
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 20 June 2022
Sec. Pituitary Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.924952
This article is part of the Research TopicPersonalized Management of AcromegalyView all 7 articles
Acromegaly is a systemic disease caused by excessive inappropriate secretion of GH and IGF-I levels, resulting in many systemic complications, including cardiovascular, respiratory, metabolic diseases, and a possible increased risk of some neoplasias. Although many studies on acromegaly and cancer remain uncertain, most data indicate that colorectal cancer (CRC) incidence is increased in this population. The exact mechanism involved in the role of GH-IGF-I axis in CRC has not been fully explained, yet it is associated with local and circulating effects of GH and IGF-I on the colon, promoting angiogenesis, cell proliferation, risk of mutation, inhibition of tumor-suppressor genes and apoptosis, thus facilitating a tumor microenvironment. Nevertheless, population-based studies present controversial findings on CRC incidence and mortality. All worldwide guidelines and expert consensuses agree with the need for colonoscopic screening and surveillance in acromegaly, although there is no consensus regarding the best period to do this. This review aims to analyze the existing data on CRC and acromegaly, exploring its pathophysiology, epidemiological studies and their limitations, colonic polyp characteristics, overall cancer and CRC incidences and mortality, risk factors for colon cancer pathophysiology, and recommendation guideline aspects.
Acromegaly is a chronic systemic disease caused by the excessive secretion of growth hormone (GH) and consequently increased insulin-like growth factor type I (IGF-I) levels. In approximately 98% of cases, acromegaly is caused by a GH-secreting pituitary adenoma (somatotropinoma) (1, 2). Almost all epidemiologic studies are consistent in that acromegaly affects both sexes equally, although a Korean study showed that its incidence is slightly higher in females (1:1.3) (3–5). Nevertheless, all studies indicate that men are younger than women by 4.5 years at diagnosis, and it usually occurs at the fourth or fifth decades of life (6). Unfortunately, there is still a delay of 5-10 years between symptom initiation and diagnosis, resulting in years of morbidity and increased mortality when not properly treated (7).
Acromegaly is associated with many systemic complications secondary to untreated chronic excess GH and IGF-I, including cardiovascular and respiratory diseases, metabolic complications, bone disease (especially vertebral fractures), arthropathy, and a possible increased risk of some neoplasias (8–10). These remarkable complications reduce the health-related quality of life and life expectancy of these patients, although the effective control of GH and IGF-I excess is able to reduce the burden of the disease and the mortality rates to normal levels observed in a general population (11–14). While cardiovascular disease has been the leading cause of mortality in the past decades, recent data suggest that cancer may be the main cause of death in acromegaly (15–17).
Although the discussion of the relationship between acromegaly and cancer dates to the last century, many uncertainties still remain in this field. Knowledge on this subject is not always homogeneous, but most studies indicate that colorectal cancer (CRC) is the main neoplasm associated with acromegaly (18, 19). Nevertheless, there is no consensus in the literature on the best approach for its screening and follow-up in these patients.
In this manuscript, we review CRC in acromegaly, discussing the GH-IGF-I axis in cancer (especially in CRC), risk factors for CRC, specific characteristics of colonic polyps, the limitations of colonoscopies in this population, data from epidemiological studies and their biases, and the different guideline recommendations for CRC screening in acromegaly.
We searched the MEDLINE/PUBMED databases up to 1979 to 2022 to identify all relevant English language medical literature for studies under the search text terms; acromegaly AND (colorectal cancer OR colon cancer OR colon polyps OR colorectal polyps).
In nonacromegaly patients, most CRCs develop as a result of a multistep transformation of normal colonic epithelium to benign adenomatous colonic polyps, severe dysplasia and, finally, an invasive and/or metastatic cancer (20–22). The process involved in the tumorigenesis of sporadic forms of CRC, which takes approximately 10–15 years, requires an accumulation of genetic and epigenetic alterations in oncogenes and tumor suppressor genes (20, 22, 23).
The first step of this classical sequence is adenomatous polyposis coli (APC) gene inactivation, a “gatekeeper” gene that regulates growth by inhibiting proliferation or promoting cell death, which causes adenoma development (20, 23). This is followed by Kirsten rat sarcoma viral oncogene homolog (KRAS) activating mutation, promoting adenoma growth; loss of heterozygosity at chromosome 18q (a tumor suppressor loci), due to chromosomal instability, a condition of malfunctioning segregation of sister chromatids during mitosis, allowing adenoma progression; and inactivation of the tumor-suppressor gene p53, which triggers the final transition to carcinoma (20, 23, 24). All these processes are associated with other genetic mutations, microsatellite instability, and epigenetic alterations, resulting in clinicopathological tumor features (20, 23).
When assessing the role of GH in oncogenesis, it is necessary to note that in addition to the endocrinological function related to pituitary production, it is also expressed in extrapituitary tissue, exercising autocrine and paracrine functions (25). In the normal colon, GH expression is low, but in conditions predisposing to colon adenoma or adenocarcinoma, it is exuberant (25). Although GH is not expressed within epithelial tumor cells in human colon adenocarcinoma, it is expressed in fibroblasts surrounding malignant colon carcinoma (25). In contrast, the GH receptor (GHR) is expressed in both colon epithelial and stromal cells (25). Currently, it has been postulated that both circulating (endocrine pattern) and local (autocrine/paracrine pattern) high GH levels, acting through the GHR, suppress p53, APC, DNA damage repair pathways and apoptosis and stimulate epithelial-mesenchymal transition by increasing the transcription of key metastasis-related genes, including proteins such as matrix metalloproteinases, cMYC (master regulator of cell cycle entry and proliferative metabolism), BCL-2 (B-cell lymphoma 2), and CHOP (C/EBP homologous protein 10), allowing a change in the normal intestinal mucosal environment in favor of a tumor microenvironment toward cell motility and invasion (25–27).
Another pathway that has been explored is that peroxisome proliferator-activated receptor gamma (PPARᵧ), a member of the nuclear hormone receptor superfamily that plays an important role in adipocyte differentiation and metabolism, also has an antiproliferative effect in several tissues, including colonic mucosa, where it is highly expressed (28). Although controversial, in vitro and animal studies suggests that PPARᵧ has an antitumor effect in CRC as its activation is associated with inhibition of cell growth and its intestinal deficiency is associated with enhanced tumorigenicity in mice small intestine and colon (28). The molecular mechanism for the antineoplastic effect of PPARᵧ activation remains incompletely enlightened. PPARᵧ ligand treatment is associated with gene expressions changes involving: induction of apoptosis, by upregulating the proapoptotic protein BAX and downregulating the antiapoptotic BCL-2; cell proliferation inhibition through the decrease in cyclin D1 expression, a downstream effector of diverse proliferative and transforming signaling pathways, leading to the arrest of cell cycle progression; induction of cellular differentiation; and inhibition of angiogenesis in CRC, by decreasing vascular endothelial growth factor (VEGF) production and inhibiting capillary endothelial cell proliferation (28). Genetic studies showed that the presence of somatic loss-of-function mutations in the gene encoding PPARᵧ contributes to colonic tumor development (29). Although some studies failed to detect any mutation of PPARᵧ of colonic tissue in patients with acromegaly, others have observed that patients with active, untreated acromegaly had lower levels of PPARᵧ expression in colonic mucosa than those with cured disease (30, 31). There is still a need for further studies, but this might have the same role of the somatic mutations in PPARᵧ, playing a role in the development and/or progression of these cancers in nonacromegaly patients (29).
In addition to the GH-IGF-I axis being able to favor tumor development, it increases the risk of mutations, stimulates cell proliferation and angiogenesis (IGF-I is expressed in endothelial cells during angiogenesis and increases vascular endothelial growth factor, the main proangiogenic factor responsible for neovascularization), invasion, and metastasis; other components of the somatotropic system, such as IGF-II and IGF binding proteins (IGFBPs), exert an antitumoral effect by stimulating apoptosis and inhibiting mitogenesis, although the strength of these actions are weaker (27, 32–35).
The final action of this axis is complex and not yet fully understood, although the data indicate that there is an imbalance in favor of neoplastic development (Figure 1).
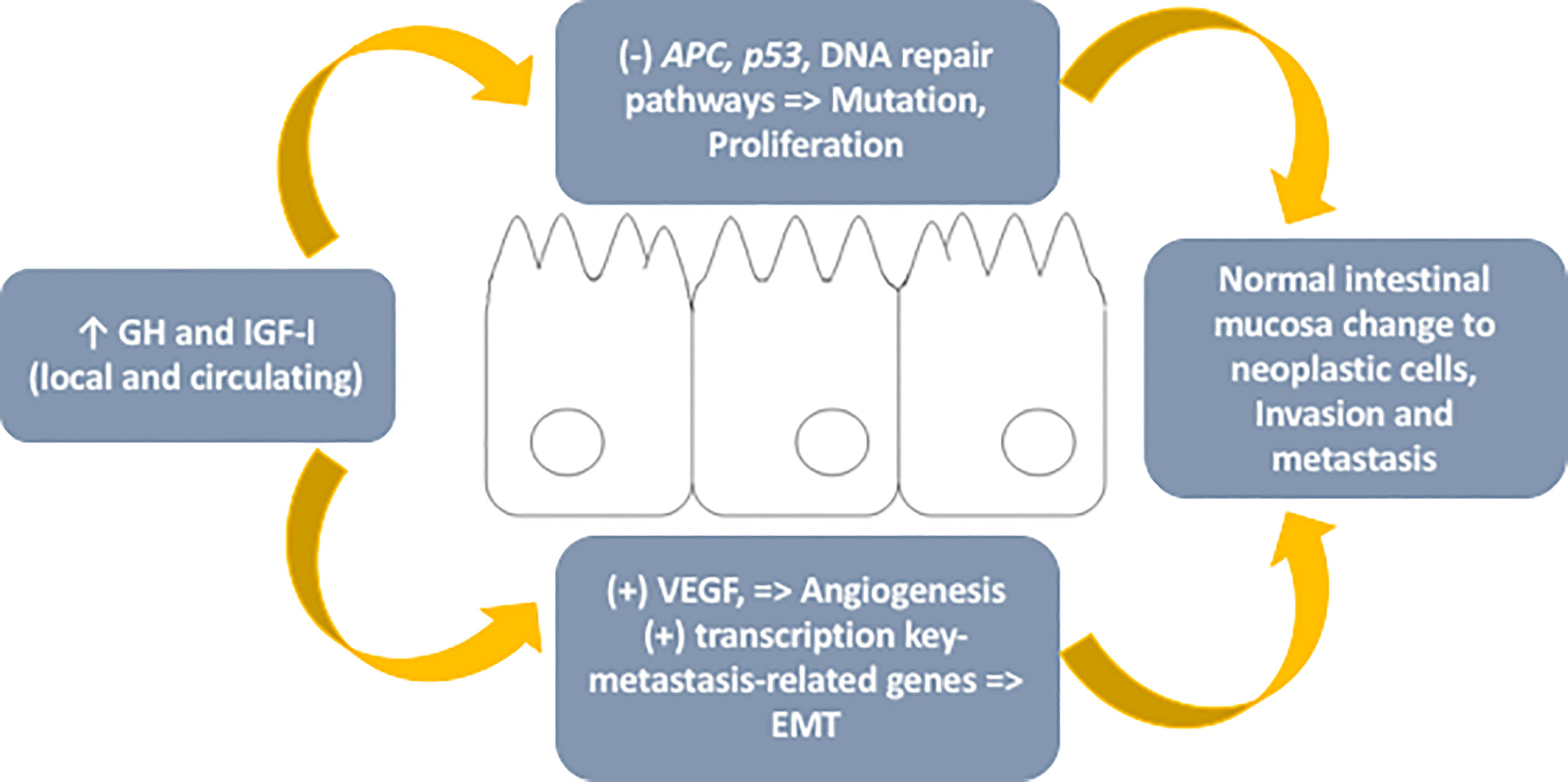
Figure 1 – Schematic representation of mechanisms involved in the role of the GH-IGF-I axis in colorectal cancer. GH, growth hormone; IGF-I, insulin-like growth factor type I; APC, adenomatous polyposis coli gene; VEGF, vascular endothelial growth factor; EMT, epithelial-mesenchymal transition.
The transformation of premalignant to malignant lesions, responsible for the development of CRC, corresponds to a complex process that occurs over years and is influenced by numerous factors (20, 36–38). Countless evidence points out that among these predisposing factors are diet, obesity, diabetes mellitus, dyslipidemia, physical inactivity, smoking, alcoholism, and genetic factors (36–38).
Among these factors, obesity, diabetes mellitus, and hypertriglyceridemia represent the ones with the most robust evidence, reflecting the main role of insulin resistance and hyperinsulinemia in association with its inflammatory markers in the carcinogenesis of the nonacromegaly population (37, 38). Although it is still debated, evidence points out that metformin has a protective role in patients with diabetes and insulin resistance as an anticancer chemoprevention agent (39, 40). The underlying mechanism of metformin antitumoral activity is not fully understood, but its action in reducing insulin levels and inhibiting phosphorylation of IGF-I receptor/insulin receptor and its pathways inhibition of cell proliferation and growth, as well as inducing apoptosis, have an important role (41, 42). Insulin promotes tumorigenesis by direct and indirect mechanisms, including one referring to the increase in circulating levels of IGF-I and IGF-II, through the reduction of IGFBPs (43). In addition, it is known that IGF-I, IGF-II, and insulin can cross-bind to each other’s receptors with a lower affinity than with their original receptor due to the homology presented between them (34). The similarity between IGF-I receptor (IGF-IR) and insulin receptor (IR) allows the formation of hybrid receptors, which have a higher affinity for IGF-I than insulin (34). The biological significance of this is not entirely known, but it may be implicated in a greater activation of the IGF-IR pathway and its consequent mitogenic activity.
In CRC, evidence points out that activation of the IR pathway by insulin promotes cell growth and proliferation and that activation of the same signaling route (IR) by IGFs contributes to the oncogenic process by decreasing apoptosis and stimulating angiogenesis, cell proliferation and migration (44, 45). These comorbidities are very often present in acromegaly, being related to disease activity and/or its treatment, and might contribute to an increased risk of CRC.
In the past 20 years, data from laboratory, animal, and human model studies indicated that GH and IGF-I are associated with cancer, although findings from population-based studies present controversial results, explained by the numerous biases that will be mentioned ahead in this review (46, 47).
A review of six nationwide cohort studies that included a standardized incidence ratio (SIR) comparing the overall cancer rate and the CRC rate in patients with acromegaly with the general population indicated that five studies pointed to an increased risk of neoplasia, albeit moderate and not always with statistical significance (Table 1) (18, 19, 48–51). This may explain why previous smaller studies (of less statistical power) present such incongruent results. Only one of these studies showed that CRC incidence was nonsignificantly lower than expected in the general population (48). This controversial result might be explained by some limitations of the work: only a small sample of the German Acromegaly Registry was used; part of cancer data was obtained by phone interviews and not always based on medical records; and 16% of patients were lost during follow-up.
Therefore, similar to overall cancer, the SIR of CRC compared to the general population has been variable, but most studies indicate an increased risk in acromegaly (18, 19, 49–51). This finding reflects the consensus of an active search for this comorbidity, although there is no uniform recommendation on how to perform this surveillance (8, 10, 13, 52–54).
Mortality in acromegaly has traditionally been related to its cardiovascular and respiratory complications (11, 51). However, current data point to a change in this paradigm, where neoplasia assumes the role of the main cause of mortality (15–17). This finding is accompanied by a general decrease in mortality when compared with older previous studies, reflecting the treatment progress made in recent years (16, 55). Thus, the decrease in overall mortality, the increase in life expectancy, and the aging of this population resulted in a consequent expected increase in the incidence of relevant age-dependent diseases, such as CRC.
Although the overall mortality rate, as well as cancer-related mortality, in controlled acromegaly are similar to that of the general population, those individuals with active disease and persistently high levels of GH and IGF-I will have higher all-cause mortality rates (between 1.5- and 2.0-fold), including those related to neoplasms, particularly CRC (15, 51, 56).
Although data related to CRC remain a subject of discussion, the increased risk for colon polyposis (either adenomatous or hyperplastic) in acromegaly is widely accepted (9). In addition, there is also a higher prevalence of diverticular disease, hemorrhoids, and the typical enlarged colon length (dolichocolon) (57).
Disease activity is directly related to the appearance of new polyps (9, 47). Excessive IGF-I levels, but not GH, and the duration of disease activity seem to correlate positively with the development of polyps in several studies (57, 58). However, there appears to be no relationship between IGF-I levels and polyp size (57).
These adenomatous polyps have some particular characteristics because, in general, they are larger, multiple, and more dysplastic than in the nonacromegaly population (59). They are also more common in men, in patients with an active disease duration greater than five years, in those with three or more skin tags, and in cases with a positive family history of colonic polyps (47, 60).
The real relationship between acromegaly and cancer remains an unsolved question (46, 47, 61). Several data have reported that GH-IGF-I axis contributes to an important role in cancer development and progression, although the excess risk seems moderate (26, 33, 46, 47, 62).
Nevertheless, the studies that associate acromegaly and cancer have controversial results, often explained by the use of different epidemiological methods that are not comparable (case–control and population-based design); the retrospective nature of these studies, especially when considering that some studies date back to an era when treatment of acromegaly was less successful, so that patients with uncontrolled disease may have died by cardiovascular morbidity, for example, before entering the age when cancer is diagnosed; or the lack of an appropriate and comparable control population, which may not adjust results for confounding factors, such as sex, age, and environmental factors (46, 47, 61, 63, 64). Another limiting factor is that many studies exclude the registration of cancer before the diagnosis of acromegaly, and as the diagnosis of this disease occurs many years after the real onset, it could have a negative impact on the actual estimate of neoplasm (47, 51). In addition, as acromegaly is a rare disease, only nationwide surveys may have the statistical power to demonstrate (or not) an excess risk of cancer (61, 64).
Another important confusing bias is related to the intensity of screening. This link between excess GH and IGF-I and the risk of cancer in some studies led to the recommendation of routine screening for neoplastic pathologies, including colorectal neoplasms, which influences the reported incidence rates (18, 26).
In addition to these study limitations, there are specific technical difficulties in assessing CRC in patients with acromegaly. In contrast to the general population, 25–40% of adenomatous polyps and 50% of adenocarcinomas in acromegaly are located in the ascending and transverse colon, so it is necessary to perform a total colonoscopy instead of a simple sigmoidoscopy (64–68). Another problem that can compromise the success of the exam is the difficulty in preparing the patient, since intestinal transit in acromegaly is slower than normal subjects. This can be explained by autonomic intestinal impairment due to vagal hypertonia, a hormonal imbalance influenced by the interactions between GH and ghrelin and the action of the increased IGF-I levels, which may stimulate the proliferation of intestinal epithelial cells (69). Gut motility disturbance can also occur in treated patients with somatostatin receptor ligands (69). Therefore, standard bowel preparations could lead to suboptimal results (70). Another major problem is that colon length and circumference are often increased (dolichocolon with megacolon), making complete intubation and identification of minor lesions more difficult (70, 71). Furthermore, there are some potentially harmful limitations inherent to this invasive procedure itself that may be enhanced in acromegaly patients, such as polypectomy bleeding (more common in the proximal colon), perforation (increased in the presence of diverticular disease – increased in acromegaly), and cardiopulmonary complications (cardiac arrythmias, hypotension, oxygen desaturation) (72).
All these considerations and technical complexity suggest the need for a trained and high-level skill endoscopist to perform this exam in patients with acromegaly.
There are numerous guidelines for the management of acromegaly patients, and practically all address the neoplasia risk. In relation to breast, prostate, lung, and other cancers, no increase in risk has been conclusively reported, and there is an agreement among all experts that surveillance should follow the same recommendations as for the general population (8, 9, 13, 47, 54). Specifically, with regard to the thyroid, current data do not support routine screening for thyroid cancer in acromegaly (13). Consistent with international guidelines, thyroid ultrasound is recommended in patients with clinically palpable nodules, and investigation with fine-needle aspiration cytology must respect the indications for the general population (8, 13, 54, 73).
However, in relation to CRC, there is no consensus regarding the best period for colonoscopic screening and surveillance during the follow-up of these patients. The most referenced guidelines on this topic in the literature are those published by the British Society of Gastroenterology (BSG) in 2010 (53); the American Association of Clinical Endocrinologists (AACE) in 2011 (54); the Pituitary Society in 2013 (10); the Endocrine Society in 2014 (13); and the Acromegaly Consensus Group (ACG) in 2019 (8).
According to the BSG, colonoscopy should begin at the age of 40 (52, 53). The AACE, Pituitary Society, Endocrine Society and ACG recommend that the first exam should be requested at the time of diagnosis, independent of the patient’s age (8, 10, 13, 54). If a patient has normal initial colonoscopy and normal IGF-I levels, all societies recommend a new exam every 10 years. Nevertheless, there is no agreement on the best interval if the initial or any subsequent colonoscopy reveals an adenoma and/or if IGF-I is uncontrolled. The AACE and Endocrine Society recommend surveillance every 5 years; BSG suggests 3-year colonoscopy; the Pituitary Society advises performing the exam more frequently if IGF-I remains persistently elevated (without specifying the precising time) and proposes to follow in accordance with clinical guidelines for the general population if colonoscopy is abnormal (8, 10, 13, 52–54). It is worth noting that only the Pituitary Society cite a positive family history for colorectal cancer as a risk-modifying agent in acromegaly (8, 10). A summary of current guideline recommendations for surveillance colonoscopy in acromegaly patients is presented in Table 2.
Special attention should be given to the degree of recommendation when assessing different opinions provided by each society, since none establishes a strong or high-quality rate of evidence. For example, the BSG expresses a degree of recommendation B, characterized by evidence obtained from at least one well-designed controlled study without randomization, evidence obtained from at least one other type of well-designed quasi-experimental study, or evidence obtained from a well-designed nonexperimental descriptive study, such as comparative studies, correlation studies, and case studies (53). The AACE presents as a weak level of evidence (Grade C) based on expert opinion and on data from experimental results and nonexperimental data (54). The Pituitary Society followed the approach recommended by the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) group (74), and it has a strong degree of recommendation for the age of onset of colonoscopy and the exam time interval if the IGF-I level persists elevated (10), but it has a discretionary recommendation if the initial colonoscopy is normal and IGF-I level is normal (10). The Endocrine Society presents its colonoscopic guideline as a weak degree of recommendation with low-quality evidence studies (13). The ACG uses the same GRADE system and has a discretionary recommendation (8).
Due to the lack of conclusive data on the real incidence of malignancy and the relationship between CRC mortality and acromegaly, there exists heterogeneity in the current surveillance recommendations for this neoplasm.
Current studies point to a higher incidence of CRC in increasingly younger nonacromegaly individuals, but screening for the general population still starts from the age of 50, as indicated by gastroenterology, oncology, and endoscopy societies (21, 36). The same guidelines recommend a more intense and earlier screening and follow-up only for patients allocated as the group at major risk for CRC, since this measure has been shown to reduce the risk of specific death for this neoplasm in this population (21, 75–79). For these individuals at increased risk, such as first-degree relatives of individuals diagnosed with CRC at young ages, the beginning of screening at younger ages is recommended (starting at age 40 years or 10 years before the youngest case in the family). For high-risk groups (familial adenomatous polyposis, hereditary nonpolyposis colon cancer, or inflammatory bowel disease), much more rigorous prevention programs are recommended, starting earlier in life (21, 75, 77, 78). It is important to note that none of these specific guidelines for CRC cite acromegaly as a screening modifying disease.
It is widely accepted that screening and follow-up colonoscopy are fundamental in acromegaly. However, the correct time for the first colonoscopy and for follow-up exams remains uncertain. There is little evidence to justify colonoscopy at the time of diagnosis in patients under the age 40, since in average-risk individuals (no prior diagnosis of CRC, adenomatous polyps, or inflammatory bowel disease; no personal diagnosis or family history of known genetic disorder that predisposes them to a high lifetime risk of CRC, such as Lynch syndrome or familial adenomatous polyposis), this exam starts at 50 years (21, 47, 80).
Although there are many biases and limitations in quantifying the overall CRC risk in the literature, current evidence suggests that acromegaly should be included in the group of factors associated with CRC, such as smoking, alcoholism, obesity, physical inactivity, diabetes mellitus, and others, which increase the risk for this neoplasm to a mild and moderate level (21, 36, 81).
Further studies on the topic are urgently needed for the adoption of a universal evidence-based guideline. In the near future, with more studies and data on this subject, the age of initial CRC screening in acromegaly and the interval between exams could possible be reviewed.
LK and BM reviewed the literature and wrote the manuscript. MG reviewed the manuscript and suggested the final changes. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ben-Shlomo A, Melmed S. Acromegaly. Endocrinol Metab Clin North Am (2008) 37(1):101–22, viii. doi: 10.1016/j.ecl.2007.10.002
2. Capatina C, Wass JA. 60 YEARS OF NEUROENDOCRINOLOGY: Acromegaly. J Endocrinol (2015) 226(2):T141–60. doi: 10.1530/JOE-15-0109
3. Park KH, Lee EJ, Seo GH, Ku CR. Risk for Acromegaly-Related Comorbidities by Sex in Korean Acromegaly. J Clin Endocrinol Metab (2020) 105(4):dgz317. doi: 10.1210/clinem/dgz317
4. Tjörnstrand A, Gunnarsson K, Evert M, Holmberg E, Ragnarsson O, Rosén T, et al. The Incidence Rate of Pituitary Adenomas in Western Sweden for the Period 2001-2011. Eur J Endocrinol (2014) 171(4):519–26. doi: 10.1530/EJE-14-0144
5. Burton T, Le Nestour E, Neary M, Ludlam WH. Incidence and Prevalence of Acromegaly in a Large US Health Plan Database. Pituitary. (2016) 19(3):262–7. doi: 10.1007/s11102-015-0701-2
6. Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L, et al. A Pituitary Society Update to Acromegaly Management Guidelines. Pituitary (2021) 24(1):1–13. doi: 10.1007/s11102-020-01091-7
7. Dal J, Feldt-Rasmussen U, Andersen M, Kristensen L, Laurberg P, Pedersen L, et al. Acromegaly Incidence, Prevalence, Complications and Long-Term Prognosis: A Nationwide Cohort Study. Eur J Endocrinol (2016) 175(3):181–90. doi: 10.1530/EJE-16-0117
8. Giustina A, Barkan A, Beckers A, Biermasz N, Biller BMK, Boguszewski C, et al. A Consensus on the Diagnosis and Treatment of Acromegaly Comorbidities: An Update. J Clin Endocrinol Metab (2019) 105(4):dgz096. doi: 10.1210/clinem/dgz096
9. Gadelha MR, Kasuki L, Lim DST, Fleseriu M. Systemic Complications of Acromegaly and the Impact of the Current Treatment Landscape: An Update. Endocr Rev (2019) 40(1):268–332. doi: 10.1210/er.2018-00115
10. Melmed S, Casanueva FF, Klibanski A, Bronstein MD, Chanson P, Lamberts SW, et al. A Consensus on the Diagnosis and Treatment of Acromegaly Complications. Pituitary (2013) 16(3):294–302. doi: 10.1007/s11102-012-0420-x
11. Sherlock M, Ayuk J, Tomlinson JW, Toogood AA, Aragon-Alonso A, Sheppard MC, et al. Mortality in Patients With Pituitary Disease. Endocr Rev (2010) 31(3):301–42. doi: 10.1210/er.2009-0033
12. Melmed S. Acromegaly Pathogenesis and Treatment. J Clin Invest (2009) 119(11):3189–202. doi: 10.1172/JCI39375
13. Katznelson L, Laws ER, Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2014) 99(11):3933–51. doi: 10.1210/jc.2014-2700
14. Crespo I, Valassi E, Webb SM. Update on Quality of Life in Patients With Acromegaly. Pituitary. (2017) 20(1):185–8. doi: 10.1007/s11102-016-0761-y
15. Ritvonen E, Löyttyniemi E, Jaatinen P, Ebeling T, Moilanen L, Nuutila P, et al. Mortality in Acromegaly: A 20-Year Follow-Up Study. Endocr Relat Cancer (2016) 23(6):469–80. doi: 10.1530/ERC-16-0106
16. Mercado M, Gonzalez B, Vargas G, Ramirez C, de los Monteros AL, Sosa E, et al. Successful Mortality Reduction and Control of Comorbidities in Patients With Acromegaly Followed at a Highly Specialized Multidisciplinary Clinic. J Clin Endocrinol Metab (2014) 99(12):4438–46. doi: 10.1210/jc.2014-2670
17. Arosio M, Reimondo G, Malchiodi E, Berchialla P, Borraccino A, De Marinis L, et al. Predictors of Morbidity and Mortality in Acromegaly: An Italian Survey. Eur J Endocrinol (2012) 167(2):189–98. doi: 10.1530/EJE-12-0084
18. Dal J, Leisner MZ, Hermansen K, Farkas DK, Bengtsen M, Kistorp C, et al. Cancer Incidence in Patients With Acromegaly: A Cohort Study and Meta-Analysis of the Literature. J Clin Endocrinol Metab (2018) 103(6):2182–8. doi: 10.1210/jc.2017-02457
19. Terzolo M, Reimondo G, Berchialla P, Ferrante E, Malchiodi E, De Marinis L, et al. Acromegaly is Associated With Increased Cancer Risk: A Survey in Italy. Endocr Relat Cancer (2017) 24(9):495–504. doi: 10.1530/ERC-16-0553
20. Muto T, Bussey HJ, Morson BC. The Evolution of Cancer of the Colon and Rectum. Cancer. (1975) 36(6):2251–70. doi: 10.1002/cncr.2820360944
21. Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology (2017) 153(1):307–23. doi: 10.1053/j.gastro.2017.05.013
22. Fearon ER, Vogelstein B. A Genetic Model for Colorectal Tumorigenesis. Cell. (1990) 61(5):759–67. doi: 10.1016/0092-8674(90)90186-I
23. Zoratto F, Rossi L, Verrico M, Papa A, Basso E, Zullo A, et al. Focus on Genetic and Epigenetic Events of Colorectal Cancer Pathogenesis: Implications for Molecular Diagnosis. Tumour Biol (2014) 35(7):6195–206. doi: 10.1007/s13277-014-1845-9
24. Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, et al. Microsatellite Instability and Loss of Heterozygosity at Chromosomal Location 18q: Prospective Evaluation of Biomarkers for Stages II and III Colon Cancer–a Study of CALGB 9581 and 89803. J Clin Oncol (2011) 29(23):3153–62. doi: 10.1200/JCO.2010.33.0092
25. Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, et al. Growth Hormone is Permissive for Neoplastic Colon Growth. Proc Natl Acad Sci U S A (2016) 113(23):E3250–9. doi: 10.1073/pnas.1600561113
26. Boguszewski CL, Boguszewski MCDS. Growth Hormone’s Links to Cancer. Endocr Rev (2019) 40(2):558–74. doi: 10.1210/er.2018-00166
27. Bustin SA, Jenkins PJ. The Growth Hormone-Insulin-Like Growth Factor-I Axis and Colorectal Cancer. Trends Mol Med (2001) 7(10):447–54. doi: 10.1016/S1471-4914(01)02104-9
28. Dai Y, Wang WH. Peroxisome Proliferator-Activated Receptor γ and Colorectal Cancer. World J Gastrointest Oncol (2010) 2(3):159–64. doi: 10.4251/wjgo.v2.i3.159
29. Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, et al. Loss-Of-Function Mutations in PPAR Gamma Associated With Human Colon Cancer. Mol Cell (1999) 3(6):799–804. doi: 10.1016/S1097-2765(01)80012-5
30. Bogazzi F, Ultimieri F, Raggi F, Costa A, Gasperi M, Cecconi E, et al. Peroxisome Proliferator Activated Receptor Gamma Expression is Reduced in the Colonic Mucosa of Acromegalic Patients. J Clin Endocrinol Metab (2002) 87(5):2403–6. doi: 10.1210/jcem.87.5.8625
31. Bogazzi F, Ultimieri F, Raggi F, Russo D, Viacava P, Cecchetti D, et al. Changes in the Expression of the Peroxisome Proliferator-Activated Receptor Gamma Gene in the Colonic Polyps and Colonic Mucosa of Acromegalic Patients. J Clin Endocrinol Metab (2003) 88(8):3938–42. doi: 10.1210/jc.2003-030273
32. Samani AA, Yakar S, LeRoith D, Brodt P. The Role of the IGF System in Cancer Growth and Metastasis: Overview and Recent Insights. Endocr Rev (2007) 28(1):20–47. doi: 10.1210/er.2006-0001
33. Weroha SJ, Haluska P. The Insulin-Like Growth Factor System in Cancer. Endocrinol Metab Clin North Am (2012) 41(2):335–50, vi. doi: 10.1016/j.ecl.2012.04.014
34. Boguszewski CL, Boguszewski MC, Kopchick JJ. Growth Hormone, Insulin-Like Growth Factor System and Carcinogenesis. Endokrynol Pol (2016) 67(4):414–26. doi: 10.5603/EP.a2016.0053
35. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-Like Growth Factor (IGF)-I, IGF Binding Protein-3, and Cancer Risk: Systematic Review and Meta-Regression Analysis. Lancet. (2004) 363(9418):1346–53. doi: 10.1016/S0140-6736(04)16044-3
36. Brenner H, Kloor M, Pox CP. Colorectal Cancer. Lancet (2014) 383(9927):1490–502. doi: 10.1016/S0140-6736(13)61649-9
37. Gallagher EJ, LeRoith D. Epidemiology and Molecular Mechanisms Tying Obesity, Diabetes, and the Metabolic Syndrome With Cancer. Diabetes Care (2013) 36 Suppl 2:S233–9. doi: 10.2337/dcS13-2001
38. Lega IC, Lipscombe LL. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr Rev (2020) 41(1):bnz014. doi: 10.1210/endrev/bnz014
39. Saraei P, Asadi I, Kakar MA, Moradi-Kor N. The Beneficial Effects of Metformin on Cancer Prevention and Therapy: A Comprehensive Review of Recent Advances. Cancer Manag Res (2019) 11:3295–313. doi: 10.2147/CMAR.S200059
40. Albertelli M, Nazzari E, Dotto A, Grasso LF, Sciallero S, Pirchio R, et al. Possible Protective Role of Metformin Therapy on Colonic Polyps in Acromegaly: An Exploratory Cross-Sectional Study. Eur J Endocrinol (2021) 184(3):419–25. doi: 10.1530/EJE-20-0795
41. Kourelis TV, Siegel RD. Metformin and Cancer: New Applications for an Old Drug. Med Oncol (2012) 29(2):1314–27. doi: 10.1007/s12032-011-9846-7
42. Salani B, Del Rio A, Marini C, Sambuceti G, Cordera R, Maggi D. Metformin, Cancer and Glucose Metabolism. Endocr Relat Cancer (2014) 21(6):R461–71. doi: 10.1530/ERC-14-0284
43. Key TJ, Appleby PN, Reeves GK, Roddam AW, Group EHaBCC. Insulin-Like Growth Factor 1 (IGF1), IGF Binding Protein 3 (IGFBP3), and Breast Cancer Risk: Pooled Individual Data Analysis of 17 Prospective Studies. Lancet Oncol (2010) 11(6):530–42. doi: 10.1016/S1470-2045(10)70095-4
44. Lu CC, Chu PY, Hsia SM, Wu CH, Tung YT, Yen GC. Insulin Induction Instigates Cell Proliferation and Metastasis in Human Colorectal Cancer Cells. Int J Oncol (2017) 50(2):736–44. doi: 10.3892/ijo.2017.3844
45. Naderi N, Zamanian Azodi M, Daskar Abkenar E, Shahidi Dadras M, Talaei R. Insulin Dysregulation Plays a Critical Role in Colon Inflammation: A Bioinformatics Approach. Gastroenterol Hepatol Bed Bench (2018) 11(Suppl 1):S85–91. doi: 10.22037/ghfbb.v0i0.1513
46. Loeper S, Ezzat S. Acromegaly: Re-Thinking the Cancer Risk. Rev Endocr Metab Disord (2008) 9(1):41–58. doi: 10.1007/s11154-007-9063-z
47. Boguszewski CL, Ayuk J. MANAGEMENT OF ENDOCRINE DISEASE: Acromegaly and Cancer: An Old Debate Revisited. Eur J Endocrinol (2016) 175(4):R147–56. doi: 10.1530/EJE-16-0178
48. Petroff D, Tönjes A, Grussendorf M, Droste M, Dimopoulou C, Stalla G, et al. The Incidence of Cancer Among Acromegaly Patients: Results From the German Acromegaly Registry. J Clin Endocrinol Metab (2015) 100(10):3894–902. doi: 10.1210/jc.2015-2372
49. Kauppinen-Mäkelin R, Sane T, Välimäki MJ, Markkanen H, Niskanen L, Ebeling T, et al. Increased Cancer Incidence in Acromegaly–a Nationwide Survey. Clin Endocrinol (Oxf) (2010) 72(2):278–9. doi: 10.1111/j.1365-2265.2009.03619.x
50. Baris D, Gridley G, Ron E, Weiderpass E, Mellemkjaer L, Ekbom A, et al. Acromegaly and Cancer Risk: A Cohort Study in Sweden and Denmark. Cancer Causes Control (2002) 13(5):395–400. doi: 10.1023/A:1015713732717
51. Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and Cancer Incidence in Acromegaly: A Retrospective Cohort Study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab (1998) 83(8):2730–4. doi: 10.1210/jc.83.8.2730
52. Dworakowska D, Gueorguiev M, Kelly P, Monson JP, Besser GM, Chew SL, et al. Repeated Colonoscopic Screening of Patients With Acromegaly: 15-Year Experience Identifies Those at Risk of New Colonic Neoplasia and Allows for Effective Screening Guidelines. Eur J Endocrinol (2010) 163(1):21–8. doi: 10.1530/EJE-09-1080
53. Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for Colorectal Cancer Screening and Surveillance in Moderate and High Risk Groups (Update From 2002). Gut. (2010) 59(5):666–89. doi: 10.1136/gut.2009.179804
54. Katznelson L, Atkinson JL, Cook DM, Ezzat SZ, Hamrahian AH, Miller KK, et al. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Acromegaly–2011 Update. Endocr Pract (2011) 17 Suppl 4:1–44. doi: 10.4158/EP.17.4.636
55. Bogazzi F, Colao A, Rossi G, Lombardi M, Urbani C, Sardella C, et al. Comparison of the Effects of Primary Somatostatin Analogue Therapy and Pituitary Adenomectomy on Survival in Patients With Acromegaly: A Retrospective Cohort Study. Eur J Endocrinol (2013) 169(3):367–76. doi: 10.1530/EJE-13-0166
56. Holdaway IM, Rajasoorya RC, Gamble GD. Factors Influencing Mortality in Acromegaly. J Clin Endocrinol Metab (2004) 89(2):667–74. doi: 10.1210/jc.2003-031199
57. Parolin M, Dassie F, Russo L, Mazzocut S, Ferrata M, De Carlo E, et al. Guidelines Versus Real Life Practice: The Case of Colonoscopy in Acromegaly. Pituitary (2018) 21(1):16–24. doi: 10.1007/s11102-017-0841-7
58. Maione L, Brue T, Beckers A, Delemer B, Petrossians P, Borson-Chazot F, et al. Changes in the Management and Comorbidities of Acromegaly Over Three Decades: The French Acromegaly Registry. Eur J Endocrinol (2017) 176(5):645–55. doi: 10.1530/EJE-16-1064
59. Lois K, Bukowczan J, Perros P, Jones S, Gunn M, James RA. The Role of Colonoscopic Screening in Acromegaly Revisited: Review of Current Literature and Practice Guidelines. Pituitary (2015) 18(4):568–74. doi: 10.1007/s11102-014-0586-5
60. Colao A, Ferone D, Marzullo P, Lombardi G. Systemic Complications of Acromegaly: Epidemiology, Pathogenesis, and Management. Endocr Rev (2004) 25(1):102–52. doi: 10.1210/er.2002-0022
61. Melmed S. Acromegaly and Cancer: Not a Problem? J Clin Endocrinol Metab (2001) 86(7):2929–34. doi: 10.1210/jcem.86.7.7635
62. Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth Hormone, the Insulin-Like Growth Factor Axis, Insulin and Cancer Risk. Nat Rev Endocrinol (2011) 7(1):11–24. doi: 10.1038/nrendo.2010.171
63. Renehan AG, Shalet SM. Acromegaly and Colorectal Cancer: Risk Assessment Should be Based on Population-Based Studies. J Clin Endocrinol Metab (2002) 87(4):1909. doi: 10.1210/jc.87.4.1909
64. Renehan AG, Bhaskar P, Painter JE, O’Dwyer ST, Haboubi N, Varma J, et al. The Prevalence and Characteristics of Colorectal Neoplasia in Acromegaly. J Clin Endocrinol Metab (2000) 85(9):3417–24. doi: 10.1210/jcem.85.9.6775
65. Rokkas T, Pistiolas D, Sechopoulos P, Margantinis G, Koukoulis G. Risk of Colorectal Neoplasm in Patients With Acromegaly: A Meta-Analysis. World J Gastroenterol (2008) 14(22):3484–9. doi: 10.3748/wjg.14.3484
66. Colao A, Balzano A, Ferone D, Panza N, Grande G, Marzullo P, et al. Increased Prevalence of Colonic Polyps and Altered Lymphocyte Subset Pattern in the Colonic Lamina Propria in Acromegaly. Clin Endocrinol (Oxf) (1997) 47(1):23–8. doi: 10.1046/j.1365-2265.1997.00253.x
67. Delhougne B, Deneux C, Abs R, Chanson P, Fierens H, Laurent-Puig P, et al. The Prevalence of Colonic Polyps in Acromegaly: A Colonoscopic and Pathological Study in 103 Patients. J Clin Endocrinol Metab (1995) 80(11):3223–6. doi: 10.1210/jcem.81.6.8964888
68. Jenkins PJ, Fairclough PD, Richards T, Lowe DG, Monson J, Grossman A, et al. Acromegaly, Colonic Polyps and Carcinoma. Clin Endocrinol (Oxf) (1997) 47(1):17–22. doi: 10.1046/j.1365-2265.1997.1911029.x
69. Inayet N, Hayat J, Bano G, Poullis A. Gastrointestinal Symptoms in Acromegaly: A Case Control Study. World J Gastrointest Pharmacol Ther (2020) 11(2):17–24. doi: 10.4292/wjgpt.v11.i2.17
70. Veysey MJ, Thomas LA, Mallet AI, Jenkins PJ, Besser GM, Wass JA, et al. Prolonged Large Bowel Transit Increases Serum Deoxycholic Acid: A Risk Factor for Octreotide Induced Gallstones. Gut. (1999) 44(5):675–81. doi: 10.1136/gut.44.5.675
71. Ron E, Gridley G, Hrubec Z, Page W, Arora S, Fraumeni JF. Acromegaly and Gastrointestinal Cancer. Cancer. (1991) 68(8):1673–7. doi: 10.1002/1097-0142(19911015)68:8<1673::AID-CNCR2820680802>3.0.CO;2-0
72. Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline From the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. (2008) 134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002
73. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
74. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
75. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, et al. American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2009 [Corrected]. Am J Gastroenterol (2009) 104(3):739–50. doi: 10.1038/ajg.2009.104
76. Davila RE, Rajan E, Baron TH, Adler DG, Egan JV, Faigel DO, et al. ASGE Guideline: Colorectal Cancer Screening and Surveillance. Gastrointest Endosc (2006) 63(4):546–57. doi: 10.1016/j.gie.2006.02.002
77. Vangala DB, Cauchin E, Balmaña J, Wyrwicz L, van Cutsem E, Güller U, et al. Screening and Surveillance in Hereditary Gastrointestinal Cancers: Recommendations From the European Society of Digestive Oncology (ESDO) Expert Discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer (2018) 104:91–103. doi: 10.1016/j.ejca.2018.09.004
78. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J Crohns Colitis (2019) 13(2):144–64. doi: 10.1093/ecco-jcc/jjy113
79. Oza VM, Frankel WL, Conwell DL. Colorectal-Cancer Incidence and Mortality After Screening. N Engl J Med (2013) 369(24):2354–5. doi: 10.1056/NEJMc1313116
80. Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. (2021) 325(19):1965–77.
Keywords: acromegaly, colon cancer, colon polyp, mortality, colonoscopy
Citation: Kasuki L, Maia B and Gadelha MR (2022) Acromegaly and Colorectal Neoplasm: An Update. Front. Endocrinol. 13:924952. doi: 10.3389/fendo.2022.924952
Received: 20 April 2022; Accepted: 18 May 2022;
Published: 20 June 2022.
Edited by:
Hidenori Fukuoka, Kobe University, JapanReviewed by:
Daniel Cuevas-Ramos, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), MexicoCopyright © 2022 Kasuki, Maia and Gadelha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leandro Kasuki, bGthc3VraUB5YWhvby5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.