- 1Department of General Practice, Yuyao People’s Hospital, Ningbo, China
- 2Department of Geriatrics, Yuyao People’s Hospital, Ningbo, China
- 3Emergency Trauma Department, Yuyao People’s Hospital, Ningbo, China
Objective: Hypertension (HTN) and type 2 diabetes (T2DM) share common risk factors and usually co-occur. This study examined the relationship between HTN history and T2DM incidence in a cohort of Chinese hypertensive subjects.
Methods: We recruited 443 cases (T2DM and HTN) and 443 sex- and age-matched controls (HTN). The history of peak systolic blood pressure (SBP) was divided into 140-159, 160-179, and ≥ 180 mmHg, and that of peak diastolic blood pressure (DBP) was divided into 90-99, 100-109, and ≥ 110 mmHg. Multiple binary logistic regression models were used to explore the association between controlled HTN status and T2DM.
Results: Creatinine concentrations were higher in the cases than in the controls (P < 0.05). The HTN duration was longer in the cases than in the controls (14.7 years vs. 13.2 years; P < 0.05). Significant differences were also found in the history of peak SBP and DBP between the cases and controls (both P < 0.05). Creatinine, HTN duration, and family history of T2DM were risk factors for T2DM in hypertensive subjects, with odds ratios (95% confidence intervals) of 1.013 (1.004-1.022), 1.025 (1.003-1.047), and 5.119 (3.266-8.026), respectively. Compared with the lowest level of peak DBP, the odds ratio for T2DM at the highest level of peak DBP was 1.757 (1.074-2.969). Subgroups analyses showed that the effect of the history of peak DBP on T2DM was significantly modified by sex (P-interaction = 0.037).
Conclusion: The highest DBP and the longest HTN duration were both independently associated with T2DM in hypertensive subjects.
Introduction
Type 2 diabetes mellitus (T2DM) is a serious public health problem worldwide due to its considerable impact on human health and quality of life. Globally, approximately 463 million individuals had T2DM in 2019, accounting for 9.3% of the world’s population (1). The prevalence of diabetes has increased rapidly, and has been predicted to further increase from 6,059/100,000 cases in 2017 to 7,059 per 100,000 people in 2030 (2). Owing to its large population, China has the largest number of T2DM cases at 114 million (2).
Several longitudinal studies have indicated that hypertension (HTN) is an important risk factor for T2DM and can play a vital role in its prognosis (3–6). HTN and T2DM share common risk factors and usually co-occur. Approximately 58% of T2DM patients also have HTN (7). The influence of high blood pressure on impaired glucose metabolism is believed to be mainly exerted via altered endothelial permeability and oxidative stress, which in turn promote insulin resistance and pancreatic β-cell dysfunction (8–10). In addition, the extent of glucose metabolism impairment due to HTN may be governed by the history of peak high blood pressure and the duration of HTN. However, no study has yet focused on the relationship between the history of HTN (i.e., the history of peak high systolic blood pressure [SBP] and diastolic blood pressure [DBP] and the duration of HTN) and the incidence of T2DM. Elucidating this relationship will provide insights into the T2DM susceptibility of hypertensive patients and can inform preventive measures, especially in China, where there is a large number of both HTN and diabetes cases.
Therefore, we conducted a community-based case–control study to examine whether the history of HTN influences the incidence of T2DM in a cohort of Chinese hypertensive subjects.
Materials and methods
Study design
This was a population-based retrospective study with 1:1 sex- and age-matched case and control groups. Individuals with HTN alone and with both HTN and T2DM were recruited from the Department of Geriatrics in Yuyao People’s Hospital, Zhejiang, China. In total, 443 cases (with both T2DM and HTN) and 443 controls (with only HTN) from the same community and matched by sex and age (within 3 years) were included in the study. Subjects who were pregnant and those with secondary HTN, serious liver or kidney failure, psychosis, cancer, Alzheimer’s disease, type 1 diabetes, or a history of ischemic stroke or coronary heart disease were excluded. All participants provided written informed consent for inclusion in this study, and the Ethical Committee of Yuyao People’s Hospital approved the study.
Hypertensive subjects were diagnosed in hospitals according to the following criteria: (i) an SBP ≥ 140 mmHg and/or a DBP ≥ 90 mmHg, based on the mean of three measurements, and (ii) the use of antihypertensive medication. Diabetes was diagnosed according to the American Diabetes Association 2010 criteria (11) when the fasting plasma glucose concentration and the 2-h post-prandial plasma glucose concentration were higher than 7.0 mmol/L and 11.1 mmol/L, respectively, or the glycated hemoglobin concentration was higher than 6.5%.
Data collection
A standard structured questionnaire was used to collect information on demographic and behavioral risk factors, including age, sex, smoking, alcohol consumption, sleeping duration, family history of HTN and T2DM, antihypertensive medication use, and HTN duration. Face-to-face interviews were conducted by trained physicians. During these interviews, the history of peak SBP and DBP was reported by the subjects, while the current SBP and DBP were measured by the physicians using a standard mercury sphygmomanometer on the right arm of the seated participants after a 5-min rest in the sitting position. The current SBP was divided into <120 mmHg, 120-139 mmHg, 140-159 mmHg, and ≥ 160 mmHg, and the current DBP was divided into <80 mmHg, 80-89 mmHg, 90-99 mmHg, and ≥ 100 mmHg. The history of peak high SBP was divided into 140-159 mmHg, 160-179 mmHg, and ≥ 180 mmHg, and the history of peak high DBP was divided into 90-99 mmHg, 100-109 mmHg, and ≥ 110 mmHg. Body mass index (BMI) was calculated as body weight (kg)/height squared (m2).
Blood samples were obtained after overnight fasting. All samples were examined for concentrations of total cholesterol, serum triglyceride (TG), uric acid (UA), low-density lipoprotein cholesterol (LDL), serum creatinine, homocysteine, and glucose. All of these clinical laboratory parameters were determined using routine laboratory methods. Total cholesterol, TG, LDL, glucose, and homocysteine concentrations were measured with the automatic biochemical analyzer HITACH 7080 using standard enzymatic methods. The serum concentrations of UA and creatinine were determined quantitatively using the uricase method and the Jaffe reaction method, respectively.
Statistical analysis
Continuous variables were summarized as means ± standard deviations. Student’s t-test was used to compare continuous variables between the case and control groups, and the chi-square test was used to compare categorical variables between the groups. Multiple binary logistic regression models were used to explore the association between controlled HTN status and T2DM incidence. The strength effect estimates were measured using odds ratios (ORs) with 95% confidence intervals (CIs). The effects of sex (men/women), smoking (yes/no), drinking (yes/no), and sleeping duration (≥8 h/<8 h) were assessed using stratified analyses. All P values were two-tailed and considered to be statistically significant at < 0.05. Statistical analysis was performed using IBM SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
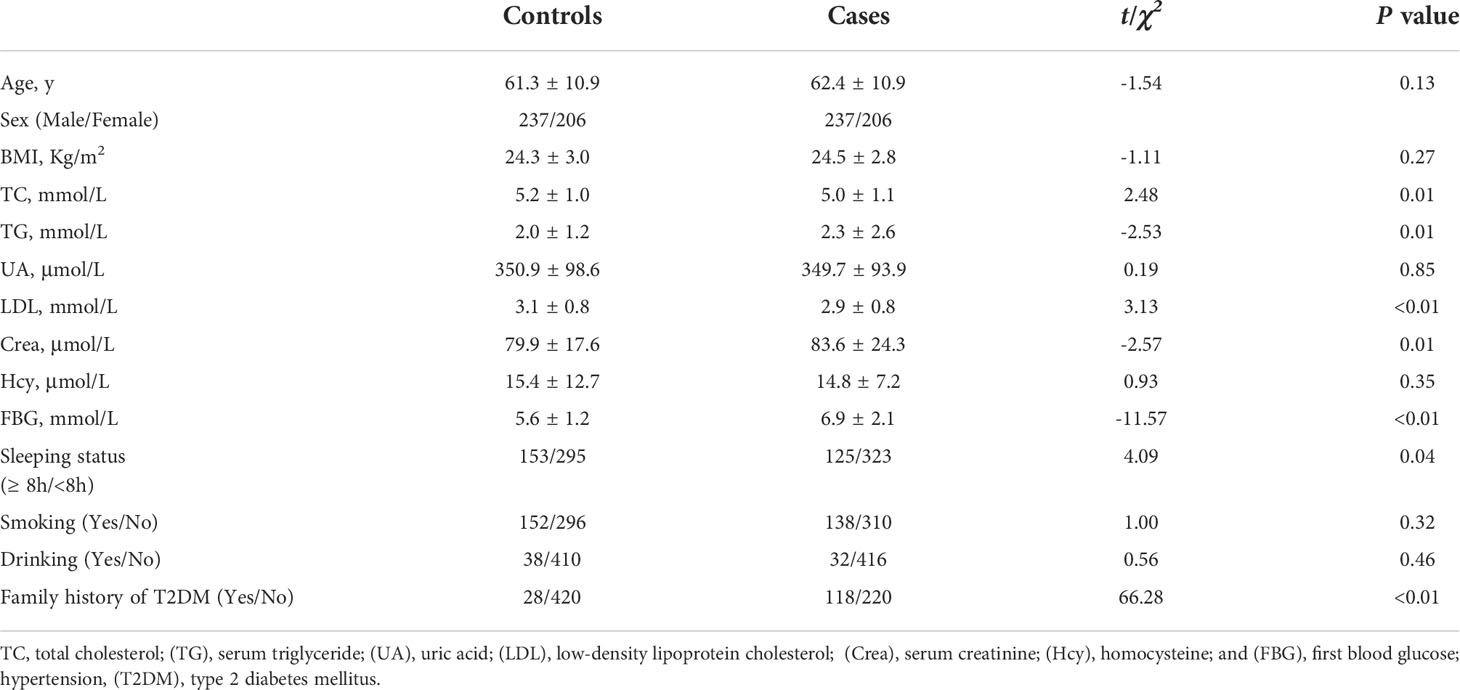
A total of 886 participants—443 cases (T2DM and HTN) and 443 controls (HTN)—were recruited in this matched case–control study. Table 1 shows the baseline characteristics of the subjects. No significant differences were found in age, sex, BMI, UA concentrations, homocysteine concentrations, smoking status, or alcohol consumption between the cases and controls. The total cholesterol and LDL concentrations were higher and the sleeping durations were longer in the controls than in the cases (all P < 0.05). The TG, creatinine, and glucose concentrations were higher and the family history of T2DM was longer in the cases than in the controls (all P < 0.05; Table 1).
The HTN durations were longer in the cases than in the controls (14.7 years vs. 13.2 years) (P < 0.05; Table 2). There were no significant differences in the status of HTN drug use or current SBP and DBP between the cases and controls (all P > 0.05; Table 2), but significant differences were found in the history of peak high SBP and DBP between the two groups (both P < 0.05; Table 2).
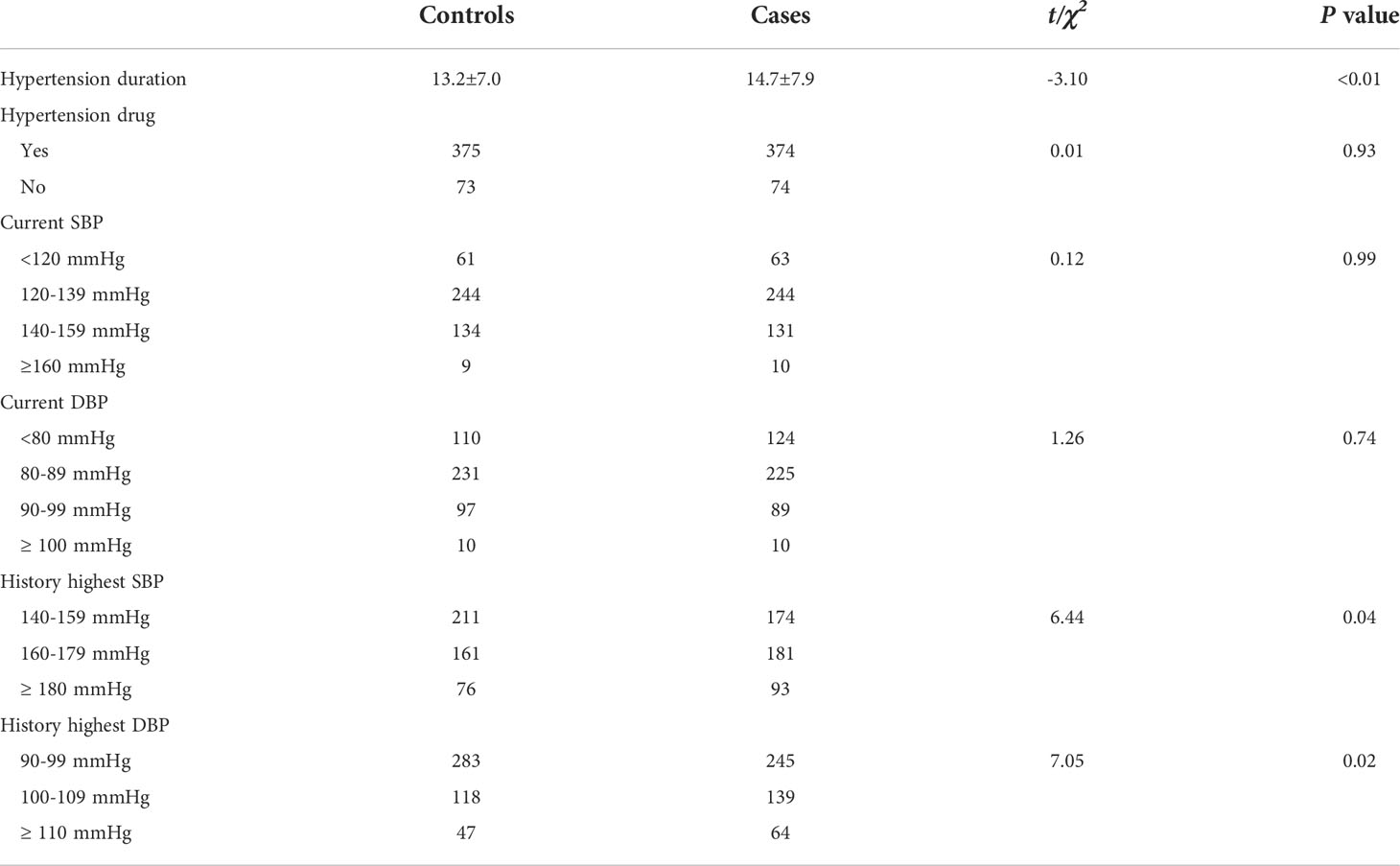
Table 2 The hypertensive characteristics of cases (T2DM and hypertension) and controls (hypertension).
Multiple binary logistic regression models
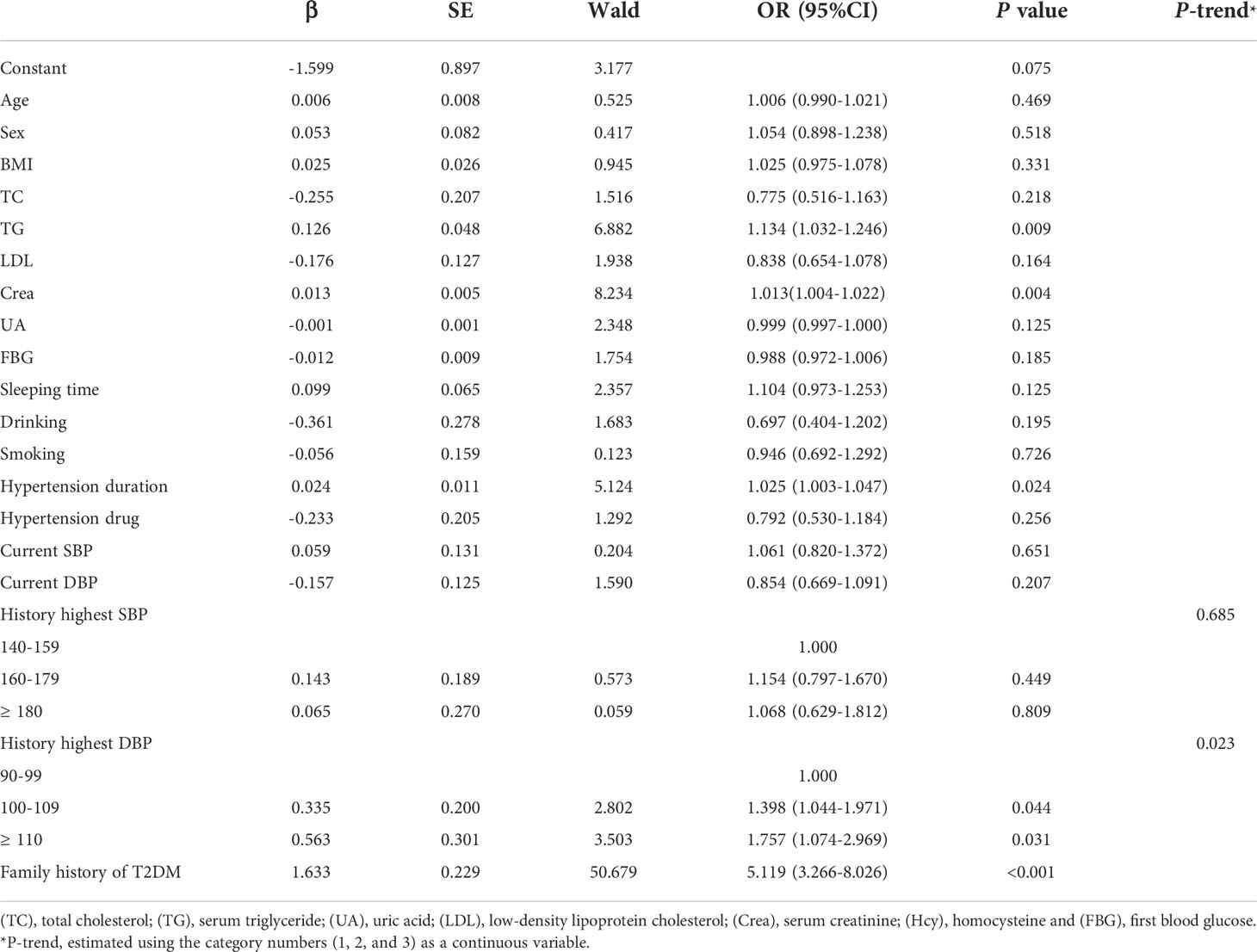
The results of multiple binary logistic regression models showed that the TG and creatinine concentrations, HTN duration, and family history of T2DM were all risk factors for T2DM in HTN (all P < 0.05, Table 3), with ORs (95% CIs) of 1.134 (1.032-1.246), 1.013 (1.004-1.022), 1.025 (1.003-1.047), and 5.119 (3.266-8.026), respectively. Analysis of the history of peak high DBP showed that compared with the low DBP category (90-99 mmHg), the ORs for T2DM in the medium (100-109 mmHg) and high (≥110 mmHg) DBP categories were 1.398 (1.044-1.971) and 1.757 (1.074-2.969), respectively. Furthermore, a linear trend was found in the association between increments in the history of peak high DBP and the risk of T2DM (P-trend = 0.023).
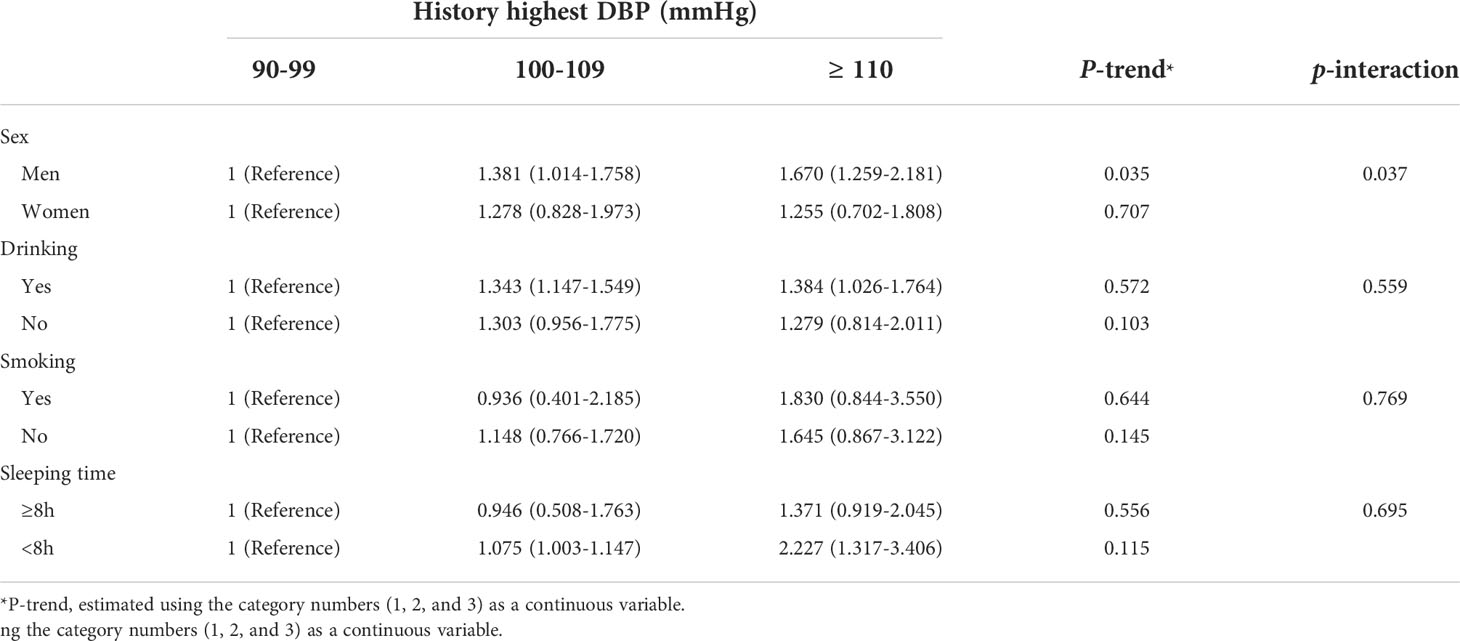
In subgroups analyses, the association between the history of peak high DBP and the incidence of T2DM was significantly modified by sex (P-interaction = 0.037). In the male subgroup, compared with the low DBP category, the ORs for T2DM in the medium and high categories of peak high DBP were 1.381 (1.014-1.758) and 1.670 (1.259-2.181), respectively (Table 4).
Discussion
This study explored the relationship between the history of HTN and the incidence of T2DM in a cohort of Chinese hypertensive subjects. After controlling for potential confounders, the history of peak high DBP and the duration of HTN were both independent risk factors for T2DM.
Studies have reported a strong association between HTN and the risk of T2DM. In particular, even normal-high SBP levels (130-139 mmHg) were shown to be significant predictors of T2DM in men (OR = 1.43) (3), after adjusting for BMI and other conventional T2DM risk factors. In a 10-year prospective study in Korea, subjects with baseline stage 2 HTN (≥ 160/100 mmHg) were at a 1.60-fold higher risk of developing diabetes than normal subjects (12). In a recent 6-year rural Chinese cohort study, restricted cubic spline analysis revealed an increased risk of T2DM with increasing mean arterial pressure among women (13). Consistent with previous studies, we discovered that the history of peak high DBP and duration of HTN were associated with T2DM in hypertensive subjects. This association remained after controlling for HTN drug use and current SBP and DBP, suggesting that the association between the history of long-term high blood pressure and the incidence of T2DM is independent of the association between the duration of HTN and the incidence of T2DM.
The direct causal link between the pathophysiological mechanisms of HTN and T2DM has not yet been completely identified, but several hypotheses have been proposed. HTN has been shown to induce microvascular changes (14) that result in insulin resistance via impaired glucose and nutrient delivery to skeletal muscle, which may in turn contribute to diabetes. Endothelial dysfunction is also related to insulin resistance and may partially account for the strong association between HTN and T2DM (15, 16). Inflammatory markers and oxidative stress also play vital roles in both HTN and diabetes (9, 17). C-reactive protein and interleukin 6 may contribute to the incidence of T2DM via interaction with the insulin signaling pathway and pancreatic β-cell function (18). Oxidative stress, which plays a critical role in pancreatic β-cell dysfunction, is also associated with high blood pressure (9).
In this study, the serum TG concentration and a family history of T2DM were found to be common risk factors for T2DM. In addition, the serum creatinine concentration was shown to be a potential risk factor for T2DM in hypertensive subjects. A recent dose–response meta-analysis of six cohort studies showed a negative association between the serum creatinine concentration and T2DM risk (19); the risk was increased by 7% with each 0.1 mg/dL decrease in the serum creatinine concentration. Serum creatinine is the only metabolite of creatine in skeletal muscle and represents the level of skeletal muscle mass. Low skeletal muscle mass can lead to insulin resistance (20, 21) and reduced glucose uptake by skeletal muscle, thereby resulting in the development of T2DM. In contrast to the finding of the previous meta-analysis, we observed a positive association between the serum creatinine concentration and T2DM risk in hypertensive subjects. The reason for this finding is not clear, but we hypothesize that the expression of creatine kinase in hypertensive patients is abnormal (22, 23). High creatine kinase activity promotes HTN through enhanced vascular contractility and sodium retention in the kidneys. Creatine kinase is tightly bound near ATPases and catalyzes the conversion of creatine to creatinine in serum (22). The specific physiological mechanisms need to be investigated in future studies.
To the best of our knowledge, this is the first study to explore the potential association between the history of peak high blood pressure and the incidence of T2DM in a cohort of Chinese hypertensive subjects. However, this study has a few limitations. First, the history of peak blood pressure was self-reported by the participants. Therefore, to reduce potential recall bias, we classified the blood pressure values into groups instead of using the specific blood pressure values. Second, the study sample included only Han Chinese adults, and thus, the findings cannot be generalized to other ethnicities in China or populations in other countries. Caution should be exercised when extrapolating our results to other populations. Finally, owing to the limitations inherent in case–control studies, longer follow-up cohort studies and intervention trials are needed to confirm our findings.
Conclusions
This study demonstrated that both the history of peak high DBP and the duration of HTN were independently associated with the incidence of T2DM in hypertensive subjects. This suggests that diabetes prevention measures should target hypertensive subjects with extremely high DBP and longer HTN duration as they are at a high risk of developing diabetes. Our findings should be verified by a longitudinal study in which a cohort of cases with HTN and a cohort of control subjects are followed up to track the development of T2DM.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by yuyao People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R. Diabetes and global ageing among 65-99-year-old adults: Findings from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract (2020) 162:108078. doi: 10.1016/j.diabres.2020.108078
2. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of type 2 diabetes - global burden of disease and forecasted trends. J Epidemiol Glob Health (2020) 10(1):107–11. doi: 10.2991/jegh.k.191028.001
3. Stahl CH, Novak M, Lappas G, Wilhelmsen L, Björck L, Hansson PO, et al. High-normal blood pressure and long-term risk of type 2 diabetes: 35-year prospective population based cohort study of men. BMC Cardiovasc Disord (2012) 12:89. doi: 10.1186/1471-2261-12-89
4. Wei GS, Coady SA, Goff DC Jr., Brancati FL, Levy D, Selvin E, et al. Blood pressure and the risk of developing diabetes in african americans and whites: ARIC, CARDIA, and the framingham heart study. Diabetes Care (2011) 34(4):873–9. doi: 10.2337/dc10-1786
5. Kim MJ, Lim NK, Choi SJ, Park HY. Hypertension is an independent risk factor for type 2 diabetes: the Korean genome and epidemiology study. Hypertens Res (2015) 38(11):783–9. doi: 10.1038/hr.2015.72
6. Raffaele Galiero AC, Vetrano E, Cesaro A, Rinaldi L, Salvatore T, Marfella R, et al. Pathophysiological mechanisms and clinical evidence of relationship between nonalcoholic fatty liver disease (NAFLD) and cardiovascular disease. Rev Cardiovasc Med (2021) 22(3):755–68. doi: 10.31083/j.rcm2203082
7. Akalu Y, Belsti Y. Hypertension and its associated factors among type 2 diabetes mellitus patients at debre tabor general hospital, Northwest Ethiopia. Diabetes Metab Syndr Obes (2020) 13:1621–31. doi: 10.2147/DMSO.S254537
8. Pinkney JH, StHTNouwer CD, Coppack SW, Yudkin JS. Endothelial dysfunction: cause of the insulin resistance syndrome. Diabetes (1997) 46 Suppl 2:S9–13. doi: 10.2337/diab.46.2.S9
9. Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? the common soil hypothesis revisited. Arterioscler Thromb Vasc Biol (2004) 24(5):816–23. doi: 10.1161/01.ATV.0000122852.22604.78
10. Na Ta ML, Wang Y, Zeng F, Nie F, Shang M, Wang X, et al. Association of polymorphisms in endothelial dysfunction-related genes with susceptibility to essential hypertension in elderly han population in liaoning province, China. Rev Cardiovasc Med (2021) 22(3):895–901. doi: 10.31083/j.rcm2203096
11. American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care (2013) 36 Suppl 1:S67–74. doi: 10.2337/dc10-S062
12. Cho NH, Kim KM, Choi SH, Park KS, Jang HC, Kim SS, et al. High blood pressure and its association with incident diabetes over 10 years in the Korean genome and epidemiology study (KoGES). Diabetes Care (2015) 38(7):1333–8. doi: 10.2337/dc14-1931
13. Guo C, Qin P, Li Q, Zhang D, Tian G, Liu D, et al. Association between mean arterial pressure and risk of type 2 diabetes mellitus: The rural Chinese cohort study. Prim Care Diabetes (2020) 14(5):448–54. doi: 10.1016/j.pcd.2020.01.007
14. Strain WD, Paldanius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol (2018) 17(1):57. doi: 10.1186/s12933-018-0703-2
15. Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA (2004) 291(16):1978–86. doi: 10.1001/jama.291.16.1978
16. Thuillez C, Richard V. Targeting endothelial dysfunction in hypertensive subjects. J Hum Hypertens (2005) 19 Suppl 1:S21–5. doi: 10.1038/sj.jhh.1001889
17. Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes (2004) 53(3):693–700. doi: 10.2337/diabetes.53.3.693
18. Kanmani S, Kwon M, Shin MK, Kim MK. Association of c-reactive protein with risk of developing type 2 diabetes mellitus, and role of obesity and hypertension: A Large population-based Korean cohort study. Sci Rep (2019) 9(1):4573. doi: 10.1038/s41598-019-40987-8
19. Qin P, Lou Y, Cao L, Shi J, Tian G, Liu D, et al. Dose-response associations between serum creatinine and type 2 diabetes mellitus risk: A Chinese cohort study and meta-analysis of cohort studies. J Diabetes (2020) 12(8):594–604. doi: 10.1111/1753-0407.13038
20. Ruegsegger GN, Creo AL, Cortes TM, Dasari S, Nair KS. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Invest (2018) 128(9):3671–81. doi: 10.1172/JCI120843
21. Mizgier ML, Casas M, Contreras-Ferrat A, Llanos P, Galgani JE. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes Rev (2014) 15(7):587–97. doi: 10.1111/obr.12166
22. Santhanam P, Khitan Z, Khthir R. Association between serum total bilirubin and serum creatinine and the effect of hypertension. J Clin Hypertens (Greenwich) (2015) 17(1):61–2. doi: 10.1111/jch.12452
Keywords: hypertension, type 2 diabetes, diastolic blood pressure, creatinine, case-control
Citation: Chen Y, Ma J, Lu D and Fang Y (2022) The risk factors of type 2 diabetes in hypertensive subjects. Front. Endocrinol. 13:901614. doi: 10.3389/fendo.2022.901614
Received: 22 March 2022; Accepted: 29 June 2022;
Published: 22 July 2022.
Edited by:
Daisuke Koya, Kusatsu General Hospital, JapanReviewed by:
Enoch ODame Anto, Kwame Nkrumah University of Science and Technology, GhanaAnthonia Ogbera, Lagos State University, Nigeria
Copyright © 2022 Chen, Ma, Lu and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yefei Fang, ZnlmMjM1ODUzMkAxMjYuY29t
 Yingqun Chen
Yingqun Chen Jiner Ma2
Jiner Ma2