
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 04 May 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.897196
This article is part of the Research Topic Endocrine and Paracrine Regulation of Spermatogenesis - A Collection of Up to Date Research Contributions on Testis Formation and Function View all 25 articles
Since their initial description by Enrico Sertoli in 1865, Sertoli cells have continued to enchant testis biologists. Testis size and germ cell carrying capacity are intimately tied to Sertoli cell number and function. One critical Sertoli cell function is signaling from Sertoli cells to germ cells as part of regulation of the spermatogenic cycle. Sertoli cell signals can be endocrine or paracrine in nature. Here we review recent advances in understanding the interplay of Sertoli cell endocrine and paracrine signals that regulate germ cell state. Although these findings have long-term implications for treating male infertility, recent breakthroughs in Sertoli cell transplantation have more immediate implications. We summarize the surge of advances in Sertoli cell ablation and transplantation, both of which are wedded to a growing understanding of the unique Sertoli cell niche in the transitional zone of the testis.
Although germ cells are the stars of spermatogenesis, Sertoli cells are the sustaining lead, without which, spermatogenesis would cease to occur. Sertoli cells provide the supportive framework within which germ cells will safely undergo rounds of mitosis and meiosis (Figure 1). This structure which includes tight junctions between adjacent Sertoli cells, divides the seminiferous epithelium into the basal and adluminal compartments, serving a protective role as the testicular region within the seminiferous tubules that is immuno-privileged (1–5). Sertoli cells act as the mediator between germ cells and endocrine signaling, from controlling spermatogenesis by hormones (follicle stimulating hormone [FSH] and testosterone [T]), originating from outside of the seminiferous tubule (6–8). Sertoli cells also have direct impacts on germ cell development through paracrine signaling (9–11). These roles are all key elements required to orchestrate the symphonic cyclicity of steady-state spermatogenesis within the adult testis. When aberrations in Sertoli cell function occur, this intricate exchange breaks down and spermatogenic failure may occur, ultimately challenging the fertility of the male. Recent research into the niche population of Sertoli cells at the transition zone between the rete testis and seminiferous tubules, as well as studies of Sertoli cell transplantation, are bringing new insights to the field. Both branches of investigation offer the promise of a deeper understanding into how Sertoli cells come to reside properly in the testis, and methods for getting functional Sertoli cells in to replace Sertoli cells that are deficient.
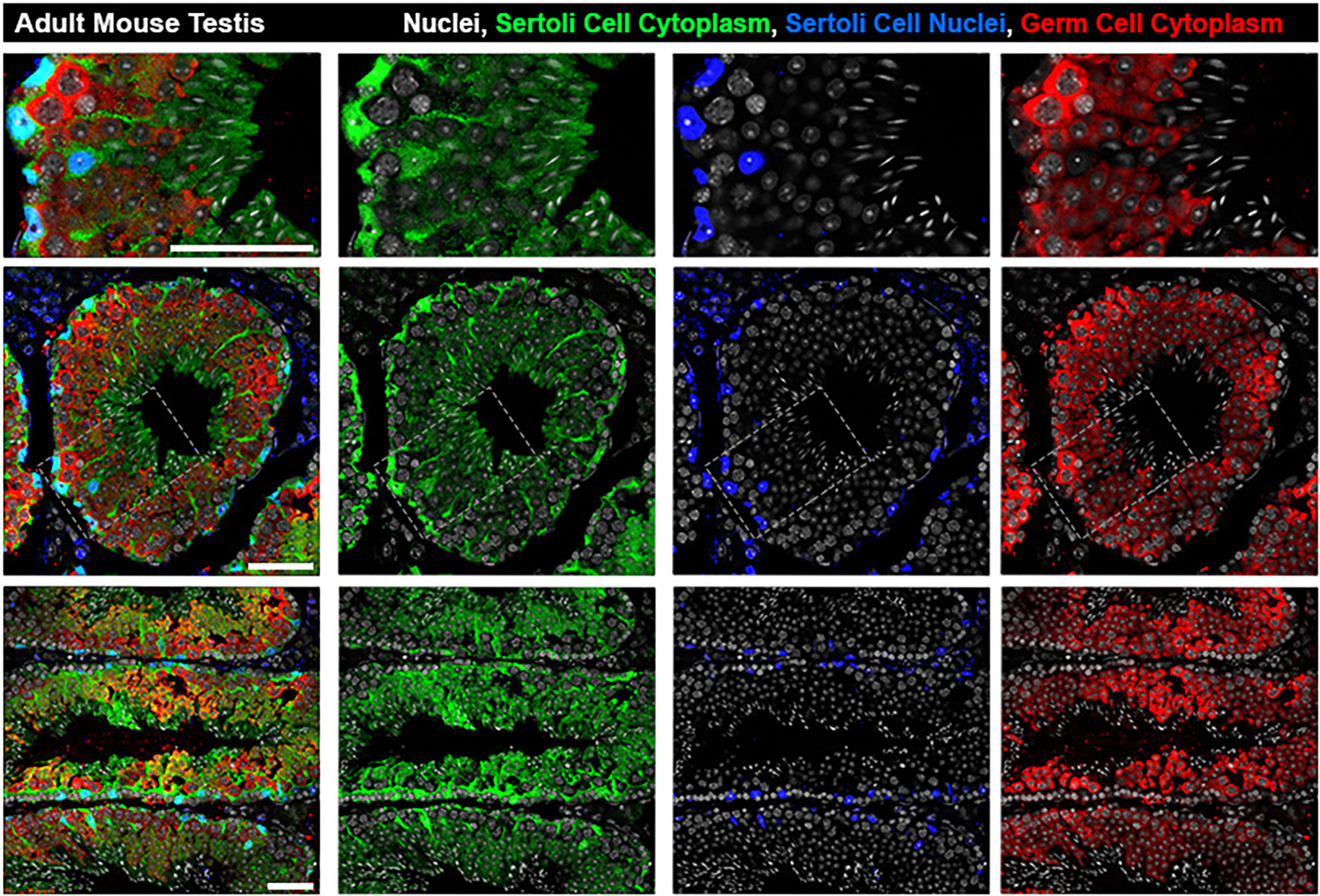
Figure 1 Architecture of Sertoli cells in the adult mouse seminiferous tubule. The bodies of Sertoli cell cytoplasm (green) can be seen engulfing germ cells (red) from basal lamina to lumen while Sertoli cell nuclei (blue) are located basally. Top row: zoomed inset from grey boxed region in Middle Row: seminiferous tubule cross section at stage V-VI. Bottom Row: Longitudinal sections showing multiple stages. All scale bars are 50µm.
Aside from the germ cell based histological staging of spermatogenesis defined by consistent cell associations present in cross-sections of the seminiferous tubule (Figure 2), generally the stages of the cycle can also be defined by unique metabolic and molecular Sertoli cell identities (22–24). Specifically in regards to the androgen signaling pathway, Sertoli cells display stage specific temporal peaks of AR expression in rodents (stages VI-VIII) (25–27) (Figure 2A), and humans (stage III) (28) (Figure 2B). For germ cells, as one progresses concentrically towards the seminiferous tubule lumen, this AR peak period coincides with: undifferentiated type A spermatogonia meiotic entry, elongating spermatid adhesion, and spermiation (29–32).
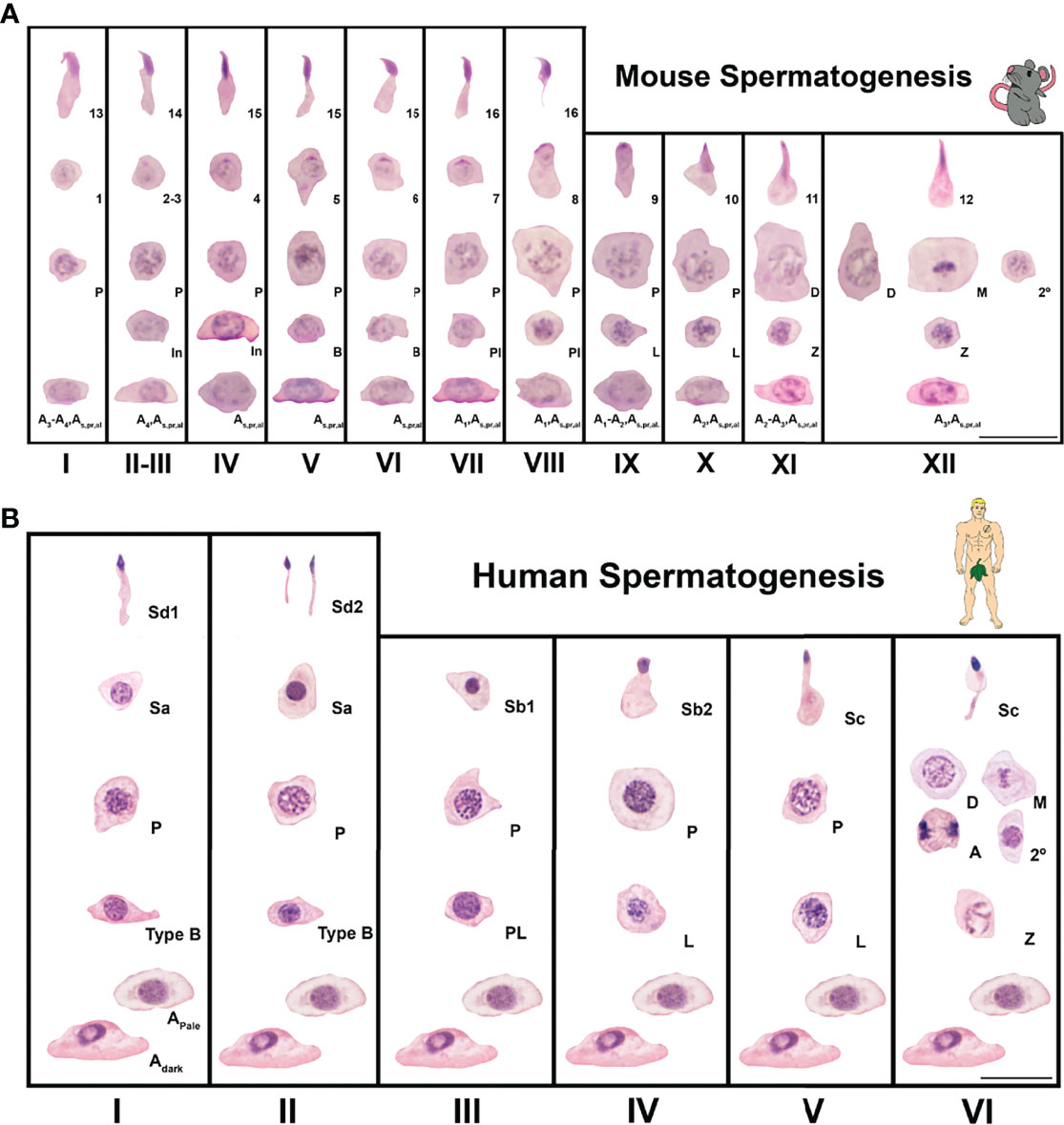
Figure 2 Seminiferous epithelial stages of mouse and human spermatogenesis as classic spermatogenesis cycle staging charts using germ cell associations and morphology. Spermatogenesis is the process of sperm development and involves phases of mitosis, meiosis, and spermiogenesis (morphological cell changes). (A) Spermatogenesis in mice is a cycle that takes ~8.6 days (12–14). The time necessary for a germ cell to go from type A spermatogonia to spermatozoa (the complete process or duration of spermatogenesis) is about 35 days (12, 13, 15). In mice, spermatogenesis is divided into 12 stages (I-XII) and 16 spermatid developmental steps. A, In, and B are type A, intermediate, and type B spermatogonia, respectively. Pl, L, Z, P, D, M, and 2º are preleptotene, leptotene, zygotene, pachytene, diplotene, meiotic, and secondary spermatocytes, respectively. Steps of spermatid development are numbered 1-16. Sections were stained with Periodic Acid Schiff’s regent-hematoxylin (PAS-H), which is a conventional staining for staging of mouse testis sections. Scale is 20μm. (B). Spermatogenesis in men is a 16 day cycle with a complete duration that was classically determined to be 64 days but modern methods show to be closer to 74 days (16–21). In humans, spermatogenesis is divided into 6 stages (I-VI) and 6 spermatid developmental steps. Adark, Apale and B are type A dark, type A pale and type B spermatogonia, respectively. Pl, L, Z, P, D, M, A and 2º are preleptotene, leptotene, zygotene, pachytene, diplotene, meiotic metaphase, meiotic anaphase and secondary spermatocytes, respectively. Steps of spermatid development are labeled Sa, Sb1, Sb2, Sc, Sd1 and Sd2. Sections were stained with Periodic Acid Schiff’s regent-hematoxylin (PAS-H), which is a conventional staining for human testis histology assessment. Scale is 20μm.
Larose et al. 2020 (33) took a more granular look at the direct impact of AR presence in Sertoli cells on germ cell meiotic progression. Using SCARKO mutant mice (Sertoli cell androgen receptor knockout) they defined a Sertoli cell-AR androgen independent period of germ cell development from meiotic initiation to early prophase. Germ cells in these mice that did not undergo apoptosis (and many germ cells did) progressed up to what, histologically, appeared to be relatively normal pachytene spermatocytes. But upon deeper investigation using scRNA-seq, the most advanced germ cells were transcriptionally defined and resembled leptotene or zygotene spermatocytes (33). This discrepancy between transcriptomic and histological cell-identity was also reported in Pdrm9 mutant germ cells (34). This finding calls into question the many definitive studies using models of androgen deficiency or receptor deletion causing a defined maturation arrest that predates the use of scRNA-seq technology and relied solely on classical histological assessment. Revisiting these classic maturation arrest studies with modern bioinformatics tools has the potential to elucidate other molecular details similar to those reported by 33.
Transcriptomic analysis on SCARKO mutant mice also identified a set of genes (including: Fabp9, Gstm5, Ybx3, Meig1, Spink2, Rsph1, Aldh1a1, Igfbps, Piwil1, Mael) regulated by AR signaling in Sertoli cells. Collectively this gene set seems to license spermatocytes for the first meiotic division, as well as for spermiogenic competency (33). Another gene, Rhox5, initially transcribed in Sertoli cells, is an androgen-inducible transcription factor (35–39). RHOX5 regulates Sertoli cell gene expression controlling cell surface and protein secretion in relation to germ cells (7, 40–43). Rhox5 has two promoters, distal and proximal. Previously, these promoters were understood to drive different tissue-specific expression, with the exception that both promoters are active in adult Sertoli cells Bhardwaj et al. 2022 defined a postnatal temporality to Rhox5 promoter activity (44). The proximal promoter is activated shortly after birth, while the distal promoter is dormant until late in the postnatal period also identified novel androgen-responsiveness for the Rhox5 distal promoter. The group then established that the proximal promoter can act as an enhancer for the distal promoter and further, that RHOX5 up-regulates its own transcription via the distal promoter (44).
Rhox5 expression in Sertoli cells is dependent on FSH signaling (36). Unlike Ar, in adult mouse Sertoli cells Fshr has a consistent expression level throughout the stages of spermatogenesis (23) and knockout experiments have shown there is a degree of added redundancy in the FSH pathway when working synergistically with the AR pathway (45, 46). Reported activity of both proximal and distal Rhox5 promoters into adulthood specifically in Sertoli cells at Stages II-V (outside AR peak) and VI-VIII (within AR peak) (44). Potentially, Rhox5 is yet another recipient of synergistic T and FSH action. This would add another layer to the evolutionary pressure postulated by 44. According to the authors, this pressure drove retention of the Rhox5 distal and proximal promoters. This evolutionary pressure was probably directed at the initial temporally-staggered promoter expression of Rhox5 postnatally. During the first wave of spermatogenesis, Ar and Fshr are known to have dynamic expression patterns in mouse Sertoli cells (24, 44).
T and FSH synergism is not limited to Sertoli cell transcription factors. A newer player in the realm of intercellular signaling is the extracellular vesicle, which can hold and transport an array of different molecules including: growth factors, cytokines, mRNAs, bioactive lipids, and microRNAs (47–49). A recent report by Mancuso et al 2015 utilized a porcine Sertoli cell culture system to define the extracellular vesicle components with FSH-alone and synergistic T+FSH stimulation (50). Proteomic analysis showed FSH-alone increased proteins generally linked to modulating the hypothalamic-pituitary axis regulating testosterone biosynthesis, the blood-testis-barrier, and spermiation (INHA, INHB, PLKA, HPT, SERA and AT1A1). While stimulation (50) with T+FSH increased proteins generally linked to blood-testis-barrier adherens junctions, and gating endocrine and paracrine regulation of spermatogenesis (INHA, INHB, TPA, EGFL8, EF1G and SERA). These extracellular vesicles also contained transcripts (Amh, Inhb, Abp, Fshr), which the authors postulate could function in loading germ cells, and other testicular cells, with mRNA that will later be translated (50).
Extracellular vesicles are generally accepted to belong to 3 categories: exosomes, microvesicles, and apoptotic bodies (51, 52). Exosomes, were recently the focus of exciting findings in the field. Aside from transporting mRNA, extracellular vesicles, specifically exosomes, can also transport microRNA (53). Paracrine signaling from Sertoli to germ cells by exosomes containing microRNA would putatively be to silence genes. Indeed, a recent report by Li et al. 2021, revealed that Sertoli exosomes contain the microRNA miR-486-5p (54). The authors used a culture system of adult Sertoli cells and P6 germ cells enriched for spermatogonial stem cells. Using this system demonstrated that Sertoli cell exosomes with miR-486-5p down-regulated spermatogonial stem cell expression of Pten by targeting of the Pten-3’UTR by miR-486-5p. The authors further identified that both Stra8. and Sycp3 were indirectly up-regulated in spermatogonial stem cells by the decrease in repressive PTEN. Ultimately this exosome exchange would seem to be part of the differentiation signal from Sertoli cells to spermatogonia (54).
The observations of Li et al. 2021 about Sertoli cell miR-486-5p containing exosomes adds to the evolving school of thought on how undifferentiated spermatogonia enter meiosis (54). Spermatogonial differentiation and meiotic entry is established to be highly dependent on retinoic acid (RA) signaling (55, 56). The commonly proposed paracrine source of germ cell stimulating RA is Sertoli cells and spermatocytes (32, 57–60). Much like AR, RA levels in the seminiferous epithelium are also cyclic and peak at stage VIII, the same stage at which undifferentiated spermatogonia commit to meiosis (61). Timing for meiotic entry is critically important, and inherent in understanding the control of this timing is the need to define how spermatogonia control RA-responsiveness. In the fetal testis CYP26B1, which catabolizes RA, is a key regulator in blocking fetal male germ cell meiotic entry (62–65). Using the first wave of spermatogenesis as a synchronized model of spermatogenesis, Velte et al. 2019 (66) showed that CYP26 also blocks meiotic entry at postnatal day 6 (P6) in undifferentiated spermatogonia that are poised to respond to RA. Spermatogonial poising for RA responsiveness is generally thought to be accomplished through RARG (RA receptor gamma) expression (66). Indeed, this model was eloquently validated by in Suzuki et al. (67), who defined two sub-populations of undifferentiated spermatogonia in the adult mouse testis. Early-undifferentiated spermatogonia did not express RARG, while late-undifferentiated spermatogonia did express RARG (67). However deeper analysis in a follow-up study further sub-divided late-undifferentiated spermatogonia into a group expressing Dppa3 (Dppa3+) and RARG that quickly transition to a differentiating spermatogonia (KIT+) state upon RA stimulation. While the other group of late-undifferentiated spermatogonia express RARG but not Dppa3 (Dppa3-) and have delayed differentiation (68). Whether or not Dppa3 transcript presence is the product of exosome-mediated microRNA silencing is still an open question.
Clinically, men can suffer from an array of Sertoli cell-origin infertility. In some cases the ligand is the issue: gonadotropin-deficient men, mutations (69) and androgen dysregulation (70). In other cases the receptor is the issue, such as complete or partial androgen insensitivity syndromes resulting from polymorphisms or deletions of the androgen receptor (71, 72). Extracellular vesicles may offer the possibility of a cell-free treatment for some forms of infertility due to specific types of Sertoli cell deficiencies. Theoretically extracellular vesicles could be injected clinically through the rete testis using the ultrasound-guided injection technique (73–76). Although these types of therapeutics are still years away, extracellular vesicles could become clinically relevant sooner due to their diagnostic potential. Two recent studies demonstrated the value of seminal exosome analysis as markers of Sertoli cell damage by varicocele (77), and predictive of testicular sperm presence in NOA men (78).
Another exciting technology that has seen a surge of progress lately is Sertoli cell transplantation. Ralph Brinster pioneered germ cell transplantation over a quarter century ago, his technique was later applied to transplant the somatic cells of the seminiferous epithelium, Sertoli cells (79). Some of the earliest reporting of Sertoli cell transplantation as a method for repairing the spermatogonial stem cell niche goes back to the early 2000’s (80, 81). A challenge to restoring Sertoli cell function through transplantation of functional Sertoli cells is what to do about clearing out the dysfunctional Sertoli cells from the seminiferous epithelium to make space. Previously transgenic lines and cadmium has been used for Sertoli cell ablation (81–84). Although effective, from a clinical perspective these methods are not feasible and pose adverse risks, respectively.
Yokonishi et al. 2020 (85) recently identified a safe alternative to cadmium, benzalkonium chloride (BC), which is an FDA-approved non-toxic agent present in over-the-counter eye drops and hair conditioner (86). The authors show that admission of 0.02% benzalkonium chloride through the mouse rete testis is sufficient to ablate Sertoli cells. Further this group defines the temporal windows for host Sertoli cell ablation, donor Sertoli cell transplantation, and donor germ cell transplantation. The window for host germ cell survival is also detailed, the method is tested with cryopreserved testicular cells, and a culture version of the method demonstrates benzalkonium chloride utility in large mammals (dog) (85). In a follow-up study the same group shower that fetal mouse gonadal cells transplanted into an ablated adult mouse testis are competent to colonize, mature, and support host germ cell spermatogenesis (87). An added level of temporality in transplanted donor Sertoli cell colonization after ablation, was recently defined in another robust ablation study. Using a transgenic system of Sertoli cell ablation, Imura-Kishi et al. 2021 showed that donor Sertoli cells first colonize the transitional zone where they resume repression of spermatogenesis. After reaching an equilibrium in the transitional zone Sertoli cells then proliferate further, repopulating the host seminiferous epithelium where the donor Sertoli cells will support host spermatogenesis (88).
The transitional zone of the testis goes by many names (Sertoli valve, transitional region, tubulis rectus, intermediate region, terminal segment) expertly reviewed in (89). Foundation papers first describing this area between the rete testis and spermatogenic seminiferous epithelium date back to the 60’s (90–97). Sertoli cells in the transitional zone are morphologically distinct having long string-like cell bodies that extend distally into the rete testis, structurally giving the zone a valve appearance histologically (98). At least a sub-population of these transitional zone Sertoli cells has been documented by multiple labs to be proliferatively competent (99–103). Specifically, because some transitional zone Sertoli cells do not express the maturation markers p27, GATA4 and AR (101). AR is not just a marker for Sertoli cell maturation and proliferative cessation (104, 105). Loss of AR has been shown to inhibit Sertoli cell maturation (106). In men and rodents, germ cells that reside in this region are exclusively spermatogonia that seem to be predominantly undifferentiating spermatogonia (88, 92, 99, 107). Collectively the transitional zone represents a unique Sertoli-germ cell niche within the testis.
During their ablation experiments, Imura-Kishi et al. 2021, identified transitional zone Sertoli cell Cyp26a1 expression that is at least partially responsible for blocking RA signaling to the spermatogonia in the transitional zone. Due to the proximity to the rete testis, the authors also showed retrograde rete derived FGF signaling may also competitively inhibit RA signal in the transitional zone (88). A separate recent report defined two sub-populations of transitional zone Sertoli cells that were KRT8+,DMRT1- or KRT8+,DMRT1+ (108). DMRT1 is essential in differentiation of Sertoli cells into a non-proliferative state (109). These studies elucidated the molecular uniqueness of the transitional zone niche, but there is still much we do not understand about cell identity and function in the transitional zone. Given the recent reports on exosomes, one cannot help but wonder if there is also a unique population of transitional zone Sertoli cell extracellular vesicles that are part of maintaining this niche.
Ablation and transplantation are done via injection through the rete testis (110). Even when done by the most skilled pair of hands, this represents a traumatic event to the surrounding tissue. The plasticity of the Sertoli cell population in the transitional zone and the robustness of this epithelium is a fortunate coincidence for this method, but also represents an intriguing source for discoveries in reversing Sertoli cell dysfunction and repopulating a Sertoli cell deficient testis. Sertoli cells in human testes partially resume proliferation after gonadotropin suppression with coincident reduction of AR (111). Continued research into maintenance and control of proliferative transitional zone Sertoli cells in conjunction with Sertoli cell transplantation has the potential to unlock new therapeutics for treatment of Sertoli cell based male infertility, and reversing the reproductive harm done by gonadotoxic cancer treatment.
VAR generated the direction for the manuscript, and produced the figures. DJL supervised the creative process providing expert feedback and insight. VAR and DJL wrote the manuscript and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
DJL serves on the Ro advisory board, and as a consultant, and has equity; and for Fellow has equity; and serves as Secretary-Treasurer for the American Board of Bioanalysts with honorarium.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Figure 1 is from unpublished immunofluorescent imaging done by VAR while training under Dr. Blanche Capel, Duke University. Figure 2 is from unpublished light microscopy imaging done by VAR while training under Dr. Monika A Ward, University of Hawai’i at Mānoa. All imaging processing and final layouts were done by VAR in FIJI (version 2.3.0/1.53f) (112) and Adobe Photoshop (version 23.1.0). Support provided by the National Institute of General Medical Sciences (F32GM129956 to VAR); the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust (DJL); the National Institute of Diabete and Digestive and Kidney Diseases (1R01DK078121 to DJL); the Small Business Innovation Research fund (1R43HD108826-01 to Inherent Bio and DJL); the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1P50HD106793-01 to The Population Council Inc and DJL); and grants 1P50HD100549- 01 (L. Levin), and 5P01HD087157 (M.M. Matzuk) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (DJL).
1. Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. St. Louis, MO: Cache River Press (1990).
3. Russell LD, Ren HP, Sinha Hikim I, Schulze W, Sinha Hikim AP. A Comparative Study in Twelve Mammalian Species of Volume Densities, Volumes, and Numerical Densities of Selected Testis Components, Emphasizing Those Related to the Sertoli Cell. Am J Anat (1990) 188:21–30. doi: 10.1002/aja.1001880104
4. Skinner MK, Tung PS, Fritz IB. Cooperativity Between Sertoli Cells and Testicular Peritubular Cells in the Production and Deposition of Extracellular Matrix Components. J Cell Biol (1985) 100:1941–7. doi: 10.1083/jcb.100.6.1941
5. Tung PS, Fritz IB. Morphogenetic Restructuring and Formation of Basement Membranes by Sertoli Cells and Testis Peritubular Cells in Co-Culture: Inhibition of the Morphogenetic Cascade by Cyclic AMP Derivatives and by Blocking Direct Cell Contact. Dev Biol (1987) 120:139–53. doi: 10.1016/0012-1606(87)90112-6
6. Smith LB, Walker WH. Hormone Signaling in the Testis. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction, 4th ed. Cambridge, MA: Elsevier Science (2014). p. 637–90.
7. Smith LB, Walker WH. The Regulation of Spermatogenesis by Androgens. Semin Cell Dev Biol (2014) 30:2–13. doi: 10.1016/j.semcdb.2014.02.012
8. Walker WH. Androgen Actions in the Testis and the Regulation of Spermatogenesis. Adv Exp Med Biol (2021) 1288:175–203. doi: 10.1007/978-3-030-77779-1_9
9. Miyaso H, Ogawa Y, Itoh M. Microenvironment for Spermatogenesis and Sperm Maturation. Histochem Cell Biol (2022) 157:273–85. doi: 10.1007/s00418-021-02071-z
10. Neto FTL, Flannigan R, Goldstein M. Regulation of Human Spermatogenesis. Adv Exp Med Biol (2021) 1288:255–86. doi: 10.1007/978-3-030-77779-1_13
11. Ni FD, Hao SL, Yang WX. Multiple Signaling Pathways in Sertoli Cells: Recent Findings in Spermatogenesis. Cell Death Dis (2019) 10:541. doi: 10.1038/s41419-019-1782-z
12. Oakberg EF. A Description of Spermiogenesis in the Mouse and its Use in Analysis of the Cycle of the Seminiferous Epithelium and Germ Cell Renewal. Am J Anat (1956) 99:391–413. doi: 10.1002/aja.1000990303
13. Oakberg EF. Duration of Spermatogenesis in the Mouse and Timing of Stages of the Cycle of the Seminiferous Epithelium. Am J Anat (1956) 99:507–16. doi: 10.1002/aja.1000990307
14. Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, et al. The First Round of Mouse Spermatogenesis is a Distinctive Program That Lacks the Self-Renewing Spermatogonia Stage. Development (2006) 133:1495–505. doi: 10.1242/dev.02316
15. Ray D, Pitts PB, Hogarth CA, Whitmore LS, Griswold MD, Ye P. Computer Simulations of the Mouse Spermatogenic Cycle. Biol Open (2015) 4:1–12. doi: 10.1242/bio.20149068
16. Clermont Y. Renewal of Spermatogonia in Man. Am J Anat (1966) 118:509–24. doi: 10.1002/aja.1001180211
17. Clermont Y. Spermatogenesis in Man. A study of the spermatogonial population. Fertil Steril (1966) 17:705–21. doi: 10.1016/S0015-0282(16)36120-9
18. Ehmcke J, Schlatt S. A Revised Model for Spermatogonial Expansion in Man: Lessons From non-Human Primates. Reproduction (2006) 132:673–80. doi: 10.1530/rep.1.01081
19. Heller CG, Clermont Y. Spermatogenesis in Man: An Estimate of its Duration. Science (1963) 140:184–6. doi: 10.1126/science.140.3563.184
20. Hess RA, Renato de Franca L. Spermatogenesis and Cycle of the Seminiferous Epithelium. Adv Exp Med Biol (2008) 636:1–15. doi: 10.1007/978-0-387-09597-4_1
21. Muciaccia B, Boitani C, Berloco BP, Nudo F, Spadetta G, Stefanini M, et al. Novel Stage Classification of Human Spermatogenesis Based on Acrosome Development. Biol Reprod (2013) 89:60. doi: 10.1095/biolreprod.113.111682
22. Chen Y, Zheng Y, Gao Y, Lin Z, Yang S, Wang T, et al. Single-Cell RNA-Seq Uncovers Dynamic Processes and Critical Regulators in Mouse Spermatogenesis. Cell Res (2018) 28:879–96. doi: 10.1038/s41422-018-0074-y
23. Green CD, Ma Q, Manske GL, Shami AN, Zheng X, Marini S, et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev Cell (2018) 46:651–667.e610. doi: 10.1016/j.devcel.2018.07.025
24. Zimmermann C, Stevant I, Borel C, Conne B, Pitetti JL, Calvel P, et al. Research Resource: The Dynamic Transcriptional Profile of Sertoli Cells During the Progression of Spermatogenesis. Mol Endocrinol (2015) 29:627–42. doi: 10.1210/me.2014-1356
25. Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical Localization of Androgen Receptors in the Rat Testis: Evidence for Stage-Dependent Expression and Regulation by Androgens. Endocrinology (1994) 135:1227–34. doi: 10.1210/endo.135.3.8070367
26. Shan LX, Zhu LJ, Bardin CW, Hardy MP. Quantitative Analysis of Androgen Receptor Messenger Ribonucleic Acid in Developing Leydig Cells and Sertoli Cells by in Situ Hybridization. Endocrinology (1995) 136:3856–62. doi: 10.1210/endo.136.9.7649092
27. Vornberger W, Prins G, Musto NA, Suarez-Quian CA. Androgen Receptor Distribution in Rat Testis: New Implications for Androgen Regulation of Spermatogenesis. Endocrinology (1994) 134:2307–16. doi: 10.1210/endo.134.5.8156934
28. Suarez-Quian CA, Martinez-Garcia F, Nistal M, Regadera J. Androgen Receptor Distribution in Adult Human Testis. J Clin Endocrinol Metab (1999) 84:350–8. doi: 10.1210/jc.84.1.350
29. Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, et al. Infertility With Defective Spermatogenesis and Hypotestosteronemia in Male Mice Lacking the Androgen Receptor in Sertoli Cells. Proc Natl Acad Sci USA (2004) 101:6876–81. doi: 10.1073/pnas.0307306101
30. De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, et al. A Sertoli Cell-Selective Knockout of the Androgen Receptor Causes Spermatogenic Arrest in Meiosis. Proc Natl Acad Sci USA (2004) 101:1327–32. doi: 10.1073/pnas.0308114100
31. Holdcraft RW, Braun RE. Androgen Receptor Function is Required in Sertoli Cells for the Terminal Differentiation of Haploid Spermatids. Development (2004) 131:459–67. doi: 10.1242/dev.00957
32. Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, et al. Retinoic Acid Induces Sertoli Cell Paracrine Signals for Spermatogonia Differentiation But Cell Autonomously Drives Spermatocyte Meiosis. Proc Natl Acad Sci USA (2012) 109:16582–7. doi: 10.1073/pnas.1214936109
33. Larose H, Kent T, Ma Q, Shami AN, Harerimana N, Li JZ, et al. Regulation of Meiotic Progression by Sertoli-Cell Androgen Signaling. Mol Biol Cell (2020) 31:2841–62. doi: 10.1091/mbc.E20-05-0334
34. Fine AD, Ball RL, Fujiwara Y, Handel MA, Carter GW. Uncoupling of Transcriptomic and Cytological Differentiation in Mouse Spermatocytes With Impaired Meiosis. Mol Biol Cell (2019) 30:717–28. doi: 10.1091/mbc.E18-10-0681
35. De Gendt K, Verhoeven G, Amieux PS, Wilkinson MF. Genome-Wide Identification of AR-Regulated Genes Translated in Sertoli Cells In Vivo Using the RiboTag Approach. Mol Endocrinol (2014) 28:575–91. doi: 10.1210/me.2013-1391
36. Lindsey JS, Wilkinson MF. Pem: A Testosterone- and LH-Regulated Homeobox Gene Expressed in Mouse Sertoli Cells and Epididymis. Dev Biol (1996) 179:471–84. doi: 10.1006/dbio.1996.0276
37. Pitman JL, Lin TP, Kleeman JE, Erickson GF, MacLeod CL. Normal Reproductive and Macrophage Function in Pem Homeobox Gene-Deficient Mice. Dev Biol (1998) 202:196–214. doi: 10.1006/dbio.1998.8978
38. Sutton KA, Maiti S, Tribley WA, Lindsey JS, Meistrich ML, Bucana CD, et al. Androgen Regulation of the Pem Homeodomain Gene in Mice and Rat Sertoli and Epididymal Cells. J Androl (1998) 19:21–30. doi: 10.1002/j.1939-4640.1998.tb02466.x
39. Verhoeven G, Willems A, Denolet E, Swinnen JV, De Gendt K. Androgens and Spermatogenesis: Lessons From Transgenic Mouse Models. Philos Trans R Soc Lond B Biol Sci (2010) 365:1537–56. doi: 10.1098/rstb.2009.0117
40. Hu Z, Dandekar D, O’Shaughnessy PJ, De Gendt K, Verhoeven G, Wilkinson MF. Androgen-Induced Rhox Homeobox Genes Modulate the Expression of AR-Regulated Genes. Mol Endocrinol (2010) 24:60–75. doi: 10.1210/me.2009-0303
41. Hu Z, MacLean JA, Bhardwaj A, Wilkinson MF. Regulation and Function of the Rhox5 Homeobox Gene. Ann New York Acad Sci (2007) 1120:72–83. doi: 10.1196/annals.1411.011
42. MacLean JA 2nd, Hu Z, Welborn JP, Song HW, Rao MK, Wayne CM, et al. The RHOX Homeodomain Proteins Regulate the Expression of Insulin and Other Metabolic Regulators in the Testis. J Biol Chem (2013) 288:34809–25. doi: 10.1074/jbc.M113.486340
43. MacLean JA 2nd, Wilkinson MF. The Rhox Genes. Reproduction (2010) 140:195–213. doi: 10.1530/REP-10-0100
44. Bhardwaj A, Sohni A, Lou CH, De Gendt K, Zhang F, Kim E, et al. Concordant Androgen-Regulated Expression of Divergent Rhox5 Promoters in Sertoli Cells. Endocrinology (2022) 163:1–17. doi: 10.1210/endocr/bqab237
45. Abel MH, Baker PJ, Charlton HM, Monteiro A, Verhoeven G, De Gendt K, et al. Spermatogenesis and Sertoli Cell Activity in Mice Lacking Sertoli Cell Receptors for Follicle-Stimulating Hormone and Androgen. Endocrinology (2008) 149:3279–85. doi: 10.1210/en.2008-0086
46. Soffientini U, Rebourcet D, Abel MH, Lee S, Hamilton G, Fowler PA, et al. Identification of Sertoli Cell-Specific Transcripts in the Mouse Testis and the Role of FSH and Androgen in the Control of Sertoli Cell Activity. BMC Genomics (2017) 18:972. doi: 10.1186/s12864-017-4357-3
47. Hoy AM, Buck AH. Extracellular Small RNAs: What, Where, Why? Biochem Soc Trans (2012) 40:886–90. doi: 10.1042/BST20120019
48. Lo Cicero A, Stahl PD, Raposo G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr Opin Cell Biol (2015) 35:69–77. doi: 10.1016/j.ceb.2015.04.013
49. Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J Extracell Vesicles (2015) 4:27066. doi: 10.3402/jev.v4.27066
50. Mancuso F, Calvitti M, Milardi D, Grande G, Falabella G, Arato I, et al. Testosterone and FSH Modulate Sertoli Cell Extracellular Secretion: Proteomic Analysis. Mol Cell Endocrinol (2018) 476:1–7. doi: 10.1016/j.mce.2018.04.001
51. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic Comparison Defines Novel Markers to Characterize Heterogeneous Populations of Extracellular Vesicle Subtypes. Proc Natl Acad Sci USA (2016) 113:E968–977. doi: 10.1073/pnas.1521230113
52. Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J Extracell Vesicles (2013) 2:1–25. doi: 10.3402/jev.v2i0.20360
53. Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell (2016) 164:1226–32. doi: 10.1016/j.cell.2016.01.043
54. Li Q, Li H, Liang J, Mei J, Cao Z, Zhang L, et al. Sertoli Cell-Derived Exosomal MicroRNA-486-5p Regulates Differentiation of Spermatogonial Stem Cell Through PTEN in Mice. J Cell Mol Med (2021) 25:3950–62. doi: 10.1111/jcmm.16347
55. Endo T, Mikedis MM, Nicholls PK, Page DC, de Rooij DG. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules (2019) 9:1–20. doi: 10.3390/biom9120775
56. Endo T, Romer KA, Anderson EL, Baltus AE, de Rooij DG, Page DC. Periodic Retinoic Acid-STRA8 Signaling Intersects With Periodic Germ-Cell Competencies to Regulate Spermatogenesis. Proc Natl Acad Sci USA (2015) 112:E2347–2356. doi: 10.1073/pnas.1505683112
57. Griswold MD. Spermatogenesis: The Commitment to Meiosis. Physiol Rev (2016) 96:1–17. doi: 10.1152/physrev.00013.2015
58. Kent T, Arnold SL, Fasnacht R, Rowsey R, Mitchell D, Hogarth CA, et al. ALDH Enzyme Expression Is Independent of the Spermatogenic Cycle, and Their Inhibition Causes Misregulation of Murine Spermatogenic Processes. Biol Reprod (2016) 94:12. doi: 10.1095/biolreprod.115.131458
59. Tong MH, Yang QE, Davis JC, Griswold MD. Retinol Dehydrogenase 10 is Indispensible for Spermatogenesis in Juvenile Males. Proc Natl Acad Sci USA (2013) 110:543–8. doi: 10.1073/pnas.1214883110
60. Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, et al. Retinoic Acid Metabolism and Signaling Pathways in the Adult and Developing Mouse Testis. Endocrinology (2006) 147:96–110. doi: 10.1210/en.2005-0953
61. Hogarth CA, Arnold S, Kent T, Mitchell D, Isoherranen N, Griswold MD. Processive Pulses of Retinoic Acid Propel Asynchronous and Continuous Murine Sperm Production. Biol Reprod (2015) 92:37. doi: 10.1095/biolreprod.114.126326
62. Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, et al. Retinoid Signaling Determines Germ Cell Fate in Mice. Science (2006) 312:596–600. doi: 10.1126/science.1125691
63. Hogarth CA, Evans E, Onken J, Kent T, Mitchell D, Petkovich M, et al. CYP26 Enzymes Are Necessary Within the Postnatal Seminiferous Epithelium for Normal Murine Spermatogenesis. Biol Reprod (2015) 93:19. doi: 10.1095/biolreprod.115.129718
64. Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M. Cyp26b1 Expression in Murine Sertoli Cells is Required to Maintain Male Germ Cells in an Undifferentiated State During Embryogenesis. PloS One (2009) 4:e7501. doi: 10.1371/journal.pone.0007501
65. MacLean G, Li H, Metzger D, Chambon P, Petkovich M. Apoptotic Extinction of Germ Cells in Testes of Cyp26b1 Knockout Mice. Endocrinology (2007) 148:4560–7. doi: 10.1210/en.2007-0492
66. Velte EK, Niedenberger BA, Serra ND, Singh A, Roa-DeLaCruz L, Hermann BP, et al. Differential RA Responsiveness Directs Formation of Functionally Distinct Spermatogonial Populations at the Initiation of Spermatogenesis in the Mouse. Development (2019) 146:1–16. doi: 10.1242/dev.173088
67. Suzuki S, McCarrey JR, Hermann BP. An Mtorc1-Dependent Switch Orchestrates the Transition Between Mouse Spermatogonial Stem Cells and Clones of Progenitor Spermatogonia. Cell Rep (2021) 34:108752. doi: 10.1016/j.celrep.2021.108752
68. Suzuki S, McCarrey JR, Hermann BP. Differential RA Responsiveness Among Subsets of Mouse Late Progenitor Spermatogonia. Reproduction (2021) 161:645–55. doi: 10.1530/REP-21-0031
69. Bashamboo A, Ferraz-de-Souza B, Lourenco D, Lin L, Sebire NJ, Montjean D, et al. Human Male Infertility Associated With Mutations in NR5A1 Encoding Steroidogenic Factor 1. Am J Hum Genet (2010) 87:505–12. doi: 10.1016/j.ajhg.2010.09.009
70. Dimitriadis F, Tsampalas S, Tsounapi P, Giannakis D, Chaliasos N, Baltogiannis D, et al. Effects of Phosphodiesterase-5 Inhibitor Vardenafil on Testicular Androgen-Binding Protein Secretion, the Maintenance of Foci of Advanced Spermatogenesis and the Sperm Fertilising Capacity in Azoospermic Men. Andrologia (2012) 44 Suppl 1:144–53. doi: 10.1111/j.1439-0272.2010.01153.x
71. Ferlin A, Vinanzi C, Garolla A, Selice R, Zuccarello D, Cazzadore C, et al. Male Infertility and Androgen Receptor Gene Mutations: Clinical Features and Identification of Seven Novel Mutations. Clin Endocrinol (Oxf) (2006) 65:606–10. doi: 10.1111/j.1365-2265.2006.02635.x
72. Hiort O, Holterhus PM, Horter T, Schulze W, Kremke B, Bals-Pratsch M, et al. Significance of Mutations in the Androgen Receptor Gene in Males With Idiopathic Infertility. J Clin Endocrinol Metab (2000) 85:2810–5. doi: 10.1210/jc.85.8.2810
73. Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of Germ Cells From Human Testicular Tissue for Low Temperature Storage and Autotransplantation. Fertil Steril (2001) 75:269–74. doi: 10.1016/S0015-0282(00)01721-0
74. Gul M, Hildorf S, Dong L, Thorup J, Hoffmann ER, Jensen CFS, et al. Review of Injection Techniques for Spermatogonial Stem Cell Transplantation. Hum Reprod Update (2020) 26:368–91. doi: 10.1093/humupd/dmaa003
75. Kaponis A, Yiannakis D, Tsoukanelis K, Tsalikis D, Tsabalas D, Baltogiannis D, et al. The Role of Ultrasonographically Guided Puncture of the Human Rete Testis in the Therapeutic Management of Nonobstructive Azoospermia. Andrologia (2003) 35:85–92. doi: 10.1046/j.1439-0272.2003.00526.x
76. Ning L, Meng J, Goossens E, Lahoutte T, Marichal M, Tournaye H. In Search of an Efficient Injection Technique for Future Clinical Application of Spermatogonial Stem Cell Transplantation: Infusion of Contrast Dyes in Isolated Cadaveric Human Testes. Fertil Steril (2012) 98:1443–1448.e1441. doi: 10.1016/j.fertnstert.2012.08.023
77. Ma Y, Zhou Y, Xiao Q, Zou SS, Zhu YC, Ping P, et al. Seminal Exosomal miR-210-3p as a Potential Marker of Sertoli Cell Damage in Varicocele. Andrology (2021) 9:451–9. doi: 10.1111/andr.12913
78. Xie Y, Yao J, Zhang X, Chen J, Gao Y, Zhang C, et al. A Panel of Extracellular Vesicle Long Noncoding RNAs in Seminal Plasma for Predicting Testicular Spermatozoa in Nonobstructive Azoospermia Patients. Hum Reprod (2020) 35:2413–27. doi: 10.1093/humrep/deaa184
79. Brinster RL, Zimmermann JW. Spermatogenesis Following Male Germ-Cell Transplantation. Proc Natl Acad Sci USA (1994) 91:11298–302. doi: 10.1073/pnas.91.24.11298
80. Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Germline Niche Transplantation Restores Fertility in Infertile Mice. Hum Reprod (2005) 20:2376–82. doi: 10.1093/humrep/dei096
81. Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Restoration of Spermatogenesis in Infertile Mice by Sertoli Cell Transplantation. Biol Reprod (2003) 68:1064–71. doi: 10.1095/biolreprod.102.009977
82. Rebourcet D, O’Shaughnessy PJ, Monteiro A, Milne L, Cruickshanks L, Jeffrey N, et al. Sertoli Cells Maintain Leydig Cell Number and Peritubular Myoid Cell Activity in the Adult Mouse Testis. PloS One (2014) 9:e105687. doi: 10.1371/journal.pone.0105687
83. Rebourcet D, O’Shaughnessy PJ, Pitetti JL, Monteiro A, O’Hara L, Milne L, et al. Sertoli Cells Control Peritubular Myoid Cell Fate and Support Adult Leydig Cell Development in the Prepubertal Testis. Development (2014) 141:2139–49. doi: 10.1242/dev.107029
84. Shinomura M, Kishi K, Tomita A, Kawasumi M, Kanezashi H, Kuroda Y, et al. A Novel Amh-Treck Transgenic Mouse Line Allows Toxin-Dependent Loss of Supporting Cells in Gonads. Reproduction (2014) 148:H1–9. doi: 10.1530/REP-14-0171
85. Yokonishi T, McKey J, Ide S, Capel B. Sertoli Cell Ablation and Replacement of the Spermatogonial Niche in Mouse. Nat Commun (2020) 11:40. doi: 10.1038/s41467-019-13879-8
86. Merchel Piovesan Pereira B, Tagkopoulos I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl Environ Microbiol (2019) 85:1–13. doi: 10.1128/AEM.00377-19
87. Yokonishi T, Capel B. Differentiation of Fetal Sertoli Cells in the Adult Testis. Reproduction (2021) 162:141–7. doi: 10.1530/REP-21-0106
88. Imura-Kishi K, Uchida A, Tsunekawa N, Suzuki H, Takase HM, Hirate Y, et al. Low Retinoic Acid Levels Mediate Regionalization of the Sertoli Valve in the Terminal Segment of Mouse Seminiferous Tubules. Sci Rep (2021) 11:1110. doi: 10.1038/s41598-020-79987-4
89. Figueiredo AFA, Hess RA, Batlouni SR, Wnuk NT, Tavares AO, Abarikwu SO, et al. Insights Into Differentiation and Function of the Transition Region Between the Seminiferous Tubule and Rete Testis. Differentiation (2021) 120:36–47. doi: 10.1016/j.diff.2021.06.002
90. Dym M. The Fine Structure of Monkey Sertoli Cells in the Transitional Zone at the Junction of the Seminiferous Tubules With the Tubuli Recti. Am J Anat (1974) 140:1–25. doi: 10.1002/aja.1001400102
91. Hermo L, Dworkin J. Transitional Cells at the Junction of Seminiferous Tubules With the Rete Testis of the Rat: Their Fine Structure, Endocytic Activity, and Basement Membrane. Am J Anat (1988) 181:111–31. doi: 10.1002/aja.1001810202
92. Lindner SG, Holstein AF. On the Morphology of the Transitional Zone of the Seminiferous Tubule and the Rete Testis in Man. Andrologia (1982) 14:352–62. doi: 10.1111/j.1439-0272.1982.tb02277.x
93. Marin-Padilla M. The Mesonephric-Testicular Connection in Man and Some Animals. Anatomical Rec (1964) 148:1–14. doi: 10.1002/ar.1091480102
94. Nykanen M. Fine Structure of the Transitional Zone of the Rat Seminiferous Tubule. Cell Tissue Res (1979) 198:441–54. doi: 10.1007/BF00234189
95. Osman DI, Ploen L. The Mammalian Tubuli Recti: Ultrastructural Study. Anatomical Rec (1978) 192:1–17. doi: 10.1002/ar.1091920102
96. Perey B, Clermont Y, Leblond CP. The Wave of the Seminiferous Epithelium of the Rat. Am J Anat (1961) 108:47–77. doi: 10.1002/aja.1001080105
97. Wrobel KH, Sinowatz F, Kugler P. The Functional Morphology of the Rete TestisTubuli Recti and Terminal Segments of the Semeniferous Tubules in the Mature Bull. Anat Histol Embryol (1978) 7:320–35. doi: 10.1111/j.1439-0264.1978.tb00671.x
98. Takahashi K, Naito M, Terayama H, Qu N, Cheng L, Tainosho S, et al. Immunomorphological Aspects of the Tubuli Recti and the Surrounding Interstitium in Normal Mice. Int J Androl (2007) 30:21–7. doi: 10.1111/j.1365-2605.2006.00704.x
99. Aiyama Y, Tsunekawa N, Kishi K, Kawasumi M, Suzuki H, Kanai-Azuma M, et al. A Niche for GFRalpha1-Positive Spermatogonia in the Terminal Segments of the Seminiferous Tubules in Hamster Testes. Stem Cells (2015) 33:2811–24. doi: 10.1002/stem.2065
100. Chui K, Trivedi A, Cheng CY, Cherbavaz DB, Dazin PF, Huynh AL, et al. Characterization and Functionality of Proliferative Human Sertoli Cells. Cell Transplant (2011) 20:619–35. doi: 10.3727/096368910X536563
101. Figueiredo AF, Franca LR, Hess RA, Costa GM. Sertoli Cells are Capable of Proliferation Into Adulthood in the Transition Region Between the Seminiferous Tubules and the Rete Testis in Wistar Rats. Cell Cycle (2016) 15:2486–96. doi: 10.1080/15384101.2016.1207835
102. Figueiredo AFA, Wnuk NT, Tavares AO, Miranda JR, Hess RA, de Franca LR, et al. Prepubertal PTU Treatment in Rat Increases Sertoli Cell Number and Sperm Production. Reproduction (2019) 158:199–209. doi: 10.1530/REP-19-0127
103. Kulibin AY, Malolina EA. Only a Small Population of Adult Sertoli Cells Actively Proliferates in Culture. Reproduction (2016) 152:271–81. doi: 10.1530/REP-16-0013
104. Hazra R, Corcoran L, Robson M, McTavish KJ, Upton D, Handelsman DJ, et al. Temporal Role of Sertoli Cell Androgen Receptor Expression in Spermatogenic Development. Mol Endocrinol (2013) 27:12–24. doi: 10.1210/me.2012-1219
105. Tan KA, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PT, et al. The Role of Androgens in Sertoli Cell Proliferation and Functional Maturation: Studies in Mice With Total or Sertoli Cell-Selective Ablation of the Androgen Receptor. Endocrinology (2005) 146:2674–83. doi: 10.1210/en.2004-1630
106. Willems A, Batlouni SR, Esnal A, Swinnen JV, Saunders PT, Sharpe RM, et al. Selective Ablation of the Androgen Receptor in Mouse Sertoli Cells Affects Sertoli Cell Maturation, Barrier Formation and Cytoskeletal Development. PloS One (2010) 5:e14168. doi: 10.1371/journal.pone.0014168
107. Nagasawa K, Imura-Kishi K, Uchida A, Hiramatsu R, Kurohmaru M, Kanai Y. Regionally Distinct Patterns of STAT3 Phosphorylation in the Seminiferous Epithelia of Mouse Testes. Mol Reprod Dev (2018) 85:262–70. doi: 10.1002/mrd.22962
108. Malolina EA, Kulibin AY. The Rete Testis Harbors Sertoli-Like Cells Capable of Expressing DMRT1. Reproduction (2019) 158:399–413. doi: 10.1530/REP-19-0183
109. Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a Gene Related to Worm and Fly Sexual Regulators, is Required for Mammalian Testis Differentiation. Genes Dev (2000) 14:2587–95. doi: 10.1101/gad.834100
110. Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of Testis Germinal Cells Into Mouse Seminiferous Tubules. Int J Dev Biol (1997) 41:111–22.
111. Tarulli GA, Stanton PG, Loveland KL, Rajpert-De Meyts E, McLachlan RI, Meachem SJ. A Survey of Sertoli Cell Differentiation in Men After Gonadotropin Suppression and in Testicular Cancer. Spermatogenesis (2013) 3:e24014. doi: 10.4161/spmg.24014
Keywords: sertoli cell (SC) niche, transitional zone (TZ), Sertoli cell ablation, Sertoli cell transplantation, Spermatogenesis, FSH signaling, AR signaling, Exosome extracellular vesicle (EV)
Citation: Ruthig VA and Lamb DJ (2022) Updates in Sertoli Cell-Mediated Signaling During Spermatogenesis and Advances in Restoring Sertoli Cell Function. Front. Endocrinol. 13:897196. doi: 10.3389/fendo.2022.897196
Received: 15 March 2022; Accepted: 31 March 2022;
Published: 04 May 2022.
Edited by:
Barry Zirkin, Johns Hopkins University, United StatesReviewed by:
Michael Griswold, Washington State University, United StatesCopyright © 2022 Ruthig and Lamb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dolores J. Lamb, ZGxhbWJAbWVkLmNvcm5lbGwuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.