
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 01 August 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.921159
Objective: To determine the impact of preexisting diabetes mellitus on cardiovascular and all-cause mortality in patients with atrial fibrillation (AF) by conducting a meta-analysis.
Methods: PubMed and Embase databases were comprehensively searched for relevant studies publishing until May 19, 2022. Cohort studies or post-hoc analyses of clinical trials that investigated the association of diabetes mellitus with cardiovascular or all-cause mortality in AF patients were included.
Results: A total of 21 studies with 526,136 AF patients were identified. The pooled prevalence of diabetes mellitus in patients with AF was 26%. The summary multivariable-adjusted risk ratio (RR) of all-cause mortality was 1.37 (95% confidence intervals [CIs] 1.23–1.53) for patients with diabetes versus those without diabetes. Moreover, diabetes mellitus was also associated with an increased risk of cardiovascular mortality (RR 1.46; 95% CI 1.34–1.58). Stratified analyses suggested that the impact of diabetes on all-cause and cardiovascular mortality was consistently observed in each named subgroup.
Conclusion: The presence of diabetes mellitus in patients with AF is associated with an increased risk of cardiovascular and all-cause mortality, even after adjustment for important confounding factors.
Atrial fibrillation (AF) is the most common type of heart rhythm disorder that can result in stroke, heart failure, myocardial infarction, and venous thromboembolism (1). The healthcare burden of atrial fibrillation is increasing because of the accelerated aging of the population worldwide (2). Despite direct-acting oral anticoagulants effectively reducing stroke risk (3), atrial fibrillation was still associated with a 1.95-fold increased risk of all-cause mortality (4). Therefore, risk stratification of survival outcomes in atrial fibrillation patients is an urgent demand for better clinical management.
Diabetes mellitus is a well-established risk factor for the development of atrial fibrillation. Patients with diabetes had approximately 28% higher risk of atrial fibrillation as compared with those without diabetes (5). Diabetes mellitus is associated with an increased risk of mortality (6). The presence of diabetes mellitus in atrial fibrillation patients may reinforce the risk of mortality. The effect of diabetes mellitus on adverse outcomes has been extensively investigated in atrial fibrillation patients (7). However, conflicting results have been reported on the association of diabetes with all-cause or cardiovascular mortality in patients with atrial fibrillation (8–11).
No previous meta-analysis has evaluated the association of diabetes mellitus with survival outcomes in patients with atrial fibrillation. To address these knowledge gaps, we performed this meta-analysis to determine the impact of diabetes mellitus on cardiovascular and all-cause mortality in atrial fibrillation patients.
We performed this meta-analysis according to the guideline of the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (12). Two authors independently searched PubMed and Embase databases until May 19, 2022, using the following combined keywords: ‘atrial fibrillation’ AND ‘diabetes’ OR ‘diabetic’ AND ‘survival’ OR ‘death’ OR ‘mortality’ AND ‘follow-up’ OR ‘follow up’ (Supplementary Text S1). Moreover, we also searched the reference lists of pertinent articles for any missing articles.
Inclusion criteria were as follows: 1) population: participants with a diagnosis of atrial fibrillation; 2) predictor: diabetes mellitus; 3) comparison: the presence of diabetes mellitus compared with those without diabetes; 4) outcomes: reported multivariable-adjusted relative risk of cardiovascular or all-cause mortality; and 5) study design: cohort studies or post-hoc analysis of clinical trials. For articles overlapping patients with another study, we only selected the article with the most complete data or the longest follow-up duration. The exclusion criteria included the following: 1) outcome measures were not of interest, 2) duplicate studies, and 3) an adjusted risk estimate was not available.
Data extraction and quality evaluation were performed by two independent authors, and disagreements were resolved through discussion. Data collected from each study included the following: the first author’s name, publication year, country, design of the study, sample sizes, gender, mean median age of patients, anticoagulant therapy, the prevalence of diabetes mellitus, outcome measures, length of follow-up, fully adjusted risk summary for diabetes versus without diabetes, and variables adjusted. To evaluate the methodological quality of included studies, we adopted a 9-point Newcastle–Ottawa Scale (NOS) for the cohort studies (13). A study with an overall score of 7 points or more was deemed high-quality.
All statistical analyses were performed using Stata 12.0 (Stata, College Station, TX, USA). The impact of diabetes mellitus on survival outcomes was summarized by pooling a fully adjusted risk ratio (RR) and 95% confidence intervals (CIs) for diabetes mellitus versus those without diabetes. Between studies, heterogeneity was explored using the I2 statistics and Cochrane Q test. A random-effects model was utilized when there was significant heterogeneity (I2 statistic >50% and/or p-value of Cochrane Q test <0.10); otherwise, we used a fixed-effects model. Publication bias was evaluated using Begg’s test, Egger’s test, and funnel plot. In case of publication bias, we run a trim-and-fill analysis to observe the impact of publication bias. Sensitivity analyses were performed by the exclusion of individual studies each time to recalculate the pooling risk summary. Subgroup analyses were conducted according to the study design, geographical region, type of atrial fibrillation, sample size, mean/median age of patients, and follow-up duration.
Briefly, a total of 1,760 articles were identified in our initial literature search, of which 614 duplicates were removed, and 1,086 articles were further excluded after scanning the titles or abstracts. Sixty full-text articles were retrieved for detailed assessment. Finally, 21 studies (8–11, 14–30) were included in the meta-analysis after applying the predefined criteria (Figure 1).
The main features of these eligible studies are summarized in Table 1. The included studies were published between 2011 and 2022. Three studies (19, 21, 27) were post-hoc analyses of randomized controlled trials, five studies (11, 14, 17, 26, 28) were retrospective designs, and others were prospective studies. Sample sizes ranged between 278 and 326,832, with 526,136 atrial fibrillation patients. The median/mean duration of follow-up ranged from 12 months to 6.3 years. Supplementary Table S1 describes the comorbidities and concomitant treatment of the included studies. Based on the criteria of the NOS, all the included studies were deemed to have high quality (Supplementary Table S2).
The prevalence of diabetes mellitus ranged from 11.2% to 44.7%. As shown in Figure 2, the pooled prevalence of diabetes mellitus across the included studies was 0.26 (95% CI 0.22–0.30) in a random-effects model with significant heterogeneity (I2 = 99.9%; p < 0.001).
The association of diabetes mellitus with all-cause mortality was reported in 19 studies (8–11, 14, 15, 17–24, 26–30). A random-effects model meta-analysis indicated that diabetes mellitus was associated with an increased risk of all-cause mortality (RR 1.37; 95% CI 1.23–1.53; I2 = 95.1%, p < 0.001; Figure 3) compared with those without diabetes. After a very large study (17) was removed, the pooled RR of all-cause mortality was 1.38 (95% CI 1.22–1.57). Leave-one-out study sensitivity analysis confirmed the robustness of the summary risk estimate (data not shown). There was no evidence of publication bias according to the results of Begg’s test (p = 0.441), Egger’s test (p = 0.964), and symmetry of the funnel plot (Supplementary Figure S1). Additionally, significant associations of diabetes mellitus with all-cause mortality were consistently observed in each subgroup (Table 2A).
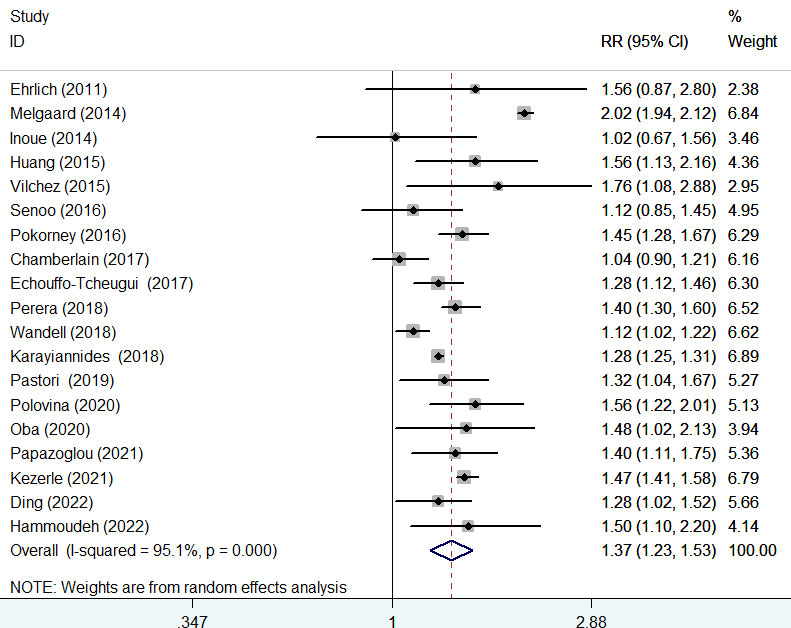
Figure 3 Forest plot showing the pooled risk ratio (RR) with 95% CI of all-cause mortality for those with diabetes versus those without diabetes.
Ten studies (9, 15, 16, 19, 20, 24–27, 29) provided data on the association of diabetes mellitus with cardiovascular mortality. A fixed-effects model meta-analysis suggested that diabetes mellitus was associated with an increased risk of cardiovascular mortality (RR 1.46; 95% CI 1.34–1.58; I2 = 0%, p = 0.594; Figure 4) compared with those without diabetes. Leave-one-out study sensitivity analysis further confirmed the robustness of the summary risk estimate (data not shown). Furthermore, significant associations between diabetes mellitus with cardiovascular mortality were consistently observed in each subgroup (Table 2B). Egger’s test (p = 0.048) but not Begg’s test (p = 0.210) suggested the likelihood of publication bias. The ‘trim-and-fill’ analysis indicated that the pooling RR of cardiovascular mortality was 1.42 (95% CI 1.05–1.91; p < 0.001) and imputed three potentially missing studies (Supplementary Figure S2).
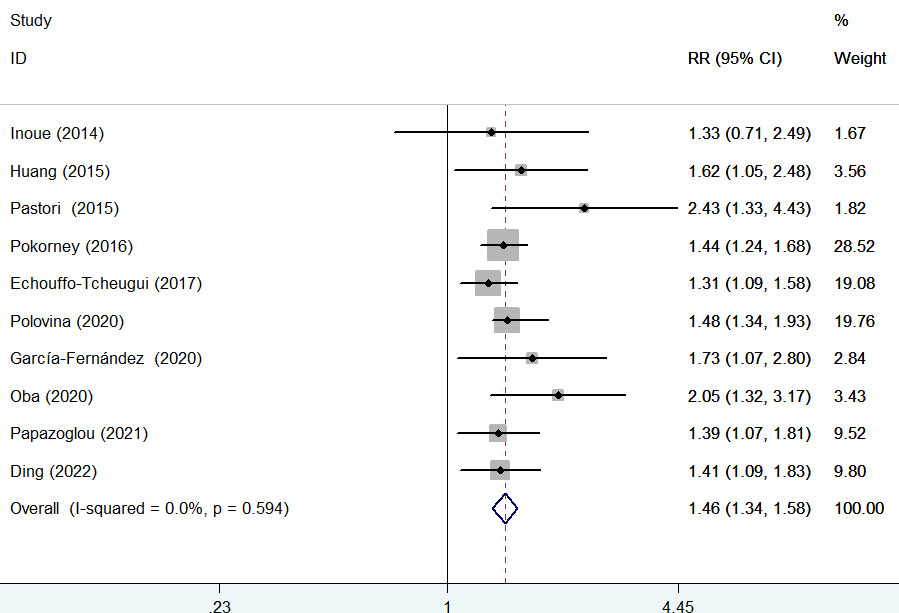
Figure 4 Forest plot showing the pooled risk ratio (RR) with 95% CI of major bleeding for those with diabetes versus those without diabetes.
This is the first meta-analysis to evaluate the impact of preexisting diabetes mellitus on cardiovascular and all-cause mortality in atrial fibrillation patients. Our meta-analysis confirmed that diabetes mellitus was associated with a higher risk of cardiovascular and all-cause mortality in patients with atrial fibrillation. Atrial fibrillation patients with preexisting diabetes had a 46% higher risk of cardiovascular mortality and 37% higher risk of all-cause mortality compared with their non-diabetic counterparts. These results indicated that the presence of diabetes mellitus in atrial fibrillation patients reinforced the mortality risk.
Apart from the cardiovascular and all-cause mortality outcomes, diabetes in atrial fibrillation patients also conferred a higher risk of heart failure (17, 24), myocardial infarction (17), stroke (17, 27), and bleeding events (17). These results suggested that diabetes is an important predictor of the risk classification of AF patients.
Atrial fibrillation and heart failure frequently coexist (31). The presence of atrial fibrillation was associated with a higher risk of mortality in heart failure patients (32). Conversely, incident heart failure conferred a particularly increased risk of mortality in patients with heart failure (33). Sodium-glucose co-transporter 2 inhibitors (SGLT2i) are a class of medication approved for the management of type 2 diabetes. Treatment with SGLT2i could significantly reduce cardiovascular or all-cause mortality in heart failure in patients with or without diabetes (34, 35). Therefore, the association of diabetes with mortality outcomes in patients with atrial fibrillation may also be biased by the use of SGLT2i.
Our subgroup analysis showed that the impact of diabetes on all-cause or cardiovascular mortality was similar in patients with age ≥75 years and those with age <75 years. This finding may be correlated with inadequate anticoagulation in many elderly atrial fibrillation patients (15). Moreover, the value of diabetes in predicting all-cause or cardiovascular mortality appeared to be stronger in patients with a non-valvular type of atrial fibrillation. This result may be explained by the higher risk of stroke and the bleeding risk associated with anticoagulant treatment in the non-valvular type of atrial fibrillation (36). However, it should be noted that the results of subgroup analysis were established on a limited number of studies.
The precise mechanisms underlying the impact of diabetes mellitus on survival in atrial fibrillation patients are not fully elucidated. First, atrial fibrillation patients with diabetes had more concomitant risk factors and comorbidities (17, 25). Second, atrial fibrillation patients with diabetes had a significant reduction in the quality of anticoagulation control (25). Third, diabetes could also cause structural, electrical, electromechanical, and autonomic remodeling (37), which could be responsible for atrial fibrillation recurrence. Finally, diabetes may promote the development of cardiomyopathy and heart failure through systemic inflammation, microvascular dysfunction, and oxidative stress (38, 39).
Our meta-analysis had important clinical implications. The prevalence of diabetes mellitus was up to 39.9% among patients with atrial fibrillation in our analyzed studies. The association between diabetes mellitus and atrial fibrillation has remained under-recognized by clinicians (40). Considering atrial fibrillation patients with diabetes had reduced survival, close monitoring of blood glucose levels and intensive glycemic control are warranted for atrial fibrillation patients with diabetes. The degree of glycemic control may affect the prognosis of atrial fibrillation patients (41). However, whether management of hyperglycemia in those with diabetes improves survival outcomes requires further study.
Several limitations should be mentioned in the current meta-analysis. First, half of the included studies were retrospective in nature, and potential selection bias may have occurred. Second, significant heterogeneity was found in pooling the prevalence of diabetes and all-cause mortality. Different types of atrial fibrillation, methods of diabetes diagnosis, or duration of follow-up may partially explain the observed heterogeneity. Third, the impact of diabetes on survival outcomes may be biased by the use of different anticoagulant agents in patients with atrial fibrillation (42). Lack of adjusted information on the use of medications (including antidiabetic drugs, anticoagulation, and heart failure-directed therapy), severity or duration of diabetes, and glycemic control may confound the pooling risk summary. Finally, the analyzed studies did not distinguish the pattern of atrial fibrillation (permanent or persistent) and type of diabetes mellitus (type 1 or type 2) in patients with atrial fibrillation. Particularly, the anticoagulant therapy was not clearly reported in the included studies. Therefore, we failed to perform a subgroup analysis based on these factors.
The presence of diabetes mellitus in patients with atrial fibrillation is associated with an increased risk of cardiovascular and all-cause mortality, even after adjustment for important confounding factors. Detection of blood glucose levels may improve risk stratification of survival outcomes in atrial fibrillation patients.
All data generated or analyzed during this study are included in this published article.
Study conception/design and interpretation of data: DG and YF. Literature search, data extraction, quality assessment, and statistical analysis: JX and YS. Writing the manuscript: JX. Revising the manuscript: YF. All authors read and approved the final manuscript.
This work is supported by 1) Jiangsu Provincial Key Research and Development Special Fund (CXTDC2016006, QNRC2016446), 2) Zhenjiang Key Research and Development Fund (SH2021038), and 3) Suqian Science and Technology Support Project Fund (K201907).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.921159/full#supplementary-material
1. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ (2016) 354:i4482. doi: 10.1136/bmj.i4482
2. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ Res (2020) 127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340
3. Abdool M, Kunutsor SK, Khunti K, Seidu S. Does the presence of diabetes mellitus confer an increased risk of stroke in patients with atrial fibrillation on direct oral anticoagulants? A systematic review and meta-analysis. Diabetes Metab Syndr (2020) 14(6):1725–33. doi: 10.1016/j.dsx.2020.08.038
4. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol (2017) 24(14):1555–66. doi: 10.1177/2047487317715769
5. Aune D, Feng T, Schlesinger S, Janszky I, Norat T, Riboli E. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J Diabetes Complications (2018) 32(5):501–11. doi: 10.1016/j.jdiacomp.2018.02.004
6. Wang Y, O'Neil A, Jiao Y, Wang L, Huang J, Lan Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med (2019) 17(1):136. doi: 10.1186/s12916-019-1355-0
7. Wang A, Green JB, Halperin JL, Piccini JPSR. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol (2019) 74(8):1107–15. doi: 10.1016/j.jacc.2019.07.020
8. Ehrlich JR, Kaluzny M, Baumann S, Lehmann R, Hohnloser SH. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol (2011) 100(11):1029–36. doi: 10.1007/s00392-011-0337-9
9. Inoue H, Atarashi H, Okumura K, Yamashita T, Origasa H, Kumagai N, et al. Impact of gender on the prognosis of patients with nonvalvular atrial fibrillation. Am J Cardiol (2014) 113(6):957–62. doi: 10.1016/j.amjcard.2013.11.057
10. Senoo K, An Y, Ogawa H, Lane DA, Wolff A, Shantsila E, et al. Stroke and death in elderly patients with atrial fibrillation in Japan compared with the united kingdom. Heart (2016) 102(23):1878–82. doi: 10.1136/heartjnl-2016-309741
11. Chamberlain AM, Alonso A, Gersh BJ, Manemann SM, Killian JM, Weston SA, et al. Multimorbidity and the risk of hospitalization and death in atrial fibrillation: A population-based study. Am Heart J (2017) 185:74–84. doi: 10.1016/j.ahj.2016.11.008
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med (2009) 151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
13. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed May 19, 2022).
14. Melgaard L, Rasmussen LH, Skjoth F, Lip GY, Larsen TB. Age dependence of risk factors for stroke and death in young patients with atrial fibrillation: A nationwide study. Stroke (2014) 45(5):1331–7. doi: 10.1161/STROKEAHA.114.004903
15. Huang B, Yang Y, Zhu J, Liang Y, Zhang H, Tian L, et al. Clinical characteristics and impact of diabetes mellitus on outcomes in patients with nonvalvular atrial fibrillation. Yonsei Med J (2015) 56(1):62–71. doi: 10.3349/ymj.2015.56.1.62
16. Pastori D, Pignatelli P, Farcomeni A, Cangemi R, Hiatt WR, Bartimoccia S, et al. Urinary 11-dehydro-thromboxane B2 is associated with cardiovascular events and mortality in patients with atrial fibrillation. Am Heart J (2015) 170(3):490–7.e1. doi: 10.1016/j.ahj.2015.05.011
17. Karayiannides S, Lundman P, Friberg L, Norhammar A. High overall cardiovascular risk and mortality in patients with atrial fibrillation and diabetes: A nationwide report. Diabetes Vasc Dis Res (2018) 15(1):31–8. doi: 10.1177/1479164117735013
18. Vilchez JA, Perez-Cuellar M, Marin F, Gallego P, Manzano-Fernandez S, Valdes M, et al. sST2 levels are associated with all-cause mortality in anticoagulated patients with atrial fibrillation. Eur J Clin Invest (2015) 45(9):899–905. doi: 10.1111/eci.12482
19. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, et al. Cause of death and predictors of all-cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: Data from ROCKET AF. J Am Heart Assoc (2016) 5(3):e002197. doi: 10.1161/JAHA.115.002197
20. Echouffo-Tcheugui JB, Shrader P, Thomas L, Gersh BJ, Kowey PR, Mahaffey KW, et al. Care patterns and outcomes in atrial fibrillation patients with and without diabetes: ORBIT-AF registry. J Am Coll Cardiol (2017) 70(11):1325–35. doi: 10.1016/j.jacc.2017.07.755
21. Perera KS, Pearce LA, Sharma M, Benavente O, Connolly SJ, Hart RG, et al. Predictors of mortality in patients with atrial fibrillation (from the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events [ACTIVE a]). Am J Cardiol (2018) 121(5):584–9. doi: 10.1016/j.amjcard.2017.11.028
22. Wandell P, Carlsson AC, Holzmann MJ, Arnlov J, Sundquist J, Sundquist K. Mortality in patients with atrial fibrillation and common co-morbidities - a cohort study in primary care. Ann Med (2018) 50(2):156–63. doi: 10.1080/07853890.2017.1407036
23. Pastori D, Antonucci E, Violi F, Palareti G, Pignatelli P, SRId d, et al. Thrombocytopenia and mortality risk in patients with atrial fibrillation: An analysis from the START registry. J Am Heart Assoc (2019) 8(21):e012596. doi: 10.1161/JAHA.119.012596
24. Polovina M, Lund LH, Dikic D, Petrovic-Dordevic I, Krljanac G, Milinkovic I, et al. Type 2 diabetes increases the long-term risk of heart failure and mortality in patients with atrial fibrillation. Eur J Heart Fail (2020) 22(1):113–25. doi: 10.1002/ejhf.1666
25. Garcia-Fernandez A, Esteve-Pastor MA, Roldan-Rabadan I, Muniz J, Ruiz Ortiz M, Cequier A, et al. Relationship of adverse events to quality of anticoagulation control in atrial fibrillation patients with diabetes: real-world data from the FANTASIIA registry. Ann Med (2020) 52(6):300–9. doi: 10.1080/07853890.2020.1778176
26. Oba K, Shinjo T, Tamashiro M, Matsuoka M, Arasaki O, Arima H, et al. Cause of death and associated factors in elderly patients with atrial fibrillation- long-term retrospective study. Circ Rep (2020) 2(9):490–8. doi: 10.1253/circrep.CR-20-0079
27. Papazoglou AS, Kartas A, Samaras A, Vouloagkas I, Vrana E, Moysidis DV, et al. Prognostic significance of diabetes mellitus in patients with atrial fibrillation. Cardiovasc Diabetol (2021) 20(1):40. doi: 10.1186/s12933-021-01232-7
28. Kezerle L, Tsadok MA, Akriv A, Senderey AB, Bachrach A, Leventer-Roberts M, et al. Pre-diabetes increases stroke risk in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol (2021) 77(7):875–84. doi: 10.1016/j.jacc.2020.12.030
29. Ding WY, Kotalczyk A, Boriani G, Marin F, Blomstrom-Lundqvist C, Potpara TS, et al. Impact of diabetes on the management and outcomes in atrial fibrillation: An analysis from the ESC-EHRA EORP-AF long-term general registry. Eur J Intern Med (2022) S0953-6205(22)00167-4. doi: 10.1016/j.ejim.2022.04.026
30. Hammoudeh A, Khader Y, Tabbalat R, Badaineh Y, Kadri N, Shawer H, et al. One-year clinical outcome in middle Eastern patients with atrial fibrillation: The Jordan atrial fibrillation (JoFib) study. Int J Vasc Med (2022) 2022:4240999. doi: 10.1155/2022/4240999
31. Ariyaratnam JP, Lau DH, Sanders P, Kalman JM. Atrial fibrillation and heart failure: Epidemiology, pathophysiology, prognosis, and management. Card Electrophysiol Clin (2021) 13(1):47–62. doi: 10.1016/j.ccep.2020.11.004
32. Odutayo A, Wong CX, Williams R, Hunn B, Emdin CA. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: A systematic review and meta-analysis. J Card Fail (2017) 23(1):56–62. doi: 10.1016/j.cardfail.2016.08.005
33. Slee A, Saksena S. Impact of initial heart failure emergence on clinical outcomes of atrial fibrillation patients in the AFFIRM trial. Am Heart J (2020) 220:1–11. doi: 10.1016/j.ahj.2019.10.005
34. Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: Systematic review and meta-analysis. ESC Heart Fail (2020) 7(6):3298–309. doi: 10.1002/ehf2.13169
35. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet (2020) 396(10254):819–29. doi: 10.1016/S0140-6736(20)31824-9
36. Tayaa S, Berrut G, de Decker L, Chevalet P. Direct oral anticoagulants in non-valvular atrial fibrillation in elderly: For a treatment adapted to patient profile. Geriatr Psychol Neuropsychiatr Vieil (2018) 16(3):229–40. doi: 10.1684/pnv.2018.0746
37. Goudis CA, Korantzopoulos P, Ntalas IV, Kallergis EM, Liu T, Ketikoglou DG. Diabetes mellitus and atrial fibrillation: Pathophysiological mechanisms and potential upstream therapies. Int J Cardiol (2015) 184:617–22. doi: 10.1016/j.ijcard.2015.03.052
38. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ Res (2018) 122(4):624–38. doi: 10.1161/CIRCRESAHA.117.311586
39. Nikolajevic Starcevic J, Janic M, Sabovic M. Molecular mechanisms responsible for diastolic dysfunction in diabetes mellitus patients. Int J Mol Sci (2019) 20(5). doi: 10.3390/ijms20051197
40. Plitt A, McGuire DK, Giugliano RP. Atrial fibrillation, type 2 diabetes, and non-vitamin K antagonist oral anticoagulants: A review. JAMA (2017) 2(4):442–8. doi: 10.1001/jamacardio.2016.5224
41. Costard-Jäckle A, Tschöpe D, Meinertz T. Cardiovascular outcome in type 2 diabetes and atrial fibrillation. Herz (2019) 44(6):522–5. doi: 10.1007/s00059-018-4704-4
Keywords: diabetes mellitus, atrial fibrillation, all-cause mortality, cardiovascular mortality, meta-analysis
Citation: Xu J, Sun Y, Gong D and Fan Y (2022) Impact of preexisting diabetes mellitus on cardiovascular and all-cause mortality in patients with atrial fibrillation: A meta-analysis. Front. Endocrinol. 13:921159. doi: 10.3389/fendo.2022.921159
Received: 15 April 2022; Accepted: 01 July 2022;
Published: 01 August 2022.
Edited by:
Marco Matteo Ciccone, University of Bari Aldo Moro, ItalyReviewed by:
Daniele Pastori, Sapienza University of Rome, ItalyCopyright © 2022 Xu, Sun, Gong and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Fan, anN6amZhbnl1QDE2My5jb20=; Dandan Gong, Z29uZ2RhbmRhbnpoakAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.