- 1Central Laboratory, Clinical Medical College and Affiliated Hospital of Chengdu University, Chengdu, China
- 2Department of Cardiology, Clinical Medical College and Affiliated Hospital of Chengdu University, Chengdu, China
- 3Clinical Medical College of Chengdu University, Chengdu, China
Background: The relationships between the rs1801282 and rs3856806 polymorphisms in nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) gene and obesity indexes as well as serum lipid levels have been extensively investigated in various studies, but the results were inconsistent and even contradictory.
Methods: PubMed, Google Scholar, Embase, Cochrane Library, Web of Science, Wanfang, CNKI and VIP databases were searched for eligible studies. The random-effTPDEects model was used, and standardized mean difference (SMD) with 95% confidence interval (CI) was calculated to estimate the differences in obesity indexes and serum lipid levels between the subjects with different genotypes in a dominant model. Heterogeneity among studies was assessed by Cochran’s x2-based Q-statistic test. Publication bias was identified by using Begg’s test.
Results: One hundred and twenty studies (70,317 subjects) and 33 studies (18,353 subjects) were identified in the analyses for the rs1801282 and rs3856806 polymorphisms, respectively. The G allele carriers of the rs1801282 polymorphism had higher levels of body mass index (SMD = 0.08 kg/m2, 95% CI = 0.04 to 0.12 kg/m2, p < 0.001), waist circumference (SMD = 0.12 cm, 95% CI = 0.06 to 0.18 cm, p < 0.001) and total cholesterol (SMD = 0.07 mmol/L, 95% CI = 0.02 to 0.11 mmol/L, p < 0.01) than the CC homozygotes. The T allele carriers of the rs3856806 polymorphism had lower levels of low-density lipoprotein cholesterol (SMD = -0.09 mmol/L, 95% CI = -0.15 to -0.03 mmol/L, p < 0.01) and higher levels of high-density lipoprotein cholesterol (SMD = 0.06 mmol/L, 95% CI = 0.02 to 0.10 mmol/L, p < 0.01) than the CC homozygotes.
Conclusions: The meta-analysis suggests that the G allele of the rs1801282 polymorphism confers an increased risk of obesity and hypercholesterolemia, while the T allele of the rs3856806 polymorphism displays a protective role against dyslipidemia, which can partly explain the associations between these polymorphisms and cardiovascular disease.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier [CRD42022319347].
Introduction
Peroxisome proliferator activated receptors (PPARs), belonging to the nuclear receptor superfamily, are ligand-inducible transcription factors (1). PPARs have three members in human beings: PPARα, PPARβ/δ and PPARγ. Of them, PPARγ is the most important one and plays an intricate role in various biological processes (2). Eight PPARγ isoforms (PPARγ1, PPARγ2, PPARγ3, etc.) have been identified in human beings according to NCBI’s reference sequence database (http://www.ncbi.nlm.nih.gov/). Upon activation by exogenous and endogenous lipid ligands, PPARγ binds to retinoid X receptor (RXR) to form a regulatory complex and is capable of stimulating adipogenesis (3), promoting adipocyte differentiation (4), and increasing insulin sensitivity (5). PPARγ is closely related to lipid disorders and obesity based on its fundamental role in lipid and glucose metabolism.
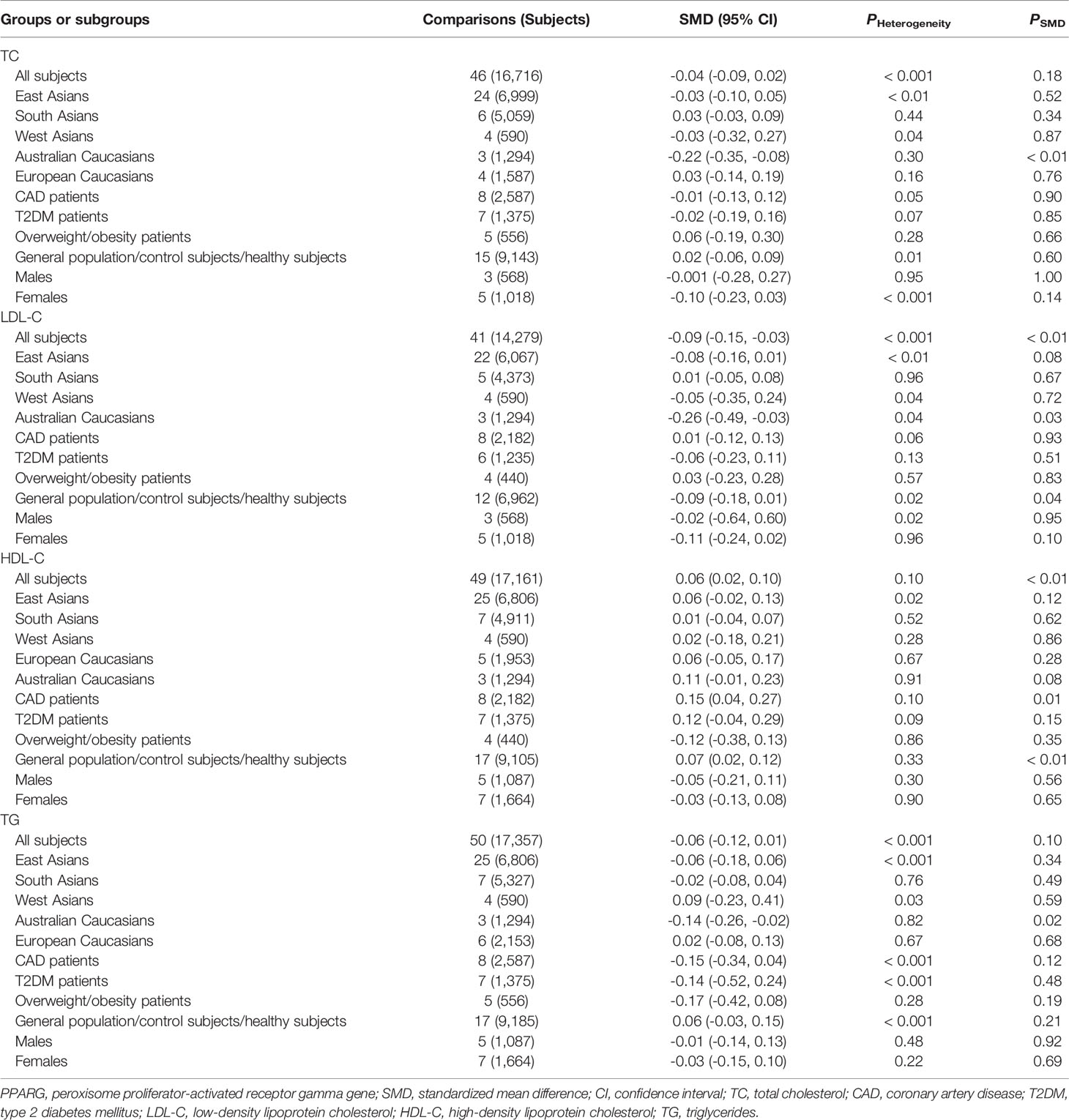
Human PPARγ gene (namely PPARG) is located on chromosome 3p25.3 and consists of nine exons: exons A1, A2, B, and 1-6 (Figure 1) (2). According to NCBI’s RefSeq database, sixteen PPARG mRNA variants have been identified so far in human beings due to alternative splicing and differential promoter usage. PPARγ gene is highly polymorphic, and thousands of genetic variants have been recorded in NCBI’s dbSNP database. Among these variants, a missense variant (rs1801282, also known as p.Pro12Ala) located in exon B has been extensively explored with regard to its significant relationships with obesity indexes and serum lipid levels (Figure 1) (2). The rs1801282 polymorphism is formed by a single-nucleotide variance from cytosine (C) to guanine (G), resulting in a proline-to-alanine substitution in PPARγ2 polypeptide. Another genetic locus, the rs3856806 polymorphism (also known as p.His477His, c.161C>T or c.1431C>T), has also been investigated widely, although not as much as the rs1801282 polymorphism. The rs3856806 polymorphism is a synonymous variant and is located in exon 6 of PPARG (Figure 1). This genetic variation is formed by a single-nucleotide variance from C to thymine (T), but the corresponding amino acid residue in PPARγ2 polypeptide does not change after nucleotide substitution.
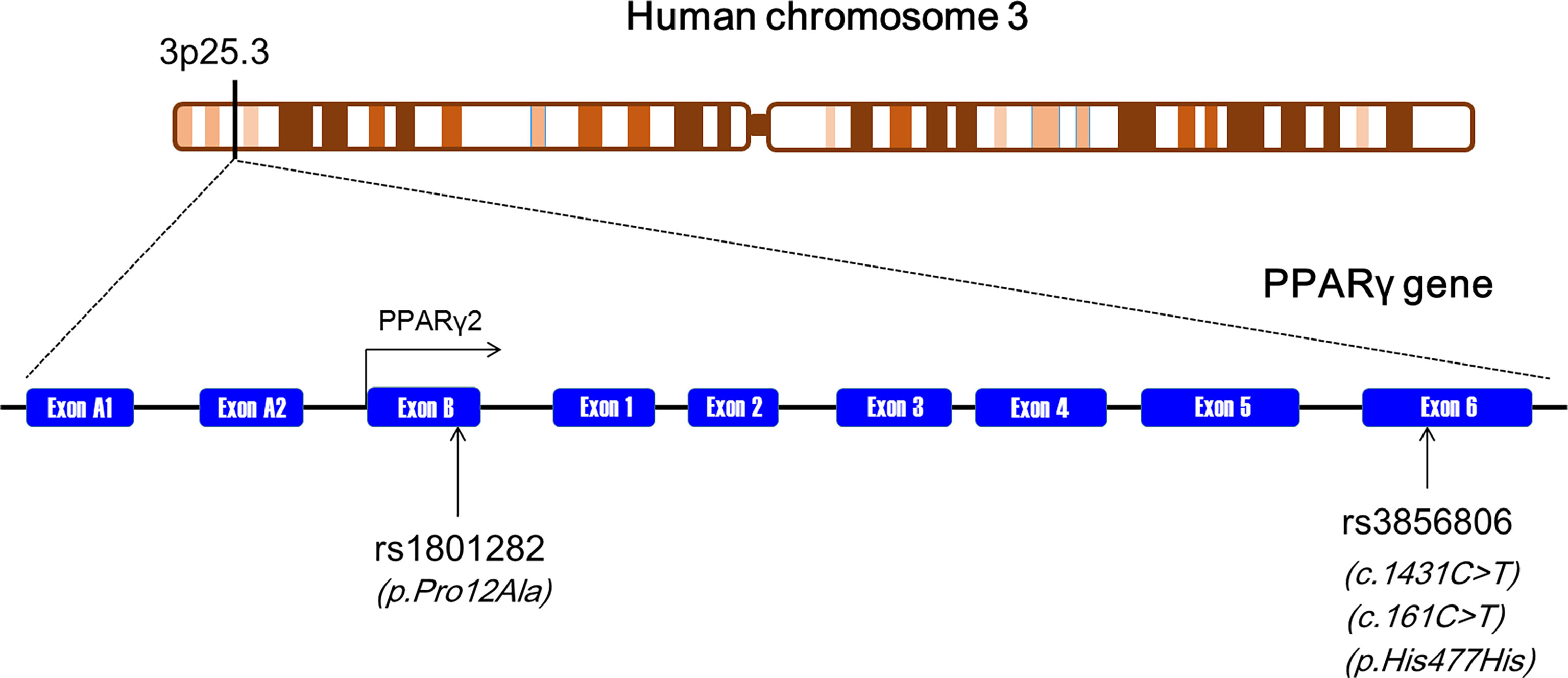
Figure 1 The genomic landscape of the rs1801282 and rs3856806 polymorphisms in PPARγ gene. PPARγ, peroxisome proliferator-activated receptor gamma.
Scientific reports of the associations between the rs1801282 and rs3856806 polymorphisms and obesity indexes as well as serum lipid levels were inconsistent and even conflicting (2). Some studies indicated that the G allele of the rs1801282 polymorphism was associated with higher levels of body mass index (BMI) (6–17), waist circumference (WC) (17–20), waist-to-hip ratio (WHR) (14–18), total cholesterol (TC) (21–27), low-density lipoprotein cholesterol (LDL-C) (24–29) and triglycerides (TG) (30–38), and lower levels of high-density lipoprotein cholesterol (HDL-C) (38–41), whereas the research data from other laboratories did not support these findings and even yielded contradictory results (42–61). There were also significant inconsistencies amongst published data in the relationships between the rs3856806 polymorphism and obesity indexes as well as serum lipid levels in various populations (62–71).
Herein, a systematic review and meta-analysis was performed based on previous publications over the past two decades to determine the relationships between the rs1801282 and rs3856806 polymorphisms and obesity indexes as well as serum lipid levels. This work can provide an opportunity to unveil the interrelationships among PPARγ gene polymorphisms, metabolic disorders and cardiovascular disease.
Methods
Literature Search Strategy
The present meta-analysis was registered in PROSPERO (registration number CRD42022319347) and conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement. PubMed, Google Scholar, Embase, Cochrane Library, Web of Science, Wanfang, CNKI and VIP databases were searched comprehensively from inception to December 2021. The keywords used for the literature searches were (“peroxisome proliferator-activated receptor gamma” or “PPARγ” or “PPARG”), (“polymorphism” or “mutation” or “variant” or “variance” or “rs1801282” or “rs3856806” or “Pro12Ala” or “1431C>T” or “161C>T” or “His477His”) and (“body mass index” or “waist circumference” or “waist-to-hip ratio” or “BMI” or “WC” or “WHR”) and (“lipid” or “total cholesterol” or “low-density lipoprotein cholesterol” or “high-density lipoprotein cholesterol” or “triglyceride” or “TC” or “LDL-C” or “HDL-C” or “TG”). The variables of this meta-analysis were limited to three obesity indexes including BMI, WC and WHR, and four serum lipid parameters including TC, LDL-C, HDL-C and TG. All articles that reported the associations of the rs1801282 and rs3856806 polymorphisms with obesity indexes and serum lipid levels were reviewed and screened.
Inclusion and Exclusion Criteria
Inclusion criteria: 1) The sample size and genotype distribution were clearly provided; 2) At least one of the seven variables (i.e., BMI, WC, WHR, TG, TC, LDL-C, and HDL-C) was presented; 3) Data were displayed as mean ± standard deviation (SD) or mean ± standard error (SE). Exclusion criteria: 1) Animal studies; 2) Incomplete data; 3) Repeatedly published articles; 4) Case reports; 5) Conference abstracts.
Data Extraction
Data were extracted independently by three reviewers. The data from each included study were as follows: first author’s name, year of publication, ethnicity, age, gender, health status, sample size, mean obesity indexes, mean lipid variables, and the SD or SE values by genotypes. SD values were calculated if SE values were given. Unit used for lipid variables was “mmol/L” in this meta-analysis, and datum conversion was conducted if data were presented as “mg/dL” or other units. All data were double-checked after extraction. Any disagreements were resolved by careful examination and group discussion.
Meta-Analysis
The STATA software package (Version 10, StataCorp, USA) was used for the present meta-analysis. A dominant model was employed because most of the included studies reported results in a dominant way (i.e., CC vs [CG + GG] for the rs1801282 polymorphism; CC vs [CT + TT] for the rs3856806 polymorphism). If there were more than one subgroup in a study (e.g., the subgroups with different ethnicities or health conditions), each subgroup was treated as an independent comparison in the meta-analysis. The subgroup analyses were performed with at least 5 comparisons for the rs1801282 polymorphism, and 3 comparisons for the rs3856806 polymorphism to ensure adequate statistical power. Standardized mean difference (SMD) and 95% confidence interval (CI) were used to assess the differences in obesity indexes and serum lipid levels between the genotypes. The random-effects model was used in the meta-analysis for the reason that it provides a more conservative result than the fixed effects model. Heterogeneity among the included studies was assessed by Cochran’s x2-based Q-statistic test. Heterogeneity was considered statistically significant if p ≤ 0.05. Furthermore, subgroup analyses and Galbraith plots were applied to detect the potential sources of heterogeneity. Subgroup analyses were conducted according to ethnicities, health conditions, genders and ages of the subjects. The subgroups classified by ethnicity included European Caucasians, American Caucasians, Australian Caucasians, East Asians, South Asians, West Asians, South Americans, and Africans. The subgroups classified by health condition included coronary artery disease (CAD), type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS), polycystic ovarian syndrome (PCOS), overweight/obesity, and general population/controls/healthy subjects; The subgroups classified by gender were males and females; The subgroups classified by age were adults (≥ 18 years) and children/adolescents (< 18 years). Publication bias was evaluated by using Begg’s test and visualized by Begg’s funnel plots, and p ≤ 0.05 the indicates the presence of a publication bias in the meta-analysis. The trim-and-fill method was used to adjust the results if a publication bias was present. All p values were two-tailed.
Results
Characteristics of the Enrolled Studies
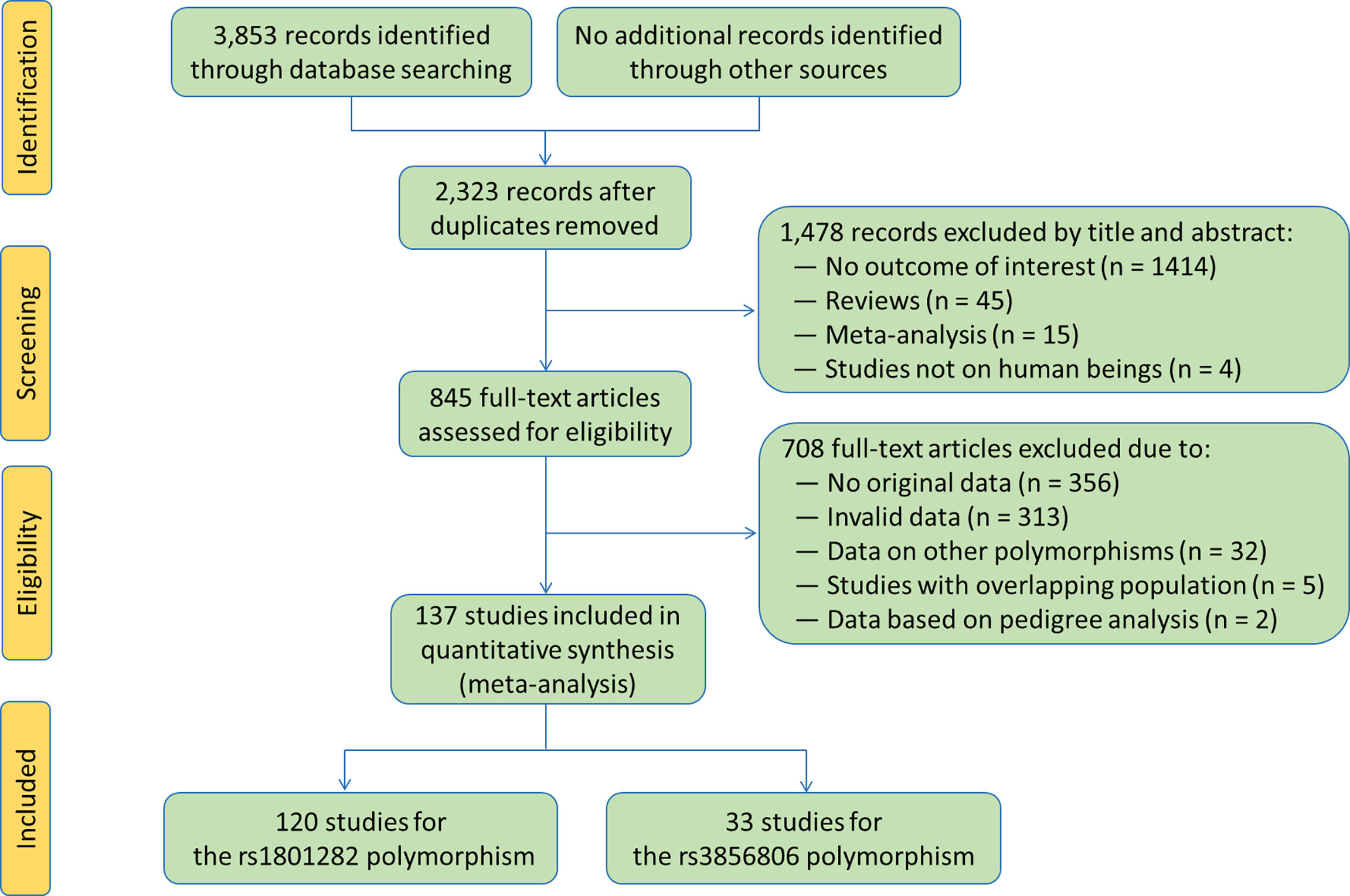
The flow diagram of the literature search process is shown in Figure 2. A total of 137 studies (6–142) were identified and included in this meta-analysis. Characteristics of the included studies are presented in Tables S1 and S2. The enrolled articles were published between 1998 and 2021, and written either in English (132 articles, 96.35%) or in Chinese (5 articles, 3.65%). Forty-eight studies, 5 studies, 2 studies, 44 studies, 9 studies, 8 studies, 7 studies, 7 studies and 7 studies involved European Caucasians, American Caucasians, Australian Caucasians, East Asians, South Asians, West Asians, South Americans, Africans and other ethnicities, respectively. Eleven studies, 29 studies, 4 studies, 10 studies, 23 studies and 77 studies involved CAD patients, T2DM patients, MetS patients, PCOS patients, overweight/obesity patients and general population/control subjects/healthy subjects, respectively. Six studies only involved males, 20 studies only involved females, and the rest studies involved both genders. One hundred and twenty-five studies involved adults, and the rest 12 studies involved children or adolescents. The subjects from 68 studies were divided into subgroups according to health conditions, genders or ethnicities, and each subgroup was considered as an independent comparison.
One hundred and twenty studies were enrolled in the meta-analysis for the rs1801282 polymorphism. Among them, 100 studies, 44 studies, 40 studies, 104 studies, 83 studies, 103 studies and 104 studies presented the data for BMI, WC, WHR, TC, LDL-C, HDL-C and TG, respectively (Tables S3 and S4). Thirty-three studies were enrolled in the meta-analysis for the rs3856806 polymorphism, and 27 studies, 10 studies, 9 studies, 28 studies, 24 studies, 30 studies and 30 studies presented the data for BMI, WC, WHR, TC, LDL-C, HDL-C and TG, respectively (Tables S5 and S6).
Summary Statistics
One hundred and seventy-six comparisons (70,137 subjects) and 53 comparisons (18,353 subjects) were distinguished for the rs1801282 and rs3856806 polymorphisms, respectively. One hundred and fifty comparisons, 168 comparisons, 54 comparisons, 142 comparisons, 117 comparisons, 146 comparisons and 151 comparisons were enrolled to compare the differences in BMI, WC, WHR, TC, LDL-C, HDL-C and TG levels for the rs1801282 polymorphism, respectively (Tables S3 and S4). Forty-five comparisons, 16 comparisons, 14 comparisons, 46 comparisons, 41 comparisons, 49 comparisons and 50 comparisons were enrolled to compare the differences in BMI, WC, WHR, TC, LDL-C, HDL-C and TG levels, respectively, for the rs3856806 polymorphism (Tables S5 and S6). For the rs1801282 polymorphism, 83.32% of the subjects had CC genotype (58,438 subjects), and 16.94% of the subjects had CG or GG genotype (11,879 subjects). Regarding the rs3856806 polymorphism, 66.06% of the subjects had CC genotype (12,124 subjects), and 33.94% of the subjects had CT or TT genotype (6,229 subjects).
Associations of the PPARG rs1801282 Polymorphism With Obesity Indexes and Serum Lipid Levels
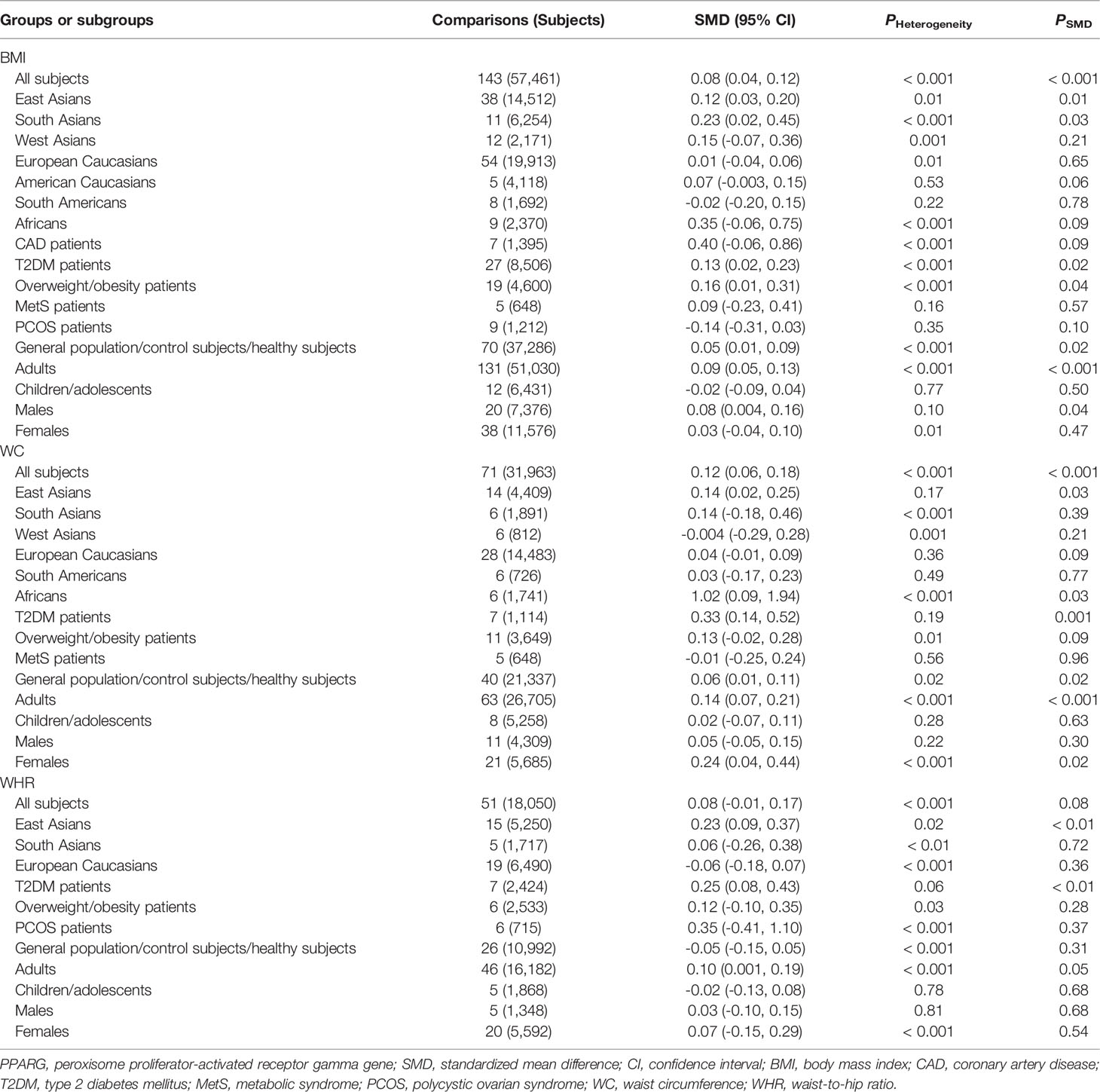
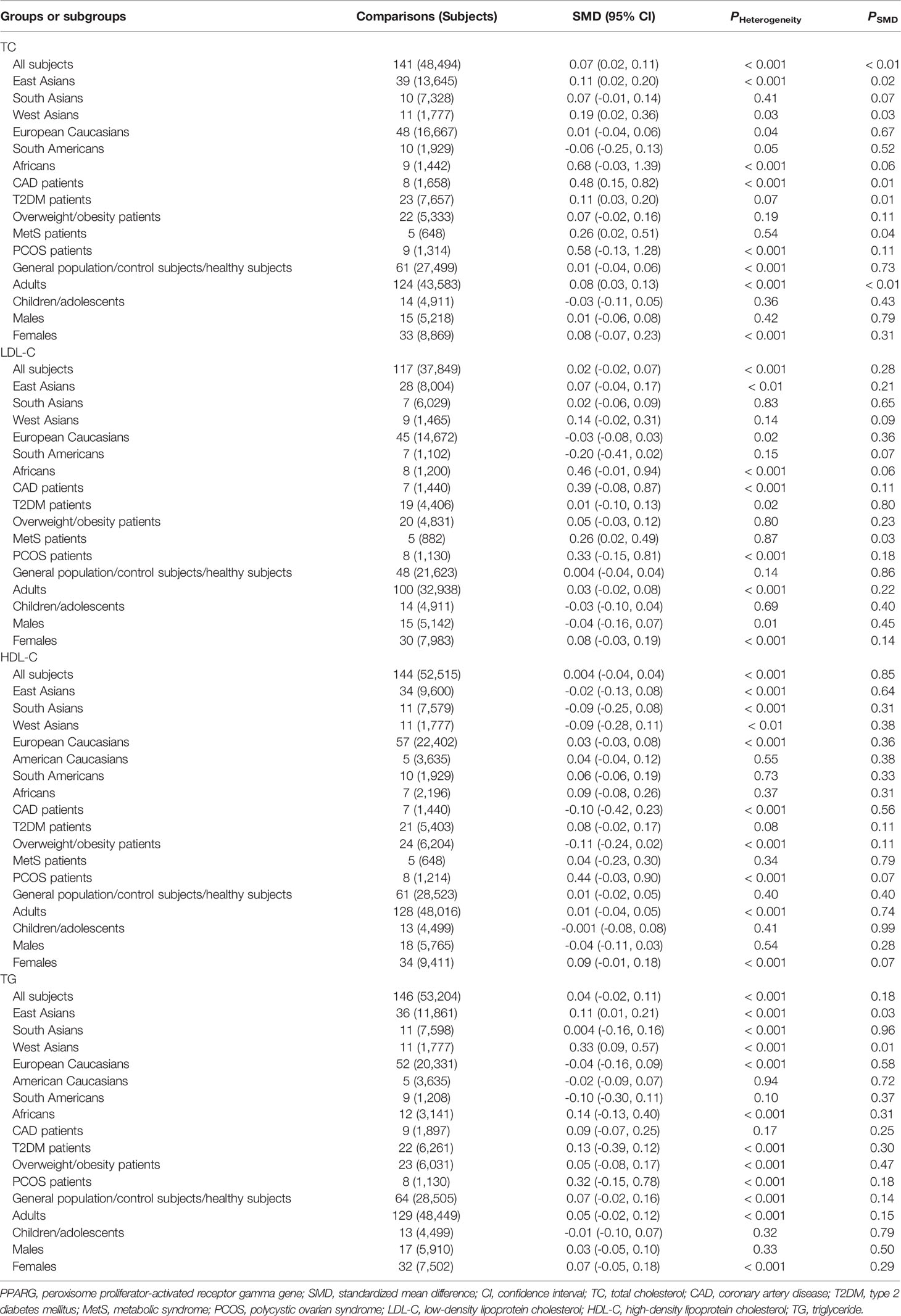
The associations between the rs1801282 polymorphism and obesity indexes are shown in Table 1. The pooled analyses in the whole population showed that the G allele carriers had significantly higher levels of BMI (SMD = 0.08 kg/m2, 95% CI = 0.04 to 0.12 kg/m2, p < 0.001) and WC (SMD = 0.12 cm, 95% CI = 0.06 to 0.18 cm, p < 0.001) than the CC homozygotes. The G allele carriers also had marginally insignificantly higher levels of WHR (SMD = 0.08, 95% CI = -0.01 to 0.17, p = 0.08) than the CC homozygotes. The associations between the rs1801282 polymorphism and serum lipid levels are shown in Table 2. The pooled analyses in the whole population showed that the G allele carriers had significantly higher levels of TC (SMD = 0.07 mmol/L, 95% CI = 0.02 to 0.11 mmol/L, p < 0.01) than the CC homozygotes. There were no significant differences in LDL-C, HDL-C or TG levels between the subjects with different genotypes of the rs1801282 polymorphism (Table 2).
Subgroup analyses were conducted according to ethnicities, health conditions, ages and genders of the subjects. In East Asians, the G allele carriers had higher levels of BMI (SMD = 0.12 kg/m2, 95% CI = 0.03 to 0.20 kg/m2, p = 0.01), WC (SMD = 0.14 cm, 95% CI = 0.02 to 0.25 cm, p = 0.03), WHR (SMD = 0.23, 95% CI = 0.09 to 0.37, p < 0.01), TC (SMD = 0.11 mmol/L, 95% CI = 0.02 to 0.20 mmol/L, p = 0.02) and TG (SMD = 0.11 mmol/L, 95% CI = 0.01 to 0.21 mmol/L, p = 0.03) than the CC homozygotes. In West Asians, the G allele carriers had higher levels of TC (SMD = 0.19 mmol/L, 95% CI = 0.02 to 0.36 mmol/L, p = 0.03) and TG (SMD = 0.33 mmol/L, 95% CI = 0.09 to 0.57 mmol/L, p = 0.01) than the CC homozygotes. The G allele carriers had higher levels of BMI (SMD = 0.23 kg/m2, 95% CI = 0.02 to 0.45 kg/m2, p = 0.03) and WC (SMD = 1.02 cm, 95% CI = 0.09 to 1.94 cm, p < 0.03) than non-carriers in South Asians and Africans, respectively. Notably, no significant associations between the rs1801282 polymorphism and obesity indexes or serum lipid levels were detected in European Caucasians and American Caucasians. In patients with T2DM, the G allele carriers had higher levels of BMI (SMD = 0.13 kg/m2, 95% CI = 0.02 to 0.23 kg/m2, p = 0.02), WC (SMD = 0.33 cm, 95% CI = 0.14 to 0.52 cm, p = 0.001), WHR (SMD = 0.25, 95% CI = 0.08 to 0.43, p < 0.01) and TC (SMD = 0.11 mmol/L, 95% CI = 0.03 to 0.20 mmol/L, p = 0.01) than the CC homozygotes. In patients with MetS, the G allele carriers had higher levels of TC (SMD = 0.26 mmol/L, 95% CI = 0.02 to 0.51 mmol/L, p = 0.04) and LDL-C (SMD = 0.26 mmol/L, 95% CI = 0.02 to 0.49 mmol/L, p = 0.03) than the CC homozygotes. The G allele carriers had higher levels of BMI (SMD = 0.16 kg/m2, 95% CI = 0.01 to 0.31 kg/m2, p = 0.04) and TC (SMD = 0.48 mmol/L, 95% CI = 0.15 to 0.82 mmol/L, p = 0.01) than non-carriers in overweight/obesity patients and CAD patients, respectively. In general population/control subjects/healthy subjects, the G allele carriers had higher levels of BMI (SMD = 0.05 kg/m2, 95% CI = 0.01 to 0.09 kg/m2, p = 0.02) and WC (SMD = 0.06 cm, 95% CI = 0.01 to 0.11 cm, p = 0.02) than the CC homozygotes.
Significant interactions between the rs1801282 polymorphism and age as well as gender on obesity indexes or serum lipid levels have been detected. The G allele carriers had higher levels of BMI (SMD = 0.09 kg/m2, 95% CI = 0.05 to 0.13 kg/m2, p < 0.001), WC (SMD = 0.14 cm, 95% CI = 0.07 to 0.21 cm, p < 0.001), WHR (SMD = 0.10, 95% CI = 0.001 to 0.19, p = 0.05) and TC (SMD = 0.08 mmol/L, 95% CI = 0.03 to 0.13 mmol/L, p < 0.01) than the CC homozygotes in adults, but not in children and adolescents. Higher levels of BMI (SMD = 0.08 kg/m2, 95% CI = 0.004 to 0.16 kg/m2, p = 0.04) in the G allele carriers than in the CC homozygotes were observed only in males, and higher levels of WC (SMD = 0.24 cm, 95% CI = 0.04 to 0.44 cm, p = 0.02) in the G allele carriers than in the CC homozygotes were present only in females.
Associations of the PPARG rs3856806 Polymorphism With Obesity Indexes and Serum Lipid Levels
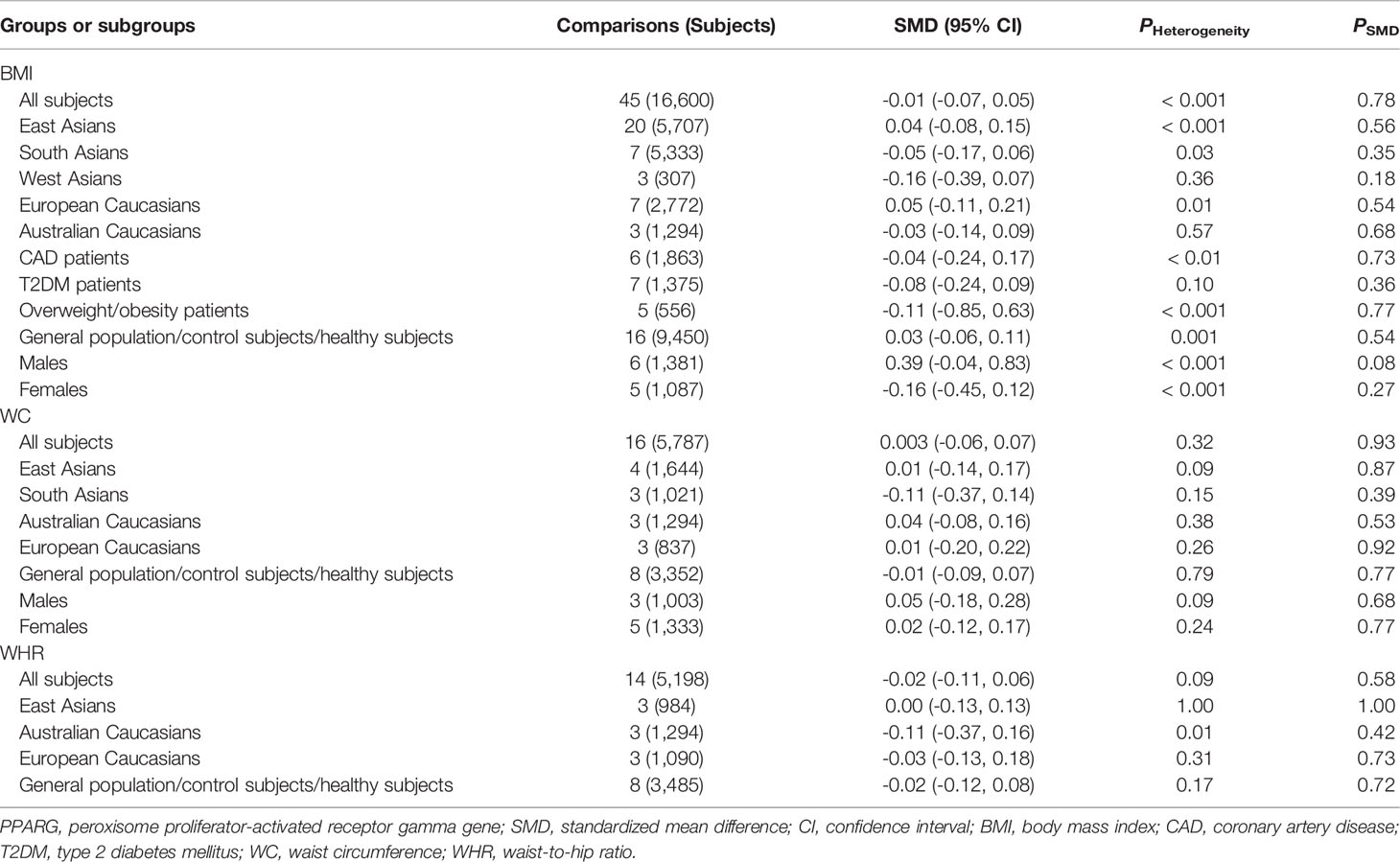
As shown in Table 3, no significant associations between the rs3856806 polymorphism and obesity indexes were found in the pooled analyses in the whole population or in the subgroups according to ethnicities, health conditions or genders of the subjects. The associations between the rs3856806 polymorphism and serum lipid levels are shown in Table 4. The pooled analyses in the whole population showed that the T allele carriers had lower levels of LDL-C (SMD = -0.09 mmol/L, 95% CI = -0.15 to -0.03 mmol/L, p < 0.01) and higher levels of HDL-C (SMD = 0.06 mmol/L, 95% CI = 0.02 to 0.10 mmol/L, p < 0.01) than the CC homozygotes. There were no significant differences in TC or TG levels between the subjects with different genotypes of the rs3856806 polymorphism (Table 4). Subgroup analyses were conducted according to ethnicities, health conditions and genders of the subjects. Reduced levels of TC (SMD = -0.22 mmol/L, 95% CI = -0.35 to -0.08 mmol/L, p < 0.01), LDL-C (SMD = -0.26 mmol/L, 95% CI = -0.49 to -0.03 mmol/L, p = 0.03) and TG (SMD = -0.14 mmol/L, 95% CI = -0.26 to -0.02 mmol/L, p = 0.02) in the T allele carriers than in the CC homozygotes were detected in Australian Caucasians, but not in European Caucasians, American Caucasians or other ethnicities. The T allele carriers had higher levels of HDL-C (SMD = 0.15 mmol/L, 95% CI = 0.04 to 0.27 mmol/L, p = 0.01) than the CC homozygotes in patients with CAD, but not in patients with other clinical symptoms or in general population/control subjects/healthy subjects.
Heterogeneity Analysis
Galbraith plots were employed to analyze the heterogeneity in the present meta-analysis. For the rs1801282 polymorphism, there was significant heterogeneity in the pooled analyses in the whole population for all three obesity indexes (Table 1) and four lipid variables (Table 2). Twelve comparisons, 7 comparisons, 6 comparisons, 6 comparisons, 6 comparisons, 11 comparisons and 18 comparisons were identified as the main contributors to the heterogeneity for the analyses of BMI, WC, WHR, TC, LDL-C, HDL-C and TG, respectively (Table S7). The heterogeneity was significantly decreased or removed after exclusion of the outlier comparisons, while the results of the pooled analyses in the whole population did not change significantly (BMI: SMD = 0.04 kg/m2, 95% CI = 0.02 to 0.07 kg/m2, PSMD < 0.01, PHeterogeneity = 0.30; WC: SMD = 0.06 cm, 95% CI = 0.02 to 0.09 cm, PSMD < 0.01, PHeterogeneity = 0.22; WHR: SMD = 0.03 kg/m2, 95% CI = -0.02 to 0.08 kg/m2, PSMD = 0.20, PHeterogeneity = 0.15; TC: SMD = 0.02 mmol/L, 95% CI = 0.01 to 0.04 mmol/L, PSMD = 0.02, PHeterogeneity = 0.44; LDL-C: SMD = 0.01 mmol/L, 95% CI = -0.02 to 0.03 mmol/L, PSMD = 0.66, PHeterogeneity = 0.64; HDL-C: SMD = 0.01 mmol/L, 95% CI = -0.01 to 0.04 mmol/L, PSMD = 0.36, PHeterogeneity = 0.84; TG: SMD = -0.02 mmol/L, 95% CI = -0.05 to 0.003 mmol/L, PSMD = 0.09, PHeterogeneity = 0.55).
Regarding the rs3856806 polymorphism, there was significant heterogeneity in the pooled analyses in the whole population for BMI, TC, LDL-C and TG (Tables 3 and 4). Five comparisons, 6 comparisons, 4 comparisons and 7 comparisons were identified as the main contributors to the heterogeneity in the association analyses in the whole population between the rs3856806 polymorphism and BMI, TC, LDL-C and TG, respectively (Table S8). The heterogeneity was significantly decreased or removed after exclusion of the outlier studies, and the pooled results in the whole population did not change significantly for BMI (SMD = -0.001 kg/m2, 95% CI = -0.04 to 0.04 kg/m2, PSMD = 0.96, PHeterogeneity = 0.18), TC (SMD = -0.02 mmol/L, 95% CI = -0.06 to 0.02 mmol/L, PSMD = 0.26, PHeterogeneity = 0.30), and LDL-C (SMD = -0.05 mmol/L, 95% CI = -0.09 to -0.01 mmol/L, PSMD < 0.01, PHeterogeneity = 0.46). However, the pooled results for TG became significant after exclusion of the outlier studies (SMD = -0.04 mmol/L, 95% CI = -0.08 to -0.003 mmol/L, PSMD = 0.04, PHeterogeneity = 0.23).
Publication Bias
Begg’s test was conducted to identify the publication bias in the present meta-analysis. No publication bias was found in the association analyses between the rs1801282 polymorphism and BMI (Z = 1.65, p = 0.10) (Figure S1), WHR (Z = 0.95, p = 0.34) (Figure S2), LDL-C (Z = 1.61, p = 0.11) (Figure S3), HDL-C, (Z = 1.60, p = 0.11) (Figure S4) or TG (Z = 1.06, p = 0.29) (Figure S5). Publication bias was observed in the association analyses between the rs1801282 polymorphism and WC (Z = 2.02, p = 0.04) (Figure S6) as well as TC (Z = 2.16, p = 0.03) (Figure S7). The trim-and-fill method was employed to adjust the publication bias, and the pooled results of both variables did not change after adjustment.
Publication bias was also evaluated for the association analyses between the rs3856806 polymorphism and obesity indexes as well as serum lipid variables, and no publication bias was detected for BMI (Z = 1.16, p = 0.24) (Figure S8), WC (Z = 0.23, p = 0.82) (Figure S9), WHR (Z = 1.31, p = 0.19) (Figure S10), TC (Z = 0.40, p = 0.69) (Figure S11), LDL-C (Z = 0.01, p = 0.99) (Figure S12), HDL-C (Z = 0.03, p = 0.98) (Figure S13) and TG (Z = 1.20, p = 0.23) (Figure S14).
Discussion
PPARγ plays an essential role in the regulation of lipid metabolism. Being activated by endogenous and exogenous lipid ligands, PPARγ exerts its function as a transcription factor and mainly up-regulates the transcription of enzymes or transporters that play key roles in lipid metabolic pathways such as reverse cholesterol transport (143, 144), cholesterol transformation (143, 144), lipogenesis (145, 146), and fatty acid oxidation (147, 148). Therefore, variations in PPARG may lead to abnormal expression of this gene and/or dysfunction of PPARγ, resulting in aberrant expressions of PPARγ-targeted genes. The relationships between the rs1801282 and rs3856806 polymorphisms and CAD have been clarified by several previous meta-analyses (149–151). Wu et al. (149) performed a meta-analysis enrolled 22 studies and 23,375 subjects, and found that the GG genotype of the rs1801282 polymorphism conferred a higher risk of CAD than the CC genotype (OR = 1.30, 95% CI = 1.01 to 1.68, p = 0.04). Qian et al. (150) did a meta-analysis enrolled 9 studies and 3,878 subjects, and the results suggested that the T allele carriers of the rs3856806 polymorphism had a lower CAD risk than the CC homozygotes (OR = 0.69; 95% CI = 0.59 to 0.82, p < 0.001). Gonzlez-Castro et al. (151) expanded the sample size to 21 studies and 15,980 subjects, and confirmed Qian’s finding that the T allele of the rs3856806 polymorphism was a protective allele against CAD (OR = 0.33, 95% CI = 0.20 to 0.52, p < 0.001).
The significant associations between the rs1801282 and rs3856806 polymorphisms and CAD prompted us to conduct the present meta-analysis to determine the relationships between these polymorphisms and obesity indexes as well as serum lipid levels since obesity and dyslipidemia are well-known risk factors for CAD (152–155). Indeed, this meta-analysis demonstrated that the G allele carriers of the rs1801282 polymorphism had significantly higher levels of BMI, WC and TC than the CC homozygotes; the T allele carriers of the rs3856806 polymorphism displayed lower levels of LDL-C, but higher levels of HDL-C than the CC homozygotes. These findings are in line with the previous meta-analyses which concluded that the G allele of the rs1801282 polymorphism was associated with an increased risk, while the T allele of the rs3856806 polymorphism was correlated with a reduced risk of CAD (149–151). To our knowledge, this is the first meta-analysis being done to date in the academic field to investigate the relationships of the rs1801282 and rs3856806 polymorphisms in PPARγ gene with obesity indexes, although there was a meta-analysis investigating the associations of the two polymorphisms with circulating lipid levels by Li and colleagues (156) in 2015. However, Li’s meta-analysis (156) mistakenly treated c.161C>T and c.1431C>T as two polymorphic loci. In fact, they are the same polymorphic locus with different names. c.161C>T was named according to the position of this variant in exon 6 of PPARγ gene since it is located at 161 bp downstream of the first nucleotide of exon 6 of PPARG (Figure 3A), and c.1431C>T was defined based on the position of this variant in PPARγ2 mRNA, as it is located at 1,431 bp downstream of the start genetic codon (Figure 3B). In addition, the present meta-analysis enrolled more studies (138 articles vs. 74 articles) and had larger sample size (78,652 vs.54,953), and thereby had a higher statistical power and more reliable results than Li’s meta-analysis (156).
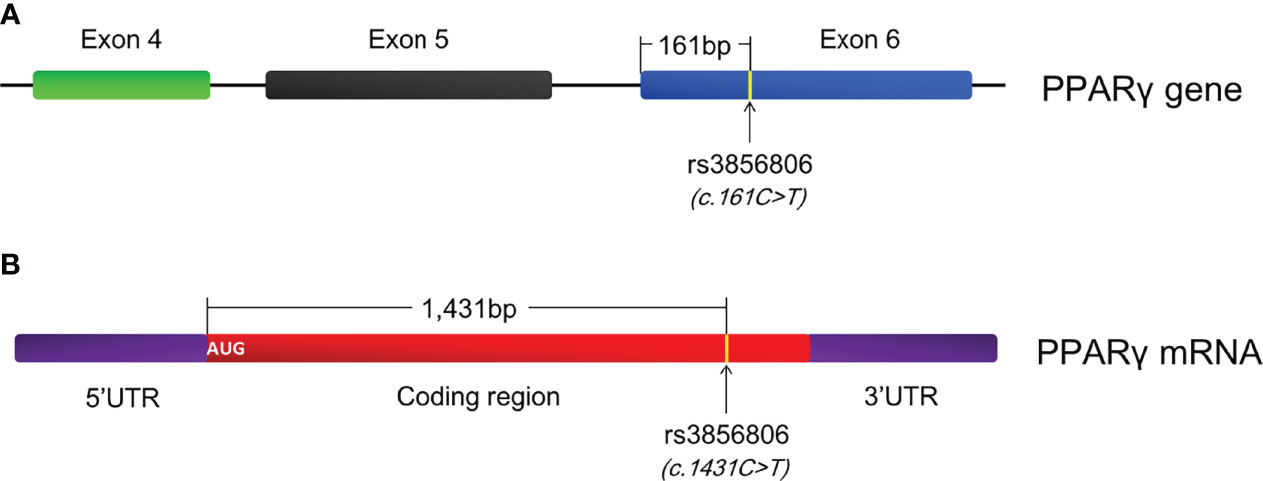
Figure 3 Annotation of the rs3856806 polymorphism in PPARγ gene. (A) c.161C>T is defined according to the position of this variant in exon 6 of PPARγ gene; (B) c.1431C>T is defined according to the position of this variant in PPARγ2 mRNA; PPARγ, peroxisome proliferator-activated receptor gamma.
In terms of the mechanisms underlying the associations between the rs1801282 and rs3856806 polymorphisms and obesity indexes as well as serum lipid levels, the first idea that comes to our mind is that the two polymorphisms lead to abnormal expression of PPARG and/or dysfunction of PPARγ, resulting in aberrant expressions of PPARγ-targeted genes. Indeed, Pihlajamäki et al. (157) examined the PPARG gene expression pattern of different genotypes of the rs1801282 polymorphism in human adipose tissues, and observed that the GG genotype was associated with a significantly higher mRNA expression level compared to the CC genotype. Other polymorphic loci in PPARγ gene have also been reported to modulate the gene expression of PPARG. The rs10865710 polymorphism (c.-681C>G) is located in the upstream promoter region of PPARγ3 gene and formed by a transversion from C to G. Lu et al. (158) observed that G allele of the rs10865710 polymorphism significantly reduced the DNA-binding activity of transcription factor CREB2 to PPARγ3 promoter. The rs948820149 polymorphism (c.-807A>C) is located in PPARγ2 promoter and C allele of this polymorphism was found to significantly down-regulate PPARγ2 expression by modulating the DNA-binding activity of transcription factor GRβ to PPARγ2 promoter (159). Another two promoter polymorphisms c.-1633C>T and c.-1572G>A in PPARG were also verified to regulate the expression efficiency of PPARG in Erhualian pigs (160). So far, there is no direct evidence that the PPARG polymorphisms affect the function of PPARγ.
Significant heterogeneity was detected in the association analyses between the rs1801282 polymorphism and obesity indexes as well as serum lipid levels. The outlier studies were identified by using Galbraith plots, and no significant changes in SMD values as well as their 95% CIs were found after excluding the outlier studies, which indicates that the associations between the rs1801282 polymorphism and the obesity indexes as well as serum lipid levels are robust. There are some limitations to the current study. First, this meta-analysis only enrolled the studies published in English and Chinese as it was difficult to get the full articles published in other languages. Second, the subgroup analyses were only conducted for ethnicities, health conditions, genders and ages of the subjects due to limitation on the amount of accessible data.
Conclusions
The G allele carriers of the PPARG rs1801282 polymorphism had higher levels of BMI, WC and TC than the CC homozygotes; the T allele carriers of the PPARG rs3856806 polymorphism had lower levels of LDL-C and higher levels of HDL-C than the CC homozygotes; the effects of the PPARG rs1801282 and rs3856806 polymorphisms on obesity indexes and/or serum lipid levels are modulated by ethnicities, health conditions, genders and ages of the subjects.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
YS, SL, and CH conceived of the systematic review and meta-analysis, participated in the design, and drafted the manuscript. HN, QP, RW, and ZZ carried out the literature searches and collected the data. YS and SL performed the statistical analyses. All authors reviewed and approved the final manuscript.
Funding
This project was supported by the Medical Science and Technology Project of Sichuan Provincial Health Commission [21PJ124], and the Scientific Research Project of Clinical Medical College and Affiliated Hospital of Chengdu University [Y2021010].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.919087/full#supplementary-material
References
1. Francque S, Szabo G, Abdelmalek MF, Byrne CD, Cusi K, Dufour JF, et al. Nonalcoholic Steatohepatitis: The Role of Peroxisome Proliferator-Activated Receptors. Nat Rev Gastroenterol Hepatol (2021) 18(1):24–39. doi: 10.1038/s41575-020-00366-5
2. Song YY, Li SJ, He C. Pparγ Gene Polymorphisms, Metabolic Disorders, and Coronary Artery Disease. Front Cardiovasc Med (2022) 9:808929. doi: 10.3389/fcvm.2022.808929
3. Fujimori K, Uno S, Kuroda K, Matsumoto C, Maehara T. Leukotriene C4 Synthase is a Novel Pparγ Target Gene, and Leukotriene C4 and D4 Activate Adipogenesis Through Cysteinyl LT1 Receptors in Adipocytes. Biochim Biophys Acta Mol Cell Res (2021) 1869(3):119203. doi: 10.1016/j.bbamcr.2021.119203
4. Lai F, Wang J, Tang H, Bian X, Lu K, He G, et al. Adipogenic Differentiation was Inhibited by Downregulation of Pparγ Signaling Pathway in Aging Tendon Stem/Progenitor Cells. J Orthop Surg Res (2021) 16(1):614. doi: 10.1186/s13018-021-02720-y
5. Liu J, Zhao H, Yang L, Wang X, Yang L, Xing Y, et al. The Role of CD36-Fabp4-Pparγ in Skeletal Muscle Involves Insulin Resistance in Intrauterine Growth Retardation Mice With Catch-Up Growth. BMC Endocr Disord (2022) 22(1):10. doi: 10.1186/s12902-021-00921-4
6. Tai ES, Corella D, Deurenberg-Yap M, Adiconis X, Chew SK, Tan CE, et al. Differential Effects of the C1431T and Pro12Ala PPARgamma Gene Variants on Plasma Lipids and Diabetes Risk in an Asian Population. J Lipid Res (2004) 45(4):674–85. doi: 10.1194/jlr.M300363-JLR200
7. Danawati CW, Nagata M, Moriyama H, Hara K, Yasuda H, Nakayama M, et al. A Possible Association of Pro12Ala Polymorphism in Peroxisome Proliferator-Activated Receptor Gamma2 Gene With Obesity in Native Javanese in Indonesia. Diabetes Metab Res Rev (2005) 21(5):465–9. doi: 10.1002/dmrr.543
8. Mattevi VS, Zembrzuski VM, Hutz MH. Effects of a PPARG Gene Variant on Obesity Characteristics in Brazil. Braz J Med Biol Res (2007) 40(7):927–32. doi: 10.1590/S0100-879X2006005000114
9. Morini E, Tassi V, Capponi D, Ludovico O, Dallapiccola B, Trischitta V, et al. Interaction Between PPARgamma2 Variants and Gender on the Modulation of Body Weight. Obes (Silver Spring) (2008) 16(6):1467–70. doi: 10.1038/oby.2008.225
10. Yaffe K, Kanaya AM, Lindquist K, Hsueh WC, Cummings SR, Beamer B, et al. Health ABC Study. PPAR-Gamma Pro12Ala Genotype and Risk of Cognitive Decline in Elders. Neurobiol Aging (2008) 29(1):78–83. doi: 10.1016/j.neurobiolaging.2006.09.010
11. Ben Ali S, Ben Yahia F, Sediri Y, Kallel A, Ftouhi B, Feki M, et al. Gender-Specific Effect of Pro12Ala Polymorphism in Peroxisome Proliferator-Activated Receptor Gamma-2 Gene on Obesity Risk and Leptin Levels in a Tunisian Population. Clin Biochem (2009) 42(16-17):1642–7. doi: 10.1016/j.clinbiochem.2009.08.019
12. Passaro A, Dalla Nora E, Marcello C, Di Vece F, Morieri ML, Sanz JM, et al. Pparγ Pro12Ala and ACE ID Polymorphisms Are Associated With BMI and Fat Distribution, But Not Metabolic Syndrome. Cardiovasc Diabetol (2011) 10:112. doi: 10.1186/1475-2840-10-112
13. Hsiao TJ, Lin E. The Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor Gamma (PPARG) Gene in Relation to Obesity and Metabolic Phenotypes in a Taiwanese Population. Endocrine (2015) 48(3):786–93. doi: 10.1007/s12020-014-0407-7
14. Kim KS, Choi SM, Shin SU, Yang HS, Yoon Y. Effects of Peroxisome Proliferator-Activated Receptor-Gamma 2 Pro12Ala Polymorphism on Body Fat Distribution in Female Korean Subjects. Metabolism (2004) 53(12):1538–43. doi: 10.1016/j.metabol.2004.06.019
15. Ramírez-Salazar M, Pérez-Luque E, Fajardo-Araujo M, Garza SM, Malacara JM. Effect of the Pro12Ala Polymorphism of the PPAR Gamma 2 Gene on Response to Pioglitazone Treatment in Menopausal Women. Menopause (2008) 15(6):1151–6. doi: 10.1097/gme.0b013e31816d5b2d
16. Fan L, Wu S, Yan Y. Association of PPAR Gamma Pro12Ala Polymorphism With Coronary Heart Disease. Clin Med China (2010) 26(10):1043–6. doi: 10.3760/CMA.J.ISSN.1008-6315.2010.10.013
17. Bhagat N, Agrawal M, Luthra K, Vikram NK, Misra A, Gupta R. Evaluation of Single Nucleotide Polymorphisms of Pro12Ala in Peroxisome Proliferator-Activated Receptor-γ and Gly308Ala in Tumor Necrosis Factor-α Genes in Obese Asian Indians: A Population-Based Study. Diabetes Metab Syndr Obes (2010) 3:349–56. doi: 10.2147/DMSO.S13514
18. Zaki M, Hassan N, El-Bassyouni HT, Kamal S, Basha W, Azmy O, et al. Association of the Pro12Ala Polymorphism With the Metabolic Parameters in Women With Polycystic Ovary Syndrome. Open Access Maced J Med Sci (2017) 5(3):275–80. doi: 10.3889/oamjms.2017.088
19. Franks PW, Jablonski KA, Delahanty L, Hanson RL, Kahn SE, Altshuler D, et al. The Pro12Ala Variant at the Peroxisome Proliferator-Activated Receptor Gamma Gene and Change in Obesity-Related Traits in the Diabetes Prevention Program. Diabetologia (2007) 50(12):2451–60. doi: 10.1007/s00125-007-0826-6
20. Franck N, Länne T, Astrand O, Engvall J, Lindström T, Ostgren CJ, et al. Cardiovascular Risk Factors Related to the Pparγ Pro12Ala Polymorphism in Patients With Type 2 Diabetes are Gender Dependent. Blood Press (2012) 21(2):122–7. doi: 10.3109/08037051.2011.623349
21. Li L, Cheng LX, Nsenga R, He MA, Wu TC. Association Between Pro12Ala Polymorphism of Peroxisome Proliferator-Activated Receptor-Gamma 2 and Myocardial Infarction in the Chinese Han Population. Clin Cardiol (2006) 29(7):300–4. doi: 10.1002/clc.4960290706
22. Ereqat S, Nasereddin A, Azmi K, Abdeen Z, Amin R. Impact of the Pro12Ala Polymorphism of the PPAR-Gamma 2 Gene on Metabolic and Clinical Characteristics in the Palestinian Type 2 Diabetic Patients. PPAR Res (2009) 2009:874126. doi: 10.1155/2009/874126
23. Fernández E, Carrizo E, Connell L, Baptista T. Pro12Ala Polymorphism of the PPAR-γ2 Gene, Metabolic Syndrome and Response to Metformin in Clozapine-Treated Patients. Schizophr Res (2012) 137(1-3):262–3. doi: 10.1016/j.schres.2012.02.005
24. Shen D, Ha D. Relationship Between Pro12ala Variant of Peroxisome Proliferator-Activated Receptor γ2 Gene and Coronary Heart Disease, or Lipids Metabolic Disorders. Med J Wuhan University (2005) 26(2):253–6. doi: 10.3969/j.issn.1671-8852.2005.02.034
25. Saeidi S, Chamaie-Nejad F, Ebrahimi A, Najafi F, Rahimi Z, Vaisi-Raygani A, et al. Pparγ Pro12Ala and C161T Polymorphisms in Patients With Acne Vulgaris: Contribution to Lipid and Lipoprotein Profile. Adv Med Sci (2018) 63(1):147–51. doi: 10.1016/j.advms.2017.09.003
26. Jiang J, Lan J, Du X. Association of Peroxisome Proliferator-Activated Receptor-Gamma 2 Variants With Coronary Heart Disease. Zhejiang Med (2016) 38(7):485–8. doi: CNKI:SUN:ZJYE.0.2016-07-013
27. Hasan NS, Kamel SA, Hamed M, Awadallah E, Rahman AHA, Musa NI, et al. Peroxisome Proliferator-Activated Receptor-γ Polymorphism (Rs1801282) Is Associated With Obesity in Egyptian Patients With Coronary Artery Disease and Type 2 Diabetes Mellitus. J Genet Eng Biotechnol (2017) 15(2):409–14. doi: 10.1016/j.jgeb.2017.08.002
28. Hung YP, Lee NY, Lin SH, Chang HC, Wu CJ, Chang CM, et al. Effects of Pparγ and RBP4 Gene Variants on Metabolic Syndrome in HIV-Infected Patients With Anti-Retroviral Therapy. PLoS One (2012) 7(11):e49102. doi: 10.1371/journal.pone.0049102
29. Vales-Villamarín C, de Dios O, Pérez-Nadador I, Gavela-Pérez T, Soriano-Guillén L, Garcés C. Pparγ2 Pro12Ala Polymorphism Is Associated in Children With Traits Related to Susceptibility to Type 2 Diabetes. Front Pharmacol (2021) 12:763853. doi: 10.3389/fphar.2021.763853
30. Barbieri M, Rizzo MR, Papa M, Acampora R, De Angelis L, Olivieri F, et al. Role of Interaction Between Variants in the PPARG and Interleukin-6 Genes on Obesity Related Metabolic Risk Factors. Exp Gerontol (2005) 40(7):599–604. doi: 10.1016/j.exger.2005.05.004
31. Hamada T, Kotani K, Tsuzaki K, Sano Y, Murata T, Tabata M, et al. Association of Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor Gamma2 Gene With Small Dense Low-Density Lipoprotein in the General Population. Metabolism (2007) 56(10):1345–9. doi: 10.1016/j.metabol.2007.05.017
32. Youssef SM, Mohamed N, Afef S, Khaldoun BH, Fadoua N, Fadhel NM, et al. A Pro 12 Ala Substitution in the Pparγ2 Polymorphism may Decrease the Number of Diseased Vessels and the Severity of Angiographic Coronary Artery. Coron Artery Dis (2013) 24(5):347–51. doi: 10.1097/MCA.0b013e328361a95e
33. Bhatt SP, Nigam P, Misra A, Guleria R, Luthra K, Pandey RM, et al. Association of Peroxisome Proliferator Activated Receptor-γ Gene With Non-Alcoholic Fatty Liver Disease in Asian Indians Residing in North India. Gene (2013) 512(1):143–7. doi: 10.1016/j.gene.2012.09.067
34. Liu F, Mei X, Zhang Y, Qi H, Wang J, Wang Y, et al. Association of Peroxisome Proliferator-Activated Receptorγ Gene Pro12Ala and C161T Polymorphisms With Cardiovascular Risk Factors in Maintenance Hemodialysis Patients. Mol Biol Rep (2014) 41(11):7555–65. doi: 10.1007/s11033-014-3645-0
35. Gu SJ, Guo ZR, Zhou ZY, Hu XS, Wu M, Zhang N. Peroxisome Proliferator Activated Receptor γ Polymorphisms as Risk Factors for Dyslipidemia. Mol Med Rep (2014) 10(5):2759–63. doi: 10.3892/mmr.2014.2553
36. Becer E, Çırakoğlu A. Effect of the Pro12Ala Polymorphism of the Peroxisome Proliferator-Activated Receptor γ2 Gene on Lipid Profile and Adipokines Levels in Obese Subjects. Balkan J Med Genet (2017) 20(1):71–80. doi: 10.1515/bjmg-2017-0007
37. Rahimi Z, Chamaie-Nejad F, Saeidi S, Rahimi Z, Ebrahimi A, Shakiba E, et al. The Association of Pparγ Pro12Ala and C161T Polymorphisms With Polycystic Ovary Syndrome and Their Influence on Lipid and Lipoprotein Profiles. Int J Fertil Steril. (2018) 12(2):147–51. doi: 10.22074/ijfs.2018.5270
38. Swarbrick MM, Chapman CM, McQuillan BM, Hung J, Thompson PL, Beilby JP. A Pro12Ala Polymorphism in the Human Peroxisome Proliferator-Activated Receptor-Gamma 2 Is Associated With Combined Hyperlipidaemia in Obesity. Eur J Endocrinol (2001) 144(3):277–82. doi: 10.1530/eje.0.1440277
39. Dedoussis GV, Theodoraki EV, Manios Y, Yiannakouris N, Panagiotakos D, Papoutsakis C, et al. The Pro12Ala Polymorphism in PPARgamma2 Gene Affects Lipid Parameters in Greek Primary School Children: A Case of Gene-to-Gender Interaction. Am J Med Sci (2007) 333(1):10–5. doi: 10.1097/00000441-200701000-00002
40. Yilmaz-Aydogan H, Kurnaz O, Kurt O, Akadam-Teker B, Kucukhuseyin O, Tekeli A, et al. Effects of the PPARG P12A and C161T Gene Variants on Serum Lipids in Coronary Heart Disease Patients With and Without Type 2 Diabetes. Mol Cell Biochem (2011) 358(1-2):355–63. doi: 10.1007/s11010-011-0987-y
41. Aberle J, Hopfer I, Beil FU, Seedorf U. Association of Peroxisome Proliferator-Activated Receptor Delta +294T/C With Body Mass Index and Interaction With Peroxisome Proliferator-Activated Receptor Alpha L162V. Int J Obes (Lond). (2006) 30(12):1709–13. doi: 10.1038/sj.ijo.0803345
42. González Sánchez JL, Serrano Ríos M, Fernández Perez C, Laakso M, Martínez Larrad MT. Effect of the Pro12Ala Polymorphism of the Peroxisome Proliferator-Activated Receptor Gamma-2 Gene on Adiposity, Insulin Sensitivity and Lipid Profile in the Spanish Population. Eur J Endocrinol (2002) 147(4):495–501. doi: 10.1530/eje.0.1470495
43. Andrulionytè L, Zacharova J, Chiasson JL, Laakso M, STOP-NIDDM Study Group. Common Polymorphisms of the PPAR-Gamma2 (Pro12Ala) and PGC-1alpha (Gly482Ser) Genes Are Associated With the Conversion From Impaired Glucose Tolerance to Type 2 Diabetes in the STOP-NIDDM Trial. Diabetologia (2004) 47(12):2176–84. doi: 10.1007/s00125-004-1577-2
44. Tavares V, Hirata RD, Rodrigues AC, Monte O, Salles JE, Scalissi N, et al. Association Between Pro12Ala Polymorphism of the PPAR-Gamma2 Gene and Insulin Sensitivity in Brazilian Patients With Type-2 Diabetes Mellitus. Diabetes Obes Metab (2005) 7(5):605–11. doi: 10.1111/j.1463-1326.2004.00453.x
45. Stefański A, Majkowska L, Ciechanowicz A, Frankow M, Safranow K, Parczewski M, et al. Association Between the Pro12Ala Variant of the Peroxisome Proliferator-Activated Receptor-Gamma2 Gene and Increased 24-H Diastolic Blood Pressure in Obese Patients With Type II Diabetes. J Hum Hypertens (2006) 20(9):684–92. doi: 10.1038/sj.jhh.1002040
46. Helwig U, Rubin D, Kiosz J, Schreiber S, Fölsch UR, Nothnagel M, et al. The Minor Allele of the PPARgamma2 Pro12ala Polymorphism Is Associated With Lower Postprandial TAG and Insulin Levels in non-Obese Healthy Men. Br J Nutr (2007) 97(5):847–54. doi: 10.1017/S0007114507665179
47. Rhee EJ, Kwon CH, Lee WY, Kim SY, Jung CH, Kim BJ, et al. No Association of Pro12Ala Polymorphism of PPAR-Gamma Gene With Coronary Artery Disease in Korean Subjects. Circ J (2007) 71(3):338–42. doi: 10.1253/circj.71.338
48. Li LL, Ma XL, Ran JX, Sun XF, Xu LM, Ren J, et al. Genetic Polymorphism of Peroxisome Proliferator-Activated Receptor-Gamma 2 Pro12Ala on Ethnic Susceptibility to Diabetes in Uygur, Kazak and Han Subjects. Clin Exp Pharmacol Physiol (2008) 35(2):187–91. doi: 10.1111/j.1440-1681.2007.04796.x
49. Lu Z, Dong B, Mo X, Chen T, Wu H, Zhang Y, et al. Pro12Ala Polymorphism in PPAR Gamma 2 Associated With Essential Hypertension in Chinese Nonagenarians/Centenarians. Exp Gerontol. (2008) 43(12):1108–13. doi: 10.1016/j.exger.2008.08.046
50. Bendlová B, Vejražková D, Včelák J, Lukášová P, Burkoňová D, Kunešová M, et al. PPARgamma2 Pro12Ala Polymorphism in Relation to Free Fatty Acids Concentration and Composition in Lean Healthy Czech Individuals With and Without Family History of Diabetes Type 2. Physiol Res (2008) 57(Suppl 1):S77–90. doi: 10.33549/physiolres.931492
51. Montagnana M, Fava C, Nilsson PM, Engström G, Hedblad B, Lippi G, et al. The Pro12Ala Polymorphism of the PPARG Gene Is Not Associated With the Metabolic Syndrome in an Urban Population of Middle-Aged Swedish Individuals. Diabetes Med (2008) 25(8):902–8. doi: 10.1111/j.1464-5491.2008.02510.x
52. Chae SJ, Kim JJ, Choi YM, Kim JM, Cho YM, Moon SY. Peroxisome Proliferator-Activated Receptor-Gamma and Its Coactivator-1alpha Gene Polymorphisms in Korean Women With Polycystic Ovary Syndrome. Gynecol Obstet Invest (2010) 70(1):1–7. doi: 10.1159/000279309
53. Chistiakov DA, Potapov VA, Khodirev DS, Shamkhalova MS, Shestakova MV, Nosikov VV. The PPARgamma Pro12Ala Variant Is Associated With Insulin Sensitivity in Russian Normoglycaemic and Type 2 Diabetic Subjects. Diabetes Vasc Dis Res (2010) 7(1):56–62. doi: 10.1177/1479164109347689
54. Jermendy A, Körner A, Kovács M, Madácsy L, Cseh K. PPAR-Gamma2 Pro12ala Polymorphism is Associated With Post-Challenge Abnormalities of Glucose Homeostasis in Children and Adolescents With Obesity. J Pediatr Endocrinol Metab (2011) 24(1-2):55–9. doi: 10.1515/jpem.2011.111
55. Garaulet M, Smith CE, Hernández-González T, Lee YC, Ordovás JM. Pparγ Pro12Ala Interacts With Fat Intake for Obesity and Weight Loss in a Behavioural Treatment Based on the Mediterranean Diet. Mol Nutr Food Res (2011) 55(12):1771–9. doi: 10.1002/mnfr.201100437
56. Guan J, Yi H, Wu X, Su K, Tao M, Yin S. Pro12Ala Polymorphism in Human Peroxisome Proliferator Activated Receptor Gamma is Associated With Hyperlipidaemia in Obstructive Sleep Apnoea Hypopnoea Syndrome. J Laryngol Otol (2011) 125(10):1042–8. doi: 10.1017/S002221511100123X
57. Domenici FA, Brochado MJ, Martinelli Ade L, Zucoloto S, da Cunha SF, Vannucchi H. Peroxisome Proliferator-Activated Receptors Alpha and Gamma2 Polymorphisms in Nonalcoholic Fatty Liver Disease: A Study in Brazilian Patients. Gene (2013) 529(2):326–31. doi: 10.1016/j.gene.2013.06.091
58. Baldani DP, Skrgatic L, Cerne JZ, Ferk P, Simunic V, Gersak K. Association of PPARG Pro12Ala Polymorphism With Insulin Sensitivity and Body Mass Index in Patients With Polycystic Ovary Syndrome. BioMed Rep (2014) 2(2):199–206. doi: 10.3892/br.2013.215
59. Stryjecki C, Peralta-Romero J, Alyass A, Karam-Araujo R, Suarez F, Gomez-Zamudio J, et al. Association Between PPAR-γ2 Pro12Ala Genotype and Insulin Resistance Is Modified by Circulating Lipids in Mexican Children. Sci Rep (2016) 6:24472. doi: 10.1038/srep24472
60. Almeida SM, Furtado JM, Mascarenhas P, Ferraz ME, Ferreira JC, Monteiro MP, et al. FTO, MC4R, and PPARG-2 Polymorphisms With Obesity Traits and Metabolic Phenotypes in School-Aged Children. Endocrine (2018) 60(3):466–78. doi: 10.1007/s12020-018-1587-3
61. Szkup M, Brodowski J, Jurczak A, Stanisławska M, Grochans E. Seeking Genetic Determinants of Selected Metabolic Disorders in Women Aged 45-60. Ann Agric Environ Med (2020) 27(3):407–12. doi: 10.26444/aaem/112579
62. Zhou X, Chen J, Xu W. Association Between C1431T Polymorphism in Peroxisome Proliferator-Activated Receptor-γ Gene and Coronary Artery Disease in Chinese Han Population. Mol Biol Rep (2012) 39(2):1863–8. doi: 10.1007/s11033-011-0931-y
63. Maeda A, Gohda T, Funabiki K, Horikoshi S, Tomino Y. Peroxisome Proliferator-Activated Receptor Gamma Gene Polymorphism Is Associated With Serum Triglyceride Levels and Body Mass Index in Japanese Type 2 Diabetic Patients. J Clin Lab Anal (2004) 18(6):317–21. doi: 10.1002/jcla.20045
64. Song Y, Raheel TM, Jia A, Dai G, Liu L, Long X, et al. Rs10865710 Polymorphism in PPARG Promoter Is Associated With the Severity of Type 2 Diabetes Mellitus and Coronary Artery Disease in a Chinese Population. Postgrad Med J (2021). postgradmedj-2021-140354. doi: 10.1136/postgradmedj-2021-140354
65. Wei WM, Wu XY, Li ST, Shen Q. PPARG Gene C161T CT/TT Associated With Lower Blood Lipid Levels and Ischemic Stroke From Large-Artery Atherosclerosis in a Han Population in Guangdong. Neurol Res (2016) 38(7):620–4. doi: 10.1080/01616412.2016.1189056
66. Wan J, Xiong S, Chao S, Xiao J, Ma Y, Wang J, et al. PPARgamma Gene C161T Substitution Alters Lipid Profile in Chinese Patients With Coronary Artery Disease and Type 2 Diabetes Mellitus. Cardiovasc Diabetol (2010) 9:13. doi: 10.1186/1475-2840-9-13
67. Wang XL, Oosterhof J, Duarte N. Peroxisome Proliferator-Activated Receptor Gamma C161→T Polymorphism and Coronary Artery Disease. Cardiovasc Res (1999) 44(3):588–94. doi: 10.1016/S0008-6363(99)00256-4
68. Chia PP, Fan SH, Say YH. Screening of Peroxisome Proliferator-Activated Receptors (PPARs) α, γ and α Gene Polymorphisms for Obesity and Metabolic Syndrome Association in the Multi-Ethnic Malaysian Population. Ethn Dis (2015) 25(4):383–90. doi: 10.18865/ed.25.4.383
69. Grygiel-Gorniak B, Mosor M, Marcinkowska J, Przyslawski J, Nowak J. Impact of the PPAR Gamma-2 Gene Polymorphisms on the Metabolic State of Postmenopausal Women. J Biosci (2016) 41(3):427–37. doi: 10.1007/s12038-016-9633-x
70. Tavares V, Hirata RD, Rodrigues AC, Monte O, Salles JE, Scallissi N, et al. Effect of the Peroxisome Proliferator-Activated Receptor-Gamma C161T Polymorphism on Lipid Profile in Brazilian Patients With Type 2 Diabetes Mellitus. J Endocrinol Invest (2005) 28(2):129–36. doi: 10.1007/BF03345355
71. Haseeb A, Iliyas M, Chakrabarti S, Farooqui AA, Naik SR, Ghosh S, et al. Single-Nucleotide Polymorphisms in Peroxisome Proliferator-Activated Receptor Gamma and Their Association With Plasma Levels of Resistin and the Metabolic Syndrome in a South Indian Population. J Biosci (2009) 34(3):405–14. doi: 10.1007/s12038-009-0047-x
72. Meirhaeghe A, Fajas L, Helbecque N, Cottel D, Lebel P, Dallongeville J, et al. A Genetic Polymorphism of the Peroxisome Proliferator-Activated Receptor Gamma Gene Influences Plasma Leptin Levels in Obese Humans. Hum Mol Genet (1998) 7(3):435–40. doi: 10.1093/hmg/7.3.435
73. Oh EY, Min KM, Chung JH, Min YK, Lee MS, Kim KW, et al. Significance of Pro12Ala Mutation in Peroxisome Proliferator-Activated Receptor-Gamma2 in Korean Diabetic and Obese Subjects. J Clin Endocrinol Metab (2000) 85(5):1801–4. doi: 10.1210/jcem.85.5.6499
74. Mori H, Ikegami H, Kawaguchi Y, Seino S, Yokoi N, Takeda J, et al. The Pro12 –>Ala Substitution in PPAR-Gamma Is Associated With Resistance to Development of Diabetes in the General Population: Possible Involvement in Impairment of Insulin Secretion in Individuals With Type 2 Diabetes. Diabetes (2001) 50(4):891–4. doi: 10.2337/diabetes.50.4.891
75. Peng D, Zhao S, Li J, Nie S. Relationship Between Pparγ C161→T Substitution and Coronary Heart Disease. Chin Circ J (2002) 17(5):370–3. doi: CNKI:SUN:ZGXH.0.2002-05-021
76. Schneider J, Kreuzer J, Hamann A, Nawroth PP, Dugi KA. The Proline 12 Alanine Substitution in the Peroxisome Proliferator–Activated Receptor-Gamma2 Gene Is Associated With Lower Lipoprotein Lipase Activity In Vivo. Diabetes (2002) 51(3):867–70. doi: 10.2337/diabetes.51.3.867
77. Vaccaro O, Mancini FP, Ruffa G, Sabatino L, Iovine C, Masulli M, et al. Fasting Plasma Free Fatty Acid Concentrations and Pro12Ala Polymorphism of the Peroxisome Proliferator-Activated Receptor (PPAR) Gamma2 Gene in Healthy Individuals. Clin Endocrinol (Oxf) (2002) 57(4):481–6. doi: 10.1046/j.1365-2265.2002.01618.x
78. Yamamoto Y, Hirose H, Miyashita K, Nishikai K, Saito I, Taniyama M, et al. PPAR(gamma)2 Gene Pro12Ala Polymorphism May Influence Serum Level of an Adipocyte-Derived Protein, Adiponectin, in the Japanese Population. Metabolism (2002) 51(11):1407–9. doi: 10.1053/meta.2002.35586
79. Song J, Sakatsume M, Narita I, Goto S, Omori K, Takada T, et al. Peroxisome Proliferator-Activated Receptor Gamma C161T Polymorphisms and Survival of Japanese Patients With Immunoglobulin A Nephropathy. Clin Genet (2003) 64(5):398–403. doi: 10.1034/j.1399-0004.2003.00154.x
80. Arashiro R, Katsuren K, Fukuyama S, Ohta T. Effect of Trp64Arg Mutation of the Beta3-Adrenergic Receptor Gene and C161T Substitution of the Peroxisome Proliferator Activated Receptor Gamma Gene on Obesity in Japanese Children. Pediatr Int (2003) 45(2):135–41. doi: 10.1046/j.1442-200X.2003.01685.x
81. Eriksson J, Lindi V, Uusitupa M, Forsén T, Laakso M, Osmond C, et al. The Effects of the Pro12Ala Polymorphism of the PPARgamma-2 Gene on Lipid Metabolism Interact With Body Size at Birth. Clin Genet (2003) 64(4):366–70. doi: 10.1034/j.1399-0004.2003.00150.x
82. Iwata E, Yamamoto I, Motomura T, Tsubakimori S, Nohnen S, Ohmoto M, et al. The Association of Pro12Ala Polymorphism in PPARgamma2 With Lower Carotid Artery IMT in Japanese. Diabetes Res Clin Pract (2003) 62(1):55–9. doi: 10.1016/S0168-8227(03)00161-X
83. Niskanen L, Lindi V, Erkkilä A, Sivenius K, Luoma J, Ylä-Herttuala S, et al. Association of the PRO12ALA Polymorphism of the PPAR-Gamma2 Gene With Oxidized Low-Density Lipoprotein and Cardiolipin Autoantibodies in Nondiabetic and Type 2 Diabetic Subjects. Metabolism (2003) 52(2):213–7. doi: 10.1053/meta.2003.50039
84. Robitaille J, Després JP, Pérusse L, Vohl MC. The PPAR-Gamma P12A Polymorphism Modulates the Relationship Between Dietary Fat Intake and Components of the Metabolic Syndrome: Results From the Québec Family Study. Clin Genet (2003) 63(2):109–16. doi: 10.1034/j.1399-0004.2003.00026.x
85. Baratta R, Di Paola R, Spampinato D, Fini G, Marucci A, Coco A, et al. Evidence for Genetic Epistasis in Human Insulin Resistance: The Combined Effect of PC-1 (K121Q) and PPARgamma2 (P12A) Polymorphisms. J Mol Med (Berl) (2003) 81(11):718–23. doi: 10.1007/s00109-003-0466-3
86. Chao TH, Li YH, Chen JH, Wu HL, Shi GY, Liu PY, et al. The 161TT Genotype in the Exon 6 of the Peroxisome-Proliferator-Activated Receptor Gamma Gene Is Associated With Premature Acute Myocardial Infarction and Increased Lipid Peroxidation in Habitual Heavy Smokers. Clin Sci (Lond) (2004) 107(5):461–6. doi: 10.1042/CS20040014
87. Orio F Jr, Palomba S, Cascella T, Di Biase S, Labella D, Russo T, et al. Lack of an Association Between Peroxisome Proliferator-Activated Receptor-Gamma Gene Pro12Ala Polymorphism and Adiponectin Levels in the Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2004) 89(10):5110–5. doi: 10.1210/jc.2004-0109
88. Pintérová D, Cerná M, Kolostová K, Novota P, Cimburová M, Romzová M, et al. The Frequency of Alleles of the Pro12Ala Polymorphism in PPARgamma2 is Different Between Healthy Controls and Patients With Type 2 Diabetes. Folia Biol (Praha) (2004) 50(5):153–6. doi: 10.1142/9789812703057_0014
89. Buzzetti R, Petrone A, Ribaudo MC, Alemanno I, Zavarella S, Mein CA, et al. The Common PPAR-Gamma2 Pro12Ala Variant Is Associated With Greater Insulin Sensitivity. Eur J Hum Genet (2004) 12(12):1050–4. doi: 10.1038/sj.ejhg.5201283
90. Pischon T, Pai JK, Manson JE, Hu FB, Rexrode KM, Hunter D, et al. Peroxisome Proliferator-Activated Receptor-γ2 P12A Polymorphism and Risk of Coronary Heart Disease in US Men and Women. Arterioscler Thromb Vasc Biol (2005) 25(8):1654–8. doi: 10.1161/01.ATV.0000171993.78135.7e
91. Hahn S, Fingerhut A, Khomtsiv U, Khomtsiv L, Tan S, Quadbeck B, et al. The Peroxisome Proliferator Activated Receptor Gamma Pro12Ala Polymorphism Is Associated With a Lower Hirsutism Score and Increased Insulin Sensitivity in Women With Polycystic Ovary Syndrome. Clin Endocrinol (Oxf) (2005) 62(5):573–9. doi: 10.1111/j.1365-2265.2005.02261.x
92. Vänttinen M, Nuutila P, Pihlajamäki J, Hällsten K, Virtanen KA, Lautamäki R, et al. The Effect of the Ala12 Allele of the Peroxisome Proliferator-Activated Receptor-Gamma2 Gene on Skeletal Muscle Glucose Uptake Depends on Obesity: A Positron Emission Tomography Study. J Clin Endocrinol Metab (2005) 90(7):4249–54. doi: 10.1210/jc.2005-0101
93. Mousavinasab F, Tähtinen T, Jokelainen J, Koskela P, Vanhala M, Oikarinen J, et al. Common Polymorphisms in the PPARgamma2 and IRS-1 Genes and Their Interaction Influence Serum Adiponectin Concentration in Young Finnish Men. Mol Genet Metab (2005) 84(4):344–8. doi: 10.1016/j.ymgme.2004.11.008
94. Zouari Bouassida K, Chouchane L, Jellouli K, Chérif S, Haddad S, Gabbouj S, et al. The Peroxisome Proliferator Activated Receptorgamma2 (PPARgamma2) Pro12Ala Variant: Lack of Association With Type 2 Diabetes in Obese and non Obese Tunisian Patients. Diabetes Metab (2005) 31(2):119–23. doi: 10.1016/S1262-3636(07)70177-5
95. Moon MK, Cho YM, Jung HS, Park YJ, Yoon KH, Sung YA, et al. Genetic Polymorphisms in Peroxisome Proliferator-Activated Receptor Gamma are Associated With Type 2 Diabetes Mellitus and Obesity in the Korean Population. Diabetes Med (2005) 22(9):1161–6. doi: 10.1111/j.1464-5491.2005.01599.x
96. Buzzetti R, Petrone A, Caiazzo AM, Alemanno I, Zavarella S, Capizzi M, et al. PPAR-Gamma2 Pro12Ala Variant Is Associated With Greater Insulin Sensitivity in Childhood Obesity. Pediatr Res (2005) 57(1):138–40. doi: 10.1203/01.PDR.0000147728.62185.21
97. Scaglioni S, Verduci E, Salvioni M, Biondi ML, Radaelli G, Agostoni C, et al. PPAR-Gamma2 Pro12Ala Variant, Insulin Resistance and Plasma Long-Chain Polyunsaturated Fatty Acids in Childhood Obesity. Pediatr Res (2006) 60(4):485–9. doi: 10.1203/01.pdr.0000238259.41560.00
98. Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, et al. Effects of Two Common Polymorphisms of Peroxisome Proliferator-Activated Receptor-Gamma Gene on Metabolic Syndrome. Arch Med Res (2006) 37(1):86–94. doi: 10.1016/j.arcmed.2005.04.008
99. Yilmaz M, Ergün MA, Karakoç A, Yurtçu E, Cakir N, Arslan M. Pro12Ala Polymorphism of the Peroxisome Proliferator-Activated Receptor-Gamma Gene in Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2006) 22(6):336–42. doi: 10.1080/09513590600733357
100. Cardona F, Morcillo S, Gonzalo-Marín M, Garrido-Sanchez L, Macias-Gonzalez M, Tinahones FJ. Pro12Ala Sequence Variant of the PPARG Gene Is Associated With Postprandial Hypertriglyceridemia in non-E3/E3 Patients With the Metabolic Syndrome. Clin Chem (2006) 52(10):1920–5. doi: 10.1373/clinchem.2006.069690
101. Liu Y, Yuan Z, Liu Y, Zhang J, Yin P, Wang D, et al. PPARgamma Gene C161T Substitution Is Associated With Reduced Risk of Coronary Artery Disease and Decreased Proinflammatory Cytokine Expression. Am Heart J (2007) 154(4):718–24. doi: 10.1016/j.ahj.2007.06.009
102. Kim K, Lee S, Valentine RJ. Association of Pro12ala Polymorphism in the Peroxisome Proliferative-Activated Receptor Gamma2 Gene With Obesity and Hypertension in Korean Women. J Nutr Sci Vitaminol (Tokyo) (2007) 53(3):239–46. doi: 10.3177/jnsv.53.239
103. Kotani K, Saiga K, Kurozawa Y, Sakane N, Sano Y, Tabata M. The Peroxisome Proliferator-Activated Receptor Gamma2 Gene Pro12Ala Polymorphism and Serum C-Reactive Protein in General Japanese Population. Clin Chim Acta (2007) 383(1-2):178–9. doi: 10.1016/j.cca.2007.05.002
104. Zafarmand MH, van der Schouw YT, Grobbee DE, de Leeuw PW, Bots ML. Peroxisome Proliferator-Activated Receptor Gamma-2 P12A Polymorphism and Risk of Acute Myocardial Infarction, Coronary Heart Disease and Ischemic Stroke: A Case-Cohort Study and Meta-Analyses. Vasc Health Risk Manage (2008) 4(2):427–36. doi: 10.2147/vhrm.s2397
105. Dongxia L, Qi H, Lisong L, Jincheng G. Association of Peroxisome Proliferator-Activated Receptorgamma Gene Pro12Ala and C161T Polymorphisms With Metabolic Syndrome. Circ J (2008) 72(4):551–7. doi: 10.1253/circj.72.551
106. Badii R, Bener A, Zirie M, Al-Rikabi A, Simsek M, Al-Hamaq AO, et al. Lack of Association Between the Pro12Ala Polymorphism of the PPAR-Gamma 2 Gene and Type 2 Diabetes Mellitus in the Qatari Consanguineous Population. Acta Diabetol (2008) 45(1):15–21. doi: 10.1007/s00592-007-0013-8
107. Hui Y, Yu-Yuan L, Yu-Qiang N, Wei-Hong S, Yan-Lei D, Xiao-Bo L, et al. Effect of Peroxisome Proliferator-Activated Receptors-Gamma and Co-Activator-1alpha Genetic Polymorphisms on Plasma Adiponectin Levels and Susceptibility of Non-Alcoholic Fatty Liver Disease in Chinese People. Liver Int (2008) 28(3):385–92. doi: 10.1111/j.1478-3231.2007.01623.x
108. Jorsal A, Tarnow L, Lajer M, Ek J, Hansen T, Pedersen O, et al. The PPAR Gamma 2 Pro12Ala Variant Predicts ESRD and Mortality in Patients With Type 1 Diabetes and Diabetic Nephropathy. Mol Genet Metab (2008) 94(3):347–51. doi: 10.1016/j.ymgme.2008.03.014
109. Evangelisti L, Attanasio M, Lucarini L, Sofi F, Marcucci R, Giglioli C, et al. PPARgamma Promoter Polymorphisms and Acute Coronary Syndrome. Atherosclerosis (2009) 205(1):186–91. doi: 10.1016/j.atherosclerosis.2008.11.009
110. Regieli JJ, Jukema JW, Doevendans PA, Zwinderman AH, van der Graaf Y, Kastelein JJ, et al. PPAR Gamma Variant Influences Angiographic Outcome and 10-Year Cardiovascular Risk in Male Symptomatic Coronary Artery Disease Patients. Diabetes Care (2009) 32(5):839–44. doi: 10.2337/dc08-1819
111. Kotronen A, Yki-Järvinen H, Aminoff A, Bergholm R, Pietiläinen KH, Westerbacka J, et al. Genetic Variation in the ADIPOR2 Gene Is Associated With Liver Fat Content and Its Surrogate Markers in Three Independent Cohorts. Eur J Endocrinol (2009) 160(4):593–602. doi: 10.1530/EJE-08-0900
112. Milewicz A, Tworowska-Bardziñska U, Dunajska K, Jêdrzejuk D, Lwow F. Relationship of PPARgamma2 Polymorphism With Obesity and Metabolic Syndrome in Postmenopausal Polish Women. Exp Clin Endocrinol Diabetes (2009) 117(10):628–32. doi: 10.1055/s-0028-1112154
113. Mirzaei H, Akrami SM, Golmohammadi T, Doosti M, Heshmat R, Nakhjavani M, et al. Polymorphism of Pro12Ala in the Peroxisome Proliferator-Activated Receptor Gamma2 Gene in Iranian Diabetic and Obese Subjects. Metab Syndr Relat Disord (2009) 7(5):453–8. doi: 10.1089/met.2008.0099
114. Xita N, Lazaros L, Georgiou I, Tsatsoulis A. The Pro12Ala Polymorphism of the PPAR-Gamma Gene Is Not Associated With the Polycystic Ovary Syndrome. Hormones (Athens) (2009) 8(4):267–72. doi: 10.1007/BF03401274
115. Ji-Rong Y, Bi-Rong D, Chang-Quan H, Zhen-Chan L, Hong-Mei W, Yan-Ling Z, et al. Pro12Ala Polymorphism in PPARgamma2 Associated With Depression in Chinese Nonagenarians/Centenarians. Arch Med Res (2009) 40(5):411–5. doi: 10.1016/j.arcmed.2009.05.005
116. Koika V, Marioli DJ, Saltamavros AD, Vervita V, Koufogiannis KD, Adonakis G, et al. Association of the Pro12Ala Polymorphism in Peroxisome Proliferator-Activated Receptor Gamma2 With Decreased Basic Metabolic Rate in Women With Polycystic Ovary Syndrome. Eur J Endocrinol (2009) 161(2):317–22. doi: 10.1530/EJE-08-1014
117. Dedoussis GV, Vidra N, Butler J, Papoutsakis C, Yannakoulia M, Hirschhorn JN, et al. Peroxisome Proliferator-Activated Receptor-Gamma (PPARgamma) Pro12Ala Polymorphism and Risk for Pediatric Obesity. Clin Chem Lab Med (2009) 47(9):1047–50. doi: 10.1515/CCLM.2009.242
118. Gao L, Wang L, Yun H, Su L, Su X. Association of the PPARgamma2 Gene Pro12Ala Variant With Primary Hypertension and Metabolic Lipid Disorders in Han Chinese of Inner Mongolia. Genet Mol Res (2010) 9(3):1312–20. doi: 10.4238/vol9-3gmr833
119. Zhang Q, Zhai C, Wang Y, Guo Y, Ding Z, Jin X. Influences of Peroxisome Proliferator-Activated Receptor Gamma 2 Genetic Polymorphism on the Effects of Dietary Intervention to the Blood Lipids Abnormalities. China J Prev Med (2010) 44(1):39–43. doi: 10.3760/cma.j.issn.0253-9624.2010.01.011
120. de Kort SW, Hokken-Koelega AC. The PPAR-Gamma Pro12Ala Polymorphism Associates With Weight Gain During GH-Treatment in Short Children Born Small for Gestational Age. Eur J Endocrinol (2010) 162(1):49–52. doi: 10.1530/EJE-09-0631
121. Liu L, Zheng T, Wang F, Wang N, Song Y, Li M, et al. Pro12Ala Polymorphism in the PPARG Gene Contributes to the Development of Diabetic Nephropathy in Chinese Type 2 Diabetic Patients. Diabetes Care (2010) 33(1):144–9. doi: 10.2337/dc09-1258
122. Hsieh MC, Lin KD, Tien KJ, Tu ST, Hsiao JY, Chang SJ, et al. Common Polymorphisms of the Peroxisome Proliferator-Activated Receptor-Gamma (Pro12Ala) and Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 (Gly482Ser) and the Response to Pioglitazone in Chinese Patients With Type 2 Diabetes Mellitus. Metabolism (2010) 59(8):1139–44. doi: 10.1016/j.metabol.2009.10.030
123. Dongiovanni P, Rametta R, Fracanzani AL, Benedan L, Borroni V, Maggioni P, et al. Lack of Association Between Peroxisome Proliferator-Activated Receptors Alpha and Gamma2 Polymorphisms and Progressive Liver Damage in Patients With Non-Alcoholic Fatty Liver Disease: A Case Control Study. BMC Gastroenterol (2010) 10:102. doi: 10.1186/1471-230X-10-102
124. Bouchard-Mercier A, Godin G, Lamarche B, Pérusse L, Vohl MC. Effects of Peroxisome Proliferator-Activated Receptors, Dietary Fat Intakes and Gene-Diet Interactions on Peak Particle Diameters of Low-Density Lipoproteins. J Nutrigenet Nutrigenomics (2011) 4(1):36–48. doi: 10.1159/000324531
125. Dedoussis GV, Manios Y, Kourlaba G, Kanoni S, Lagou V, Butler J, et al. An Age-Dependent Diet-Modified Effect of the Pparγ Pro12Ala Polymorphism in Children. Metabolism (2011) 60(4):467–73. doi: 10.1016/j.metabol.2010.04.007
126. Ramakrishnan L, Sachdev HS, Sharma M, Abraham R, Prakash S, Gupta D, et al. Relationship of APOA5, Pparγ and HL Gene Variants With Serial Changes in Childhood Body Mass Index and Coronary Artery Disease Risk Factors in Young Adulthood. Lipids Health Dis (2011) 10:68. doi: 10.1186/1476-511X-10-68
127. Chen CH, Lu ML, Kuo PH, Chen PY, Chiu CC, Kao CF, et al. Gender Differences in the Effects of Peroxisome Proliferator-Activated Receptor γ2 Gene Polymorphisms on Metabolic Adversity in Patients With Schizophrenia or Schizoaffective Disorder. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35(1):239–45. doi: 10.1016/j.pnpbp.2010.11.014
128. Estivalet AA, Leiria LB, Dora JM, Rheinheimer J, Bouças AP, Maia AL, et al. D2 Thr92Ala and Pparγ2 Pro12Ala Polymorphisms Interact in the Modulation of Insulin Resistance in Type 2 Diabetic Patients. Obes (Silver Spring) (2011) 19(4):825–32. doi: 10.1038/oby.2010.231
129. Bhatt SP, Misra A, Sharma M, Luthra K, Guleria R, Pandey RM, et al. Ala/Ala Genotype of Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor-γ2 Gene Is Associated With Obesity and Insulin Resistance in Asian Indians. Diabetes Technol Ther (2012) 14(9):828–34. doi: 10.1089/dia.2011.0277
130. Curti ML, Rogero MM, Baltar VT, Barros CR, Siqueira-Catania A, Ferreira SR. FTO T/A and Peroxisome Proliferator-Activated Receptor-γ Pro12Ala Polymorphisms But Not ApoA1 -75 are Associated With Better Response to Lifestyle Intervention in Brazilians at High Cardiometabolic Risk. Metab Syndr Relat Disord (2013) 11(3):169–76. doi: 10.1089/met.2012.0055
131. Yang J, Gong H, Liu W, Tao T. The Association of Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor-Gamma2 Gene With the Metabolic Characteristics in Chinese Women With Polycystic Ovary Syndrome. Int J Clin Exp Pathol (2013) 6(9):1894–902. doi: 10.1155/2013/416870
132. Pei Q, Huang Q, Yang GP, Zhao YC, Yin JY, Song M, et al. PPAR-γ2 and PTPRD Gene Polymorphisms Influence Type 2 Diabetes Patients' Response to Pioglitazone in China. Acta Pharmacol Sin (2013) 34(2):255–61. doi: 10.1038/aps.2012.144
133. Arnaiz-Villena A, Fernández-Honrado M, Areces C, Enríquez-de-Salamanca M, Abd-El-Fatah-Khalil S, Coca C, et al. Amerindians Show No Association of PPAR-γ2 Gene Ala12 Allele and Obesity: An "Unthrifty" Variant Population Genetics. Mol Biol Rep (2013) 40(2):1767–74. doi: 10.1007/s11033-012-2230-7
134. Chehaibi K, Nouira S, Mahdouani K, Hamdi S, Rouis M, Slimane MN. Effect of the Pparγ C161T Gene Variant on Serum Lipids in Ischemic Stroke Patients With and Without Type 2 Diabetes Mellitus. J Mol Neurosci (2014) 54(4):730–8. doi: 10.1007/s12031-014-0326-3
135. Rocha RM, Barra GB, Rosa ÉC, Garcia ÉC, Amato AA, Azevedo MF. Prevalence of the Rs1801282 Single Nucleotide Polymorphism of the PPARG Gene in Patients With Metabolic Syndrome. Arch Endocrinol Metab (2015) 59(4):297–302. doi: 10.1590/2359-3997000000086
136. Rotter I, Skonieczna-Żydecka K, Kosik-Bogacka D, Adler G, Rył A, Laszczyńska M. Relationships Between FTO Rs9939609, MC4R Rs17782313, and Pparγ Rs1801282 Polymorphisms and the Occurrence of Selected Metabolic and Hormonal Disorders in Middle-Aged and Elderly Men - a Preliminary Study. Clin Interv Aging (2016) 11:1723–32. doi: 10.2147/CIA.S120253
137. Li X, Zhang BL, Zhang XG, Su XL. Correlation Between PPARg2 Gene Pro12Ala Polymorphism and Cerebral Infarction in an Inner Mongolian Han Chinese Population. Genet Mol Res (2016) 15(2):gmr7332. doi: 10.4238/gmr.15027332
138. Priya SS, Sankaran R, Ramalingam S, Sairam T, Somasundaram LS. Genotype Phenotype Correlation of Genetic Polymorphism of PPAR Gamma Gene and Therapeutic Response to Pioglitazone in Type 2 Diabetes Mellitus- A Pilot Study. J Clin Diagn Res (2016) 10(2):FC11–4. doi: 10.7860/JCDR/2016/16494.7331
139. Zheng JS, Chen J, Wang L, Yang H, Fang L, Yu Y, et al. Replication of a Gene-Diet Interaction at CD36, NOS3 and PPARG in Response to Omega-3 Fatty Acid Supplements on Blood Lipids: A Double-Blind Randomized Controlled Trial. EBioMedicine (2018) 31:150–6. doi: 10.1016/j.ebiom.2018.04.012
140. Chmurzynska A, Muzsik A, Krzyżanowska-Jankowska P, Mądry E, Walkowiak J, Bajerska J. PPARG and FTO Polymorphism can Modulate the Outcomes of a Central European Diet and a Mediterranean Diet in Centrally Obese Postmenopausal Women. Nutr Res (2019) 69:94–100. doi: 10.1016/j.nutres.2019.08.005
141. García-Ricobaraza M, García-Bermúdez M, Torres-Espinola FJ, Segura Moreno MT, Bleyere MN, Díaz-Prieto LE, et al. Association Study of Rs1801282 PPARG Gene Polymorphism and Immune Cells and Cytokine Levels in a Spanish Pregnant Women Cohort and Their Offspring. J BioMed Sci (2020) 27:101. doi: 10.1186/s12929-020-00694-3
142. Carrillo-Venzor MA, Erives-Anchondo NR, Moreno-González JG, Moreno-Brito V, Licón-Trillo A, González-Rodríguez E, et al. Pro12Ala PPAR-γ2 and +294T/C PPAR-δ Polymorphisms and Association With Metabolic Traits in Teenagers From Northern Mexico. Genes (Basel) (2020) 11(7):776. doi: 10.3390/genes11070776
143. Lv O, Wang L, Li J, Ma Q, Zhao W. Effects of Pomegranate Peel Polyphenols on Lipid Accumulation and Cholesterol Metabolic Transformation in L-02 Human Hepatic Cells via the Pparγ-ABCA1/CYP7A1 Pathway. Food Funct (2016) 7(12):4976–83. doi: 10.1039/C6FO01261B
144. Zhang F, Liu P, He Z, Zhang L, He X, Liu F, et al. Crocin Ameliorates Atherosclerosis by Promoting the Reverse Cholesterol Transport and Inhibiting the Foam Cell Formation via Regulating Pparγ/LXR-α. Cell Cycle (2022) 21(2):202–18. doi: 10.1080/15384101.2021.2015669
145. Jie J, Ling L, Yi Y, Tao L, Liao X, Gao P, et al. Tributyltin Triggers Lipogenesis in Macrophages via Modifying Pparγ Pathway. Environ pollut (2021) 271:116331. doi: 10.1016/j.envpol.2020.116331
146. Schubert M, Becher S, Wallert M, Maeß MB, Abhari M, Rennert K, et al. The Peroxisome Proliferator-Activated Receptor (PPAR)-γ Antagonist 2-Chloro-5-Nitro-N-Phenylbenzamide (GW9662) Triggers Perilipin 2 Expression via Pparδ and Induces Lipogenesis and Triglyceride Accumulation in Human THP-1 Macrophages. Mol Pharmacol (2020) 97(3):212–25. doi: 10.1124/mol.119.117887
147. Legchenko E, Chouvarine P, Borchert P, Fernandez-Gonzalez A, Snay E, Meier M, et al. Pparγ Agonist Pioglitazone Reverses Pulmonary Hypertension and Prevents Right Heart Failure via Fatty Acid Oxidation. Sci Transl Med (2018) 10(438):eaao0303. doi: 10.1126/scitranslmed.aao0303
148. Sikder K, Shukla SK, Patel N, Singh H, Rafiq K. High Fat Diet Upregulates Fatty Acid Oxidation and Ketogenesis via Intervention of PPAR-γ. Cell Physiol Biochem (2018) 48(3):1317–31. doi: 10.1159/000492091
149. Wu Z, Lou Y, Jin W, Liu Y, Lu L, Lu G. The Pro12Ala Polymorphism in the Peroxisome Proliferator-Activated Receptor Gamma-2 Gene (Pparγ2) is Associated With Increased Risk of Coronary Artery Disease: A Meta-Analysis. PLoS One (2012) 7(12):e53105. doi: 10.1371/journal.pone.0053105
150. Qian Y, Li P, Zhang J, Shi Y, Chen K, Yang J, et al. Association Between Peroxisome Proliferator-Activated Receptor-Alpha, Delta, and Gamma Polymorphisms and Risk of Coronary Heart Disease: A Case-Control Study and Meta-Analysis. Medicine (Baltimore) (2016) 95(32):e4299. doi: 10.1097/MD.0000000000004299
151. González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, Hernández-Díaz Y, López-Narváez ML, Rodríguez-Pérez C, et al. PON2 and PPARG Polymorphisms as Biomarkers of Risk for Coronary Heart Disease. biomark Med (2018) 12(3):287–97. doi: 10.2217/bmm-2017-0227
152. Baghbani-Oskouei A, Gholampourdehaki M. Anthropometric Measures and the Risk of Coronary Artery Disease. Caspian J Intern Med (2020) 11(2):183–90. doi: 10.22088/cjim.11.2.183
153. Solymanzadeh F, Rokhafroz D, Asadizaker M, Dastoorpoor M. Prediction of Risk of Coronary Artery Disease Based on Framingham Risk Score in Association With Shift Work Among Nurses. Int J Occup Saf Ergon (2022) 1–6. doi: 10.1080/10803548.2021.2024403
154. Agrawal S, Klarqvist MDR, Emdin C, Patel AP, Paranjpe MD, Ellinor PT, et al. Selection of 51 Predictors From 13,782 Candidate Multimodal Features Using Machine Learning Improves Coronary Artery Disease Prediction. Patterns (N Y) (2021) 2(12):100364. doi: 10.1016/j.patter.2021.100364
155. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of Potentially Modifiable Risk Factors Associated With Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet (2004) 364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9
156. Li Q, Chen R, Bie L, Zhao D, Huang C, Hong J. Association of the Variants in the PPARG Gene and Serum Lipid Levels: A Meta-Analysis of 74 Studies. J Cell Mol Med (2015) 19(1):198–209. doi: 10.1111/jcmm.12417
157. Pihlajamäki J, Schwab U, Kaminska D, Ågren J, Kuusisto J, Kolehmainen M, et al. Dietary Polyunsaturated Fatty Acids and the Pro12Ala Polymorphisms of PPARG Regulate Serum Lipids Through Divergent Pathways: A Randomized Crossover Clinical Trial. Genes Nutr (2015) 10(6):43. doi: 10.1007/s12263-015-0493-z
158. Lu H, Wen D, Sun J, Zeng L, Du J, Du D, et al. Enhancer Polymorphism Rs10865710 Associated With Traumatic Sepsis is a Regulator of PPARG Gene Expression. Crit Care (2019) 23(1):430. doi: 10.1186/s13054-019-2707-z
159. Wu L, Song Y, Zhang Y, Liang B, Deng Y, Tang T, et al. Novel Genetic Variants of Pparγ2 Promoter in Gestational Diabetes Mellitus and Its Molecular Regulation in Adipogenesis. Front Endocrinol (Lausanne) (2021) 11:499788. doi: 10.3389/fendo.2020.499788
160. Wang H, Xiong K, Sun W, Fu Y, Jiang Z, Yu D, et al. Two Completely Linked Polymorphisms in the PPARG Transcriptional Regulatory Region Significantly Affect Gene Expression and Intramuscular Fat Deposition in the Longissimus Dorsi Muscle of Erhualian Pigs. Anim Genet (2013) 44(4):458–62. doi: 10.1111/age.12025
Keywords: peroxisome proliferator-activated receptor gamma, polymorphism, rs1801282, rs3856806, obesity, dyslipidemia
Citation: Li S, He C, Nie H, Pang Q, Wang R, Zeng Z and Song Y (2022) G Allele of the rs1801282 Polymorphism in PPARγ Gene Confers an Increased Risk of Obesity and Hypercholesterolemia, While T Allele of the rs3856806 Polymorphism Displays a Protective Role Against Dyslipidemia: A Systematic Review and Meta-Analysis. Front. Endocrinol. 13:919087. doi: 10.3389/fendo.2022.919087
Received: 13 April 2022; Accepted: 30 May 2022;
Published: 29 June 2022.
Edited by:
Alexandre Gabarra Oliveira, São Paulo State University, BrazilReviewed by:
Cassiano Merussi Neiva, São Paulo State University, BrazilIrene Leal-Berumen, Universidad Autónoma de Chihuahua, Mexico
Copyright © 2022 Li, He, Nie, Pang, Wang, Zeng and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyan Song, c29uZ3lvbmd5YW5AY2R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Shujin Li1†
Shujin Li1† Yongyan Song
Yongyan Song