- 1Department of Medicine, Division of Endocrinology, Pituitary Center and Center for Endocrine Tumors, Leiden University Medical Center, Leiden, Netherlands
- 2Department of Medicine, Center for Endocrine Tumours Leiden, Leiden University Medical Center, Leiden, Netherlands
- 3Department of Neurosurgery, Leiden University Medical Center, University Neurosurgical Center Holland, Leiden, Netherlands
- 4Directorate of Finances, Leiden University Medical Center, Leiden, Netherlands
- 5Department of Endocrinology and Metabolism, Amsterdam University Medical Center, Meibergdreef 9, Amsterdam, Netherlands
Purpose: Value-based healthcare (VBHC) provides a framework to improve care by improving patient outcomes and reducing healthcare costs. To support value-based decision making in clinical practice we evaluated healthcare costs and cost drivers in perioperative care for pituitary tumour patients.
Methods: We retrospectively assessed financial and clinical data for surgical treatment up to the first year after surgery of pituitary tumour patients treated between 2015 and 2018 in a Dutch tertiary referral centre. Multivariable regression analyses were performed to identify determinants of higher costs.
Results: 271 patients who underwent surgery were included. Mean total costs (SD) were €16339 (13573) per patient, with the following cost determinants: surgery time (€62 per minute; 95% CI: 50, 74), length of stay (€1331 per day; 95% CI 1139, 1523), admission to higher care unit (€12154 in total; 95% CI 6413, 17895), emergency surgery (€10363 higher than elective surgery; 95% CI: 1422, 19305) and postoperative cerebrospinal fluid leak (€14232; 95% CI 9667, 18797). Intradural (€7128; 95% CI 10421, 23836) and combined transsphenoidal/transcranial surgery (B: 38494; 95% CI 29191, 47797) were associated with higher costs than standard. Further, higher costs were found in these baseline conditions: Rathke’s cleft cyst (€9201 higher than non-functioning adenoma; 95% CI 1173, 17230), giant adenoma (€19106 higher than microadenoma; 95% CI 12336, 25877), third ventricle invasion (€14613; 95% CI 7613, 21613) and dependent functional status (€12231; 95% CI 3985, 20477). In patients with uncomplicated course, costs were €8879 (3210) and with complications €17551 (14250).
Conclusions: Length of hospital stay, and complications are the main drivers of costs in perioperative pituitary tumour healthcare as were some baseline features, e.g. larger tumors, cysts and dependent functional status. Costs analysis may correspond with healthcare resource utilization and guide further individualized care path development and capacity planning.
1 Introduction
Clinically apparent pituitary adenomas are rare with an incidence of 3.9-7.4 persons per 100.000 per year and a prevalence of 1 per 1000 persons (1). Patients present with a variety of symptoms due to mass effects (e.g., chiasm compression, hypopituitarism) and/or hormonal hypersecretion, depending on the adenoma subtype. Preferred treatment in most cases is transsphenoidal resection (2–4), with or without a combination of medical therapy. Radiotherapy is used as a last resort in selective cases.
There are disease-specific treatment guidelines (2, 3, 5–7), however, in clinical practice the evidence base of optimal management choices is rather limited. Patients show highly variable clinical outcomes and health-related quality of life after treatment (8, 9). The Leiden University Medical Centre aims to improve pituitary tumour healthcare by adapting and implementing a value-based healthcare (VBHC) approach with prospective real-time outcome evaluations. VBHC demonstrates a framework to restructure healthcare with the ultimate goal of improving outcomes and/or reducing (unnecessary) costs, and thus increase value for patients (10, 11). Accordingly, our team designed and published a value-based framework to measure perioperative outcomes in pituitary tumour patients, allowing clinicians to evaluate and individualize healthcare (8). Subsequently, we expect that assessment of healthcare costs, which reflects in-hospital resource utilization, results in development of cost reduction initiatives and better individualized care pathways, while maintaining or improving clinical outcomes.
A comprehensive cost evaluation of perioperative care with extensive analyses of cost drivers for pituitary tumour patients is currently lacking. Previous cost evaluation studies in pituitary healthcare were limited to the surgical procedure (12), index hospitalization (12–14), complications or readmissions (15, 16), instead of encompassing the entire perioperative trajectory. Other studies focussed on a single diagnosis, limiting comparisons between pituitary tumour types and other case mix variables and prohibiting evaluation of the care pathways and the required capacity within the pituitary healthcare team (17–29). Moreover, many studies assessed predictors of healthcare costs instead of cost determinants (24, 28) with predictors not being cost drivers per se. Therefore, this study aims to evaluate 1) total in-hospital costs during pituitary tumour surgery, and up to the first year after; 2) how total costs are attributable to different cost domains (i.e., costs for surgery, hospitalization, irradiation and diagnostic investigations); and 3) which determinants are drivers of total costs. Based on our results and prior knowledge we will propose strategies for improving VBHC care pathways.
2 Materials and Methods
2.1 Study Design
This retrospective study was performed using data of consecutive surgically treated pituitary tumour patients between January 2015 and December 2018 at the Leiden University Medical Centre (LUMC), a tertiary referral centre for pituitary surgery in the Netherlands. Patients were eligible if diagnosed with a pituitary adenoma, Rathke’s cleft cyst (RCC) or craniopharyngioma. Patients with other tumours in the (para)sellar region (e.g., meningioma, cerebral metastases, chordoma, and chondrosarcoma) or uncertain diagnosis were excluded. Clinical data were collected from electronic patient charts and in-hospital costs data were extracted from a financial database constructed for VBHC research. Data of patients were obtained after approval of the scientific committee of the department, after which a waiver was obtained from the institution medical ethics committee, local study number (G19·011).
2.2 Perioperative Care Trajectory
Preoperative evaluation of all patients includes imaging (MRI and CT-scan), comprehensive endocrine work-up of all pituitary axes, and a neuro-ophthalmological assessment when indicated. During a combined consultation with an endocrinologist and neurosurgeon, the main indication for surgery (e.g., hypersecretion, visual symptoms), aim of surgery (e.g., complete resection, gross total resection (GTR), chiasm decompression) and intended effect (e.g. biochemical remission, recovery of visual function, lowering medication dosage) are established, depending on the patient’s symptoms and surgical feasibility of pituitary tumour resection. Based on last preoperative imaging all pituitary tumours, including RCC’s and craniopharyngioma’s, were classified as micro- (<10mm), macro- (10-40mm) or giant (>40mm) adenoma for comparability. Third ventricle invasion was established and cavernous sinus invasion was defined as a KNOSP-score of ≥3 (30). Patient characteristics and comorbidities were determined during hospital admission. Hormonal hypersecretion and hypopituitarism are defined according to current guidelines and hormone replacement therapy was initiated when indicated, especially in case of corticotropic or thyrotropic insufficiency [8, 16, 33, 34]. Prior treatment with pharmacotherapy (e.g., cabergoline, metyrapone, octreotide), surgery, or irradiation was established.
Endoscopic transsphenoidal surgery is generally performed by two neurosurgeons and open microscopic transcranial surgery by one surgeon. Patients with complex nasal anatomy or extensive skull base destruction are operated together with an ENT-surgeon. Most surgical resections were performed endoscopically transsphenoidal. When necessary, an extended or intradural approach was performed. In some cases of giant adenomas or post-operative apoplexy in a suprasellar adenoma remnant, a craniotomy or combined endoscopic transsphenoidal and microscopic transcranial surgery was performed. We described our surgical approach in detail in a recent review (31).
Following surgery, patients were discharged at postoperative day 2 or 3 when eligible for a short-stay protocol, instead of at postoperative day 5 (32). Length of stay (LOS) was measured from the day of surgery until discharge. Admission to a high care unit was defined as hospitalization at the medium- or intensive care unit. Subsequently, HPA-axis function was evaluated within one week after surgery through fasting cortisol or dynamic testing (33). Patients were daily monitored by a case manager for occurrence of complications in the first 2 weeks postoperatively and in case of postoperative hyponatraemia readmission was considered If fluid restriction in the home situation was not sufficient. Diabetes insipidus (DI) was evaluated and classified based on duration. Accordingly, patients with DI lasting for shorter or longer than 2 weeks were classified as 1-2 or 3-4, respectively (34). An outpatient clinic visit for evaluation of treatment outcomes was performed at 6 months, or sooner in case of complications or complaints, which consists of evaluation of residual tumour on MRI, ophthalmological examination (visual acuity and visual fields testing through static perimetry), and assessment of hormone levels. Remission of hormonal hypersecretion, hypopituitarism, and vision were defined according to guidelines and recent literature (5–7, 35, 36).
2.3 Costs Analyses
Our costs analyses adhere to the Consolidated Health Economic Evaluation Report Standards (37). Direct costs were derived from an institutional perspective, conform the most recent national guidelines for in-hospital cost assessment (38). Costs are displayed in euros and can be converted into American dollars by applying the mean purchasing power parity (PPP), as reported by the Organisation for Economic Co-operation and Development (OECD). The mean PPP between 2015 and 2018 was 0,791 per dollar (39). The reported costs are not corrected for inflation and a 0% discount rate was applied. In line with the study’s perspective, we calculated direct cost as opposed to indirect costs, because they are directly related to healthcare utilization and so a reflection of care capacity. The costs were attributed to units using cost allocation keys, meaning that costs for resources were activity-adjusted, e.g., costs for surgery or consultations were calculated based on the estimated mean duration of the activity.
Total costs during follow-up and the distribution of total costs into cost domains (i.e., costs for surgery, hospitalization, irradiation and diagnostic investigations or consultations) were established for the total cohort. Surgical costs were based on costs for resources during the surgical procedure, including surgery materials, surgeons, nurses and monitoring devices. Hospitalization costs comprised expenditures for the general ward, ICU, physician visits and nurses, including readmissions. Diagnostic investigations or consultations costs consisted of laboratory tests, radiology, pathology and (para)medic consultations. Prescribed medication at our hospital and expenditures at other facilities than the LUMC, such as the general practitioner, were not included.
2.4 Statistical Analyses
Data analyses were performed using the SPSS Statistics Version 25. For categorical variables, the number of patients and corresponding percentage of the total population were calculated. For continuous variables, the mean with range and standard deviation are reported. Missing data were excluded from analyses using pairwise deletion.
First of all we report the mean total costs and how these costs are distributed amongst different cost domains (i.e., surgery, hospitalization, irradiation, diagnostic investigations or consultations) of the total cohort. For each cost domain, both absolute cost data and percentage of total costs are reported. Subsequently, univariable and multivariable linear regression analyses were performed to identify factors associated with higher costs. For each assessed determinant, a separate multivariable analysis was performed, estimating the association between the determinant of interest and total costs, corrected for the relevant confounders. Confounders were defined as variables associated with both the determinant and the outcomes, but not being caused by the determinant, i.e., not laying within the causal path between the determinant and outcomes (40). Confounders were chosen for each determinant following this definition and using prior clinical knowledge. The effect sizes of the regression analyses are reported in unstandardized regression coefficients and 95% confidence intervals (95% CI) (41), with P-values lower than 0,05 considered significant.
Furthermore, we analyse how total costs of determinants were distributed amongst different cost domains, expecting that some determinants would increase costs among a specific cost domain. For example, larger tumour size may require prolonged, complex surgery and therefore increased surgery costs may the main cost driver. Complications may prolong length of stay and mainly increase hospitalization costs. By analysing how each cost domain contributes to total costs we aim to investigate whether increased total costs are explained by specific parts of the healthcare pathways, which may serve as target for future value-based healthcare initiatives.
3 Results
3.1 Study Population
A total of 271 patients with a pituitary tumour met the inclusion criteria (Figure 1). Demographics, clinical characteristics, perioperative factors and outcomes are displayed in Table 1. Mean (SD) age was 50 (17) years, 147 patients were female (54%), mean BMI was 27,6 (4,9) and 18% were currently smoking. Patients were diagnosed with non-functioning adenoma (NFA) (n=118; 44%), acromegaly (n=42; 16%), prolactinoma (n=39; 13%), Cushing’s disease (n=36; 13%) craniopharyngioma (n=20; 7%), RCC (n=10; 4%) FSH-adenoma (n=3; 1%) or thyrotropinoma (n=3; 1%). The majority of patients had a macroadenoma (n=188; 69%), 51 (19%)a microadenoma, 21 (8% a giant adenoma and in 11 (4%) of patients no clear adenoma was visible. Comorbidities included hypertension (n=80; 30%) diabetes mellitus (n=24; 9%), COPD (n=3; 1%) and congestive heart failure (n=1; <1%), and 11 (4%) patients were functionally dependent. Based on these comorbidities, the mFI-5 score of patients were 0 (n=174; 64%), 1 (n=79; 29%), 2 (n=15; 6%), 3 (n=2; 1%) or 4 (n=1; <1%). Prior to surgery, 28 (11%) had cortisol deficiency, 73 (27%) had hypopituitarism amongst other axes and 37 (14%) had panhypopituitarism. Vision loss or VFD was established in 116 (44%) and a cranial nerve deficit in 18 (7%). The tumour invaded the third ventricle or cavernous sinus in 17 (7%) and 46 (17%) of patients, respectively. In 27 (10%) apoplexy was determined. Some patients had undergone prior treatment; 89 patients (33%) had received pharmacological treatment, 52 patients (19%) had previous surgery and 2 patients (1%) received prior radiotherapy.
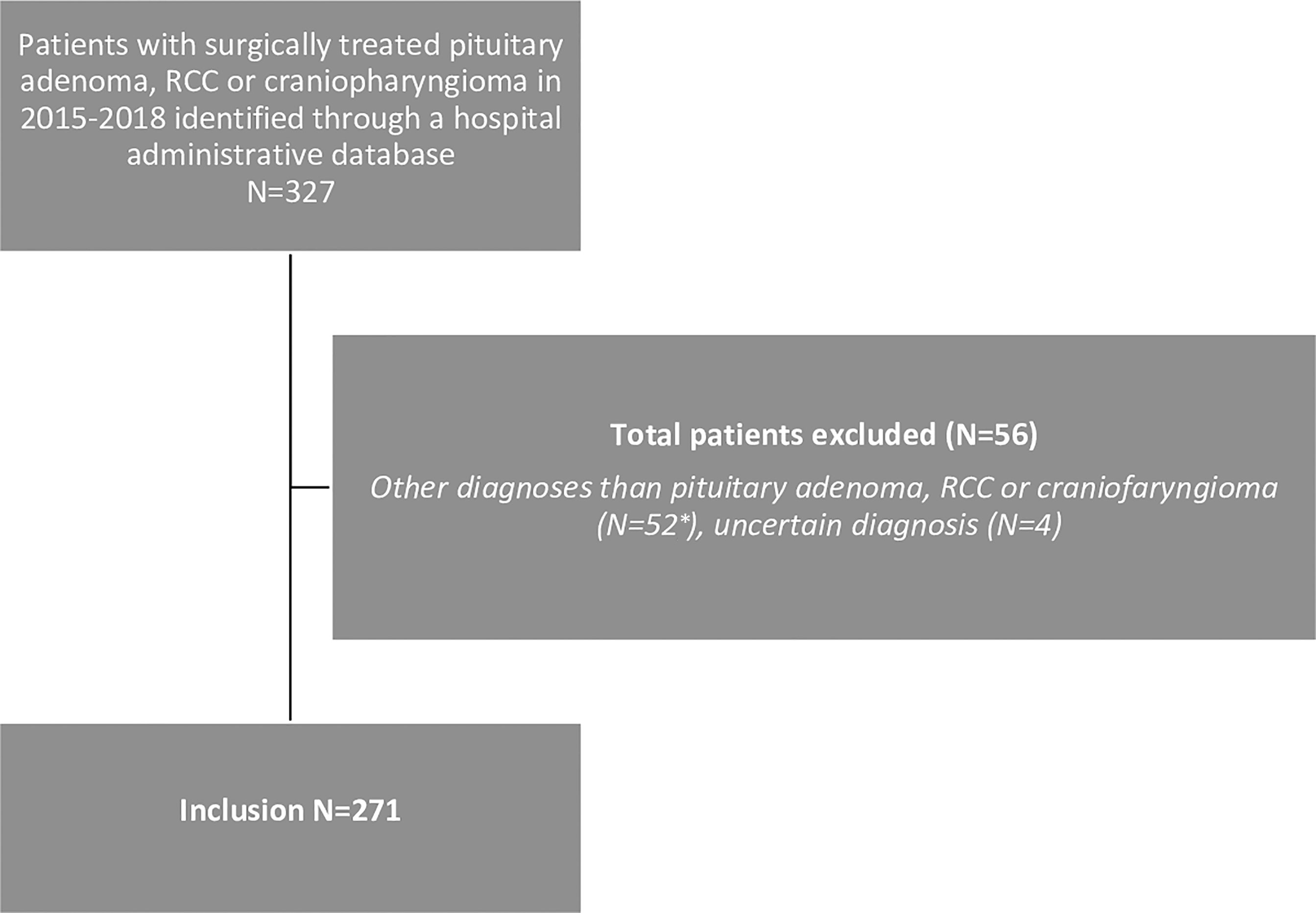
Figure 1 Flow chart of patients’ selection. * Excluded diagnoses: meningioma (N=31), metastasis (N=5), arachnoid cyst (N=1), chordoma (N=5) chondrosarcoma (N=3), Schwannoma (N=2), glioblastoma (N=1), medulloblastoma (N=1), giant cell tumour (N=1), plasmacytoma (N=1), pituicytoma (N=1).
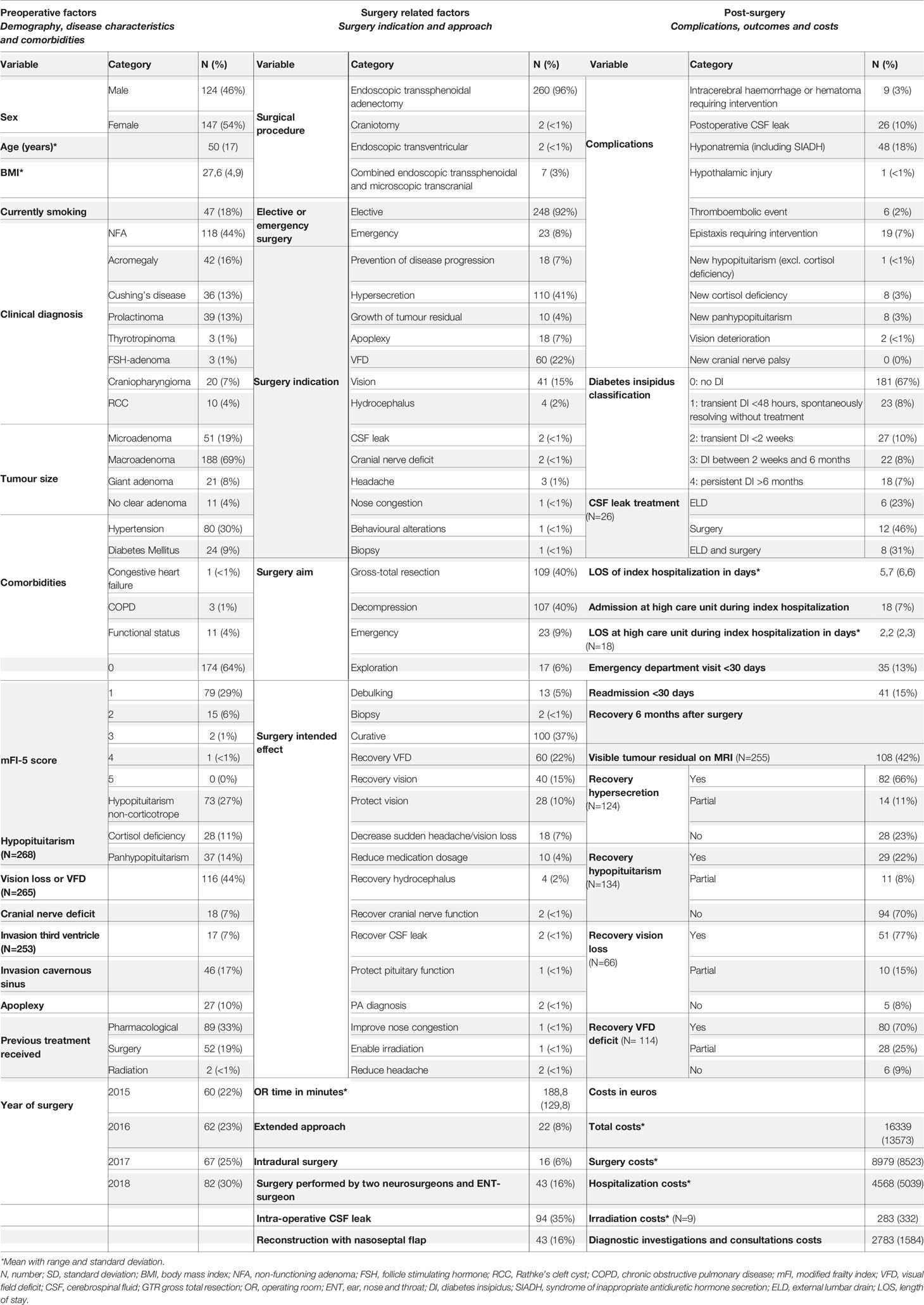
Table 1 Baseline characteristics displayed in number of patients with corresponding percentage or mean with standard deviation.
The indication of surgery was hormonal hypersecretion (n=110; 41), VFD (n=60; 22%), vision loss (n=41; 15%), apoplexy (n=18; 7%), prevention of disease progression (n=18; 7%), growth of tumour residual (n=10; 4%), hydrocephalus (n=4; 2%), headache (n=3; 1%), CSF leak (n=2; <1%), cranial nerve deficit (n=2; <1%), nose congestion (n=1; <1%), behavioural alterations (n=1; <1%) or to perform a biopsy (n=1; <1%). The surgical aim was GTR (n=109; 40%), decompression (n=107; 40%), emergency (n=23, 9%), exploration (n=17; 6%), debulking (n=13; 5%) or biopsy (n=2; <1%). The intended effect was curative or remission (n=100; 37%), recover VFD (n=66; 22%), recover vision loss (n=40; 15%) prevent vision loss (n=18; 7%), reduce mediation dosage (n=10; 4%), recover cranial nerve function (n=2; <1%), recover CSF leak (n=2; <1%), protect pituitary function (n=1; <1%), obtain histopathological diagnosis (n=2; <1%), improve nose congestion (n=1; <1%) or reduce headache.
Patients underwent surgery in 2018 (n=82; 30%), 2017 (n=67; 25%), 2016 (n=62; 23%) or 2015 (n=60; 22%) on an elective basis (n=248; 92%) or as an emergency (n=23; 8%) with a mean surgery duration of 189 (130) minutes. Endoscopic trans-sphenoidal surgery was performed in 260 (96%) patients, of which 22 patients (8%) underwent extended endoscopic surgery and in 16 cases (6%) intradural trans-sphenoidal surgery was performed. Other surgical procedures included endoscopic transventricular (n=2; 1%), transcranial (n=2; 1%) and combined endoscopic transsphenoidal/microscopic transcranial surgery (n=7; 3%). Most procedures were performed by two neurosurgeons, in 43 (16%) surgery was performed together with an ENT-surgeon. Intra-operative CSF leak occurred in 94 (35%) and in 43 (16%) skull base reconstruction with nasoseptal flap was performed.
Mean length of stay (LOS) was 6 (7) days. 18 patients (7%) were admitted to the medium or intensive care (IC) unit during index hospitalization with a mean LOS of 2 (2) days. Postoperative complications were hyponatremia or SIADH (n=48; 18%), CSF leak (n=26; 10%), epistaxis (n=19; 7%), intracerebral haemorrhage or hematoma (n=9; 3%), cortisol deficiency (n=8; 3%), panhypopituitarism (n=8; 3%), thromboembolic event (n=6; 2%), vision deterioration (n=2; <1%), hypothalamic injury (n=1; <1%) or other (i.e., no cortisol deficiency) hypopituitarism (n=1; <1%). Diabetes insipidus (DI) occurred in 90 (33%) and resolved within 48 hours (DI 1; n=23; 8%), 2 weeks (DI 2; n=27; 10%) or 6 months (DI 3; n=22; 8%). In 18 (7%) DI persisted for over 6 months (DI 4). CSF leak was treated with surgery (n=12; 46%), ELD (n=6; 23%) or both ELD and surgery (n=8; 31%).
3.2 Healthcare Costs
Mean total costs were €16339 (13573) per patient for surgery and the first-year post-operative care. Total costs comprised mean costs for surgery (€8979, SD 8523), hospitalization (€4568, SD 5039), irradiation (n=9; €283, SD 332) and diagnostic investigations or consultations (€2783, SD 1584). For the total cohort, costs for surgical care account for 55% of the total costs, hospitalizations for 28%, diagnostic investigations or consultations for 17%. Costs for irradiation comprise less than 1% of total costs with only few (n=9) subjected to radiotherapy in this study period (data not shown). Costs stratified for different pituitary tumour diagnoses and tumour size are presented in Figure 2. Mean costs (SD) for patients with pituitary adenoma are €14861 (11962) for NFA, €11560 (5824) for prolactinoma, €14496 (3471) for FSH-adenoma, €8649 (1566) for thyrotropinoma, €14973 (11556) for acromegaly and €15868 (8055) for Cushing’s disease. Mean total costs for patients with RCC or craniopharyngioma are €23263 (24788) and €36129 (21388), respectively. Mean total costs separated per tumour size are €11344 (5513) for microadenomas, €16478 (12383) for macroadenomas and €28707 (26926) for giant adenomas. Mean costs for patients with no clear adenoma on MRI (comprising patients with Cushing’s disease) are €13516 (6875).
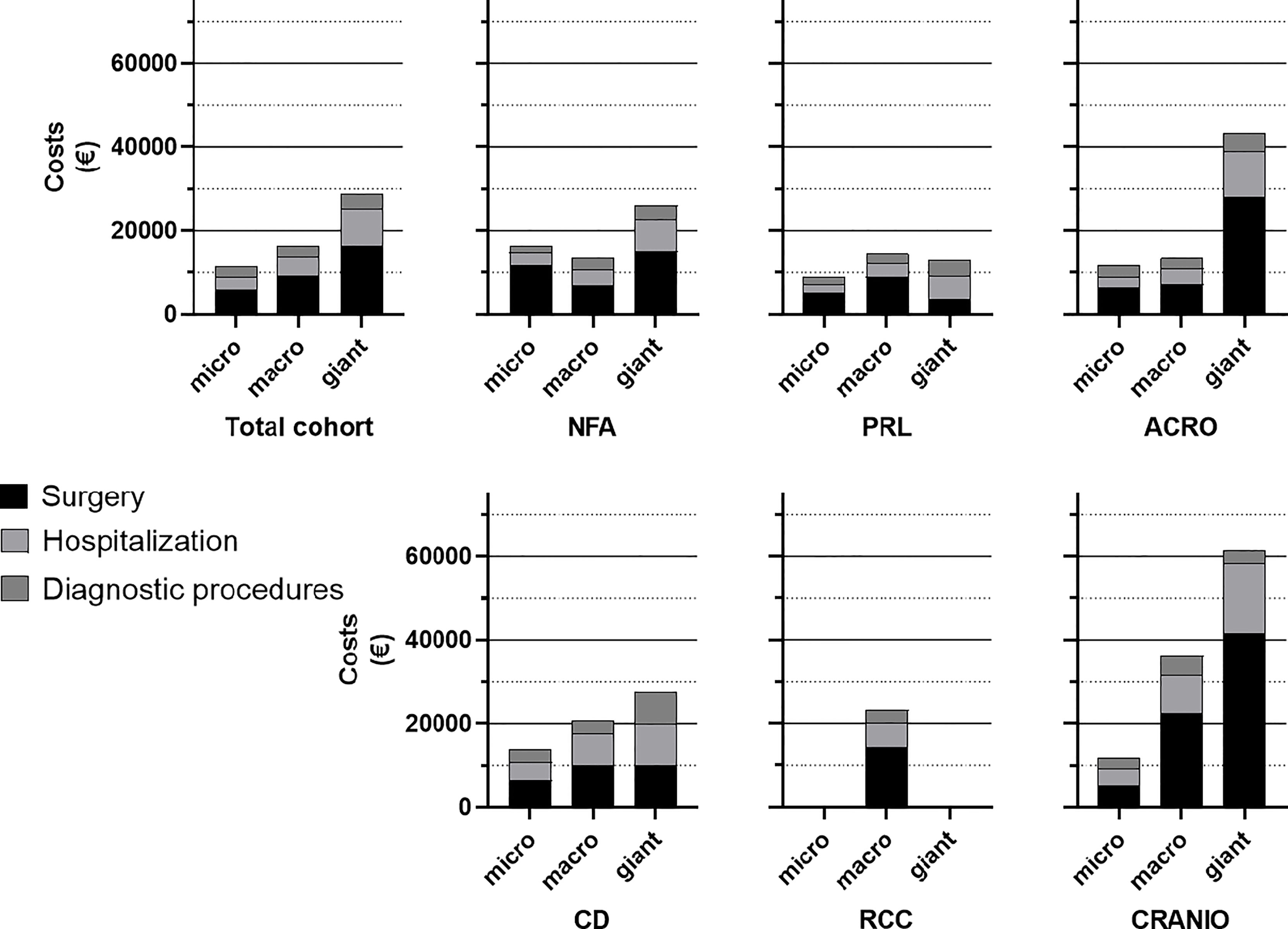
Figure 2 Stacked bars displaying mean total costs of patients with different diagnoses and tumour size. For comparability, tumour size of patients with RCC or craniopharyngioma were also categorized as micro, macro or giant tumours. In most groups, surgery costs account for >50% of total costs and total costs increase with tumour size. NFA, non-functioning adenoma; PRL, Prolactinoma; ACRO, acromegaly; CD, Cushing’s disease; RCC, Rathke’s cleft cyst; CRANIO, craniopharyngioma.
3.3 Determinants of Total Costs
Results are displayed in Table 2 and summarized in Figure 3. Univariable and multivariable analyses with confounders applied for each determinant can be found in Supplementary Table S1. None of the demographic factors were associated with total costs. Preoperative factors associated with higher costs were patients with RCC (B: 9201; 95% CI: 1173, 17230) compared to NFA, macroadenoma (B: 4627; 95% CI 210, 9044) or giant adenoma (B: 19106; 95% CI 12336, 25877) compared to microadenoma. Preoperative panhypopituitarism (B: 5750; 95% CI 1052, 10447), reduced visual acuity (B: 6048; 95% CI 2537, 9560) and third ventricle invasion (B: 14613; 95% CI 7613, 21613) were also associated with increased costs, compared to patients without these characteristics. Patients with pituitary adenoma apoplexy were related to lower costs (B: -27404; 95% CI -36081, -18728) compared to patients without apoplexy. Finally, patients with dependent functional status were associated with higher costs (B: 12231; 95% CI 3985, 20477) compared to functionally independent patients, while none of the comorbidities was related to increased costs.
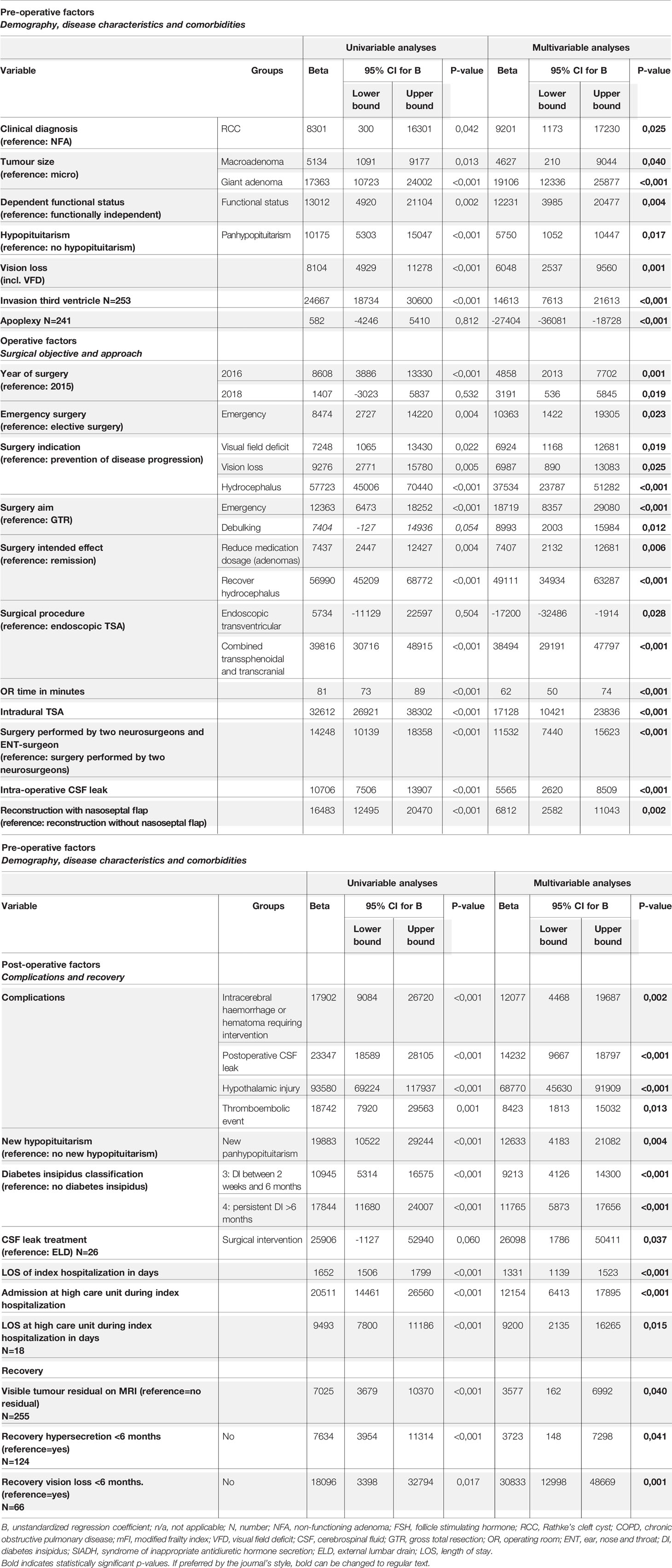
Table 2 Univariable and multivariable linear regression analyses displaying significant determinants of total costs.
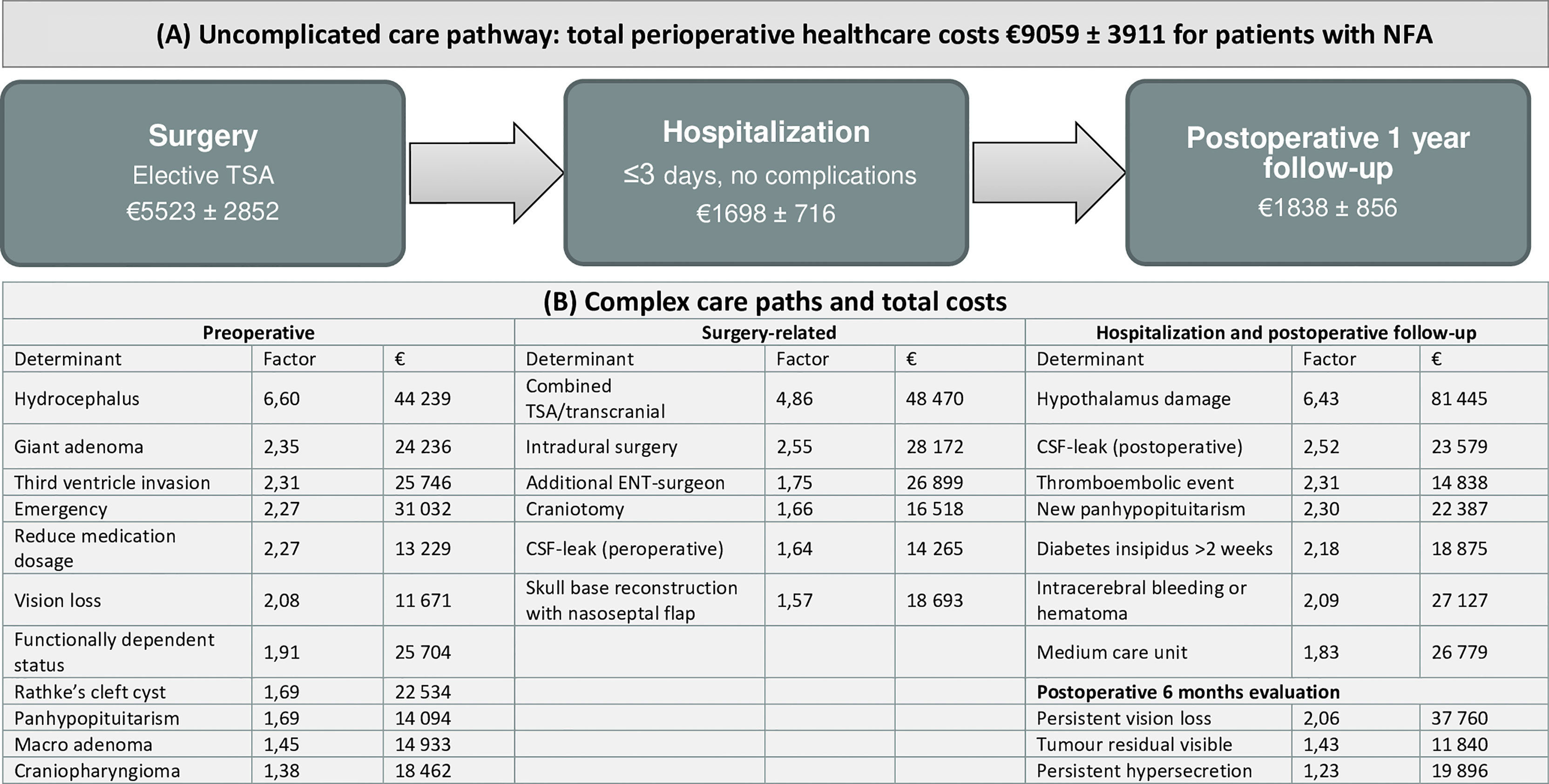
Figure 3 Overview of perioperative healthcare costs for patients undergoing pituitary surgery. (A) To illustrate, the care trajectory of patients with non-functioning micro- or macroadenoma undergoing transsphenoidal adenectomy is shown as an example for an uneventful case. (B) Cost determinants are summarized in coefficients (calculated by dividing total costs of the determinant by total costs of the reference group) and total perioperative costs, based on multivariable analyses (Supplemental Table S1) E.g., total healthcare costs for patients with postoperative CSF leak is 2,52 times higher compared to patients without CSF leak. NFA, non-functioning adenoma; TSA, transsphenoidal adenectomy; ENT, ear, nose and throat; CSF, cerebrospinal fluid.
Surgical factors associated with higher costs were emergency surgery, e.g. for hydrocephalus and reduced visual acuity, (B: 10363; 95% CI 1422, 19305) compared to elective surgery. One additional minute in OR was associated with a €62 increase in costs (95% CI 50, 74). Surgery indications VFD (B: 6924; 95% CI 1168, 12681), reduced visual acuity (B: 6987; 95% CI 890, 13083) or hydrocephalus (B: 37534; 95% CI 23787, 51282) were associated with increased costs, compared to a more elective indication or preventive surgery (e.g. growing mass close to the optic chiasm without VFD yet). In reference to GTR as surgical aim, emergency surgery (B: 18719; 95% CI 8357, 29080) and debulking (B: 8993; 95% CI 2003, 15984) were also related to higher costs. When the intended effect of surgery was to lower medication dosage or alleviate hydrocephalus, costs were €7407 (95% CI 2132, 12681) and €49111 (95% CI 34934, 63287) higher than achieving remission, respectively. Compared to endoscopic trans-sphenoidal surgery, combined transsphenoidal/transcranial (B: 38494; 95% CI 29191, 47797) and intradural surgery (B: 17128; 95% CI 10421, 23836) were associated with higher costs, whereas endoscopic transventricular surgery was related to lower costs (B: -17200; 95% CI -32486, -1914). Furthermore, surgery performed by two neurosurgeons and ENT surgeon together (B: 11532; 95% CI 7440, 15623) compared to two neurosurgeons, intra-operative CSF leak (B: 5565; 95% CI 2620, 8509) and skull base reconstruction with nasoseptal flap (B: 6812; 95% CI 2582, 11043) compared to reconstruction methods without nasoseptal flap (e.g., with abdominal fat, fibrin glue, free mucosa graft and/or fascia lata) were associated with higher costs.
As expected, LOS in days (B: 1331; 95% CI 1139, 1523), high care unit admission (B: 12154; 95% CI 6413, 17895) and LOS in days at the high care unit (B: 9200; 95% CI 2135, 16265) were associated with higher cost. Complications including intracerebral haemorrhage or hematoma (B: 12077; 95% CI 4468, 19687), postoperative CSF leak (B: 14232; 95% CI 9667, 18797), hypothalamic injury (B: 68770; 95% CI 45630, 91909), thromboembolic event (B: 8423; 95% CI 1813, 15032), new panhypopituitarism (B: 12633; 95% CI 4183, 21082) and diabetes insipidus class 3 (B: 9213; 95% CI 4126, 14300) or 4 (B: 11765; 95% CI 5873, 17656) compared to no diabetes insipidus were also related to higher costs. Of patients with postoperative CSF leak, surgical treatment (B: 26098; 95% CI 1786, 50411) was associated with higher costs compared to external lumbar drain (ELD) placement. Surprisingly, LOS was shorter in patients with ELD compared to surgically treated patients (data not shown). Visiting the emergency department or being readmitted within 30 days after surgery were not significantly related to increased costs. Patients with persistent hypersecretion (B: 3723; 95% CI 148, 7298), reduced visual acuity (B: 30833; 95% CI 12998, 48669) or a visible tumour residual on MRI (B: 3577; 95% CI 162, 6992) were also associated with higher costs compared to remission of hormonal hypersecretion, normalisation of visual acuity and complete resection of tumour mass.
We performed additional multivariable analyses to compare the effect of cost outliers on the association between clinical diagnosis and total costs. We excluded cases with >€80.000 (n=3) and this changed the association of clinical diagnoses and total costs for patients with RCC (B: 7081; 95% CI -4347, 7976), craniopharyngioma (B: 7080; 95% CI 1887, 12274) and Cushing’s disease (B: 5672; 95% CI 1455, 9890) (data not shown).
Based on cost determinants, we analysed total costs, OR duration and LOS of patients with uneventful or complex course. Uneventful cases were defined as patients with micro- or macroadenoma undergoing elective TSA performed by two neurosurgeons, without (post)operative complications and a mean LOS of ≤ 3 days. Likewise, patients were complex if at least one cost determinant was present (e.g., patients with giant adenoma, emergency surgery, tumours invading the third ventricle) (Supplemental Table S2 and Supplemental Figure S3). In the total cohort, patients with uneventful and complicated course had mean costs of €8879 (3210) and €17551 (14250), respectively. For patients with NFA, costs were €9059 (3911) for uneventful cases and €16190 (12778) for complex cases. Total costs were €6766 for one uneventful case with RCC, while all other patients with RCC or craniopharyngioma had a complicated course with mean total costs €33157 (23170). Mean costs of specific cost domains, OR duration and LOS are provided in Supplemental Table S2.
4 Discussion
This study evaluated the in-hospital direct costs (€16380) for the year after surgery, with higher mean costs in patients with RCCs (€23263) and craniopharyngiomas (€36129) compared to pituitary adenomas (€14462). In line with previous studies, total costs were mostly attributable to direct costs of the surgical procedure (55%) and to a lesser extent to hospitalization (28%), irradiation, and diagnostic investigations or consultations (17%) (14, 42, 43). Additionally, we identified factors associated with higher total costs, that are generally well explained by more complex patient and tumour characteristics, or a more complex clinical course (Figure 3). So, increased costs were logically related to healthcare utilization (e.g., longer OR time, LOS, ICU), in concurrence with previous reports (5, 14–16, 24, 28, 44–50). Additionally, we found that patients with RCCs, dependent functional status, tumours invading the third ventricle, specific surgical approaches (i.e., combined transsphenoidal/transcranial, intradural, involvement of ENT-surgeon, nasoseptal flap skull base reconstruction) were increased costs. In patients with postoperative CSF leak, we found that surgical re-intervention was particularly related to higher cost compared with ELD placement.
Interestingly, we found that patients with apoplexy had lower costs. We attempted to address this finding by exploring differences in patients’ characteristics. In patients with apoplexy, we found that mean OR time was 30 min shorter compared to the total cohort, but other cost-driving factors (i.e. surgical approach, LOS and complications) did not explain lower costs. Thus, the explanation for lower costs in patients with apoplexy remains speculative, e.g. shared follow-up with regional centre, incomplete diagnostic workup preoperatively. It is also important to note that higher costs in patients undergoing emergency surgery are not attributable to apoplexy, but to patients having CSF leak or hydrocephalus.
4.1 General Relevance and Implications
Costs evaluations are necessary to improve care paths in line with the VBHC approach. Structural changes can be made within healthcare trajectories to limit the use of expensive or unnecessary interventions that do not improve outcome or add value for patients (e.g., prolonged LOS in select patients). Moreover, cost determinants can be used as proxy for healthcare utilization. Therefore, insights in costs enable physicians to consider costs and outcomes simultaneously in clinical decision making and make more individualized paths. For the first time, this study provides a costs baseline, since reference data for European patients with pituitary tumours was lacking, and enables us to identify cost-drivers, relate costs to clinical outcomes and detect changes in cost over time. By doing so, we can evaluate the effectiveness of future value-improving initiatives during different parts of the care trajectory.
4.2 Healthcare Costs in Perspective
It is difficult to compare our outcomes to those of earlier studies, because of different healthcare systems and cost assessment methods. Economic evaluations for patients with pituitary tumours undergoing surgery have been mainly performed in the USA (12–16, 42–45, 48), reporting costs ranging from $20000 to $35000 (14, 23, 42, 51). However, the USA has a different healthcare system than most European countries (52). European economic evaluations, reported lower annual direct costs of €8000-12000 (17, 29, 53) for patients with acromegaly (17, 18, 29, 54), €2000 for prolactinoma (20) and €3000 (19) for NFA. However, these studies included yearly chronic care instead of the year of surgical intervention associated with a peak in costs (18–21, 26). Nevertheless, surgery may be cost-effective in the long-term for patients with acromegaly or prolactinoma compared to pharmacotherapy, particularly when remission is achieved since costs of drugs will decrease after intervention (29, 55, 56).
4.3 Differences With Other Studies
In contrast to previous studies, we did not find significant associations between costs and age (19), smoking status (14, 47), comorbidities (23, 28, 47), MFI-5 score (51), Cushing’s disease (24, 44, 45) or readmissions (44, 45). Age was not related to higher costs (19), which may be explained by the higher proportion of complex cases at younger age, compared to more patients with NFAs at higher age. Together, our results indicate that pituitary tumour characteristics particularly contribute to increased costs, while patient characteristics influence costs only to a lesser extent. Therefore, stratifying care paths based on tumour characteristics may be suitable for improving VBHC.
4.4 Implications for VBHC Initiatives
The previously mentioned cost determinants can be used for value-improving initiatives. However, not all determinants may reduce costs effectively, because they are not subject to preventive measures or extremely rare. For example, preoperative panhypopituitarism may be associated with higher costs, but preventing panhypopituitarism is already an important treatment objective. Regardless, these unmodifiable determinants may be used as a sign of more complex disease course and may be relevant in-patient counselling. Likewise, though hypothalamic injury was the strongest cost-driver, this complication was present in only one case and therefore care adjustments likely have a low impact on overall healthcare costs.
4.4.1 LOS
LOS is a clear cost driver of in-hospital costs (24, 45) with an €1331 increase in costs per day. We previously evaluated the effect of reducing LOS to 2-3 days in selected patients and showed that reducing LOS was safe with no significant decrease in costs (32). However, this analysis was based on a small number of patients and used a different cost-analysis methodology. In the current study, using clinical practice data of a large group of patients, we do find that a decrease of LOS indeed reduces costs. In analogy, other studies reported significant cost reduction after reducing LOS to 1-2 days after brain tumour surgery (57–59). Cost data reported in this study will serve as reference point to evaluate the cost-effectiveness of further LOS reduction in select patients.
4.4.2 Functionally Dependent Patients
Higher costs in functionally dependent patients are likely explained by longer LOS and more complications after surgery (60–62). Despite the risk, patients may benefit from brain tumour surgery and recover functional independence (60). Therefore, the risks and benefits of surgery and alternative treatment options should be carefully weighed, while considering individual values. Prehabilitation programs have been designed to improve functional status and general health prior to elective surgery and have shown improved outcomes (63–69). However, these studies focussed on thoracic, abdominal and orthopaedic surgery and therefore results may not be applicable to pituitary surgery patients. Besides, the cost-effectiveness of such interventions remains unclear (70, 71). In the current cohort the total effect of prehabilitation on costs is likely limited, due to the small proportion of functional dependent patients (n=11, 4%).
4.4.3 Tumours Related to the Third Ventricle, RCCs and Craniopharyngiomas
Some unmodifiable determinants of cost are tumours related to the third ventricle, RCCs and craniopharyngiomas, as they are associated with more complications. These patients showed higher, more varying costs, OR time and LOS (Supplemental Table S2), possibly reflecting more complex disease course, unpredictable outcomes and more complications (72, 73). Though preferred surgical approach is debated, an experienced surgeon and team is needed for optimal outcomes, but still complications are frequently seen (74). Opting for GTR may be risky, as surrounding structures may be damaged and hypopituitarism may occur. However, subtotal resection may lead to recurrences. As illustrated by our results, complex tumours, extensive surgical approaches and complications are highly prevalent in patients with RCC or craniopharyngioma compared to pituitary adenomas, indicating that a separate treatment trajectory for these patients may be justified. Careful preoperative planning is mandatory and additional imaging techniques could aid choosing the best surgical approach and improve outcomes (75–77). Furthermore, this trajectory may incorporate an earlier postoperative MRI to assess residual tumour volume or recurrence and provide counselling concerning increased risk for panhypopituitarism. Future studies are needed to explain more specifically why patients with RCC or craniopharyngioma are associated with higher costs and which factors increase risks for complications, so that care pathways can be adjusted accordingly.
4.4.4 Postoperative CSF Leak
Identifying patients at risk for postoperative CSF leak and implementing preventive measures likely improves outcomes and reduce costs, thereby improving value for patients and allocating resources (i.e., labour, OR capacity) more efficiently. Postoperative CSF leak was related to over €14000 additional costs per case and occurred in 9,6% and 7,5% in the total cohort or in patients with pituitary adenoma, respectively. Compared to previous studies reporting a prevalence between 0,9 and 5,2% (14, 15, 48, 78, 79) after transsphenoidal pituitary surgery, the prevalence of postoperative CSF leak in the present study was high. However, this needs to be placed in perspective with the complexity of our case-mix in a tertiary referral centre and potential selection bias (i.e., more severe cases undergo surgical intervention rather than ELD placement). Known risk groups for postoperative CSF leak are patients with higher BMI, third ventricle invasion, craniopharyngioma, previous skull base irradiation, prior surgery and intra-operative CSF leak (74, 78, 80–82). For these patients particularly, tailored skull base reconstruction methods are critical to prevent and/or manage CSF leak optimally (83). Also, preventive ELD placement may result in more efficient use of resources and indirectly lower costs, however it will also increase LOS in short-stay protocols. As illustrated by our results, more extensive reconstruction methods (i.e., using nasoseptal flap reconstruction) and ELD placement are costly interventions. However, they may be cost effective when CSF leak or additional surgical intervention is prevented (48, 74, 81, 84).
4.5 Strengths and Limitations
Despite previous cost evaluations, this study is to our knowledge the first European study providing a comprehensive overview of the cost of perioperative healthcare for patients with pituitary tumours in a tertiary referral centre. Though, this study has several limitations. First, this study is retrospective, with all inherent limitations. Another limitation is the single centre-nature, with our centre being a tertiary referral centre and both nationally and internationally endorsed pituitary expertise centre, receiving referrals from throughout the country, including more complex cases (e.g., more macroprolactinoma and RCC). Hence, accurate assessment of healthcare usage is subject to hospital registration data, which differs in different hospitals. Consequently, differences in costs between Dutch tertiary referral centres may be partially accountable to the quality of in-hospital costs registration.
Limitations in our costs assessment were that we did not correct for inflation, which may have confounded our cost results across the years. Also, we only included direct costs incurred at our institution, thereby neglecting indirect (e.g., administrative) costs and costs incurred at other institutions. Finally, the costs for medication were not included in this analysis since these are not included in hospital costs. This likely results in underestimating the costs of patients receiving healthcare at other facilities or pharmacological treatment (e.g., patients with functioning adenoma or growth hormone replacement therapy). We encourage future studies to adhere to cost evaluation guidelines to promote interpretability of results (37). Cost-evaluations including costs for medical treatment and hormone replacement therapy are warranted to pursue a good evaluation of management strategies. A limitation in our statistical analyses using pairwise deletion instead of multiple imputation for missing data, which may have biased our results.
It is also important to point out that this study focussed on perioperative healthcare, thereby disregarding the costs of the preoperative trajectory and follow-up care after one year. During of the preoperative care different factors might be profound contributors to higher costs. For example, functional imaging for Cushing’s disease or frequent consultations in prolactinoma patients might be associated with higher costs in preoperative healthcare. Finally, we did not exclude patients with inordinately high costs due to a complicated course which possibly confounded the effect size of some determinants. The two most expensive patients in our cohort costed over €100.000 each. They both presented with hydrocephalus and had a complicated course with surgically treated postoperative CSF leak. One of these patients had a NFA and also experienced intracerebral haemorrhage, multiple organ failure, several infections and hypothalamic damage and was admitted for 69 days. The other patient was functionally dependent, and surgery was complicated by phlebitis and hematemesis secondary to stage IV oesophagitis, being admitted for 44 days. Though we attempted to alleviate confounding effects in the multivariable analyses, we cannot preclude these patients confounded the effect sizes of some cost determinants (hydrocephalus, dependent functional status, surgical treatment of CSF leak, cerebral haemorrhage and hypothalamic syndrome), and as shown in analyses with or without cost outliers, cost outliers influence the significance of some cost determinants (i.e., Cushing’s disease, RCC and craniopharyngioma) (data not shown).
5 Conclusion
Insight in healthcare costs and their determinants is necessary to curtail costs and facilitate VBHC initiatives. The present study provides a concise overview of costs and its determinants, which will serve as reference point for future value-improving initiatives and as a proxy for resource utilization. With this information, future studies can investigate costs and outcomes more specifically (e.g., predict complications), so that healthcare pathways can be adjusted strategically according to the VBHC framework. For example, differentiated healthcare pathways (Figure 3) for patients with uncomplicated disease course and predictable costs or patients with a complicated course (i.e., presence of one or more cost determinants) and higher, more varying costs. Consequently, developing value-improving initiatives for patients with giant tumours, non-adenomatous aetiology, dependent functional status, third ventricle invasion or extensive surgical approaches may result in reduced costs and improved outcomes. Also, further reduction of LOS seems viable and safe in select patients. In this way, we endeavour to improve value-based healthcare for patients with pituitary tumours.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by LUMC medical ethics committee, local study number G19·011. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AD, FV, and EH collected the data, and AD and FV conducted the data analysis. The study was performed under supervision of NB. AD and FV wrote the primary version of the manuscript. AZ, MV, AP, WF, and NB supervised the data analysis, contributed to the interpretation of the results, and reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.905019/full#supplementary-material
References
1. Daly A, Beckers A. The Epidemiology of Pituitary Adenomas. Endocrinol Metab Clinics North America (2020) 49(3):347–55. doi: 10.1016/j.ecl.2020.04.002
2. Gatto F, Perez-Rivas LG, Olarescu NC, Khandeva P, Chachlaki K, Trivellin G, et al. Diagnosis and Treatment of Parasellar Lesions. Neuroendocrinology (2020) 110(9-10):728–39. doi: 10.1159/000506905
3. Chanson P, Raverot G, Castinetti F, Cortet-Rudelli C, Galland F, Salenave S, et al. Management of Clinically Non-Functioning Pituitary Adenoma. Ann Endocrinol (Paris) (2015) 76(3):239–47. doi: 10.1016/j.ando.2015.04.002
4. Molitch ME. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA (2017) 317(5):516–24. doi: 10.1001/jama.2016.19699
5. Katznelson L, Laws ER Jr., Melmed S, Molitch ME, Murad MH, Utz A, et al. Acromegaly: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2014) 99(11):3933–51. doi: 10.1210/jc.2014-2700
6. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96(2):273–88. doi: 10.1210/jc.2010-1692
7. Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The Diagnosis of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2008) 93(5):1526–40. doi: 10.1210/jc.2008-0125
8. Lobatto DJ, Zamanipoor Najafabadi AH, de Vries F, Andela CD, van den Hout WB, Pereira AM, et al. Toward Value Based Health Care in Pituitary Surgery: Application of a Comprehensive Outcome Set in Perioperative Care. Eur J Endocrinol (2019) 181(4):375–87. doi: 10.1530/EJE-19-0344
9. Esposito V, Santoro A, Minniti G, Salvati M, Innocenzi G, Lanzetta G, et al. Transsphenoidal Adenomectomy for Gh-, Prl- and Acth-Secreting Pituitary Tumours: Outcome Analysis in a Series of 125 Patients. Neurol Sci (2004) 25(5):251–6. doi: 10.1007/s10072-004-0351-z
10. Porter ME. Value-Based Health Care Delivery. Ann Surg (2008) 248(4):503–9. doi: 10.1097/SLA.0b013e31818a43af
11. Porter ME. A Strategy for Health Care Reform–toward a Value-Based System. New Engl J Med (2009) 361(2):109–12. doi: 10.1056/NEJMp0904131
12. Reese JC, Twitchell S, Wilde H, Azab MA, Guan J, Karsy M, et al. Analysis of Treatment Cost Variation Among Multiple Neurosurgical Procedures Using the Value-Driven Outcomes Database. World Neurosurg (2019) 126:e914–e20. doi: 10.1016/j.wneu.2019.03.010
13. Parasher AK, Workman AD, Kidwai SM, Goljo E, Signore AD, Iloreta AM, et al. Costs in Pituitary Surgery: Racial, Socioeconomic, and Hospital Factors. J Neurol Surg B Skull Base (2018) 79(6):522–7. doi: 10.1055/s-0038-1635081
14. Parasher AK, Lerner DK, Glicksman JT, Miranda SP, Dimentberg R, Ebesutani D, et al. Drivers of in-Hospital Costs Following Endoscopic Transphenoidal Pituitary Surgery. Laryngoscope (2020) 131(4):760–4 doi: 10.1002/lary.29041
15. Rizvi ZH, Ferrandino R, Luu Q, Suh JD, Wang MB. Nationwide Analysis of Unplanned 30-Day Readmissions After Transsphenoidal Pituitary Surgery. Int Forum Allergy Rhinol (2019) 9(3):322–9. doi: 10.1002/alr.22241
16. Hendricks BL, Shikary TA, Zimmer LA. Causes for 30-Day Readmission Following Transsphenoidal Surgery. Otolaryngology–Head Neck Surg (2015) 154(2):359–65. doi: 10.1177/0194599815617130
17. Kamusheva M, Vandeva S, Mitov K, Rusenova Y, Elenkova A, Zacharieva S, et al. New Epidemiological, Clinical and Economic Data for Patients With Acromegaly in Bulgaria. Front Public Health (2020) 8:147. doi: 10.3389/fpubh.2020.00147
18. Lesen E, Granfeldt D, Houchard A, Dinet J, Berthon A, Olsson DS, et al. Comorbidities, Treatment Patterns and Cost-Of-Illness of Acromegaly in Sweden: A Register-Linkage Population-Based Study. Eur J Endocrinol (2017) 176(2):203–12. doi: 10.1530/EJE-16-0623
19. Lobatto DJ, van den Hout WB, Zamanipoor Najafabadi AH, Steffens ANV, Andela CD, Pereira AM, et al. Healthcare Utilization and Costs Among Patients With Non-Functioning Pituitary Adenomas. Endocrine (2019) 64(2):330–40. doi: 10.1007/s12020-019-01847-7
20. van der Meulen M, Zamanipoor Najafabadi AH, Lobatto DJ, van den Hout WB, Andela CD, Zandbergen IM, et al. Healthcare Utilization and Costs Among Prolactinoma Patients: A Cross-Sectional Study and Analysis of Determinants. Pituitary (2020) 24(1):79–95. doi: 10.1007/s11102-020-01089-1
21. Broder MS, Neary MP, Chang E, Cherepanov D, Ludlam WH. Burden of Illness, Annual Healthcare Utilization, and Costs Associated With Commercially Insured Patients With Cushing Disease in the United States. Endocr Pract (2015) 21(1):77–86. doi: 10.4158/EP14126.OR
22. Broder MS, Neary MP, Chang E, Ludlam WH. Incremental Healthcare Resource Utilization and Costs in Us Patients With Cushing's Disease Compared With Diabetes Mellitus and Population Controls. Pituitary (2015) 18(6):796–802. doi: 10.1007/s11102-015-0654-5
23. Broder MS, Neary MP, Chang E, Cherepanov D, Katznelson L. Treatments, Complications, and Healthcare Utilization Associated With Acromegaly: A Study in Two Large United States Databases. Pituitary (2014) 17(4):333–41. doi: 10.1007/s11102-013-0506-0
24. Little AS, Chapple K. Predictors of Resource Utilization in Transsphenoidal Surgery for Cushing Disease. J Neurosurg (2013) 119(2):504–11. doi: 10.3171/2013.1.JNS121375
25. Swearingen B, Wu N, Chen S-Y, Pulgar S, Biller BM. Health Care Resource Use and Costs Among Patients With Cushing Disease. Endocrine Pract (2011) 17(5):681–90. doi: 10.4158/EP10368.OR
26. Van Uum S, Hurry M RP, Koch C, Dranitsaris G, Lacroix A. Management of Patients With Cushing's Disease: A Canadian Cost of Illness Analysis. J Popul Ther Clin Pharmacol (2014) 21(3):e508–e17.
27. Placzek H, Xu Y, Mu Y, Begelman S, Fisher M. Clinical and Economic Burden of Commercially Insured Patients Withacromgaly in the Us a Retrospective Study. J Managed Care Specialty Pharm (2015) 21(12):1106–14c. doi: 10.18553/jmcp.2015.21.12.1106
28. Zaidi HA, Chapple K, Little AS. National Treatment Trends, Complications, and Predictors of in-Hospital Charges for the Surgical Management of Craniopharyngiomas in Adults From 2007 to 2011. Neurosurgical Focus (2014) 37(5):E6. doi: 10.3171/2014.8.focus14366
29. Didoni G, Grottoli S, Gasco V, Battistini M, Ferone D, Giusti M, et al. Cost-Of-Illness Study in Acromegalic Patients in Italy. J Endocrinol Invest (2004) 27:1034–9. doi: 10.1007/BF03345306
30. Knosp E, Steiner E, Kitz K, Matula C. Pituitary Adenomas With Invasion of the Cavernous Sinus Space: A Magnetic Resonance Imaging Classification Compared With Surgical Findings. Neurosurgery (1993) 33(4):610–8. doi: 10.1227/00006123-199310000-00008
31. van Furth WR, de Vries F, Lobatto DJ, Kleijwegt MC, Schutte PJ, Pereira AM, et al. Endoscopic Surgery for Pituitary Tumors. Endocrinol Metab Clin North Am (2020) 49(3):487–503. doi: 10.1016/j.ecl.2020.05.011
32. Lobatto DJ, Vliet Vlieland TPM, van den Hout WB, de Vries F, de Vries AF, Schutte PJ, et al. Feasibility, Safety, and Outcomes of a Stratified Fast-Track Care Trajectory in Pituitary Surgery. Endocrine (2020) 69(1):175–87. doi: 10.1007/s12020-020-02308-2
33. de Vries F, Lobatto DJ, Bakker LEH, van Furth WR, Biermasz NR, Pereira AM. Early Postoperative Hpa-Axis Testing After Pituitary Tumor Surgery: Reliability and Safety of Basal Cortisol and Crh Test. Endocrine (2020) 67(1):161–71. doi: 10.1007/s12020-019-02094-6
34. de Vries F, Lobatto DJ, Verstegen MJT, van Furth WR, Pereira AM, Biermasz NR. Postoperative Diabetes Insipidus: How to Define and Grade This Complication? Pituitary (2020) 24(2):284–91. doi: 10.1007/s11102-020-01083-7
35. Pelsma ICM, Verstegen MJT, de Vries F, Notting IC, Broekman MLD, van Furth WR, et al. Quality of Care Evaluation in Non-Functioning Pituitary Adenoma With Chiasm Compression: Visual Outcomes and Timing of Intervention Clinical Recommendations Based on a Systematic Literature Review and Cohort Study. Pituitary (2020) 23(4):417–29. doi: 10.1007/s11102-020-01044-0
36. Fleseriu M, Hashim IA, Karavitaki N, Melmed S, Murad MH, Salvatori R, et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2016) 101(11):3888–921. doi: 10.1210/jc.2016-2118
37. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (Cheers) Statement. Eur J Health Econ (2013) 14(3):367–72. doi: 10.1007/s10198-013-0471-6
38. Kaljouw MJ. De Nederlandse Zorgautoriteit: Regeling Registratie En Aanlevering Kostprijzen Zorgproducten Medisch-Specialistische Zorg The Netherlands(2020). Available at: https://puc.overheid.nl/nza/doc/PUC_318906_22/1/.
39. OECD. Organisation for Economic Co-Operation and Development. In: Purchasing Power Parities (2021). Available from: https://data.oecd.org/chart/6IQK.
40. van Diepen M, Ramspek CL, Jager KJ, Zoccali C, Dekker FW. Prediction Versus Aetiology: Common Pitfalls and How to Avoid Them. Nephrol Dial Transplant (2017) 32(suppl_2):ii1–5. doi: 10.1093/ndt/gfw459
41. le Cessie S, Goeman JJ, Dekkers OM. Who Is Afraid of Non-Normal Data? Choosing Between Parametric and Non-Parametric Tests. Eur J Endocrinol (2020) 182(2):E1–3. doi: 10.1530/EJE-19-0922
42. McLaughlin N, Martin NA, Upadhyaya P, Bari AA, Buxey F, Wang MB, et al. Assessing the Cost of Contemporary Pituitary Care. Neurosurg Focus (2014) 37(5):E7. doi: 10.3171/2014.8.FOCUS14445
43. Karsy M, Brock AA, Guan J, Bisson EF, Couldwell WT. Assessment of Cost Drivers in Transsphenoidal Approaches for Resection of Pituitary Tumors Using the Value-Driven Outcome Database. World Neurosurg (2017) 105:818–23. doi: 10.1016/j.wneu.2017.05.148
44. Guan J, Karsy M, Bisson EF, Couldwell WT. Patient-Level Factors Influencing Hospital Costs and Short-Term Patient-Reported Outcomes After Transsphenoidal Resection of Sellar Tumors. Neurosurgery (2018) 83(4):726–31. doi: 10.1093/neuros/nyx471
45. Little AS, Chapple K, Jahnke H, White WL. Comparative Inpatient Resource Utilization for Patients Undergoing Endoscopic or Microscopic Transsphenoidal Surgery for Pituitary Lesions. J Neurosurg (2014) 121(1):84–90. doi: 10.3171/2014.2.JNS132095
46. Oosmanally N, Paul JE, Zanation AM, Ewend MG, Senior BA, Ebert CS Jr. Comparative Analysis of Cost of Endoscopic Endonasal Minimally Invasive and Sublabial-Transseptal Approaches to the Pituitary. Int Forum Allergy Rhinol (2011) 1(4):242–9. doi: 10.1002/alr.20048
47. Kidwai SM, Yang A, Gray ML, McKee S, Iloreta AM, Shrivastava R, et al. Hospital Charge Variability Across New York State: Sociodemographic Factors in Pituitary Surgery. J Neurol Surg B Skull Base (2019) 80(6):612–9. doi: 10.1055/s-0038-1676839
48. Parikh A, Adapa A, Sullivan SE, McKean EL. Predictive Factors, 30-Day Clinical Outcomes, and Costs Associated With Cerebrospinal Fluid Leak in Pituitary Adenoma Resection. J Neurol Surg B Skull Base (2020) 81(1):43–55. doi: 10.1055/s-0039-1679896
49. Spinazzi EF, Pines MJ, Fang CH, Raikundalia MD, Baredes S, Liu JK, et al. Impact and Cost of Care of Venous Thromboembolism Following Pituitary Surgery. Laryngoscope (2015) 125(7):1563–7. doi: 10.1002/lary.25161
50. Luque-Ramírez M, Paramo C, Verela da Costa C, Garcia-Mayor R. Cost of Management of Invasive Growth Hormone-Secreting Macroadenoma. J Endocrinol Invest (2007) 30(7):541–5. doi: 10.1007/BF03346346
51. Khalafallah AM, Shah PP, Huq S, Jimenez AE, Patel PP, London NR Jr., et al. The 5-Factor Modified Frailty Index Predicts Health Burden Following Surgery for Pituitary Adenomas. Pituitary (2020) 23(6):630–40. doi: 10.1007/s11102-020-01069-5
52. Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income Countries. JAMA (2018) 319(10):1024–39. doi: 10.1001/jama.2018.1150
53. Douven RBM, Burger M, Schut E. Grote Prijsverschillen Ziekenhuiszorg, Ondanks Concurrentie. ESB Gezondheidszorg (2018) 103(4762):276–9.
54. Elbaum M, Mizera L, Bolanowski M. The Real Costs of Acromegaly: Analysis of Different Therapies. Endokrynol Pol (2019) 70(1):74–85. doi: 10.5603/EP.a2018.0080
55. Zygourakis CC, Imber BS, Chen R, Han SJ, Blevins L, Molinaro A, et al. Cost-Effectiveness Analysis of Surgical Versus Medical Treatment of Prolactinomas. J Neurol Surg B Skull Base (2017) 78(2):125–31. doi: 10.1055/s-0036-1592193
56. Jethwa PR, Patel TD, Hajart AF, Eloy JA, Couldwell WT, Liu JK. Cost-Effectiveness Analysis of Microscopic and Endoscopic Transsphenoidal Surgery Versus Medical Therapy in the Management of Microprolactinoma in the United States. World Neurosurg (2016) 87:65–76. doi: 10.1016/j.wneu.2015.10.090
57. Neville IS, Urena FM, Quadros DG, Solla DJF, Lima MF, Simoes CM, et al. Safety and Costs Analysis of Early Hospital Discharge After Brain Tumour Surgery: A Pilot Study. BMC Surg (2020) 20(1):105. doi: 10.1186/s12893-020-00767-y
58. Richardson AM, McCarthy DJ, Sandhu J, Mayrand R, Guerrero C, Rosenberg C, et al. Predictors of Successful Discharge of Patients on Postoperative Day 1 After Craniotomy for Brain Tumor. World Neurosurg (2019) 126:e869–e77. doi: 10.1016/j.wneu.2019.03.004
59. Thomas JG, Gadgil N, Samson SL, Takashima M, Yoshor D. Prospective Trial of a Short Hospital Stay Protocol After Endoscopic Endonasal Pituitary Adenoma Surgery. World Neurosurg (2014) 81(3-4):576–83. doi: 10.1016/j.wneu.2013.11.014
60. Stienen MN, Zhang DY, Broggi M, Seggewiss D, Villa S, Schiavolin S, et al. The Influence of Preoperative Dependency on Mortality, Functional Recovery and Complications After Microsurgical Resection of Intracranial Tumors. J Neurooncol (2018) 139(2):441–8. doi: 10.1007/s11060-018-2882-9
61. Dasenbrock HH, Yan SC, Chavakula V, Gormley WB, Smith TR, Claus EB, et al. Unplanned Reoperation After Craniotomy for Tumor: A National Surgical Quality Improvement Program Analysis. Neurosurgery (2017) 81(5):761–71. doi: 10.1093/neuros/nyx089
62. Ball T, Oxford BG, Alhourani A, Ugiliweneza B, Williams BJ. Predictors of Thirty-Day Mortality and Length of Stay in Operative Subdural Hematomas. Cureus (2019) 11(9):e5657. doi: 10.7759/cureus.5657
63. Wahl TS, Graham LA, Hawn MT, Richman J, Hollis RH, Jones CE, et al. Association of the Modified Frailty Index With 30-Day Surgical Readmission. JAMA Surg (2017) 152(8):749–57. doi: 10.1001/jamasurg.2017.1025
64. Gillis C, Buhler K, Bresee L, Carli F, Gramlich L, Culos-Reed N, et al. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterology (2018) 155(2):391–410.e4. doi: 10.1053/j.gastro.2018.05.012
65. Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal Prehabilitation Improves Functional Capacity Before and After Colorectal Surgery for Cancer: A Five-Year Research Experience. Acta Oncol (2017) 56(2):295–300. doi: 10.1080/0284186X.2016.1268268
66. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Deans DC, Skipworth RJ. Prehabilitation Before Major Abdominal Surgery: A Systematic Review and Meta-Analysis. World J Surg (2019) 43(7):1661–8. doi: 10.1007/s00268-019-04950-y
67. Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation Versus Rehabilitation: A Randomized Control Trial in Patients Undergoing Colorectal Resection for Cancer. Anesthesiology (2014) 121(5):937–47. doi: 10.1097/ALN.0000000000000393
68. Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A Systematic Review and Meta-Analysis of Physical Exercise Prehabilitation in Major Abdominal Surgery (Prospero 2017 Crd42017080366). J Gastrointestinal Surg (2020) 24(6):1375–85. doi: 10.1007/s11605-019-04287-w
69. Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-Week Multimodal Prehabilitation Program Improves Perioperative Functional Capability in Patients Undergoing Thoracoscopic Lobectomy for Lung Cancer: A Randomized Controlled Trial. Anesthesia Analgesia (2020) 131(3):840–9. doi: 10.1213/ANE.0000000000004342
70. Nunns M, Shaw L, Briscoe S, Thompson Coon J, Hemsley A, McGrath JS, et al. Multicomponent Hospital-Led Interventions to Reduce Hospital Stay for Older Adults Following Elective Surgery: A Systematic Review. Health Serv Deliv Res (2019) 7(40):1–178. doi: 10.3310/hsdr07400
71. Nielsen PR, Andreasen J, Asmussen M, Tønnesen H. Costs and Quality of Life for Prehabilitation and Early Rehabilitation After Surgery of the Lumbar Spine. BMC Health Serv Res (2008) 8(1):1–7. doi: 10.1186/1472-6963-8-209
72. Giese H, Haenig B, Haenig A, Unterberg A, Zweckberger K. Neurological and Neuropsychological Outcome After Resection of Craniopharyngiomas. J Neurosurg (2019) 132(5):1425–34. doi: 10.3171/2018.10.JNS181557
73. Rotman LE, Alford EN, Davis MC, Vaughan TB, Woodworth BA, Riley KO. Preoperative Radiographic and Clinical Factors Associated With the Visualization of Intraoperative Cerebrospinal Fluid During Endoscopic Transsphenoidal Resection of Pituitary Adenomas. Surg Neurol Int (2020) 11:59. doi: 10.25259/SNI_24_2020
74. Algattas H, Setty P, Goldschmidt E, Wang EW, Tyler-Kabara EC, Snyderman CH, et al. Endoscopic Endonasal Approach for Craniopharyngiomas With Intraventricular Extension: Case Series, Long-Term Outcomes, and Review. World Neurosurg (2020) 144:e447–e59. doi: 10.1016/j.wneu.2020.08.184
75. Cossu G, Jouanneau E, Cavallo LM, Elbabaa SK, Giammattei L, Starnoni D, et al. Surgical Management of Craniopharyngiomas in Adult Patients: A Systematic Review and Consensus Statement on Behalf of the Eans Skull Base Section. Acta Neurochir (Wien) (2020) 162(5):1159–77. doi: 10.1007/s00701-020-04265-1
76. Fan J, Liu Y, Pan J, Peng Y, Peng J, Bao Y, et al. Endoscopic Endonasal Versus Transcranial Surgery for Primary Resection of Craniopharyngiomas Based on a New Qst Classification System: A Comparative Series of 315 Patients. J Neurosurg (2021), 1–12. doi: 10.3171/2020.7.JNS20257
77. MacFarlane J, Bashari WA, Senanayake R, Gillett D, van der Meulen M, Powlson AS, et al. Advances in the Imaging of Pituitary Tumors. Endocrinol Metab Clin North Am (2020) 49(3):357–73. doi: 10.1016/j.ecl.2020.06.002
78. Ozawa H, Sekimizu M, Saito S, Nakamura S, Mikoshiba T, Watanabe Y, et al. Risk Factor for Cerebrospinal Fluid Leak After Endoscopic Endonasal Skull Base Surgery: A Single-Center Experience. Acta Otolaryngol (2021) 141(6):621–5. doi: 10.1080/00016489.2021.1900600
79. Ament JD, Yang Z, Khatchadourian V, Strong EB, Shahlaie K. Cost-Effectiveness of Endoscopic Versus Microscopic Transsphenoidal Surgery for Pituitary Adenoma. World Neurosurg (2018) 110:e496–503. doi: 10.1016/j.wneu.2017.11.046
80. Lobatto DJ, de Vries F, Zamanipoor Najafabadi AH, Pereira AM, Peul WC, Vliet Vlieland TPM, et al. Preoperative Risk Factors for Postoperative Complications in Endoscopic Pituitary Surgery: A Systematic Review. Pituitary (2018) 21(1):84–97. doi: 10.1007/s11102-017-0839-1
81. Xue H, Wang X, Yang Z, Bi Z, Liu P. Risk Factors and Outcomes of Cerebrospinal Fluid Leak Related to Endoscopic Pituitary Adenoma Surgery. Br J Neurosurg (2020) 34(4):447–52. doi: 10.1080/02688697.2020.1754336
82. Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic Skull Base Surgery: A Comprehensive Comparison With Open Transcranial Approaches. Br J Neurosurg (2012) 26(5):637–48. doi: 10.3109/02688697.2012.654837
83. Khan DZ, Ali AMS, Koh CH, Dorward NL, Grieve J, Layard Horsfall H, et al. Skull Base Repair Following Endonasal Pituitary and Skull Base Tumour Resection: A Systematic Review. Pituitary (2021) 24(5):698–713. doi: 10.1007/s11102-021-01145-4
84. Zwagerman NT, Wang EW, Shin SS, Chang YF, Fernandez-Miranda JC, Snyderman CH, et al. Does Lumbar Drainage Reduce Postoperative Cerebrospinal Fluid Leak After Endoscopic Endonasal Skull Base Surgery? A Prospective, Randomized Controlled Trial. J Neurosurg (2018) 131:172–8. doi: 10.3171/2018.4.JNS172447
Keywords: pituitary tumour, pituitary adenoma, pituitary surgery, value-based healthcare, cost analysis, transsphenoidal surgery
Citation: Dekkers AJ, de Vries F, Zamanipoor Najafabadi AH, van der Hoeven EM, Verstegen MJT, Pereira AM, van Furth WR and Biermasz NR (2022) Costs and Its Determinants in Pituitary Tumour Surgery. Front. Endocrinol. 13:905019. doi: 10.3389/fendo.2022.905019
Received: 26 March 2022; Accepted: 04 May 2022;
Published: 07 July 2022.
Edited by:
Marek Bolanowski, Wroclaw Medical University, PolandReviewed by:
Ivana Ságová, National Institute of Diabetes and Endocrinology, SlovakiaLuiz Augusto Casulari, University of Brasilia, Brazil
Copyright © 2022 Dekkers, de Vries, Zamanipoor Najafabadi, van der Hoeven, Verstegen, Pereira, van Furth and Biermasz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alies J. Dekkers, YS5qLmRla2tlcnNAbHVtYy5ubA==
 Alies J. Dekkers
Alies J. Dekkers Friso de Vries1,2
Friso de Vries1,2 Amir H. Zamanipoor Najafabadi
Amir H. Zamanipoor Najafabadi Nienke R. Biermasz
Nienke R. Biermasz