- Department of Nuclear Medicine, All India Institute of Medical Sciences, New Delhi, India
Radioiodine-refractory differentiated thyroid cancer (RAIR-DTC), though uncommon, presents a considerable therapeutic challenge with poor long-term outcomes. Currently, tyrosine kinase inhibitors are the mainstay of treatment for advanced RAIR-DTC patients. However, these agents are associated with a multitude of adverse events with resultant deterioration in the quality-of-life of the patients. Targeted theranostic approaches with radiolabelled integrin binders and fibroblast activation protein- (FAP)-inhibitors seem to have a promising role in the management of such patients. This mini-review focuses on these novel theranostic strategies in RAIR-DTC, with emphasis on recent advances, existing challenges, and future directions.
Introduction
Thyroid cancer, accounting for 586,000 cases worldwide, was ranked as the ninth most commonly incident cancer in the year 2020 (1). Differentiated thyroid cancers (DTCs), comprising ~90% of all thyroid cancers, usually have favourable long-term prognosis following surgery+/-radioiodine treatment, with mortality rates ranging from 0.3-0.5/100,000 population (1, 2). Nevertheless, the entity of radioiodine-refractory DTC (RAIR-DTC) presents a challenge to the treating physicians, with 10-year survival rate of ~10% from the time of detection of metastatic lesion(s) (3). The 2015 American Thyroid Association (ATA) guidelines classify RAIR-DTC into four categories: de-novo RAIR-DTC, i.e. the tumor never concentrated radioiodine; loss of ability to concentrate radioiodine; disease heterogeneity, i.e. some lesions concentrating radioiodine, while not others; and, lastly, progressive disease despite radioiodine concentration. Molecular imaging with 2-deoxy-2-[18F]fluoro-D-glucose-positron-emission-tomography/computed-tomography (2-[18F]FDG-PET/CT) is widely used to assess disease extent and progression in patients with RAIR-DTC. While asymptomatic indolent RAIR-DTC can be followed up with suppressive levothyroxine dose alone, symptomatic, progressive oligometastatic disease may benefit from directed therapies, namely, surgery, external radiotherapy, or thermal ablation. The major challenge lies with symptomatic, rapidly progressive, inoperable locally advanced/widely metastatic RAIR-DTC, which requires systemic therapies (4). Treatment options in this setting have been so far limited to only three tyrosine kinase inhibitors (TKIs) approved by the Food and Drugs Administration (FDA).
Role of Tyrosine Kinase Inhibitors
The use of multikinase inhibitors has proven to be an effective strategy in the treatment of RAIR-DTC due to their action on the PI3K/Akt/mTOR- and MAPK-signaling pathways (5). However, given their toxicity profile, their use should be considered only for symptomatic, rapidly progressive, inoperable locally advanced/widely metastatic RAIR-DTC (4).
Sorafenib, an inhibitor of VEGFR, PDGFR, cKIT, and RET, was first approved for this indication based on the results of the DECISION trial (5). The trial randomized 417 patients to sorafenib or placebo, and reported a progression-free survival (PFS) benefit of 5.0 months. However, objective response rate (ORR) with sorafenib was modest at only 12.2% (6).
The results of the SELECT trial led to the approval of lenvatinib in RAIR-DTC. Lenvatinib inhibits FGFR, VEGFR, PDGFR, cKIT, and RET, and is administered orally at a dosage of 24 mg once daily in adults (5). The SELECT trial reported a significant PFS benefit of 14.7 months, as well as a remarkable ORR of 64.8% (7).
Cabozantinib is the latest drug that has been approved for RAIR-DTC patients, who have progressed on up to two prior VEGFR inhibitors. The COSMIC-311 trial reported a PFS benefit of 9.1 months with cabozantinib arm while the ORR was 18%. The recommended dosage is 60 mg orally once daily in adults (8).
Despite these encouraging results, the use of TKIs is associated with a wide spectrum of adverse events (AEs), such as hypertension, mucocutaneous toxicities, prolonged QTc interval, raised liver enzymes, proteinuria, haematological toxicities, and pancreatitis. Further, patients with prior cardiovascular events, poorly-controlled hypertension, prolonged QTc interval, or baseline deranged organ function are ineligible for treatment with these drugs (4). In this scenario, there is an unmet need for novel, effective and safe therapeutic strategies for patients with RAIR-DTC.
Theranostics in RAIR-DTC
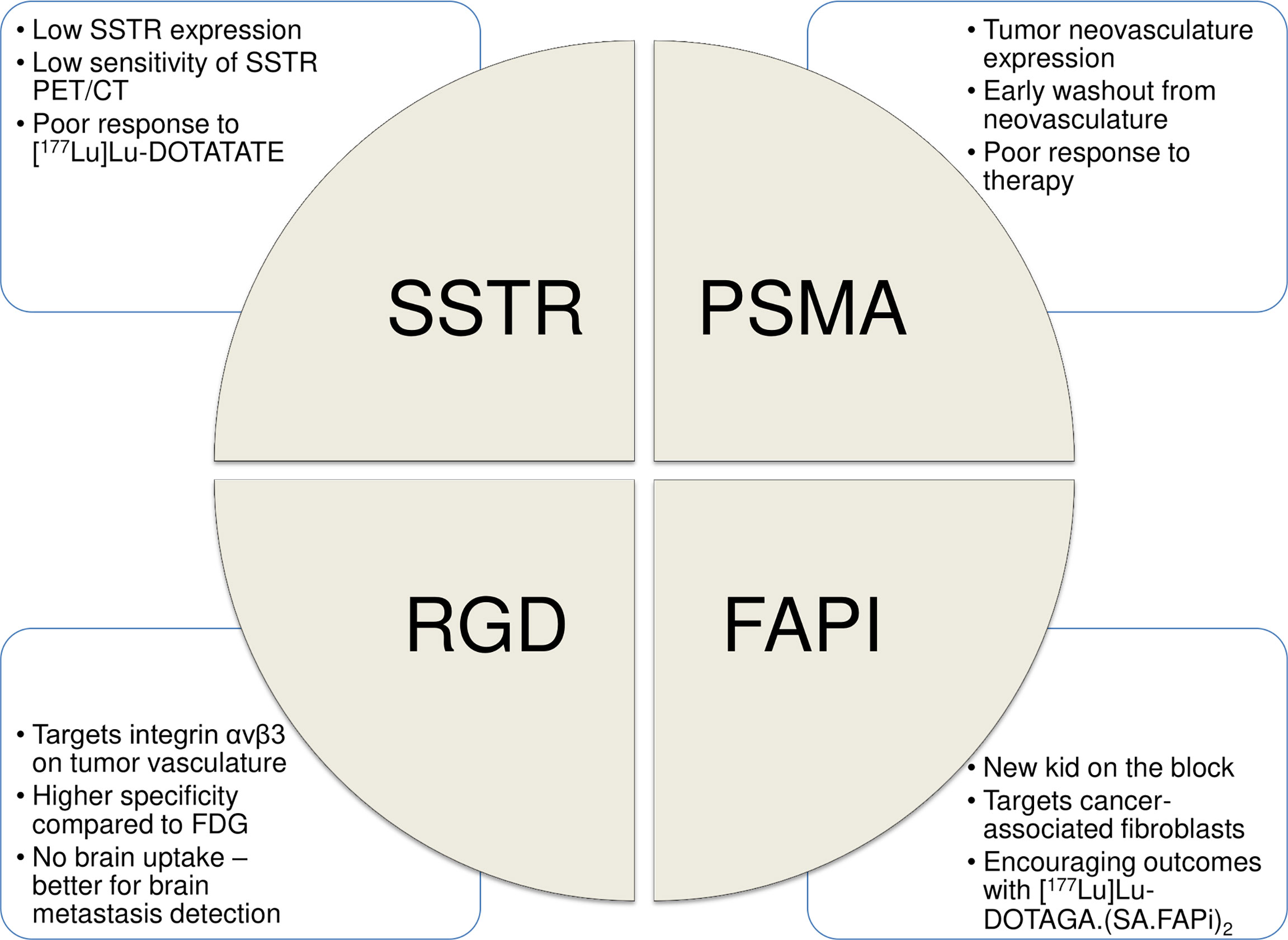
The field of “Theranostics” involves the combined approach of using same or similar radiopharmaceuticals for diagnostic and therapeutic purposes. In the past decade, use of theranostic pairs, targeted to somatostatin receptors (SSTRs) and prostate-specific membrane antigen (PSMA), has significantly altered the treatment paradigm in advanced neuroendocrine tumors (NETs) and prostate cancer (PCa), respectively. Herein, we explore the role of theranostics in RAIR-DTC, with emphasis on recent advances, existing challenges, and future directions. Figure 1 enumerates the currently available theranostic strategies for RAIR-DTC.
68Ga/177Lu-Somatostatin Analogues
SSTRs are G-protein-coupled-receptors, overexpressed in well-differentiated NETs. Radiolabelled somatostatin analogues, namely, Gallium-68-(68Ga)-DOTANOC/DOTATOC/DOTATATE target these SSTRs, and have high sensitivity and specificity for the detection of well-differentiated NETs (9). The therapeutic counterpart, Lutetium-177-(177Lu)-DOTATATE was FDA-approved for advanced, progressive gastroenteropancreatic NETs (GEP-NETs) following the NETTER trial, which showed significant improvement in the PFS with [177Lu]Lu-DOTATATE (10). Subsequently, [177Lu]Lu-DOTATATE has also been tried successfully in the first-line setting in NETs (11, 12).
The expression of SSTRs in thyroid cells has been used to identify metastatic lesions and to select patients for peptide receptor radionuclide therapy (PRRT). One study enrolled 41 patients with progressive RAIR-DTC, and reported SSTR positivity in 24/41 patients (59%) compared to FDG positivity in 34/41 (83%) patients (13). In another study with 62 such patients, the authors reported SSTR-positive disease in 40/62 (65%) patients versus FDG-positive disease in 45/62 (72%) patients. Per-patient sensitivity and specificity of [68Ga]Ga-DOTANOC PET-CT was 78.4% and 100%, compared to 86.3% and 90.9%, respectively for 2-[18F]FDG-PET/CT (14). Overall, the sensitivity of SSTR-PET/CT was seen to be lower, and hence, unlikely to replace 2-[18F]FDG-PET/CT as the first-line imaging modality in RAIR-DTC.
Treatment-wise, PRRT with [177Lu]Lu-DOTATATE has been even less successful in RAIR-DTC. In a study comprising five RAIR-DTC patients with SSTR-positive disease on [68Ga]Ga-DOTATATE-PET/CT, the investigators administered 2–4 cycles of [177Lu]Lu-DOTATATE therapy (mean injected dose: 7.0± 0.7 gigabecquerels, GBq) at 12 weeks intervals. The results were poor with only one out of five patients having partial response, while three patients had disease progression (15). Similar results were reported in another study from India, where 6/8 (75%) patients with thyroglobulin-elevated negative iodine scintigraphy (TENIS) syndrome had radiological disease progression on [177Lu]Lu-DOTATATE. The reason for such modest responses could be the low degree of SSTR expression in RAIR-DTC, with corresponding high FDG avidity, which is suggestive of aggressive tumor behaviour (16). Notably, [177Lu]Lu-DOTATATE PRRT has also been tried with limited success in patients with advanced medullary thyroid cancer. Even in these cases, [177Lu]Lu-DOTATATE could mostly achieve stable disease, and not complete response or partial response (17, 18).
68Ga/177Lu-PSMA Ligands
PSMA is a type II transmembrane glycoprotein that is overexpressed in majority of the PCa cells, thereby making it an ideal target for diagnosis and therapy in PCa. PET/CT with the small molecule PSMA inhibitor, [68Ga]Ga-PSMA-11, has been shown to be useful in detecting involved sites in patients with metastatic PCa (19). In the landmark VISION trial, its therapeutic counterpart, [177Lu]Lu-PSMA-617, has been proven to improve overall survival, when added to standard-of-care, in patients with end-stage metastatic castration-resistant PCa (mCRPC) (20). Another phase-2 trial has also shown non-inferior outcomes with [177Lu]Lu-PSMA-617 compared to docetaxel in chemotherapy-naïve mCRPC (21).
Apart from the PCa cells, PSMA is also overexpressed in the endothelial cells of the neovasculature of various malignancies (19). This phenomenon has been sought to be explored in RAIR-DTC as well. In one of the earliest case series, comprising six patients with iodine-negative and FDG-positive metastasized DTC, the investigators reported PSMA-expressing disease in 5/6 (83%) patients. Of these, three patients had intense uptake on [68Ga]Ga-PSMA-11-PET/CT (22). However, in another series comprising nine patients with TENIS syndrome, the authors reported PSMA-positive disease in only 5/9 (56%) patients compared to FDG-positive disease in 8/9 (89%) patients. Further, out of a total of 14 lesions, [68Ga]Ga-PSMA-11-PET/CT detected less lesions compared to 2-[18F]FDG-PET/CT (9/14 versus 11/14, respectively) (23). Given the lower detection rates, the role of [68Ga]Ga-PSMA-11-PET/CT in RAIR-DTC lies primarily in patient selection for [177Lu]Lu-PSMA-617 therapy, and not for diagnostic purposes.
The first-in-human results of [177Lu]Lu-PSMA-617 in RAIR-DTC were reported in a series of five patients. Three patients were found to be eligible for [177Lu]Lu-PSMA-617, based on tracer uptake on [68Ga]Ga-PSMA-11-PET/CT, and of these, two patients underwent treatment. Two cycles of [177Lu]Lu-PSMA-617 (6.0 GBq per cycle) were administered to each patient at 6-11 weeks intervals. While one patient showed rapid disease progression one month later, the other patient showed partial response. Nevertheless, the response was not durable, with the patient eventually progressing seven months after treatment (24). The localization of PSMA expression in the neovasculature of RAIR-DTC instead of tumor cells as in PCa could potentially explain the relatively poor outcomes with [177Lu]Lu-PSMA-617 therapy in DTC compared to PCa.
68Ga/177Lu-RGD Analogues
Integrins are a group of heterodimer, transmembrane glycoprotein cell-adhesion molecules that are involved in cellular interactions and carcinogenesis. In particular, integrin αvβ3 is reported to be associated with tumor neoangiogenesis. It is also important in regulating the metastatic potential of tumor cells through its interactions with the extracellular matrix (25). Consequently, integrin αvβ3 is overexpressed in the tumor vasculature of various malignancies, and is an attractive target for diagnostic and therapeutic applications. More interestingly, in thyroid cancer, the αvβ3 integrin is reported to be overexpressed on both the tumor endothelial cells as well as the tumor cell surface (26, 27).
The Arg-Gly-Asp (RGD) tripeptide sequence has high affinity and specificity towards the integrin αvβ3 (28). Radiolabelled RGD analogues can, thus, be used for theranostic purposes in RAIR-DTC. This was demonstrated in a single-centre prospective study which enrolled 44 patients with RAIR-DTC for 2-[18F]FDG- and [68Ga]Ga‐DOTA‐RGD2-PET/CT studies. [68Ga]Ga‐DOTA‐RGD2-PET/CT detected 123 lesions, with an overall sensitivity, specificity and accuracy of 82.3%, 100%, and 86.4%, respectively. In contrast, 2-[18F]FDG-PET/CT detected 144 lesions, with overall sensitivity, specificity and accuracy of 82.3%, 50%, and 75%, respectively. Notably, [68Ga]Ga‐DOTA‐RGD2-PET/CT had similar sensitivity with much higher specificity compared to 2-[18F]FDG-PET/CT. This can be explained by the non-specific FDG accumulation in inflammatory/infective sites, in comparison to the more specific integrin localization in the tumor neovasculature (29). Similar results have also been reported with other RGD analogues, such as 18F-galacto-RGD, 18F-FPP-RGD2, and alfatide. Further, owing to the low background uptake in the normal brain tissue, radiolabelled RGD analogues have incremental role over 2-[18F]FDG-PET/CT in detecting brain metastases (30).
In the study by Parihar et al., the authors also reported that 82.1% patients positive on [68Ga]Ga‐DOTA‐RGD2-PET/CT had lesional radiotracer uptake higher than that of normal liver, and hence, suitable for potential treatment with [177Lu]Lu-RGD analogues (29). This is in stark contrast to the predominantly low-grade uptake with SSTR-analogues and PSMA-inhibitors. Further, [177Lu]Lu‐DOTA‐RGD2 is also reported to have favourable pharmacokinetics, as evident from biodistribution studies in mice with melanoma showing 80% clearance of radioactivity by 30 minutes after tracer administration, and 96% clearance by 72 hours. The rapid radiotracer clearance from the blood pool could potentially minimize the absorbed dose to the marrow. The tumor radioactivity retention was also good, with the highest tracer activity in the whole‐body at 72 hours being noted at the tumor site, i.e. 1.5% injected activity per gram of tissue (IA/g) (31). In a first-in-human study, Parihar et al. treated a 54-year-old female, having progressive TENIS syndrome with a single cycle of 5.5 GBq of [177Lu]Lu-DOTA‐RGD2. The patient had disseminated disease, and showed high tracer uptake on the baseline [68Ga]Ga‐DOTA‐RGD2-PET/CT. Four months after treatment, she experienced significant clinical as well as radiological response (32) (Table 1). Phase-1/2 trials with [177Lu]Lu-DOTA‐RGD2 are now warranted to determine the optimum radioactivity per cycle, while maximizing efficacy and minimizing toxicity.
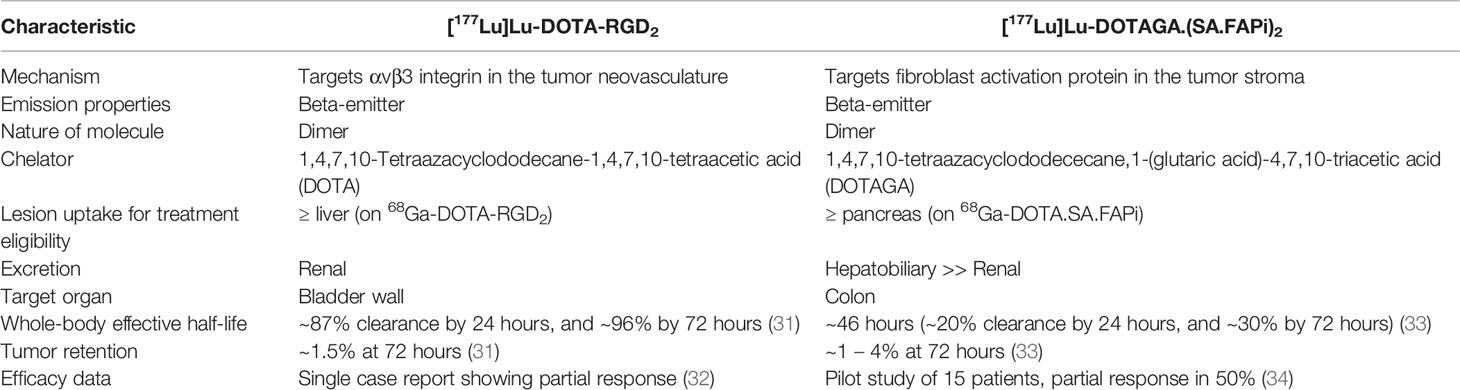
Table 1 Salient features of [177Lu]Lu-DOTA-RGD2 and [177Lu]Lu-DOTAGA.(SA.FAPi)2 theranostic strategies.
68Ga/177Lu-FAP Inhibitors
Fibroblast activation protein (FAP) is a type II transmembrane serine protease that is overexpressed in cancer-associated fibroblasts (CAFs), and plays a key role in modulating the tumor microenvironment, angiogenesis, and chemotherapy resistance. FAP is overexpressed in a variety of tumors, such as colon, pancreatic, ovarian, hepatocellular carcinoma, etc (35). Several radiolabelled small molecule FAP inhibitors (FAPIs), namely, [68Ga]Ga-FAPI-02, -04, -46, and -DOTA.SA.FAPi have been tried for imaging purposes in various malignancies (35–37). The lack of FAP expression in normal tissues results in a low background uptake with high image contrast. This has consequently led to [68Ga]Ga-FAPI-PET/CT outperforming 2-[18F]FDG-PET/CT in sensitivity and specificity for primary, nodal, and metastatic lesion evaluation across tumor types (38). Radiolabelled FAP inhibitors, therefore, present an interesting avenue for diagnostic and therapeutic applications in RAIR-DTC.
Fu et al. demonstrated intense FAPI uptake in local recurrent and distant metastatic sites in a patient with TENIS syndrome. In a subsequent case of RAIR-DTC, the same group detected additional metastatic lesions on [68Ga]Ga-FAPI-PET/CT, which were not positive on 2-[18F]FDG-PET/CT. This was attributed to the much better tumor-to-background ratio in [68Ga]Ga-FAPI-PET/CT compared to 2-[18F]FDG-PET/CT (39, 40). While adequately powered studies comparing the two modalities in RAIR-DTC are currently lacking, the results from these initial experiences as well as those extrapolated from other tumor types clearly favour an incremental role of [68Ga]Ga-FAPI-PET/CT in this setting.
In the field of therapeutics, 177Lu-labelled FAPI has witnessed considerable initial success. A major challenge with the initial small molecule FAPIs was their relatively lower tumor residence time owing to faster clearance. This was overcome by Moon et al. by developing a homodimeric system having two squaramide-conjugated FAP inhibitors connected by a central, bifunctional chelator (DOTAGA) to increase the tumor residence time, i.e. DOTAGA.(SA.FAPi)2. PET studies in six patients revealed significantly higher tumor uptake and longer tumor retention for [68Ga]Ga-DOTAGA.(SA.FAPi)2 compared to [68Ga]Ga-DOTA.SA.FAPi (41). Subsequent dosimetry studies also reported substantially higher median whole-body effective half-life (Te) (46.2 hours versus 23.1 hours, respectively), tumor Te (86.6 hours versus 14 hours, respectively), and tumor absorbed dose (6.7 Gy/GBq versus 0.6 Gy/GBq, respectively) with [177Lu]Lu-DOTAGA.(SA.FAPi)2 in comparison to [177Lu]Lu-DOTA.SA.FAPi (33). Building on these initial encouraging results, the authors prospectively enrolled 19 RAIR-DTC patients, who had prior progression on sorafenib/lenvatinib. Fifteen/19 (79%) patients had intense tracer uptake (≥pancreatic uptake) on the baseline [68Ga]Ga-DOTAGA.SA.FAPi-PET/CT, and underwent treatment with 2-3 cycles of [177Lu]Lu- DOTAGA.(SA.FAPi)2 at 8 weeks intervals (cumulative activity, 8.2 ± 2.7 GBq). On follow-up, the patients experienced significant improvement in the performance scores and serum thyroglobulin levels, with no grade 3/4 AEs. Most notably, of the eight patients evaluated for morphological response, four (50%) achieved partial response and three (38%) had stable disease (disease control rate, 88%) (34) (Table 1). The results of this pilot study suggest [177Lu]Lu- DOTAGA.(SA.FAPi)2 to be a safe and effective strategy for the management of RAIR-DTC patients, particularly for those who have exhausted or are intolerable to the existing treatment options.
Discussion
Diagnostic and treatment options in RAIR-DTC have come a long way over the last decade. While 2-[18F]FDG-PET/CT remains the modality of choice in the initial evaluation of RAIR-DTC patients, newer targeted approaches, such as [68Ga]Ga‐DOTA‐RGD2 and [68Ga]Ga-FAPI-PET/CT seem to have an incremental role. The ability to use these molecules for therapeutic purposes further adds to the armamentarium of treatment options in this setting. With encouraging results from pilot studies, these molecules hold considerable promise for patients with advanced RAIR-DTC.
Theranostic approaches have certain distinctive advantages over the current standard-of-care in advanced RAIR-DTC, i.e. TKIs. Given their non-specific nature of action, TKIs are associated with various AEs, affecting multiple systems (4, 5). In contrast, a targeted approach with radiotheranostics has limited off-site action, and hence, a high degree of safety margin, as evident from the preliminary studies (33, 34). Further, with the exception of lenvatinib, the existing TKIs mostly cause stable disease on follow-up (6–8). However, extrapolation of data from SSTR and PSMA theranostics in advanced NETs and mCRPC respectively, as well as results from the pilot studies with [177Lu]Lu-DOTA‐RGD2 and [177Lu]Lu- DOTAGA.(SA.FAPi)2 in RAIR-DTC, suggest higher partial response rates with the theranostic approaches (10, 20, 32, 34). This, in turn, can potentially result in increased PFS and overall survival for these patients, and the same remains to be validated in future studies.
Another major limitation with the currently approved TKIs is their high costs, especially in low-to-medium income countries. A prolonged duration of treatment with these agents causes significant financial burden on the patients, with many discontinuing treatment. Radionuclide therapy can, thus, prove to be an effective alternative for such patients. Since these therapies are administered at 2-3 monthly intervals, and up to a limited number of cycles, the financial burden on the patients could be relatively lower. This, coupled with their efficacy and safety, can potentially lead to improved quality-of-life for such patients. Interestingly, in a cost-consequence analysis for patients with pancreatic NETs, [177Lu]Lu-DOTATATE was associated with lower costs per progression-free month compared to the TKI, sunitinb (€2989 versus €5337, respectively) (42).
Despite the initial successes with theranostics in RAIR-DTC, few challenges remain. At the outset, SSTR and PSMA theranostics seem to have limited role here and only useful in selected individuals. In contrast, high-grade lesional uptake is noted in ~80% of patients on [68Ga]Ga‐DOTA‐RGD2 and [68Ga]Ga-FAPI-PET/CT, making these suitable for routine applications (29, 34). Nevertheless, human dosimetry data for [177Lu]Lu‐DOTA‐RGD2 is currently lacking, and its efficacy and safety remain to be validated in a patient cohort. Though [177Lu]Lu- DOTAGA.(SA.FAPi)2 has crossed these initial hurdles, few key problems persist. While dimerization of the FAPI molecule increased its intratumoral accumulation, it also prolonged its clearance from the blood pool, thereby potentially causing increased marrow toxicity. Although the initial results do not suggest major grade 3/4 AEs, the sample size is small and further studies are warranted to clearly demonstrate its safety. Further, the dimer FAPI molecule is predominantly excreted by the hepatobiliary route, thereby resulting in the colon being the target organ and receiving the highest absorbed dose (33, 34). Since significant radiotracer concentration is noted in the colon for the initial 48 hours, laxatives could be administered to accelerate its clearance and reduce toxicity. Another potential problem with FAPI theranostics could be that the targeting of the CAFs by the radiolabelled molecule may result in destruction of the tumor stroma only, and not tumor cells per se, owing to the low tissue path length of 177Lu beta particles.
In conclusion, novel theranostic strategies with RGD analogues and FAPI hold immense promise to alter the treatment paradigm in advanced RAIR-DTC. Adequately powered phase-2/3 trials are now required to generate further data on their efficacy and safety, including long-term outcomes. Targeted alpha therapy using these molecules could also be considered for refractory patients, and is an interesting topic for future research.
Author Contributions
SS: Literature search, article selection, data extraction, manuscript writing. CB: Conception, literature search, article selection, data extraction, interpretation, manuscript refinement, and final approval. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. Diagnosis and Treatment of Patients With Thyroid Cancer. Am Health Drug Benefits (2015) 8:30–40.
3. Schmidt A, Iglesias L, Klain M, Pitoia F, Schlumberger MJ. Radioactive Iodine-Refractory Differentiated Thyroid Cancer: An Uncommon But Challenging Situation. Arch Endocrinol Metab (2017) 61:81–9. doi: 10.1590/2359-3997000000245
4. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26:1–133. doi: 10.1089/thy.2015.0020
5. Porter A, Wong DJ. Perspectives on the Treatment of Advanced Thyroid Cancer: Approved Therapies, Resistance Mechanisms, and Future Directions. Front Oncol (2021) 10:592202. doi: 10.3389/fonc.2020.592202
6. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in Radioactive Iodine-Refractory, Locally Advanced or Metastatic Differentiated Thyroid Cancer: A Randomised, Double-Blind, Phase 3 Trial. Lancet (2014) 384:319–28. doi: 10.1016/S0140-6736(14)60421-9
7. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib Versus Placebo in Radioiodine-Refractory Thyroid Cancer. N Engl J Med (2015) 372:621–30. doi: 10.1056/NEJMoa1406470
8. Brose MS, Robinson B, Sherman SI, Krajewska J, Lin CC, Vaisman F, et al. Cabozantinib for Radioiodine-Refractory Differentiated Thyroid Cancer (COSMIC-311): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2021) 22:1126–38. doi: 10.1016/S1470-2045(21)00332-6
9. Hofman MS, Lau WF, Hicks RJ. Somatostatin Receptor Imaging With 68Ga DOTATATE PET/CT: Clinical Utility, Normal Patterns, Pearls, and Pitfalls in Interpretation. Radiographics (2015) 35:500–16. doi: 10.1148/rg.352140164
10. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med (2017) 376:125–35. doi: 10.1056/NEJMoa1607427
11. Satapathy S, Mittal BR, Sood A, Sood A, Kapoor R, Gupta R. Peptide Receptor Radionuclide Therapy as First-Line Systemic Treatment in Advanced Inoperable/Metastatic Neuroendocrine Tumors. Clin Nucl Med (2020) 45:e393–9. doi: 10.1097/RLU.0000000000003170
12. Satapathy S, Mittal BR, Sood A, Sood A, Kapoor R, Gupta R, et al. 177Lu-DOTATATE Plus Radiosensitizing Capecitabine Versus Octreotide Long-Acting Release as First-Line Systemic Therapy in Advanced Grade 1 or 2 Gastroenteropancreatic Neuroendocrine Tumors: A Single-Institution Experience. JCO Glob Oncol (2021) 7:1167–75. doi: 10.1200/GO.21.00103
13. Versari A, Sollini M, Frasoldati A, Fraternali A, Filice A, Froio A, et al. Differentiated Thyroid Cancer: A New Perspective With Radiolabeled Somatostatin Analogues for Imaging and Treatment of Patients. Thyroid (2014) 24:715–26. doi: 10.1089/thy.2013.0225
14. Kundu P, Lata S, Sharma P, Singh H, Malhotra A, Bal C. Prospective Evaluation of (68)Ga-DOTANOC PET-CT in Differentiated Thyroid Cancer Patients With Raised Thyroglobulin and Negative (131)I-Whole Body Scan: Comparison With (18)F-FDG PET-Ct. Eur J Nucl Med Mol Imaging (2014) 41:1354–62. doi: 10.1007/s00259-014-2723-9
15. Roll W, Riemann B, Schäfers M, Stegger L, Vrachimis A. 177Lu-DOTATATE Therapy in Radioiodine-Refractory Differentiated Thyroid Cancer: A Single Center Experience. Clin Nucl Med (2018) 43:e346–51. doi: 10.1097/RLU.0000000000002219
16. Basu S, Parghane RV, Naik C. Clinical Efficacy of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy in Thyroglobulin-Elevated Negative Iodine Scintigraphy: A "Not-So-Promising" Result Compared to GEP-NETs. World J Nucl Med (2020) 19:205–10. doi: 10.4103/wjnm.WJNM_21_19
17. Beukhof CM, Brabander T, van Nederveen FH, van Velthuysen MF, de Rijke YB, Hofland LJ, et al. Peptide Receptor Radionuclide Therapy in Patients With Medullary Thyroid Carcinoma: Predictors and Pitfalls. BMC Cancer (2019) 19:325. doi: 10.1186/s12885-019-5540-5
18. Satapathy S, Mittal BR, Sood A, Verma R, Panda N. Efficacy and Safety of Concomitant 177Lu-DOTATATE and Low-Dose Capecitabine in Advanced Medullary Thyroid Carcinoma: A Single-Centre Experience. Nucl Med Commun (2020) 41:629–35. doi: 10.1097/MNM.0000000000001205
19. Bois F, Noirot C, Dietemann S, Mainta IC, Zilli T, Garibotto V, et al. [68Ga]Ga-PSMA-11 in Prostate Cancer: A Comprehensive Review. Am J Nucl Med Mol Imaging (2020) 10:349–74.
20. Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med (2021) 385:1091–103. doi: 10.1056/NEJMoa2107322
21. Satapathy S, Mittal BR, Sood A, Das CK, Mavuduru RS, Goyal S, et al. 177Lu-PSMA-617 Versus Docetaxel in Chemotherapy-Naïve Metastatic Castration-Resistant Prostate Cancer: A Randomized, Controlled, Phase 2 non-Inferiority Trial. Eur J Nucl Med Mol Imaging (2022) 49:1754–64. doi: 10.1007/s00259-021-05618-3
22. Lütje S, Gomez B, Cohnen J, Umutlu L, Gotthardt M, Poeppel TD, et al. Imaging of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68ga-HBED-CC-PSMA PET/Ct. Clin Nucl Med (2017) 42:20–5. doi: 10.1097/RLU.0000000000001454
23. Verma P, Malhotra G, Meshram V, Chandak A, Sonavane S, Lila AR, et al. Prostate-Specific Membrane Antigen Expression in Patients With Differentiated Thyroid Cancer With Thyroglobulin Elevation and Negative Iodine Scintigraphy Using 68ga-PSMA-HBED-CC PET/Ct. Clin Nucl Med (2021) 46:e406–9. doi: 10.1097/RLU.0000000000003655
24. de Vries LH, Lodewijk L, Braat AJAT, Krijger GC, Valk GD, Lam MGEH, et al. 68Ga-PSMA PET/CT in Radioactive Iodine-Refractory Differentiated Thyroid Cancer and First Treatment Results With 177Lu-PSMA-617. EJNMMI Res (2020) 10:18. doi: 10.1186/s13550-020-0610-x
25. Chen X. Multimodality Imaging of Tumor Integrin Alphavbeta3 Expression. Mini Rev Med Chem (2006) 6:227–34. doi: 10.2174/138955706775475975
26. Hoffmann S, Maschuw K, Hassan I, Reckzeh B, Wunderlich A, Lingelbach S, et al. Differential Pattern of Integrin Receptor Expression in Differentiated and Anaplastic Thyroid Cancer Cell Lines. Thyroid (2005) 15:1011–20. doi: 10.1089/thy.2005.15.1011
27. Cheng W, Feng F, Ma C, Wang H. The Effect of Antagonizing RGD-Binding Integrin Activity in Papillary Thyroid Cancer Cell Lines. Onco Targets Ther (2016) 9:1415–23. doi: 10.2147/OTT.S99166
28. Liu S. Radiolabeled Cyclic RGD Peptides as Integrin Alpha(V)Beta(3)-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjug Chem (2009) 20:2199–213. doi: 10.1021/bc900167c
29. Parihar AS, Mittal BR, Kumar R, Shukla J, Bhattacharya A. 68Ga-DOTA-RGD2 Positron Emission Tomography/Computed Tomography in Radioiodine Refractory Thyroid Cancer: Prospective Comparison of Diagnostic Accuracy With 18F-FDG Positron Emission Tomography/Computed Tomography and Evaluation Toward Potential Theranostics. Thyroid (2020) 30:557–67. doi: 10.1089/thy.2019.0450
30. Klubo-Gwiezdzinska J, Chen X. Targeting Integrins With Radiolabeled RGD Analogues for Radiotheranostics of Metastatic Radioactive Iodine Nonresponsive Thyroid Cancer: New Avenues in Personalized Medicine. Thyroid (2020) 30:476–8. doi: 10.1089/thy.2020.0169
31. Chakraborty S, Sarma HD, Vimalnath KV, Pillai MR. Tracer Level Radiochemistry to Clinical Dose Preparation of (177)Lu-Labeled Cyclic RGD Peptide Dimer. Nucl Med Biol (2013) 40:946–54. doi: 10.1016/j.nucmedbio.2013.05.011
32. Parihar AS, Sood A, Kumar R, Bhusari P, Shukla J, Mittal BR. Novel Use of 177Lu-DOTA-RGD2 in Treatment of 68Ga-DOTA-RGD2-Avid Lesions in Papillary Thyroid Cancer With TENIS. Eur J Nucl Med Mol Imaging (2018) 45:1836–7. doi: 10.1007/s00259-018-4036-x
33. Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [177Lu]Lu-DOTA.SA.FAPi and [177Lu]Lu-DOTAGA.(SA.FAPi)2. Pharm (Basel) (2021) 14:1212. doi: 10.3390/ph14121212
34. Ballal S, Yadav MP, Moon ES, Roesch F, Kumari S, Agarwal S, et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid (2022) 32:65–77. doi: 10.1089/thy.2021.0412
35. Lindner T, Loktev A, Altmann A, Giesel F, Kratochwil C, Debus J, et al. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J Nucl Med (2018) 59:1415–22. doi: 10.2967/jnumed.118.210443
36. Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J Nucl Med (2020) 61:1171–7. doi: 10.2967/jnumed.119.236786
37. Ballal S, Yadav MP, Moon ES, Kramer VS, Roesch F, Kumari S, et al. Biodistribution, Pharmacokinetics, Dosimetry of [68Ga]Ga-DOTA. SA.FAPi head-to-head comparison [18F]F-FDG PET/CT patients various cancers Eur J Nucl Med Mol Imaging (2021) 48:1915–31. doi: 10.1007/s00259-020-05132-y
38. Taveira M. Comparison of 68Ga-FAPI Versus 18F-FDG PET/CT for Initial Cancer Staging. Radiol Imaging Cancer (2021) 3:e219007. doi: 10.1148/rycan.2021219007
39. Fu H, Fu J, Huang J, Su X, Chen H. 68Ga-FAPI PET/CT in Thyroid Cancer With Thyroglobulin Elevation and Negative Iodine Scintigraphy. Clin Nucl Med (2021) 46:427–30. doi: 10.1097/RLU.0000000000003569
40. Fu H, Fu J, Huang J, Pang Y, Chen H. 68Ga-FAPI PET/CT Versus 18f-FDG PET/CT for Detecting Metastatic Lesions in a Case of Radioiodine-Refractory Differentiated Thyroid Cancer. Clin Nucl Med (2021) 46:940–42. doi: 10.1097/RLU.0000000000003730
41. Moon ES, Ballal S, Yadav MP, Bal C, Van Rymenant Y, Stephan S, et al. Fibroblast Activation Protein (FAP) Targeting Homodimeric FAP Inhibitor Radiotheranostics: A Step to Improve Tumor Uptake and Retention Time. Am J Nucl Med Mol Imaging (2021) 11:476–91.
42. Spada F, Campana D, Lamberti G, Laudicella R, Dellamano R, Dellamano L, et al. [177lu]Lu-DOTA-TATE Versus Standard of Care in Adult Patients With Gastro-Enteropancreatic Neuroendocrine Tumours (GEP-NETs): A Cost-Consequence Analysis From an Italian Hospital Perspective. Eur J Nucl Med Mol Imaging (2022) 49:2037–48. doi: 10.1007/s00259-021-05656-x
Keywords: radioiodine-refractory differentiated thyroid cancer, RAIR-DTC, theranostic, integrin binders, RGD, FAPI
Citation: Satapathy S and Bal C (2022) Theranostic Options for Radioiodine-Refractory Differentiated Thyroid Carcinoma: Recent Advances, Challenges, and Road Ahead. Front. Endocrinol. 13:924841. doi: 10.3389/fendo.2022.924841
Received: 20 April 2022; Accepted: 17 June 2022;
Published: 12 July 2022.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Sergio Baldari, University of Messina, ItalyYan-Song Lin, Peking Union Medical College Hospital, China
Copyright © 2022 Satapathy and Bal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chandrasekhar Bal, Y3NiYWxAaG90bWFpbC5jb20=
 Swayamjeet Satapathy
Swayamjeet Satapathy Chandrasekhar Bal
Chandrasekhar Bal