- 1Reproductive Medicine, Department of Obstetrics and Gynecology, Baruch-Padeh Medical Center, Poriya, Israel
- 2Azrieili Faculty of Medicine in Galilee, Safed, Bar-Ilan University, Safed, Israel
Introduction
Endometriosis is a worldwide, widespread, chronic, and inflammatory gynecological disease that affects 1 in 10 women during their reproductive years. Up to 50% of women with endometriosis have infertility. However, the possible pathophysiologic mechanisms leading to endometriosis-associated infertility remain poorly comprehended, explicitly in cases where no apparent mechanical factor is involved. Reduced fecundity in patients with endometriosis has been related to a wide variety of reasons acting at the level of the pelvic cavity, ovaries, and the uterus (1, 2). In addition to pelvic adhesions, other mechanisms were suggested, including pelvic inflammation, increased oxidative stress, dysregulation of the immune system, disturbed folliculogenesis, ovulatory dysfunction, and defective implantation.
Ovarian endometriosis, specifically endometriotic cyst or endometrioma, is a distinctive variation of the disease identified in up to 44% of affected women (3). It represents the most pathognomonic and diagnosed form due to ultrasound technology advancement (4). Due to its anatomical locus, ovarian endometriosis may negatively impact folliculogenesis and oocyte competence, possibly more than the disease’s superficial or deep infiltrating forms. Therefore, endometrioma per se may impair oocyte quality and lead to deficient fertilization, sub-optimal embryo development, and flawed implantation. Furthermore, studying separately the impact of different clinical forms of endometriosis may reveal new insights and pave the way for understanding the composite mechanisms leading to low fecundity and endometriosis-associated infertility.
Although in-vitro and basic science studies have suggested mechanisms that may explain endometriosis-associated oocyte quality and embryo development impairment, the evidence is inconclusive (5, 6). In the clinical setting, comparable rates of blastocyst aneuploidy were reported in a large series of women with endometriosis equivalent to their age-matched peers of IVF controls, implying that spindle apparatus alterations and oocyte meiotic errors are not implicated in endometriosis-associated infertility (7). Therefore, other oocyte-related pathophysiological mechanisms should be explored.
Assisted reproductive technologies (ART) is an impeccable and convenient clinical setting where inquiries into oocyte quality and development in endometriosis cases and its association to pregnancy achievement could be investigated in well-planned and targeted studies. The following perspective examines contemporary clinical evidence available to evaluate oocyte quality in patients with endometriosis undergoing ART treatment. Studies that assess ovarian endometriosis on oocyte competence will be emphasized. Numerous aspects of oocyte quality or impacted by oocyte competence will be explored, including morphology, embryology, morphokinetics, survival following vitrification, clinical pregnancy, and live birth rates.
To properly achieve this objective, a search of the published English literature was done on Pubmed.com from January 2011 to December 2021, with the keywords ‘endometriosis’, ‘endometrioma’, ‘ovarian endometriosis’, ‘IVF’, ‘ICSI’, ‘oocyte morphology’, ‘oocyte maturation’, ‘fertilization’, ‘top quality embryo’, blastulation’, ‘time-lapse’, ‘morphokinetics’, ‘oocyte vitrification’, ‘clinical pregnancy, ‘live birth’ and meta-analysis. The relevance of attained publications was evaluated following reading the abstract. A manual search of review articles and cross-references completed the search. Articles with an unsuitable design were excluded.
Oocyte Morphology
Few studies in the ART setting recently targeted oocyte morphology in women with endometriosis with contrasting results (8–10). All three published studies had a retrospective and controlled design (Table 1). None targeted women exclusively with ovarian endometriosis, although some cases had a verified endometrioma (9, 10). Of interest and most comprehensive is the study of Robin et al, published recently, that targeted oocyte morphology as the primary outcome measure on large oocyte cohorts. Furthermore, it comprised a thorough oocyte morphology evaluation to detect a wide range of abnormalities relying on validated scores. The researchers investigated 175 women with endometriosis and 401 controls and evaluated 2016 and 4073 oocytes, respectively (10). Among women with endometriosis, 48% had an endometrioma. In this study, the average oocyte quality index and metaphase II oocyte morphological scoring system was not different, refuting the negative impact of endometriosis on oocyte morphology in the ART setting.
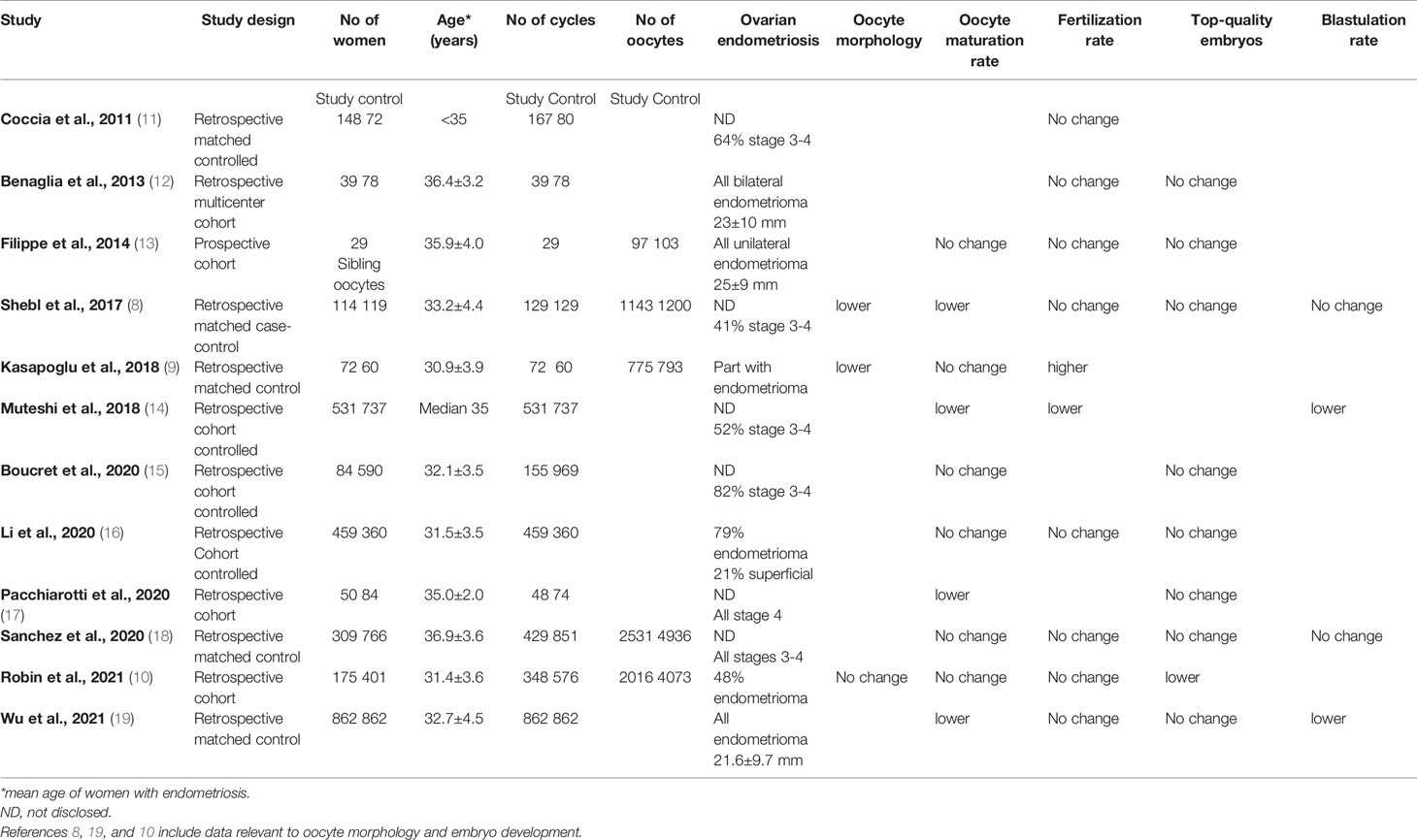
Table 1 Oocyte quality and embryo development in infertile women with endometriosis as compared to controls.
Embryo Development
Searching the literature for contemporary studies that targeted embryo development in women with endometriosis undergoing IVF treatment revealed 12 such studies published in the last decade (8–19). All but one study (13) were retrospective in design and were controlled. However, their results concerning oocyte maturation, fertilization, top-embryo quality, and blastulation were contrasted (Table 1). This inconsistency may highlight the controversy in the clinical setting regarding the contribution of oocyte quality and competence to the pathophysiological mechanisms of endometriosis-associated infertility.
Among the 12 studies, three targeted only women with ovarian endometrioma (12, 13, 19). Filippe et al. in a prospective study including 29 women with an intact endometrioma of 25 ± 9 mm, studying sibling oocytes, did not find an adverse impact on oocyte maturation, fertilization, and top embryo quality rates (13). Similarly, Benaglia et al, in a retrospective study of 39 women with intact bilateral endometrioma of a mean diameter of 23 ± 10 mm, did not find an adverse impact on oocyte quality, fertilization rate, or top embryo rate compared to controls (12).
Conversely, a recent retrospective study that evaluated infertile women with an endometrioma of 22 ± 10 mm matched to controls, each comprising 826 cases, showed lower maturation and blastulation rates, though similar fertilization and top embryo quality rates in the study group as compared to controls (19). Of notice, women with an endometrioma included were before (n=293) or after (n=569) endometriotic cystectomy. Women following ovarian surgery showed better oocyte maturation, fertilization, and top-quality embryo rates. Nevertheless, multivariate logistic regression analysis showed no correlation between endometrioma per se and live birth after adjusting for the number of top-quality embryos transferred and embryo transfer stage.
Another study by Li et al. retrospectively designed deserves special attention since a unique comparison was made between ovarian (n=363) and superficial (n=96) endometriosis on embryo development (16). Although comparable maturation, fertilization, cleavage, and high-quality embryo rates were found between endometriosis patients (n=459) and controls (n=360), these same parameters were superior in the ovarian compared to the superficial endometriosis cases, suggesting that the peritoneal form of the disease is responsible for the oocyte quality impairment (16).
Collectively, most data on oocyte fertilization and development in cases with ovarian endometriosis come from retrospective controlled studies that may contain several confounders, such as the size and laterality of endometrioma and ovarian surgery, which preclude definite conclusions. Available evidence from one prospective and a few retrospective studies suggest that oocyte quality is not impaired in cases with ovarian endometriosis. Furthermore, low-level evidence suggests that oocyte quality impairment may be attributed to superficial endometriosis. Prospective targeted studies are to be performed to corroborate these findings.
Time-Lapse Morphokinetics
Time-lapse morphokinetic analysis may predict embryo development, implantation, and live birth in the ART setting, minimizing environmental influences (20–22), rendering this technology potentially a surrogate measure of oocyte quality. However, to the best of our knowledge, only five low-scale reports (23–27), four retrospective, and one prospective (n=20-126) have been published so far, examining morphokinetics in women with endometriosis compared to controls and reaching contrasting results (Table 2). While some found morphokinetic delay in embryos developing from endometriosis women than controls (24, 25, 27), suggesting a poorer oocyte quality, others did not (23, 26). Of interest, one prospective observational study that targeted ovarian endometriosis examined sibling oocytes in twenty women with an endometrioma (23). Sixty-nine retrieved from an ovary with an intact endometrioma compared to 59 embryos from the contralateral normal ovary showed no significant change in morphokinetic parameters.
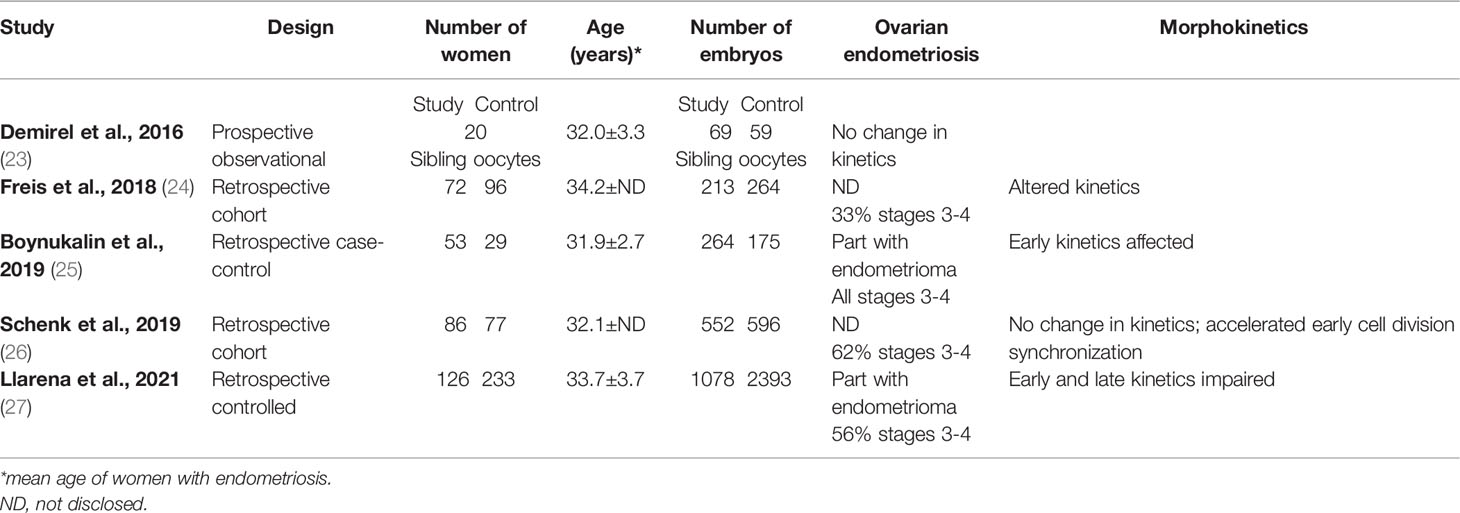
Table 2 Summary of clinical studies evaluating embryos by time-lapse morphokinetics in endometriosis patients as compared to controls.
Altogether, few studies in the ART setting were published examining the impact of endometriosis on embryo development morphokinetics, including a few cases and reaching contrasting results. In addition, only one small-scale prospective study examined exclusively ovarian endometriosis with no impact on embryo kinetics and presumably oocyte quality. Therefore, further targeted studies should be conducted to examine oocyte quality in this model.
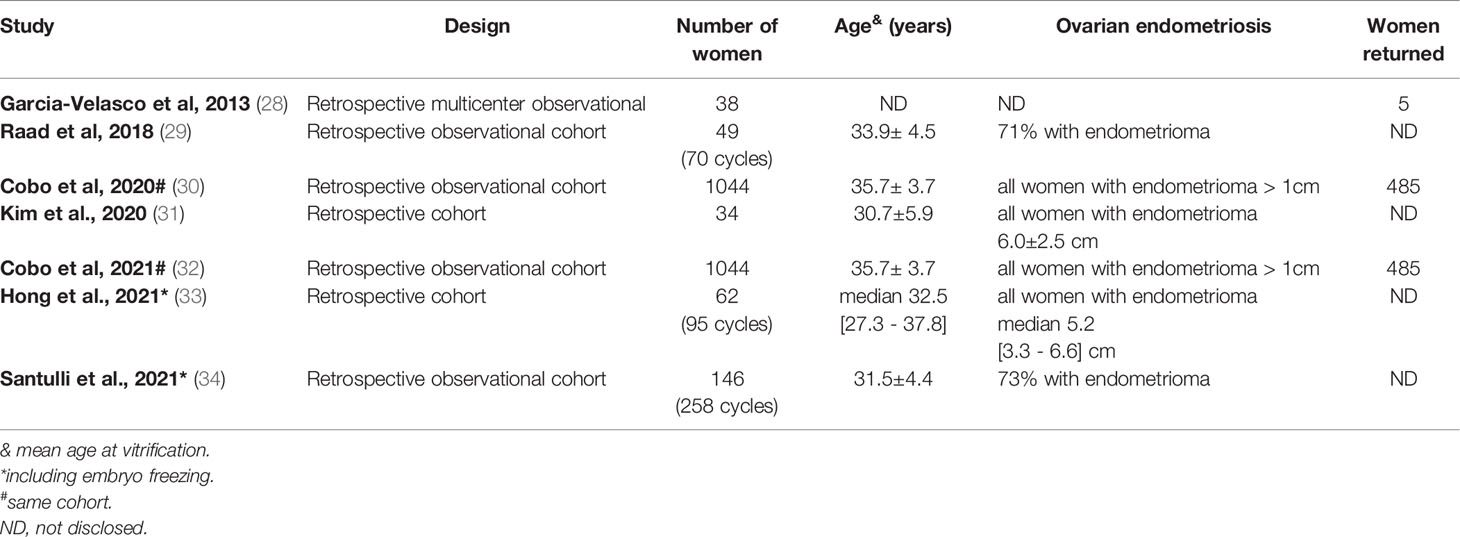
Oocyte Survival Following Vitrification
Few cohort studies on oocyte vitrification in women with endometriosis have been published (Table 3), all retrospectively conducted (28–34). It seems that the most comprehensive comes from two centers of the IVI group in Spain (30, 32), especially due to the relatively high number of cases (n=1044) and high rate of women coming back (43%) to thaw their gametes. All women in this study had established ovarian endometriosis with an endometrioma > 1 cm in diameter. Other inclusion criteria were age < 42 years, AMH > 0.5 ng/mL, and AFC > 3.
Oocyte survival following vitrification and worming could be considered a surrogate measure of oocyte quality. Elective oocyte preservation for age-related fertility decline (social freezing) supports this notion. The survival rate of vitrified oocytes is significantly higher in women ≤35 (n=123) as compared to women >35 years of age (n=518), corresponding to 91.4% and 82.1%, respectively (35). However, in the same ART setting employing historical controls, women ≤35 years of age with endometriosis (n=260) had a significantly lower oocyte survival rate as compared to the same age groups of oocyte donors (n=15,899) or social freezing (n=123), corresponding to 85.1%, 92.3%, and 91.4%, respectively (30, 35).
Collectively, available evidence comes mainly from one complex of ART centers summarizing their clinical practice retrospectively. Within the vitrification model, oocyte quality in patients with endometriosis, all with ovarian endometriosis, seems slightly impaired, especially in women below 35 years. Further data from additional centers are required to substantiate these findings.
Clinical Pregnancy and Live Birth Rates
Clinical pregnancy and live birth rates are complex and multifactorial events that are not considered direct oocyte quality and competence markers. Nevertheless, they are impacted by and interrelated to oocyte quality, fertilization, and development. Upraised clinical pregnancy and live births may be regarded as the eventual goals of high-quality oocytes in the ART setting.
Several systematic reviews and meta-analyses were published in the last decade examining the impact of ovarian endometriosis, specifically endometriotic cyst, on clinical pregnancy and live-birth rates (36–42). Generally, two methodologies were implemented in these studies (Table 4). The first compares women with an intact endometrioma to women with a normal ovary or no endometrioma (36–38). The second compares women with an intact endometrioma to women following endometriotic cystectomy (36, 39–41). Both methodologies in the ART setting showed comparable clinical pregnancy and live birth rates in all published systematic reviews and meta-analyses. These results imply that oocyte quality from an ovary with an intact endometrioma is similar to that retrieved from a normal ovary (or an ovary with no endometrioma). Furthermore, endometriotic cystectomy does not seem to improve oocyte quality compared to oocytes retrieved from an ovary with an intact endometrioma. Nonetheless, it is essential to note that most eligible reports pooled into these published systematic reviews and meta-analyses originated from retrospectively designed studies dipping their level of evidence.
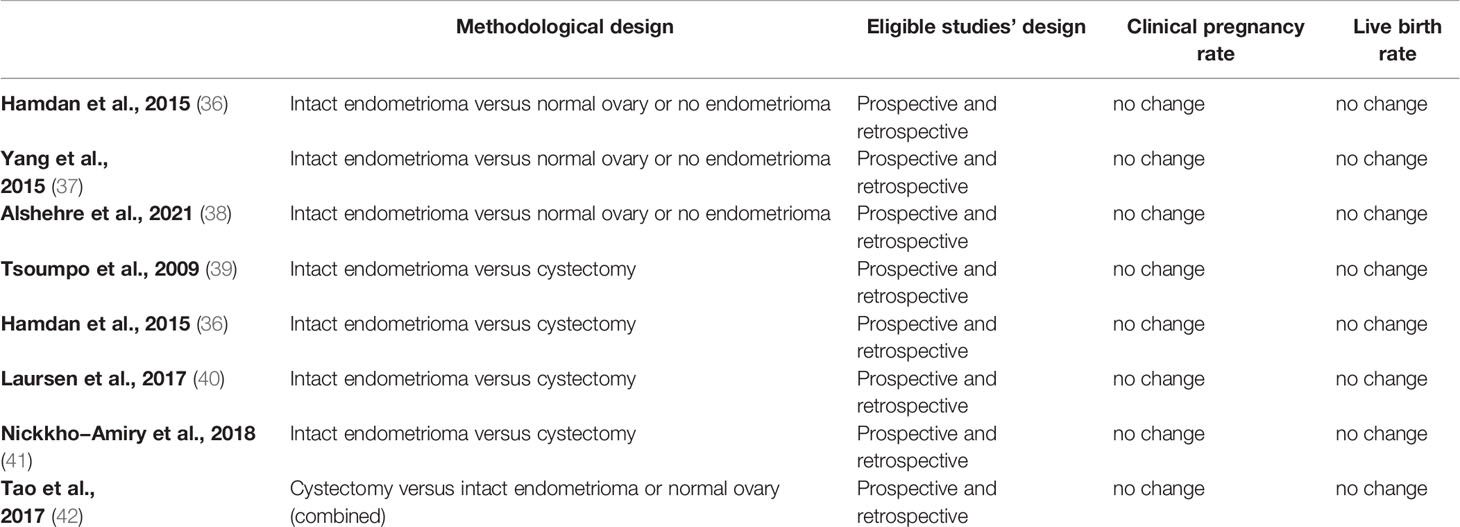
Table 4 Summary of meta-analyses examining the impact of an intact endometrioma and endometriotic cystectomy on clinical pregnancy and live birth rates.
Taken together, clinical pregnancy and live birth rates do not seem to be compromised in cases with an intact endometrioma nor improved following endometriotic cystectomy, suggesting that oocyte quality in ovarian endometriosis cases is not impaired within the ART setting. However, since most evidence originates from retrospective studies, further prospective targeted studies need to increase the level of evidence.
Discussion
Most available studies on oocyte quality target endometriosis as a single distinct disorder with no differentiation between ovarian endometriosis and other forms of the disease. While it is challenging to target exclusively ovarian endometriosis, there is no conclusive evidence within the clinical setting to show that endometrioma per se impairs oocyte quality. Available current literature in cases with an intact endometrioma does not establish an adverse impact on oocyte morphology, embryo development, and morphokinetics. Although there is some data for slight oocyte quality impairment in the vitrification worming model, available evidence relies on retrospective historical controls. Furthermore, the latest systematic reviews and meta-analyses do not provide evidence to support the notion that endometrioma may harm oocyte quality since neither an intact endometrioma (36–38) nor endometriotic cystectomy (36, 39–41) impact clinical pregnancy rate and live birth rates in the ART setting.
Although in vivo studies and basic research has suggested mechanisms for oocyte quality impairment in women with ovarian endometriosis, most studies in the clinical ART setting, reviewed in this second look appraisal, do not substantiate these findings. One possible explanation might be that the in-vivo reduced fecundity in patients with endometriosis results from the toxic pelvic cavity encountered in these cases. The ART setting may neutralize this adverse influence (43, 44). It is also possible that other forms of the disease, probably superficial endometriosis, could contribute more to the toxic pelvic environment leading to natural fertility dysfunction and infertility.
Since most outcomes encountered and presented in this opinion paper rely on retrospective studies, including available systematic reviews, prospective, targeted, and well-controlled studies are essential to substantiate these findings and increase the level of evidence. Furthermore, while some studies included women following ovarian surgery, others excluded them, which should also be accounted for. Future studies, preclinical and clinical, should target different forms of the disease, especially cases with ovarian and superficial endometriosis and their direct impact on oocyte quality and competence. This strategy may allow a more comprehensiveness of the disease’s pathophysiology, which may have broader implications for targeted treatment.
Author Contributions
The author contributed to the conception and design of the manuscript, acquisition of data, analyses, and interpretation, drafted the article, and approved it.
Conflict of Interest
The authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Ziegler D, Borghese B, Chapron C. Endometriosis and Infertility: Pathophysiology and Management. Lancet (2010) 376:730–8. doi: 10.1016/S0140-6736(10)60490-4
2. Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking Mechanisms, Diagnosis and Management of Endometriosis. Nat Rev Endocrinol (2019) 15:666–82. doi: 10.1038/s41574-019-0245-z
3. Busacca M, Vignali M. Ovarian Endometriosis: From Pathogenesis to Surgical Treatment. Curr Opin Obstet Gynecol (2003) 15:321–6. doi: 10.1097/01.gco.0000084247.09900.4f
4. Van Holsbeke C, Van Calster B, Guerriero S, Savelli L, Paladini D, Lissoni AA, et al. Endometriomas: Their Ultrasound Characteristics. Ultrasound Obstet Gynecol (2010) 35:730–40. doi: 10.1002/uog.7668
5. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The Distinguishing Cellular and Molecular Features of the Endometriotic Ovarian Cyst: From Pathophysiology to the Potential Endometrioma-Mediated Damage to the Ovary. Hum Reprod Update (2014) 20:217–30. doi: 10.1093/humupd/dmt053
6. Simopoulou M, Rapani A, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, et al. Getting to Know Endometriosis-Related Infertility Better: A Review on How Endometriosis Affects Oocyte Quality and Embryo Development. Biomedicines (2021) 9:273. doi: 10.3390/biomedicines9030273
7. Juneau C, Kraus E, Werner M, Franasiak J, Morin S, Patounakis G, et al. Patients With Endometriosis Have Aneuploidy Rates Equivalent to Their Age-Matched Peers in the In Vitro Fertilization Population. Fertil Steril (2017) 108:284–8. doi: 10.1016/j.fertnstert.2017.05.038
8. Shebl O, Sifferlinger I, Habelsberger A, Oppelt P, Mayer RB, Petek E, et al. Oocyte Competence in In Vitro Fertilization and Intracytoplasmic Sperm Injection Patients Suffering From Endometriosis and its Possible Association With Subsequent Treatment Outcome: A Matched Case-Control Study. Acta Obstet Gynecol Scand (2017) 96:736–44. doi: 10.1111/aogs.12941
9. Kasapoglu I, Kuspinar G, Saribal S, Turk P, Avcı B, Uncu G. Detrimental Effects of Endometriosis on Oocyte Morphology in Intracytoplasmic Sperm Injection Cycles: A Retrospective Cohort Study. Gynecol Endocrinol (2018) 34:206–11. doi: 10.1080/09513590.2017.1391203
10. Robin C, Uk A, Decanter C, Behal H, Collinet P, Rubod C, et al. Impact of Endometriosis on Oocyte Morphology in IVF-ICSI: Retrospective Study of a Cohort of More Than 6000 Mature Oocytes. Reprod Biol Endocrinol (2021) 19:160. doi: 10.1186/s12958-021-00798-x
11. Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Impact of Endometriosis on In Vitro Fertilization and Embryo Transfer Cycles in Young Women: A Stage-Dependent Interference. Acta Obstet Gynecol Scand (2011) 90:1232–8. doi: 10.1111/j.1600-0412.2011.01247.x
12. Benaglia L, Bermejo A, Somigliana E, Faulisi S, Ragni G, Fedele L, et al. In Vitro Fertilization Outcome in Women With Unoperated Bilateral Endometriomas. Fertil Steril (2013) 99:1714–9. doi: 10.1016/j.fertnstert.2013.01.110
13. Filippi F, Benaglia L, Paffoni A, Restelli L, Vercellini P, Somigliana E, et al. Ovarian Endometriomas and Oocyte Quality: Insights From In Vitro Fertilization Cycles. Fertil Steril (2014) 101:988–93. doi: 10.1016/j.fertnstert.2014.01.008
14. Muteshi CM, Ohuma EO, Child T, Becker CM. The Effect of Endometriosis on Live Birth Rate and Other Reproductive Outcomes in ART Cycles: A Cohort Study. Hum Reprod Open (2018) 2018:hoy016. doi: 10.1093/hropen/hoy016
15. Boucret L, Bouet PE, Riou J, Legendre G, Delbos L, Hachem HE, et al. Endometriosis Lowers the Cumulative Live Birth Rates in IVF by Decreasing the Number of Embryos But Not Their Quality. J Clin Med (2020) 9:2478. doi: 10.3390/jcm9082478
16. Li A, Zhang J, Kuang Y, Yu C. Analysis of IVF/ICSI-FET Outcomes in Women With Advanced Endometriosis: Influence on Ovarian Response and Oocyte Competence. Front Endocrinol (Lausanne) (2020) 11:427. doi: 10.3389/fendo.2020.00427
17. Pacchiarotti A, Iaconianni P, Caporali S, Vitillo M, Meledandri M, Monaco G, et al. Severe Endometriosis: Low Value of AMH Did Not Affect Oocyte Quality and Pregnancy Outcome in IVF Patients. Eur Rev Med Pharmacol Sci (2020) 24:11488–95. doi: 10.26355/eurrev_202011_23790
18. Sanchez AM, Pagliardini L, Cermisoni GC, Privitera L, Makieva S, Alteri A, et al. Does Endometriosis Influence the Embryo Quality and/or Development? Insights From a Large Retrospective Matched Cohort Study. Diagn (Basel) (2020) 10:83. doi: 10.3390/diagnostics10020083
19. Wu Y, Yang R, Lan J, Lin H, Jiao X, Zhang Q. Ovarian Endometrioma Negatively Impacts Oocyte Quality and Quantity But Not Pregnancy Outcomes in Women Undergoing IVF/ICSI Treatment: A Retrospective Cohort Study. Front Endocrinol (Lausanne) (2021) 12:739228. doi: 10.3389/fendo.2021.739228
20. Meseguer M, Herrero J, Tejera A, Hilligsoe KM, Ramsing NB, Remohi J. The Use of Morphokinetics as a Predictor of Embryo Implantation. Hum Reprod (2011) 26:2658–71. doi: 10.1093/humrep/der256
21. Cruz M, Garrido N, Herrero J, Perez-Cano I, Munoz M, Meseguer M. Timing of Cell Division in Human Cleavage-Stage Embryos is Linked With Blastocyst Formation and Quality. Reprod BioMed Online (2012) 25:371–81. doi: 10.1016/j.rbmo.2012.06.017
22. Barberet J, Bruno C, Valot E, Antunes-Nunes C, Jonval L, Chammas J, et al. Can Novel Early non-Invasive Biomarkers of Embryo Quality be Identified With Time-Lapse Imaging to Predict Live Birth? Hum Reprod (2019) 34:1439–49. doi: 10.1093/humrep/dez085
23. Demirel C, Bastu E, Aydogdu S, Donmez E, Benli H, Tuysuz G, et al. The Presence of Endometrioma Does Not Impair Time-Lapse Morphokinetic Parameters and Quality of Embryos: A Study On Sibling Oocytes. Reprod Sci (2016) 23:1053–7. doi: 10.1177/1933719116630426
24. Freis A, Dietrich JE, Binder M, Holschbach V, Strowitzki T, Germeyer A. Relative Morphokinetics Assessed by Time-Lapse Imaging Are Altered in Embryos From Patients With Endometriosis. Reprod Sci (2018) 25:1279–85. doi: 10.1177/1933719117741373
25. Boynukalin FK, Serdarogullari M, Gultomruk M, Coban O, Findikli N, Bahceci M. The Impact of Endometriosis on Early Embryo Morphokinetics: A Case-Control Study. Syst Biol Reprod Med (2019) 65:250–7. doi: 10.1080/19396368.2019.1573275
26. Schenk M, Kröpfl JM, Hörmann-Kröpfl M, Weiss G. Endometriosis Accelerates Synchronization of Early Embryo Cell Divisions But Does Not Change Morphokinetic Dynamics in Endometriosis Patients. PLoS One (2019) 14:e0220529. doi: 10.1371/journal.pone.0220529
27. Llarena NC, Hur CE, Yao M, Schwartz K, Falcone T, Desai N. The Impact of Endometriosis on Embryo Morphokinetics: Embryos From Endometriosis Patients Exhibit Delayed Cell Cycle Milestones and Decreased Blastulation Rates. J Assist Reprod Genet (2022) 39:619–28. doi: 10.1007/s10815-022-02406-2
28. Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five Years' Experience Using Oocyte Vitrification to Preserve Fertility for Medical and Nonmedical Indications. Fertil Steril (2013) 99:1994–9. doi: 10.1016/j.fertnstert.2013.02.004
29. Raad J, Sonigo C, Tran C, Sifer C, Durnerin IC, Grynberg M. Oocyte Vitrification for Preserving Fertility in Patients With Endometriosis: First Observational Cohort Study and Many Unresolved Questions. Letter to the Editor. Eur J Obstet Gynecol Reprod Biol (2018) 220:140–1. doi: 10.1016/j.ejogrb.2017.12.001
30. Cobo A, Giles J, Paolelli S, Pellicer A, Remohí J, García-Velasco JA. Oocyte Vitrification for Fertility Preservation in Women With Endometriosis: An Observational Study. Fertil Steril (2020) 113:836–44. doi: 10.1016/j.fertnstert.2019.11.017
31. Kim SJ, Kim SK, Lee JR, Suh CS, Kim SH. Oocyte Cryopreservation for Fertility Preservation in Women With Ovarian Endometriosis. Reprod BioMed Online (2020) 40:827–34. doi: 10.1016/j.rbmo.2020.01.028
32. Cobo A, Coello A, de Los Santos MJ, Giles J, Pellicer A, Remohí J, et al. Number Needed to Freeze: Cumulative Live Birth Rate After Fertility Preservation in Women With Endometriosis. Reprod BioMed Online (2021) 42:725–32. doi: 10.1016/j.rbmo.2020.12.013
33. Hong YH, Lee HK, Kim SK, Lee JR, Suh CS. The Significance of Planned Fertility Preservation for Women With Endometrioma Before an Expected Ovarian Cystectomy. Front Endocrinol (Lausanne) (2021) 12:794117. doi: 10.3389/fendo.2021.794117
34. Santulli P, Bourdon M, Koutchinsky S, Maignien C, Marcellin L, Maitrot-Mantelet L, et al. Fertility Preservation for Patients Affected by Endometriosis Should Ideally Be Carried Out Before Surgery. Reprod BioMed Online (2021) 43:853–63. doi: 10.1016/j.rbmo.2021.08.023
35. Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and Onco-Fertility Preservation: Factors Related to IVF Outcomes. Hum Reprod (2018) 33:2222–31. doi: 10.1093/humrep/dey321
36. Hamdan M, Dunselman G, Li TC, Cheong Y. The Impact of Endometrioma on IVF/ICSI Outcomes: A Systematic Review and Meta-Analysis. Hum Reprod Update (2015) 21:809–25. doi: 10.1093/humupd/dmv035
37. Yang C, Geng Y, Li Y, Chen C, Gao Y. Impact of Ovarian Endometrioma on Ovarian Responsiveness and IVF: A Systematic Review and Meta-Analysis. Reprod BioMed Online (2015) 31:9–19. doi: 10.1016/j.rbmo.2015.03.005
38. Alshehre SM, Narice BF, Fenwick MA, Metwally M. The Impact of Endometrioma on In Vitro Fertilisation/Intra-Cytoplasmic Injection IVF/ICSI Reproductive Outcomes: A Systematic Review and Meta-Analysis. Arch Gynecol Obstet (2021) 303:3–16. doi: 10.1007/s00404-020-05796-9
39. Tsoumpou I, Kyrgiou M, Gelbaya TA, Nardo LG. The Effect of Surgical Treatment for Endometrioma on In Vitro Fertilization Outcomes: A Systematic Review and Meta-Analysis. Fertil Steril (2009) 92:75–87. doi: 10.1016/j.fertnstert.2008.05.049
40. Laursen JB, Schroll JB, Macklon KT, Rudnicki M. Surgery Versus Conservative Management of Endometriomas in Subfertile Women. A Systematic Review. Acta Obstet Gynecol Scand (2017) 96:727–35. doi: 10.1111/aogs.13154
41. Nickkho-Amiry M, Savant R, Majumder K, Edi-O'sagie E, Akhtar M. The Effect of Surgical Management of Endometrioma on the IVF/ICSI Outcomes When Compared With No Treatment? A Systematic Review and Meta-Analysis. Arch Gynecol Obstet (2018) 297:1043–57. doi: 10.1007/s00404-017-4640-1
42. Tao X, Chen L, Ge S, Cai L. Weigh the Pros and Cons to Ovarian Reserve Before Stripping Ovarian Endometriomas Prior to IVF/ICSI: A Meta-Analysis. PLoS One (2017) 12:e0177426. doi: 10.1371/journal.pone.0177426
43. Younis JS, Laufer N. Peritoneal Fluid in the Pouch of Douglas: Strategically Located and Affecting Reproductive Events. Fertil Steril (2015) 104:831–2. doi: 10.1016/j.fertnstert.2015.08.010
Keywords: endometriosis, endometrioma, oocyte quality, oocyte competence, morphokinetics, vitrification
Citation: Younis JS (2022) Is Oocyte Quality Impaired in Cases With Ovarian Endometriosis? A Second Look Into the Clinical Setting. Front. Endocrinol. 13:921032. doi: 10.3389/fendo.2022.921032
Received: 15 April 2022; Accepted: 06 June 2022;
Published: 30 June 2022.
Edited by:
Gedis Grudzinskas, Self-employed, London, United KingdomReviewed by:
Takashi Kajitani, Sakura no Seibo Junior College, JapanCopyright © 2022 Younis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johnny S. Younis, anlvdW5pc0Bwb3JpYS5oZWFsdGguZ292Lmls
 Johnny S. Younis
Johnny S. Younis