
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 02 June 2022
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.883827
This article is part of the Research TopicThyroid Endocrine DisruptorsView all 10 articles
Objectives: Triclosan is an antibacterial agent suspected to disrupt the endocrine system. The aim of this study was to investigate the influence of triclosan on the human thyroid system through a systematic literature review of human studies.
Methods: Eligibility criteria and method of analysis were registered at Prospero (registration number: CRD42019120984) before a systematic search was conducted in Pubmed and Embase in October 2020. Seventeen articles were found eligible for inclusion. Thirteen studies were observational, while four had a triclosan intervention. Participants consisted of pregnant women in eight studies, of men and non-pregnant women in seven studies and of chord samples/newborns/children/adolescents in six studies. The outcomes were peripheral thyroid hormones and thyroid-stimulating hormone (TSH) in blood samples.
Results: Several studies found a negative association between triclosan and triiodothyronine and thyroxine, and a positive association with TSH; however, the opposite associations or no associations were also found. In general, the studies had limited measurement timepoints of thyroid outcomes, and the interventional studies used low concentrations of triclosan. Thus, study design limitations influence the quality of the dataset and it is not yet possible to conclude whether triclosan at current human exposure levels adversely affects the thyroid hormone system.
Conclusions: Further larger studies with more continuity and more elaborate outcome measurements of thyroid function are needed to clarify whether triclosan, at current exposure levels, affects the human thyroid hormone system.
Systematic Review Registration: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42019120984, identifier PROSPERO (CRD42019120984).
A large number of environmental endocrine disrupting chemicals can affect the thyroid hormone system (1–3). Chemicals can reduce circulating levels of thyroid hormones by a number of mechanisms, including inhibition of iodide uptake and thyroid hormone synthesis in the thyroid gland. However, these are not the only mechanisms of thyroid hormone system disruption and an increasing number of chemicals have been shown to interfere with the thyroid hormone receptor, enzymes or with serum distribution proteins and transporters that play important roles in mediating thyroid hormone action (1–3) (Figure 1).
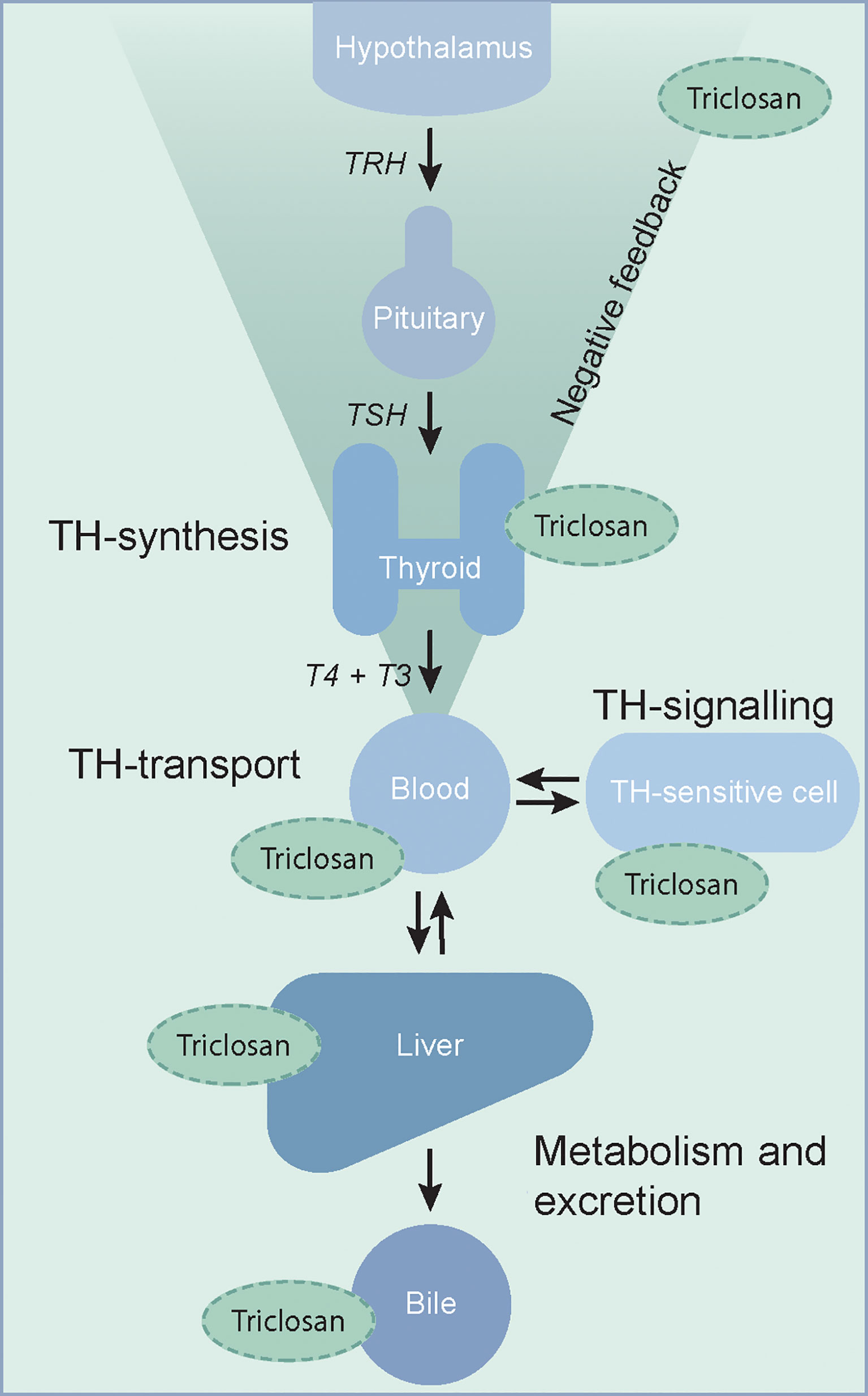
Figure 1 The thyroid hormone system is a complex endocrine system with many potential targets of thyroid hormone system disrupting chemicals. Putative sites of interference by triclosan, as discussed in this review, are indicated. TH, thyroid hormone; T4, thyroxine; T3, 3,3´,5-tri-iodothyronine; TSH, thyroid stimulating hormone; TRH, thyrotropin releasing hormone.
Research endeavours have been intensive within the area and the importance of an optimal thyroid function throughout life for development and maintenance of normal metabolic functions as well as neurological and brain development both in foetal life, and later, has been acknowledged. Nevertheless, the field is still lacking sufficient understanding of the effects on the endocrine systems from the numerous chemicals utilized in everyday household and cosmetic products.
Triclosan (5-Chloro-2-(2,4-dichlorophenoxy)phenol) has been under investigation for its possible endocrine disrupting effects in humans through the last decades (4, 5). Triclosan is an antibacterial agent used in industrial, personal and household products, such as deodorants and toothpaste (4) and it can be detected in the urine of 97.1% of young Danish men (6), although with a decreasing tendency over 8 years (7). This decreasing tendency was in agreement with results from The National Health and Nutrition Examination Survey (NHANES) of nationally representative sample of about 5,000 persons each year from different places of United States of America. However, in the NHANES study covering data from 2013-14 (8) the concentration was about three to ten times higher in young American compared to the young Danish men.
Structurally, triclosan resembles thyroxine (T4), (Figure 2) and it can disrupt the thyroid hormone system (9–12). Thus, in rodents, triclosan consistently reduces serum T4 concentrations, probably by increasing hepatic catabolism of thyroid hormones (9, 13, 14).
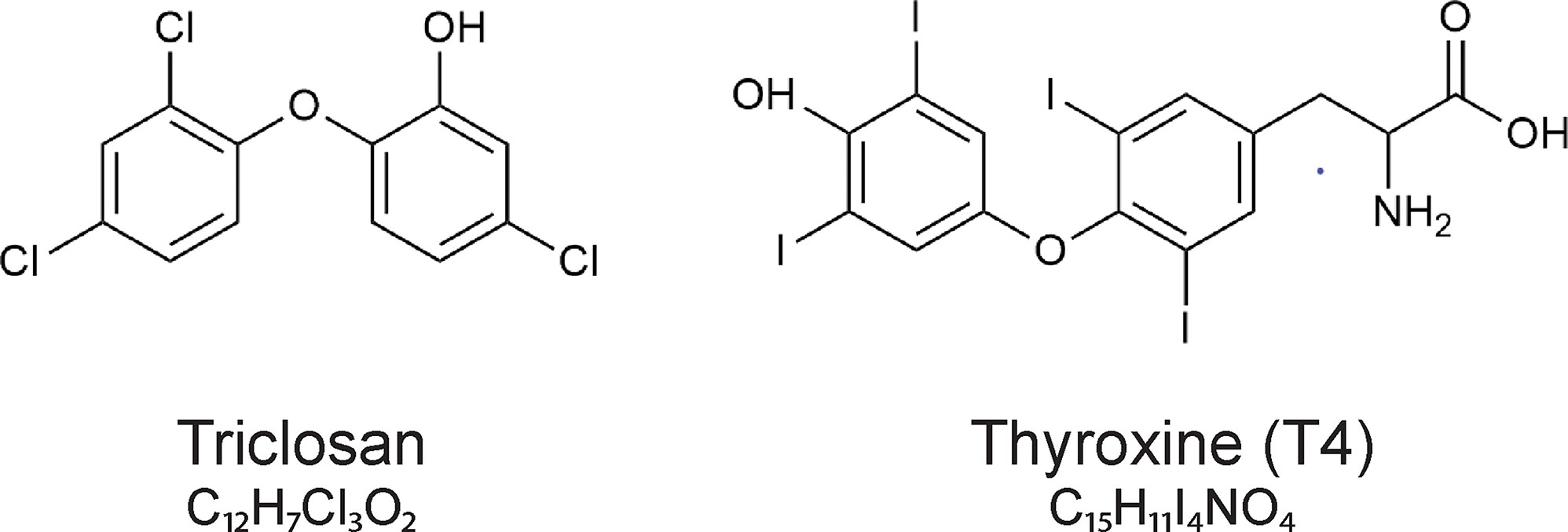
Figure 2 The chemical structures of triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol)) and the thyroid hormone thyroxine (T4).
In the European Union triclosan is registered under the REACH (Registration, Evaluation, Authorization and Restriction of Chemical substances) regulation and it is under evaluation as an endocrine disruptor (15, 16). Triclosan is currently not being manufactured in or imported to the European Economic Area (16) but exposure appears to be continuing with triclosan still being found in the urine of young Danish men (7). The aim of this study was to investigate the effect of triclosan on the human thyroid hormone system through a systematic literature review of human studies.
As guidance in the process of this systematic review, we used the PRISMA statement for reporting systematic reviews (17) and Cochrane Handbook for systematic reviews.
The method of analysis and criteria have been specified in advance and registered in two protocols concerning respectively human and animal studies at Prospero (registration number: CRD42019120984). Here we report the results from the protocol concerning the human studies. All human studies concerning the effect of triclosan on thyroid function, thyroid growth and thyroid cell morphology directly were included in the search. The studies had to be in English and with original data. No restrictions were imposed concerning publication date, type of participants or type of outcome measures, except that details of the method used must be reported. The only restriction toward type of intervention was exclusion in case of deliberate exposure to other potential endocrine disruptors in combination with triclosan (supplementary material).
Studies were identified by systematic searches on Pubmed (Medline/US National Library of Medicine), 1946 to present) and Embase (1974 to present, provider: Ovid). The searches were done in October 2020.
Search terms were selected through MeSH and “search-tools” search in PubMed and Embase, respectively. The full search strategy can be seen in the supplementary material.
One author (MH) screened titles and abstracts for reports that matched eligibility criteria. If doubt of inclusion, full text was read, and a second author (ÅKR or UFR) was consulted. A data-extraction sheet was developed by MH and data was collected regarding study design, participant characteristics, tissue studied and thyroid endpoints, primary outcome of the study, timing of outcome measurement and method used, other relevant outcomes, confounders and information to assess risk of bias. Further data for interventional studies was collected regarding intervention groups, route of administration, dose and frequency of exposure.
If the authors of more studies were the same and study participants were similar, the study characteristics and outcomes were compared to avoid replicates.
The risk of bias in the included studies was assessed by guidance of the Cochrane Collaboration’s tool for assessing risk of bias.
When searching the two databases 247 records were identified, and 154 remained after removal of duplicates. Additional four articles were found through other sources. Screening of the 158 titles and abstracts led to exclusion of 129 reports. The remaining 29 reports were read in full text and assessed for eligibility. Twelve reports did not match eligibility criteria and were excluded. Finally, a total of 17 reports met the criteria and were included in the review (Figure 3).
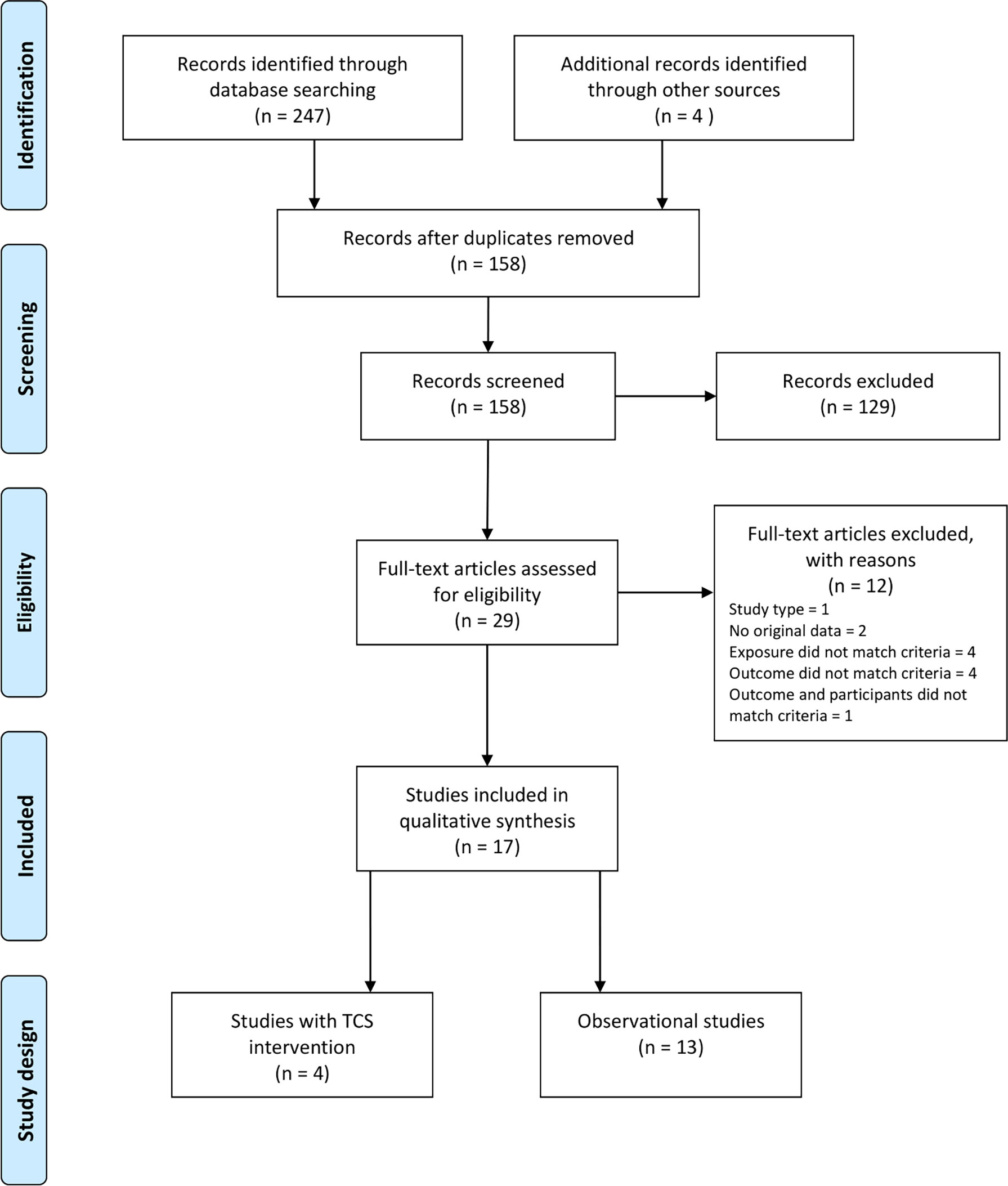
Figure 3 PRISMA diagram of selection of eligible studies (4 studies of triclosan intervention and 13 observational studies) included in the search for studies of triclosan exposure and human thyroid function.
The study characteristics of the 17 included human studies are summarised in Table 1. Thirteen studies were observational, while four had a triclosan intervention.
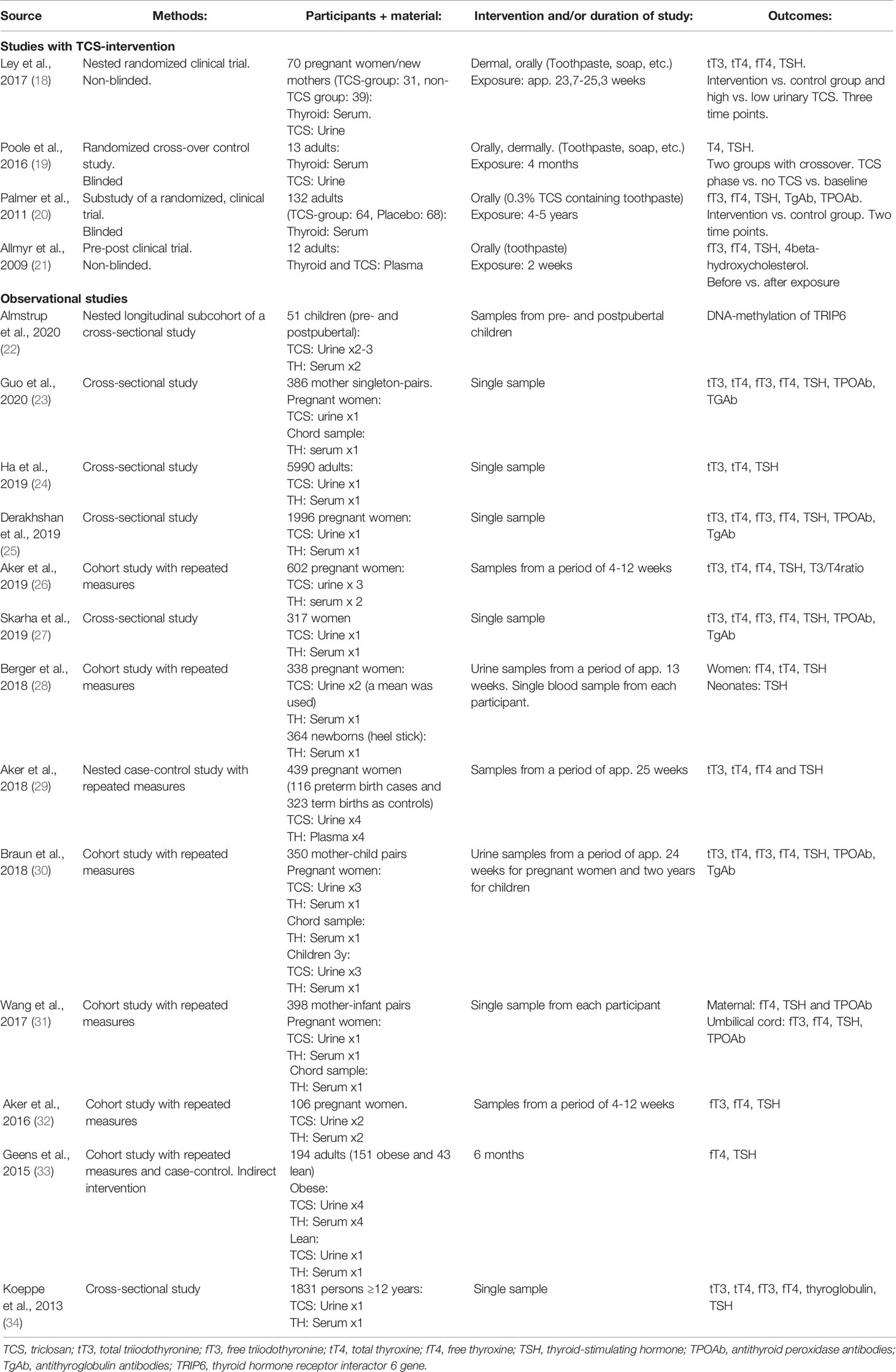
Table 1 Characteristics of the articles (four studies with triclosan intervention and 13 observational studies) obtained from searches at PubMED (1946 to Oct 2020) and Embase (1974 to Oct 2020) of human studies concerning the effect of triclosan on the thyroid hormone system.
All 17 human studies measured thyroid function outcomes in blood samples and when the triclosan concentration was assessed, it was measured in urine samples. Eight of the studies used serum from pregnant women (18, 25, 26, 28–32). Three studies used chord serum samples (23, 30, 31) and four used serum from newborns/children/adolescents (22, 28, 30, 34). Seven studies investigated men and non-pregnant women (19–21, 24, 27, 33, 34).
One interventional study was randomized (n=70) (18), two were randomized and blinded trials (n=132, n=13) (19, 20), while one study compared the outcome before and after the triclosan intervention (n=12) (21).
The triclosan interventions consisted of household and personal care products containing triclosan in two of the studies (18, 19) and only triclosan containing toothpaste in the other two (20, 21). Three of the interventional studies measured the triclosan concentration in blood or urine after exposure, but only one assessed the association between the actual measured triclosan concentration and thyroid endpoints (18). The exposure time varied between two weeks and five years.
The four intervention studies were assessed for risk of bias by using a modified version of the Cochrane Collaboration’s “Risk of Bias” tool. Because the majority of included studies were cross sectional, we chose to use JBI Critical appraisal tool specifically for cross sectional studies (35, 36) (Supplementary Material, Tables 3, 4).
In general, the observational studies informed about eligibility criteria, study population, confounding factors, exposure and outcome measurements. Most of the interventional studies took risk of bias into account in their study design. However, unclear information about randomization method, blinding and handling of incomplete outcome data prevented thorough risk of bias assessment.
The results of the included studies are summarised in Tables 2 and 3.
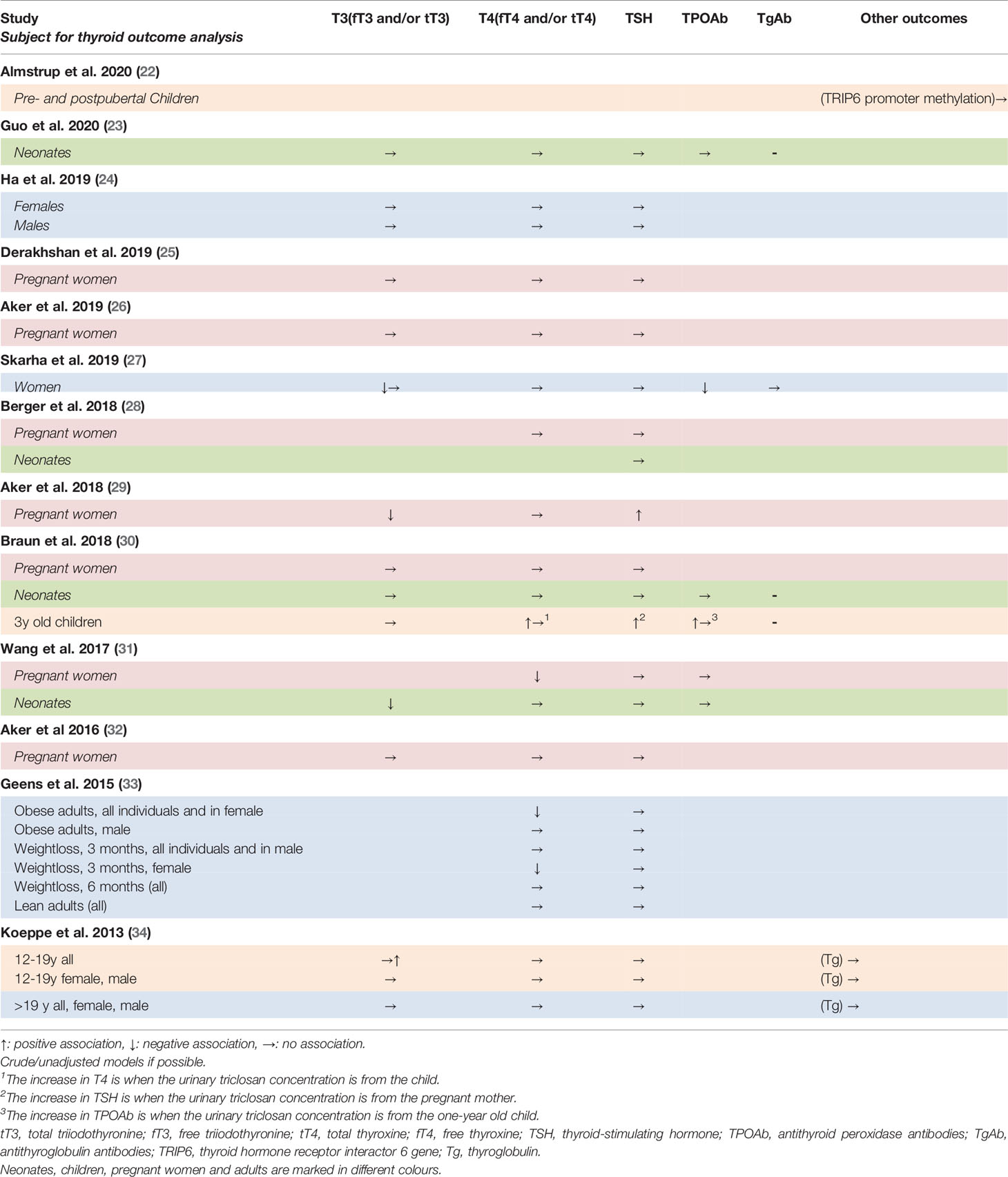
Table 2 Summary of findings of associations between triclosan concentrations and thyroid hormone system variables in observational studies of adult humans.
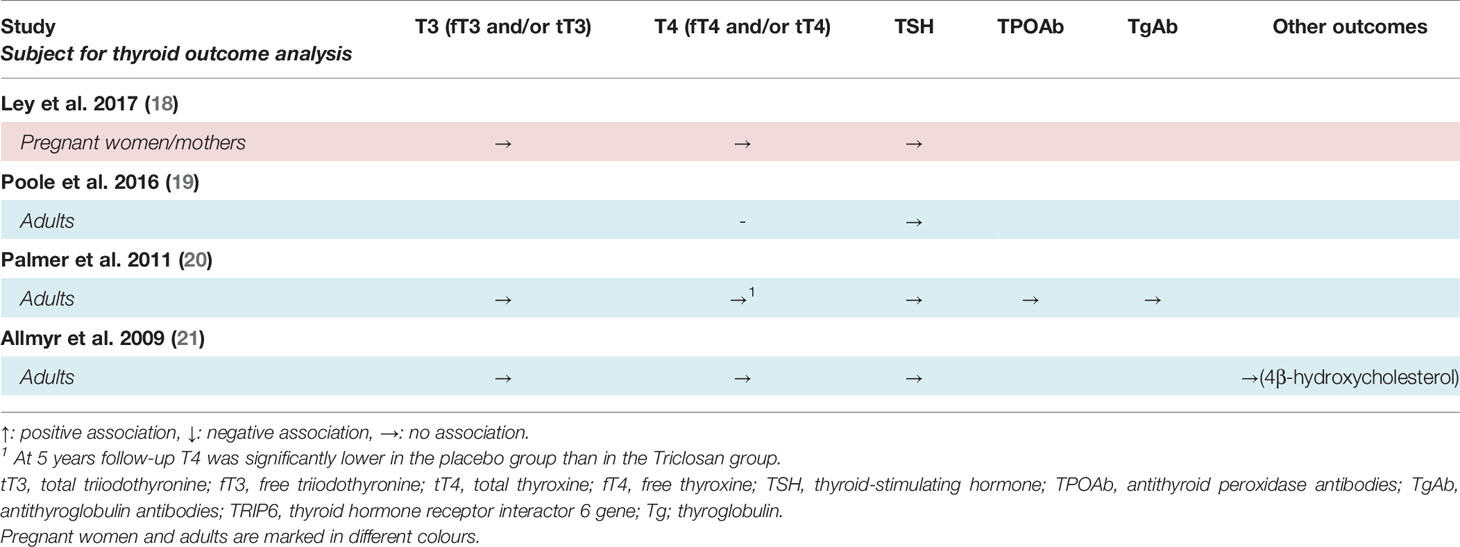
Table 3 Summary of findings of associations between triclosan concentrations and thyroid gland variables in interventional studies of human adults.
Four studies investigated the effect of triclosan on thyroid hormones by interventional experiments and none found a significant effect (18, 19, 21), except for one study observing a decrease in free T4 (fT4) in the placebo group (20).
The two studies who exposed participants through triclosan-containing household and/or care products observed a significant increase in urinary triclosan after the intervention (18, 19). However, none of the two studies observed a significant association between the intervention arm and T4 and thyroid-stimulating hormone (TSH), respectively after four months, nor of total triiodothyronine (T3), total T4, fT4 and TSH after approximately 23 weeks of exposure. There was no association between urinary triclosan concentration and serum thyroid hormones (18).
Two studies investigated the effects of triclosan-containing toothpaste (20, 21). Palmer and co-workers did not find an association between triclosan and free T3 (fT3), TSH, antithyroglobulin antibodies (TgAb) nor antithyroid peroxidase antibodies (TPOAb) when comparing the intervention and control group after 4 years of exposure. However, fT4 was significantly decreased after 4 years use of placebo, while the triclosan-exposed participants had an unchanged concentration (20). The other study, on the use of toothpaste, did not find an association between triclosan and fT3, fT4, TSH nor of 4beta-hydroxycholesterol, respectively, before and after 2 weeks of exposure, although the plasma triclosan concentration was significantly increased (21).
Ten studies assessed the associations between urinary triclosan concentrations and serum T3 while 12 studies measured T4. The associations to TSH were investigated in 12 studies while that on TPOAb and TgAb in 5 and 4 studies, respectively.
Ten studies assessed the associations of triclosan to the concentration of T3 (23–27, 29–32, 34). Four of them found a significant association between triclosan and T3 - three found triclosan associated with a decrease in T3, while one found an increase. The decrease in T3 was seen in studies of 317 women with no history of hypo- or hyperthyroidism seeking medically assisted reproductive treatment, 439 pregnant women and 398 mother-infant pairs (27, 29, 31), while the increase was seen in adolescents (34).
Several models were adjusted for demographic factors and other confounders; however, it did not lead to any major changes in the results or the conclusions.
Nine of the twelve studies on the associations between triclosan and T4 did not find a significant effect (23–29, 32, 34), while three did (30, 31, 33). Two of these three studies found an association between triclosan and a decrease in T4, while the last found triclosan related to an increase (30).
One study followed pregnant women and their children until the age of three and found a positive association between childhood urinary triclosan and T4 concentrations in 3-year olds, however similar associations were not found in pregnant women or neonates (30). Another study of pregnant women found that triclosan was associated with a decrease in T4, while no association was found between maternal urinary triclosan and T4 in cord serum samples (31). A study of obese persons undergoing a weight loss found a negative association between fT4 and urinary triclosan before the weight loss. After three months of weight loss this association was only seen in females (33).
Several models were further adjusted for demographic factors and other confounders, but no substantial changes were seen, apart from one study of pregnant women describing a decrease in T4 after adjustments for maternal age, education, country of birth, poverty index at baseline and similarly when further adjusted for benzophenone-3, triclosan and the sum of dialkyl phosphate metabolites (data not shown) (28).
Moving to the pituitary response to peripheral thyroid hormones, ten of 12 studies did not find an association between triclosan and TSH (23–28, 31–34) while two studies demonstrated an increase in TSH with increasing triclosan concentration in the urine (29, 30). In one of the studies including pregnant women, this increase disappeared when the models were stratified by gestational age (29). The other study followed pregnant women and their children until the age of three. They found a positive association between gestational urinary triclosan and serum TSH in the children at age three, but not in the pregnant women nor in the neonates (30). The association in children was not significant when the model was adjusted for demographic factors (data not shown), however, no changes were observed in the remaining studies after adjustment for gestational age, body mass index etc.
Five studies measured thyroid autoantibodies (25, 27, 30, 31, 37). Two of these found a significant association between TPOAb and triclosan (27, 30), while one assessed TPOAb and TgAb as confounders in a cross sectional study of 1996 pregnant women (25). They described that the association of triclosan with TSH or T4 did not differ according to TPOAb status (p>0.05). No calculations were mentioned for TgAb.
A cross-sectional study of 317 women described a negative association between TPOAb and urinary triclosan (27). Another study followed pregnant women and their children until the age of three (30). Here, 328% higher (95% CI: 18, 1457) TPOAb levels in three years olds were positively associated with each 10-fold urinary triclosan level at the age of one. No association was found with gestational or other childhood triclosan concentrations, nor with TPOAb levels at delivery (30).
Serum thyroglobulin concentrations were measured in a single study of 1831 men and women at the age of 12 and older. Here, there were no statistically significant associations with triclosan in any analyses (34). Another study investigated the association between triclosan and the promoter methylation of the thyroid hormone receptor interactor 6 gene (TRIP6) in 51 pubertal children and did not find any association (22).
This review explores the potential effects of triclosan on thyroid parameters in humans. The outcomes investigated in nearly all studies were the pituitary hormone TSH and the thyroid hormones, T4 and T3. T4 is produced and released from the thyroid gland in larger quantities than the more active T3. Most of the T4 is deiodinated to T3 or the inactive reverse T3 in peripheral tissue cells, where T3 can bind to, and activate nuclear receptors. T4 is thus considered a prohormone. Measurement of the hormones in serum can be as total T4 and T3 measurements: the free hormones plus the hormones bound to the binding proteins thyroxine-binding globulin, albumin and transthyretin are measured. Alternatively, measurements can be made as an estimate of the free hormone concentrations (fT4 and fT3), which comprises a very small fraction: of the hormones in the circulation. These small amounts result in a high variability in measurements partly due to the low concentrations, partly to the protein binding and risk of disturbance of equilibrium by physiological, pathophysiological and pharmacological interferences (38). It has not been established, if triclosan can disturb this equilibrium and influence measurements of free thyroid hormone in urine and plasma although it is possible due to the ability of triclosan to bind the serum distribution protein transthyretin (39–43).
The 13 observational studies were all judged to have a low risk of bias (Supplementary Material, Table 3). Of the 4 intervention studies, two of them lacked blinding (18, 21) and in two it was impossible to judge attrition bias because of missing information on incomplete outcome data (18, 19). Despite the more positive judgement of risk of bias in the observational than in the interventional studies, observational studies should nevertheless in general be evaluated more critically than randomized controlled clinical trials.
Based on the 17 included studies, it is not possible to determine the potential effect of triclosan on the human thyroid system at current exposure levels. Although several studies found a negative association between triclosan and T3 and T4, and a positive association with TSH; the opposite associations as well as no association in the majority of the studies were found. The results are thus still ambiguous. Most of the included studies investigated women and children, both of whom are more prone to thyroid diseases than men. During development adequate and timely supplies of the thyroid hormones are essential for fetal and childhood development, particularly for the nervous system and cognitive function, but also for growth (44). However, it is difficult to interpret thyroid status in pregnant women due to pronounced physiological changes during pregnancy, including a necessary physiological increase in thyroid hormone binding proteins, which by definition changes measurements and conclusions for both total and free hormones (45). The final interpretation of thyroid hormone concentrations are therefore in each case best based on a combination of TSH, total T4 and T3 and fT4 and fT3 estimates as well as thyroid binding protein concentrations (46).
One of the limitations of this study was that the majority of included studies were cross sectional or repeated measures studies with two to four samples. This is a result of the strict inclusion criteria and partly a consequence of the questions addressed in this review. Ethically, it is not possible to administer high amounts of triclosan in a randomized clinical trial. Furthermore, a satisfactory exclusion of other confounders in the form of potential presence of other endocrine disrupting chemicals (EDCs) is very difficult without a high degree of isolation from everyday products. This makes good randomized clinical trials difficult to execute. Based on observational studies we cannot establish causal relationships between triclosan exposure and measured outcome, and exposure to other substances as well as other confounders may influence the results.
Four of the included studies were clinical trials exposing participants to triclosan via the daily use of personal care products such as toothpaste and soap. The concentration of triclosan in the intervention products was low and exposure reflected a realistic triclosan exposure from the use of a single triclosan-containing product, but this compromised distinction from other EDCs and exposure to triclosan from multiple sources. Furthermore, the numbers of participants were small. The strength of evidence in the interventional studies is therefore not clearly higher than in the cross-sectional studies.
To use a single or few blood and urine samples to assess the association between triclosan and the thyroid system, does not elaborate the possible effects of triclosan adequately. Both because of the possible changes in the association over time and because of the numerous interactions in the endocrine system and with other EDCs. Investigations of limited parts of the system are consequently uncertain (Figure 1). The applicability of the studies is especially debatable when regarding the eight studies of pregnant women, since the thyroid hormone homeostasis is particularly dynamic during pregnancy. In the beginning of a pregnancy, the amount of thyroxine-binding globulin rises drastically because of estrogen stimulation of the hepatic production (47). At the same time, the thyroid hormone requirement is increasing as a consequence of fetal thyroid hormone consumption, plasma volume expansion, thyroid hormone metabolism, and increase in renal clearance of iodide, and finally conversion of T4 to reverse T3 in the placenta by deiodinase 3 also increases (47, 48). This requires the thyroid gland to increase thyroid hormone synthesis, although changes in the measured serum hormone concentrations are rather small. Still, this renders the pregnant thyroid hormone system vulnerable to exogenous stressors. Total T3 and T4 reach a plateau around gestational week 20, but until this point, T4 and thyroxine-binding globulin levels are constantly changing and exhibit wide individual variation (49, 50). Therefore, the associations may vary considerably according to the measurement time point and it is possible that the effect of triclosan also varies over the course of pregnancy (44, 51).
One study included measurements throughout pregnancy and collected blood and urine in all three trimesters, this study found no associations between triclosan and fT4, total T4, total T3 or TSH at any time point (29). However, most of the included observational studies of pregnant women had only one or two measurement timepoints. Thus, variations between individuals and their pregnancy timing can reduce the statistical power of the studies and the differences in study designs impairs comparisons between the studies. Although the thyroid hormone balance is more stable in non-pregnant individuals the limited measurement timepoints are also in this respect critical for the power of the studies.
Aker and co-workers suggested in their study from 2019 (26) that the decreased total T3 could be a result of the structural similarity between triclosan and T3/T4. This corresponds with the speculations of Wang et al, that triclosan acts as negative feedback on the hypothalamic-pituitary-thyroid axis with inhibition of TSH secretion as a result (31). This could occur by imitating the effect of T3/T4 in the negative feedback loop. The thyrotrophs monitor the intracellular T3 concentration, where already formed T3 enters from plasma and deiodinase type II transforms T4 to T3. T3 subsequently decreases the number of thyrotropin releasing hormone (TRH) receptors on the surface of the thyrotrophs and also inhibits the synthesis of TSH (52). Another explanation could be through inhibition of the TRH-receptor or the successive phospholipase c pathway which upon stimulation increases synthesis and release of effect of TSH (52). Wang et al. found an association between a reduction of serum TSH and the medium tertile of urinary triclosan concentrations (31), which could be in keeping with above theories.
Conversely, Aker et al. demonstrated an increase in TSH in their study from 2018 (29) and argued that if triclosan was responsible for the decrease in T3, then TSH would increase given the negative feedback loop. This consequently implies an assumption that triclosan does not inhibit the TSH release. An alternative explanation could be that triclosan inhibits the effect of TSH effects in the thyrocytes, which would reduce the T3 and T4 synthesis and release, and lead to a rise in TSH. However, many other mechanisms act on the feedback loop, among other somatostatin and dopamine (52).
Most included studies found a decrease in either T3 or T4, however Koeppe et al. (34) and Braun et al. (30) found increases in T3 and T4, respectively. Sulfation is the rate-limiting step for T3 and glucuronidation for T4 in biliary excretion of thyroid hormones (53), and triclosan has been shown to inhibit both enzymes, which could explain the increase (54, 55). Paul and co-workers oppositely found increased T4-glucuronidation, and phase II enzymes responsible for thyroid hormone catabolism in the liver were upregulated in rodents (9).
The mechanisms by which triclosan potentially disrupts the thyroid hormone system has been assessed in numerous in vitro studies of human cell lines and cytosol. Butt and coworkers (55) investigated the effect of triclosan on deiodinases from human liver cells (56). Only type 1 of the outer ring-deiodinases is present in the liver, and triclosan inhibited its transformation of T4 to T3. Type 2 deiodinase is present in the CNS, where it transforms T4 to T3 and thereby affects the negative feedback loop of thyroid hormones and TSH. It could be interesting to investigate whether triclosan also inhibits type 2 deiodinase. The inhibition of type 1 deiodinase alone would lead to a decrease in fT3 without affecting the TSH concentration, while a simultaneous inhibition of type 2 deiodinase would lead to a rise in TSH. No effect of triclosan has been observed on the inactivation of T4 to reverse T3 by inner ring deiodination (56).
Triclosan can also inhibit iodotyrosine deiodinase, also known as iodotyrosine dehalogenase 1 or DEHAL1 (57). Iodotyrosine deiodinase action normally results in the release of iodide and tyrosine from the two products 3-iodo-l-tyrosine and 3,5-diiodo-l-tyrosine, which are released along with T3 and T4 during the thyroglobulin proteolysis. This can lead to a lack of adequate iodide retention and a decrease in T3 and T4 (57).
Several studies found that triclosan could bind to transthyretin receptors and were capable of displacing T4 (39–43). It is suggested that this displacement leads to a larger amount of available free T4 for hepatic uptake, conjugation and biliary elimination (43). This mechanism may be more relevant for rodents, since humans have a large reserve of T4 (75%) stably bound to thyroid binding globulin (58), however it is suggested that the disruption of transthyretin binding will lead to a change in the delivery of free T4 to target cells (43). Furthermore, transthyretin is important in humans because of its role in mediating the delivery of T4 to the human foetus’ through the placenta and across the blood brain barrier and in transport of thyroid hormones within the brain (39).
Several experimental animal studies have shown that triclosan reduces the level of T4 in rodents (14), but without an effect on TSH (9, 12, 59). Thus, a concern for potential human health effects of triclosan exposure is warranted, also in the wake of equivocal human evidence. This, in particular because of the challenges in performing epidemiological studies of chemical exposure and the thyroid hormone system and due to the combined human exposure to environmental chemicals.
This systematic literature review has investigated the potential effects of triclosan on the human thyroid system at current exposure levels. Several studies found a negative association between triclosan and T3 and T4, and a positive association with TSH, however, opposite observations were also seen, and many of the studies did not find any association at all. Thus, our conclusion is that it is still equivocal whether triclosan, at current human exposure levels, affects the human thyroid hormone system. This is in agreement with several earlier published studies in the field. Most of the included studies assessed the thyroid outcome through few blood samples which decreases the applicability of the studies, especially concerning pregnant women who have a particularly dynamic thyroid hormone homeostasis. Since triclosan potentially causes harm to human health, further and more extensive cohort studies with numerous measurement timepoints for the thyroid outcomes are needed, as are studies with designs that take mixture effects of multiple endocrine disrupting chemicals into account.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
UF-R, ÅKR, and MH contributed to conception and design of the study. LR was consultant. MH wrote the first draft of the manuscript. UF-R and ÅKR wrote sections of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
MH received unrestricted grants from The Novo Nordisk Foundation (grant number: NNF18OC0052576), and ‘Musikforlæggerne Agnes og Knut Mørks Fond’. UF-R’s research salary was sponsored by a donation from The Kirsten and Freddy Johansen’s Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Professor Niels Erik Skakkebaek and Senior Researcher Hanne Frederiksen are thanked for inspiration to do research within the area of Endocrine Disrupting Chemicals and particularly Thyroid Disruptors.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.883827/full#supplementary-material
1. Boas M, Main KM, Feldt-Rasmussen U. Environmental Chemicals and Thyroid Function: An Update. Curr Opin Endocrinol Diabetes Obes (2009) 16:385–91. doi: 10.1097/MED.0b013e3283305af7
2. Zoeller TR. Environmental Chemicals Targeting Thyroid. Hormones (2010) 9:28–40. doi: 10.14310/horm.2002.1250
3. Boas M, Feldt-Rasmussen U, Main KM. Thyroid Effects of Endocrine Disrupting Chemicals. Mol Cell Endocrinol (2012) 355:240–8. doi: 10.1016/j.mce.2011.09.005
4. Milanović M, Đurić L, Milošević N, Milić N. Comprehensive Insight Into Triclosan—From Widespread Occurrence to Health Outcomes. Environ Sci Pollut Res (2021) 1–22. doi: 10.1007/s11356-021-17273-0
5. Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Zajdel R, et al. Triclosan Exposure and In Vitro Fertilization Treatment Outcomes in Women Undergoing In Vitro Fertilization. Environ Sci Pollut Res (2021) 28:12993–9. doi: 10.1007/s11356-020-11287-w
6. Frederiksen H, Jensen TK, Jørgensen N, Kyhl HB, Husby S, Skakkebæk NE, et al. Human Urinary Excretion of non-Persistent Environmental Chemicals: An Overview of Danish Data Collected Between 2006 and 2012. Reproduction (2013) 147(4):555–65. doi: 10.1530/rep-13-0522
7. Frederiksen H, Nielsen O, Koch HM, Skakkebaek NE, Juul A, Jørgensen N, et al. Changes in Urinary Excretion of Phthalates, Phthalate Substitutes, Bisphenols and Other Polychlorinated and Phenolic Substances in Young Danish Men; 2009–2017. Int J Hyg Environ Health (2020) 223:93–105. doi: 10.1016/j.ijheh.2019.10.002
8. Centers for Disease Control and Prevention National Center for Environmental Health. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables (2019). Available at: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf (Accessed 2022 Jan 21).
9. Paul KB, Hedge JM, DeVito MJ, Crofton KM. Short-Term Exposure to Triclosan Decreases Thyroxine In Vivo via Upregulation of Hepatic Catabolism in Young Long-Evans Rats. Toxicol Sci (2010) 113:367–79. doi: 10.1093/toxsci/kfp271
10. Dann AB, Hontela A. Triclosan: Environmental Exposure, Toxicity and Mechanisms of Action. J Appl Toxicol (2011) 31:285–311. doi: 10.1002/jat.1660
11. Hua X, Cao X-Y, Wang X-L, Sun P, Chen L. Exposure of Pregnant Mice to Triclosan Causes Insulin Resistance via Thyroxine Reduction. Toxicol Sci (2017) 160:150–60. doi: 10.1093/toxsci/kfx166
12. Abd-Elhakim YM, Mohammed AT, Ali HA. Impact of Subchronic Exposure to Triclosan and/or Fluoride on Estrogenic Activity in Immature Female Rats: The Expression Pattern of Calbindin-D9k and Estrogen Receptor Alpha Genes. J Biochem Mol Toxicol (2018) 32(2). doi: 10.1002/jbt.22027
13. Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, et al. Developmental Triclosan Exposure Decreases Maternal, Fetal, and Early Neonatal Thyroxine: A Dynamic and Kinetic Evaluation of a Putative Mode-of-Action. Toxicology (2012) 300:31–45. doi: 10.1016/j.tox.2012.05.023
14. Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, et al. Application of the Navigation Guide Systematic Review Methodology to the Evidence for Developmental and Reproductive Toxicity of Triclosan. Environ Int (2016) 92–93:716–28. doi: 10.1016/j.envint.2016.03.009
15. Available at: https://echa.europa.eu/da/substance-information/-/substanceinfo/100.020.167.
16. The Danish Environmental Protection Agency. Endocrine Disruptor List (2021). Available at: https://edlists.org/the-ed-lists/list-ii-substances-under-eu-investigation-endocrine-disruption?page=4 (Accessed 2022 Jan 21).
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1371/journal.pmed.1000100
18. Ley C, Pischel L, Parsonnet J. Triclosan and Triclocarban Exposure and Thyroid Function During Pregnancy—A Randomized Intervention. Reprod Toxicol (2017) 74:143–9. doi: 10.1016/j.reprotox.2017.09.005
19. Poole AC, Pischel L, Goodrich JK, Ley RE, Suh G, Haggerty TD, et al. Crossover Control Study of the Effect of Personal Care Products Containing Triclosan on the Microbiome. mSphere (2016) 1:1–10. doi: 10.1128/mSphere.00056-15
20. Palmer JE, Cullinan MP, Carle AD, Seymour GJ, West MJ. Long Term Use of Triclosan Toothpaste and Thyroid Function. Sci Total Environ (2011) 416:75–9. doi: 10.1016/j.scitotenv.2011.11.063
21. Allmyr M, Panagiotidis G, Sparve E, Diczfalusy U, Sandborgh-Englund G. Human Exposure to Triclosan via Toothpaste Does Not Change CYP3A4 Activity or Plasma Concentrations of Thyroid Hormones. Basic Clin Pharmacol Toxicol (2009) 105:339–44. doi: 10.1111/j.1742-7843.2009.00455.x
22. Almstrup K, Frederiksen H, Andersson AM, Juul A. Levels of Endocrine-Disrupting Chemicals are Associated With Changes in the Peri-Pubertal Epigenome. Endocr Connect (2020) 9:845–57. doi: 10.1530/EC-20-0286
23. Guo J, Wu C, Zhang J, Li W, Lv S, Lu D, et al. Maternal and Childhood Urinary Phenol Concentrations, Neonatal Thyroid Function, and Behavioral Problems at 10 Years of Age: The SMBCS Study. Sci Total Environ (2020) 743:140678. doi: 10.1016/j.scitotenv.2020.140678
24. Ha NY, Kim DH, Ryu JY. Relationship Between Triclosan Exposure and Thyroid Hormones: The Second Korean National Environmental Health Survey (2012-2014). Ann Occup Environ Med (2019) 31:1–9. doi: 10.35371/aoem.2019.31.e22
25. Derakhshan A, Shu H, Peeters RP, Kortenkamp A, Lindh CH, Demeneix B, et al. Association of Urinary Bisphenols and Triclosan With Thyroid Function During Early Pregnancy. Environ Int (2019) 133(August):105123. doi: 10.1016/j.envint.2019.105123
26. Aker AM, Ferguson KK, Rosario ZY, Mukherjee B, Alshawabkeh AN, Calafat AM, et al. A Repeated Measures Study of Phenol, Paraben and Triclocarban Urinary Biomarkers and Circulating Maternal Hormones During Gestation in the Puerto Rico PROTECT Cohort. Environ Heal A Glob Access Sci Source (2019) 18:1–13. doi: 10.1186/s12940-019-0459-5
27. Skarha J, Minguez-Alarcon L, Williams PL, Korevaar TIM, de Poortere RA, Broeren MAC, et al. Cross-Sectional Associations Between Urinary Triclosan and Serum Thyroid Function Biomarker Concentrations in Women. Environ Int (2019) 122:256–62. doi: 10.1016/j.envint.2018.11.015
28. Berger K, Gunier RB, Chevrier J, Calafat AM, Ye X, Eskenazi B, et al. Associations of Maternal Exposure to Triclosan, Parabens, and Other Phenols With Prenatal Maternal and Neonatal Thyroid Hormone Levels. Environ Res (2018) 165:379–86. doi: 10.1016/j.envres.2018.05.005
29. Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations Between Maternal Phenol and Paraben Urinary Biomarkers and Maternal Hormones During Pregnancy: A Repeated Measures Study. Environ Int (2018) 113:341–9. doi: 10.1016/j.envint.2018.01.006
30. Braun JM, Chen A, Hoofnagle A, Papandonatos GD, Jackson-Browne M, Hauser R, et al. Associations of Early Life Urinary Triclosan Concentrations With Maternal, Neonatal, and Child Thyroid Hormone Levels. Horm Behav (2018) 101:77–84. doi: 10.1016/j.yhbeh.2017.11.009
31. Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J. Maternal Urinary Triclosan Concentration in Relation to Maternal and Neonatal Thyroid Hormone Levels: A Prospective Study. Environ Health Perspect (2017) 125(6):067017. doi: 10.1289/ehp500
32. Aker AM, Watkins DJ, Johns LE, Ferguson KK, Soldin OP, Anzalota Del Toro LV, et al. Phenols and Parabens in Relation to Reproductive and Thyroid Hormones in Pregnant Women. Environ Res (2016) 151:30–7. doi: 10.1016/j.envres.2016.07.002
33. Geens T, Dirtu AC, Dirinck E, Malarvannan G, Van Gaal L, Jorens PG, et al. Daily Intake of Bisphenol A and Triclosan and Their Association With Anthropometric Data, Thyroid Hormones and Weight Loss in Overweight and Obese Individuals. Environ Int (2015) 76:98–105. doi: 10.1016/j.envint.2014.12.003
34. Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship Between Urinary Triclosan and Paraben Concentrations and Serum Thyroid Measures in NHANES 2007-2008. Sci Total Environ (2013) 445–446:299–305. doi: 10.1016/j.scitotenv.2012.12.052
35. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic Reviews of Etiology and Risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI (2020). Available at: https://synthesismanual.jbi.global. doi: 10.46658/JBIMES-20-08
36. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What are They and Which is Better? Mil Med Res (2020) 7:1–11. doi: 10.1186/s40779-020-00238-8
37. Guo J, Ito S, Nguyen HT, Yamamoto K, Tanoue R, Kunisue T, et al. Effects of Prenatal Exposure to Triclosan on the Liver Transcriptome in Chicken Embryos. Toxicol Appl Pharmacol (2018) 347:23–32. doi: 10.1016/j.taap.2018.03.026
38. Feldt-Rasmussen U, Klose M. Clinical Strategies in the Testing of Thyroid Function. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al, editors. South Dartmouth (MA: MDText.com, Inc. (2000). Available at: www.endotext.org.
39. Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, et al. Biosensor Discovery of Thyroxine Transport Disrupting Chemicals. Toxicol Appl Pharmacol (2008) 232:150–60. doi: 10.1016/j.taap.2008.06.014
40. Moon TW, Kleywegt S, Topp E, Letcher RJ, Trudeau VL, Wagh P, et al. A Multi-Assay Screening Approach for Assessment of Endocrine-Active Contaminants in Wastewater Effluent Samples. Sci Total Environ (2013) 454–455:132–40. doi: 10.1016/j.scitotenv.2013.02.074
41. Weiss JM, Andersson PL, Zhang J, Simon E, Leonards PEG, Hamers T, et al. Tracing Thyroid Hormone-Disrupting Compounds: Database Compilation and Structure-Activity Evaluation for an Effect-Directed Analysis of Sediment. Anal Bioanal Chem (2015) 407:5625–34. doi: 10.1007/s00216-015-8736-9
42. Cavanagh J-AE, Trought K, Mitchell C, Northcott G, Tremblay LA. Assessment of Endocrine Disruption and Oxidative Potential of Bisphenol-A, Triclosan, Nonylphenol, Diethylhexyl Phthalate, Galaxolide, and Carbamazepine, Common Contaminants of Municipal Biosolids. Toxicol In Vitro (2018) 48:342–9. doi: 10.1016/j.tiv.2018.02.003
43. Hamers T, Kortenkamp A, Scholze M, Molenaar D, Cenijn PH, Weiss JM. Transthyretin-Binding Activity of Complex Mixtures Representing the Composition of Thyroid-Hormone Disrupting Contaminants in House Dust and Human Serum. Environ Health Perspect (2020) 128:1–15. doi: 10.1289/EHP5911
44. De Escobar GM, Obregón MJ, del Rey FE. Maternal Thyroid Hormones Early in Pregnancy and Fetal Brain Development. Best Pract Res Clin Endocrinol Metab (2004) 18:225–48. doi: 10.1016/j.beem.2004.03.012
45. Feldt-Rasmussen U, Mathiesen ER. Endocrine Disorders in Pregnancy: Physiological and Hormonal Aspects of Pregnancy. Best Pract Res Clin Endocrinol Metab (2011) 2:875–84. doi: 10.1016/j.beem.2011.07.004
46. Feldt-Rasmussen U. Laboratory Measurement of Thyroid-Related Hormones, Proteins, and Autoantibodies in Serum. In: Braverman LE, Cooper DS, Kopp PA, editors. Werner & Ingbar’s The Thyroid A Fundamental and Clinical Text. Philadelphia, PA: Lippincott Williams & Wilkins (2021). p. 267–99.
47. Delitala AP, Capobianco G, Cherchi PL, Dessole S, Delitala G. Thyroid Function and Thyroid Disorders During Pregnancy: A Review and Care Pathway. Arch Gynecol Obstet (2019) 299:327–38. doi: 10.1007/s00404-018-5018-8
48. Medici M, Korevaar TIM, Visser WE, Visser TJ, Peeters RP. Thyroid Function in Pregnancy: What Is Normal? Clin Chem (2015) 61:704–13. doi: 10.1373/clinchem.2014.236646
49. Glinoer D. The Regulation of Thyroid Function in Pregnancy: Pathways of Endocrine Adaptation From Physiology to Pathology. Endocr Rev (1997) 18:404–33. doi: 10.1210/edrv.18.3.0300
50. Krassas GE, Poppe K, Glinoer D. Thyroid Function and Human Reproductive Health. Endocr Rev (2010) 31:702–55. doi: 10.1210/er.2009-0041
51. Andersen SL, Andersen S. Hyperthyroidism in Pregnancy: Evidence and Hypothesis in Fetal Programming and Development. Endocr Connect (2021) 10:R77–86. doi: 10.1530/EC-20-0518
52. Boron WF, Boulpaep EL. Medical Physiology. Philadelphia, PA: Elsevir Saunders (2012) p. 1052–1056 p.
53. Klaassen CD, Watkins JB. Casarett & Doull’s Essentials of Toxicology. New York: McGraw-Hill Companies, Inc (2010).
54. Butt CM, Stapleton HM. Inhibition of Thyroid Hormone Sulfotransferase Activity by Brominated Flame Retardants and Halogenated Phenolics. Chem Res Toxicol (2013) 26:1692–702. doi: 10.1021/tx400342k
55. Wang LQ, Falany CN, James MO. Triclosan as a Substrate and Inhibitor of 3′-Phosphoadenosine 5′-Phosphosulfate-Sulfotransferase and UDP-Glucuronosyl Transferase in Human Liver Fractions. Drug Metab Dispos (2004) 32:1162–9. doi: 10.1124/dmd.104.000273
56. Butt CM, Wang D, Stapleton HM. Halogenated Phenolic Contaminants Inhibit the In Vitro Activity of the Thyroid-Regulating Deiodinases in Human Liver. Toxicol Sci (2011) 124:339–47. doi: 10.1093/toxsci/kfr117
57. Shimizu R, Yamaguchi M, Uramaru N, Kuroki H, Ohta S, Kitamura S, et al. Structure-Activity Relationships of 44 Halogenated Compounds for Iodotyrosine Deiodinase-Inhibitory Activity. Toxicol (2013) 314:22–9. doi: 10.1016/j.tox.2013.08.017
58. Pappa T, Ferrara AM, Refetoff S. Inherited Defects of Thyroxine-Binding Proteins. Best Pract Res Clin Endocrinol Metab (2015) 29:735–47. doi: 10.1016/j.beem.2015.09.002
Keywords: triclosan, thyroid, endocrine disrupting chemicals, health, thyroid toxicity, environment
Citation: Homburg M, Rasmussen ÅK, Ramhøj L and Feldt-Rasmussen U (2022) The Influence of Triclosan on the Thyroid Hormone System in Humans - A Systematic Review. Front. Endocrinol. 13:883827. doi: 10.3389/fendo.2022.883827
Received: 25 February 2022; Accepted: 11 March 2022;
Published: 02 June 2022.
Edited by:
Leonidas H. Duntas, National University Of Athens, GreeceReviewed by:
Miloš Žarković, University of Belgrade, SerbiaCopyright © 2022 Homburg, Rasmussen, Ramhøj and Feldt-Rasmussen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulla Feldt-Rasmussen, dWZlbGR0QHJoLmRr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.