
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 09 September 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.897728
This article is part of the Research Topic Diabetes and Aging: Glycemic Control, Insulin Regulation, and the Subsequent Effects View all 12 articles
 Yinjiao Zhao1†
Yinjiao Zhao1† Peiyu Song1†
Peiyu Song1† Chan Zhu1†
Chan Zhu1† Lingyun Zhang1
Lingyun Zhang1 Xiaoyu Chen2
Xiaoyu Chen2 Hui Zhang1
Hui Zhang1 Peipei Han2
Peipei Han2 Wei Ding3
Wei Ding3 Jianying Niu4
Jianying Niu4 Junli Zhao5
Junli Zhao5 Xiang Shao6
Xiang Shao6 Liming Zhang7
Liming Zhang7 Chen Yu8
Chen Yu8 Jia Xu9
Jia Xu9 Chenghu Fang1
Chenghu Fang1 Qi Guo1,2*
Qi Guo1,2*Objective: The purpose of this study was to observe the relationship between physical performance and mild cognitive impairment (MCI) in the presence or absence of type 2 diabetes in elderly hemodialysis patients.
Methods: In this multicenter cross-sectional study, 396 clinically stable and aged ≥60 years hemodialysis patients (255 men; mean age: 68.3 ± 5.9 years) were included from seven dialysis units in Shanghai, China. The Chinese version of the Modified Mini-Mental State Examination (MMSE) and the Instrumental Activities of Daily Living (IADL) scale were utilized to assess MCI. The performance-based assessments consisted of three physical tests, grip strength (GS), Timed Up and Go Test (TUGT), and 4-m walking test, which respectively represent muscle strength, mobility, and walking speed (WS). Logistic regression and multivariate linear regression were used for analysis.
Results: Hemodialysis patients with diabetes had a high prevalence of MCI (20.6%). The odds ratio (OR) of MCI for the interacted items [(TUGT) * (diabetes) and (WS) * (diabetes)] was significant (p < 0.05). In diabetes patients, TUGT was positively associated with MCI, and WS was negatively associated with MCI after adjusting covariates [OR = 0.129; 95% confidence interval (CI) = 0.028–0.704, p = 0.021]. However, no significant association was found between physical performance and MCI in the non-diabetes hemodialysis patients (p > 0.05). Further analysis showed that TUGT was negatively associated with attention and calculation and language. WS was positively associated with recall and language in diabetic hemodialysis patients.
Conclusions: Physical performance was associated with MCI in diabetic hemodialysis patients rather than the non-diabetes group. Whether increasing mobility or WS can positively influence MCI in individuals with type 2 diabetes requires further study.
Mild cognitive impairment (MCI) represents a transitional stage between normal age-related decline in cognitive function and dementia and is more prevalent in the elderly population and hemodialysis patients than the general population (1). As a therapeutic window, the latest guidelines show that MCI patients are still more likely to improve or maintain cognitive function (2). The decline in cognitive function is often influenced by many factors, such as age, education, vascular diseases, and chronic diseases (diabetes, hypertension) (3). Considering that chronic kidney disease (CKD) patients are usually accompanied by the protein-energy wasting and metabolic disorders that lead to impaired muscle mass and a decline in physical performance (4), the relationship between physical performance and MCI in elderly hemodialysis patients deserves further in-depth study.
Diabetes is considered to be a major cause of end-stage renal disease and appears to be increasing rapidly (5). Having prediabetes and diabetes was significantly associated with lower health-related quality of life relative to normal glucose tolerance (6). Data from a well-functioning population showed that compared with those without diabetes, those with diabetes exhibited lower performance on objective measures of lower-extremity function (7). The latest study showed a strong and significant correlation between 5-m gait speed and glycemia (8). Physical activity and sedentary behavior are associated with biomarkers of endothelial dysfunction, and the associations were stronger in (pre)diabetes than in normal glucose metabolism (9). In addition, a previous study has indicated a link of diabetes to an increased risk of MCI (10) and shown that the risk of incident MCI is higher in people with type 2 diabetes than that in those without diabetes (11). Pasquale et al. found a significant correlation between 5-m gait speed test and Montreal Cognitive Assessment (MoCA) score in frail diabetic older adults (12). Due to insufficient insulin secretion or insulin resistance, insulin-stimulated glucose uptake is markedly reduced in skeletal muscle and a hyperglycemic condition leads to endothelial and cerebral microvascular dysfunction (13–15), which may affect both physical performance and cognitive function. Previous and our studies have reported that poor physical performance is significantly associated with MCI in community-dwelling older adults (16–18). However, whether the presence of diabetes alters the relationship between physical performance and MCI is not yet known.
Therefore, this study aimed to explore the relationship between physical performance and MCI in elderly hemodialysis patients with and without diabetes. According to the above indications, we hypothesized that the presence of diabetes would lead to poorer physical performance and high prevalence of MCI, and different conditions may influence the association between physical performance and MCI. Moreover, it also investigated the association between physical performance [muscle strength, mobility, and walking speed (WS)] and specific cognitive functions in the presence or absence of type 2 diabetes to provide evidence for clinicians to effectively manage MCI in hemodialysis patients.
The multicenter cross-sectional study recruited patients who underwent hemodialysis in seven dialysis units in Shanghai, China [ChiCTR1900027039] between July 2020 and April 2021. Hemodialysis is a process in which blood is drained outside the body through a circulatory line, exchanged through a dialyzer, and the purified blood is returned to the body. Vascular access modalities for hemodialysis included fistulas and catheters, and dialyzer models included F14, LOPS15, FX80, etc. Patients aged 60 years or older who were on hemodialysis for 4 h per session, three times a week, and for more than 3 months were included in the study. Participants with the following conditions were excluded: 1) unable to communicate with interviewers or grant informed consent; 2) unable to complete the physical performance test; 3) had a known diagnosis of dementia, psychiatric disorders, or other neurodegenerative diseases; and 4) no blood sample collection. Following these exclusions, the final analyzed population comprised 396 subjects (255 men, 141 women). All participants are required to complete an annual health screening and a detailed questionnaire on lifestyle and disease history. The study was approved by the Ethics Committee of the Shanghai University of Medicine and Health Sciences, and the methods were carried out in accordance with the principles of the Declaration of Helsinki. All participants were informed and signed consent prior to enrollment in the study.
Demographic characteristics (including age, gender, education level, registered residence, and marital status) and health behaviors (including smoking, drinking, and sleep duration) were obtained from a standardized questionnaire by face-to-face interview. Physical activity was assessed using the short form of the International Physical Activity Questionnaire (IPAQ) (19), and depressive symptoms were assessed using the Patient Health Questionnaire 9 (PHQ9) (20). Nutritional status was assessed using the Malnutrition Inflammation Score (MIS) (21). Charlson Comorbidity Index (CCI) was used to assess the comorbidity risk associated with several conditions (22). We collected biochemical data including serum albumin, hemoglobin, calcium, phosphate, and parathyroid hormone (PTH) within 3 months of physical assessment. Dialysis adequacy was defined as the total fractional clearance index for urea (Kt/V) and urea reduction ratio (URR).
Access to diabetes information was based on subjects’ self-reports, and we again carefully checked the fasting plasma glucose (FPG) data through electronic medical records. According to the American Diabetes Association 2021 criteria, FPG level ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L during an oral glucose tolerance test or HbA1c ≥6.5% was considered as diabetes (23).
Performance-based assessment consisted of grip strength (GS), Timed Up and Go Test (TUGT), and 4-m WS test. GS was measured using a dynamometer (GRIP-D; Takei Ltd., Niigata, Japan). Participants were allowed to exert maximum efforts twice using the dominant hand, and the average value was calculated from two attempts. TUGT assessed the seconds of standing up from a chair, walking 3 m at usual pace past a line on the floor, turning around, walking back to the chair, and then sitting down on the chair. The WS test consists of participants being timed while walking 4 m at their usual pace and they were allowed to use a gait-assistive device. Participants completed the test twice, and the mean gait speed (m/s) was calculated (17). Higher GS values, shorter TUGT, and faster WS represent better physical performance. All tests were monitored by corresponding professional physical therapists.
This study adopted the MCI diagnostic criteria based on Petersen’s definitions with modifications (24): 1) memory complaints (self-reported or reported by family members or caregivers); 2) objective cognitive impairment, as assessed by the Mini-Mental State Examination (MMSE); 3) intact or only mildly impaired daily living ability, as assessed by the Instrumental Activities of Daily Living (IADL) Scale; 4) no clear dementia, as evaluated by the Chinese version of the Dementia Rating Scale (CDRS); 5) no abnormal memory impairment for age. The MMSE score ranges from 0 to 30 points, with the higher scores indicating better cognitive performance. It has been reported that the Chinese version of the MMSE indicates MCI for scores ≤17, 20, and 24 in people with the educational level of illiteracy, primary school, and middle school or higher, respectively (25). The IADL Scale includes eight items, and the score ranges from 0 to 8 points, with the higher scores indicating better daily living ability. IADL scored ≥6 indicates intact or only mildly impaired daily living ability (26). The MMSE includes a broad set of cognitive functions that measure the following: orientation (10 points), registration (3 points), attention and calculation (5 points), recall (3 points), and language (9 points).
The baseline characteristics of participants were presented according to the classification of diabetes and MCI. Continuous variables were presented as means ± standard deviation (SD), and categorical variables were expressed as numbers and percentages. Baseline sociodemographic characteristics were analyzed using t test, Pearson’s chi-square test, and Mann–Whitney U test. The interaction effect between the component of physical performance and diabetes was tested by adding three interacted items (GS * diabetes; TUGT * diabetes; WS * diabetes) in the logistic regression analysis. Binary logistic regression analysis was used to analyze the relationship between physical performance and MCI in hemodialysis patients in the non-diabetic and diabetic groups. MCI was used as the dependent variable, each component of physical performance (GS, TUGT, WS) was used as an independent variable, and several confounding factors [age, gender, body mass index (BMI), year, widowhood, living alone, illiteracy, smoking, alcohol consumption, sleep duration, IPAQ, depression, number of medications, and CCI] were adjusted as covariates. Linear regression models were used to analyze the relationship between GS, TUGT, WS, and various cognitive functions. All of the statistical analyses were performed with the SPSS V26.0 software, and differences were defined as significant when p < 0.05.
Figure 1 shows the flow of hemodialysis participants with subgroups. The analysis sample consisted of 396 study participants (255 men; mean age: 68.3 ± 5.9 years). Baseline characteristics of the subjects were presented in Table 1. Among all participants, 204 (51.5%) reported diabetes and 74 (18.7%) had MCI. Compared to non-MCI, MCI patients with or without diabetes were prone to be widowed (p < 0.05). Compared to non-diabetes, hemodialysis patients with diabetes were prone to be men, have a shorter vintage, and have a higher CCI level. As shown in Figure 2, it is noteworthy that in the diabetes group, MCI patients’ physical performance (TUGT and WS) was significantly worse than that of the cognitively normal group. The TUGT of patients with diabetes was significantly longer than that of those without diabetes (p < 0.05), indicating poorer mobility. However, in the non-diabetes group, there was no significant difference in physical performance between the MCI group and the cognitively normal group.
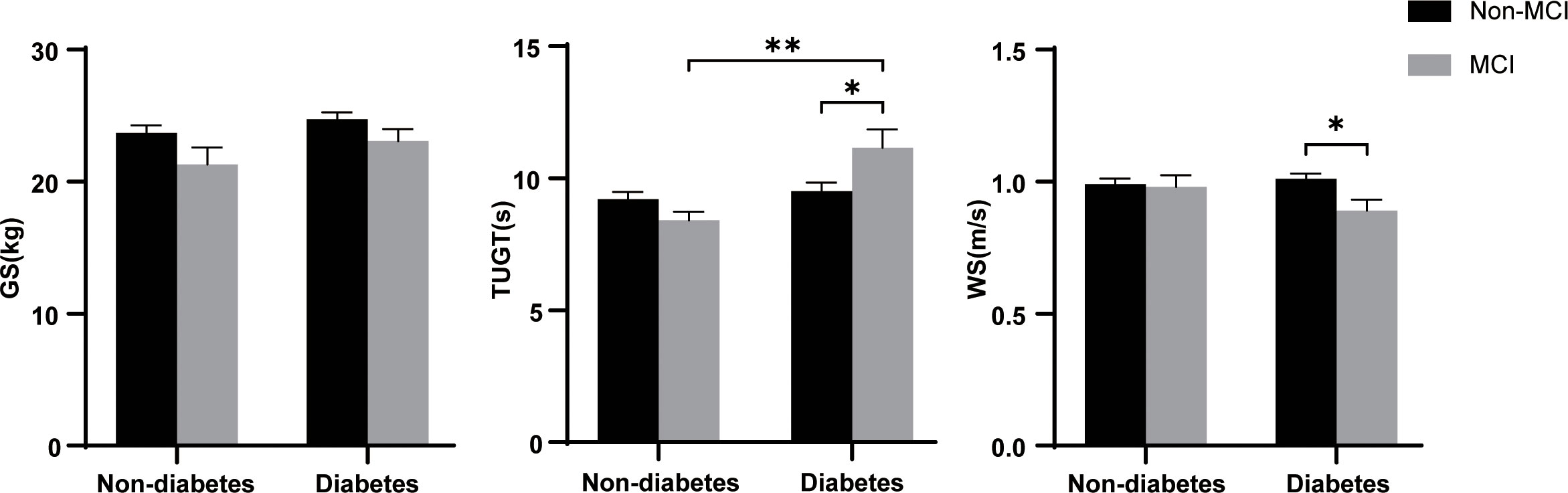
Figure 2 Difference of physical performance between the different groups. GS, grip strength; TUGT, Timed Up and Go Test; WS, 4-m walking speed; MCI, mild cognitive impairment. Data are presented as mean ± SD using t test. * represents p < 0.05, ** represents p < 0.01.
As the main findings, we investigated the association between physical performance and MCI, and the interactive effects of physical performance and diabetes were evaluated by adding the interacted items using logistic regression analysis (Table 2). The odds ratio (OR) of MCI for the interacted items [(TUGT) * (diabetes) and (WS) * (diabetes)] were significant (1.044, 95% confidence interval [CI] 1.002–1.087, p = 0.040; 0.905, 95% CI 0.826–0.991, p = 0.032; Table 2), suggesting a diabetes-dependent effect of mobility and WS. In the subgroup analysis, the crude model showed that TUGT and WS were associated with the risk of MCI in the diabetes group, and ORs (and 95% CIs) were 1.077 (1.005–1.155) and 0.181 (0.048–0.681), respectively (p < 0.05), indicating that longer TUGT was associated with a higher risk of MCI, and faster WS was associated with a lower risk of MCI, respectively. In the adjusted model (age, sex, BMI, vintage, widowed, living alone, illiteracy, smoking, drinking, sleep duration, IPAQ, depression, number of drugs, and CCI), only WS was negatively associated with MCI (p = 0.021). However, whether crude or adjusted, this association did not exist in the non-diabetes group (all p > 0.05).
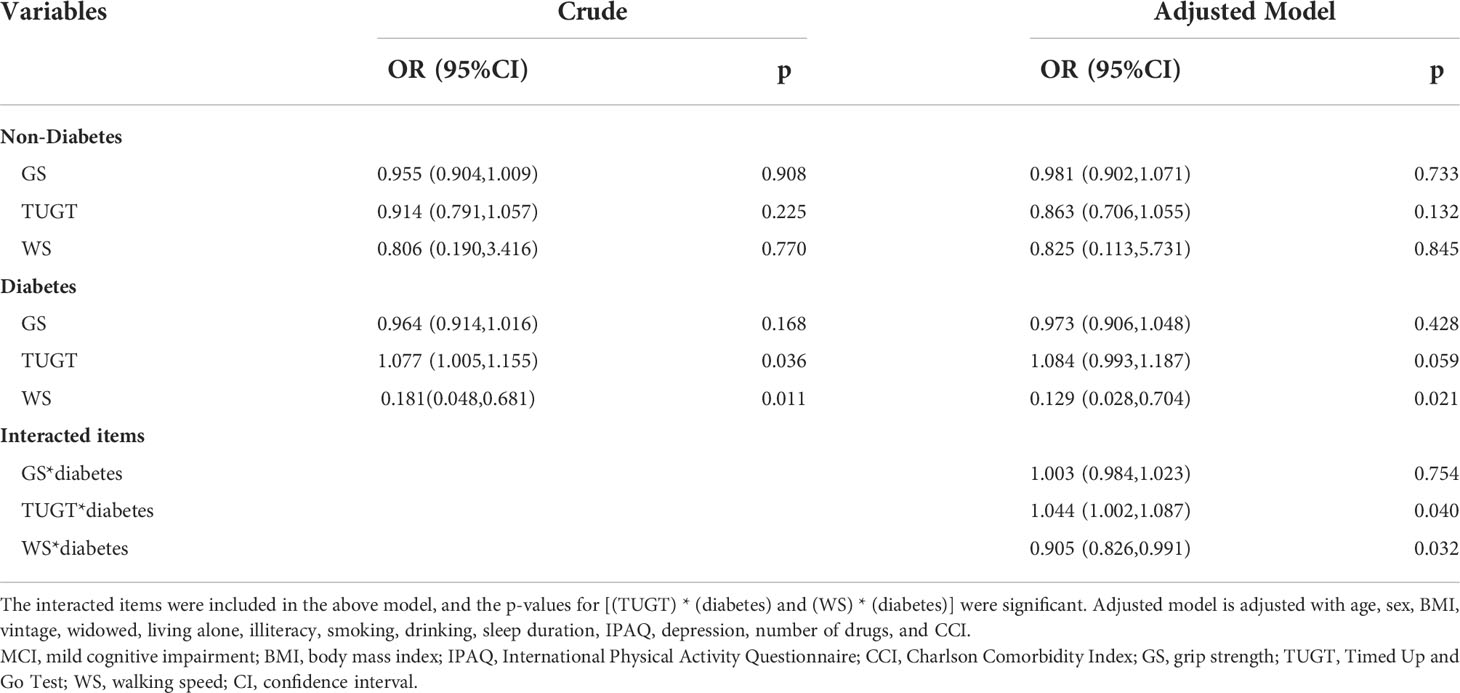
Table 2 Logistic regression analysis of physical performance and MCI in the non-diabetic and diabetic hemodialysis patients.
Then, we performed multivariate linear regression analysis of the association between different physical performance components and cognitive functions in the non-diabetes and diabetes hemodialysis patients (Table 3). In the fully adjusted model, the TUGT was negatively associated with overall cognition, attention and calculation, and language, and the WS was positively associated with overall cognition, recall, and language in the diabetes group (p < 0.05), while only WS was positively associated with attention and calculation in the non-diabetes group (p = 0.044). Whether in the non-diabetes or diabetes group, GS was not associated with any of the cognitive functions (all p > 0.05).
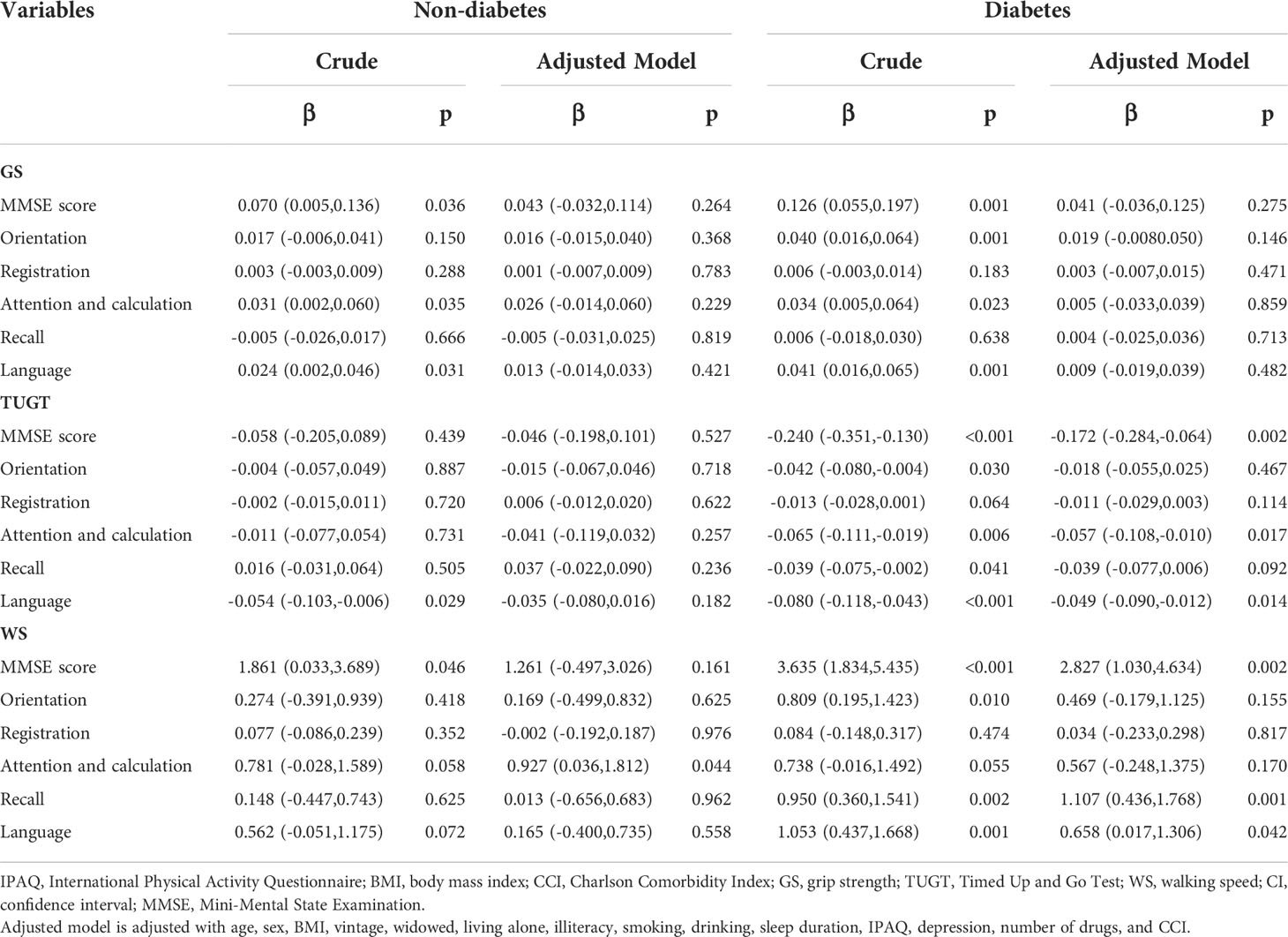
Table 3 Multivariate linear regression analysis of the association between physical performance and cognitive functions in the non-diabetic and diabetic hemodialysis patients.
The main findings of our current study showed that diabetic hemodialysis patients with MCI performed worse mobility than the non-diabetes group. Further analysis found that the interaction between mobility/WS and diabetes is significant. In hemodialysis patients with diabetes, those with MCI performed worse WS than those without MCI, whereas no association was found for patients without diabetes. Moreover, multivariate linear regression analysis showed that TUGT was negatively associated with attention and calculation and language. WS was positively associated with recall and language in diabetic hemodialysis patients.
Our previous studies have shown that physical performance was significantly different based on MCI status in Chinese older adults with an average age of 72.6 years (17). Poor health outcomes in diabetes are closely linked to physical activity and dietary patterns, which are also risk factors for CKD (27–29). Therefore, we compared physical performance in hemodialysis populations grouped by diabetes and MCI and explored the relationship between physical performance and MCI in hemodialysis patients with and without diabetes in our study. It is worth noting that we found, whether compared with MCI in the non-diabetes group or non-MCI in the diabetes group, diabetic patients with MCI have poor mobility (Figure 2, p < 0.05). This finding is unprecedented. Patients with CKD experience substantial loss of muscle mass, and skeletal muscle dysfunction leads to mobility limitation (30). Kestenbaum et al. (31) demonstrated a 25% reduced leg muscle mitochondrial oxidative capacity in participants with CKD and that leg muscle oxidative capacity is a significant predictor of mobility. Moreover, a history of diabetes also imparted nearly the same magnitude of reduction in mitochondrial function (31). Therefore, altered metabolic transcriptional networks and defective mitochondrial function are likely to be major mechanistic factors in the progression of CKD caused by diabetes that impairs physical function (32). In a slight departure from our previous study, we did not find an association between GS and MCI; the reason may be that our hemodialysis population cohort is relatively younger (with an average age of 68.3 years) than the elderly population cohort. Although another comparison of physical performance between the groups according diabetes showed lower physical performance in the diabetes group than the non-diabetes group of hemodialysis and peritoneal dialysis patients (33), however, so far, no research has shown that physical activity is significantly worse in the coexistence of diabetes and MCI diseases.
Furthermore, results of our study found that the prevalence of MCI in diabetic hemodialysis patients was high (20.6%). This finding was similar to the AGES–Reykjavik study (34) that showed that individuals with type 2 diabetes had poorer performance on cognitive tests than individuals without type 2 diabetes. There are several possible mechanisms for the result. First, the accumulation of glycosylation end products triggers vascular endothelial dysfunction (35), and multiple risk factors including oxidative stress, inflammation, vascular calcification, and insulin-like growth factor-1 also play roles in the development and progression of MCI (36). Second, neurodegenerative mechanisms have been proposed for the association of diabetes with MCI. The hippocampus, entorhinal formation, and frontal cortex are potential target regions in the brain that are known to have insulin receptors through which insulin-related effects may affect cognitive function (37). Diabetes may adversely affect amyloid processing and increase brain intraneuronal β-amyloid deposition (38) and tau hyperphosphorylation (39) in target regions, which is a sign of cognitive impairment. Therefore, it is reasonable to believe that diabetes in end-stage renal disease patients receiving hemodialysis may be an important risk factor for the development of MCI.
In previous studies, the relationship between physical performance and MCI in hemodialysis patients has not been fully established. In the current study, we found a significant interaction between mobility/WS and diabetes in hemodialysis patients, while the interaction between GS and diabetes was not significant. It is possible that the mechanism underlying this interaction is multifactorial. For instance, diabetes and its primary risk factors (hypertension, heart disease, and obesity) are both strongly associated with impaired mobility function and WS. Secondly, WS is associated with factors such as inflammation, neuropathy, and vascular function, which are common pathways to cognitive and physical function. However, current evidence of the association between GS and diabetes is controversial, and a study has shown no significant association between them (40). In addition, WS in physical performance was negatively associated with MCI in diabetic hemodialysis patients; however, no significant association was found in the non-diabetic group. The possible reasons are as follows: for patients with diabetes, they are significantly associated with poor physical performance, the mean TUGT was also longer in the diabetes group than that in the non-diabetes group (11.15 vs. 8.41, p < 0.05), and the mean WS was slower in the diabetes group than that in the non-diabetes group in our study (0.89 vs. 0.98) but did not show a significant difference probably due to the small sample size. Moreover, a study has shown that hyperglycemia is associated with the development of frailty and incident mobility limitations, potentially mediated by loss of muscle (41). This is also consistent with previous findings supporting a role of specific cardiovascular risk factor contributors in the association between physical performance and cognitive decline (42). Therefore, it is reasonable to believe that poor physical performance due to diabetes may be an important risk factor for the development of MCI. This finding takes our pinpointing of amenable factors for MCI a step further, and physical performance interventions in more precise populations may be useful for early prevention and control of MCI progression.
Moreover, we found that TUGT was negatively associated with not only global cognitive function but also several specific functions, including attention and calculation and language. WS was positively associated with recall and language even after adjusting for potential confounding factors. Recent studies have revealed a strong relationship between gait and executive functions in healthy and pathological aging. The main negative correlations were found between time of TUGT and total score (r = –0.476) and language domain (r = –0.448) in the MCI group (43). McGough etal. (44) found that slow gait was associated with registration, attention and calculation, and executive performance. This is consistent with our findings that showed that WS was positively associated with attention and calculation (p = 0.044) in the non-diabetes group, and TUGT was negatively associated with language in the diabetes group. The following clinically relevant links can explain our results: cognitive function is related to the dorsolateral frontal cortex and hippocampus, which affect the executive function, attention and calculation, and recall of individuals. On the flip side, gait decline increases the risk of cognitive decline and dementia, and poor mobility outcomes were reliably associated with reduced gray and white matter volume (45). At present, although many consistent studies showed the relationship between physical activity and cognitive functions, there are still some inconsistent results (16, 44). Future studies should focus on the different cognition changes in the weak physical population, and more well-designed cohort studies need to be carried out to verify the relationship between physical performance and different cognitive functions. Generally, our finding gives us some inspiration on how to manage physical activity and interfere with MCI in hemodialysis patients, especially those with diabetes.
The strengths of our study included the following: It is the first multicenter study to examine the relationship between physical performance and MCI among hemodialysis patients across different diabetic states. Secondly, the study assessed the association between physical performance and multiple cognitive functions in hemodialysis patients with and without diabetes. Furthermore, most recognized confounders were taken into account in regression models to analyze the independent association of physical performance and MCI in this study. However, some limitations also exist. First, all participants in the present study come from one city, which means that this study has a certain degree of regional limitation. Second, this study is based on a cross-sectional design, so it is not possible to determine causal relationships. To clarify this issue, a further longitudinal study with a large sample size is needed to explore the new onset risk of MCI in the hemodialysis population with diabetes.
In this study, we found that physical performance was associated with MCI in diabetic hemodialysis patients rather than the non-diabetes group. Further analysis showed the relationship between physical performance and specific cognitive functions. This study provides some key considerations for physicians that poor mobility and WS in diabetic hemodialysis patients are more associated with MCI. Further research is required to confirm the direction of causality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was reviewed and approved by the Ethics Committee of Shanghai University of Medicine and Health Sciences and the methods were carried out in accordance with the principles of the Declaration of Helsinki. The patients/participants provided their written informed consent to participate in this study.
YZ, PS and CZ contributed equally to this work and should be considered as the co-first authors. XC, PS and YZ conceived the concept and design of the study. WD, JN, JZ, XS, LMZ, CY and JX provided the study materials or patients. CZ, LYZ and YZ collected and assembled the data. PS, CF, PH and HZ analyzed and interpreted the data. YZ, PS and CZ drafted the article or revising it critically for important intellectual content. QG provided administrative support. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 82172552) and the Key Clinical Support Specialty Construction Project of Shanghai Hongkou District (HKZK 2020B02).
We thank all the medical staff at the multi-center dialysis for their generous technical assistance and guidance. We also thank all the study participants for their kind participation and cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sarnak MJ, Tighiouart H, Scott TM, Lou KV, Sorensen EP, Giang LM, et al. Frequency of and risk factors for poor cognitive performance in hemodialysis patients. Neurology (2013) 80:471–80. doi: 10.1212/WNL.0b013e31827f0f7f
2. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: Mild cognitive impairment: Report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
3. Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
4. Siew ED, Ikizler TA. Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial (2010) 23:378–82. doi: 10.1111/j.1525-139X.2010.00763.x
5. Pepin M, Villain C. Chronic kidney disease and cognitive impairment. Geriatr Psychol Neuropsychiatr Vieil (2020) 18:429–35. doi: 10.1684/pnv.2020.0897
6. Leal J, Becker F, Feenstra T, Pagano E, Jensen TM, Vistisen D, et al. Health-related quality of life for normal glycaemia, prediabetes and type 2 diabetes mellitus: Cross-sectional analysis of the ADDITION-PRO study. Diabetes Med (2022) 39:e14825. doi: 10.1111/dme.14825
7. De Rekeneire N, Resnick HE, Schwartz AV, Shorr RI, Kuller LH, Simonsick EM, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the health, aging, and body composition study. Diabetes Care (2003) 26:3257–63. doi: 10.2337/diacare.26.12.3257
8. Pansini A, Lombardi A, Morgante M, Frullone S, Marro A, Rizzo M, et al. Hyperglycemia and physical impairment in frail hypertensive older adults. Front Endocrinol (Lausanne) (2022) 13:831556. doi: 10.3389/fendo.2022.831556
9. Vandercappellen EJ, Koster A, Savelberg H, Eussen S, Dagnelie PC, Schaper NC, et al. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation-relevance for (pre)diabetes: The maastricht study. Diabetologia (2022) 65:777–89. doi: 10.1007/s00125-022-05651-3
10. Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement (2014) 10:18–26. doi: 10.1016/j.jalz.2013.01.001
11. Rawlings AM, Sharrett AR, Albert MS, Coresh J, Windham BG, Power MC, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: The ARIC study. Diabetes Care (2019) 42:1248–54. doi: 10.2337/dc19-0120
12. Mone P, Gambardella J, Lombardi A, Pansini A, De Gennaro S, Leo AL, et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc Diabetol (2022) 21:10. doi: 10.1186/s12933-021-01442-z
13. Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol (2015) 3:75–89. doi: 10.1016/S2213-8587(14)70148-2
14. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol (2015) 14:329–40. doi: 10.1016/S1474-4422(14)70249-2
15. Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ, et al. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care (2013) 36:137–44. doi: 10.2337/dc12-0493
16. Hooghiemstra AM, Ramakers I, Sistermans N, Pijnenburg YAL, Aalten P, Hamel REG, et al. Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: Findings from the 4C study. J Gerontol A Biol Sci Med Sci (2017) 72:846–54. doi: 10.1093/gerona/glx003
17. Liu Y, Ma W, Li M, Han P, Cai M, Wang F, et al. Relationship between physical performance and mild cognitive impairment in Chinese community-dwelling older adults. Clin Interv Aging (2021) 16:119–27. doi: 10.2147/CIA.S288164
18. Wu X, Hou G, Han P, Yu X, Chen X, Song P, et al. Association between physical performance and cognitive function in Chinese community-dwelling older adults: Serial mediation of malnutrition and depression. Clin Interv Aging (2021) 16:1327–35. doi: 10.2147/CIA.S315892
19. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
20. Levis B, Benedetti A, Thombs BD, Collaboration DESD. Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ (2019) 365:l1476. doi: 10.1136/bmj.l1476
21. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis (2001) 38:1251–63. doi: 10.1053/ajkd.2001.29222
22. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol (2011) 173:676–82. doi: 10.1093/aje/kwq433
23. American Diabetes Association. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care (2021) 44:S15–33. doi: 10.2337/dc21-S002
24. Su X, Shang L, Xu Q, et al. Prevalence and predictors of mild cognitive impairment in xi’an: a community-based study among the elders. PloS One (2014) 9:e83217. doi: 10.1371/journal.pone.0083217
25. Katzman R, Zhang MY, Ouang Ya Q, Wang ZY, Liu WT, Yu E, et al. A Chinese version of the mini-mental state examination; impact of illiteracy in a shanghai dementia survey. J Clin Epidemiol (1988) 41:971–8. doi: 10.1016/0895-4356(88)90034-0
26. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
27. Bach KE, Kelly JT, Palmer SC, Khalesi S, Strippoli GFM, Campbell KL. Healthy dietary patterns and incidence of CKD: A meta-analysis of cohort studies. Clin J Am Soc Nephrol (2019) 14:1441–9. doi: 10.2215/CJN.00530119
28. Rampersad C, Brar R, Connelly K, Komenda P, Rigatto C, Prasad B, et al. Association of physical activity and poor health outcomes in patients with advanced CKD. Am J Kidney Dis (2021) 78:391–8. doi: 10.1053/j.ajkd.2020.12.018
29. Sasso FC, Pafundi PC, Gelso A, Bono V, Costagliola C, Marfella R, et al. Relationship between albuminuric CKD and diabetic retinopathy in a real-world setting of type 2 diabetes: Findings from no blind study. Nutr Metab Cardiovasc Dis (2019) 29:923–30. doi: 10.1016/j.numecd.2019.05.065
30. Roshanravan B, Gamboa J, Wilund K. Exercise and CKD: Skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis (2017) 69(6):837–52.
31. Kestenbaum B, Gamboa J, Liu S, Ali AS, Shankland E, Jue T, et al. Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight (2020) 5(5):e133289. doi: 10.1172/jci.insight.133289
32. Bittel DC, Bittel AJ, Varadhachary AS, Pietka T, Sinacore DR. Deficits in the skeletal muscle transcriptome and mitochondrial coupling in progressive diabetes-induced CKD relate to functional decline. Diabetes (2021) 70:1130–44. doi: 10.2337/db20-0688
33. Silva MZC, Antonio KJ, Reis JMS, Alves LS, Caramori JCT, Vogt BP. Age, diabetes mellitus, and dialysis modality are associated with risk of poor muscle strength and physical function in hemodialysis and peritoneal dialysis patients. Kidney Res Clin Pract (2021) 40:294–303. doi: 10.23876/j.krcp.20.159
34. Qiu C, Sigurdsson S, Zhang Q, Jonsdottir MK, Kjartansson O, Eiriksdottir G, et al. Diabetes, markers of brain pathology and cognitive function: the age, Gene/Environment susceptibility-Reykjavik study. Ann Neurol (2014) 75:138–46. doi: 10.1002/ana.24063
35. Chen G, Cai L, Chen B, Liang J, Lin F, Li L, et al. Serum level of endogenous secretory receptor for advanced glycation end products and other factors in type 2 diabetic patients with mild cognitive impairment. Diabetes Care (2011) 34:2586–90. doi: 10.2337/dc11-0958
36. Liu P, Cui L, Liu B, Liu W, Hayashi T, Mizuno K, et al. Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol Behav (2020) 213:112689. doi: 10.1016/j.physbeh.2019.112689
37. Craft S. Insulin resistance and alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res (2007) 4:147–52. doi: 10.2174/156720507780362137
38. Wakabayashi T, Yamaguchi K, Matsui K, Sano T, Kubota T, Hashimoto T, et al. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of alzheimer’s disease. Mol Neurodegener (2019) 14:15. doi: 10.1186/s13024-019-0315-7
39. Sun Y, Xiao Q, Luo C, Zhao Y, Pu D, Zhao K, et al. High-glucose induces tau hyperphosphorylation through activation of TLR9-P38MAPK pathway. Exp Cell Res (2017) 359:312–8. doi: 10.1016/j.yexcr.2017.07.032
40. Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr., Orlandini A, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet (2015) 386:266–73. doi: 10.1016/S0140-6736(14)62000-6
41. Kalyani RR, Tian J, Xue QL, Walston J, Cappola AR, Fried LP, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc (2012) 60:1701–7. doi: 10.1111/j.1532-5415.2012.04099.x
42. Montero-Odasso MM, Barnes B, Speechley M, Muir Hunter SW, Doherty TJ, Duque G, et al. Disentangling cognitive-frailty: Results from the gait and brain study. J Gerontol A Biol Sci Med Sci (2016) 71:1476–82. doi: 10.1093/gerona/glw044
43. Mumic de Melo L, Hotta Ansai J, Giusti Rossi P, Carvalho Vale FA, Pires de Andrade L. Performance of an adapted version of the timed up-and-Go test in people with cognitive impairments. J Mot Behav (2019) 51:647–54. doi: 10.1080/00222895.2018.1552917
44. McGough EL, Cochrane BB, Pike KC, Logsdon RG, McCurry SM, Teri L. Dimensions of physical frailty and cognitive function in older adults with amnestic mild cognitive impairment. Ann Phys Rehabil Med (2013) 56:329–41. doi: 10.1016/j.rehab.2013.02.005
Keywords: mild cognitive impairment, diabetes, physical performance, walking speed, hemodialysis
Citation: Zhao Y, Song P, Zhu C, Zhang L, Chen X, Zhang H, Han P, Ding W, Niu J, Zhao J, Shao X, Zhang L, Yu C, Xu J, Fang C and Guo Q (2022) Relationship between physical performance and mild cognitive impairment in elderly hemodialysis patients is modified by the presence of diabetes: A multicenter cross-sectional study. Front. Endocrinol. 13:897728. doi: 10.3389/fendo.2022.897728
Received: 18 March 2022; Accepted: 16 August 2022;
Published: 09 September 2022.
Edited by:
Raffaele Marfella, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Antonella Pansini, Local Health Authority Avellino, ItalyCopyright © 2022 Zhao, Song, Zhu, Zhang, Chen, Zhang, Han, Ding, Niu, Zhao, Shao, Zhang, Yu, Xu, Fang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Guo, Z3VvcWlqcEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.