- 1Department of General Surgery, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
Background: Distant metastasis (DM) is a rare event and has a negative effect on the prognosis for papillary thyroid carcinoma (PTC). The relationship between cervical lymph node metastasis and DM is complicated and unclear. This study aimed to evaluate the impact of N stage subclassification on different distant metastasis sites based on age stratification, especially for patients with papillary thyroid microcarcinoma.
Methods: A total of 28,712 patient with PTC cases between 2010 and 2018 were extracted from the Surveillance, Epidemiology, and End Results database. Multivariable logistic regression analysis was utilized to adjust for confounding variables. Risk stratification, including positive lymph node number and lymph node ratio, was established by receiver operating characteristic curves to help predict DM.
Results: Lung was the most common metastatic site regardless of N0, N1a disease, or N1b disease. As the N stage increased, the higher the rate of DM identified. After age stratification, only N1b disease significantly increased the risk of lung metastasis (LM; odds ratio, OR = 20.45, P < 0.001) rather than bone metastasis (BM; OR = 3.46, P > 0.05) in younger patients. However, in older patients, N1b disease significantly increased the risk of both LM (OR = 4.10, P < 0.001) and BM (OR = 2.65, P = 0.007). In patients with papillary thyroid microcarcinoma (PTMC), N1a disease did not increase the risk of DM, LM, and BM compared with N0 disease (P > 0.05). Furthermore, combined N stage with risk stratification has well performance in predicting DM (area under the curve, AUC = 0.761). Similar results were shown in PTC patients with LM (AUC = 0.770) and BM (AUC = 0.729).
Conclusion: Overall, the incidence of DM significantly increased with the progress of N disease after age stratification. N1a disease did not increase the risk of DM in PTMC patients, regardless of LM or BM. Combined N stage with risk stratification may be beneficial for DM prediction.
Introduction
Papillary thyroid carcinoma (PTC) was the most frequent histological subtype of thyroid malignancy which has a rapidly rising incidence worldwide in the last few decades (1, 2). In total, 20.7–62% of patients with PTC present cervical lymph node metastasis (LNM) at initial diagnosis, which was associated with a poor prognosis (3, 4), yet the recently updated 8th edition of the TNM staging system eliminates the difference between central (N1a) and lateral (N1b) LNM, and N1b disease is not adopted for the final staging classification (5–7). These changes have been questioned, and concern arises in such a way that ignoring the metastatic lymph node sites may underestimate recurrence and mortality in patients with PTC.
Distant metastasis (DM), including lung metastasis (LM), bone metastasis (BM), brain metastasis, and liver metastasis, contributes to the leading cause of thyroid cancer-related death (8), and the rates of 5- and 10-year cancer-specific survival (CSS) for PTC patients with DM were reported to be 35 and 25%, respectively (9, 10). Previous studies pointed out that LNM was considered as the strongest predictor of DM (11–13), especially in younger patients (<55 years) (14). Vuong and his colleagues (15) demonstrated that N1b disease was associated with a significant risk for DM. In contrast, N1a disease could not increase the risk of DM in patients with PTC. Surprisingly, further investigations on this relationship conducted by Wang (8) proved that LNM had no significant influence on DM. The conflicting results were partly attributable to neglecting the influence of potential confounding factors (advanced age, male gender, tumor size, and BRAF-V600E) on DM and simply exploring the risk factors that affect the overall DM without considering the heterogeneity of different metastatic sites. Thus, the role of LNM impact on DM has not reach an agreement (16), and there is no reference study focused on the effect of N stages subclassification on different metastatic sites.
The current study was undertaken to evaluate the effect of N classification on DM based on age stratification in patients with PTC using a large thyroid cancer cohort, especially for LM and BM. In the meantime, in order to guide the active surveillance (AS) for patients with papillary thyroid microcarcinoma (PTMC), we explore whether N1a disease can significantly increase the risk of DM. Furthermore, a DM predictive model based on N stages, positive lymph node number (PLNN), and lymph node ratio (LNR) was established to identify asymptomatic DM early. Therefore, the present results may provide guidance to personalize the therapeutic approaches and follow-up strategies.
Materials and Methods
Study Population
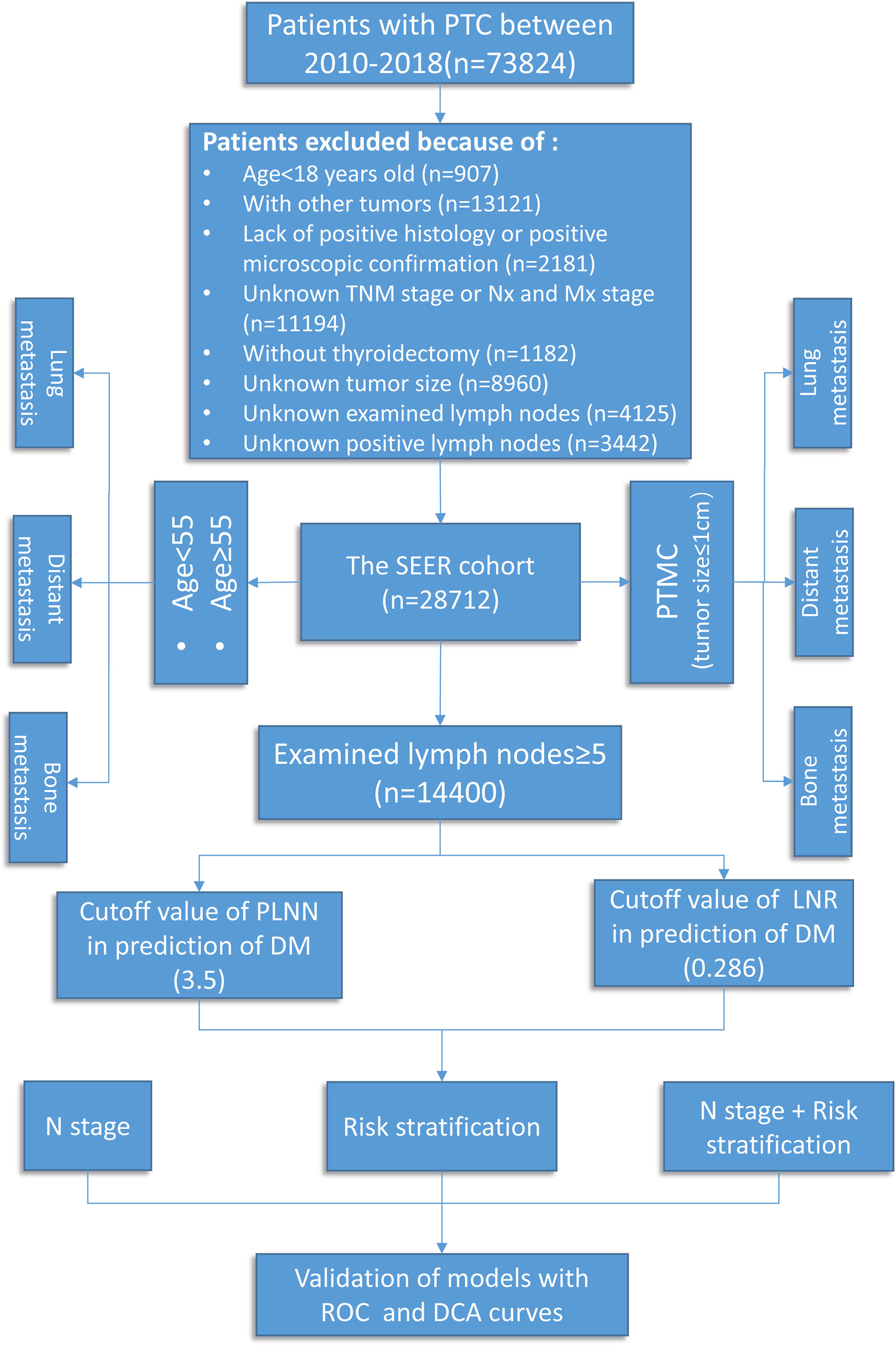
All data were obtained from the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov, access date: April 2021), a database that covered approximately 30% of the United States citizens and contained detailed demographics, therapy information, survival data, and clinicopathological characteristics (17). The International Classification of Diseases for Oncology code was used to identify patients diagnosed with PTC (8460, 8453, 8450, 8453, 8050, 8340-8344, and 8260) from the SEER 21 registry during the period 2010–2018. Data on age at diagnosis, T/N/M stage, grade (I, well differentiated; II, moderately differentiated; III, poorly differentiated; IV, undifferentiated, anaplastic), primary tumor size, distant metastatic sites, number of examined lymph nodes, PLNN, and radiation therapy (radioactive iodine therapy and external beam radiation therapy) were retrieved for further analysis. The inclusion criteria were as follows: (I) histologically confirmed PTC, (II) age ≥18 years, (III) clearly denoted N stage and distant metastatic sites (diagnosed by ultrasound, SPECT/CT, MRI, and post-treatment I131 whole-body scan during the 8 years of follow-up), and (IV) performed thyroidectomy with neck lymph node dissection. Patients with unknown or insufficient clinicopathologic profile were excluded. In total, 28,712 patients with PTC were enrolled in this study (Figure 1).
Study Design
According to the 8th edition TNM-N classification system, the 28,712 PTC patients were classified into three groups: N0 (no evidence of LNM), N1a (metastasis to level VI or VII), and N1b (metastasis to retropharyngeal lymph nodes or level I – V) (18). The impact of N classification on DM was evaluated, especially for LM and BM. Recently, the revised 8th TNM stage updated the age cutoff from 45 to 55 years old; the effect of LNM on DM was analyzed by using age stratification, with a cutoff of 55 years. All patients were classified into the younger group (<55 years) and the older group (≥55 years) for further analysis. Finally, to evaluate the value of PLNN and LNR on DM, patients with retrieved lymph nodes less than 5 during neck lymph node dissection were ruled out to reduce the selection bias. LNR was defined as metastatic lymph nodes to total retrieved lymph nodes.
Statistical Analysis
Statistical analyses were performed using R software (version 4.1.0). Continuous variables were presented as means ± standard deviations, while categorical variables were displayed as percentages and numbers. Univariate analysis was performed using t-test for continuous variables and chi-square test for categorical variables. To screen the independent risk factors affecting DM, continuous variables—including age and tumor size—and categorical variables—including T stage, race, sex, radiation therapy, grade, and laterality—were assessed by logistic regression analysis. In addition, odds ratios (OR) with 95% confidence intervals (CI) were calculated precisely. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to determine the most appropriate cutoff points for LNR and PLNN. Furthermore, a predictive model was constructed to categorize the patients into low-, medium-, and high-risk groups. Decision curve analysis (DCA) and AUC were utilized to assess the clinical performance and predictive accuracy. All P-values reported are two-sided, and those less than 0.05 were considered statistically significant.
Results
Baseline Clinical and Pathologic Characteristics
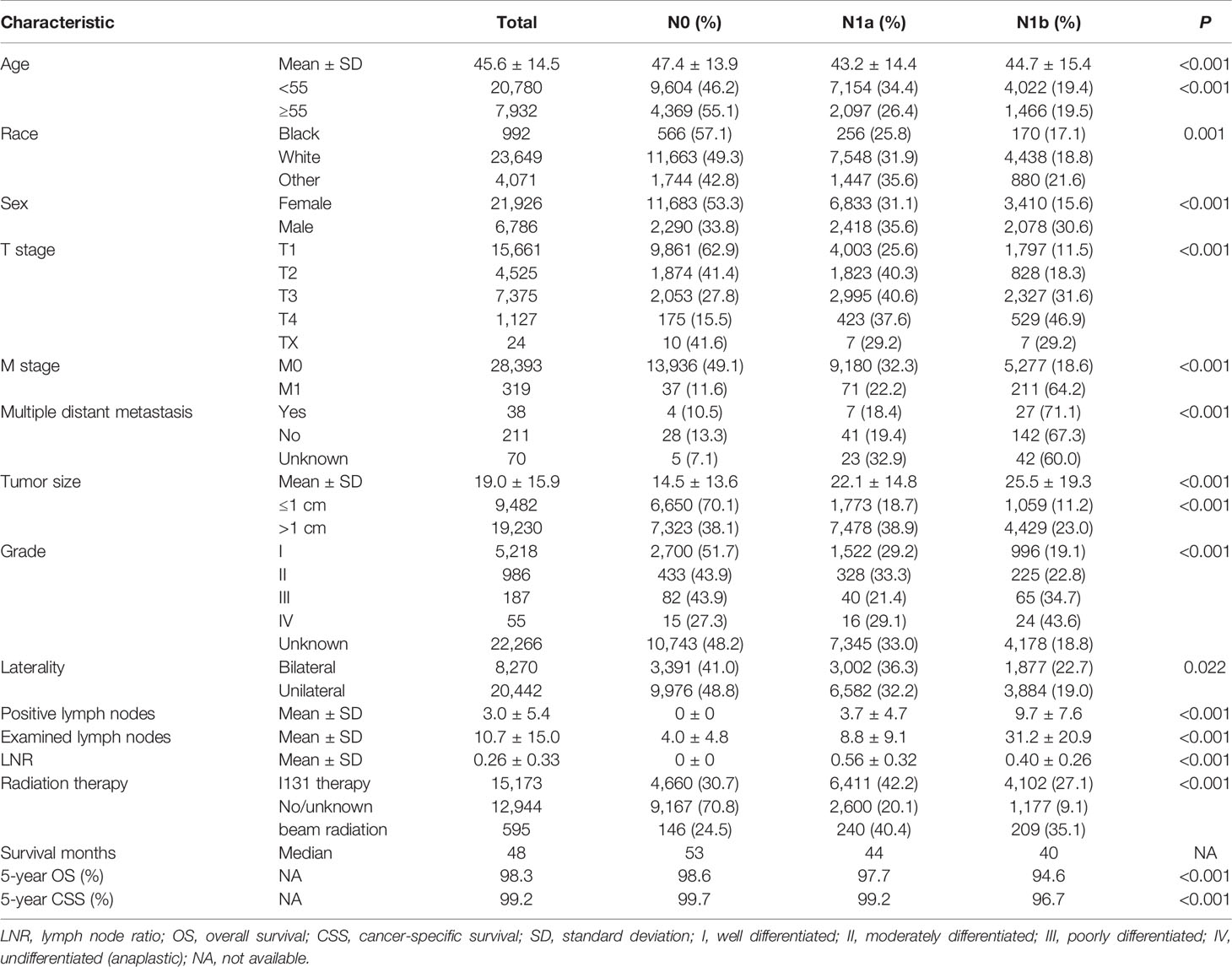
A total of 28,712 PTC patients were enrolled in this study, and the patient characteristics are presented in Table 1. The distribution of N classification was 13,973 (48.7%) with N0 stage, 9,251 (32.2%) with N1a stage, and 5,488 (19.1%) with N1b stage. Among these patients, 21,926 (76.4%) were female, and 72.4% were younger than 55 years old; the median age was 45.6 ± 14.5 years. Unilateral tumors, pathological tumor size ≤1 cm, and T1 stage were found in 20,442 (71.2%), 9,482 (33.0%), and 15,661 (54.5%) of the patients, respectively. About 38 (0.13%) patients underwent multiple distant metastasis. Besides this, patients with grade I/II (6,204) were significantly more numerous than patients with grade III/IV (242). The mean number of retrieved lymph nodes and PLNN were 10.7 ± 15.0 and 3.0 ± 5.4, respectively. About 15,173 (52.8%) patients underwent I131 therapy. The median follow-up duration was 61 months (range, 1–107 months), the 5-year overall survival and CSS were 98.3 and 99.2%, respectively. Besides this, DM was found in 319 (1.1%) patients; of them, 189 patients (0.65%) had LM, 63 (0.22%) had BM, 10 (0.03%) had liver metastases, and 9 (0.03%) had brain metastases. Supplementary Figure S1 shows that lung is the most common metastatic sites regardless of N0, N1a disease, or N1b disease. As the stage increased, the higher the rate of DM was identified. Age, gender, grade, race, tumor size, PLNN, LNR, examined lymph nodes, T stage, M stage, radiation therapy, and survival time were significant difference among N classification (P <0.05).
The Impact of N Classification on Distant Metastasis
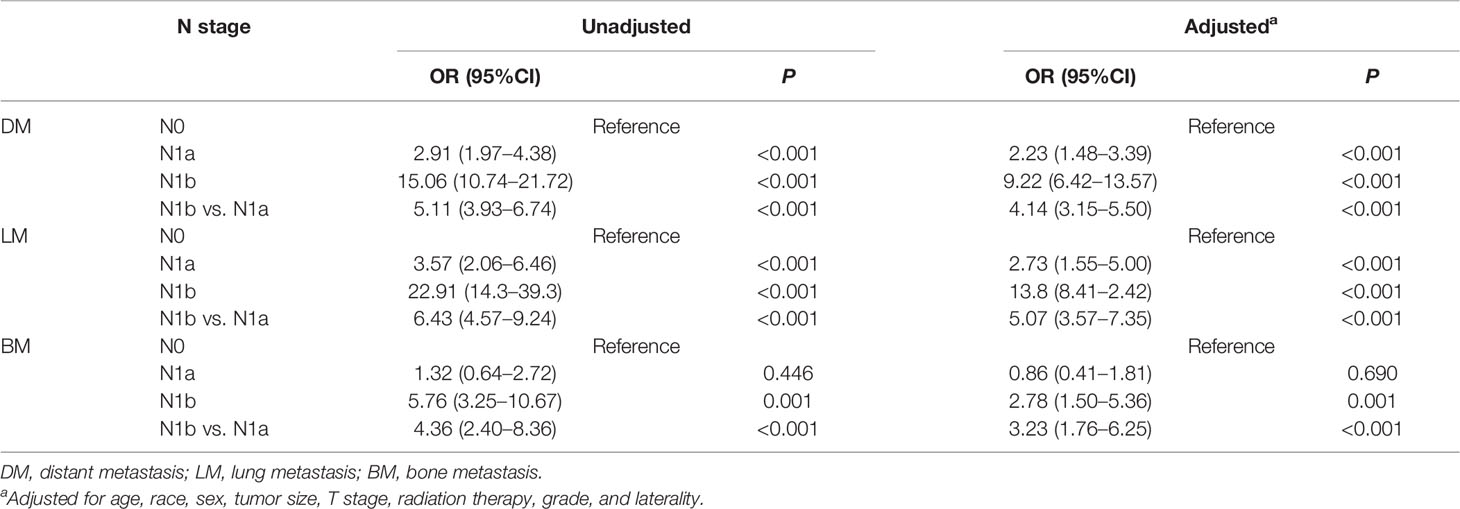
Overall, with the increase of N stage, the prevalence of DM also elevates, including LM and BM. After adjustment for age, race, sex, tumor size, T stage, laterality, radiation therapy, and grade, N1a (OR = 2.23; P < 0.001) and N1b disease (OR = 9.22; P < 0.001) were significantly associated with DM compared with N0 disease. Similar results were revealed in PTC patients with LM. However, there was no significant difference between N0 and N1a disease in PTC with BM (P = 0.69). In contrast, N1b disease was associated with a higher risk of BM compared with N0 disease (OR = 2.78, 95%CI: 1.50–5.36, P < 0.001) (Table 2). These abovementioned results indicate that LNM significantly increase the incidence of DM, especially for N1b disease.
The Impact of N Classification on Distant Metastasis Based on Age Stratification
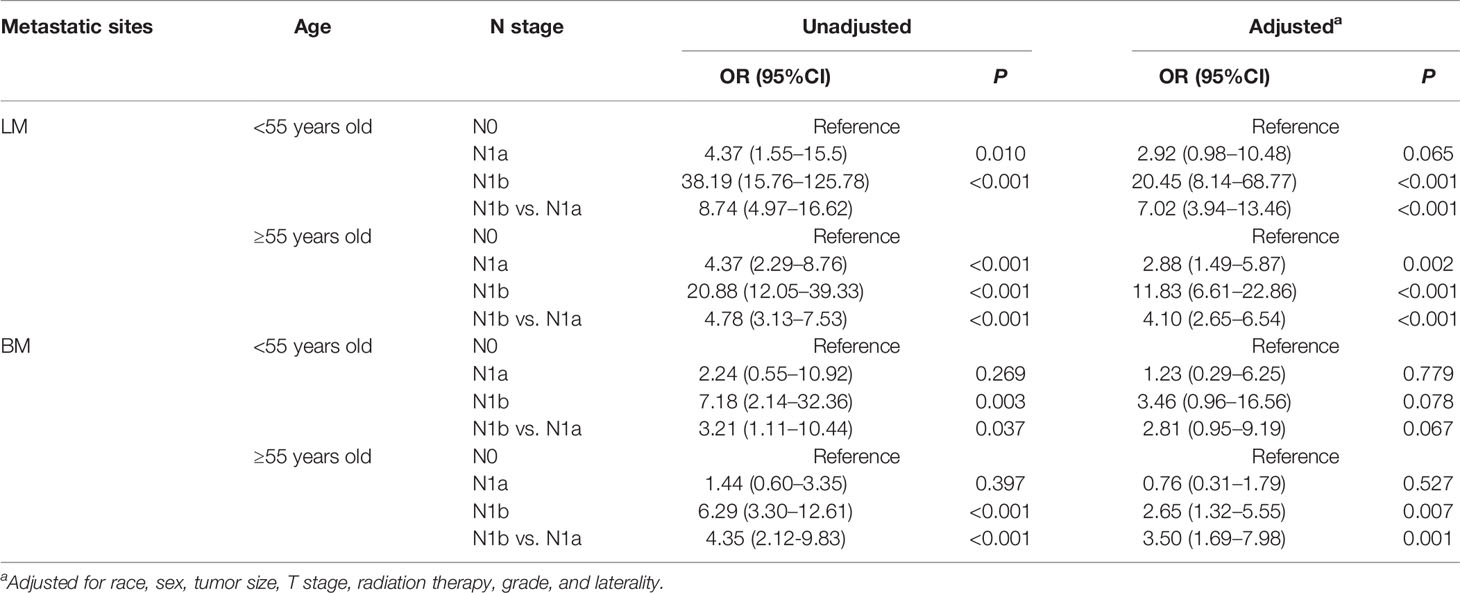
Patient’s age at diagnosis has been reported to be associated with prognosis (19). The incidence of DM significantly increased with the progress of N disease regardless of older patients or younger patients (P < 0.001) (Supplementary Table S1). Subsequently, we further explored the influence of N classification on different metastatic sites. In older patients with LM, the results were consistent with the abovementioned observations. In younger patients with LM, N1b disease (OR = 20.45, 95%CI: 8.14–68.77, P < 0.001), instead of N1a disease (OR = 2.92, 95%CI: 0.98–10.48, P = 0.065), boosts the incidence of LM after adjusting for all confounding variables (Table 3). Surprisingly, we discovered that the N classification had no significant effect on BM in younger patients, and only N1b disease (OR = 2.65, 95%CI: 1.32–5.55, P = 0.007) elevated the rate of BM in older patients (Table 3). These findings imply that more emphasis should be devoted to the impact of LNM on LM rather than BM in younger patients with PTC. However, in older patients, N1b disease significantly increases the risk of both LM and BM.
Impact of N Classification on Distant Metastasis in Patients With PTMC
Compared with N0 disease, N1a disease does not increase the risk of DM (P = 0.069), LM (P = 0.058), and BM (P = 0.678) after adjusting for age, race, sex, T stage, laterality, grade, and treatment approaches. Meanwhile, the risks of DM (OR = 6.14, 95%CI: 2.25–11.79, P < 0.001) and LM (OR = 20.43, 95%CI: 3.37–47.30, P = 0.002) were significant higher in patients with N1b disease than patients with N0 disease. Nevertheless, N1b disease did not have a significantly higher risk of BM than those with N0 disease (P = 0.550) (Table 4). These results reveal that N1a disease does not increase the risk of DM, regardless of LM or BM.
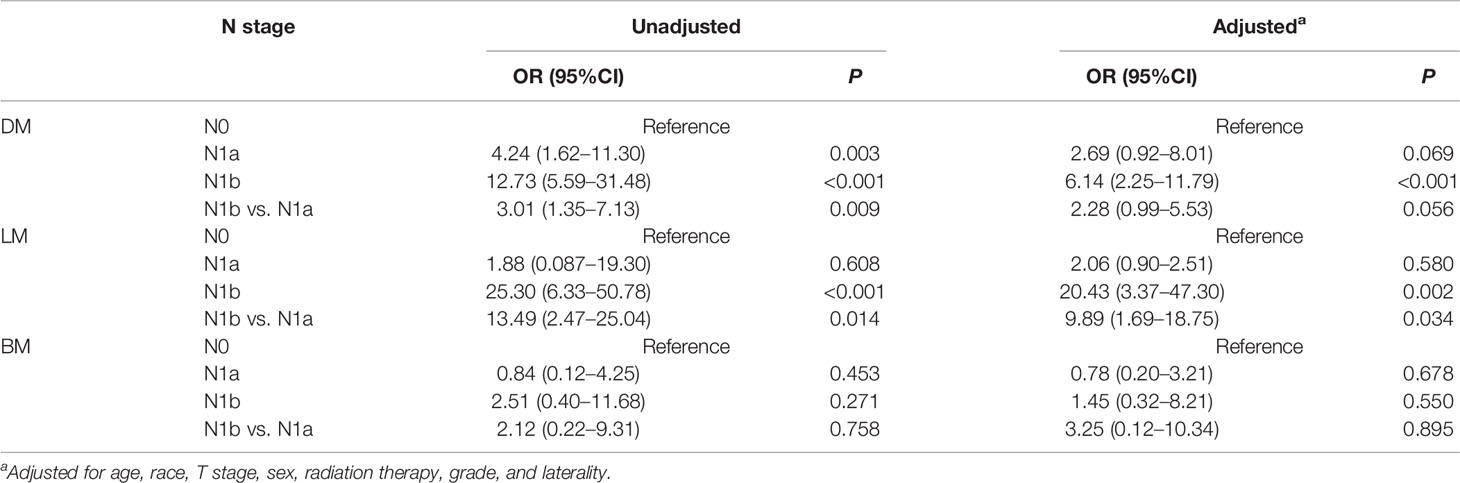
Table 4 Impact of N classification on distant metastasis in patients with papillary thyroid microcarcinoma.
Establishment of a Model to Predict Distant Metastasis
In addition, prior studies have reported that the PLNN and LNR played a vital role in predicting DM (20). The clinical implications and appropriate cutoff points have not been comprehensively studied at the same time. To improve the predictive reliability of the PLNN and LNR, the total retrieved lymph nodes less than 5 had been excised. First, we used ROC curves to determine that the cutoff of PLNN and LNR were 3.5 (AUC = 0.725) and 0.286 (AUC = 0.636), respectively (Supplementary Figure S2). Thereafter, the enrolled patients were classified into high-risk (PLNN ≥4 and LNR ≥0.286), medium-risk (PLNN >4 and LNR <0.286 or PLNN <4 and LNR ≥0.286), and low- risk (PLNN <4 and LNR <0.286) groups. The statistics model combined N stage with risk stratification and has a well preference in predicting DM (AUC = 0.761). Similar results were shown in PTC patients with LM (AUC = 0.770) and BM (AUC = 0.729). The DCA also revealed that the combined model had an excellent performance in daily clinical practice (Figure 2).
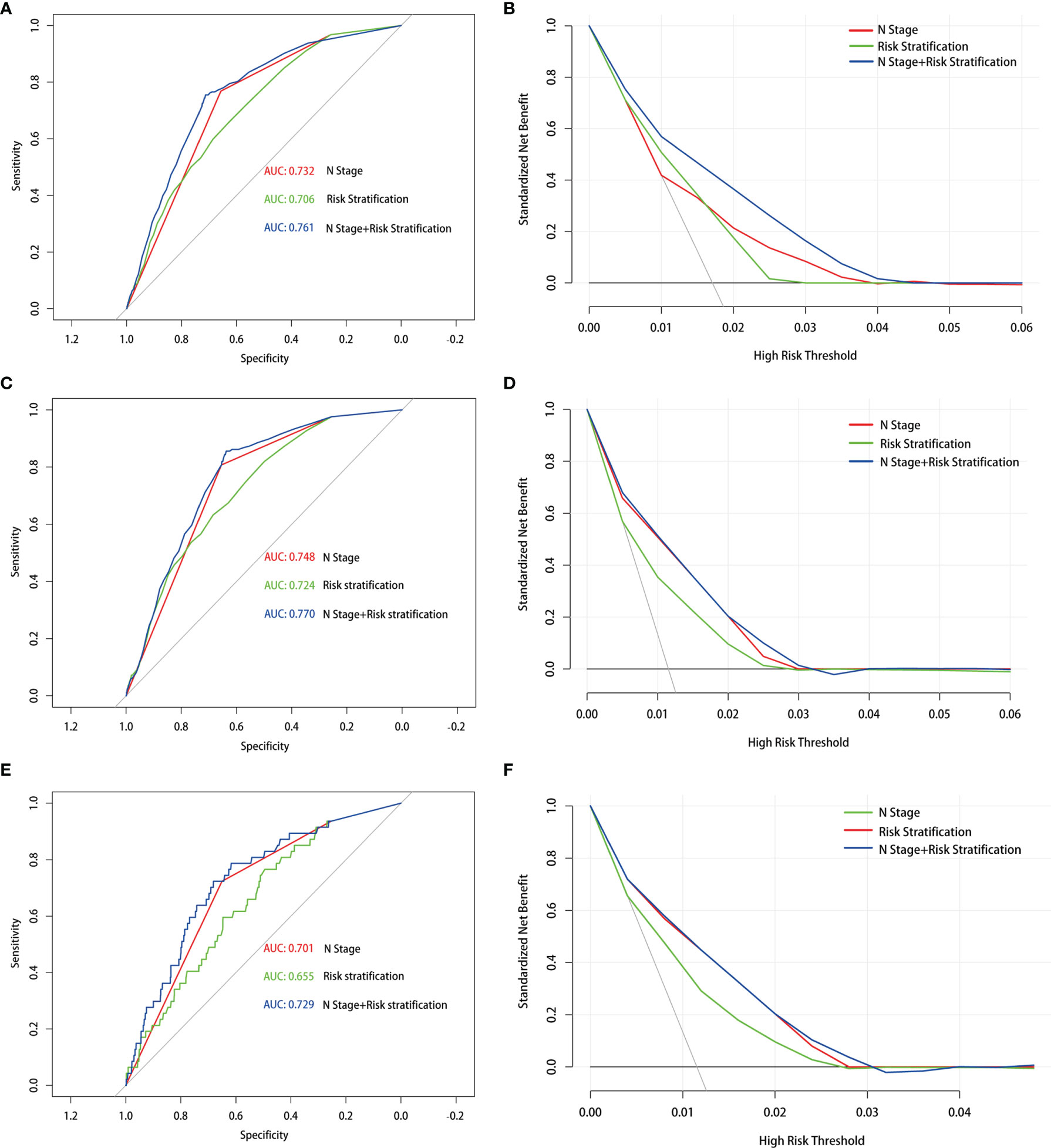
Figure 2 Receiver operating characteristic and decision curve analysis curves with different models in the prediction of distant metastasis (A, B), lung metastasis (C, D) and bone metastasis (E, F) in papillary thyroid carcinoma patients with 5 or more lymph nodes examined.
Discussion
This is, to our knowledge, the largest analysis to specifically assess the impact of N classification on different distant metastasis sites. The results have shown that lung was the most frequent metastatic site regardless of N0, N1a, or N1b disease. As the N stage increased, the higher the rate of DM that was identified in the whole cohort even after age stratification. Overall, N1a disease does not increase the risk of DM in PTMCs, and combined N stage with PLNN and LNR has well performance in predicting DM. All these results will help clinicians to develop tailored treatment and follow-up strategies.
Patient’s age at diagnosis has been reported to be a strong prognostic factor in PTC patients (19, 21, 22). Numerous studies have focused on the analysis of prognosis based on age stratification. Our previous research demonstrated that the effect of tumor size on the aggressiveness of PTC varied with the patient’s age (23). Additionally, a study indicated that BRAF mutation was more common in PTC patients over 55 years old (24). It therefore follows that age stratification is critical in assessing unfavorable events (DM, LNM, and gross extrathyroidal extension), although there were some studies about the association between LNM and clinical prognosis in PTC patients (25, 26). To the best of our knowledge, the influence of LNM on DM based upon the age threshold of 55 years has not been fully elucidated. Consequently, this present study filled this gap. Generally, an increase in N stage was associated with a higher risk of DM in both younger and older patients. Unexpectedly, after a more detailed analysis of LM and BM separately, LNM was regarded as a risk factor of LM, but not BM, in younger patients, while LNM was significantly correlated with both LM and BM in older patients. Taken together, the dissimilarities regarding different metastatic sites and variant ages must be considered when exploring the effect of LNM on DM.
More recently, the management of patients with PTMC has become an important issue since over-diagnosis of low-risk PTMCs is a widespread global problem resulting in overtreatment in many cases (27). In this situation, many researchers, including Japanese scholars, suggested that AS rather than immediate surgery could be more beneficial for low-risk PTMC patients (28–31). Nevertheless, the potential risks of AS are increasingly being reported. Approximately 8 to 23.2% of patients experienced an increase in tumor volume during AS (29, 30, 32, 33), and more concerns arise in the occurrence of LNM in PTMC (34–37). Researchers from Kuma Hospital demonstrated that the rate of LNM among PTMC patients who underwent AS could reach 1.2–2.1% (19, 38). In stark contrast to AS, a study conducted in Italy found that none of the low-risk PTMC patients had a lymph node recurrence after surgery (39). Thereafter, Oda et al. observed that the incidence of LNM in AS patients (0.5%) was discrepant to that in immediate surgery patients (0.2%) (40). As demonstrated in these studies, LNM had great implications for treatment decision-making in PTMC. Clinically, LNM seems to be involved in DM (16, 25, 41), an important prognostic factor associated with survival in PTMC (42, 43). Consequently, we sought to ascertain whether the occurrence of central LNM increased the probability of DM during the implementation of AS in PTMC. In this study, it is worth noting that N1a disease did not increase the risk of DM, regardless of LM or BM. Therefore, from the perspective of DM, AS can be chosen for PTMC because it is not too late to perform therapeutic neck lymph node dissection after the occurrence of a central LNM. Accordingly, given the small effect of N1a disease on DM, our study provided clinical evidence to support AS for PTMC patients.
Besides this, the PLNN and LNR were supposed to be strongly associated with the prognosis (44–46). However, the cutoff value was not defined. Schneider DF et al. found that the cutoff values of LNR for predicting CSS and DFS were 0.7 and 0.42, respectively (47, 48). In addition, Machens A et al.suggested that the LNM categories of 0, 1–20, and more than 20 associated better with LM than the current TNM-N categories in PTC (49). Nevertheless, insufficient sample size is a common shortcoming in existing studies. Moreover, the role of PLNN and LNR in predicting the overall DM of PTC was not captured in these studies. Herein 14,400 PTC patients were evaluated, and then it was demonstrated that LNR (cutoff value: 0.286) and PLNN (cutoff value: 4) could be used for risk stratification of DM. Furthermore, the present evidence proved that combined risk stratification with N classification had the best predictive effect on DM, which provides a new perspective for the accurate prediction of DM in PTC.
Although this study revealed numerous important findings, it must be noted that there are several limitations in our study. Firstly, biases, such as selection bias, could have been introduced due to the retrospective design of the study. Secondly, data on the recurrence of DM are lacking in the SEER database, and some other clinical factors associated with DM, such as the size of metastatic nodes, extrathyroidal extension, TERT promoter mutation, multifocality, dose of radioactive iodine therapy, and BRAF V600E, are not available in the SEER database. Thirdly, few patients with PTC presented with DM, limiting the power of the study and making type II errors very likely. Finally, the extension of cervical lymph node dissection and the proportion of prophylactic lymph node dissection in patients are unknown in the SEER database. Nevertheless, data mining from SEER databases can help minimize biases caused by a single-center study. In addition, strict inclusion and exclusion criteria were followed to avoid potential bias in this study.
Conclusion
In summary, this study based on a large cohort of PTC patients demonstrated that the N stage increased and the higher risks of DM was identified, including LM and BM. However, after age stratification, the incidence of LM, instead of BM, significantly increased with the progress of N disease in younger patients. Meantime, only N1b disease could significantly increase the risk of both LM and BM in older patients. Thus, such discrepancy should be taken into consideration when making treatment strategies. Moreover, N1a disease did not increase the risk of DM, regardless of LM or BM, which indicated that AS was safe for the management of low-risk PTMCs. In addition, we found that combining N stage with PLNN and LNR could individualize the DM estimates for PTC.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and institutional requirements.
Author Contributions
YD and WW contributed equally to this study. WW and YD conceptualized the project. XL and WJ were responsible for the whole administration. YD and WW analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81672885) and the Innovative Foundation for Graduate Students of Hunan Province (grant number 2020zzts259).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the SEER databases for providing important data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.917794/full#supplementary-material
Supplementary Figure 1 | The number (A) and percentage (B) of different types of distant metastasis in the N0, N1a, and N1b stages of the cohort.
Supplementary Figure 2 | Receiver operating characteristic curves with positive lymph nodes number (A) and lymph nodes ratio (B) in the prediction of distant metastasis.
References
1. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer Incidence and Mortality in China, 2015. J Natl Cancer Center (2021) 1(1):2–11. doi: 10.1016/j.jncc.2020.12.001
2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
3. Choi SM, Kim JK, Lee CR, Lee J, Jeong JJ, Nam KH, et al. Completion Total Thyroidectomy Is Not Necessary for Papillary Thyroid Microcarcinoma With Occult Central Lymph Node Metastasis: A Long-Term Serial Follow-Up. Cancers (Basel) (2020) 12(10):3032. doi: 10.3390/cancers12103032
4. Wang W, Zhang Z, Zhao Y, Xue W, Xia F, Li X. Management of Lateral Multiple-Level Metastasis in N1b Papillary Thyroid Microcarcinoma. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01586
5. Nam SH, Bae MR, Roh JL, Gong G, Cho KJ, Choi SH, et al. A Comparison of the 7th and 8th Editions of the AJCC Staging System in Terms of Predicting Recurrence and Survival in Patients With Papillary Thyroid Carcinoma. Oral Oncol (2018) 87:158–64. doi: 10.1016/j.oraloncology.2018.11.003
6. Kim M, Jeon MJ, Oh HS, Park S, Song DE, Sung TY, et al. Prognostic Implication of N1b Classification in the Eighth Edition of the Tumor-Node-Metastasis Staging System of Differentiated Thyroid Cancer. Thyroid (2018) 28(4):496–503. doi: 10.1089/thy.2017.0473
7. Zhi J, Wu Y, Hu L, Zhao J, Liu H, Ruan X, et al. Assessment of the Prognostic Value and N1b Changes of the Eighth TNM/AJCC Staging System for Differentiated Thyroid Carcinoma. Int J Clin Oncol (2020) 25(1):59–66. doi: 10.1007/s10147-019-01522-x
8. Wang W, Shen C, Zhao Y, Sun B, Bai N, Li X. Identification and Validation of Potential Novel Biomarkers to Predict Distant Metastasis in Differentiated Thyroid Cancer. Ann Transl Med (2021) 9(13):1053. doi: 10.21037/atm-21-383
9. Martin Schlumberger SL. Treatment of Distant:Metastases From Follicular Cell-Derived Thyroid Cancer. F1000Prime Rep (2015) 22(7):1–9. doi: 10.12703/P7-22
10. Jung CK, Jung SH, Jeon S, Jeong YM, Kim Y, Lee S, et al. Risk Stratification Using a Novel Genetic Classifier Including PLEKHS1 Promoter Mutations for Differentiated Thyroid Cancer With Distant Metastasis. Thyroid (2020) 30(11):1589–600. doi: 10.1089/thy.2019.0459
11. Khan U, Al Afif A, Aldaihani A, MacKay C, Rigby MH, Rajaraman M, et al. Patient and Tumor Factors Contributing to Distant Metastasis in Well-Differentiated Thyroid Cancer: A Retrospective Cohort Study. J Otolaryngol Head Neck Surg (2020) 49(1):78. doi: 10.1186/s40463-020-00469-8
12. Liu Z, Chen S, Huang Y, Hu D, Zeng W, Wang M, et al. Synergic Effects of Histology Subtype, Tumor Size, and Lymph Node Metastasis on Distant Metastasis in Differentiated Thyroid Cancer. Ann Transl Med (2019) 7(20):533. doi: 10.21037/atm.2019.09.137
13. Chen D, Huang L, Chen S, Huang Y, Hu D, Zeng W, et al. Innovative Analysis of Distant Metastasis in Differentiated Thyroid Cancer. Oncol Lett (2020) 19(3):1985–92. doi: 10.3892/ol.2020.11304
14. Liu Z, Hu D, Huang Y, Chen S, Zeng W, Zhou L, et al. Factors Associated With Distant Metastasis in Pediatric Thyroid Cancer: Evaluation of the SEER Database. Endocr Connect (2019) 8(2):78–85. doi: 10.1530/EC-18-0441
15. Vuong HG, Duong UNP, Pham TQ, Tran HM, Oishi N, Mochizuki K, et al. Clinicopathological Risk Factors for Distant Metastasis in Differentiated Thyroid Carcinoma: A Meta-Analysis. World J Surg (2018) 42(4):1005–17. doi: 10.1007/s00268-017-4206-1
16. Ho AS, Luu M, Shafqat I, Mallen-St Clair J, Chen MM, Chen Y, et al. Predictive Impact of Metastatic Lymph Node Burden on Distant Metastasis Across Papillary Thyroid Cancer Variants. Thyroid (2021) 31(10):1549–57. doi: 10.1089/thy.2021.0131
17. Doll KM, Rademaker A, Sosa JA. Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database. JAMA Surg (2018) 153(6):588–9. doi: 10.1001/jamasurg.2018.0501
18. Kim M, Kim HK, Kim HI, Kim EH, Jeon MJ, Yi HS, et al. : Modification of the Eight-Edition Tumor-Node-Metastasis Staging System With N1b for Papillary Thyroid Carcinoma: A Multi-Institutional Cohort Study. Oral Oncol (2018) 86:48–52. doi: 10.1016/j.oraloncology.2018.09.008
19. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient Age is Significantly Related to the Progression of Papillary Microcarcinoma of the Thyroid Under Observation. Thyroid (2014) 24(1):27–34. doi: 10.1089/thy.2013.0367
20. Barbosa MP, Momesso D, Bulzico DA, Farias T, Dias F, Lima RA, et al. Metastatic Lymph Node Characteristics as Predictors of Recurrence/Persistence in the Neck and Distant Metastases in Differentiated Thyroid Cancer. Arch Endocrinol Metab (2017) 61(6):584–9. doi: 10.1590/2359-3997000000307
21. Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of Age and Disease Status as Predictors of Thyroid Cancer-Specific Mortality Using the Surveillance, Epidemiology, and End Results Database. Thyroid (2015) 25(1):125–32. doi: 10.1089/thy.2014.0116
22. van Velsen EFS, Visser WE, Stegenga MT, Mäder U, Reiners C, van Kemenade FJ, et al. Finding the Optimal Age Cutoff for the UICC/AJCC TNM Staging System in Patients With Papillary or Follicular Thyroid Cancer. Thyroid (2021) 31(7):1041–9. doi: 10.1089/thy.2020.0615
23. Meng C, Wang W, Zhang Y, Li X. The Influence of Nodule Size on the Aggressiveness of Thyroid Carcinoma Varies With Patient's Age. Gland Surg (2021) 10(3):961–72. doi: 10.21037/gs-20-747
24. Subash A, Sinha P, Singh A. BRAF Mutation and Age in Differentiated Thyroid Cancer Risk Stratification: Two Sides of the Same Coin. Oral Oncol (2020) 106:104732. doi: 10.1016/j.oraloncology.2020.104732
25. Zhang J, Cheng X, Shen L, Wang X, Wang L, Sun X, et al. The Association Between Lymph Node Stage and Clinical Prognosis in Thyroid Cancer. Front Endocrinol (Lausanne) (2020) 11:90. doi: 10.3389/fendo.2020.00090
26. Lee YS, Lim YS, Lee JC, Wang SG, Kim IJ, Son SM, et al. Clinical Implications of Bilateral Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Cancer: A Risk Factor for Lung Metastasis. Ann Surg Oncol (2011) 18(12):3486–92. doi: 10.1245/s10434-011-1763-7
27. Krajewska J, Kukulska A, Oczko-Wojciechowska M, Kotecka-Blicharz A, Drosik-Rutowicz K, Haras-Gil M, et al. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front Endocrinol (Lausanne) (2020) 11:571421. doi: 10.3389/fendo.2020.571421
28. Xue S, Wang P, Hurst ZA, Chang YS, Chen G. Active Surveillance for Papillary Thyroid Microcarcinoma: Challenges and Prospects. Front Endocrinol (Lausanne) (2018) 9:736. doi: 10.3389/fendo.2018.00736
29. Jeon MJ, Kim WG, Chung KW, Baek JH, Kim WB, Shong YK. Active Surveillance of Papillary Thyroid Microcarcinoma: Where Do We Stand? Eur Thyroid J (2019) 8(6):298–306. doi: 10.1159/000503064
30. Oh HS, Ha J, Kim HI, Kim TH, Kim WG, Lim DJ, et al. Active Surveillance of Low-Risk Papillary Thyroid Microcarcinoma: A Multi-Center Cohort Study in Korea. Thyroid (2018) 28(12):1587–94. doi: 10.1089/thy.2018.0263
31. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and Strategy for Active Surveillance of Adult Low-Risk Papillary Thyroid Microcarcinoma: Consensus Statements From the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid (2021) 31(2):183–92. doi: 10.1089/thy.2020.0330
32. Sakai T, Sugitani I, Ebina A, Fukuoka O, Toda K, Mitani H, et al. Active Surveillance for T1bN0M0 Papillary Thyroid Carcinoma. Thyroid (2019) 29(1):59–63. doi: 10.1089/thy.2018.0462
33. Kwon H, Oh HS, Kim M, Park S, Jeon MJ, Kim WG, et al. Active Surveillance for Patients With Papillary Thyroid Microcarcinoma: A Single Center's Experience in Korea. J Clin Endocrinol Metab (2017) 102(6):1917–25. doi: 10.1210/jc.2016-4026
34. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph Node Metastasis From 259 Papillary Thyroid Microcarcinomas: Frequency, Pattern of Occurrence and Recurrence, and Optimal Strategy for Neck Dissection. Ann Surg (2003) 237(3):399–407. doi: 10.1097/01.SLA.0000055273.58908.19
35. Roh JL, Kim JM, Park CI. Central Lymph Node Metastasis of Unilateral Papillary Thyroid Carcinoma: Patterns and Factors Predictive of Nodal Metastasis, Morbidity, and Recurrence. Ann Surg Oncol (2011) 18(8):2245–50. doi: 10.1245/s10434-011-1600-z
36. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive Factors for Lymph Node Metastasis in Papillary Thyroid Microcarcinoma. Ann Surg Oncol (2016) 23(9):2866–73. doi: 10.1245/s10434-016-5225-0
37. Medas F, Canu GL, Cappellacci F, Boi F, Lai ML, Erdas E, et al. Predictive Factors of Lymph Node Metastasis in Patients With Papillary Microcarcinoma of the Thyroid: Retrospective Analysis on 293 Cases. Front Endocrinol (Lausanne) (2020) 11:551. doi: 10.3389/fendo.2020.00551
38. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An Observational Trial for Papillary Thyroid Microcarcinoma in Japanese Patients. World J Surg (2010) 34(1):28–35. doi: 10.1007/s00268-009-0303-0
39. Durante C, Attard M, Torlontano M, Ronga G, Monzani F, Costante G, et al. Identification and Optimal Postsurgical Follow-Up of Patients With Very Low-Risk Papillary Thyroid Microcarcinomas. J Clin Endocrinol Metab (2010) 95(11):4882–8. doi: 10.1210/jc.2010-0762
40. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid (2016) 26(6):807–15. doi: 10.1089/thy.2015.0429
41. Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, et al. Features Predictive of Distant Metastasis in Papillary Thyroid Microcarcinomas. Thyroid (2016) 26(1):161–8. doi: 10.1089/thy.2015.0375
42. Sugino K, Nagahama M, Kitagawa W, Ohkuwa K, Uruno T, Matsuzu K, et al. Distant Metastasis in Pediatric and Adolescent Differentiated Thyroid Cancer: Clinical Outcomes and Risk Factor Analyses. J Clin Endocrinol Metab (2020) 105(11):e3981–8. doi: 10.1210/clinem/dgaa545
43. Weng HY, Yan T, Qiu WW, Xi C, Hou LY, Yang ZL, et al. Long-Term Outcomes and Prognostic Factors in Papillary Thyroid Microcarcinoma Patients With Distant Metastases. Endocrine (2021) 75(2):495-507. doi: 10.21203/rs.3.rs-734874/v1
44. Lee J, Song Y, Soh EY. Prognostic Significance of the Number of Metastatic Lymph Nodes to Stratify the Risk of Recurrence. World J Surg (2014) 38(4):858–62. doi: 10.1007/s00268-013-2345-6
45. Rajeev P, Ahmed S, Ezzat TM, Sadler GP, Mihai R. The Number of Positive Lymph Nodes in the Central Compartment has Prognostic Impact in Papillary Thyroid Cancer. Langenbecks Arch Surg (2013) 398(3):377–82. doi: 10.1007/s00423-012-1041-6
46. Mansour J, Sagiv D, Alon E, Talmi Y. Prognostic Value of Lymph Node Ratio in Metastatic Papillary Thyroid Carcinoma. J Laryngol Otol (2018) 132(1):8–13. doi: 10.1017/S0022215117002250
47. Schneider DF, Mazeh H, Chen H, Sippel RS. Lymph Node Ratio Predicts Recurrence in Papillary Thyroid Cancer. Oncologist (2013) 18(2):157–62. doi: 10.1634/theoncologist.2012-0240
48. Schneider DF, Chen H, Sippel RS. Impact of Lymph Node Ratio on Survival in Papillary Thyroid Cancer. Ann Surg Oncol (2013) 20(6):1906–11. doi: 10.1245/s10434-012-2802-8
Keywords: lymph node metastasis, papillary thyroid carcinoma, distant metastasis, N stage, age stratification
Citation: Wang W, Ding Y, Jiang W and Li X (2022) Can Cervical Lymph Node Metastasis Increase the Risk of Distant Metastasis in Papillary Thyroid Carcinoma? Front. Endocrinol. 13:917794. doi: 10.3389/fendo.2022.917794
Received: 11 April 2022; Accepted: 20 May 2022;
Published: 24 June 2022.
Edited by:
Fabio Monzani, University of Pisa, ItalyReviewed by:
Fausto Bogazzi, University of Pisa, ItalySana Ghaznavi, University of Calgary, Canada
Gabriela Brenta, Dr. César Milstein Care Unit, Argentina
Copyright © 2022 Wang, Ding, Jiang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Jiang, Mjk1NTcyOTFAcXEuY29t; Xinying Li, bGl4aW55aW5nY25AMTI2LmNvbQ==
†ORCID: Xinying Li, b3JjaWQub3JnLzAwMDAtMDAwMi0yNjg2LTg4MDc=
‡These authors have contributed equally to this work
 Wenlong Wang
Wenlong Wang Ying Ding
Ying Ding Wei Jiang
Wei Jiang Xinying Li
Xinying Li