- 1Department of Internal Medicine, University of California, Los Angeles, Los Angeles, CA, United States
- 2Department of Medicine, Garden City Hospital, Garden City, MI, United States
- 3Department of Gastroenterology, Greater Los Angeles Veterans Affairs, Los Angeles, CA, United States
- 4Vatche & Tamar Manoukian Division of Digestive Diseases, Los Angeles, CA, United States
Treatment of obesity, an ongoing global epidemic, is challenging, as weight-loss efforts require a multidisciplinary approach addressing both behavioral and biologic needs that are not completely understood. Recent studies of the gut microbiome may provide better insight into the condition, and ultimately serve to advance more effective therapies. Research in this field has shifted from analyzing microbiome compositional differences to investigating functional changes that affect disease pathophysiology and outcome. Bacteria-derived metabolites are a way to bridge compositional changes to functional consequences. Through the production of metabolites, such as short chain fatty acids, tryptophan derivatives and bile acids, and interactions with peripheral and central signaling pathways, the gut microbiome may alter the body’s metabolic and behavioral responses to food. Here, we summarize these mechanisms driven by gut-derived metabolites, through which the microbiome is thought to contribute to obesity, as well as review recent investigations of interventions related to these metabolites. Limitations of existing research, primarily due to paucity of causal studies in humans, are also discussed in this review.
Introduction
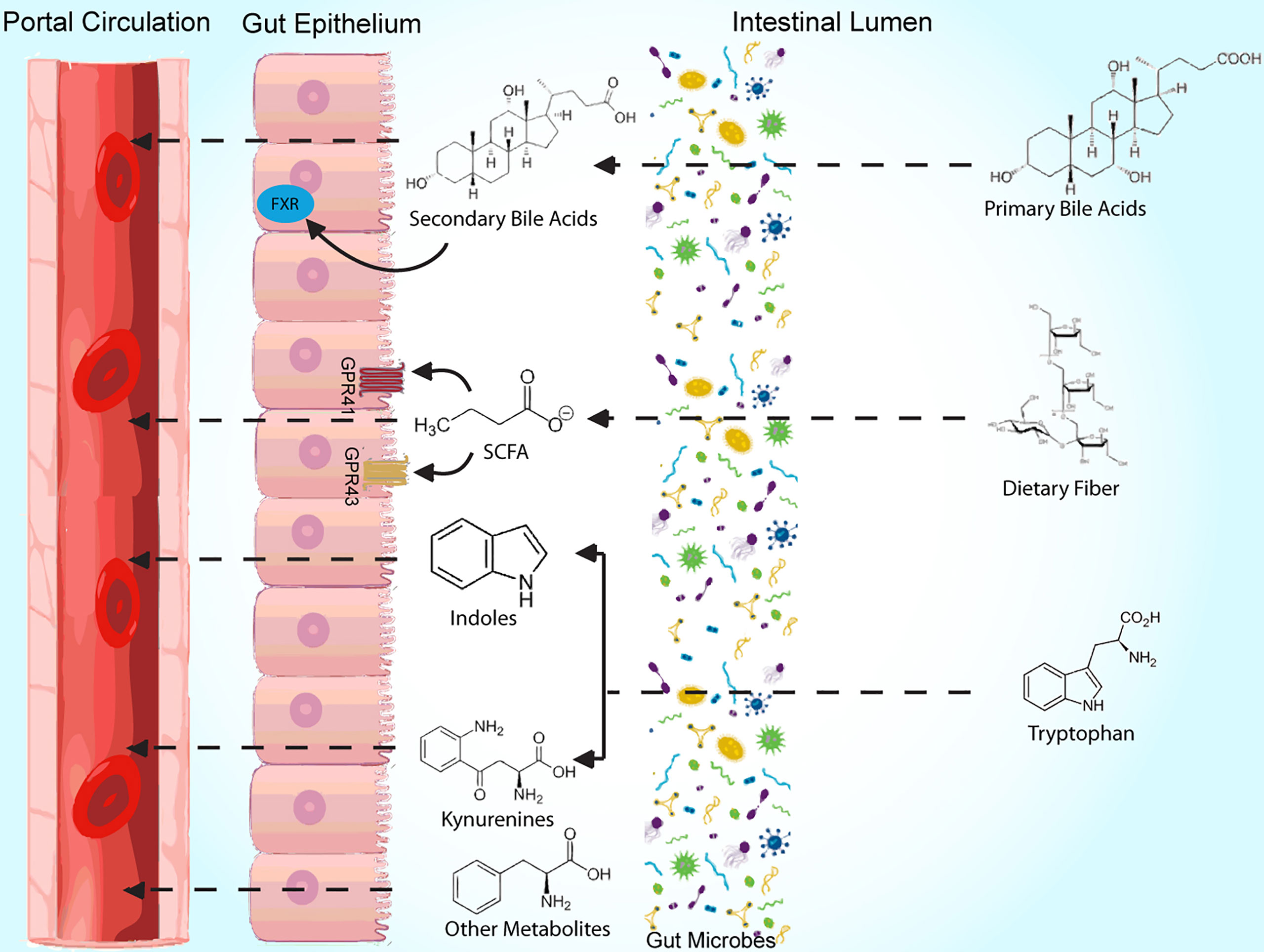
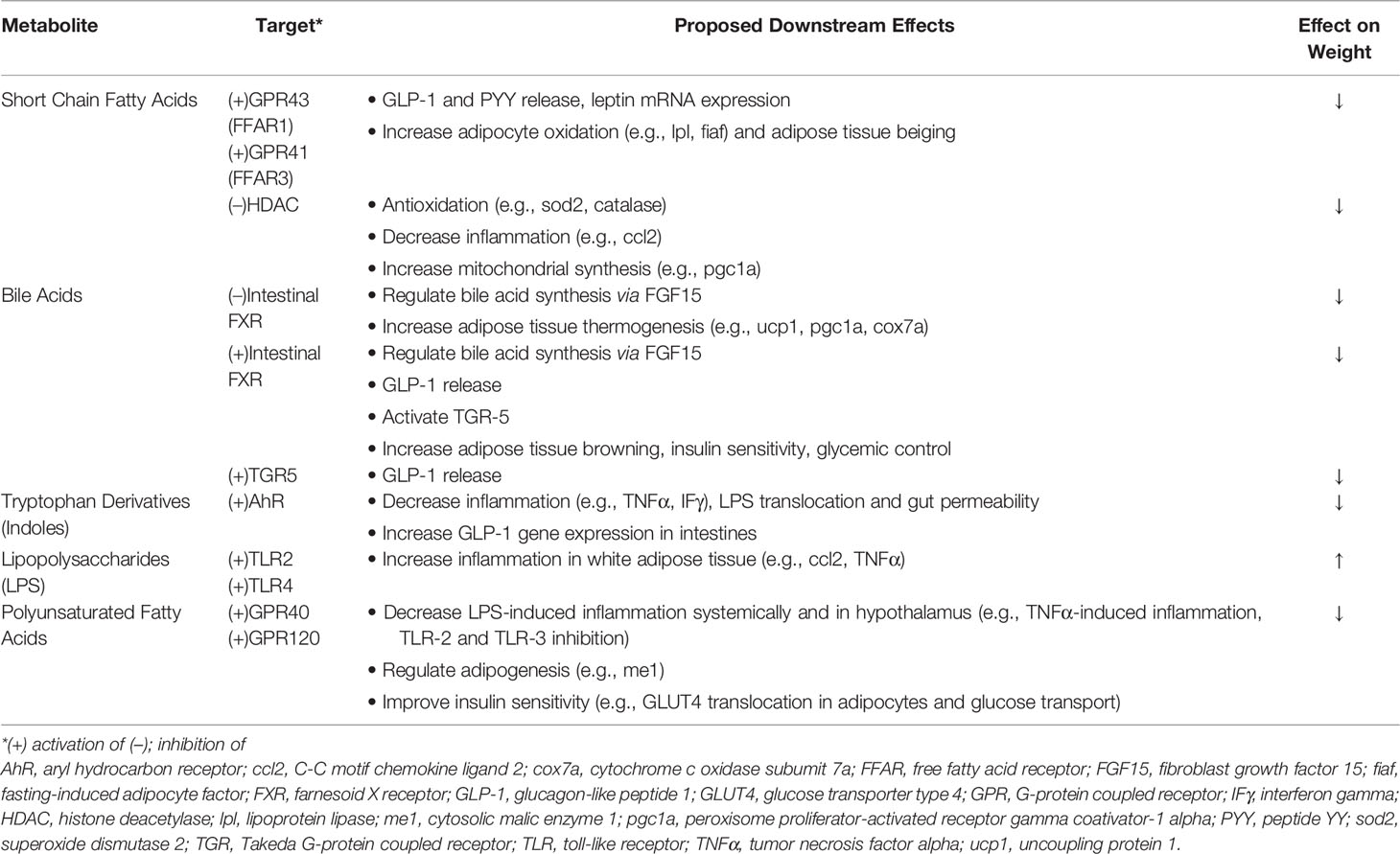
The obesity epidemic, which affects greater than a third of Americans (1), is a global health issue involving 650 million adults worldwide (2). Successful treatment of obesity has historically been difficult (3), likely from insufficient knowledge of its pathophysiology, and microbial colonizers of the gut, which have gained recognition for their role in metabolic disease, may serve as the missing link. Early research of the gut microbiome’s role in weight regulation has largely involved correlational studies of microbial composition, and differences in microbiome content have been reported among groups with varying genetic and environmental backgrounds (4, 5). Further, studies have associated obesity with taxonomic changes of the microbiome in response to antibiotic use (6, 7); similar investigations of the microbiome have been performed to evaluate various dietary interventions (8–11). However, in recent studies, there has been a shift prioritizing assessment of microbial function over composition in an effort to better guide its use in treatment of obesity. In this review, we summarize key and recent literature related to mechanisms of several commonly studied metabolites involved in microbiome-mediated pathways resulting in obesity, including short chain fatty acids, bile acids, and tryptophan derivatives, in obesity development (Figure 1, Table 1). Clinical applications, as they relate to these metabolites, are also discussed. We searched for original research articles in PubMed and Google Scholar using combinations of the following key words: gut microbiome, obesity, short chain fatty acid, bile acids, tryptophan, LPS.
Mechanism of Action
Short Chain Fatty Acids
The most abundant microbial metabolites are short-chain fatty acids (SCFAs), which are breakdown products of carbohydrates that occur from bacterial fermentation in the gut. Numerous studies have investigated the role of various SCFAs in mediating the gut microbiota’s effects on metabolic syndrome. In general, SCFAs have been associated with beneficial effects on metabolic health. For instance, higher levels of fecal SCFAs, including butyrate, acetate, and propionate, in humans have been correlated with markers indicative of improved insulin resistance, obesity and food intake (12–14).
To better understand these gut-derived metabolites, studies have investigated the role of G protein-coupled receptors, namely GPR43 and GPR41 (alternatively known as free fatty acid receptors 1 and 3, respectively), which are both activated by SCFAs (15). Kimura et al. found that mice with knockout genes (KO) for GPR43 gained weight without a high fat diet (HFD), while GPR43 overexpression prevented obesity despite a HFD (16). Results from such studies serve as evidence of the receptor’s involvement in obesity development, which may in part be mediated by the release of satiety hormones glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) from enteroendocrine cells (17), free fatty acid oxidation of adipose tissue (18), and control of energy expenditure (16). Additionally, GPR41 expression on vagal sensory neurons suggests that the receptor may influence centrally mediated effects on normal eating behavior, which was altered in GPR41 KO mice (19). SCFAs may also directly communicate with the central nervous system, as evidenced by carbon-labeled uptake by the brain of intraperitoneally administered acetate in a PET-CT imaging study (14).
Alternatively, SCFAs may act by inhibiting histone deacetylase (HDAC), a crucial enzyme in DNA transcription, given reduced HDAC activity measured in butyrate-treated enterocytes (20). Further, reduction of weight gain with butyrate treatment in HFD-fed mice was lost in HDAC KO mice, highlighting the significance of this enzyme in mediating SCFA’s effects (20). Butyrate-mediated HDAC inhibition has been associated with increased gene expression for PYY (21), as well as for various antioxidants and mitochondrial synthesis (22), which reduce metabolic dysfunction. In summary, SCFA’s favorable effects on obesity are likely channeled through several pathways.
Of note, data supporting beneficial effects of SCFAs have been met with some skepticism, as results from an early study of obese mice were suggestive of greater fecal energy extraction in association with higher levels of SCFAs (23). In subsequent studies, higher fecal concentrations of both various and total SCFAs have been linked to obesity (24–26), and further, circulating levels of SCFAs have been positively associated with weight (27, 28). More studies are needed to elucidate the mechanisms and clinical effects of SCFAs.
Bile Acids
The function of bile acids (BAs) within the gut not only lies in intestinal lipid absorption, but also in mediating the metabolic effects of the microbiome. From cholesterol, primary BAs are formed and subsequently conjugated in the liver; when released into the gut, microbiota mediate deconjugation and metabolism to secondary BAs (29). In a correlational study of microbiome from both mice and human subjects, levels of the BAs ursodeoxycholate (UDCA), chenodeoxycholate, and lithocholate (LCA) were reduced in obesity (30), suggesting beneficial effects of non-12-hydroxylated BAs. Similarly, mice that had improvements in metabolic markers, such as weight, after Parabacateroides distasonis administration that elevated UDCA and LCA (31). Further, antibiotic treatment leading to altered microbiome in mice produced both an improved metabolic phenotypes and increased tauro-β-muricholic acid (TBMCA) levels (32).
BA mediated outcomes on metabolic syndrome involve the farnesoid X receptor (FXR), whose activation modulates expression of genes regulating metabolism and BA synthesis (33, 34). FXR antagonism by BAs such as glycine-β-muricholic acid (Gly-MCA) and TBMCA in intestinal cells has been associated with prevention of obesity (32, 35), with similar effects seen from mice with intestinal FXR KO genes (36). The microbiome’s link to this pathway was strengthened in a study demonstrating more obesity in conventionally raised, compared to GF mice but no difference in weight when mice were FXR KO (36). However, intestine-specific FXR agonists such as fexaramine also generated favorable metabolic profiles that were associated with BA compositional changes (37–39). These mixed results of FXR-mediated activity are not completely understood and require further investigation.
In addition to FXR, Takeda G-protein coupled receptor 5 (TGR5) is also essential in the BA pathway, where its agonism and overexpression in HFD-fed mice were both linked to metabolic improvement (40). These outcomes may result from TGR5-stimulated release of GLP-1, and receptor activation likely plays a role in both peripherally-mediated metabolic and centrally-mediated eating/behavioral mechanisms influencing obesity (40–42). Effective use of BAs in combating obesity may need to involve both receptors, which have been shown to produce distinct downstream effects from one another (43).
Tryptophan Derivatives
In addition to its many roles as an essential amino acid, tryptophan also modulates the microbiome’s effects on weight and metabolism (44). After dietary ingestion, processing by the gut can follow one of three pathways forming either serotonin, kynurenine (Kyn) or indole metabolites (44). The link between tryptophan derivatives and the gut microbiome was strengthened in several correlation studies (45), including one that associated changes in microbial composition with levels of tryptophan-derived neurotransmitters and neurotransmitter transporters in piglets administered antibiotics (46). Further, the presence of spore forming bacteria were linked to higher levels of serotonergic metabolites in mice (47). Obesity development has also been attributed to activity of indoleamine 2,3 dioxygenase (IDO), yielding higher levels of kynurenine, as well as with lower circulating levels of indole products (48–50). When mice on a HFD were either administered indole or genetically lacked IDO, weight gain was mitigated (48, 50).
Trypophan-derived metabolites likely exert their effects in part through aryl hydrocarbon receptors (AhR) (33, 51), given positive associations seen among indole metabolites, AhR activation and improved metabolic markers. For instance, diet induced obese (DIO) mice that had lower fecal indole derivative concentrations also had decreased AhR activity, and several metabolic effects (such as insulin sensitivity) were reversed with use of an AhR agonist (52). Effects are thought to be mediated by the microbiome, as indole metabolite levels were altered in conventional, but not in GF, mice on HFD (53). However, AhR antagonism was also associated with beneficial metabolic effects in a study using both ligands antagonizing AhR and AhR genetic deletions in mice (54); given that this inconsistent response was thought to be mediated by Kyn rather than indole, future studies may be needed to evaluate differences in response from various AhR ligands.
Downstream effects of microbial tryptophan metabolism have been correlated to the central nervous system as part of the brain-gut axis. In trials of obese humans, indolepropionic acid (IPA) levels inversely correlated with food addiction and magnetic resonance imaging (MRI) derived activity in reward centers of the brain (55), with the latter finding seen again in relation to fecal tryptophan levels (56). In addition, central inhibitory control positively correlated with IPA levels in obese humans; mice with microbial transplants from obese humans with impaired inhibitory control not only exhibited similar behavior but also altered prefrontal cortex activity as measured by metabolic gene expression (57). Tryptophan and its metabolites, as part of the brain-gut axis, have gained recognition for their integral role in obesity development.
Miscellaneous Metabolites
Metabolic influence of the gut microbiome may also involve the immune system. In a study of rats continuously infused lipopolysaccharide (LPS), often a virulent component of gram-negative bacterial cell walls (58), chronic LPS exposure led to hyperphagia and leptin resistance (59). However, virulence and endotoxic response from LPS may vary, contingent on the bacterium from which it is derived (60). Toll-like receptors (TLR) may mediate these effects. Caesar et al. reported greater weight gain and activation of LPS receptor, TLR4, from serum of lard-fed mice, with weight preservation in mice with KO genes for TLR adaptor molecules (61). Further, serum bacterial DNA levels were similar between obese and nonobese mice, suggesting a more direct role for molecular signaling than systemic bacterial infiltration in inducing these effects (61). TLR4 activation is linked to increases in inflammatory markers within adipose tissue, which in turn produces phenotypes associated with metabolic syndrome (61).
Polyunsaturated fatty acid (PUFA)-derived metabolites have also been linked to metabolic health. The microbiome was implicated in transforming PUFAs to their metabolites in a study that reported lower fecal levels of PUFA metabolites in GF, compared to conventional, mice (62). Further, FMTs from HFD-fed mice supplemented with a PUFA metabolite enhanced glycemic control compared to those from mice without supplementation (63). These gut-derived substances may act through their activation of GPR40 and GPR120, producing favorable outcomes such as reduction of inflammation, lipogenesis, and glucose intolerance (64–66). Overall, results from studies involving these metabolites have been encouraging.
Clinical Applications
In this section, we discuss potential novel options for oral treatment of obesity involving the gut microbiome, in the context of alterations to metabolites and mechanisms discussed in our review.
Direct Microbiome Involvement
Two of the most common classes of therapies targeted directly at changing composition of the gut include prebiotics and probiotics. Though outcomes from studies measuring probiotic efficacy in treating obesity have been mixed, recent clinical trials of Akkermansia muciniphila have shown promising results. Obese patients who received A. muciniphila supplementation achieved more weight loss compared to those who received placebo, and metabolic changes in the treatment group were associated with decrease in plasma LPS levels; however, a difference was not seen with levels of GLP-1 (67). Mouse organoids exposed to A. muciniphila experienced greater modulation of genes, such as HDAC and Gpr43, than those for Faecalibacterium prausnitzii (68), which may suggest more efficacy of A. muiniphila use. In addition, various prebiotics, which are fibers digested by the gut microbiome, have been associated with changes in microbial composition (69–71), as well as with weight and metabolic activity (70, 71). Despite some encouraging data, no formal recommendations on use of A. muciniphila or prebiotic formulations exist.
Polyphenols are plant metabolites often poorly absorbed in the gut, resulting in delivery to gut microbiome for processing and essentially functioning as prebiotics (72). Several studies of polyphenols, such as resveratrol and various fruit extracts have correlated levels with improved metabolic markers (73–75) and altered microbial bile acid composition, TGR5 expression and TLR4 activation (76, 77), indicative of microbiome-induced metabolic changes. However, poor bioavailability has limited its clinical applicability, and additional studies of human use are warranted to develop effective formulations of polyphenol ingredients (78, 79).
Indirect Microbiome Involvement
Other potential therapies are aimed at pathways involving the gut microbiome. For example, synthetic forms of metabolites from microbial processing have been studied in instances when poor drug delivery restricts effective use. Clinical use of butyrate is limited by its short half-life; instead, its prodrug, tributyrin may provide metabolic benefit with an improved pharmacokinetic profile (80). Additionally, Gly-MCA, bile acid derivative, effectively inhibited intestinal FXR to reduce obesity in mice, while resisting hydrolytic activity by bile salt hydrolase, whose activity could hinder its use in vivo (35). Alternatively, treatment with obeticholic acid, an FXR agonist with better delivery compared to its less lipophilic bile acid derivative, has been associated with improved hepatic steatosis in humans (81, 82).
Therapeutic agents for obesity may also target associated pathways of gut-microbial action. Use of BA sequestrants reducing intracolonic BA levels may have downstream, suppressive activity on FXR and has been linked to GLP-1 secretion and improved glycemic control (83). Perhaps the most well-recognized instance of microbiome-induced pathway modulation involves the use of GLP-1 receptor agonists, such as semaglutide (84) whose success and widely accepted use in both diabetes and obesity treatment is unsurprising, given the extent of data associating GLP-1 release with healthier phenotypes. In summary, modulations to microbial metabolites and their complex pathways present considerable opportunities for obesity treatment, which require further investigation.
Discussion and Limitations
As we deepen our understanding of the gut’s role in metabolism and advance our knowledge of precision medicine, it becomes more evident that incorporating the microbiome will be critical to both prognostication and treatment of obesity. While recent research has focused on characterizing function over composition of the microbiome, an interplay between the two inherently exists, and treatment should integrate knowledge of both entities. For instance, we believe that microbiome-based therapies will be an adjunct to established treatments for obesity, as well as a tool for personalized medicine in the future. Oral probiotic or prebiotic supplements aimed at altering the composition and function of the microbiome, in conjunction with dietary and lifestyle modifications, can reach separate targets and increase the likelihood of success for weight loss. Furthermore, systemic and fecal concentrations of molecular signals from the microbiome’s functional domain may serve as biomarkers to predict outcomes, including risk of obesity, development of metabolic syndrome, or likelihood to respond to therapies for obesity, such as specialized diets (e.g., keto, Mediterranean, high protein) or bariatric surgery.
While the gut microbiome holds promise in improving treatment options for obesity, several challenges remain. A significant proportion of data stems from studies involving mice, which may be less accurate when applied to humans (85). Further, trials involving human subjects may also present difficulties. First, variations in human behavior may restrict the ability to conduct controlled studies. For example, compliance with an intervention such as diet or exercise may vary between subjects and often relies on the participant’s disclosure, which may be inaccurate. Given the impact of patient-dependent factors, such as diet, on the gut microbiome, a wide spectrum of baseline microbial composition and diversity likely exists, which may affect outcomes. Additionally, unlike studies of GF and genetically altered mice, trials involving humans have mostly produced data suggestive of correlational, rather than causative, relationships.
There are also limitations to the existing knowledge in the field. Well-defined roles for specific microbes of the gut have yet to be defined, and there are varied results in the literature. This is also true of several molecules, such as FXR and AhR, whose activation has been associated with both beneficial and harmful effects on metabolic disease. We may still have limited understanding of complex signaling pathways, which should continue to be investigated. Further, there should be increased efforts to evaluate and discover clinical applications of the gut microbiome in obesity treatment.
In summary, a rapidly expanding body of research suggests that the gut microbiome is essential in the development of metabolic diseases, including obesity. This is likely mediated through several gut-derived metabolites and their downstream effects on both central and peripheral pathways, which require further research and understanding.
Author Contributions
AHL: literature review, manuscript preparation. AM: literature review, manuscript preparation. TD: manuscript revisions and revision, tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief (2020) 360):1–8.
2. Organization WH. Obesity and Overweight (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
3. Nordmo M, Danielsen YS, Nordmo M. The Challenge of Keeping It Off, a Descriptive Systematic Review of High-Quality, Follow-Up Studies of Obesity Treatments. Obes Rev (2020) 21(1):e12949. doi: 10.1111/obr.12949
4. Deschasaux M, Bouter KE, Prodan A, Levin E, Groen AK, Herrema H, et al. Depicting the Composition of Gut Microbiota in a Population With Varied Ethnic Origins But Shared Geography. Nat Med (2018) 24(10):1526–31. doi: 10.1038/s41591-018-0160-1
5. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment Dominates Over Host Genetics in Shaping Human Gut Microbiota. Nature (2018) 555(7695):210–5. doi: 10.1038/nature25973
6. Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the Intestinal Microbiota During a Critical Developmental Window Has Lasting Metabolic Consequences. Cell (2014) 158(4):705–21. doi: 10.1016/j.cell.2014.05.052
7. Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, et al. Prenatal Exposure to Antibiotics, Cesarean Section and Risk of Childhood Obesity. Int J Obes (Lond) (2015) 39(4):665–70. doi: 10.1038/ijo.2014.180
8. Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab (2017) 26(4):672–85.e4. doi: 10.1016/j.cmet.2017.08.019
9. Fabbiano S, Suárez-Zamorano N, Chevalier C, Lazarević V, Kieser S, Rigo D, et al. Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Cell Metab (2018) 28(6):907–21.e7. doi: 10.1016/j.cmet.2018.08.005
10. Kahleova H, Rembert E, Alwarith J, Yonas WN, Tura A, Holubkov R, et al. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships With Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients (2020) 12(10):2917. doi: 10.3390/nu12102917
11. Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean Diet Intervention in Overweight and Obese Subjects Lowers Plasma Cholesterol and Causes Changes in the Gut Microbiome and Metabolome Independently of Energy Intake. Gut (2020) 69(7):1258–68. doi: 10.1136/gutjnl-2019-320438
12. Sanna S, van Zuydam NR, Mahajan A, Kurilshikov A, Vich Vila A, Võsa U, et al. Causal Relationships Among the Gut Microbiome, Short-Chain Fatty Acids and Metabolic Diseases. Nat Genet (2019) 51(4):600–5. doi: 10.1038/s41588-019-0350-x
13. Li Z, Yi CX, Katiraei S, Kooijman S, Zhou E, Chung CK, et al. Butyrate Reduces Appetite and Activates Brown Adipose Tissue via the Gut-Brain Neural Circuit. Gut (2018) 67(7):1269–79. doi: 10.1136/gutjnl-2017-314050
14. Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, et al. The Short-Chain Fatty Acid Acetate Reduces Appetite via a Central Homeostatic Mechanism. Nat Commun (2014) 5:3611. doi: 10.1038/ncomms4611
15. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G Protein-Coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J Biol Chem (2003) 278(13):11312–9. doi: 10.1074/jbc.M211609200
16. Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The Gut Microbiota Suppresses Insulin-Mediated Fat Accumulation via the Short-Chain Fatty Acid Receptor GPR43. Nat Commun (2013) 4:1829. doi: 10.1038/ncomms2852
17. Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, et al. The Short Chain Fatty Acid Propionate Stimulates GLP-1 and PYY Secretion via Free Fatty Acid Receptor 2 in Rodents. Int J Obes (Lond) (2015) 39(3):424–9. doi: 10.1038/ijo.2014.153
18. Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short Chain Fatty Acids Prevent High-Fat-Diet-Induced Obesity in Mice by Regulating G Protein-Coupled Receptors and Gut Microbiota. Sci Rep (2016) 6:37589. doi: 10.1038/srep37589
19. Cook TM, Gavini CK, Jesse J, Aubert G, Gornick E, Bonomo R, et al. Vagal Neuron Expression of the Microbiota-Derived Metabolite Receptor, Free Fatty Acid Receptor (FFAR3), Is Necessary for Normal Feeding Behavior. Mol Metab (2021) 54:101350. doi: 10.1016/j.molmet.2021.101350
20. Whitt J, Woo V, Lee P, Moncivaiz J, Haberman Y, Denson L, et al. Disruption of Epithelial HDAC3 in Intestine Prevents Diet-Induced Obesity in Mice. Gastroenterology (2018) 155(2):501–13. doi: 10.1053/j.gastro.2018.04.017
21. Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble FM, Reimann F, et al. SCFAs Strongly Stimulate PYY Production in Human Enteroendocrine Cells. Sci Rep (2018) 8(1):74. doi: 10.1038/s41598-017-18259-0
22. Chriett S, Dąbek A, Wojtala M, Vidal H, Balcerczyk A, Pirola L. Prominent Action of Butyrate Over β-Hydroxybutyrate as Histone Deacetylase Inhibitor, Transcriptional Modulator and Anti-Inflammatory Molecule. Sci Rep (2019) 9(1):742. doi: 10.1038/s41598-018-36941-9
23. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An Obesity-Associated Gut Microbiome With Increased Capacity for Energy Harvest. Nat (2006) 444(7122):1027–31. doi: 10.1038/nature05414
24. de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher Fecal Short-Chain Fatty Acid Levels Are Associated With Gut Microbiome Dysbiosis, Obesity, Hypertension and Cardiometabolic Disease Risk Factors. Nutrients (2018) 11(1):51. doi: 10.3390/nu11010051
25. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TM. Evidence for Greater Production of Colonic Short-Chain Fatty Acids in Overweight Than Lean Humans. Int J Obes (Lond) (2014) 38(12):1525–31. doi: 10.1038/ijo.2014.46
26. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, Gut Microbiota and Faecal Short Chain Fatty Acids Are Linked in Adult Humans. Nutr Diabetes (2014) 4(6):e121. doi: 10.1038/nutd.2014.23
27. Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate Mediates a Microbiome-Brain-β-Cell Axis to Promote Metabolic Syndrome. Nat (2016) 534(7606):213–7. doi: 10.1038/nature18309
28. Serena C, Ceperuelo-Mallafré V, Keiran N, Queipo-Ortuño MI, Bernal R, Gomez-Huelgas R, et al. Elevated Circulating Levels of Succinate in Human Obesity are Linked to Specific Gut Microbiota. Isme J (2018) 12(7):1642–57. doi: 10.1038/s41396-018-0068-2
29. Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk Between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab (2016) 24(1):41–50. doi: 10.1016/j.cmet.2016.05.005
30. Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, et al. A Dysregulated Bile Acid-Gut Microbiota Axis Contributes to Obesity Susceptibility. EBioMedicine (2020) 55:102766. doi: 10.1016/j.ebiom.2020.102766
31. Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, et al. Parabacteroides Distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep (2019) 26(1):222–35.e5. doi: 10.1016/j.celrep.2018.12.028
32. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. Gut Microbiota Regulates Bile Acid Metabolism by Reducing the Levels of Tauro-Beta-Muricholic Acid, a Naturally Occurring FXR Antagonist. Cell Metab (2013) 17(2):225–35. doi: 10.1016/j.cmet.2013.01.003
33. Agus A, Clément K, Sokol H. Gut Microbiota-Derived Metabolites as Central Regulators in Metabolic Disorders. Gut (2021) 70(6):1174–82. doi: 10.1136/gutjnl-2020-323071
34. Song X, Wang L, Liu Y, Zhang X, Weng P, Liu L, et al. The Gut Microbiota-Brain Axis: Role of the Gut Microbial Metabolites of Dietary Food in Obesity. Food Res Int (2022) 153:110971. doi: 10.1016/j.foodres.2022.110971
35. Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, et al. Intestine-Selective Farnesoid X Receptor Inhibition Improves Obesity-Related Metabolic Dysfunction. Nat Commun (2015) 6:10166. doi: 10.1038/ncomms10166
36. Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, et al. Microbiota-Induced Obesity Requires Farnesoid X Receptor. Gut (2017) 66(3):429–37. doi: 10.1136/gutjnl-2015-310283
37. Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, et al. Farnesoid X Receptor Induces Takeda G-Protein Receptor 5 Cross-Talk to Regulate Bile Acid Synthesis and Hepatic Metabolism. J Biol Chem (2017) 292(26):11055–69. doi: 10.1074/jbc.M117.784322
38. Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, et al. Intestine Farnesoid X Receptor Agonist and the Gut Microbiota Activate G-Protein Bile Acid Receptor-1 Signaling to Improve Metabolism. Hepatology (2018) 68(4):1574–88. doi: 10.1002/hep.29857
39. Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, et al. Intestinal FXR Agonism Promotes Adipose Tissue Browning and Reduces Obesity and Insulin Resistance. Nat Med (2015) 21(2):159–65. doi: 10.1038/nm.3760
40. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab (2009) 10(3):167–77. doi: 10.1016/j.cmet.2009.08.001
41. Bensalem A, Murtaza B, Hichami A, Khan AS, Oulamara H, Merlen G, et al. Bile Acid Receptor TGR5 Is Critically Involved in Preference for Dietary Lipids and Obesity. J Nutr Biochem (2020) 76:108298. doi: 10.1016/j.jnutbio.2019.108298
42. Castellanos-Jankiewicz A, Guzmán-Quevedo O, Fénelon VS, Zizzari P, Quarta C, Bellocchio L, et al. Hypothalamic Bile Acid-TGR5 Signaling Protects From Obesity. Cell Metab (2021) 33(7):1483–92.e10. doi: 10.1016/j.cmet.2021.04.009
43. Jadhav K, Xu Y, Xu Y, Li Y, Xu J, Zhu Y, et al. Reversal of Metabolic Disorders by Pharmacological Activation of Bile Acid Receptors TGR5 and FXR. Mol Metab (2018) 9:131–40. doi: 10.1016/j.molmet.2018.01.005
44. Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr (2020) 11(3):709–23. doi: 10.1093/advances/nmz127
45. Menni C, Hernandez MM, Vital M, Mohney RP, Spector TD, Valdes AM. Circulating Levels of the Anti-Oxidant Indoleproprionic Acid Are Associated With Higher Gut Microbiome Diversity. Gut Microbes (2019) 10(6):688–95. doi: 10.1080/19490976.2019.1586038
46. Gao K, Pi Y, Mu CL, Peng Y, Huang Z, Zhu WY. Antibiotics-Induced Modulation of Large Intestinal Microbiota Altered Aromatic Amino Acid Profile and Expression of Neurotransmitters in the Hypothalamus of Piglets. J Neurochem (2018) 146(3):219–34. doi: 10.1111/jnc.14333
47. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous Bacteria From the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell (2015) 161(2):264–76. doi: 10.1016/j.cell.2015.02.047
48. Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, et al. Genetic Deficiency of Indoleamine 2,3-Dioxygenase Promotes Gut Microbiota-Mediated Metabolic Health. Nat Med (2018) 24(8):1113–20. doi: 10.1038/s41591-018-0060-4
49. Cussotto S, Delgado I, Anesi A, Dexpert S, Aubert A, Beau C, et al. Tryptophan Metabolic Pathways Are Altered in Obesity and Are Associated With Systemic Inflammation. Front Immunol (2020) 11:557. doi: 10.3389/fimmu.2020.00557
50. Virtue AT, McCright SJ, Wright JM, Jimenez MT, Mowel WK, Kotzin JJ, et al. The Gut Microbiota Regulates White Adipose Tissue Inflammation and Obesity via a Family of microRNAs. Sci Transl Med (2019) 11(496):eaav1892. doi: 10.1126/scitranslmed.aav1892
51. de Vos WM, Tilg H, Van Hul M, Cani PD. Gut Microbiome and Health: Mechanistic Insights. Gut (2022) 71(5):1020–32. doi: 10.1136/gutjnl-2021-326789
52. Natividad JM, Agus A, Planchais J, Lamas B, Jarry AC, Martin R, et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab (2018) 28(5):737–49.e4. doi: 10.1016/j.cmet.2018.07.001
53. Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep (2018) 23(4):1099–111. doi: 10.1016/j.celrep.2018.03.109
54. Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, et al. Inhibition of the Aryl Hydrocarbon Receptor Prevents Western Diet-Induced Obesity. Model for AHR Activation by Kynurenine via Oxidized-LDL, TLR2/4, Tgfβ, and IDO1. Toxicol Appl Pharmacol (2016) 300:13–24. doi: 10.1016/j.taap.2016.03.011
55. Dong TS, Mayer EA, Osadchiy V, Chang C, Katzka W, Lagishetty V, et al. A Distinct Brain-Gut-Microbiome Profile Exists for Females With Obesity and Food Addiction. Obes (Silver Spring) (2020) 28(8):1477–86. doi: 10.1002/oby.22870
56. Dong TS, Guan M, Mayer EA, Stains J, Liu C, Vora P, et al. Obesity Is Associated With a Distinct Brain-Gut Microbiome Signature That Connects Prevotella and Bacteroides to the Brain's Reward Center. Gut Microbes (2022) 14(1):2051999. doi: 10.1080/19490976.2022.2051999
57. Arnoriaga-Rodríguez M, Mayneris-Perxachs J, Contreras-Rodríguez O, Burokas A, Ortega-Sanchez JA, Blasco G, et al. Obesity-Associated Deficits in Inhibitory Control Are Phenocopied to Mice Through Gut Microbiota Changes in One-Carbon and Aromatic Amino Acids Metabolic Pathways. Gut (2021) 70(12):2283–96. doi: 10.1136/gutjnl-2020-323371
58. Maldonado RF, Sá-Correia I, Valvano MA. Lipopolysaccharide Modification in Gram-Negative Bacteria During Chronic Infection. FEMS Microbiol Rev (2016) 40(4):480–93. doi: 10.1093/femsre/fuw007
59. de La Serre CB, de Lartigue G, Raybould HE. Chronic Exposure to Low Dose Bacterial Lipopolysaccharide Inhibits Leptin Signaling in Vagal Afferent Neurons. Physiol Behav (2015) 139:188–94. doi: 10.1016/j.physbeh.2014.10.032
60. Anhê FF, Barra NG, Cavallari JF, Henriksbo BD, Schertzer JD. Metabolic Endotoxemia Is Dictated by the Type of Lipopolysaccharide. Cell Rep (2021) 36(11):109691. doi: 10.1016/j.celrep.2021.109691
61. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk Between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation Through TLR Signaling. Cell Metab (2015) 22(4):658–68. doi: 10.1016/j.cmet.2015.07.026
62. Druart C, Bindels LB, Schmaltz R, Neyrinck AM, Cani PD, Walter J, et al. Ability of the Gut Microbiota to Produce PUFA-Derived Bacterial Metabolites: Proof of Concept in Germ-Free Versus Conventionalized Mice. Mol Nutr Food Res (2015) 59(8):1603–13. doi: 10.1002/mnfr.201500014
63. Miyamoto J, Igarashi M, Watanabe K, Karaki SI, Mukouyama H, Kishino S, et al. Gut Microbiota Confers Host Resistance to Obesity by Metabolizing Dietary Polyunsaturated Fatty Acids. Nat Commun (2019) 10(1):4007. doi: 10.1038/s41467-019-11978-0
64. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell (2010) 142(5):687–98. doi: 10.1016/j.cell.2010.07.041
65. Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, et al. Dysfunction of Lipid Sensor GPR120 Leads to Obesity in Both Mouse and Human. Nat (2012) 483(7389):350–4. doi: 10.1038/nature10798
66. Dragano NRV, Solon C, Ramalho AF, de Moura RF, Razolli DS, Christiansen E, et al. Polyunsaturated Fatty Acid Receptors, GPR40 and GPR120, Are Expressed in the Hypothalamus and Control Energy Homeostasis and Inflammation. J Neuroinflammation (2017) 14(1):91. doi: 10.1186/s12974-017-0869-7
67. Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation With Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat Med (2019) 25(7):1096–103. doi: 10.1038/s41591-019-0495-2
68. Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, et al. Differential Modulation by Akkermansia Muciniphila and Faecalibacterium Prausnitzii of Host Peripheral Lipid Metabolism and Histone Acetylation in Mouse Gut Organoids. mBio (2014) 5(4):e01438–14. doi: 10.1128/mBio.01438-14
69. Delannoy-Bruno O, Desai C, Raman AS, Chen RY, Hibberd MC, Cheng J, et al. Evaluating Microbiome-Directed Fibre Snacks in Gnotobiotic Mice and Humans. Nat (2021) 595(7865):91–5. doi: 10.1038/s41586-021-03671-4
70. Nicolucci AC, Hume MP, Martínez I, Mayengbam S, Walter J, Reimer RA. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology (2017) 153(3):711–22. doi: 10.1053/j.gastro.2017.05.055
71. Hiel S, Gianfrancesco MA, Rodriguez J, Portheault D, Leyrolle Q, Bindels LB, et al. Link Between Gut Microbiota and Health Outcomes in Inulin -Treated Obese Patients: Lessons From the Food4Gut Multicenter Randomized Placebo-Controlled Trial. Clin Nutr (2020) 39(12):3618–28. doi: 10.1016/j.clnu.2020.04.005
72. Man AWC, Zhou Y, Xia N, Li H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction With Polyphenol in Host Immunometabolism. Nutrients (2020) 12(10):3054. doi: 10.3390/nu12103054
73. Wang P, Gao J, Ke W, Wang J, Li D, Liu R, et al. Resveratrol Reduces Obesity in High-Fat Diet-Fed Mice via Modulating the Composition and Metabolic Function of the Gut Microbiota. Free Radic Biol Med (2020) 156:83–98. doi: 10.1016/j.freeradbiomed.2020.04.013
74. Wang P, Li D, Ke W, Liang D, Hu X, Chen F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int J Obes (Lond) (2020) 44(1):213–25. doi: 10.1038/s41366-019-0332-1
75. Kim TT, Parajuli N, Sung MM, Bairwa SC, Levasseur J, Soltys CM, et al. Fecal Transplant From Resveratrol-Fed Donors Improves Glycaemia and Cardiovascular Features of the Metabolic Syndrome in Mice. Am J Physiol Endocrinol Metab (2018) 315(4):E511–e9. doi: 10.1152/ajpendo.00471.2017
76. Han X, Guo J, Yin M, Liu Y, You Y, Zhan J, et al. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol Nutr Food Res (2020) 64(10):e2000149. doi: 10.1002/mnfr.202000149
77. Ye Y, Warusawitharana H, Zhao H, Liu Z, Li B, Wu Y, et al. Tea Polyphenols Attenuates Inflammation via Reducing Lipopolysaccharides Level and Inhibiting TLR4/NF-κb Pathway in Obese Mice. Plant Foods Hum Nutr (2022) 77(1):105–11. doi: 10.1007/s11130-021-00937-0
78. Morissette A, Kropp C, Songpadith JP, Junges Moreira R, Costa J, Mariné-Casadó R, et al. Blueberry Proanthocyanidins and Anthocyanins Improve Metabolic Health Through a Gut Microbiota-Dependent Mechanism in Diet-Induced Obese Mice. Am J Physiol Endocrinol Metab (2020) 318(6):E965–e80. doi: 10.1152/ajpendo.00560.2019
79. Li Q, Liu F, Liu J, Liao S, Zou Y. Mulberry Leaf Polyphenols and Fiber Induce Synergistic Antiobesity and Display a Modulation Effect on Gut Microbiota and Metabolites. Nutrients (2019) 11(5):1017. doi: 10.3390/nu11051017
80. Sato FT, Yap YA, Crisma AR, Portovedo M, Murata GM, Hirabara SM, et al. Tributyrin Attenuates Metabolic and Inflammatory Changes Associated With Obesity Through a GPR109A-Dependent Mechanism. Cells (2020) 9(9):2007. doi: 10.3390/cells9092007
81. Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic Acid for the Treatment of Non-Alcoholic Steatohepatitis: Interim Analysis From a Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. Lancet (2019) 394(10215):2184–96. doi: 10.1016/S0140-6736(19)33041-7
82. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X Nuclear Receptor Ligand Obeticholic Acid for non-Cirrhotic, Non-Alcoholic Steatohepatitis (FLINT): A Multicentre, Randomised, Placebo-Controlled Trial. Lancet (2015) 385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4
83. Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, et al. Farnesoid X Receptor Inhibits Glucagon-Like Peptide-1 Production by Enteroendocrine L Cells. Nat Commun (2015) 6:7629. doi: 10.1038/ncomms8629
84. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-Weekly Semaglutide in Adults With Overweight or Obesity. N Engl J Med (2021) 384(11):989. doi: 10.1056/NEJMoa2032183
Keywords: gut, microbiome, microbiota, obesity, weight
Citation: Lee AH, Manly A and Dong TS (2022) Leveraging the Microbiome for Obesity: Moving From Form to Function. Front. Endocrinol. 13:918923. doi: 10.3389/fendo.2022.918923
Received: 12 April 2022; Accepted: 13 June 2022;
Published: 07 July 2022.
Edited by:
Konstantinos Tziomalos, Aristotle University of Thessaloniki, GreeceReviewed by:
Amanda Cuevas-Sierra, IMDEA Food Institute, SpainCopyright © 2022 Lee, Manly and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna H. Lee, QW5uYUxlZUBtZWRuZXQudWNsYS5lZHU=
 Anna H. Lee
Anna H. Lee Amanda Manly2
Amanda Manly2 Tien S. Dong
Tien S. Dong