- 1Department of Rheumatology, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Internal Medicine 3, Rheumatology and Immunology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität (FAU) Erlangen-Nürnberg, Erlangen, Germany
- 3Drug Discovery, Shanghai Huaota Biopharmaceutical Co. Ltd., Shanghai, China
Bone marrow adipocytes (BMAs) represent 10% of the total fat mass of the human body and serve as an energy reservoir for the skeletal niche. They function as an endocrine organ by actively secreting fatty acids, cytokines, and adipokines. The volume of BMAs increases along with age, osteoporosis and/or obesity. With the rapid development of multi-omic analysis and the advance in in vivo imaging technology, further distinct characteristics and functions of BMAs have been revealed. There is accumulating evidence that BMAs are metabolically, biologically and functionally unique from white, brown, beige and pink adipocytes. Bone metastatic disease is an uncurable complication in cancer patients, where primary cancer cells spread from their original site into the bone marrow. Recent publications have highlighted those BMAs could also serve as a rich lipid source of fatty acids that can be utilized by the cancer cells during bone metastasis, particularly for breast, prostate, lung, ovarian and pancreatic cancer as well as melanoma. In this review, we summarize the novel progressions in BMAs metabolism, especially with multi-omic analysis and in vivo imaging technology. We also update the metabolic role of BMAs in bone metastasis, and their potential new avenues for diagnosis and therapies against metastatic cancers.
Introduction
Several types of cancer cells evade clinical treatment by niching into the bone, such as cancer of the breat, prostate, lung and melanoma. Additionally, the bone marrow is a depot for fat-storing adipocytes, which poses a highly dynamic and metabolically active organ. Therefore, the role of bone marrow adipocytes (BMAs) and their effect on niching tumor cells and subsequent tumor growth are of clinical interest. Several studies have demonstrated that BMAs could function as an energy reservoir for the skeletal niche and serve as an endocrine organ secreting fatty acids, cytokines, and adipokines, supporting cancer cells to niche and grow within the bone marrow microenvironment. Because BMAs are deeply embedded in the bone marrow niche, the isolation of sufficient numbers of BMAs from rodent or human bone marrow remains a challenge. In contrast to white adipose tissues embedded in a matrix consisting of collagen, fibronectin and laminin, BMAs are distributed as single cells or patches in the bone marrow (1). Moreover, their large size and buoyancy do not facilitate their isolation by pelleting or cell sorting. The previous histomorphometric methods could only supply limited descriptions about these cells at the cellular level, such as alterations in structure and organelles, but no further information about molecular changes. Therefore, the characterization of BMA functions in bone metastasis is currently challenging.
Nevertheless, these gaps in understanding the underlying mechanisms have been largely filled in the recent decade due to the rapid development of multi-omic analysis and in vivo imaging. Technologies as RNA-seq, single-cell RNA-seq (scRNA-seq), gas chromatography-mass spectrometry (GC-MS), or liquid chromatography-mass spectrometry (LC-MS), gave insights into the transcriptomic, proteomic, and metabolic depth of BMAs. Using lineage tracing, fate mapping technologies and positron emission tomography-computed tomography (PET/CT) with 18F-fluorodeoxyglucose ([18F]FDG), distinct characteristics and functions of BMAs have been revealed in both rodents and humans. Recent findings demonstrate the importance of BMAs as metabolically, biologically, and functionally unique adipocyte subsets distinct from white, brown, beige and pink adipocytes. Here, we summarize the novel research on BMAs, especially the unique metabolic specificity and their potential function in supporting bone metastasis.
Anatomy
In the human body, BMAs are mainly located in the arms, legs, and sternum but rarely in the clavicle, ribs, and vertebrae (2). Meanwhile BMAs can also be observed in caudal (tail) vertebrae but not in thoracic or lumbar vertebrae (3). Interestingly, in human adults, BMAs represent around 10% of the total adipose tissue mass (4). By the age of 25 years, around 70% of the bone marrow volume in healthy adults is filled with BMAs (5). These cells can mainly be found in long bones in early adulthood. However, around 60 years of age and over, BMAs display age-associated increases in the axial skeleton (6). In long bones, BMAs dwell among the trabecular bone of the epiphysis and metaphysis or close to the endosteal surface of the diaphysis (7). BMAs have been historically overlooked and were considered “fillers” of the inert space for a long time (7). However, with the increasing interest in immunometabolism, they have raised more attention, especially for their distinct metabolic process and the consequent functional alterations.
As early as 1976, Tavassoli has discovered two distinct populations of BMAs in the bone marrow: the performic acid-Schiff (PFAS) – positively stained BMAs in red marrow and the PFAS-negatively stained BMAs in yellow marrow. The two populations also respond differently during the expansion of hematopoiesis (8). In 2015, using the osmium tetroxide staining, Scheller et al. defined for the first time regulated bone marrow adipocytes (rBMAs or red marrow BMAs) and constitutive bone marrow adipocytes (cBMAs or yellow marrow BMAs) (9). cBMAs develop after birth, are large in size and localized in close proximity to each other with a lack of hematopoietic cells in between (10). Their lipid storages mainly contain unsaturated fatty acids. In contrast, the smaller rBMAs develop throughout life and contain mostly saturated fatty acids. In steady state, rBMAs are single cells distributed within areas of active hematopoiesis.
Several environmental factors have been reported to promote the dynamic changes of BMAs. In several publications and our own data, high-calorie feeding such as high-fat diet increases number and size of BMAs. Here, mostly rBMAs localized in the metaphysis of the proximal tibia expand as response to changes in diet and diseases (11, 12). The special location of fat induced expansion of BMAs was confirmed in humans suffering from obesity, diabetes and/or osteoporosis (13, 14). In mice, irradiation and activation of the adipocyte differentiation pathway Peroxisome proliferator-activated receptor gamma (PPARγ) leads to a steady induction of BMA expansion (15). Additionally, expansion of BMAs can be observed in murine models of aging or ovariectomy-induced osteoporosis similar to the observations in patients (16, 17). Intriguingly, caloric deprivation in patients also increases the number of BMAs with gender difference regarding their localization, in L4 vertebra for men and at the femoral metaphysis for women (13). In addition, the psychiatric disease anorexia nervosa paradoxically leads to expanded bone marrow adipose tissue, while other fat depots in the body are reduced in size (18).
Origin
The origin of BMAs has been investigated for decades and is still updating thanks to the development of advanced technologies. In 1976, BMAs were first depicted as derived from a unique progenitor distinct from white adipocytes (19). Nevertheless, due to the limited technical conditions, the differences between BMAs and their extramedullary counterpart were only described roughly according to their morphology. Nowadays, lineage tracing reporter mice and the large-scale, single-cell RNA-sequencing (scRNA-seq) have helped to delineate their features in more details.
BMAs are thought to be derived from Sca1+ CD45− CD31− or LepR+ CD45− CD31− mesenchymal stem cells (MSCs) in the bone marrow (20, 21). Using in vivo cell lineage tracing of the dTomato+ in Vav1-Cre: mT/mG mice, BMAs are further confirmed to be originated from MSCs but not hematopoietic stem cells (HSCs) (22, 23). Pathway enrichment analysis also displayed that BMAs are closer to bone marrow mesenchymal stem cells (BMSCs) than to white adipocytes (24). Moreover, in contrast to brown adipocytes, BMAs are all dTomato- in Myf5-Cre: mT/mG mice (25, 26). This indicates that BMAs do not share the same progenitors as brown adipocytes. Further studies demonstrated that BMA progenitors can express Prx1 and Osx1, two markers labelling mesenchymal-osteogenic cells, while white and brown adipocytes cannot be traced in Osx1-Cre reporter mice (27, 28). In another study, using the lineage tracing of AdipoqCre+/mTmG+ and UCP1Cre+/mTmG+ mice, BMAs were demonstrated to not express UCP1 during development or upon the stimulation of β3-adrenergic agonist CL316,243 (29). These results indicate that BMAs derive from a mesenchymal-osteogenic lineage, and are genetically distinct from white, beige or brown adipocytes. Most recently, with the help of AdipoqCre+/DTA+/mTmG+ triple mutant mice, a defined cluster of adiponectin-negative stromal progenitors has been shown in the bone marrow of fat-free mice. This population was able to differentiate into ectopic BMAs with age and metabolic diseases. These BMAs have increased lipid storage and are not thermogenic as they are unresponsive to cold stress or β3-adrenergic stimulation (30). Despite that adiponectin is an essential adipocyte specific cytokine, the discovery of adiponectin-independent BMA subsets allows to speculate that further origins of BMAs remain to be revealed. Indeed, Zhong et al. have already defined a new population in the bone marrow from their scRNA-seq data, termed marrow adipogenic lineage precursors (MALPs) (31). This subpopulation expresses typical adipocyte markers as Pparg, Cebpa, Adipoq, Apoe, and Lpl, but not Plin1, thus containing no lipid droplets. They are not proliferative precursors for adipocytes but are essential for maintaining marrow vasculature and promoting pathologic bone loss in a RANKL-dependent manner (32, 33). Together, these data have vastly enriched the framework between MSCs and mature adipocytes, bringing more directions for future investigations.
The differentiation fate of BMAs from MSCs is also rigorously regulated by transcriptional cascades (34) (Figure 1). The transcription factors CCAAT/enhancer-binding protein CEBPβ and δ are induced primarily during early adipogenesis. Then they activate the expression of two critical adipogenic transcription factors: PPARγ and CEBPα (37). Expression of Cepba and Cebpb are selectively elevated in cBMAs of rats compared to rBMAs and subcutaneous white adipocytes (9). In addition, the tug-of-war between adipocytes and osteoblast differentiation in the bone marrow is also determined by many pathways such as Wnt/β-catenin and Leptin/LepR signaling. Wnt/β-catenin signaling promotes a cell fate shift from adipocytes to pre-osteoblasts (35, 36), while Leptin/LepR signaling facilitates adipogenesis and inhibits osteogenesis (21) (Figure 1).
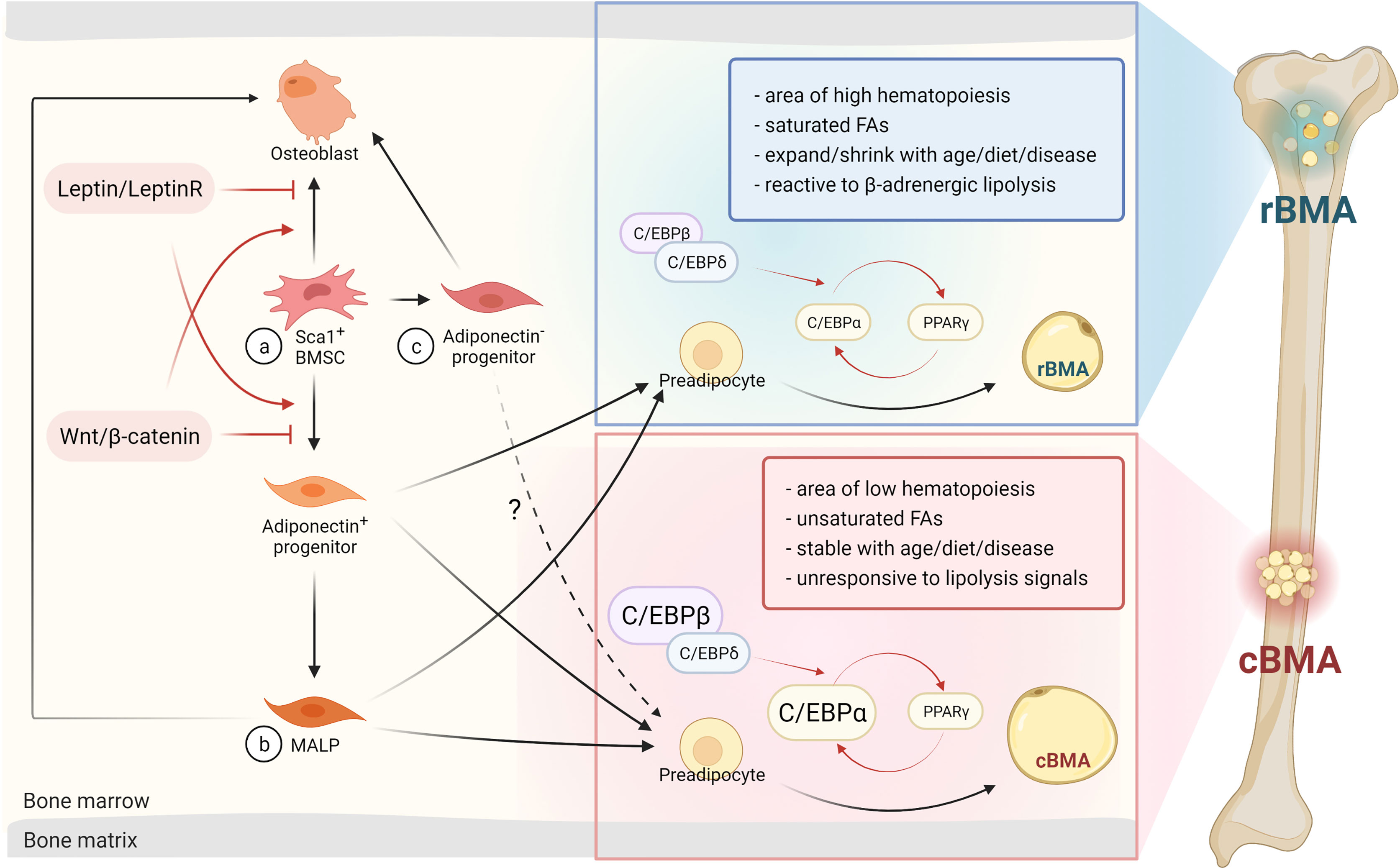
Figure 1 BMAs arise from BMSCs and can differentiate via osteogenic or adipogenic progenitors into rBMAs or cBMAs. (A) BMAs or osteoblasts originate from Sca1+ BMSCs modulated by the Leptin/LeptinR or Wnt/β-catenin signaling pathways (20–23, 35, 36). (B) MALPs are a newly defined primarily adipogenic sub-population that arises from adiponectin+ progenitors. Factors like acute injury and aging can trigger osteogenic differentiation of MALPs (30, 31). (C) Adiponectin- progenitors are predominantly of the osteogenic lineage, but are also able to differentiate into BMAs in metabolic disorders or in aging adults. This population elicits similar properties as cBMAs (30). BMSC, bone mesenchymal stem cells; rBMA, regulated bone marrow adipocyte; cBMA, constitutive bone marrow adipocyte; MALP, marrow adipogenic lineage precursor; C/EBP, CCAAT/enhancer-binding protein; FA, fatty acid; PPARγ, peroxisome proliferator-activated receptor gamma. Red arrows indicate transcription factors and signaling pathways. Dark arrows represent the consecutive stage of differentiation. The dashed arrow emphasizes similarities in cBMAs and adiponectin- progenitor-derived BMAs. Designed by Biorender.
In addition to the rigorous regulation of transcription cascades of BMA differentiation, the dynamic and complex bone marrow microenvironment could also be an essential contributor. Osteocyte-derived sclerostin, a glycoprotein encoded by SOst gene, could promote the expression of the adipogenic transcription factors Pparγ and Cebpα in primary MSCs from both humans and mice in vitro. As a consequence, the adipocyte differentiation via inhibition of the canonical Wnt signaling pathway was enhanced. In vivo studies also found decreased BMA formation in both sclerostin knock-out mouse models and wild-type mice treated with a sclerostin neutralizing antibody (38–40). These studies demonstrated a role for SOst and osteocyte-derived sclerostin in regulating fate determination of BMA progenitors. Bone morphogenetic proteins (BMPs) could also promote adipogenesis by promoting the expression of Pparγ and Cebpα (41). Bajaj and colleagues reported that BMP4 was highly expressed and secreted especially by T cells and stromal cells in response to irradiation. Thereby, the adipogenic commitment of the M2-10B4 cell line and primary murine MSCs were promoted. This could probably be one of the causes of marrow adipogenesis post-myelosuppression (42). These extrinsic factors generated by the marrow microenvironment may contribute to the distinct metabolic features and function of BMAs compared to white adipocytes, even though much still remains to be further elucidated.
Metabolic Features
Recent technologies have also unveiled numerous novel metabolic features of BMAs. Attané and colleagues compared the proteomic and lipidomic features of BMAs with subcutaneous fat tissue and concluded that BMAs display a distinct lipid metabolism contrary to classical white adipocytes (45). Pathway enrichment in proteomic results displayed elevated cholesterol metabolism in BMAs, which was further confirmed by a 1.5-fold increase in free cholesterol content and decreased lipolytic activity in BMAs. Moreover, more sphingosine, fewer ceramides and sphingomyelin were observed in the lipid profiles of BMAs compared to subcutaneous white adipocytes. The monoacylglycerol lipase (MGLL) expression is reduced with monoacylglycerol (MG) species elevated in BMAs, implying on an impaired MG hydrolysis compared to subcutaneous fat tissues. The altered lipid metabolism is also corroborated in another study, delineating that human BMAs possess distinct gene expression profiles, especially in regulating lipid metabolism, stemness genes, and browning pathways compared to subcutaneous adipose tissue (24). The overall steady state molecular signature of BMAs was described more comparable to brown adipocytes. In contrast, BMA expansion by aging or diabetes leads to a steady energy storing, white adipocyte-resembling metabolic signature (46). Scheller et al. also reported the diminished lipid hydrolysis in BMAs compared to white adipose tissue in response to β-adrenergic stimulation, mainly in distal regions (47). Transcriptomic analysis in rabbits also revealed decreased glycerol content, insulin resistance, reduced lipid synthesis, and transport, decreased fatty acid metabolism, and decreased thermoregulation in BMAs compared to white adipocytes. Reduction in fatty acid β-oxidation (FAO) and oxidative phosphorylation were also found in BMAs (29).
The glucose metabolism in BMAs and their role in systemic glucose homeostasis are also unique. The transcriptome analysis in rabbits and humans both revealed an altered glucose metabolism and diminished insulin responsiveness in BMAs compared to white adipocytes, while markers of brown or beige adipocytes were enriched. Using PET/CT and [18F] FDG, it was recently demonstrated that BMAs possess high basal glucose uptake both in rodents and humans but are unresponsive to insulin, cold exposure and glucocorticoids (2). However, in another clinical trial, Tam et al. as well used PET/CT and [18F] FDG to characterize the glucose uptake (GU) in human femoral and vertebral BMAs, found that insulin enhances GU in human femoral BMAs (48). These two conflicting results indicate that different species (rodents vs. human) and different sites (distal tibia BMAs vs. femur BMAs) vary significantly in BMA metabolism.
Metabolic programming also plays an important role in regulating BMA differentiation. BMA progenitors display higher insulin-dependent glucose utilization, enhanced capacity for oxidative phosphorylation (OXPHOS) and lipid storage, while osteoblast progenitors exhibit diminished insulin signaling, glycolysis-prone energy production, and reduced lipid storage (49). Moreover, metabolic changes in diseases such as obesity, diabetes and anorexia nervosa could also affect the formation of BMAs. Dyslipidemia caused by overnutrition in obesity facilitates BMA expansion and BMAs could then buffer extra energy in the form of triglycerides (50). The impaired lipid metabolism of type 2 diabetes (T2D) is characterized by the elevated low-density lipoprotein (LDL) cholesterol and free fatty acids, high concentration of plasma triglyceride and decreased high-density lipoprotein (HDL) cholesterol (51). This kind of hyperlipidemia could probably be associated with the enhanced adiposity of the bone marrow, for fatty acids could bind and activate PPARγ (52). In addition, hyperglycemia could induce expression of PPARγ by activating PI3K/Akt pathway and therefore enhance the adipogenicity of MSCs (53). The production of reactive oxygen species (ROS) resulting from the increased glucose levels in T2D could also promote the expression of genes associated with adipogenesis (54, 55). Starvation or fasting caused by anorexia nervosa also leads to hyperlipidemia (56), which could probably partly explain the expanded BMAs mentioned before. Collectively, BMA formation seems to be much closer to serum lipid levels than the type of diseases.
The number of BMAs and osteoblasts might be reciprocal, since they are competing for the same original stem cells. However, BMAs could also interfere with skeletal homeostasis and bone remodeling via its metabolic activities (57). The maintenance of bone mass depends on the dynamic and precise coordination of osteoclast-dominated bone resorption and osteoblast-mediated bone formation (58, 59). Studies in rats and dogs indicated reduced osteoblast activity, osteoclast numbers and increased bone loss at sites with higher BMA numbers (57). As osteoblasts are highly dependent on fatty acids for their glycolytic energy production, taking up to 80%, intact BMAs could be of importance for osteoblast function (60, 61). Moreover, Fatty acids, cholesterol, phospholipids and endogenous metabolites have been proven to regulate numerous signaling pathways mediating the proliferation and function of local osteoclasts and osteoblasts (62). Besides energy resources, BMAs may also protect osteoblasts from lipotoxicity (63). Other studies have shown the existence of BMA-derived exosomes filled with adipogenic factors and anti-osteoblastic miRNAs that are able to alter osteoblast function (64). Nevertheless, the role of BMA metabolism on bone cell survival and function remains poorly understood, and would require further investigation.
Metabolic Role in Bone Metastasis
Bone is one of the main organs for metastasis by various tumors. Hernandez et al. have retrospectively analyzed the real world electronic medical record data from oncology practices in the US and estimated the cumulative incidence of bone metastasis among patients with various solid tumors (65). The prostate cohort had the highest risk of bone metastasis with an incidence of 18.0% at one year, 20.4% at two years, 24.5% at five years, and 29.2% at ten years followed by lung (10.4-12.9%), renal (5.8-9.9%), breast (3.4-8.1%), gastrointestinal (2.3-3.6%), malignant melanoma (1.6-3.0%) and other tumors. In addition, the incidence of bone metastasis increased by the stage at diagnosis in all studied tumors. Another retrospective population-based study using data (2010-2015) from Surveillance, Epidemiology, and End Results (SEER) program, has reported that 5.7% of cancer patients suffer from bone metastasis (66). The third most vital factor for cancer is obesity, while smoking and infection pose number 1 and 2, respectively (67). Indeed, approximately 40% of cancers are associated with the excess of body weight (68). Researchers were able to show, that the risk of metastasis formation in obese breast cancer patients is increased by 46% (69). Overall, the link between the expansion of adipose tissue and metastasis formation has become evident in the recent decade, while the mechanism underlying bone metastases and BMAs remains unclear to date.
The novel findings in the metabolism of BMAs could be of vital importance for the understanding of tumor cell niching and growth in the bone marrow (Figure 2). In our previous work, we were able to observe that increased numbers of BMAs lead to accelerated melanoma tumor growth in the bone marrow and can be abrogated by inhibiting the adipocyte differentiation via PPARγ with the pharmacological compound bisphenol-A-diglycidylether (BADGE) (11, 12). Further experiments demonstrated that increased adipogenic differentiation of pre-adipocytes boosted by melanoma cell-derived factors led to the increase of BMAs at the early stage of bone metastasis, which further favored the tumor cells to niche and proliferate (70). Moreover, it is known that upregulated number of BMAs after chemotherapy and radiotherapy can correlate with tumor evasion (71). Finally, the facts that bone metastasis occurs preferentially in older people who have a higher portion of adipocytes in the bone marrow, and that BMAs rapidly expand (9-32%) in tumor patients over one year (72) further confirmed the close connection between BMAs and bone metastasis. As a result, the involvement of BMAs in the “vicious cycle” of tumor cells and bone cells seems to accelerate tumor growth. However, recent starving therapies have obtained a gratified result in eliciting an anti-tumor response (73, 74), while BMAs were also observed elevated in these fasting-like conditions. The ambiguous results may depend on the type of the tumor cells and stage of the disease, or the individual state of BMA subsets.
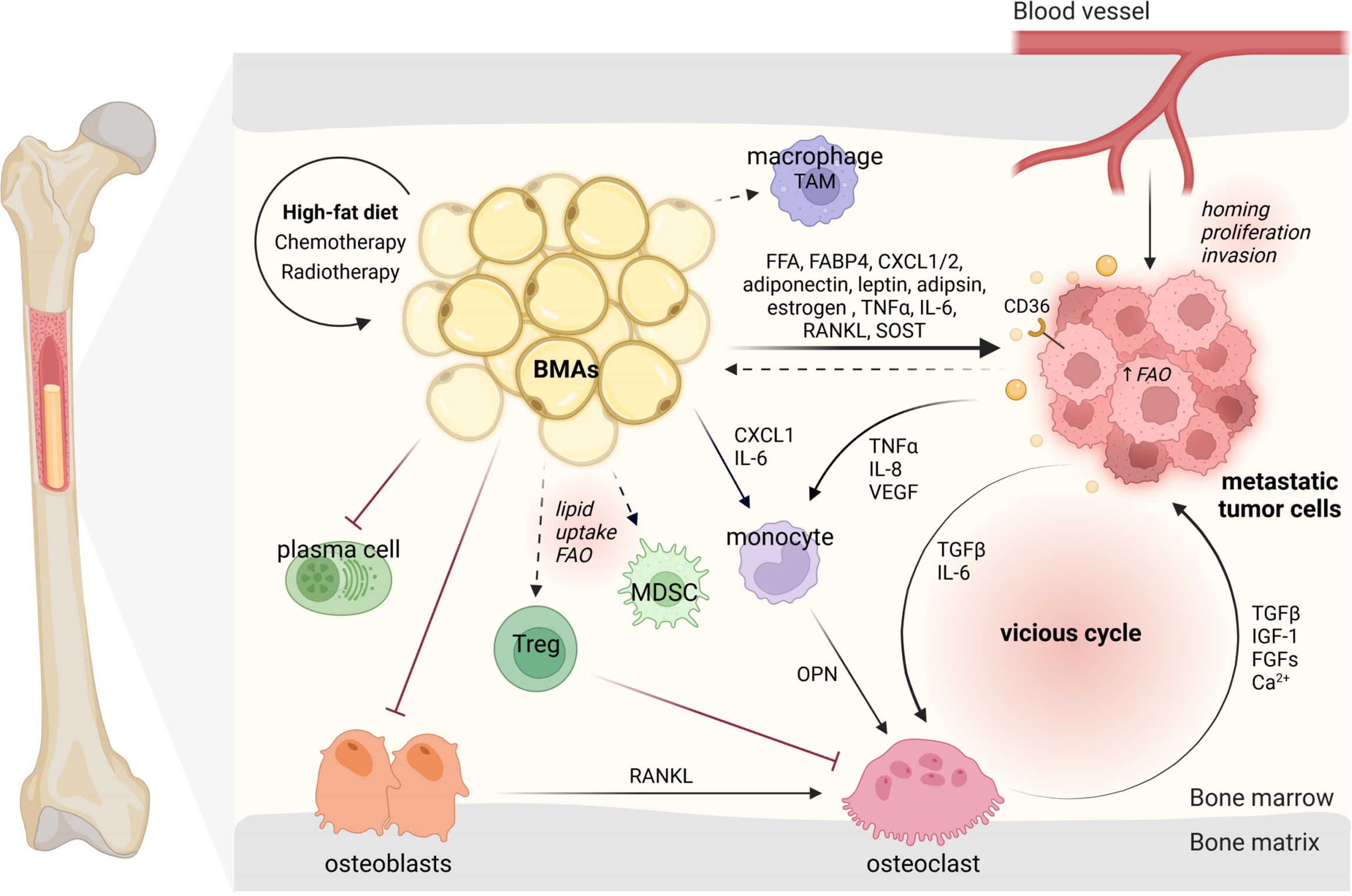
Figure 2 BMAs modulate their surrounding microenvironment and interact with niching tumor cells and bone marrow-resident cells. BMAs, bone marrow adipocytes; TAM, tumor-associated macrophage; FFA, free fatty acid; FABP4, fatty acid-binding protein 4; CXCL1/2, C-X-C motif ligand 1/2; FAO, fatty acid oxidation; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; TGFβ, transforming growth factor beta; IGF-1, insulin-like growth factor-1; FGFs, fibroblast growth factors; OPN, osteopontin; RANKL, receptor activator of NF-κB ligand; Tregs, regulatory T cells. The dark arrows indicate relationships, while dashed arrows represent potential links (43, 44). Designed by Biorender.
In general, tumor cells metastasize to rBMAs-enriched regions (proximal femur, hip, and lumbar spine) which contain smaller and less stable adipocytes (75). This preference may be directly connected to the distribution of blood vessels allowing distinct distribution of nutrition and oxygen concentration (76). The mechanisms underlying the pro-tumor effects of BMAs have attracted considerable attention. Many publications have discussed the importance of adipokines released by BMAs in bone metastasis, such as adiponectin (71), leptin (77), adipsin (78) and estrogen (79). Others have focused on the pro-inflammatory cytokines released by BMAs like TNFα, IL-6 and RANKL or target on BMAs like sclerostin (75, 80). But the metabolic functions of BMAs during bone metastasis have been less reviewed.
BMAs are a direct power station for tumor cells via lipolysis and lipid transfer (81). Using the vibrational spectroscopic technique-Fourier transform infrared (FTIR) microspectroscopy, Ehsan and his colleagues demonstrated that prostate cancer cells take up isotopically labeled FA [deuterated palmitic acid (D(31)-PA)] from human MSC-derived adipocytes (82). Furthermore, they also observed the lipid uptake of prostate cancer cells from nearby BMAs in the bone metastases specimens from patients, providing direct evidence of BMAs inducing tumor growth (83). BMAs could also shape tumor cell metabolism, contributing to their growth and metastasis. Podgorski and colleagues demonstrated that lipids from BMAs could fuel prostate tumor cells by upregulating CD36, FABP4, and Perilipin 2, supporting fatty acid transport (84). They also proved that BMAs drive metabolic reprogramming of tumor cells via an oxygen-independent mechanism of HIF-1α activation (85). CD36 is a scavenger receptor found on tumor cells, which was shown to be vital for metastasis formation and is currently considered as a potential therapeutic target (86, 87). It can be activated by free fatty acids secreted by BMAs and thus promote cancer growth (88). For prostate cancer bone metastases, researchers were able to show that the oxidative and endoplasmic reticulum (ER) stress pathways activated in BMAs can upregulate the secretion of survivin and heme oxygenase 1 to facilitate tumor cell survival (89). Other studies have demonstrated BMAs to drive FAO in tumor cells embedded in the bone marrow (88). As a parallel research field, bone cancers show similar indications for BMA mediated FAO. In acute monocytic leukemia, BMAs promote the cell survival by facilitating FAO via the stress response-associated AMP-activated protein kinase (AMPK). Thus, FAO in BMAs could also be considered as potential therapeutic target in the fight against bone metastases (90). The investigation of adipocyte-rich tissues revealed that ovarian, pancreatic and breast tumor cells can reprogram adipocytes to cancer-associated adipocytes (CAA). This phenotype aids the tumor growth by adipocyte dedifferentiation and release of their lipids, thereby promoting migration, proliferation, survival and chemoresistance (91–93). In this context, Liu et al. were able to show that BMAs can be reprogrammed to support myeloma-induced bone disease (94). Nevertheless, it remains unclear whether BMAs can dedifferentiate into the same tumor-aiding phenotype as found in other adipose tissues. Regarding overall lipid metabolism, researchers have shown that caprylic acid (C8:0) was increased in prostate cancer patients with diagnosed bone metastases (95). These results open a novel research avenue to study the various fatty acid-influenced molecular actions in the BMA-tumor cell interplays.
BMAs may also shape the microenvironment in the bone marrow in aid of tumor cell colonization (96). An expansion of BMAs with age was shown to be associated with a decreased bone mineral density (BMD) in patients (97). Similarly, experiments in mice demonstrated that high-calorie diets induce a shift from osteoblast to adipocyte differentiation, while increasing parameters for osteoclast activity (12). In addition, BMAs can promote osteoclastogenesis by mediation of osteoblast-secreted RANKL (98). These phenotypes are contributing to the severity of BMA-induced tumor burden, by driving osteoclastogenesis and thereby osteolytic lesion formation via IL-6 or indirectly via CXCL1 and osteopontin (OPN) (11). The CXCL1 and CXCL2 derived from BMAs were shown to promote prostate cancer survival and stiffen the overall tumor immune response (88, 99). Along this line, these chemokines could potentially attract macrophages and attribute to the distinct BMA-altered microenvironment. Studies in omental adipocytes have demonstrated to induce tumor-associated-macrophage polarization by upregulation of Pparb expression (88). Further research is needed to define the specific role of monocyte and macrophage sub-populations dependent on the presence of BMAs on the growth of tumor cells. Concerning the B cell lineage, BMAs were shown to overall impair the function of plasma cells compared to other adipocytes in humans (100). However, B cells in bone tumor niches remain an untouched area of research. Nevertheless, BMAs seem to play a pivotal role in the bone niche allowing the tumor cells to move in and grow.
While the fact that BMA-induced direct metabolic alterations on tumor cells poses a relatively wide scientific base, the effect on the metabolism of other resident cells and metastatic tumor progression remains to be largely under-studied. Researchers have shown the importance of metabolism in various tumor microenvironments. Therefore, it stands to reason that BMAs could influence their microenvironment in a similar way. For instance in other murine tumor tissues, it was shown that lipid uptake and FAO in myeloid-derived suppressor cells (MDSCs) facilitate their inhibitory role on anti-tumor T cells and promote tumor cell growth and migration (101, 102). Researchers could also show that tumor regulatory T cells (Tregs) suppress anti-tumor responses. At the same time the lipid metabolism supports the survival and function of Tregs within the hypoxic tumor microenvironment (103, 104). As Tregs also modulate osteoclasts, a potential link should be investigated (105). Moreover, in obese mice, creatine is a key metabolite linking adipocytes and breast tumors (106). Even though it is still unknown whether this fits for BMAs and the skeletal metastatic cells, creatine has been reported to promote the antitumor immune activity of CD8+ T cells and reduce the proliferation of subcutaneous tumors (107). Altogether, BMAs and their contribution to bone metastasis growth need to be further elucidated.
Limitations and Perspectives
Taken together, BMAs are distinct from other adipocyte fat depots, especially in the context of transcriptome, metabolism and functions to direct tumor growth. With novel emerging technologies, more information beneath the tip of the BMA iceberg has been unveiled, and BMAs might be considered as potential target to counteract the bone metastasis in a manner of individual treatment. However, their functions are still puzzling and would require further investigation.
While BMAs in their regulated or constitutive form are better characterized in rodents, this topic remains under-studied in patients. As humans have higher bone marrow adiposity than rodents, the role of BMAs in altering the surrounding environment may differ as well. Studies have shown contradicting conclusions regarding GU and insulin response in rodents versus humans. Here, the lack of receptors or other dissimilarities of the different species have to be taken in account. In humans, the insulin-producing beta cells lack a part of the G-protein-coupled receptor as compared to mice (108). Another dissimilarity observed between the species, was the sex-specific increased rBMA content in female versus male mice (9, 109). Overall, it has to be further elucidated whether the sexual dimorphism in rodent BMAs as well as human white adipose tissue is also reflected on human BMAs (110, 111). It is also important to mention that most of the findings are based on in vitro co-culture of diverse tumor cells with isolated bone marrow mesenchymal cells-induced adipocytes or differentiated pre-adipocyte cell lines (e.g. 3T3-L1). However, the reduced lipolytic activity in BMAs in vivo could not be recapitulated in vitro using these bone marrow mesenchymal stem cells (24, 45). It is always questionable to call in vitro differentiated adipocytes real BMAs, as the underlying microenvironmental factors distinguishing them from non-BMAs are lacking. Thus, future studies should rely on the direct in vivo evidence between BMAs and tumor cells. Also, the different metabolic or functional manners between BMAs and other adipocyte fat depots in supporting tumor cells colonization should be separately delineated.
Nevertheless, the animal models precisely tracing and locating rBMAs and cBMAs in vivo are also what we desperately need in future studies. The animal models will be beneficial for the investigations of BMA subpopulations. Exploit of Ptrf knockout initiates the first step towards establishing the rBMAs ablation model (9). Simultaneously, we are also confident that more and preciser markers of these adipocyte subpopulations will emerge in the future due to the utilization of large-scale scRNA-seq analyses. Advanced in-depth analyzing strategies may further help eliminate the contamination of BMAs surrounding cells such as osteoblasts and hematopoietic cells (45).
In addition, future studies need to explore the site-dependent lipid types (rBMAs vs. cBMAs) (9), cellular source and subcellular localization of the altered fatty acids. These investigations will help to quantify the impact of BMAs on local and systemic metabolism, and their function in steady-state or with tumor burden. Thus, the pro-tumor and anti-tumor roles of BMAs will be defined further in the future.
Author Contributions
XC, YZ and AB designed this review. YL, SC and AG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81771729, 81971534). This project was also supported by the German Research Foundation (DFG) priority program SPP2084 μBONE. BO-3811/5–1; BO-3811/6–1, Collaborative Research Centre 1181 project A01, Interdisciplinary Center for Clinical Research grant A77 and J76, The European Research Council consolidator grant ODE.
Conflict of Interest
Author YZ is employed by company Shanghai Huaota Biopharmaceutical Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ruiz-Ojeda FJ, Mendez-Gutierrez A, Aguilera CM, Plaza-Diaz J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int J Mol Sci (2019) 20(19):4888. doi: 10.3390/ijms20194888
2. Suchacki KJ, Tavares AAS, Mattiucci D, Scheller EL, Papanastasiou G, Gray C, et al. Bone Marrow Adipose Tissue Is a Unique Adipose Subtype With Distinct Roles in Glucose Homeostasis. Nat Commun (2020) 11(1):3097. doi: 10.1038/s41467-020-16878-2
3. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-Marrow Adipocytes as Negative Regulators of the Haematopoietic Microenvironment. Nature (2009) 460(7252):259–63. doi: 10.1038/nature08099
4. Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside Out: Bone Marrow Adipose Tissue as a Source of Circulating Adiponectin. Adipocyte (2016) 5(3):251–69. doi: 10.1080/21623945.2016.1149269
5. Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow Fat and Bone–New Perspectives. J Clin Endocrinol Metab (2013) 98(3):935–45. doi: 10.1210/jc.2012-3634
6. Baum T, Rohrmeier A, Syvari J, Diefenbach MN, Franz D, Dieckmeyer M, et al. Anatomical Variation of Age-Related Changes in Vertebral Bone Marrow Composition Using Chemical Shift Encoding-Based Water-Fat Magnetic Resonance Imaging. Front Endocrinol (Lausanne) (2018) 9:141. doi: 10.3389/fendo.2018.00141
7. Morris EV, Edwards CM. Bone Marrow Adipose Tissue: A New Player in Cancer Metastasis to Bone. Front Endocrinol (Lausanne) (2016) 7:90. doi: 10.3389/fendo.2016.00090
8. Tavassoli M. Marrow Adipose Cells Histochemical Identification of Labile and Stable Components. Arch Pathol Lab Med (1976) 100(1):16–8.
9. Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-Specific Variation in the Properties of Skeletal Adipocytes Reveals Regulated and Constitutive Marrow Adipose Tissues. Nat Commun (2015) 6:7808. doi: 10.1038/ncomms8808
10. Craft CS, Li Z, MacDougald OA, Scheller EL. Molecular Differences Between Subtypes of Bone Marrow Adipocytes. Curr Mol Biol Rep (2018) 4(1):16–23. doi: 10.1007/s40610-018-0087-9
11. Chen GL, Luo Y, Eriksson D, Meng X, Qian C, Bauerle T, et al. High Fat Diet Increases Melanoma Cell Growth in the Bone Marrow by Inducing Osteopontin and Interleukin 6. Oncotarget (2016) 7(18):26653–69. doi: 10.18632/oncotarget.8474
12. Gaculenko A, Gregoric G, Popp V, Seyler L, Ringer M, Kachler K, et al. Systemic Ppargamma Antagonism Reduces Metastatic Tumor Progression in Adipocyte-Rich Bone in Excess Weight Male Rodents. J Bone Miner Res (2021) 36(12):2440–52. doi: 10.1002/jbmr.4422
13. Fazeli PK, Bredella MA, Pachon-Pena G, Zhao W, Zhang X, Faje AT, et al. The Dynamics of Human Bone Marrow Adipose Tissue in Response to Feeding and Fasting. JCI Insight (2021) 6(12):e138636. doi: 10.1172/jci.insight.138636
14. Veldhuis-Vlug AG, Rosen CJ. Clinical Implications of Bone Marrow Adiposity. J Intern Med (2018) 283(2):121–39. doi: 10.1111/joim.12718
15. Sebo ZL, Rendina-Ruedy E, Ables GP, Lindskog DM, Rodeheffer MS, Fazeli PK, et al. Bone Marrow Adiposity: Basic and Clinical Implications. Endocr Rev (2019) 40(5):1187–206. doi: 10.1210/er.2018-00138
16. Kajkenova O, Lecka-Czernik B, Gubrij I, Hauser SP, Takahashi K, Parfitt AM, et al. Increased Adipogenesis and Myelopoiesis in the Bone Marrow of Samp6, a Murine Model of Defective Osteoblastogenesis and Low Turnover Osteopenia. J Bone Miner Res (1997) 12(11):1772–9. doi: 10.1359/jbmr.1997.12.11.1772
17. Kurabayashi T, Tomita M, Matsushita H, Honda A, Takakuwa K, Tanaka K. Effects of a Beta 3 Adrenergic Receptor Agonist on Bone and Bone Marrow Adipocytes in the Tibia and Lumbar Spine of the Ovariectomized Rat. Calcif Tissue Int (2001) 68(4):248–54. doi: 10.1007/s002230001203
18. Fazeli PK, Klibanski A. The Paradox of Marrow Adipose Tissue in Anorexia Nervosa. Bone (2019) 118:47–52. doi: 10.1016/j.bone.2018.02.013
19. Tavassoli M. Ultrastructural Development of Bone Marrow Adipose Cell. Acta Anat (Basel) (1976) 94(1):65–77. doi: 10.1159/000144545
20. Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte Accumulation in the Bone Marrow During Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell (2017) 20(6):771–84.e6. doi: 10.1016/j.stem.2017.02.009
21. Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell (2016) 18(6):782–96. doi: 10.1016/j.stem.2016.02.015
22. Zmuidzinas A, Fischer KD, Lira SA, Forrester L, Bryant S, Bernstein A, et al. The Vav Proto-Oncogene Is Required Early in Embryogenesis But Not for Hematopoietic Development in Vitro. EMBO J (1995) 14(1):1–11. doi: 10.1002/j.1460-2075.1995.tb06969.x
23. Katzav S, Martin-Zanca D, Barbacid M. Vav, a Novel Human Oncogene Derived From a Locus Ubiquitously Expressed in Hematopoietic Cells. EMBO J (1989) 8(8):2283–90. doi: 10.1002/j.1460-2075.1989.tb08354.x
24. Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, et al. Bone Marrow Adipocytes Support Hematopoietic Stem Cell Survival. J Cell Physiol (2018) 233(2):1500–11. doi: 10.1002/jcp.26037
25. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. Prdm16 Controls a Brown Fat/Skeletal Muscle Switch. Nature (2008) 454(7207):961–7. doi: 10.1038/nature07182
26. Horowitz MC, Berry R, Holtrup B, Sebo Z, Nelson T, Fretz JA, et al. Bone Marrow Adipocytes. Adipocyte (2017) 6(3):193–204. doi: 10.1080/21623945.2017.1367881
27. Chen J, Shi Y, Regan J, Karuppaiah K, Ornitz DM, Long F. Osx-Cre Targets Multiple Cell Types Besides Osteoblast Lineage in Postnatal Mice. PLoS One (2014) 9(1):e85161. doi: 10.1371/journal.pone.0085161
28. Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the Developing Mouse Limb Bud Driven by a Prxl Enhancer. Genesis (2002) 33(2):77–80. doi: 10.1002/gene.10092
29. Craft CS, Robles H, Lorenz MR, Hilker ED, Magee KL, Andersen TL, et al. Bone Marrow Adipose Tissue Does Not Express Ucp1 During Development or Adrenergic-Induced Remodeling. Sci Rep (2019) 9(1):17427. doi: 10.1038/s41598-019-54036-x
30. Zhang X, Robles H, Magee KL, Lorenz MR, Wang Z, Harris CA, et al. A Bone-Specific Adipogenesis Pathway in Fat-Free Mice Defines Key Origins and Adaptations of Bone Marrow Adipocytes With Age and Disease. Elife (2021) 10:e66275. doi: 10.7554/eLife.66275
31. Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, et al. Single Cell Transcriptomics Identifies a Unique Adipose Lineage Cell Population That Regulates Bone Marrow Environment. Elife (2020) 9:e54695. doi: 10.7554/eLife.54695
32. Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z, et al. Bone Marrow Adipogenic Lineage Precursors Promote Osteoclastogenesis in Bone Remodeling and Pathologic Bone Loss. J Clin Invest (2021) 131(2):e140214. doi: 10.1172/JCI140214
33. Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H, et al. Rankl From Bone Marrow Adipose Lineage Cells Promotes Osteoclast Formation and Bone Loss. EMBO Rep (2021) 22(7):e52481. doi: 10.15252/embr.202152481
34. Rosen ED, MacDougald OA. Adipocyte Differentiation From the Inside Out. Nat Rev Mol Cell Biol (2006) 7(12):885–96. doi: 10.1038/nrm2066
35. Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, et al. A Wnt-Mediated Transformation of the Bone Marrow Stromal Cell Identity Orchestrates Skeletal Regeneration. Nat Commun (2020) 11(1):332. doi: 10.1038/s41467-019-14029-w
36. Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of Wnt/Beta-Catenin Signaling Causes Cell Fate Shift of Preosteoblasts From Osteoblasts to Adipocytes. J Bone Miner Res (2012) 27(11):2344–58. doi: 10.1002/jbmr.1694
37. Cao Z, Umek RM, McKnight SL. Regulated Expression of Three C/Ebp Isoforms During Adipose Conversion of 3t3-L1 Cells. Genes Dev (1991) 5(9):1538–52. doi: 10.1101/gad.5.9.1538
38. Wang J, Liu R, Wang F, Hong J, Li X, Chen M, et al. Ablation of Lgr4 Promotes Energy Expenditure by Driving White-To-Brown Fat Switch. Nat Cell Biol (2013) 15(12):1455–63. doi: 10.1038/ncb2867
39. Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res (2017) 32(2):373–84. doi: 10.1002/jbmr.3001
40. Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The Skeletal Cell-Derived Molecule Sclerostin Drives Bone Marrow Adipogenesis. J Cell Physiol (2018) 233(2):1156–67. doi: 10.1002/jcp.25976
41. Tang QQ, Lane MD. Adipogenesis: From Stem Cell to Adipocyte. Annu Rev Biochem (2012) 81:715–36. doi: 10.1146/annurev-biochem-052110-115718
42. Bajaj MS, Kulkarni RS, Ghode SS, Limaye LS, Kale VP. Irradiation-Induced Secretion of Bmp4 by Marrow Cells Causes Marrow Adipogenesis Post-Myelosuppression. Stem Cell Res (2016) 17(3):646–53. doi: 10.1016/j.scr.2016.11.015
43. Lamora A, Talbot J, Mullard M, Brounais-Le Royer B, Redini F, Verrecchia F. Tgf-Beta Signaling in Bone Remodeling and Osteosarcoma Progression. J Clin Med (2016) 5(11):96. doi: 10.3390/jcm5110096
44. Wang M, Xia F, Wei Y, Wei X. Molecular Mechanisms and Clinical Management of Cancer Bone Metastasis. Bone Res (2020) 8(1):30. doi: 10.1038/s41413-020-00105-1
45. Attane C, Esteve D, Chaoui K, Iacovoni JS, Corre J, Moutahir M, et al. Human Bone Marrow Is Comprised of Adipocytes With Specific Lipid Metabolism. Cell Rep (2020) 30(4):949–58.e6. doi: 10.1016/j.celrep.2019.12.089
46. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone Marrow Fat Has Brown Adipose Tissue Characteristics, Which Are Attenuated With Aging and Diabetes. Bone (2012) 50(2):546–52. doi: 10.1016/j.bone.2011.06.016
47. Scheller EL, Khandaker S, Learman BS, Cawthorn WP, Anderson LM, Pham HA, et al. Bone Marrow Adipocytes Resist Lipolysis and Remodeling in Response to Beta-Adrenergic Stimulation. Bone (2019) 118:32–41. doi: 10.1016/j.bone.2018.01.016
48. Pham TT, Ivaska KK, Hannukainen JC, Virtanen KA, Lidell ME, Enerback S, et al. Human Bone Marrow Adipose Tissue Is a Metabolically Active and Insulin-Sensitive Distinct Fat Depot. J Clin Endocrinol Metab (2020) 105(7):2300–10. doi: 10.1210/clinem/dgaa216
49. Tencerova M, Rendina-Ruedy E, Neess D, Faergeman N, Figeac F, Ali D, et al. Metabolic Programming Determines the Lineage-Differentiation Fate of Murine Bone Marrow Stromal Progenitor Cells. Bone Res (2019) 7:35. doi: 10.1038/s41413-019-0076-5
50. Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High-Fat Diet-Induced Obesity Promotes Expansion of Bone Marrow Adipose Tissue and Impairs Skeletal Stem Cell Functions in Mice. J Bone Miner Res (2018) 33(6):1154–65. doi: 10.1002/jbmr.3408
51. Mooradian AD. Dyslipidemia in Type 2 Diabetes Mellitus. Nat Clin Pract Endocrinol Metab (2009) 5(3):150–9. doi: 10.1038/ncpendmet1066
52. Varga T, Czimmerer Z, Nagy L. Ppars Are a Unique Set of Fatty Acid Regulated Transcription Factors Controlling Both Lipid Metabolism and Inflammation. Biochim Biophys Acta (2011) 1812(8):1007–22. doi: 10.1016/j.bbadis.2011.02.014
53. Chuang CC, Yang RS, Tsai KS, Ho FM, Liu SH. Hyperglycemia Enhances Adipogenic Induction of Lipid Accumulation: Involvement of Extracellular Signal-Regulated Protein Kinase 1/2, Phosphoinositide 3-Kinase/Akt, and Peroxisome Proliferator-Activated Receptor Gamma Signaling. Endocrinology (2007) 148(9):4267–75. doi: 10.1210/en.2007-0179
54. Atashi F, Modarressi A, Pepper MS. The Role of Reactive Oxygen Species in Mesenchymal Stem Cell Adipogenic and Osteogenic Differentiation: A Review. Stem Cells Dev (2015) 24(10):1150–63. doi: 10.1089/scd.2014.0484
55. Giacco F, Brownlee M. Oxidative Stress and Diabetic Complications. Circ Res (2010) 107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545
56. Devlin MJ. Why Does Starvation Make Bones Fat? Am J Hum Biol (2011) 23(5):577–85. doi: 10.1002/ajhb.21202
57. Scheller EL, Rosen CJ. What's the Matter With Mat? Marrow Adipose Tissue, Metabolism, and Skeletal Health. Ann N Y Acad Sci (2014) 1311:14–30. doi: 10.1111/nyas.12327
58. Hadjidakis DJ, Androulakis II. Bone Remodeling. Ann N Y Acad Sci (2006) 1092:385–96. doi: 10.1196/annals.1365.035
59. Wang L, You X, Zhang L, Zhang C, Zou W. Mechanical Regulation of Bone Remodeling. Bone Res (2022) 10(1):16. doi: 10.1038/s41413-022-00190-4
60. Kushwaha P, Wolfgang MJ, Riddle RC. Fatty Acid Metabolism by the Osteoblast. Bone (2018) 115:8–14. doi: 10.1016/j.bone.2017.08.024
61. Lee WC, Ji X, Nissim I, Long F. Malic Enzyme Couples Mitochondria With Aerobic Glycolysis in Osteoblasts. Cell Rep (2020) 32(10):108108. doi: 10.1016/j.celrep.2020.108108
62. During A, Penel G, Hardouin P. Understanding the Local Actions of Lipids in Bone Physiology. Prog Lipid Res (2015) 59:126–46. doi: 10.1016/j.plipres.2015.06.002
63. Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of Palmitate-Induced Lipotoxicity in Human Osteoblasts. Endocrinology (2014) 155(1):108–16. doi: 10.1210/en.2013-1712
64. Martin PJ, Haren N, Ghali O, Clabaut A, Chauveau C, Hardouin P, et al. Adipogenic Rnas Are Transferred in Osteoblasts Via Bone Marrow Adipocytes-Derived Extracellular Vesicles (Evs). BMC Cell Biol (2015) 16:10. doi: 10.1186/s12860-015-0057-5
65. Hernandez RK, Wade SW, Reich A, Pirolli M, Liede A, Lyman GH. Incidence of Bone Metastases in Patients With Solid Tumors: Analysis of Oncology Electronic Medical Records in the United States. BMC Cancer (2018) 18(1):44. doi: 10.1186/s12885-017-3922-0
66. Ryan C, Stoltzfus KC, Horn S, Chen H, Louie AV, Lehrer EJ, et al. Epidemiology of Bone Metastases. Bone (2022) 158:115783. doi: 10.1016/j.bone.2020.115783
67. Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body Mass Index and Survival in Women With Breast Cancer-Systematic Literature Review and Meta-Analysis of 82 Follow-Up Studies. Ann Oncol (2014) 25(10):1901–14. doi: 10.1093/annonc/mdu042
68. Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, et al. Vital Signs: Trends in Incidence of Cancers Associated With Overweight and Obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep (2017) 66(39):1052–8. doi: 10.15585/mmwr.mm6639e1
69. Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, et al. Effect of Obesity on Prognosis After Early-Stage Breast Cancer. J Clin Oncol (2011) 29(1):25–31. doi: 10.1200/JCO.2010.29.7614
70. Wang J, Chen GL, Cao S, Zhao MC, Liu YQ, Chen XX, et al. Adipogenic Niches for Melanoma Cell Colonization and Growth in Bone Marrow. Lab Invest (2017) 97(6):737–45. doi: 10.1038/labinvest.2017.14
71. Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone Marrow Adipose Tissue Is an Endocrine Organ That Contributes to Increased Circulating Adiponectin During Caloric Restriction. Cell Metab (2014) 20(2):368–75. doi: 10.1016/j.cmet.2014.06.003
72. Hui SK, Arentsen L, Sueblinvong T, Brown K, Bolan P, Ghebre RG, et al. A Phase I Feasibility Study of Multi-Modality Imaging Assessing Rapid Expansion of Marrow Fat and Decreased Bone Mineral Density in Cancer Patients. Bone (2015) 73:90–7. doi: 10.1016/j.bone.2014.12.014
73. Nencioni A, Caffa I, Cortellino S, Longo VD. Fasting and Cancer: Molecular Mechanisms and Clinical Application. Nat Rev Cancer (2018) 18(11):707–19. doi: 10.1038/s41568-018-0061-0
74. Butler M, van der Meer LT, van Leeuwen FN. Amino Acid Depletion Therapies: Starving Cancer Cells to Death. Trends Endocrinol Metab (2021) 32(6):367–81. doi: 10.1016/j.tem.2021.03.003
75. Luo G, He Y, Yu X. Bone Marrow Adipocyte: An Intimate Partner With Tumor Cells in Bone Metastasis. Front Endocrinol (Lausanne) (2018) 9:339. doi: 10.3389/fendo.2018.00339
76. Gruneboom A, Hawwari I, Weidner D, Culemann S, Muller S, Henneberg S, et al. A Network of Trans-Cortical Capillaries as Mainstay for Blood Circulation in Long Bones. Nat Metab (2019) 1(2):236–50. doi: 10.1038/s42255-018-0016-5
77. Maroni P. Leptin, Adiponectin, and Sam68 in Bone Metastasis From Breast Cancer. Int J Mol Sci (2020) 21(3):1051. doi: 10.3390/ijms21031051
78. Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature Adipocytes in Bone Marrow Protect Myeloma Cells Against Chemotherapy Through Autophagy Activation. Oncotarget (2015) 6(33):34329–41. doi: 10.18632/oncotarget.6020
79. Limonard EJ, Veldhuis-Vlug AG, van Dussen L, Runge JH, Tanck MW, Endert E, et al. Short-Term Effect of Estrogen on Human Bone Marrow Fat. J Bone Miner Res (2015) 30(11):2058–66. doi: 10.1002/jbmr.2557
80. Liu C, Zhao Q, Yu X. Bone Marrow Adipocytes, Adipocytokines, and Breast Cancer Cells: Novel Implications in Bone Metastasis of Breast Cancer. Front Oncol (2020) 10:561595. doi: 10.3389/fonc.2020.561595
81. Diedrich JD, Herroon MK, Rajagurubandara E, Podgorski I. The Lipid Side of Bone Marrow Adipocytes: How Tumor Cells Adapt and Survive in Bone. Curr Osteoporos Rep (2018) 16(4):443–57. doi: 10.1007/s11914-018-0453-9
82. Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct Evidence of Lipid Translocation Between Adipocytes and Prostate Cancer Cells With Imaging Ftir Microspectroscopy. J Lipid Res (2007) 48(8):1846–56. doi: 10.1194/jlr.M700131-JLR200
83. Gazi E, Dwyer J, Lockyer NP, Gardner P, Shanks JH, Roulson J, et al. Biomolecular Profiling of Metastatic Prostate Cancer Cells in Bone Marrow Tissue Using Ftir Microspectroscopy: A Pilot Study. Anal Bioanal Chem (2007) 387(5):1621–31. doi: 10.1007/s00216-006-1093-y
84. Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, et al. Bone Marrow Adipocytes Promote Tumor Growth in Bone Via Fabp4-Dependent Mechanisms. Oncotarget (2013) 4(11):2108–23. doi: 10.18632/oncotarget.1482
85. Diedrich JD, Rajagurubandara E, Herroon MK, Mahapatra G, Huttemann M, Podgorski I. Bone Marrow Adipocytes Promote the Warburg Phenotype in Metastatic Prostate Tumors Via Hif-1alpha Activation. Oncotarget (2016) 7(40):64854–77. doi: 10.18632/oncotarget.11712
86. Wang J, Li Y. Cd36 Tango in Cancer: Signaling Pathways and Functions. Theranostics (2019) 9(17):4893–908. doi: 10.7150/thno.36037
87. Liang Y, Han H, Liu L, Duan Y, Yang X, Ma C, et al. Cd36 Plays a Critical Role in Proliferation, Migration and Tamoxifen-Inhibited Growth of Er-Positive Breast Cancer Cells. Oncogenesis (2018) 7(12):98. doi: 10.1038/s41389-018-0107-x
88. Cha YJ, Koo JS. Roles of Omental and Bone Marrow Adipocytes in Tumor Biology. Adipocyte (2019) 8(1):304–17. doi: 10.1080/21623945.2019.1643189
89. Herroon MK, Rajagurubandara E, Diedrich JD, Heath EI, Podgorski I. Adipocyte-Activated Oxidative and Er Stress Pathways Promote Tumor Survival in Bone Via Upregulation of Heme Oxygenase 1 and Survivin. Sci Rep (2018) 8(1):40. doi: 10.1038/s41598-017-17800-5
90. Tabe Y, Yamamoto S, Saitoh K, Sekihara K, Monma N, Ikeo K, et al. Bone Marrow Adipocytes Facilitate Fatty Acid Oxidation Activating Ampk and a Transcriptional Network Supporting Survival of Acute Monocytic Leukemia Cells. Cancer Res (2017) 77(6):1453–64. doi: 10.1158/0008-5472.CAN-16-1645
91. John B, Naczki C, Patel C, Ghoneum A, Qasem S, Salih Z, et al. Regulation of the Bi-Directional Cross-Talk Between Ovarian Cancer Cells and Adipocytes by Sparc. Oncogene (2019) 38(22):4366–83. doi: 10.1038/s41388-019-0728-3
92. Takehara M, Sato Y, Kimura T, Noda K, Miyamoto H, Fujino Y, et al. Cancer-Associated Adipocytes Promote Pancreatic Cancer Progression Through Saa1 Expression. Cancer Sci (2020) 111(8):2883–94. doi: 10.1111/cas.14527
93. Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res (2011) 71(7):2455–65. doi: 10.1158/0008-5472.CAN-10-3323
94. Liu H, He J, Koh SP, Zhong Y, Liu Z, Wang Z, et al. Reprogrammed Marrow Adipocytes Contribute to Myeloma-Induced Bone Disease. Sci Transl Med (2019) 11(494):eaau9087. doi: 10.1126/scitranslmed.aau9087
95. Wang C, Wang J, Chen K, Pang H, Li X, Zhu J, et al. Caprylic Acid (C8:0) Promotes Bone Metastasis of Prostate Cancer by Dysregulated Adipo-Osteogenic Balance in Bone Marrow. Cancer Sci (2020) 111(10):3600–12. doi: 10.1111/cas.14606
96. Hofbauer LC, Bozec A, Rauner M, Jakob F, Perner S, Pantel K. Novel Approaches to Target the Microenvironment of Bone Metastasis. Nat Rev Clin Oncol (2021) 18(8):488–505. doi: 10.1038/s41571-021-00499-9
97. Hardouin P, Rharass T, Lucas S. Bone Marrow Adipose Tissue: To Be or Not to Be a Typical Adipose Tissue? Front Endocrinol (Lausanne) (2016) 7:85. doi: 10.3389/fendo.2016.00085
98. Wu JB, Yin L, Shi C, Li Q, Duan P, Huang JM, et al. Maoa-Dependent Activation of Shh-Il6-Rankl Signaling Network Promotes Prostate Cancer Metastasis by Engaging Tumor-Stromal Cell Interactions. Cancer Cell (2017) 31(3):368–82. doi: 10.1016/j.ccell.2017.02.003
99. Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Marrow Adipocyte-Derived Cxcl1 and Cxcl2 Contribute to Osteolysis in Metastatic Prostate Cancer. Clin Exp Metastasis (2015) 32(4):353–68. doi: 10.1007/s10585-015-9714-5
100. Miggitsch C, Meryk A, Naismith E, Pangrazzi L, Ejaz A, Jenewein B, et al. Human Bone Marrow Adipocytes Display Distinct Immune Regulatory Properties. EBioMedicine (2019) 46:387–98. doi: 10.1016/j.ebiom.2019.07.023
101. Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, et al. Exogenous Lipid Uptake Induces Metabolic and Functional Reprogramming of Tumor-Associated Myeloid-Derived Suppressor Cells. Oncoimmunology (2017) 6(10):e1344804. doi: 10.1080/2162402X.2017.1344804
102. Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res (2015) 3(11):1236–47. doi: 10.1158/2326-6066.CIR-15-0036
103. Field CS, Baixauli F, Kyle RL, Puleston DJ, Cameron AM, Sanin DE, et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab (2020) 31(2):422–37.e5. doi: 10.1016/j.cmet.2019.11.021
104. Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid Signalling Enforces Functional Specialization of Treg Cells in Tumours. Nature (2021) 591(7849):306–11. doi: 10.1038/s41586-021-03235-6
105. Zhu L, Hua F, Ding W, Ding K, Zhang Y, Xu C. The Correlation Between the Th17/Treg Cell Balance and Bone Health. Immun Ageing (2020) 17:30. doi: 10.1186/s12979-020-00202-z
106. Maguire OA, Ackerman SE, Szwed SK, Maganti AV, Marchildon F, Huang X, et al. Creatine-Mediated Crosstalk Between Adipocytes and Cancer Cells Regulates Obesity-Driven Breast Cancer. Cell Metab (2021) 33(3):499–512.e6. doi: 10.1016/j.cmet.2021.01.018
107. Di Biase S, Ma X, Wang X, Yu J, Wang YC, Smith DJ, et al. Creatine Uptake Regulates Cd8 T Cell Antitumor Immunity. J Exp Med (2019) 216(12):2869–82. doi: 10.1084/jem.20182044
108. Amisten S, Atanes P, Hawkes R, Ruz-Maldonado I, Liu B, Parandeh F, et al. A Comparative Analysis of Human and Mouse Islet G-Protein Coupled Receptor Expression. Sci Rep (2017) 7:46600. doi: 10.1038/srep46600
109. Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A. Marrow Adipose Tissue: Skeletal Location, Sexual Dimorphism, and Response to Sex Steroid Deficiency. Front Endocrinol (Lausanne) (2017) 8:188. doi: 10.3389/fendo.2017.00188
110. Chang E, Varghese M, Singer K. Gender and Sex Differences in Adipose Tissue. Curr Diabetes Rep (2018) 18(9):69. doi: 10.1007/s11892-018-1031-3
Keywords: bone marrow adipocytes, lineage-tracing, metabolism, bone metastasis, multi-omic analysis
Citation: Li Y, Cao S, Gaculenko A, Zhan Y, Bozec A and Chen X (2022) Distinct Metabolism of Bone Marrow Adipocytes and their Role in Bone Metastasis. Front. Endocrinol. 13:902033. doi: 10.3389/fendo.2022.902033
Received: 22 March 2022; Accepted: 06 May 2022;
Published: 21 June 2022.
Edited by:
Guanwu Li, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2022 Li, Cao, Gaculenko, Zhan, Bozec and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxiang Chen, eGlhb3hpYW5nMDcyMUAxMjYuY29t
†These authors have contributed equally to this work
 Yixuan Li
Yixuan Li Shan Cao
Shan Cao Anastasia Gaculenko
Anastasia Gaculenko Yifan Zhan
Yifan Zhan Aline Bozec
Aline Bozec Xiaoxiang Chen
Xiaoxiang Chen