors for DPN: >66 years of age (OR 2.647; 95% CI 1.469–4.770; p = 0.001), hypertension (OR 1.829; 95% CI 1.146–2.920; p = 0.011), NE > 4.0 × 109/L (OR 0.256; 95% CI 0.162–0.405; p < 0.0001), LY > 3.0 × 109/L (OR 7.173; 95% CI 4.258–12.086; p < 0.0001), HbA1c > 7.7% (OR 3.151; 95% CI 1.959–5.068; p < 0.0001), and FT3 > 4.4 pmol/L (OR 0.417; 95% CI 0.263–0.662; p < 0.0001). The Hosmer–Lemeshow test showed adequate fitness (χ2 = 3.612; p = 0.890).
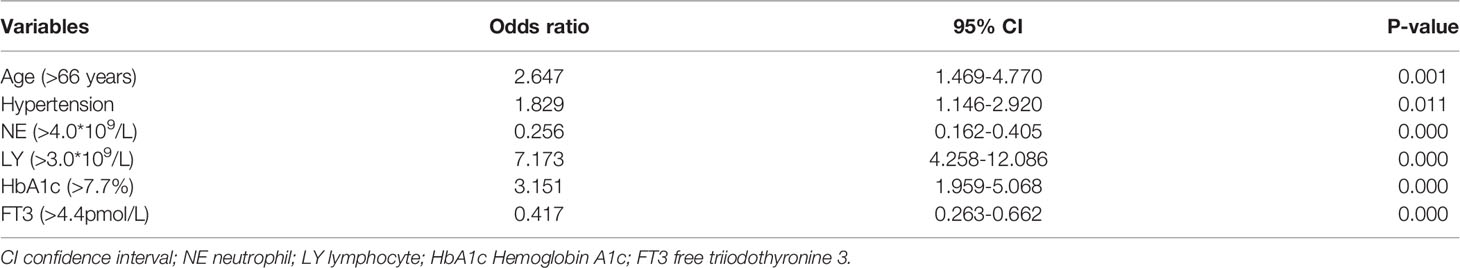
Table 4 Multivariate analysis (Multiple logistic regression analysis) of factors associated with diabetic peripheral neuropathy.
In this study, a large-sample retrospective cohort was applied to address the epidemiologic characteristics of diabetic patients with DPN. We found that the overall prevalence of diabetic patients with DPN was 72.7%. Six predictive factors, namely, age (>66 years), hypertension, NE (>4.0 × 109/L), LY (>3.0 × 109/L), HbA1c (>7.7%), and FT3 (>4.4pmol/L), were independently correlated with DPN.
The overall prevalence of DPN among diabetic patients analyzed was 793/1,091 (72.7%). Our study result was similar to the studies reported from Iran (75.1%) and Italy (82%) (15, 16). However, our prevalence was higher than other studies reported from the USA, Malaysia, and Ethiopia where the prevalence were found to be 51%, 50.7%, and 53.6%, respectively (17, 18) and even higher than a study in Jordan, which was 39.5% (19). Besides the disparities in genetic predisposition, in healthcare qualities, and in study settings and designs, the high prevalence of DPN in diabetic patients indicates poor patient’s self-conception of symptom of DPN and deficits of patient’s education, which should be stressed in future medical service.
Age, as a predictor for DPN, has been reported previously (18, 20, 21). Patients > 66 years old were 2.65 times more likely to develop DPN than those < 66 years old. Three cross-sectional studies revealed that age > 40 years old and >50 years old were independent predictors for DPN (18, 21, 22). In the study of Abdissa et al. (18), patients > 40 years old and >50 years old were 4.57 times and 6.5 times more likely to develop DPN compared to patients whose age was less than 30 years old, respectively. DPN is a chronic complication needing time to develop, which may be the reason for this association between age and DPN.
HbA1c value was used to assess the quality of the glycemic control. According to the present study, there was a significant association between DPN and HbA1c > 7.7% (OR 3.151; 95% CI 1.959–5.068; p < 0.001). This association between HbA1c and DPN has been reported previously by Darivemula et al. (23), showing that compared to patients with normal HbA1c levels (<7%), those with high HbA1c (>7%) were 4.81 times more likely to develop DPN. One percent increase in HbA1c levels was positively related to microvascular rather than macrovascular complications (24). It is reported that high level of blood glucose is correlated with the severity of DPN (25).
Hypertension has been identified as a strong independent risk factor for DPN in patients with DM (26–32). Our finding is consistent with previous studies. In contrast, treatment of hypertension in patients with T2DM shows a significant reduction in the prevalence of DPN, which shows that vascular dysfunction plays an essential role in the pathogenesis of DPN. In the study of Ponirakis et al. (32), no effect on neuropathy hypertension was found in cases without diabetes. With respect to the underlying pathophysiology behind hypertension, loss of myogenic tone and vascular hypertrophy have been previously revealed in resistance vessels of diabetic patients with DPN (33), which was partially alleviated after improved glycemic control (34, 35). Further studies are needed to clarify the physiologic link between hypertension and DPN in diabetic patients.
LY showed the most powerful relationship with DPN in diabetic patients. Diabetic patients with LY >3.0 × 109/L are 7.17 times (95% CI 4.258–12.086; p < 0.001) more prone to DPN than those with LY < 3.0 × 109/L. Both T1DM and T2DM, as well as their complications, had been identified as inflammatory disease and a dysfunction status of the immune system (36, 37). White blood cell (WBC) count and its subtypes are inflammatory indicators. Neutrophils are closely associated with ongoing inflammation, and lymphocytes present the state of the immune system (38). A retrospective study conducted by Liu et al. (39) demonstrated that T2DM patients with higher neutrophil-to-lymphocyte ratio (NLR) levels are more likely to develop DPN. Accompanied by CD8+ T cells, NK cells play an essential role in the immune response to peripheral neuropathy (40), including not only their cytotoxicity, but also the selective pruning of damaged sprouting peripheral axons.
Moreover, our study has revealed two protective factors for DPN in diabetic patients. To the best of our knowledge, the present study is the first to identify NE (>4.0 × 109/L) as a protective factor for DPN. Neutrophils are multifaceted cells acting either protectively or harmfully, depending on the specific disease condition, the activation state of neutrophils, and the body compartment affected (41). Besides their effects on tissue injury, neutrophils showed effects on tissue repair by releasing growth factors (42). A set of studies has found the relationship between neutrophils and DM. Elevated phagocytic activity of neutrophils had been revealed to be improved due to treatment of diabetic foot infections (43). Insulin can increase neutrophil count and function in diabetic patients following cardiac surgery (44, 45). Whether neutrophils alleviate or aggravate DPN calls for more studies.
Neutrophils are traditionally regarded as a simple infantry of the innate immune system, with a limited set of pro-inflammatory functions. It is clear that neutrophils are actually complex cells with a large number of special functions (46). In our study, we found that the increase of neutrophils may be a protective factor of DPN, which is not consistent with our usual cognition. Neutrophils play a variety of roles in acute viral infection. They limit virus replication and spread through phagocytosis, threshing, respiratory burst, secretion of cytokines, and release of extracellular traps of neutrophils, and activate adaptive immune response, thus playing a protective role in tissue damage (47). Studies have found that neutrophils have been observed to prevent and promote the establishment of infection, and neutrophils may affect the persistence of chronic parasites in a mouse model of leishmaniasis (48). The pathogenesis of DPN is still unknown. There is a question about whether this interesting finding in our study, contrary to previous perceptions, will further advance our understanding of DPN.
An FT3 level of more than 4.4 pmol/L showed a protective effect on DPN patients. In the study of Fernandez-Real et al., an intrinsic link between thyroid function tests and insulin resistance had been demonstrated (49). Sendi et al. conducted a multicenter cross-sectional study and found a significant association between DNP and comorbidities including thyroid diseases (50). However, Yuan et al. considered that FT3 or FT4 alone may not be the best predictor to truly reflect the integral alteration of free thyroid hormone metabolism, and they found that a low FT3/FT4 ratio level is an independent risk factor in euthyroid patients with three-vessel disease (51). Park et al. found that the high FT3/FT4 ratio is associated with insulin resistance, and the elevated FT3/FT4 ratio could be the result of adapting to adverse insulin resistance. Further studies are needed to investigate this relationship between DPN and FT3. In our study, male gender and history of smoking were not significantly associated with DPN in diabetic patients. In our study, we found that an FT3 level higher than 4.4 pmol/L has a protective effect on DPN. A large number of previous studies have also shown that FT3 has an important link to non-thyroid diseases. Hypothyroidism, including a decrease in FT3, has been found to be associated with nephrotic syndrome. This is considered to be a common feature of primary and secondary glomerular disease, with loss of protein in urine and increased urinary excretion of thyroid hormone and thyroxine-binding globulin (52). The level of plasma free thyroid hormone and FT3 decreased rapidly in acute critically ill patients, which was found to be related to the decrease of myocardial function. After administration of T3/T4, myocardial dysfunction was rapidly reversed (53). A meta-study of suicidal tendencies found that 2,807 participants were involved. The study found that patients with suicidal behavior had lower levels of FT3 than patients without suicidal behavior, and the average levels of FT3 and TT4 in patients with suicidal behavior were significantly lower than those without suicidal behavior (54). These studies focus more on the relationship between the reduction of FT3 and disease. Our results suggest that the increase of FT3 may be a protective factor, which may be an important research direction for us to further explore DPN in the future.
In the study of thyroid hormone and aging, more and more researchers pay attention to the important role of FT3/FT4. A study of 672 well-defined Italian subjects found that FT3 levels and the FT3/FT4 ratio decreased, while FT4 and TSH increased in an age-dependent manner (55). Another study recruited 593 well-defined Italian subjects, including 180 centenarians, the offspring of 276 centenarians, and 137 age-matched controls. Their results found a link between thyroid hormone levels and weakness in centenarians, which is the basis of the important role of thyroid in aging and longevity (56). Further studies found a possible correlation between the decrease of FT3/FT4 (an indirect marker of thyroxine deiodination disorder) and the debilitating state and survival rate of hospitalized elderly patients (57).
We acknowledge some limitations to our study. It involved only one institution, and a retrospective cohort study cannot imply cause and effect. Multicenter and prospective studies should be conducted to investigate the prevalence and risk factors of DPN in diabetic patients to confirm our findings. Nonetheless, a total of 1,091 patients, a relatively large simple capacity, were analyzed in our study. We performed 4 years of follow-up for DPN. Additionally, 13 continuous variables were evaluated by ROC curve analysis to identify the most sensitive cutoff value.
We found that the prevalence of DPN in diabetic patients was 72.7%. Age (>66 years old), hypertension, NE (>4.0 × 109/L), LY (>3.0 × 109/L), HbA1c (>7.7%), and FT3 (>4.4 pmol/L) were six significant predictors for the occurrence of DPN. We hope that these factors are useful for the individualized assessment, risk stratification, and development of targeted prevention programs.
As a cross-sectional study, we can only preliminarily study the significance of some blood parameters in the first diagnosis of DPN patients. In the long course of disease of patients with DM, many influencing factors are not fully included. Current research lacks the most important variables related to the occurrence of DPN. This is also the limitation of our research.
The data analyzed during the current study is available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by the Ethical Decision Committee of the Research Administration at First Affiliated Hospital of Wenzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
YC, WC, and JZ were responsible for data statistics and writing the paper. JW, XL, and QW collected data. QL provided resources and designed the study. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all the patients and their caregivers for their cooperation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.934020/full#supplementary-material
1. Grubelnik V, Zmazek J, Markovič R, Gosak M, Marhl M. Mitochondrial Dysfunction in Pancreatic Alpha and Beta Cells Associated With Type 2 Diabetes Mellitus. Life (Basel Switzerland) (2020) 10:348. doi: 10.3390/life10120348
2. Zhu Z, Liao H. Impact of Cognitive Impairment and Systemic Vascular Comorbidities on Risk of All-Cause and Cardiovascular Mortality: National Health and Nutrition Examination Survey 1999 to 2002. Int J Cardiol (2020) 300:255–61. doi: 10.1016/j.ijcard.2019.11.131
3. Kalra S, Coetzee A, Kalra PA, Saldaña JR, Kilov G. Rubrometabolic Syndrome. Minerva Endocrinol (2020). doi: 10.23736/S0391-1977.20.03353-2
4. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and Global Ageing Among 65-99-Year-Old Adults: Findings From the International Diabetes Federation Diabetes Atlas, 9(Th) Edition. Diabetes Res Clin Pract (2020) 162:108078. doi: 10.1016/j.diabres.2020.108078
5. Zilliox LA, Russell JW. Physical Activity and Dietary Interventions in Diabetic Neuropathy: A Systematic Review. Clin Autonomic Res Off J Clin Autonomic Res Soc (2019) 29:443–55. doi: 10.1007/s10286-019-00607-x
6. Chen Y, Gong Y, Cai K. Correlations of Cardiovascular Autonomic Neuropathy With Urinary Albumin Excretion Rate and Cardiac Function in Patients With Type 2 Diabetes Mellitus. Minerva Endocrinol (2021). doi: 10.23736/S2724-6507.21.03358-7
7. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic Neuropathies: Update on Definitions, Diagnostic Criteria, Estimation of Severity, and Treatments. Diabetes Care (2010) 33:2285–93. doi: 10.2337/dc10-1303
8. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic Interventions for Painful Diabetic Neuropathy: An Umbrella Systematic Review and Comparative Effectiveness Network Meta-Analysis. Ann Internal Med (2014) 161:639–49. doi: 10.7326/M14-0511
9. Low PA, Benrud-Larson LM, Sletten DM, Opfer-Gehrking TL, Weigand SD, O'Brien PC, et al. Autonomic Symptoms and Diabetic Neuropathy: A Population-Based Study. Diabetes Care (2004) 27:2942–7. doi: 10.2337/diacare.27.12.2942
10. Tentolouris N, Marinou K, Kokotis P, Karanti A, Diakoumopoulou E, Katsilambros N. Sudomotor Dysfunction is Associated With Foot Ulceration in Diabetes. Diabetic Med J Br Diabetic Assoc (2009) 26:302–5. doi: 10.1111/j.1464-5491.2009.02677.x
11. Carrillo-Larco RM, Luza-Dueñas AC, Urdániga-Hung M, Bernabé-Ortiz A. Diagnosis of Erectile Dysfunction can be Used to Improve Screening for Type 2 Diabetes Mellitus. Diabetic Med J Br Diabetic Assoc (2018) 35:1538–43. doi: 10.1111/dme.13783
12. Pfeifer MA, Schumer MP. Clinical Trials of Diabetic Neuropathy: Past, Present, and Future. Diabetes (1995) 44:1355–61. doi: 10.2337/diab.44.12.1355
13. Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic Peripheral Neuropathic Pain: Clinical and Quality-of-Life Issues. Mayo Clin Proc (2006) 81:S3–11. doi: 10.1016/S0025-6196(11)61474-2
14. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care (2017) 40:136–54. doi: 10.2337/dc16-2042
15. Rota E, Quadri R, Fanti E, Isoardo G, Poglio F, Tavella A, et al. Electrophysiological Findings of Peripheral Neuropathy in Newly Diagnosed Type II Diabetes Mellitus. J Peripheral Nervous System JPNS (2005) 10:348–53. doi: 10.1111/j.1085-9489.2005.00046.x
16. Janghorbani M, Rezvanian H, Kachooei A, Ghorbani A, Chitsaz A, Izadi F, et al. Peripheral Neuropathy in Type 2 Diabetes Mellitus in Isfahan, Iran: Prevalence and Risk Factors. Acta Neurol Scandinavica (2006) 114:384–91. doi: 10.1111/j.1600-0404.2006.00716.x
17. Pop-Busui R, Lu J, Lopes N, Jones TL. Prevalence of Diabetic Peripheral Neuropathy and Relation to Glycemic Control Therapies at Baseline in the BARI 2D Cohort. J Peripheral Nervous System JPNS (2009) 14:1–13. doi: 10.1111/j.1529-8027.2009.00200.x
18. Abdissa D, Hamba N, Kene K, Bedane DA, Etana G, Muleta D, et al. Prevalence and Determinants of Peripheral Neuropathy Among Type 2 Adult Diabetes Patients Attending Jimma University Medical Center, Southwest Ethiopia, 2019, an Institutional-Based Cross-Sectional Study. J Diabetes Res (2020) 2020:9562920. doi: 10.1155/2020/9562920
19. Khawaja N, Abu-Shennar J, Saleh M, Dahbour SS, Khader YS, Ajlouni KM. Correction to: The Prevalence and Risk Factors of Peripheral Neuropathy Among Patients With Type 2 Diabetes Mellitus; the Case of Jordan. Diabetol Metab Syndrome (2018) 10:43. doi: 10.1186/s13098-018-0336-3
20. Kiani J, Moghimbeigi A, Azizkhani H, Kosarifard S. The Prevalence and Associated Risk Factors of Peripheral Diabetic Neuropathy in Hamedan, Iran. Arch Iranian Med (2013) 16:17–9.
21. Jember G, Melsew YA, Fisseha B, Sany K, Gelaw AY, Janakiraman B. Peripheral Sensory Neuropathy and Associated Factors Among Adult Diabetes Mellitus Patients in Bahr Dar, Ethiopia. J Diabetes Metab Disord (2017) 16:16. doi: 10.1186/s40200-017-0295-5
22. D'Souza M, Kulkarni V, Bhaskaran U, Ahmed H, Naimish H, Prakash A, et al. Diabetic Peripheral Neuropathy and its Determinants Among Patients Attending a Tertiary Health Care Centre in Mangalore, India. J Public Health Res (2015) 4:450. doi: 10.4081/jphr.2015.450
23. Darivemula S, Nagoor K, Patan SK, Reddy NB, Deepthi CS, Chittooru CS. Prevalence and Its Associated Determinants of Diabetic Peripheral Neuropathy (DPN) in Individuals Having Type-2 Diabetes Mellitus in Rural South India. Indian J Community Med Off Publ Indian Assoc Prev Soc Med (2019) 44:88–91. doi: 10.4103/ijcm.IJCM_207_18
24. Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, et al. Vascular Complications in Patients With Type 2 Diabetes: Prevalence and Associated Factors in 38 Countries (the DISCOVER Study Program). Cardiovasc Diabetol (2018) 17:150. doi: 10.1186/s12933-018-0787-8
25. Zhang C, Tang M, Lu X, Zhou Y, Zhao W, Liu Y, et al. Relationship of Ankle-Brachial Index, Vibration Perception Threshold, and Current Perception Threshold to Glycemic Variability in Type 2 Diabetes. Medicine (2020) 99:e19374. doi: 10.1097/MD.0000000000019374
26. Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a Risk Factor for Diabetic Neuropathy: A Prospective Study. Diabetes (1997) 46:665–70. doi: 10.2337/diab.46.4.665
27. Ruggenenti P, Lauria G, Iliev IP, Fassi A, Ilieva AP, Rota S, et al. Effects of Manidipine and Delapril in Hypertensive Patients With Type 2 Diabetes Mellitus: The Delapril and Manidipine for Nephroprotection in Diabetes (DEMAND) Randomized Clinical Trial. Hypertension (Dallas Tex: 1979) (2011) 58:776–83. doi: 10.1161/HYPERTENSIONAHA.111.174474
28. Gregory JA, Jolivalt CG, Goor J, Mizisin AP, Calcutt NA. Hypertension-Induced Peripheral Neuropathy and the Combined Effects of Hypertension and Diabetes on Nerve Structure and Function in Rats. Acta Neuropathol (2012) 124:561–73. doi: 10.1007/s00401-012-1012-6
29. De Visser A, Hemming A, Yang C, Zaver S, Dhaliwal R, Jawed Z, et al. The Adjuvant Effect of Hypertension Upon Diabetic Peripheral Neuropathy in Experimental Type 2 Diabetes. Neurobiol Dis (2014) 62:18–30. doi: 10.1016/j.nbd.2013.07.019
30. Sanada LS, Tavares MR, Sato KL, Ferreira Rda S, Neubern MC, Castania JA, et al. Association of Chronic Diabetes and Hypertension in Sural Nerve Morphometry: An Experimental Study. Diabetol Metab Syndrome (2015) 7:9. doi: 10.1186/s13098-015-0005-8
31. Yang CP, Lin CC, Li CI, Liu CS, Lin WY, Hwang KL, et al. Cardiovascular Risk Factors Increase the Risks of Diabetic Peripheral Neuropathy in Patients With Type 2 Diabetes Mellitus: The Taiwan Diabetes Study. Medicine (2015) 94:e1783. doi: 10.1097/MD.0000000000001783
32. Ponirakis G, Petropoulos IN, Alam U, Ferdousi M, Asghar O, Marshall A, et al. Hypertension Contributes to Neuropathy in Patients With Type 1 Diabetes. Am J Hypertension (2019) 32:796–803. doi: 10.1093/ajh/hpz058
33. Schofield I, Malik R, Izzard A, Austin C, Heagerty A. Vascular Structural and Functional Changes in Type 2 Diabetes Mellitus: Evidence for the Roles of Abnormal Myogenic Responsiveness and Dyslipidemia. Circulation (2002) 106:3037–43. doi: 10.1161/01.CIR.0000041432.80615.A5
34. Malik RA, Schofield IJ, Izzard A, Austin C, Bermann G, Heagerty AM. Effects of Angiotensin Type-1 Receptor Antagonism on Small Artery Function in Patients With Type 2 Diabetes Mellitus. Hypertension (Dallas Tex: 1979) (2005) 45:264–9. doi: 10.1161/01.HYP.0000153305.50128.a1
35. Greenstein AS, Price A, Sonoyama K, Paisley A, Khavandi K, Withers S, et al. Eutrophic Remodeling of Small Arteries in Type 1 Diabetes Mellitus is Enabled by Metabolic Control: A 10-Year Follow-Up Study. Hypertension (Dallas Tex: 1979) (2009) 54:134–41. doi: 10.1161/HYPERTENSIONAHA.109.129718
36. Boitard C. T-Lymphocyte Recognition of Beta Cells in Type 1 Diabetes: Clinical Perspectives. Diabetes Metab (2013) 39:459–66. doi: 10.1016/j.diabet.2013.08.001
37. Zheng H, Sun W, Zhang Q, Zhang Y, Ji L, Liu X, et al. Proinflammatory Cytokines Predict the Incidence of Diabetic Peripheral Neuropathy Over 5 Years in Chinese Type 2 Diabetes Patients: A Prospective Cohort Study. EClinicalMedicine (2021) 31:100649. doi: 10.1016/j.eclinm.2020.100649
38. Rajakariar R, Lawrence T, Bystrom J, Hilliard M, Colville-Nash P, Bellingan G, et al. Novel Biphasic Role for Lymphocytes Revealed During Resolving Inflammation. Blood (2008) 111:4184–92. doi: 10.1182/blood-2007-08-108936
39. Liu S, Zheng H, Zhu X, Mao F, Zhang S, Shi H, et al. Neutrophil-To-Lymphocyte Ratio is Associated With Diabetic Peripheral Neuropathy in Type 2 Diabetes Patients. Diabetes Res Clin Pract (2017) 130:90–7. doi: 10.1016/j.diabres.2017.05.008
40. Davies AJ, Rinaldi S, Costigan M, Oh SB. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front Neurosci (2020) 14:142. doi: 10.3389/fnins.2020.00142
41. Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury. PloS Pathog (2015) 11:e1004651. doi: 10.1371/journal.ppat.1004651
42. Wang J. Neutrophils in Tissue Injury and Repair. Cell Tissue Res (2018) 371:531–9. doi: 10.1007/s00441-017-2785-7
43. Top C, Yildiz S, Oncül O, Qydedi T, Cevikbaş A, Soyogul UG, et al. Phagocytic Activity of Neutrophils Improves Over the Course of Therapy of Diabetic Foot Infections. J Infect (2007) 55:369–73. doi: 10.1016/j.jinf.2007.06.003
44. Rassias AJ, Marrin CA, Arruda J, Whalen PK, Beach M, Yeager MP. Insulin Infusion Improves Neutrophil Function in Diabetic Cardiac Surgery Patients. Anesth Analgesia (1999) 88:1011–6. doi: 10.1097/00000539-199905000-00008
45. Rassias AJ, Givan AL, Marrin CA, Whalen K, Pahl J, Yeager MP. Insulin Increases Neutrophil Count and Phagocytic Capacity After Cardiac Surgery. Anesth Analgesia (2002) 94:1113–9. doi: 10.1097/00000539-200205000-00010
46. Kolaczkowska E, Kubes P. Neutrophil Recruitment and Function in Health and Inflammation. Nat Rev Immunol (2013) 13:159–75. doi: 10.1038/nri3399
47. Ma Y, Zhang Y, Zhu L. Role of Neutrophils in Acute Viral Infection. Immun Inflammation Dis (2021) 9:1186–96. doi: 10.1002/iid3.500
48. Carlsen ED, Liang Y, Shelite TR, Walker DH, Melby PC, Soong L. Permissive and Protective Roles for Neutrophils in Leishmaniasis. Clin Exp Immunol (2015) 182:109–18. doi: 10.1111/cei.12674
49. Fernández-Real JM, López-Bermejo A, Castro A, Casamitjana R, Ricart W. Thyroid Function is Intrinsically Linked to Insulin Sensitivity and Endothelium-Dependent Vasodilation in Healthy Euthyroid Subjects. J Clin Endocrinol Metab (2006) 91:3337–43. doi: 10.1210/jc.2006-0841
50. Sendi RA, Mahrus AM, Saeed RM, Mohammed MA, Al-Dubai SAR. Diabetic Peripheral Neuropathy Among Saudi Diabetic Patients: A Multicenter Cross-Sectional Study at Primary Health Care Setting. J Family Med Primary Care (2020) 9:197–201. doi: 10.4103/jfmpc.jfmpc_927_19
51. Yuan D, Zhang C, Jia S, Liu Y, Jiang L, Xu L, et al. Predictive Value of Free Triiodothyronine (FT3) to Free Thyroxine (FT4) Ratio in Long-Term Outcomes of Euthyroid Patients With Three-Vessel Coronary Artery Disease. Nutrition Metabol Cardiovasc Dis NMCD (2021) 31:579–86. doi: 10.1016/j.numecd.2020.10.011
52. Mario FD, Pofi R, Gigante A, Rivoli L, Rosato E, Isidori AM, et al. Hypothyroidism and Nephrotic Syndrome: Why, When and How to Treat. Curr Vasc Pharmacol (2017) 15:398–403. doi: 10.2174/1570161115999170207114706
53. Novitzky D, Cooper DK. Thyroid Hormone and the Stunned Myocardium. J Endocrinol (2014) 223:R1–8. doi: 10.1530/JOE-14-0389
54. Toloza FJK, Mao Y, Menon L, George G, Borikar M, Thumma S, et al. Association of Thyroid Function With Suicidal Behavior: A Systematic Review and Meta-Analysis. Med (Kaunas Lithuania) (2021) 57:714. doi: 10.3390/medicina57070714
55. Ostan R, Monti D, Mari D, Arosio B, Gentilini D, Ferri E, et al. Heterogeneity of Thyroid Function and Impact of Peripheral Thyroxine Deiodination in Centenarians and Semi-Supercentenarians: Association With Functional Status and Mortality. J Gerontol Ser A Biol Sci Med Sci (2019) 74:802–10. doi: 10.1093/gerona/gly194
56. Arosio B, Monti D, Mari D, Passarino G, Ostan R, Ferri E, et al. Thyroid Hormones and Frailty in Persons Experiencing Extreme Longevity. Exp Gerontol (2020) 138:111000. doi: 10.1016/j.exger.2020.111000
Keywords: type-2 diabetes mellitus, diabetic peripheral neuropathy, determinants, clinical significance, prevalence, risk factor
Citation: Cheng Y, Cao W, Zhang J, Wang J, Liu X, Wu Q and Lin Q (2022) Determinants of Diabetic Peripheral Neuropathy and Their Clinical Significance: A Retrospective Cohort Study. Front. Endocrinol. 13:934020. doi: 10.3389/fendo.2022.934020
Received: 02 May 2022; Accepted: 22 June 2022;
Published: 26 July 2022.
Edited by:
Fabio Monzani, University of Pisa, ItalyReviewed by:
Giovanni Vitale, Italian Auxological Institute (IRCCS), ItalyCopyright © 2022 Cheng, Cao, Zhang, Wang, Liu, Wu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingxia Lin, MzgwMzY0NjEzQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.