
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 22 July 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.899000
This article is part of the Research Topic Safety and Child Health of Assisted Reproduction Technology (ART) View all 28 articles
 Ming-Xing Chen1,2†
Ming-Xing Chen1,2† Xiang-Qian Meng3†
Xiang-Qian Meng3† Zhao-Hui Zhong1,4
Zhao-Hui Zhong1,4 Xiao-Jun Tang4
Xiao-Jun Tang4 Tian Li5
Tian Li5 Qian Feng6
Qian Feng6 Enoch Appiah Adu-Gyamfi2
Enoch Appiah Adu-Gyamfi2 Yan Jia7
Yan Jia7 Xing-Yu Lv3
Xing-Yu Lv3 Li-Hong Geng7
Li-Hong Geng7 Lin Zhu8
Lin Zhu8 Wei He8*
Wei He8* Qi Wan3*
Qi Wan3* Yu-Bin Ding1,2*
Yu-Bin Ding1,2*Background: The GnRH agonist long-acting protocol and GnRH antagonist protocol are widely used in ovarian stimulation. Which protocol eliciting higher live birth rate for IVF/ICSI patients with different ages, different ovarian reserves and different body mass index (BMI) has not been studied. However, among these protocols, the one that elicits higher live birth in IVF/ICSI patients with different ages, ovarian reserves and body mass indexes (BMI) has not been identified.
Methods: This was a retrospective cohort study about 8579 women who underwent the first IVF-ET from January, 2018 to August, 2021. Propensity Score Matching (PSM) was used to improve the comparability between two protocols.
Results: After PSM, significant higher live birth rates were found in the GnRH agonist long-acting protocol compared to GnRH antagonist protocol (44.04% vs. 38.32%) (p<0.001). Stratified analysis showed that for those with AMH levels between 3 ng/ml and 6 ng/ml, with BMI ≥ 24 kg/m2 and were aged ≥ 30 years old, and for those women with BMI < 24kg/m2 and were aged ≥30 years whose AMH levels were ≤ 3ng/ml, the GnRH agonist long-acting protocol was more likely to elicit live births [OR (95%CI), 2.13(1.19,3.80)], [OR (95%CI), 1.41(1.05,1.91)]. However, among women with BMI ≥ 24kg/m2 and were aged ≥30 years whose AMH levels were ≤ 3ng/ml, the GnRH agonist long-acting protocol had a lower possibility of eliciting live births [OR (95%CI), 0.54(0.32,0.90)]. Also, among women with AMH levels between 3 ng/ml and 6 ng/ml, with BMI ≥ 24 kg/m2 and with age < 30 years and for those with AMH levels between 3 ng/ml and 6 ng/ml, regardless of age, and with BMI<24kg/m2,, the possibility of live births was similar between the two protocols [OR (95%CI), 1.06(0.60,1.89)], [OR (95%CI), 1.38(0.97,1.97)], [OR (95%CI), 0.99(0.72,1.37)]. Among the women with AMH levels ≤ 3 ng/ml and with were aged < 30years, regardless of BMI, the possibility of live birth was similar between the two protocols [OR (95%CI), 1.02(0.68,1.54)], [OR (95%CI), 1.43(0.68,2.98)]. Moreover, among women with AMH levels ≥ 6ng/ml, the possibility of live birth was similar between the two protocols [OR (95%CI),1.42(0.75,2.69)], [OR (95%CI),1.02(0.19,5.35)], [OR (95%CI), 1.68(0.81,3.51)], [OR (95%CI), 0.51(0.10,2.55)].
Conclusions: The suitability of the GnRH agonist long-acting protocol or GnRH antagonist protocol to infertility patients is dependent on specific biological characteristics of the patients.
In vitro fertilization and embryo transfer (IVF-ET) is the most commonly patronized treatment option for women experiencing infertility. This is attributable to the increase in pregnancy rates of patients undergoing IVF-ET. A key to the improvement in pregnancy rate is the application of the controlled ovarian stimulation (COS) protocols (1, 2). Among the COS protocols that have been developed are the gonadotropin-releasing hormone (GnRH) agonist long protocol and the GnRH antagonist protocol (2, 3). The GnRH agonist long-acting protocol is one of the mainstream protocols of COS in China because of its advantages such as effectively improving endometrial receptivity and increasing the clinical pregnancy rate of fresh IVF cycles (4, 5). The GnRH antagonist protocol, on the other hand, is widely used because of its shorter duration of stimulation and its association with a low incidence of ovarian hyperstimulation syndrome (OHSS) (5–7).
Since both protocols are advantageous to some extent, clinicians have become indecisive about which one to fully rely upon. Previous studies that compared both protocols on live birth rates yielded seemingly conflicting findings. Yang et al. reported (8) that live birth rate, clinical pregnancy rate and implantation rate of the GnRH agonist long-acting protocol are significantly higher than those of the antagonist protocol. However, Wang et al. found (9) that there is no significant difference in live birth rate between both protocols in patients with normal ovarian reserves. Li et al. (10) observed that in patients with poor ovarian response, the GnRH agonist long-acting protocol is associated with higher live birth rates than the GnRH-antagonist protocol. These seemingly conflicting reports, together with the confounding factors such as variation in the basic characteristics of women, make it difficult to decide on which of the two protocols is optimal for IVF women. Hence, it is necessary to implement individualized COS protocols in accordance with the specific characteristics of the patients.
An important clinical feature of female infertility is ovarian reserve, which is also a crucial factor used in selecting the most appropriate COS protocol (11–13). Several studies have shown that AMH is a reliable marker of ovarian reserve (14–18), and has a significant correlation with age (19, 20). Due to this, AMH, combined with age, is commonly used to evaluate ovarian reserve in clinical practice.
It has been found that increased body mass index (BMI) affects the success of IVF (21, 22) as well as live births following IVF (23). Also, it has been observed that serum AMH is positively correlated with BMI in normal weight women with normal ovarian reserve (24). However, in women with polycystic ovary syndrome (PCOS), serum AMH was observed to correlate negatively with BMI (25). These findings indicate that BMI and AMH serum levels should be taken into account when establishing an individualized COS protocol. Thus, in this study, we retrieved the data of infertile women who had been exposed to the GnRH agonist long-acting protocol or the antagonist protocol, and assessed their live birth rate by combining the basic characteristics: age, BMI and AMH levels. Our findings would provide reference for clinical guidance and treatment of female infertility.
Women who had undergone their first IVF/ICSI cycles between January, 2018 and August, 2021 at the Chengdu Xinan Gynecology Hospital and Chengdu Jinjiang Hospital for Women’s and Children’s Health were retrospectively identified in the institutional database. Only women who received COS with GnRH agonist long-acting protocol or the GnRH antagonist protocol and received fresh embryo transfer were included in this study. Exclusion criteria were abnormal results on parental karyotyping, missing lab data, and incomplete live birth information. Patients' flow chart detailing the whole process is shown in Figure 1.
Each woman received a GnRH agonist (Diphereline, 3.75mg, Beaufort-Ipson, France) on the 2nd to 4th day of menstruation (follicular phase). Serum levels of sex hormones and ultrasound assessment of developing follicles were monitored on the 28th to the 35th day after GnRH agonist administration. The following criteria were used: (a) endometrial thickness < 5mm, (b) estradiol (E2) < 50pmol/L, luteinizing hormone (LH) < 5IU/L follicle-stimulating hormone (FSH) <5 IU/L, progesterone (P) of <1 ng/ml, (c) no functional cyst, (d) follicle size 3–5 mm under ultrasound. In accordance with the patient’s age, BMI, antral follicle number (AFC) and AMH levels, we determined the initial dose of gonadotropin (Gn) that could control ovulation. The dosage was adjusted continually according to the patient’s ovarian reaction and follicular growth. 250 µg of recombinant human chorionic gonadotropin (rhCG, Merck Schlano, Germany) were given to each woman until two to three ovarian follicles were, at least, 17–18 mm in diameter. Oocyte retrieval was performed 36 hours post-hCG injection.
In accordance with the patient’s age, BMI, antral follicle number (AFC) and AMH levels, recombinant FSH 100 ~ 300 IU/d (rFSH, Gonal-F, Merck Serono S.A., Switzerland) administration was done from the 2nd to the 4th day of the menstrual cycle. This was followed by Gn administration. The Gn dosage was adjusted as the follicles developed. A daily dose of 0.25 mg GnRH-ant (Ganerik acetate, Merck Serono, Switzerland) was started either on the 6th day of rFSH stimulation until the hCG injection or when the dominant follicle’s diameter was ≥ 12-14 mm. The induction of ovulation was performed by administering the women with 250 µg of rhCG (Merck Schlano, Germany) or with the 0.2 mg of Decapeptyl either alone or in combination with, 2000 IU of urinary hCG [Merck Schlano]). This was done during the period when two to three ovarian follicles were, at least, 17–18 mm in diameter. Oocyte retrieval was performed 36 hours after the ovulation induction.
On the 3rd to 5th day after fertilization, 1 to 2 of grade I-II high-quality embryos were selectively transferred. Embryo grading was done in accordance with the proceedings of the Istanbul consensus (26). The luteal phase support was started on the day when the oocytes were retrieved with 200 mg intravaginal progesterone soft capsules for 8 hours/times. 20mg of dydrogesterone (Dupbaston, Dutch) was taken twice on each day.
The primary outcome measure was the live birth which was defined as the delivery of any living baby at or after 28 weeks of pregnancy during the first embryo transfer. Live birth rate = number of live birth cycles/number of embryo transfer cycles. The secondary outcomes were biochemical pregnancy rate, clinical pregnancy rate, incidence of ovarian hyperstimulation syndrome (OHSS), number of retrieved oocytes, number of metaphase II (MII) oocytes, and number of 2 pronuclear (2PN) embryos. The biochemical pregnancy was defined as the serum β-HCG>25U/L 14 days after embryo transfer Clinical pregnancy, defined as the presence of gestational sac or fetal heart, was confirmed with transvaginal ultrasound 28 days after embryo transfer. The OHSS was defined according to the Golan et al’ criteria (27).
Propensity Score Matching (PSM) was used in data analysis to balance the baseline and improve the comparability between GnRH agonist long-acting protocol group and GnRH antagonist protocol group. The variables in PSM model included female age, BMI, duration of infertility, type of infertility, basal sex hormone (E2, P, FSH, LH), AFC, AMH, insemination methods, the number of good quality embryos transferred and the type of embryos transferred. A 1:1 nearest neighbor matching method with caliper (0.1) was used to match data between groups.
Continuous variables are expressed as mean ± SD or median (IQR); and Categorical variables are expressed as number (n) and percentage (%). Normality was checked through Shapiro-Wilk normality test. Mann-Whitney U test or Student’s t-tests were used for continuous variables and the Chi-square test was used for categorical variables.
Multivariate logistic regression analysis was performed to compare the live birth rates between the two protocols. Additional analyses were performed after stratification of the participants by age (age<30 years vs age≥30 years) (28), BMI (BMI<24 kg/m2 vs BMI≥24 kg/m2), AMH levels (AMH ≤ 3ng/ml vs 3ng/ml<AMH<6ng/ml vs AMH≥6ng/ml) (29) and also after combining the above three parameters. All analyses were performed using the Statistical Package for the Social Sciences (Version 25.0; SPSS, Chicago, IL). P <0.05 was used to indicate a significant statistical difference.
The demographic characteristics, cycle characteristics and pregnancy outcomes of the study participants before and after PSM are shown in Tables 1, 2. Before PSM, a total of, 8579 cycles were included in this study. Significant differences in the comparison of baseline characteristics were observed between two groups in age, BMI, AMH, AFC, basal FSH, basal LH, basal E2, basal P, Gn dose, duration of Gn, number of good quality embryos transferred., 4972 of the cycles used the GnRH agonist long-acting protocol and generated 45.09% of live birth rate while, 3607 of the cycles used the GnRH antagonist protocol and generated 38.70% of live birth rate. After 1:1 matching, a total of, 6608 cycles were analyzed in this study. There were no significant differences in age, BMI, basal FSH, basal LH, and the number of good quality embryos transferred between the two groups. However, the GnRH agonist long protocol group still received a higher gonadotropin dosage (1875IU vs, 1800IU) and longer gonadotropin exposure duration (12 vs 9) than the antagonist protocol group., 3304 of the cycles used the GnRH agonist long-acting protocol and generated 44.04% of live birth rate while, 3304 of the cycles used the GnRH antagonist protocol and generated 38.32% of live birth rate. The live birth rate of the GnRH agonist long-acting protocol group was significantly higher than that of the GnRH antagonist protocol group before and after matching(P<0.001).
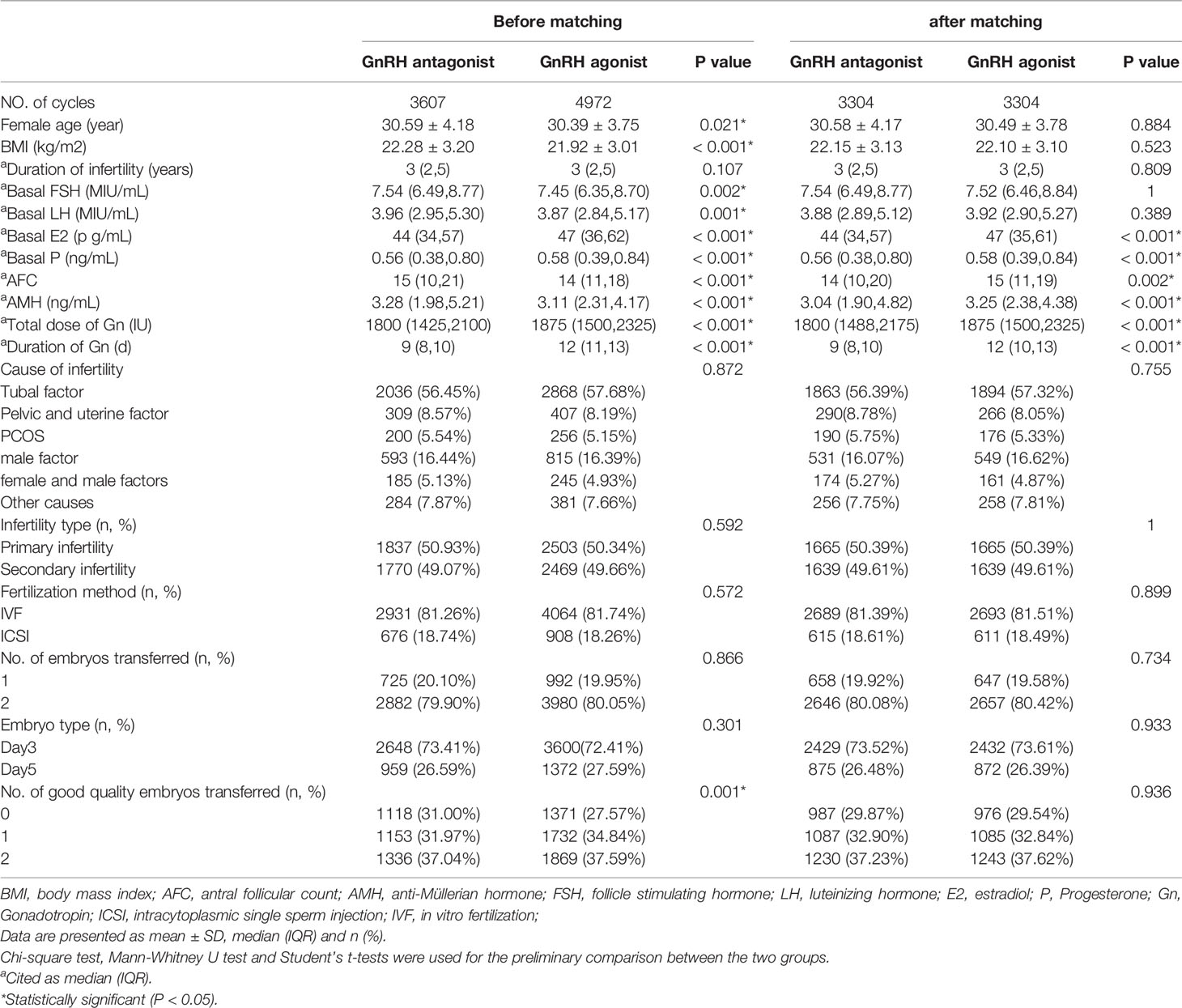
Table 1 Comparison of baseline parameters between the GnRH agonist long-acting protocol and GnRH antagonist protocol and after PS matching.
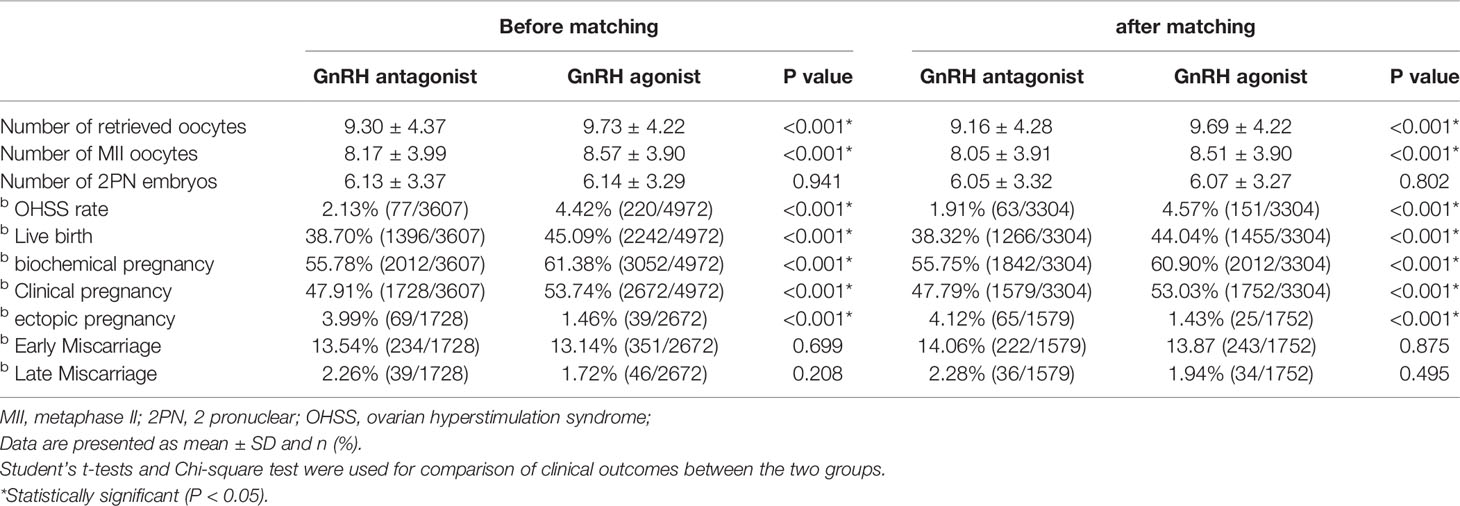
Table 2 Comparison of clinical outcomes between the GnRH agonist long-acting protocol and GnRH antagonist protocol and after PS matching.
After matching, the number of oocytes retrieved (9.69 ± 4.22 vs 9.16 ± 4.28), the mature eggs number (8.51 ± 3.90 vs 8.05 ± 3.91), the biochemical pregnancy rate (60.90% vs 55.75%), the clinical pregnancy rate (53.03% vs 47.79%) and the incidence of OHSS (4.57% vs 1.91%) were higher in the GnRH agonist long-acting protocol group than in the antagonist protocol group. Nonetheless, the ectopic pregnancy rates (1.43% vs 4.12%) in the GnRH agonist long-acting protocol group were significantly lower than those of the GnRH antagonist protocol group. There was no significant difference in the two-Pro-Nuclei (2PN) fertilized eggs number (6.07 ± 3.27 vs 6.05 ± 3.32), early miscarriage (13.87% vs 14.06%) and late miscarriage rate (1.94% vs 2.28%) between two groups (Table 2).
Before and after matching, and after adjusting for potential confounding factors (such as age, BMI, AMH, AFC, basal FSH, basal LH, basal E2, basal P, Gn dose, duration of Gn, number of good quality embryos transferred), the multivariate logistic regression analysis showed that the GnRH agonist long-acting protocol was associated with a higher possibility of having live birth than that of the GnRH antagonist protocol [OR (95%CI), 1.25(1.01,1.53)], P<0.001; [OR (95%CI), 1.20(1.00,1.43)], P=0.002 (Table 3).
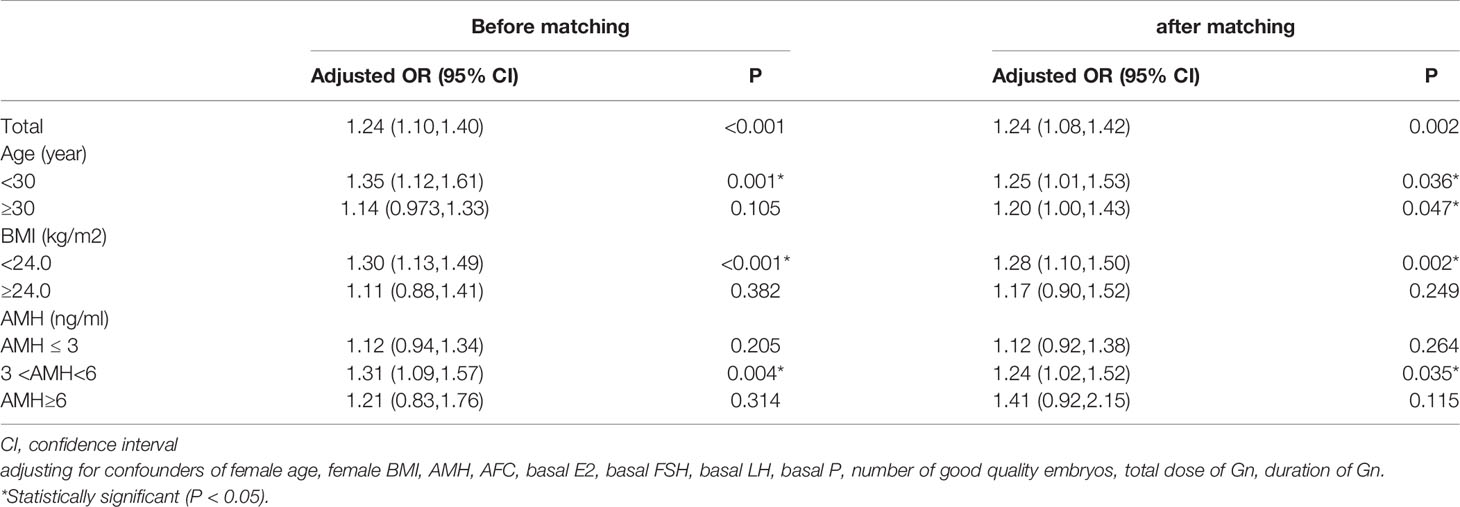
Table 3 Comparison of live birth rate of the GnRH agonist long-acting protocol and GnRH antagonist protocol using multivariable logistic regression analysis in subgroup women with different BMI, AMH and age and after PS matching. (the GnRH antagonist protocol as a reference).
To find the live birth rate of the GnRH agonist long or antagonist protocols in patients with different characteristics, we carried out a further analysis by stratifying the patients according to their ages, BMIs and AMH levels. After matching, the multivariate logistic regression analysis showed a significantly higher possibility of having live births of each layer stratified by age in the GnRH agonist long protocol group than in the GnRH antagonist protocol group [OR (95%CI), 1,24(1.10,1.40)], [OR (95%CI), 1.24(1.08,1.42)]. For women with BMI <24kg/m2, the GnRH agonist long-acting protocol was associated with a higher opportunity of getting live births [OR (95%CI), 1.28(1.10,1.50)]; for women with overweight (BMI≥24kg/m2), the two protocols had similar live birth rates [OR (95%CI),1.17(0.90,1.52)]. When the population was stratified by AMH, for women with normal ovarian reserves (3ng/ml<AMH<6ng/ml), we found a significantly higher possibility of live birth in the GnRH agonist long protocol group than in the GnRH antagonist protocol group [OR (95%CI), 1.24(1.02,1.52)]; Among women with AMH≥ 3ng/ml or AMH≥ 6ng/ml, the chances of getting live births were similar between the two groups. [OR (95%CI),1.12(0.92,1.38)], [OR (95%CI), 1.41(0.92,2.15)] (Table 3).
After matching, the study population was divided into 12 groups according to the combination of AMH levels, age and BMI (Table 4). The multivariate logistic regression analysis showed that for younger women (age<30 years old), regardless of their BMI and ovarian reserves, the GnRH agonist long-acting protocol was more likely to elicit live births than the antagonist protocol, although the difference was not statistically significant. However, among women who were above 30 years old and who had normal ovarian reserves (3ng/ml<AMH<6ng/ml) and variable BMI, the abilities of the two protocols to elicit live births may differ significantly. For women who had AMH levels from 3ng/ml to 6ng/ml (3ng/ml<AMH<6ng/ml), were aged ≥ 30 years old and had BMI ≥ 24kg/m2, the GnRH agonist long-acting protocol was more likely to have live births than the antagonist protocol [OR (95%CI), 2.13(1.19,3.80)]; while among the women with normal ovarian reserves, were aged ≥30 years old and had BMI < 24kg/m2, the chances to have live births were similar between the two protocol groups [OR (95%CI),0.99(0.72,1.37)]. Among women with AMH ≤ 3ng/ml, aged ≥ 30 years old and with BMI < 24kg/m2, the GnRH agonist long-acting protocol had a higher possibility to live births than the antagonist protocol [OR (95%CI), 1.41(1.05,1.91)]. Interestingly, for women with AMH ≤ 3ng/ml, age ≥ 30 years old and BMI≥ 24kg/m2, the GnRH agonist long-acting protocol had a lower possibility of live births the antagonist protocol [OR (95%CI), 0.54(0.32,0.90)]. Among the women who had AMH level ≥ 6ng/ml, aged ≥ 30 years old and had BMI < 24kg/m2, the possibilities to have live births were similar between the two protocols [OR (95%CI), 1.68(0.81,3.51)] However, among the women who had AMH level ≥ 6ng/ml, aged ≥ 30 years old and with BMI≥ 24kg/m2, the GnRH agonist long-acting protocol had a lower possibility of eliciting live birth than the antagonist protocol [OR (95%CI), 0.51(0.10,2.55)]. Before matching, and after adjusting potential confounding factors, the multivariate logistic regression analysis showed that for younger women (age<30 years old), who had normal ovarian reserves and with BMI < 24kg/m2, the GnRH agonist long-acting protocol was more likely to elicit live births than the antagonist protocol [OR (95%CI),1.58(1.16, 2.16)] (Supplemental Table 1).
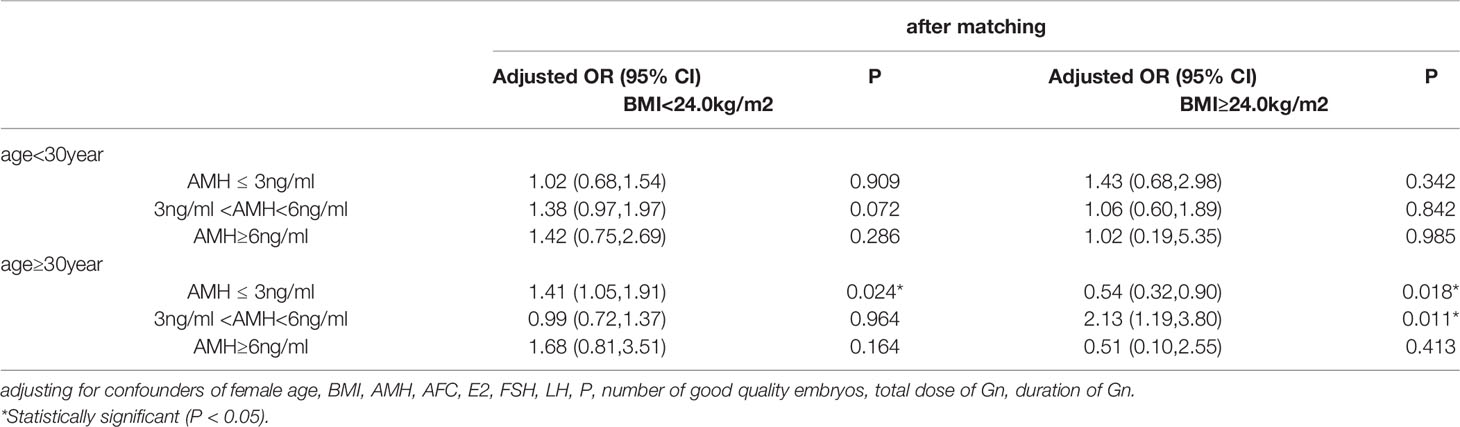
Table 4 Multivariable logistic regression analysis of live birth rate of the GnRH agonist long-acting protocol and GnRH antagonist protocol for women with different AMH, Age and BMI (after PS matching) (the GnRH antagonist protocol group as a reference).
Providing an individualized IVF-ET protocol, via individual characteristics, so as to maximize the rate of pregnancy and live births while reducing OHSS and adverse pregnancy outcomes, is still a big challenge in clinical medicine. In this study, we first analyzed the variables of the participants without any special stratification; and observed that the GnRH agonist long-acting protocol group had higher live birth rates, biochemical pregnancy rates and clinical pregnancy rates than the antagonist protocol group (Tables 2, 3). This is consistent with the findings of other studies (4, 30) which showed that in the fresh cycle, the GnRH agonist long-acting protocol group had a higher clinical pregnancy rate and implantation rate than the GnRH antagonist protocol group. The mRNA and protein levels of HOXA10, MEIS1 and LIF, which are markers of uterine development and endometrial receptivity (31, 32), were found to be higher in the GnRH agonist long-acting protocol group than in the antagonist protocol group. This indicates that the GnRH agonist long-acting protocol, unlike the antagonist protocol, may have a less association with the impairment of the patients’ endometrial receptivity. In addition, we found that the GnRH agonist long-acting protocol was associated with a higher risk of OHSS (4.57% vs 1.91%), which is consistent with Toftager et al’s results (33). These findings indicate that the GnRH agonist long-acting protocol, rather than the GnRH antagonist protocol, may be more beneficial to women who undergo ART therapy.
To date, there is no single COS solution that works for all infertile women. Zhang et al. (34) indicated that the choice of COS protocol is highly dependent on ovarian reserve and age. Marci et al. (35) reported that high BMI could impair the ovarian response to exogenous gonadotropins. However, it is not a common practice to combine these factors to select a COS protocol for infertile women. Therefore, to explore whether women with different characteristics are more suitable for any protocol, we divided the study population into several groups according to the ages, AMH levels and BMI of the study participants. We found that among women with normal ovarian reserve, BMI < 24kg/m2 and age <30 years old, the GnRH agonist long-acting protocol was associated with a higher possibility of having live birth than that of the GnRH antagonist protocol [OR (95%CI),1.58(1.16,2.16)] (Supplemental Table 1). Grow et al. (36) reported that good-prognosis patients had higher live birth rate with the GnRH agonist long-acting protocol than with the antagonist protocol [OR (95%CI),1.13(1.03,1.25)]. The results of this study are consistent with our findings. Additionally, in overweight women (BMI ≥ 24 kg/m2) with normal ovarian reserve, the women aged ≥ 30 years old had higher live birth rates with the GnRH agonist long-acting protocol than with the antagonist protocol [OR (95%CI), 2.13(1.19,3.80)]. Also, our results showed that a higher number of oocytes was retrieved in the GnRH agonist long-acting protocol group than in the antagonist protocol group. Since a decline in the number of oocyte as well as the increase of age, old age (37) and embryo aneuploidy (38) are crucial factors of infertility, the GnRH agonist long-acting protocol is recommended for infertile women with normal ovarian reserve, who have BMI<24kg/m2 and are aged <30 years old as well as those who have normal ovarian reserve have BMI ≥ 24kg/m2 and are aged ≥ 30 years.
Further, in women with normal ovarian reserve (3ng/ml < AMH < 6ng/ml), with BMI < 24kg/m2 and are aged ≥30 years old or with BMI ≥ 24 kg/m2 and with ages < 30 years old, the possibilities to have live births were similar between the two protocols [OR (95%CI), 0.99(0.72,1.37)], [OR (95%CI), 1.06(0.60,1.89)]. Our results are consistent with that of a meta-analysis (9) which showed no difference between the agonist protocol group and the antagonist protocol group of women with normal ovarian reserves (OR [95% CI] = 0.95 [0.74, 1.09], P = 0.27). Al-Inany et al. [35] found that compared to the GnRH agonist long-acting protocol, the antagonist protocol significantly reduced the incidence of any grade of OHSS (OR 0.61, 95% C 0.51 to 0.72; 36 RCTs, n = 7944, I2 = 31%, moderate quality evidence) without affecting the live birth rate (OR 1.02, 95% CI 0.85 to 1.23; 12 RCTs, n = 2303, I2 = 27%, moderate quality evidence). Therefore, the antagonist protocol is recommended for infertile women with normal ovarian reserve, with BMI < 24kg/m2 and with ages ≥30 years or with BMI ≥ 24kg/m2 and with ages < 30 years.
Other studies (33, 39–41) have reported that the GnRH antagonist protocol is safer for women with a low and high ovarian reserve, just that live birth rates are similar in both protocols. Our study with larger sample size further revealed that, regardless of age and BMI, among women with relatively high ovarian reserve (AMH ≥ 6 ng/ml), the two protocols had similar live birth rates. Particularly, in women with relatively high ovarian reserve (AMH ≥ 6 ng/ml), with BMI ≥ 24kg/m2 and have ages ≥30 years, the possibility of getting live birth in the GnRH agonist protocol was lower although the difference was not significant [OR (95%CI), 0.54(0.32,0.90)]. Moreover, among younger (age <30 years) women with relatively low ovarian reserve (AMH≤ 3ng/ml), regardless of BMI, the live birth rate was similar in the two protocols. Therefore, the GnRH antagonist protocol is strongly recommended for women with the above characteristics.
Li et al. (10) reported that among women in POSEIDON group 4 of advanced age and have diminished ovarian reserves, the GnRH agonist long-acting protocol and the antagonist protocol achieved comparable live birth rates. However, our study found that among the women with relatively low ovarian reserve (AMH ≤ 3ng/ml), with ages ≥ 30 years old and with BMI < 24kg/m2, the GnRH agonist long-acting protocol was more likely to have live births than the antagonist protocol [OR (95%CI), 1.41(1.05,1.91)]; while among women with relatively low ovarian reserve (AMH ≤ 3ng/ml), with age ≥ 30 years old and with BMI ≥ 24kg/m2, the GnRH agonist long-acting protocol had a lower possibility of live birth than the antagonist protocol [OR (95%CI), 0.54(0.32,0.90)]. These indicate that BMI is a vital factor to be considered in a personalized COS protocol. Unfortunately, to the best of our knowledge, there have been no studies comparing the GnRH agonist long-acting protocol and the GnRH antagonist protocol in women who have low ovarian reserve and who have different BMIs. Rabinson et al. (42) showed that in general women with BMI < 25kg/m2, the GnRH agonist protocol had a higher pregnancy rate. Although the ovarian reserve of women included in the study was not selected, the trend of their results was consistent with ours. These findings show that the GnRH agonist long-acting protocol may be more suitable for women with relatively low ovarian reserve (AMH ≤ 3ng/ml), with ages ≥ 30 years old and with BMI < 24kg/m2. Nevertheless, among women with relatively low ovarian reserve (AMH ≤ 3ng/ml), with age ≥ 30 years old and with BMI ≥ 24kg/m2, the GnRH antagonist protocol is recommended since it can help avoid the excessive suppression of the pituitary-gonadal axis and the concentrations of endogenous FSH and LH (43).
To our knowledge, this is the first study to compare the live birth rates of the GnRH agonist long-acting protocol and antagonist protocol in women with different characteristics by combining BMI with ovarian reserve markers. In spite of all the efforts to control bias, this study is inherently limited by the review of a retrospectively collected data set. In addition, this study did not follow up to the frozen embryo cycle, and could not provide relevant indicators such as cumulative live birth rate.
Among infertile women who receive fresh embryo transfer after the first IVF treatment, the GnRH agonist long-acting protocol is recommended for women with normal ovarian reserve (3ng/ml < AMH < 6ng/ml), with BMI<24 kg/m2 and with ages<30 years, and for those with normal ovarian reserve (3ng/ml < AMH < 6ng/ml), with BMI ≥ 24 kg/m2 and are aged above 30 years. It is also recommended for women with BMI < 24kg/m2 and with ages<30 years whose AMH levels are ≤ 3ng/ml. However, among the remaining infertile women in the cohort, the antagonist protocol may suite them because of the lower incidence of ovarian hyperstimulation syndrome, duration and dosage of Gn. Taken together, our results may provide a personalized recommendation in COS protocol selection. The recommendation of two protocols for women in different characters is shown in Supplemental Table 2.
The data used in this article were obtained from the Chengdu Xinan Gynecology Hospital and Chengdu Jinjiang Hospital for Women’s and Children’s Health by request. Upon data request, the corresponding author would obtain permission from the Chengdu Xinan Gynecology Hospital and Chengdu Jinjiang Hospital for Women’s and Children’s Health before sharing them.
M-XC contributed to study design, data collection, statistical analysis and drafting of the manuscript. QW assisted with data collection and interpretation. X-JT reviewed the analyzed results. Z-HZ reviewed the analyzed results and revised the manuscript. X-QM, TL, QF, YJ, X-YL, L-HG, LZ and QW provided ART-related clinical theory and technical support. Y-BD and Enoch Appiah Adu-Gyamfi critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the the National Natural Science Foundation of China (Grant No. 81971391), National Key Research and Development Program of China (2017YFC1002001), and the Chongqing Science and Technology Bureau (Grant No. cstc2019jxjl130030).
The authors have no conflict of interest to declare.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to all the physicians, nurses and laboratory staff (at the Chengdu Xinan Gynecology Hospital and Chengdu Jinjiang Hospital for Women’s and Children’s Health) who contributed to this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.899000/full#supplementary-material
1. Bosch E, Ezcurra D. Individualised Controlled Ovarian Stimulation (Icos): Maximising Success Rates for Assisted Reproductive Technology Patients. Reprod Biol Endocrinol (2011) 9:82. doi: 10.1186/1477-7827-9-82
2. Racca A, Drakopoulos P, Neves AR, Polyzos NP. Current Therapeutic Options for Controlled Ovarian Stimulation in Assisted Reproductive Technology. Drugs (2020) 80(10):973–94. doi: 10.1007/s40265-020-01324-w
3. Pacchiarotti A, Selman H, Valeri C, Napoletano S, Sbracia M, Antonini G, et al. Ovarian Stimulation Protocol in Ivf: An Up-to-Date Review of the Literature. Curr Pharm Biotechnol (2016) 17(4):303–15. doi: 10.2174/1389201017666160118103147
4. Geng Y, Xun Y, Hu S, Lai Q, Jin L. Gnrh Antagonist Versus Follicular-Phase Single-Dose Gnrh Agonist Protocol in Patients of Normal Ovarian Responses During Controlled Ovarian Stimulation. Gynecol Endocrinol (2019) 35(4):309–13. doi: 10.1080/09513590.2018.1528221
5. Depalo R, Jayakrishan K, Garruti G, Totaro I, Panzarino M, Giorgino F, et al. Gnrh Agonist Versus Gnrh Antagonist in In Vitro Fertilization and Embryo Transfer (Ivf/Et). Reprod Biol Endocrinol (2012) 10:26. doi: 10.1186/1477-7827-10-26
6. Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-Releasing Hormone Antagonists for Assisted Conception: A Cochrane Review. Reprod Biomedicine Online (2007) 14(5):640–9. doi: 10.1016/s1472-6483(10)61059-0
7. Zhang Y, Liu L, Qin J, Huang H, Xue L, Wang S, et al. Evaluation of Gnrh Antagonist Pretreatment Before Ovarian Stimulation in a Gnrh Antagonist Protocol in Normal Ovulatory Women Undergoing Ivf/Icsi: A Randomized Controlled Trial. Reprod Biol Endocrinol (2021) 19(1):158. doi: 10.1186/s12958-021-00836-8
8. Yang R, Guan Y, Perrot V, Ma J, Li R. Comparison of the Long-Acting Gnrh Agonist Follicular Protocol With the Gnrh Antagonist Protocol in Women Undergoing In Vitro Fertilization: A Systematic Review and Meta-Analysis. Adv Ther (2021) 38(5):2027–37. doi: 10.1007/s12325-020-01612-7
9. Wang R, Lin S, Wang Y, Qian W, Zhou L. Comparisons of Gnrh Antagonist Protocol Versus Gnrh Agonist Long Protocol in Patients With Normal Ovarian Reserve: A Systematic Review and Meta-Analysis. PLoS One (2017) 12(4):e0175985. doi: 10.1371/journal.pone.0175985
10. Li F, Ye T, Kong H, Li J, Hu L, Jin H, et al. Efficacies of Different Ovarian Hyperstimulation Protocols in Poor Ovarian Responders Classified by the Poseidon Criteria. Aging (2020) 12(10):9354–64. doi: 10.18632/aging.103210
11. Haahr T, Esteves SC, Humaidan P. Individualized Controlled Ovarian Stimulation in Expected Poor-Responders: An Update. Reprod Biol Endocrinol (2018) 16(1):20. doi: 10.1186/s12958-018-0342-1
12. Pilsgaard F, Grynnerup AG, Lossl K, Bungum L, Pinborg A. The Use of Anti-Mullerian Hormone for Controlled Ovarian Stimulation in Assisted Reproductive Technology, Fertility Assessment and -Counseling. Acta Obstet Gynecol Scand (2018) 97(9):1105–13. doi: 10.1111/aogs.13334
13. Vazquez AC, Rodriguez J, Algara ALC, Garcia JDM. Correlation Between Biochemical, Ultrasonographic and Demographic Parameters With Ovarian Response to Ivf/Icsi Treatments in Mexican Women. JBRA Assist Reprod (2021) 25(1):4–9. doi: 10.5935/1518-0557.20200040
14. Alipour F, Rasekhjahromi A, Maalhagh M, Sobhanian S, Hosseinpoor M. Comparison of Specificity and Sensitivity of Amh and Fsh in Diagnosis of Premature Ovarian Failure. Dis Markers (2015) 2015:585604. doi: 10.1155/2015/585604
15. Elgindy EA, El-Haieg DO, El-Sebaey A. Anti-Mullerian Hormone: Correlation of Early Follicular, Ovulatory and Midluteal Levels With Ovarian Response and Cycle Outcome in Intracytoplasmic Sperm Injection Patients. Fertility sterility (2008) 89(6):1670–6. doi: 10.1016/j.fertnstert.2007.05.040
16. Kotlyar AM, Seifer DB. Ethnicity/Race and Age-Specific Variations of Serum Amh in Women-A Review. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.593216
17. Celik H, Bildircin D, Guven D, Cetinkaya MB, Alper T, Batuoglu AS. Random Anti-Mullerian Hormone Predicts Ovarian Response in Women With High Baseline Follicle-Stimulating Hormone Levels : Anti-Mullerian Hormone in Poor Responders in Assisted Reproductive Treatment. J assisted Reprod Genet (2012) 29(8):797–802. doi: 10.1007/s10815-012-9794-y
18. Tal R, Seifer DB. Ovarian Reserve Testing: A User's Guide. Am J Obstet Gynecol (2017) 217(2):129–40. doi: 10.1016/j.ajog.2017.02.027
19. Cui L, Qin Y, Gao X, Lu J, Geng L, Ding L, et al. Antimullerian Hormone: Correlation With Age and Androgenic and Metabolic Factors in Women From Birth to Postmenopause. Fertility Sterility (2016) 105(2):481–5.e1. doi: 10.1016/j.fertnstert.2015.10.017
20. Kedem A, Yung Y, Yerushalmi GM, Haas J, Maman E, Hanochi M, et al. Anti Müllerian Hormone (Amh) Level and Expression in Mural and Cumulus Cells in Relation to Age. J Ovarian Res (2014) 7:113. doi: 10.1186/s13048-014-0113-3
21. Koning AM, Kuchenbecker WK, Groen H, Hoek A, Land JA, Khan KS, et al. Economic Consequences of Overweight and Obesity in Infertility: A Framework for Evaluating the Costs and Outcomes of Fertility Care. Hum Reprod Update (2010) 16(3):246–54. doi: 10.1093/humupd/dmp053
22. Chen R, Chen S, Liu M, He H, Xu H, Liu H, et al. Pregnancy Outcomes of Pcos Overweight/Obese Patients After Controlled Ovarian Stimulation With the Gnrh Antagonist Protocol and Frozen Embryo Transfer. Reprod Biol Endocrinol (2018) 16(1):36. doi: 10.1186/s12958-018-0352-z
23. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female Obesity Is Negatively Associated With Live Birth Rate Following Ivf: A Systematic Review and Meta-Analysis. Hum Reprod Update (2019) 25(4):439–51. doi: 10.1093/humupd/dmz011
24. Albu D, Albu A. The Relationship Between Anti-Mullerian Hormone Serum Level and Body Mass Index in a Large Cohort of Infertile Patients. Endocrine (2019) 63(1):157–63. doi: 10.1007/s12020-018-1756-4
25. Kriseman M, Mills C, Kovanci E, Sangi-Haghpeykar H, Gibbons W. Antimullerian Hormone Levels Are Inversely Associated With Body Mass Index (Bmi) in Women With Polycystic Ovary Syndrome. J assisted Reprod Genet (2015) 32(9):1313–6. doi: 10.1007/s10815-015-0540-0
26. Alpha Scientists in Reproductive M, Embryology ESIGo. The Istanbul Consensus Workshop on Embryo Assessment: Proceedings of an Expert Meeting. Hum Reprod (Oxford England) (2011) 26(6):1270–83. doi: 10.1093/humrep/der037
27. Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian Hyperstimulation Syndrome: An Update Review. Obstet Gynecol Surv (1989) 44(6):430–40. doi: 10.1097/00006254-198906000-00004
28. Rosen MP, Johnstone E, McCulloch CE, Schuh-Huerta SM, Sternfeld B, Reijo-Pera RA, et al. A Characterization of the Relationship of Ovarian Reserve Markers With Age. Fertility Sterility (2012) 97(1):238–43. doi: 10.1016/j.fertnstert.2011.10.031
29. Keane K, Cruzat VF, Wagle S, Chaudhary N, Newsholme P, Yovich J. Specific Ranges of Anti-Mullerian Hormone and Antral Follicle Count Correlate to Provide a Prognostic Indicator for Ivf Outcome. Reprod Biol (2017) 17(1):51–9. doi: 10.1016/j.repbio.2016.12.002
30. Xia M, Zheng J. Comparison of Clinical Outcomes Between the Depot Gonadotrophin-Releasing Hormone Agonist Protocol and Gonadotrophin-Releasing Hormone Antagonist Protocol in Normal Ovarian Responders. BMC Pregnancy Childbirth (2021) 21(1):372. doi: 10.1186/s12884-021-03849-8
31. Xu B, Zhou M, Wang J, Zhang D, Guo F, Si C, et al. Increased Aif-1-Mediated Tnf-Alpha Expression During Implantation Phase in Ivf Cycles With Gnrh Antagonist Protocol. Hum Reprod (Oxford England) (2018) 33(7):1270–80. doi: 10.1093/humrep/dey119
32. Li F, Zhang M, Zhang Y, Liu T, Qu X. Gnrh Analogues May Increase Endometrial Hoxa10 Promoter Methylation and Affect Endometrial Receptivity. Mol Med Rep (2015) 11(1):509–14. doi: 10.3892/mmr.2014.2680
33. Toftager M, Bogstad J, Bryndorf T, Lossl K, Roskaer J, Holland T, et al. Risk of Severe Ovarian Hyperstimulation Syndrome in Gnrh Antagonist Versus Gnrh Agonist Protocol: Rct Including 1050 First Ivf/Icsi Cycles. Hum Reprod (Oxford England) (2016) 31(6):1253–64. doi: 10.1093/humrep/dew051
34. Zhang W, Xie D, Zhang H, Huang J, Xiao X, Wang B, et al. Cumulative Live Birth Rates After the First Art Cycle Using Flexible Gnrh Antagonist Protocol Vs. Standard Long Gnrh Agonist Protocol: A Retrospective Cohort Study in Women of Different Ages and Various Ovarian Reserve. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.00287
35. Marci R, Lisi F, Soave I, Lo Monte G, Patella A, Caserta D, et al. Ovarian Stimulation in Women With High and Normal Body Mass Index: Gnrh Agonist Versus Gnrh Antagonist. Gynecol Endocrinol (2012) 28(10):792–5. doi: 10.3109/09513590.2012.664192
36. Grow D, Kawwass JF, Kulkarni AD, Durant T, Jamieson DJ, Macaluso M. Gnrh Agonist and Gnrh Antagonist Protocols: Comparison of Outcomes Among Good-Prognosis Patients Using National Surveillance Data. Reprod Biomedicine Online (2014) 29(3):299–304. doi: 10.1016/j.rbmo.2014.05.007
37. Steiner AZ, Jukic AM. Impact of Female Age and Nulligravidity on Fecundity in an Older Reproductive Age Cohort. Fertility Sterility (2016) 105(6):1584–8.e1. doi: 10.1016/j.fertnstert.2016.02.028
38. Fritz R, Jindal S. Reproductive Aging and Elective Fertility Preservation. J Ovarian Res (2018) 11(1):66. doi: 10.1186/s13048-018-0438-4
39. Nardo LG, Bosch E, Lambalk CB, Gelbaya TA. Controlled Ovarian Hyperstimulation Regimens: A Review of the Available Evidence for Clinical Practice. Produced on Behalf of the Bfs Policy and Practice Committee. Hum Fertil (Camb) (2013) 16(3):144–50. doi: 10.3109/14647273.2013.795385
40. Oudshoorn SC, van Tilborg TC, Eijkemans MJC, Oosterhuis GJE, Friederich J, van Hooff MHA, et al. Individualized Versus Standard Fsh Dosing in Women Starting Ivf/Icsi: An Rct. Part 2: The Predicted Hyper Responder. Hum Reprod (Oxford England) (2017) 32(12):2506–14. doi: 10.1093/humrep/dex319
41. Ovarian Stimulation T, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. Eshre Guideline: Ovarian Stimulation for Ivf/Icsi(Dagger). Hum Reprod Open (2020) 2020(2):hoaa009. doi: 10.1093/hropen/hoaa009
42. Rabinson J, Meltcer S, Zohav E, Gemer O, Anteby EY, Orvieto R. Gnrh Agonist Versus Gnrh Antagonist in Ovarian Stimulation: The Influence of Body Mass Index on In Vitro Fertilization Outcome. Fertility sterility (2008) 89(2):472–4. doi: 10.1016/j.fertnstert.2007.03.007
Keywords: GnRH agonist long-acting protocol, GnRH antagonist protocol, live birth rate, ovarian reserve, body mass index
Citation: Chen M-X, Meng X-Q, Zhong Z-H, Tang X-J, Li T, Feng Q, Adu-Gyamfi EA, Jia Y, Lv X-Y, Geng L-H, Zhu L, He W, Wan Q and Ding Y-B (2022) An Individualized Recommendation for Controlled Ovary Stimulation Protocol in Women Who Received the GnRH Agonist Long-Acting Protocol or the GnRH Antagonist Protocol: A Retrospective Cohort Study. Front. Endocrinol. 13:899000. doi: 10.3389/fendo.2022.899000
Received: 18 March 2022; Accepted: 17 June 2022;
Published: 22 July 2022.
Edited by:
Yimin Zhu, Zhejiang University, ChinaReviewed by:
Yong-Jiang Zhou, Hainan Medical University, ChinaCopyright © 2022 Chen, Meng, Zhong, Tang, Li, Feng, Adu-Gyamfi, Jia, Lv, Geng, Zhu, He, Wan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei He, YW55aGV3ZWlAMTYzLmNvbQ==; Qi Wan, d2FucWkxMjNAMTYzLmNvbQ==; Yu-Bin Ding, ZGluZ3liQGNxbXUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.