
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 July 2022
Sec. Pediatric Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.910119
This article is part of the Research Topic Precocious Puberty: Central, Peripheral, and Exogenous. New Insights and Implications on Clinical Management View all 6 articles
 Yawen Zhang1†
Yawen Zhang1† Jianmin Ni1†
Jianmin Ni1† Lei Zhang1
Lei Zhang1 Tingting Yu2,3
Tingting Yu2,3 Xiaoqing Li2,3
Xiaoqing Li2,3 Peng Xue2,3
Peng Xue2,3 Yifan Liu4
Yifan Liu4 Bo Gao5
Bo Gao5 Xinwen Xue6
Xinwen Xue6 Huijun Kong1*
Huijun Kong1* Shijian Liu2,3*
Shijian Liu2,3*Objective: We sought to investigate the prevalence of precocious puberty in children aged 6–10 years in Qufu City, Shandong Province, China.
Methods: A total of 5,169 primary school students from grades 1–3 were recruited by population-based multi-stage stratified cluster random sampling. Professional pediatricians conducted physical examinations in October 2020. Precocious puberty is defined as the onset of secondary sexual characteristics in boys aged < 9 years or girls < 8 years or menstruation in girls < 10 years old. Tanner staging was used to record the development of breast and pubic hair in girls and testicular volume and pubic hair in boys. According to the standards of the International Obesity Task Force, we diagnosed overweight, obesity, or severe obesity. In obese girls, a combination of palpation and ultrasound was used to evaluate breast development. The difference was tested by chi-squared test, and all data were statistically analyzed using IBM SPSS version 25.0.
Results: The unadjusted and adjusted prevalence rates of precocious puberty were 5.01% (11.53% for girls and 1.41% for boys) and 6.29% (14.23% for girls and 1.54% for boys), respectively. The prevalence of precocious puberty in urban (5.34%) dwellers was much higher than that in suburban residents (2.36%, P < 0.05). The prevalence of precocious puberty in the overweight (21.43% for girls and 1.97% for boys), obesity (35.48% for girls and 4.6% for boys), and severe obesity (32.35% for girls and 3.38% for boys) groups were higher than that in the normal weight group (4% for girls and 0.54% for boys, P < 0.05).
Conclusion: The prevalence of precocious puberty is high, and overweight and obesity are related to precocious puberty in Qufu, Shandong Province, China.
The prevalence of precocious puberty has increased in recent decades. In Denmark, a study based on a national registration system showed that from 1998–2017 the incidence of precocious puberty in girls increased from 2.6/10000 to 14.6/10000, while the incidence in boys increased from 0.1/10000 to 2.1/10000 (1). A survey in South Korea found that the incidence of central precocious puberty (CPP) in girls increased from 0.33/10000 to 5.04/10000, while that in boys increased from 0.03/10000 to 0.12/10000, from 2004–2010 (2), the overall incidence and prevalence of central precocious puberty from 2008 to 2014 was 12.28/10000 persons (girls, 26.28; boys, 0.7) and 19.32/10000 persons (girls, 41.06; boys, 1.09), respectively (3). An epidemiological survey of precocious puberty in Taiwan showed that the crude prevalence of boys and girls increased from 0.99/10000 to 7.01/10000 and 13.56/10000 to 110.95/10000, respectively, from 2000–2013 (3). A nationwide study in France determined that the incidence rate of idiopathic central precocious puberty from 2011–2013 was 2.68/10000 in girls < 9 years old and 0.24/10000 in boys < 10 years old (4). Finally, a school-based population study showed that the prevalence of precocious puberty was 11.47% for girls and 3.26% for boys in Zhongshan City, Guangdong Province, China, in 2021 (5).
Studies have shown that many factors can affect the occurrence of precocious puberty, including both genetic and environmental factors. Height (6), mother’s age at menarche (7), and diet (8, 9) all have an impact on the occurrence of precocious puberty. Environmental endocrine disruptors can lead to earlier menarche and earlier breast and pubic hair development in adolescent girls (10). Precocious puberty is more likely to occur in obese girls (11). Overweight, obesity, and severe obesity also advance puberty (12, 13), but the effects of overweight, obesity, and severe obesity on puberty in boys and girls are not consistent. In girls, a higher pre-adolescent body mass index (BMI) is positively correlated with precocious puberty (14), while, in boys, this effect is not observed. Studies have shown that puberty onset in obese boys occurs earlier than that in normal-weight boys (15). Another study showed that there was no significant relationship between BMI and the average age of genital development (16).
Most existing studies on precocious puberty employed the registration data of research centers or clinical units, which often underestimate the actual prevalence. At present worldwide, there are few cross-sectional surveys of precocious puberty from school-based children. In the present study, the prevalence of precocious puberty was investigated in China’s northern city of Qufu via physical examination of school children aged 6–10 years conducted by pediatricians. Based on the collected data, the relationship between obesity and precocious puberty was also investigated.
We investigated children in grades 1–3 (6–10 years old) of primary school in October 2020. According to the preliminary survey results, we estimated a sample size of 6,201 participants. The sampling survey process is shown in Figure 1. Using stratified cluster random sampling, we first selected 3 of 4 urban areas and 2 of 8 suburban areas in Qufu City, Shandong Province, northern China, and then randomly selected 6,474 children from 12 of 41 primary schools in the selected area, including 8 schools from 3 urban areas and 4 schools from 2 suburban areas. A total of 5,169 (79.84%) guardians agreed to the children’s participation in the study, and 4,712 participated in physical examination. The inclusion criteria were as follows: (1) the case group met the diagnostic criteria of girls’ precocious puberty, i.e., girls presented with secondary sexual signs at < 8 years old (Tanner stage ≥ II) or menarche at < 10 years old, or boys presented with secondary sexual signs at < 9 years old (17); and (2) girls or boys in the control group met the screening criteria of normal weight or obesity of girls or boys without precocious puberty aged 6–10 years. According to the Group of China Obesity Task Force BMI cutoff-point standard for obesity screening of girls aged 2–18 years old, the BMI of obese girls aged 7, 8, 9 years old is 18.8, 19.5, 20.4 kg/m2, the BMI of obese boys aged 7, 8, 9, 10 years old is 19.2, 20.1, 21.1, and 22.2 kg/m2, respectively (18). According to the questionnaire filled by the guardians, the following individuals were excluded: (1) patients with obesity caused by various pathological factors, including obesity in girls caused by hormone use; (2) patients with secondary precocious puberty caused by a pituitary tumor or adrenal tumor; and (3) patients with congenital adrenocortical hyperplasia, androgen-secreting adrenocortical tumors or ovarian tumors, or precocious puberty caused by exogenous androgen intake.
To facilitate obtaining informed consent, we included the informed consent form in an electronic survey, and the head teacher forwarded the two-dimensional code of the electronic questionnaire to the class WeChat group (social media) for guardians to access. The informed consent form had an independent option, which could be filled in only after consent was given. If guardians chose not to agree, the questionnaire automatically jumped to the end, and there was no need to fill in and participate in the subsequent physical examination. Finally, 5,169 of 6,474 (79.84%) parents agreed to participate and then completed the questions regarding the child’s health and family environment. The main contents of the questionnaire included the child’s and parents’ basic information and physical examination findings, including height, weight, waist circumference, and secondary sexual characteristics. This study was approved by the ethics committee of Shanghai Children’s Medical Center and Qufu People’s Hospital.
All children were examined by professional pediatricians. The items of interest included height, weight, and waist circumference, which were measured with unified calibrated instruments. During height measurement, children were asked to take off their shoes and stand upright, and their height was recorded accurately to 0.1 cm. During weight measurement, each child was asked to wear close-fitting clothes and bare feet, and the data was recorded accurately to 0.1 kg. When measuring waist circumference, the child was asked to adopt a standing position and their waist was measured horizontally at the midpoint of the connecting line between the lower edge of the ribs and the upper edge of the iliac bone and recorded accurately to 0.1 cm. The calculation of BMI (kg/m2) used the square of the weight (kg) divided by the height (m). According to the BMI cutoffs of sex and age of 2–18 years old according to the IOTF standard, we divided the subjects into the following groups: thinness, normal weight, overweight, obesity, and severe obesity (19)
Precocious puberty is defined by the onset of secondary sexual signs (Tanner stage ≥ II) of girls before 8 years old and boys before 9 years old. Precocious puberty in girls involves breast tissue development before 8 years old or menstruation before 10 years old, Breast Stage 2 means appearance of breast bud and elevation of breast and papilla as a small mound, enlargement of areola diameter. Meanwhile, the testicular enlargement of boys with precocious puberty is ≥ 4 mL before 9 years old (20). In this study, professional pediatricians conducted physical examinations of boys and girls. In order to protect their privacy, boys and girls were examined by doctors of the same sex in separate rooms. During the physical examination, each child entered separately, and their development was recorded according to Tanner’s stages. In the case of doubt or uncertainty, two doctors discussed the findings. When the two doctors’ opinions remained inconsistent, a third doctor participated in the evaluation (21, 22). Female doctors inspected the development of girls’ breasts and pubic hair through visual inspection, and overweight and obese girls also underwent portable breast ultrasound imaging (model M9CV; Shenzhen Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) to distinguish breast and adipose tissues (23). Male doctors measured the testicular volume by palpation and a Prader testicular meter and examined the development of pubic hair.
Obese girls, girls with precocious puberty, and girls with normal weights underwent extraction of 2–3 mL of venous blood. The levels of sex hormones (luteinizing hormone [LH]/follicle-stimulating hormone [FSH]) were detected by chemiluminescence (Cobase 411; Roche Diagnostics GmbH, Mannheim, Germany). An LH cutoff value of 0.3 IU/L or above was used to suggest the occurrence of precocious puberty (24).
All data were recorded and proofread by two researchers. We used stratified cluster sampling method, adjusted prevalence by two-step inverse probability weighting (see Supplements 1 and 2 for the correction process), and used SPSS version 25.0 (IBM Corporation, Armonk, NY, USA) for statistical analysis. The prevalence rates between the two groups were compared by chi-squared test. A two-sided test was performed, and P < 0.05 indicated the statistical difference.
Figure 1 shows the flowchart of the survey. A total of 5,169 parents or guardians filled in the questionnaire, and 4,712 children completed the physical examination. Excluding 67 questionnaires with missing values, 53 questionnaires with invalid values, and 14 respondents with notable medical histories, 4,565 children were included in the final analysis. The basic characteristics of the study population are shown in Table 1. We enrolled a total of 4,565 children, including 2,433 boys (53.30%) and 2,132 girls (46.70%). Mean age was 7.25 ± 0.89 years and mostly living in urban areas (88.67%). Most participants had a normal weight (60.83%); 17.11% were overweight, 8.61% were obese, and 4.53% were severely obese.
The prevalence of precocious puberty according to sex is shown in Table 2. A total of 176 children with precocious puberty were diagnosed. The unadjusted and adjusted prevalence of Tanner stage ≥ II was 5.01% (11.53% for girls and 1.41% for boys) and 6.29% (14.23% for girls and 1.54% for boys), respectively. The unadjusted and adjusted prevalence of precocious puberty in girls calculated by Tanner staging combined with ultrasound was 8.33% and 11.18%, respectively.
The prevalence rates of precocious puberty in urban and suburban areas are shown in Table 3. The prevalence of precocious puberty for girls in urban and suburban areas according to Tanner Stage was 12.21% and 5.93%, respectively. The prevalence of precocious puberty for boys in urban and suburban areas according to Tanner Stage was 1.54% and 0.40%, respectively. After adjustment, the total prevalence of precocious puberty in urban and suburban areas was 1.65% and 1.36%, respectively; that is, the prevalence of precocious puberty in suburban areas was lower than that in urban areas (P < 0.05).
Table 4 shows the distribution of BMI and the prevalence of precocious puberty in urban and suburban areas. In urban areas, the prevalence of precocious puberty in children diagnosed with overweight, obesity, or severe obesity was 10.81%, 13.26%, and 13.55%, respectively, all of which were significantly higher than that of 2.69% in the normal-weight group (P < 0.05). The prevalence of precocious puberty was 3.51%, 4.55%, and 11.11% in suburban children diagnosed with overweight, obesity, or severe obesity, respectively, which were significantly higher than that of 1.21% in normal-weight children (P < 0.05). These findings suggest that BMI is positively correlated with the prevalence of precocious puberty (Figure 2).
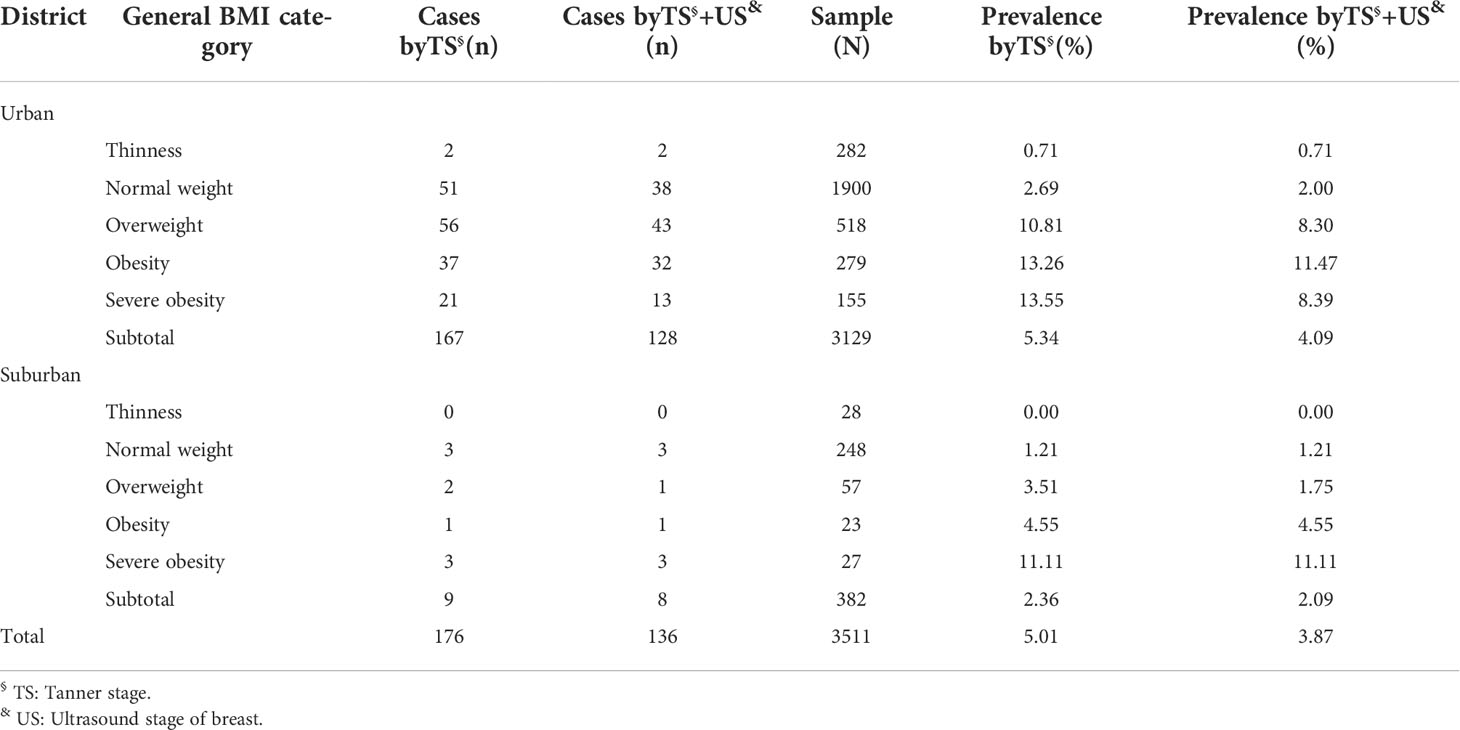
Table 4 Prevalence of precocious puberty in urban and suburban districts according to general BMI category.
Table 5 shows the effect of BMI on precocious puberty in boys and girls. According to BMI, the prevalence of precocious puberty in children diagnosed with overweight (29.76%), obesity (43.55%), and severe obesity (55.88%) was higher than that in normal-weight children (5.53%, P < 0.05), and the distribution trend was similar in boys. The percentage of girls with precocious puberty (LH ≥ 0.3 IU/L) is higher than that of girls with normal puberty (10.87% vs 5.80%, P =0.298); similarly, the percentage of boys with precocious puberty (LH ≥ 0.3 IU/L) is higher than that of boys with normal puberty (18.75% vs 11.75%, P=0.652, Table S1); the percentage of obese girls with precocious puberty (LH ≥ 0.3 IU/L) is higher than that of girls with normal puberty (13.89% vs 7.84%, P =0.388, Table S2).
In this study, we investigated children aged 6–10 years in 12 primary schools in Qufu City, and we found that the adjusted prevalence of precocious puberty in Qufu was 6.29% (1.54% for boys and 14.23% for girls), or 5.56% in urban and 7.66% in suburban areas, respectively. The prevalence of precocious puberty in normal-weight children was 2.52%, that in overweight children was 10.09%, that in obese children was 12.62%, and that in severely obese children was 13.19%. The prevalence of precocious puberty was positively correlated with BMI, especially in girls.
The prevalence of precocious puberty in this study, performed in China, was higher than that in previous studies conducted in other countries. Because the physical examinations of children in our study was performed by pediatricians, and school-based populations were examined, including children with central or peripheral precocious puberty, the detection rate of precocious puberty may be higher compared with other studies. Moore et al. in France (4) used data from the national insurance database, which could not exclude the difference in medical level and geographical factors, and thus potentially excluded cases with precocious puberty without consultation or diagnosis at the hospital and therefore may have underestimated the prevalence of precocious puberty. In Denmark, a study (1) used statistics from 1998–2017 that were classified according to the immigration group. The International Classification of Diseases (ICD), 10th revision, diagnostic code used therein was not verified for the medical records, and this may have affected the number of cases involved, thus underestimating the true prevalence. In Taiwan (3), a study on the prevalence of children with precocious puberty did not include patients who were not diagnosed at medical institutes, so the prevalence of precocious puberty was likely underestimated. The difference in the prevalence of precocious puberty between our study and studies in other countries may also be related to different ethnic and social backgrounds (25, 26). Meanwhile, compared to the Liu YF’s study in Zhongshan city, Guangdong province, China (6), the adjusted prevalence of precocious puberty in the present study is slightly higher than that in girls (14.23% vs 13.43%), however, it was lower than that in boys (1.54% vs 3.26%), furthermore, it was lower than that in urban girls (8.63% vs 11.30%) and higher than that in suburban girls (15.90% vs 14.16%).
In this study, we found that overweight and obesity are related to the prevalence of precocious puberty. Precocious puberty is a form of early sexual maturity wherein the clinical signs are inconsistent with the age. It seriously damages the growth potential of children, easily causes damage to children’s height, and even leads to pediatric psychological diseases (27). There is increasing evidence that obesity is closely related to precocious puberty. A study in Shanghai showed that early puberty is associated with obesity, especially centripetal obesity (28). An investigation of the influence of adolescent obesity and body composition on puberty in Chongqing (13) found that a high BMI and high body fat rate can advance the puberty development of girls.
The mechanism of obesity leading to early puberty is still inconclusive, and there are different opinions. First, leptin secreted by adipose tissue may be the reason for the early puberty of obese girls. This regulates the release of adult-releasing hormone, which directly affects the ovaries and plays an important role in the beginning and progression of puberty (29). Second, adipose tissue has aromatase activity, which can promote the conversion of androgen into estrogen. This increase in androgen concentration in obese girls can promote the increase of gonadotrophin-releasing hormone secretion and lead to early puberty (30, 31).
This study has several advantages. First, we used stratified cluster random sampling to select schools from different regions with different economic backgrounds, which reduces the impact of different regions and environments and ensures better population representation. Second, children’s secondary sexual characteristics were directly examined by pediatricians, and the physical examinations were objective and reliable. Third, the breast examination of overweight and obese children with ultrasound was helpful to accurately distinguish between breast and adipose tissue. Furthermore, the children involved in the survey were dynamically followed up, and the dynamic changes of growth and development and living habits of the children were fully observed, which was helpful for evaluating the influencing factors of precocious puberty.
However, our research also has many limitations. First, this cross-sectional study cannot rule out other factors affecting the prevalence of precocious puberty. Second, we used BMI to define obesity, which cannot completely screen out obese children among all subjects (32–34), and there is a certain false-negative rate. Third, this study could not distinguish between central or peripheral precocious puberty, the prevalence of precocious puberty may be bit high due to the ages of their study members being nearly puberty. A sex hormone stimulation test cannot be carried out in school children, but we provided the physical examination results to the guardians and suggested they go to the local hospital for a further examination. Finally, it was impossible to carry out pituitary imaging and color ultrasound examination for every child, and it was impossible to completely rule out precocious puberty caused by pituitary abnormalities, tumors, and other reasons.
The prevalence of precocious puberty in overweight and obese children is higher than that in normal-weight children, and there is a sex difference in this effect, which is more obvious in girls.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Shanghai Children’s Medical Center and Qufu People’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian.
YZ drafted the manuscript. JN, LZ, TY, PX, BG, XX, and XL performed physical examinations and collected the data. YL conducted statistical analysis. and SL and HK designed the study. All authors contributed to the article and approved the submitted version.
This study is supported by the National Natural Science Foundation of China [grant nos. 81872637 and 82173534].
We appreciate the help and guidance of Shanghai Children’s Medical Center and Shanghai Jiao Tong University School of Medicine and the support of Qufu Education Commission and the students and their parents of participating schools.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.910119/full#supplementary-material
Supplementary Table 1 | The number of children with basal LH≥0.3IU/L.
Supplementary Table 2 | The number of obese girls with basal LH≥0.3IU/L.
1. Brauner EV, Busch AS, Eckert-Lind C, Koch T, Hickey M, Juul A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Netw Open (2020) 13(10):e2015665. doi: 10.1001/jamanetworkopen.2020.15665
2. Kim SH, Huh K, Won S, Lee KW, Park MJ. A significant increase in the incidence of central precocious puberty among Korean girls from 2004 to 2010. PloS One (2015) 10(11):e0141844. doi: 10.1371/journal.pone.0141844
3. Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, et al. Incidence and prevalence of central precocious puberty in Korea: An epidemiologic study based on a national database. J Pediatr (2019) 208:221–8. doi: 10.1016/j.jpeds.2018.12.022
4. Su PH, Huang JY, Li CS, Chang HP. The age distribution among children seeking medical treatment for precocious puberty in Taiwan. Int J Environ Res Public Health (2020) 17(18):6765. doi: 10.3390/ijerph17186765
5. Le Moal J, Rigou A, Le Tertre A, De Crouy-Channel P, Léger J, Carel J. Marked geographic patterns in the incidence of idiopathic central precocious puberty: a nationwide study in France. Eur J Endocrinol (2018) 178(1):33–41. doi: 10.1530/EJE-17-0379
6. Liu YF, Yu TT, Li XQ, Pan DX, Lai X, Chen Y, et al. Prevalence of precocious puberty among Chinese children: a school population-based study. Endocrine (2021) 72(2):573–81. doi: 10.1007/s12020-021-02630-3
7. Upners EN, Busch AS, Almstrup K, Petersen JH, Assens M, Main KM, et al. Does height and IGF-I determine pubertal timing in girls? Pediatr Res (2021) 90(1):176–83. doi: 10.1038/s41390-020-01215-6
8. Yang B, Ostbye T, Huang X, Li Y, Fang B, Wang H, et al. Maternal age at menarche and pubertal timing in boys and girls: A cohort study from chongqing, China. J Adolesc Health (2021) 68(3):508–16. doi: 10.1016/j.jadohealth.2020.06.036
9. Duan R, Qiao T, Chen Y, Chen MX, Xue HM, Zhou X, et al. The overall diet quality in childhood is prospectively associated with the timing of puberty. Eur J Nutr (2021) 60(5):2423–34. doi: 10.1007/s00394-020-02425-8
10. Valsamakis G, Arapaki A, Balafoutas D, Charmandari E, Vlahos NF. Diet-induced hypothalamic inflammation, phoenixin,and subsequent precocious puberty. Nutrients (2021) 13(10):3460. doi: 10.3390/nu13103460
11. Roy J, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans–a review. Med Sci Monit (2009) 15(6):RA137–145.
12. Delemarre-van de Waal HA. Environmental factors influencing growth and pubertal development. Environ Health Perspect (1993) 101(Suppl 2):39–44. doi: 10.1289/ehp.93101s239
13. Brix N, Ernst A, Lauridsen LLB, Parner ET, Arah OA, Olsen J, et al. Childhood overweight and obesity and timing of puberty in boys and girls: Cohort and sibling-matched analyses. Int J Epidemiol (2020) 49(3):834–44. doi: 10.1093/ije/dyaa056
14. He F, Guan P, Liu Q, Crabtree D, Peng L, Wang H. The relationship between obesity and body compositions with respect to the timing of puberty in chongqing adolescents: A cross-sectional study. BMC Public Health (2017) 17(1):664. doi: 10.1186/s12889-017-4681-1
15. Li W, Liu Q, Deng X, Chen Y, Yang B, Huang X. Association of prepubertal obesity with pubertal development in Chinese girls and boys: A longitudinal study. Am J Hum Biol (2018) 30(6):e23195. doi: 10.1002/ajhb.23195
16. Busch AS, Hojgaard B, Hagen CP, Teilmann G. Obesity is associated with earlier pubertal onset in boys. J Clin Endocrinol Metab (2020) 105(4):dgz222. doi: 10.1210/clinem/dgz222
17. Abou El Ella SS, Barseem NF, Tawfik MA, Ahmed AF. BMI relationship to the onset of puberty: Assessment of growth parameters and sexual maturity changes in Egyptian children and adolescents of both sexes. J Pediatr Endocrinol Metab (2020) 33(1):121–8. doi: 10.1515/jpem-2019-0119
18. Chen M, Eugster EA. Central precocious puberty: Update on diagnosis and treatment. Paediatr Drugs (2015) 17(4):273–81. doi: 10.1007/s40272-015-0130-8
19. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes (2012) 7(4):284–94. doi: 10.1111/j.2047-6310.2012.00064.x
20. Cheuiche AV, da Silveira LG, de Paula LCP, Lucena IRS, Silveiro SP. Diagnosis and management of precocious sexual maturation: An updated review. Eur J Pediatr (2021) 180(10):3073–87. doi: 10.1007/s00431-021-04022-1
21. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child (1969) 44:291. doi: 10.1136/adc.44.235.291
22. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child (1970) 45:13. doi: 10.1136/adc.45.239.13
23. Bruserud IS, Roelants M, Oehme NHB, Madsen A, Eide GE, Bjerknes R, et al. References for ultrasound staging of breast maturation, tanner breast staging, pubic hair, and menarche in Norwegian girls. J Clin Endocrinol Metab (2020) 105(5):1599–607. doi: 10.1210/clinem/dgaa107
24. Wankanit S, Mahachoklertwattana P, Pattanaprateep O, Poomthavorn P. Basal serum luteinising hormone cut-off, and its utility and cost-effectiveness for aiding the diagnosis of the onset of puberty in girls with early stages of breast development. Clin Endocrinol (Oxf) (2020) 92(1):46–54. doi: 10.1111/cen.14124
25. Soriano-Guillen L, Corripio R, Labarta JI, Canete R, Castro-Feijoo L, Espino R, et al. Central precocious puberty in children living in Spain: Incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab (2010) 95(9):4305–13. doi: 10.1210/jc.2010-1025
26. Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev (2003) 24(5):668–93. doi: 10.1210/er.2002-0019
27. Zurita-Cruz JN, Torres-Tamayo M, Aguilar-Herrera BE, Miranda-Lora AL, Rivera-Hernández AJ, Calzada-León R, et al. Monitoring during the treatment of precocious puberty: Clinical guideline for the diagnosis and treatment of precocious puberty. Bol Med Hosp Infant Mex (2020) 77(Supl 1):29–34. doi: 10.24875/BMHIM.20000086
28. Chen C, Zhang YT, Sun WQ, Chen Y, Jiang YR, Song YJ, et al. Investigating the relationship between precocious puberty and obesity: A cross-sectional study in shanghai, China. BMJ Open (2017) 7(4):e014004. doi: 10.1136/bmjopen-2016-014004
29. Nieuwenhuis D, Pujol-Gualdo N, Arnoldussen IAC, Kiliaan AJ. Adipokines: A gear shift in puberty. Obes Rev (2020) 21(6):e13005. doi: 10.1111/obr.13005
30. Mans F, Remer T. Role of nutritional status in the regulation of adrenarche. J Clin Endocrinol Metab (1999) 84(11):3936–44. doi: 10.1210/jcem.84.11.6093
31. Huang A, Reinehr T, Roth CL. Connections between obesity and puberty: Invited by Manuel tena-sempere, Cordoba. Curr Opin Endocr Metab Res (2020) 14:160–8. doi: 10.1016/j.coemr.2020.08.004
32. Javed A, Jumean M, Murad MH, Okorodudu D, Kumar S, Somers VK, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: A systematic review and meta-analysis. Pediatr Obes (2015) 10(3):234–44. doi: 10.1111/ijpo.242
33. Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: Systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr (1996) 63(4):500–6. doi: 10.1093/ajcn/63.4.500
Keywords: precocious puberty, prevalence, children, cross-sectional survey, obesity
Citation: Zhang Y, Ni J, Zhang L, Yu T, Li X, Xue P, Liu Y, Gao B, Xue X, Kong H and Liu S (2022) The prevalence of precocious puberty among children in Qufu City, Shandong Province, China, a population-based study. Front. Endocrinol. 13:910119. doi: 10.3389/fendo.2022.910119
Received: 31 March 2022; Accepted: 29 June 2022;
Published: 25 July 2022.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Anna Grandone, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Zhang, Ni, Zhang, Yu, Li, Xue, Liu, Gao, Xue, Kong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijian Liu, bGl1c2hpamlhbkBzY21jLmNvbS5jbg==; Huijun Kong, aHVpanVuMTk3NUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.