- 1Reproductive Medicine Center, Department of Reproductive Endocrinology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 2Institute of Food Science and Engineering, Hangzhou Medical College, Hangzhou, China
A randomized sibling-embryo pilot trial investigated whether two ways of laser-assisted hatching result in different blastulation and clinical outcomes after extended in vitro culture process of highly fragmented day-3 cleavage embryos. From 92 couples, a total of 315 highly fragmented day-3 embryos (the fragmentation >25%) were recruited and randomized into laser-assisted zona thinning (LAT, n=157) and opening (LAO, n=158) groups, and then underwent a blastocyst culture in vitro. The main endpoint measurements including blastocyst formation and grading as well as the clinical pregnancy after blastocyst transfer were obtained during the treatment procedure of in vitro fertilization and embryo transfer, and then analyzed with generalized estimating equation (GEE) and/or time-to blastocyst analysis models. A total of 166 day-3 embryos developed into blastocyst stage (52.70%), of which 97 were viable blastocysts (30.79%), and 42 top-quality ones (13.33%). LAT did not have any inferior or superior to LAO in the endpoints of either total, viable, top-quality or hatched blastocyst formation, with the ORs (95%CI) from GEE model as 0.89 (0.55-1.45), 0.71 (0.42-1.21), 1.12 (0.56-2.25) and 0.68 (0.42-1.12) respectively for LAT treatment. And the time-to-blastocyst analysis showed a similar result. Additionally, no difference in clinical outcomes after blastocyst transfer was found between the two groups. The author concluded that when applying the LAHs during the extended culture of highly fragmented embryos, both LAT and LAO can generate a promising clinical outcome, and the LAT operation be equivalent to the LAO. Future well-designed, multiple-center, larger-sample investigations are required to ascertain above conclusion.
Introduction
Embryonic fragmentation, one of the most common morphological defections in cleavage embryos, is considered as a dominant impairment to oocyte utilization during IVF procedure, and unanimously as a major stubborn challenge faced by embryologists (1, 2). Despite poor developing potential and inappropriateness for direct transfer, heavily fragmented cleavage embryos are still subject to extended in vitro culture in practice, with the purpose of vitality discrimination (3, 4). Currently nevertheless, the blastulation of the fragmented embryos remains really inferior, even though various means have been attempted, such as mechanical aspiration of the cellular debris and addition of some cytokines such as granulocyte colony-stimulating factor (G-CSF) (5, 6). This highlights the necessity that additional modifications be considered for present blastocyst culture procedure
Laser technology is now being widely used in embryo laboratory operations such as intracytoplasmic sperm injection, embryo biopsy, sperm immobilization/selection, assisted hatching and others, and reaches a grate convenience and consistency (7). Laser-assisted hatching (LAH), which refers to etching the zona pellucida of embryos to facilitate the hatching process, has been regarded as the most frequent form of its applications (7, 8). A beneficial effect of LAH application on in vitro blastulation process has been implicated by abundant evidence that zona ablations by laser in cleavage embryos result in a better implantation potential and clinical pregnancy, especially for the cases of poor prognosis (9, 10). And the direct evidence related to this benefit has been reported in our and other’s studies (11, 12). All these findings encourage the addition of LAH operation during the extended culture process for untransferable cleavage embryos to promote the utilization of low-graded embryos, especially for the fragmented ones. Our previous report has identified an improved blastocyst outcome when a LAH operation was performed in low-graded cleavage embryos at day 4 (3). This is consistent with a previous study conducted on fragmented embryos using a AH method with acidic tyrode’s solution (13).
When performing LAH, two major types of zona ablation, zona thinning (LAT) and zona opening (LAO), are usually chosen (7, 14). Despite their clinical efficacy during ART treatment has been extensively studied and recognized, it remains elusive which one may bring about a better outcome (15). Some population studies showed that the two ways have a similar efficacy in the endpoints of implantation and clinical pregnancy (9, 16, 17), but others proposed that the former be associated with a better clinical outcome (18, 19). Evidence from animal studies show that LAO may result in a higher rate of hatched blastocysts than LAT (quarter zonal-thinning), while the blastocyst formation be similar (20, 21). Therefore, for a more promising prognosis, details especially the types of laser ablation should be explored furtherly when performing the LAH for the fragmented embryos. For this purpose, we conducted this prospective study with a randomized control sibling-embryo design to compare the clinical efficacy of the two ways of LAH during the extended culture of highly fragmented cleavage embryos.
Materials and Methods
Study Participants
This is a prospective randomized sibling-oocyte study on the IVF-ET couples from June, 2020 to April, 2021 at the Reproductive Center of Zhejiang Provincial People’s Hospital, China. The study protocol was approved by the Ethics Committee of Zhejiang Provincial People’s Hospital.
We recruited the participants from the infertile couples who underwent their first IVF-ET cycles. The inclusion criteria were defined as follows (1): female partner aged <40 years (2); those couples with more than 2 highly fragmented day-3 cleavage embryos (specified as embryos originating from 2PN zygote, with fragment rate >25% and at least 4 blastomeres) (3); receiving extended in vitro culture. The exclusion criteria were (1) abnormal karyotypes of any partner (2); embryos originating from assisted oocyte activating or in vitro maturation procedure (3); familial infertility of any partner. All recruited couples signed the written inform consents.
Blastocyst Culture and Laser Operations
Controlled ovarian stimulation, oocyte retrieval and insemination were conducted according to the regular procedure of our routine clinical IVF-ET program. The zygotes formed were put into Sydney IVF Cleavage medium (CM, COOK MEDICAL, Australia) for further culture, and the forming embryos scored in light of the parameters of blastomere amount, blastomere evenness as well as fragment rate (22). The recruited day-3 fragmented embryos were arranged randomly into zona opening (LAO) or zona thinning (LAT) groups with a computer randomization method. Randomized embryos were put into blastocyst culture in parallel with the resting ones in 60 μL microdrops of Sydney IVF Blastocyst medium (Cook Medical, Australia) under routine conditions, 37°C, 6% CO2 and 5% O2.
At day 4, a laser dissection was performed for the two randomized groups as described previously (23). Briefly, in LAT group, a series of successive ablations of 2.6 ms duration, which covered quarter of the zona circumference, were performed at the outer edge of the zona to form a defect occupying about 60% zona thickness, while in LAO group, two juxtaposed rows of ablations with 2.6 ms duration were exerted perpendicular to the zona to create a single full thickness opening (about 10μm diameter) through the zona. Zona operation was carried out at the area that is farthest from the blastomeres both in the LAT and LAO groups with OCTAX Laser Shot system (MTG Medical Technology, Altdorf, Germany). At the morning day 5 and 6, the blastulation and hatching state was checked, and the quality of blastocysts were evaluated according to the Gardner score system (24). Specifically, those staged over 3 with inner cell mass or trophectoderm grade above ‘B’ were regarded as viable blastocysts, while those staged over 3 at day 5 or over 4 at day 6 and graded above ‘B’ for both inner cell mass and trophectoderm as good-quality ones. All viable and good-quality blastocysts were cryopreserved on day 5 or day 6 according to the instructions provided by the vitrification reagent manufacturer (Vitrolife, Sweden).
Endometrial Preparation, Frozen Blastocyst Transfer and Pregnancy Check
Receptive endometrium was prepared through either a hormone replacement (HRT) or natural cycle. For HRT cycles, an oral dose of 6 mg/day estradiol (estradiol valerate, Delpharm Lille S.A.S, France) was given from cycle day 3 onwards, and if endometrial lining remained <7mm 7 days after HRT initiation, additional dose (5g/day) of transvaginal estradiol gel (Besins Manufacturing Belgium) was administrated for another 5-7 days. When endometrial thickness reached a satisfactory level, daily 40 mg progesterone was injected. For natural cycles, the progesterone treatment started from ovulation day or 2 days after LH surge. One or two blastocysts were thawed, and transferred under ultrasound guidance. Once pregnancy was achieved, the progesterone and estradiol supplementation were continued until 10 weeks of gestation.
Pregnancy was checked by serum hCG test 10 days after transfer, and ascertained under ultrasound examination 5 weeks after transfer. Clinical pregnancy referred to the presence of a gestational sac under ultrasound. An implantation rate was defined as the ratio of the number of gestational sacs visualized under ultrasound to that of embryos transferred, and early miscarriage rate as the spontaneous loss of a clinical pregnancy before 12th weeks of gestation.
Statistics
The distribution of each continuous variable was checked by the Shapiro-Wilk test. If in normal distribution, the continuous variables were expressed as mean ± standard deviation, (±SD) or else, d as median (inter-quartile Range, IQR). All category variables were described as n (%). Data analysis was performed with the SPSS statistical software (version 21.0, IBM Corp., USA). To compare the study endpoints and related covariates such as blastomere amount and fragments between the two groups, non-parameter Kruskal-Wallis test and Fisher’s exact test were conducted for continuous variables and category ones respectively. Importantly, a cluster-weighted generalized estimating equation (GEE) models were used to account for within person correlations between the fragmented embryos from the same participant, since the contribution of the cluster size (here refers to number of fragmented embryos) is nonignorable (25, 26). During GEE models, a binomial distribution with log link function were specified, and the inverse of the number of recruited embryos taken as the weighting coefficient. OR values were calculated after adjusting for the covariates of female age, BMI, primary infertility, intracytoplasmic sperm injection insemination (ICSI) as well as day-3 embryos parameters. Considering the fact that blastulation time be a determinant for further development and implantation potential, we also performed a time-to-blastocyst analysis by fitting conventional survival analysis, and assigned the blastocyst cryopreservation as the censoring event. A Cox proportional hazards regression model was specified for total and viable blastocyst formation, while a fine & Gray model for top-quality and hatched ones given the competitive relationship between cryopreserved blastocysts at day 5 and top-quality or hatched blastocysts formed at day 6. A two-tailed P<0.05 was considered as statistical significance.
Results
Recruited Couples and Embryos
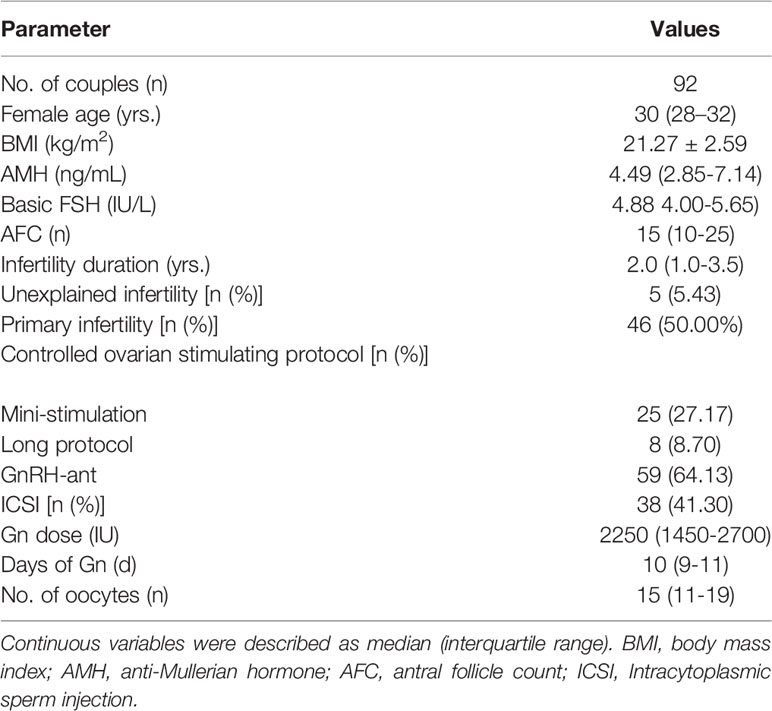
A total of 92 couples and 315 fragmented day-3 embryos were recruited into present study. All participants were of Han ethnicity. The median age of female partners was 30 years [IQR: 28-32], average BMI 21.27 ± 2.59 kg/m2, median serum AMH level 4.49 [IQR: 2.85-7.14] ng/mL, and median retrieved oocytes 15 (IQR: 11-19). Other baseline characteristics of female partners were summarized in Table 1. Of all randomized day-3 embryos, the median number of blastomere was 5 [IQR:5-6], and median fragments 30% (IQR: 25%-35%). Finally, a total of 166 day-3 embryos developed into blastocyst stage (52.70%), of which 97 were viable blastocysts (30.79%), and 42 top-quality ones (13.33%). The blastulation outcomes here were obviously higher than those for untreated embryos as reported in our previous study (40.2%, 18.6% and 9.2%, respectively) under the same cultural conditions (3).
Blastocyst Cultural Outcomes
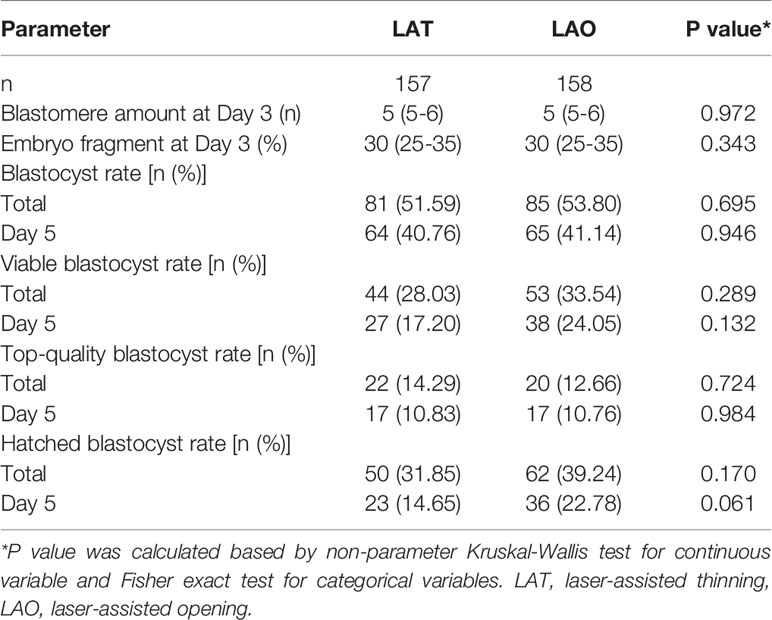
Of all recruited day-3 embryos, 157 were arranged to the LAT group, and 158 to the LAO. The distribution of blastomere amount and fragments were described in Figure 1 and Table 1, and showed quite equivalent between the two groups, indicating a considerable comparability for the comparison. Consequently, total blastulation rates were 51.59% and 53.80%, viable blastocyst 28.03% and 33.54%, top-quality blastocyst 14.29% and 12.66%, and hatching blastocyst 31.85% and 31.24% for LAT and LAO treatments respectively. No significance between the two groups was observed in any above endpoints. At day 5, despite a seemingly lower viable and hatched blastocyst rate was identified in LAO group (17.20% vs 24.50%, and 14.65% and 22.78%), the difference did not reach a significance (Table 2).
Figure 1 Distribution of blastomere number and fragments between the two groups. (A) blastomere number, and (B) embryo fragments. LAT, laser-assisted thinning; LAO, laser-assisted opening.
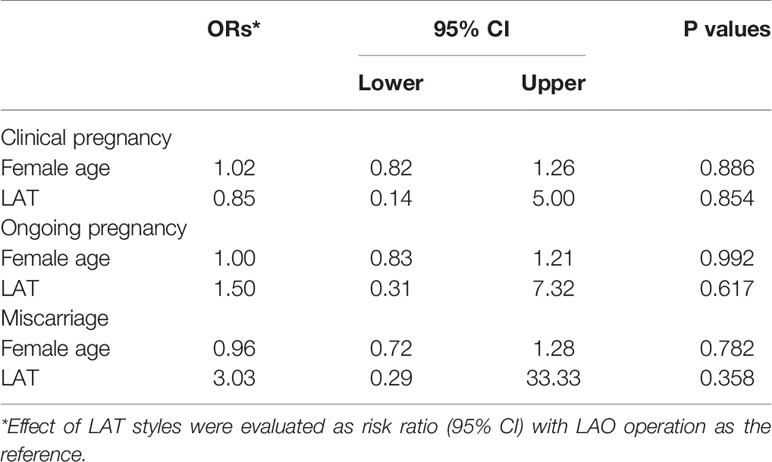
Considering the risk that between-embryos correlation might impair the robustness of our study, a multi-variate analysis with cluster-weighted GEE (CWGEE) model were used to ascertain above results, and showed that difference in LAH ways have no influence on either total, viable, top-quality or hatched blastocyst formation, with the ORs (95%CI) from GEE model as 0.89 (0.55-1.45), 0.71 (0.42-1.21), 1.12 (0.56-2.25) and 0.68 (0.42-1.12) respectively for LAT treatment as compared with the LAO (Table 3). Tentatively, a time-to-blastocyst analysis grown out of survival analysis was conducted, and still showed a similar null result (ORs were shown in Table 3). Collectively, our results showed that zona thinning and opening have a similar blastulation outcome when used in fragmented day-3 embryos. Additionally, when analyzing the covariates taken into our multivariate models, we identified a striking association of blastomere amount and fragment rates with the blastulation outcomes in both CWGEE and time-to-blastocyst analysis (Table 3).
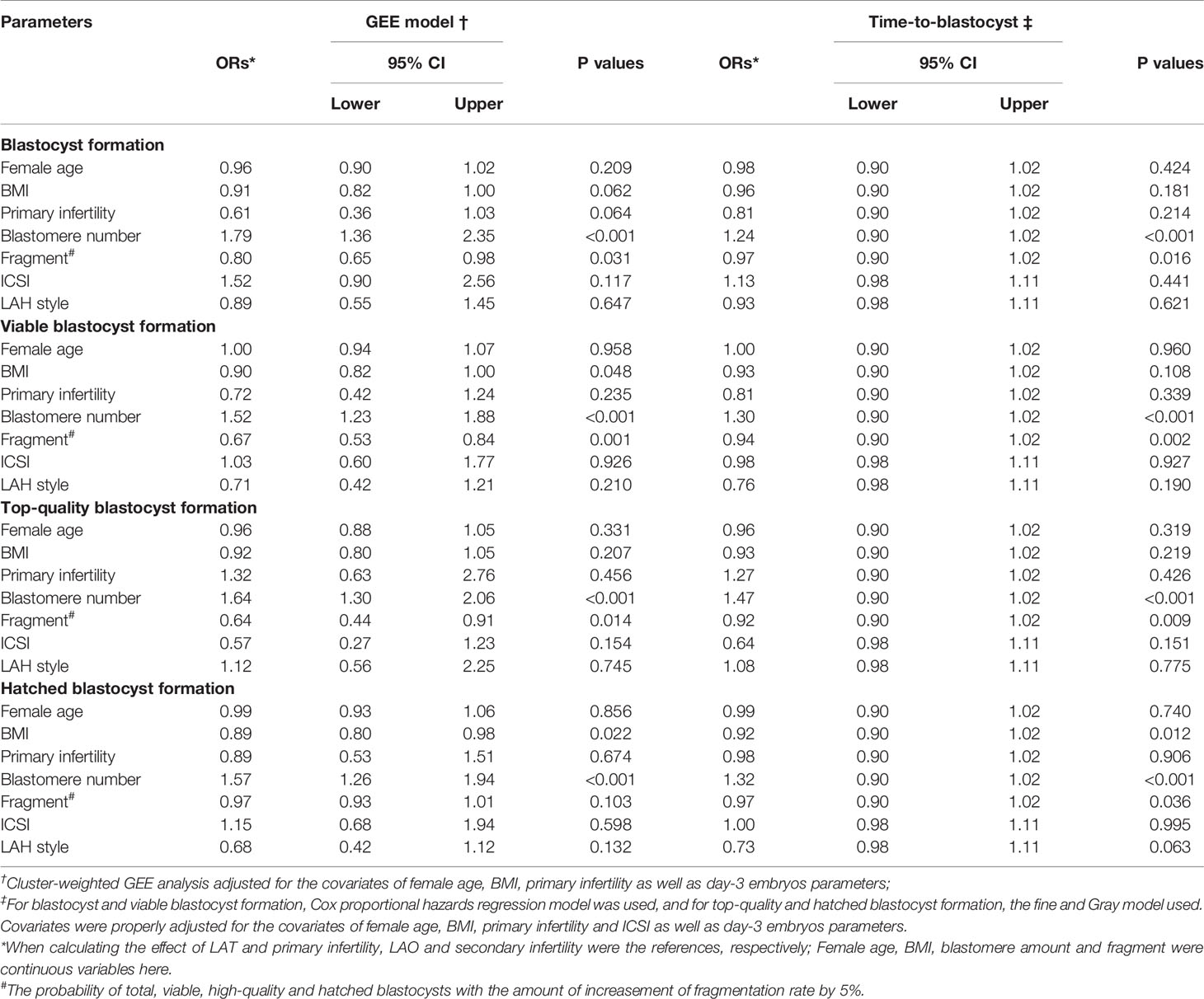
Table 3 GEE analysis and time-to-blastocyst analysis on the blastocyte culture outcomes of fragmented day-3 embryos.
Clinical Outcomes After Blastocyst Transfer
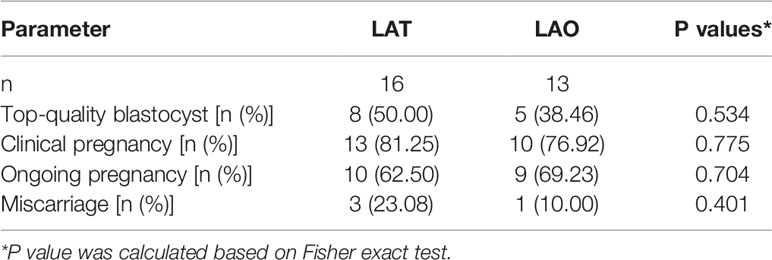
To further compare the blastocyst development and implantation potential between the two groups, we also investigated the clinical outcomes after blastocyst transfer. Of all blastocysts derived from the recruited fragmented embryos, 29 were finally transferred, of which 16 were from the LAT group and 13 from the LAO. Clinical rates were 81.25% and 76.92%, live birth rates 62.50% and 69.23%, and miscarriage rates 23.08% and 10.00% for LAT and LAO groups respectively (Table 4). No significance was identified in any endpoints, either with univariate or GEE analysis models (Tables 4, 5).
Discussion
Improving the utilization of highly fragmented embryos, the most common low-graded day-3 embryos, is conducive for the prognosis of current ART procedures. Our previous evidence has proven that LAH application may play certain beneficial role during the blastocyst culture of low-graded cleavage embryos, but which types of LAH may give a better outcome remains unknown. This randomized controlled study is the first to compare the blastulation and clinical outcomes of two types of LAH, quarter zona thinning (LAT) and single point opening (LAO) in the fragmented day-3 embryos. Our results showed that the LAT treatment have an equivalent value to LAO in terms of total, viable, top-quality and hatched blastocyst formation, as well as the clinical outcomes after blastocyst transfer. Both LAO and LAT have better blastulation outcomes compared to those of non-treated low-graded embryos, as reported in our previous study (3).
Fragments in early human embryos are generally considered as anucleate cytoplasmic structures, which usually occur during the cytokinetic phase of blastomeres (27, 28). General consensuses have been already reached on the poor prognosis of the fragmented embryos (particularly when the fragmentation >25%) (1, 4, 29, 30). For the cost-effective consideration, an extended culture is usually recommended to screen out the pitifully few survivals which have an acceptable developmental potential. In this study, we chose day 4 instead of day 3 to operate LAHs, because a larger perivitelline space can be obtained in day-4 compact embryos, which facilitates the operation and reduces the risk of damage to embryos. This prolonged process also provides a window period for certain interventions intended for a better blastulation, such as aspiration of the fragments, addition of some cytokines as well as LAHs. As shown in present and our previous study, application of LAHs in low-graded or highly fragmented cleavage embryos are related to an appreciable blastulation outcome (transferable blastocyst >30%), substantially better than that in the control (3).
Some mechanisms have been supposed for the harmful effect of embryo fragments. Deleterious microenvironment related to the fragments may cause a constant and permanent damage to the neighboring blastomeres. It is believed that fragments within embryos release some toxic substances through certain pathophysiological processes such as necrosis and apoptosis (31–33), which construct a deleterious microenvironment containing high reactive oxygen species and proapoptotic factors such as caspase-3 (32, 34). Ultrastructural observation showed that the blastomere adjacent to fragments present signs of degeneration, suggesting induced apoptosis indeed occur in peripheral cells (35). On the other hand, communication between blastomeres, mainly mediated by gap junction, has been recognized as the key regulator during blastomere radial polarization and compaction, and disturbance to this cellular event may impair the foundation for the initial cell fate decisions and morphogenetic movements of embryogenesis (36, 37). In highly fragmented embryos, the fragments occupying the space among blastomeres, may hamper that relationship, and consequently, impair morula formation, embryo cavitation and blastocyst formation (38). From this perspective, it seems that LAO might be superior to LAT when applied during extended culture of fragmented embryos, since an opening hole by LAO may promote the efflux of localized harmful substances such as ROS, proapoptotic factors, and even the fragment itself. Furthermore, an artificial opening through zona may facilitate the exchange of nutrients and other bioactive substances, which is requisite for embryo development and blastulation (3). Nevertheless, in present study, we found an equivalent beneficial effect for the two treatments. Similarly, despite some retrospective studies showed that the defragmentation operation on highly fragmented embryos may result in an equivalent clinical outcome to that of high-grade, non-defragmented embryos (2, 39), a randomized controlled study ascertained that fragment removal operation cannot increase the embryo’s ability to undergo compaction, morula formation, or blastocyst formation (5). These findings suggested that alleviating local deleterious environment or reducing the existence of debris alone have no, or minimal (if any) contribution the beneficial effect of LAHs in fragmented embryos.
Another likely explanation for the advantage of LAHs relies on advanced escape from encapsule of zona and shorter coexistence of the developing embryos with the adverse environments. Indeed, a bulk of evidence have proven that both LAO and LAT be able to accelerate the process of hatching, and some considered that these methods can increase the blastocyst formation (11, 12, 14, 40). In view of this, relieving the developing embryos from a deleterious circumstance caused by fragmentation might be the common pathway for the two LAHs, and explain the equal beneficial effect for LAO and LAT. Lastly, even though LAT cannot render additional benefits from thorough opening through the zona, it presents some unique advantages. There is less risk of blastomere damage with zona thinning due to the laser beam being positioned further away from the cells than LAO. Additionally, LAT can retain the remnant opportunity of blastocyst expansion and physiological zona thinning during extended culture, which has been supposed to boost the proliferation of blastocyst cells and then generate a blastocyst with putative higher grade (14, 20). In present study, we thinned outer layer of zona in the LAT group consistent with many other researchers (14, 40), retaining the more elastic inner layer. Taken together, the similarity in the blastulation outcomes between the two groups should be attributed to the comprehensive consequence of multiple mechanisms.
A multivariate weighted GEE model, which has been thought to be competent in handling the data of cluster type (25, 26), was adopt in present study to adjust for the confounding from certain covariates including female age, BMI, primary infertility, blastomere amount and fragment rate and ICSI operation, and meanwhile, control the close correlation within embryos from the same patient. Furthermore, the time-to-blastocyst endpoint were also analyzed with a Cox proportional hazards regression or fine and Gray model, which has been the first application of this statical method in the ART scene (41, 42). Both the cluster-weighted GEE and time-to-blastocyst analysis showed a consistent conclusion with that of univariate analysis, indicating a considerable robustness of our result. Additionally, when analyzing the covariates with the multivariate statistical models, a substantial contribution from both blastomere amount and fragments rates has been identified. The probability of total, viable, high-quality and hatched blastocysts increased by 79%, 52%, 64% and 57% respectively with the blastomere amount increased by 1, while decreased by 20%, 33%, 36% and 3% respectively with a 5% increasement in fragmentation. This finding may be of great implication for the future studies intended to estimate the difference in developing potential of fragmented embryos.
In summary, this study proposed that both LAT and LAO have an evident beneficial effect during the extended culture of highly fragmented day-3 cleavage embryos, and LAT be not inferior to LAO during this procedure. However, due to the strict inclusion criteria as well as priority of embryo selection for transfer in practice, even fewer recruited patients accomplishing a transfer with the blastocyst harvested in this study, the sample sizes for both cultural and transfer cycles remained even small, which might impair the statistical force. Thus, the conclusion derived from this study remains preliminary and should be treated with caution. Future well-designed, multiple-center, larger-sample investigations are required to provide a more solid conclusion.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Zhejiang Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LingZ and Y-EZ conducted the cultural manipulation, statistical analysis, and manuscript drafting. Y-JW, L-MW, S-SL, LinZ, ZJ, and C-YS contributed to data collection and critical discussion. W-HX and JS designed the study and edited the manuscript. The authors read and approved the final manuscript.
Funding
This study was supported by the General Research Program for Medicine and Health of Zhejiang Province (2021KY065, LZ), ‘New Century 151 Talent Program’ of Zhejiang Province (LZ) and Adjunct Talent Fund of Zhejiang Provincial People’s Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ebner T, Yaman C, Moser M, Sommergruber M, Pölz W, Tews G. Embryo Fragmentation In Vitro and Its Impact on Treatment and Pregnancy Outcome. Fertil Steril (2001) 76(2):281–5. doi: 10.1016/S0015-0282(01)01904-5
2. Keltz MD, Skorupski JC, Bradley K, Stein D. Predictors of Embryo Fragmentation and Outcome After Fragment Removal in In Vitro Fertilization. Fertil Steril (2006) 86(2):321–4. doi: 10.1016/j.fertnstert.2006.01.048
3. Xu W, Zhang L, Zhang L, Jin Z, Wu L, Li S, et al. Laser-Assisted Hatching in Lower Grade Cleavage Stage Embryos Improves Blastocyst Formation: Results From a Retrospective Study. J Ovarian Res (2021) 14(1):94. doi: 10.1186/s13048-021-00844-7
4. Stone BA, Greene J, Vargyas JM, Ringler GE, Marrs RP. Embryo Fragmentation as a Determinant of Blastocyst Development In Vitro and Pregnancy Outcomes Following Embryo Transfer. Am J obstet gynecol (2005) 192(6):2014–9 discussion 9-20. doi: 10.1016/j.ajog.2005.02.048
5. Keltz M, Fritz R, Gonzales E, Ozensoy S, Skorupski J, Stein D. Defragmentation of Low Grade Day 3 Embryos Resulted in Sustained Reduction in Fragmentation, But did Not Improve Compaction or Blastulation Rates. Fertil Steril (2010) 94(6):2406–8. doi: 10.1016/j.fertnstert.2010.03.014
6. Halvaei I, Khalili MA, Esfandiari N, Safari S, Talebi AR, Miglietta S, et al. Ultrastructure of Cytoplasmic Fragments in Human Cleavage Stage Embryos. J Assisted Reprod Genet (2016) 33(12):1677–84. doi: 10.1007/s10815-016-0806-1
7. Davidson LM, Liu Y, Griffiths T, Jones C, Coward K. Laser Technology in the ART Laboratory: A Narrative Review. Reprod BioMed Online. (2019) 38(5):725–39. doi: 10.1016/j.rbmo.2018.12.011
8. Obruca A, Strohmer H, Sakkas D, Menezo Y, Kogosowski A, Barak Y, et al. Fertilization and Early Embryology: Use of Lasers in Assisted Fertilization and Hatching. Hum Reproduction. (1994) 9(9):1723–6. doi: 10.1093/oxfordjournals.humrep.a138781
9. Ma B, Wang Y, Zhang H, Zhang X. A Study Comparing Three Different Laser-Assisted Hatching Techniques. Clin Exp Obstet Gynecol. (2014) 41(1):37–40. doi: 10.12891/ceog16092014
10. González-Ortega C, Cancino-Villarreall P, Anaya-Torres FJ, Pérez-Peña E, Gutiérrez-Gutiérrez AM. [Impact of Laser-Assisted Hatching (Quarter Technique) in Poor Prognosis Patients]. Ginecologia y obstetricia Mexico (2015) 83(11):670–9.
11. Wang EH, Wang AC, Wang BS, Li B. Outcomes of Vitrified-Warmed Cleavage-Stage Embryo Hatching After In Vitro Laser-Assisted Zona Pellucida Thinning in Patients. Biomed Rep (2016) 5(3):376–82. doi: 10.3892/br.2016.716
12. Lee YS, Park MJ, Park SH, Koo JS, Moon HS, Joo BS. Effect of Laser-Assisted Multi-Point Zona Thinning on Development and Hatching of Cleavage Embryos in Mice. Clin Exp Reprod Med (2015) 42(2):51–7. doi: 10.5653/cerm.2015.42.2.51
13. Magli MC, Gianaroli L, Ferraretti AP, Fortini D, Aicardi G, Montanaro N. Rescue of Implantation Potential in Embryos With Poor Prognosis by Assisted Zona Hatching. Hum Reproduction. (1998) 13(5):1331–5. doi: 10.1093/humrep/13.5.1331
14. Blake DA, Forsberg AS, Johansson BR, Wikland M. Laser Zona Pellucida Thinning—an Alternative Approach to Assisted Hatching. Hum Reproduction. (2001) 16(9):1959–64. doi: 10.1093/humrep/16.9.1959
15. De Vos A, Van Steirteghem A. Zona Hardening, Zona Drilling and Assisted Hatching: New Achievements in Assisted Reproduction. Cells Tissues Organs (2000) 166(2):220–7. doi: 10.1159/000016734
16. Le MT, Nguyen TTA, Nguyen TTT, Nguyen VT, Le DD, Nguyen VQH, et al. Thinning and Drilling Laser-Assisted Hatching in Thawed Embryo Transfer: A Randomized Controlled Trial. Clin Exp Reprod Med (2018) 45(3):129–34. doi: 10.5653/cerm.2018.45.3.129
17. Lee J-W, Cha J-H, Shin S-H, Kim Y-J, Lee S-K, C-k P, et al. Effects of Laser-Assisted Thinning Versus Opening on Clinical Outcomes According to Maternal Age in Patients With Repeated Implantation Failure. Lasers Med Science (2019) 34(9):1889–95. doi: 10.1007/s10103-019-02787-4
18. Ng EHY, Lau EYL, Yeung WSB, Cheung TM, Tang OS, Ho PC. Randomized Double-Blind Comparison of Laser Zona Pellucida Thinning and Breaching in Frozen Thawed Embryo Transfer at the Cleavage Stage. Fertil Steril (2008) 89(5):1147–53. doi: 10.1016/j.fertnstert.2007.05.016
19. Mantoudis E, Podsiadly BT, Gorgy A, Venkat G, Craft IL. A Comparison Between Quarter, Partial and Total Laser Assisted Hatching in Selected Infertility Patients. Hum Reprod (Oxford England). (2001) 16(10):2182–6. doi: 10.1093/humrep/16.10.2182
20. Chailert C, Sanmee U, Piromlertamorn W, Samchimchom S, Vutyavanich T. Effects of Partial or Complete Laser-Assisted Hatching on the Hatching of Mouse Blastocysts and Their Cell Numbers. Reprod Biol Endocrinol (2013) 11(1):21. doi: 10.1186/1477-7827-11-21
21. Tinney GM, Windt M-L, Kruger TF, Lombard CJ. Use of a Zona Laser Treatment System in Assisted Hatching: Optimal Laser Utilization Parameters. Fertil Steril (2005) 84(6):1737–41. doi: 10.1016/j.fertnstert.2005.05.048
22. Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul Consensus Workshop on Embryo Assessment: Proceedings of an Expert Meeting†. Hum Reproduction. (2011) 26(6):1270–83. doi: 10.1016/j.rbmo.2011.02.001
23. Yano K, Kubo T, Ôhashi I, Yano C. Assisted Hatching Using a 1.48-µm Diode Laser: Evaluation of Zona Opening and Zona Thinning Techniques in Human Embryos. Reprod Med Biol (2006) 5(3):221–6. doi: 10.1111/j.1447-0578.2006.00145.x
24. Gardner DK, Schoolcraft WB. Culture and Transfer of Human Blastocysts. Curr Opin Obstet Gynec (1999) 11(3):307–11. doi: 10.1097/00001703-199906000-00013
25. Missmer SA, Pearson KR, Ryan LM, Meeker JD, Cramer DW, Hauser R. Analysis of Multiple-Cycle Data From Couples Undergoing In Vitro Fertilization: Methodologic Issues and Statistical Approaches. Epidemiology (2011) 22(4):497–504. doi: 10.1097/EDE.0b013e31821b5351
26. Williamson JM, Kim H-Y, Warner L. Weighting Condom Use Data to Account for Nonignorable Cluster Size. Ann Epidemiol (2007) 17(8):603–7. doi: 10.1016/j.annepidem.2007.03.008
27. Fujimoto VY, Browne RW, Bloom MS, Sakkas D, Alikani M. Pathogenesis, Developmental Consequences, and Clinical Correlations of Human Embryo Fragmentation. Fertil Steril (2011) 95(4):1197–204. doi: 10.1016/j.fertnstert.2010.11.033
28. Stensen MH, Tanbo TG, Storeng R, Åbyholm T, Fedorcsak P. Fragmentation of Human Cleavage-Stage Embryos is Related to the Progression Through Meiotic and Mitotic Cell Cycles. Fertil Steril (2015) 103(2):374–81.e4. doi: 10.1016/j.fertnstert.2014.10.031
29. Alikani M, Calderon G, Tomkin G, Garrisi J, Kokot M, Cohen J. Cleavage Anomalies in Early Human Embryos and Survival After Prolonged Culture in-Vitro. Hum Reprod (Oxford England). (2000) 15(12):2634–43. doi: 10.1093/humrep/15.12.2634
30. Hardy K, Stark J, Winston RML. Maintenance of the Inner Cell Mass in Human Blastocysts From Fragmented Embryos. Biol Reproduction. (2003) 68(4):1165–9. doi: 10.1095/biolreprod.102.010090
31. Alikani M. Epithelial Cadherin Distribution in Abnormal Human Pre-Implantation Embryos. Hum Reprod (2005) 20(12):3369–75. doi: 10.1093/humrep/dei242
32. Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of Reactive Oxygen Species (ROS) and Apoptosis in Human Fragmented Embryos. Hum Reproduction. (1998) 13(4):998–1002. doi: 10.1093/humrep/13.4.998
33. Van Blerkom J, Davis P, Alexander S. A Microscopic and Biochemical Study of Fragmentation Phenotypes in Stage-Appropriate Human Embryos. Hum Reprod (2001) 16(4):719–29. doi: 10.1093/humrep/16.4.719
34. Jurisicova A, Antenos M, Varmuza S, Tilly JL, Casper RF. Expression of Apoptosis-Related Genes During Human Preimplantation Embryo Development: Potential Roles for the Harakiri Gene Product and Caspase-3 in Blastomere Fragmentation. Mol Hum Reprod (2003) 9(3):133–41. doi: 10.1093/molehr/gag016
35. Antczak M, Van Blerkom J. Temporal and Spatial Aspects of Fragmentation in Early Human Embryos: Possible Effects on Developmental Competence and Association With the Differential Elimination of Regulatory Proteins From Polarized Domains. Hum Reprod (Oxford England). (1999) 14(2):429–47. doi: 10.1093/humrep/14.2.429
36. Bevilacqua A, Loch-Caruso R, Erickson RP. Abnormal Development and Dye Coupling Produced by Antisense RNA to Gap Junction Protein in Mouse Preimplantation Embryos. Proc Natl Acad Sci (1989) 86(14):5444–8. doi: 10.1073/pnas.86.14.5444
37. Nance J. Getting to Know Your Neighbor: Cell Polarization in Early Embryos. J Cell Biol (2014) 206(7):823–32. doi: 10.1083/jcb.201407064
38. Alikani M, Cohen J, Tomkin G, Garrisi GJ, Mack C, Scott RT. Human Embryo Fragmentation In Vitro and its Implications for Pregnancy and Implantation. Fertil Steril (1999) 71(5):836–42. doi: 10.1016/S0015-0282(99)00092-8
39. Kim S-G, Kim Y-Y, Park J-Y, Kwak S-J, Yoo C-S, Park I-H, et al. Early Fragment Removal on In Vitro Fertilization Day 2 Significantly Improves the Subsequent Development and Clinical Outcomes of Fragmented Human Embryos. Clin Exp Reprod Med (2018) 45(3):122–8. doi: 10.5653/cerm.2018.45.3.122
40. Huang Z, Liu J, Gao L, Zhuan Q, Luo Y, Zhu S, et al. The Impacts of Laser Zona Thinning on Hatching and Implantation of Vitrified-Warmed Mouse Embryos. Lasers Med Science (2019) 34(5):939–45. doi: 10.1007/s10103-018-2681-8
41. Austin PC, Fine JP. Practical Recommendations for Reporting Fine-Gray Model Analyses for Competing Risk Data. Stat Med (2017) 36(27):4391–400. doi: 10.1002/sim.7501
Keywords: blastocyst, fragmentation, laser-assisted hatching (LAH), in vitro fertilisation and embryo transfer, oocyte utilization
Citation: Zhang L, Zhou Y-e, Wu Y-j, Wu L-m, Li S-s, Zhang L, Jin Z, Shu C-y, Xu W-h and Shu J (2022) Thinning or Opening: A Randomized Sibling-Embryo Pilot Trial on the Efficacy of Two Laser-Assisted Hatching Modes During the Extended Culture of Highly Fragmented Cleavage Embryos. Front. Endocrinol. 13:927834. doi: 10.3389/fendo.2022.927834
Received: 25 April 2022; Accepted: 25 May 2022;
Published: 27 June 2022.
Edited by:
Sijia Lu, Yikon Genomics, ChinaReviewed by:
Zhang Zhiguo, First Affiliated Hospital of Anhui Medical University, ChinaBin Wang, IVF Canada, Canada
Copyright © 2022 Zhang, Zhou, Wu, Wu, Li, Zhang, Jin, Shu, Xu and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-hai Xu, eHdoNDgxN0AxNjMuY29t; Jing Shu, c2h1amluZ0BobWMuZWR1LmNu
†These authors have contributed equally to this work
 Ling Zhang
Ling Zhang Yi-er Zhou1†
Yi-er Zhou1† Yue-jin Wu
Yue-jin Wu Jing Shu
Jing Shu