
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 03 June 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.876231
This article is part of the Research Topic Endocrine and Metabolic Consequences of Childhood Obesity View all 16 articles
 Rapheeporn Khwanchuea*
Rapheeporn Khwanchuea* Chuchard Punsawad
Chuchard PunsawadBackground: Serum leptin levels reflects one’s degree of obesity and can affect vitamin D levels. The relationship between body fat, leptin, and 25-hydroxyvitamin D (25(OH)D) has not been extensively studied in adolescents. This study aimed to investigate the correlations between body composition and leptin and 25(OH)D levels in boys and girls.
Methods: Participants aged 12–14 years (n = 205) were grouped according to sex. After body composition was recorded using bioelectrical impedance analysis, they were classified into three groups according to body fat percentage (%BF) (< 30, ≥ 30 and < 40, and ≥ 40). Serum leptin and 25(OH)D levels were measured using the enzyme-linked immunosorbent assay (ELISA). Correlations between all variables were analyzed according to sex and the percentage of BF groups.
Results: Boys and girls with %BF ≥ 30 showed no difference in body mass index (BMI), %BF, and leptin and 25(OH)D, while other variables of body composition were more common in boys than in girls. The %BF, body fat mass (BFM), and 25(OH)D of both sexes with %BF ≥ 30, and leptin levels of boys with %BF ≥ 40 increased with an increase in %BF. A negative correlation between leptin and 25(OH)D levels was found in boys with %BF < 40 and girls with %BF < 30. In the %BF ≥ 30 and < 40 groups, there were negative correlations between leptin, BFM, free fat mass, and muscle mass (MM); between leptin, 25(OH)D, and height in boys; and between 25(OH)D, body weight, BMI, and MM in girls.
Conclusion: A negative correlation between leptin and 25(OH)D levels varied according to sex, while for body composition, it was evident at 30 and 40% BF.
Obesity is defined as an excessive proportion of body fat relative to lean body mass due to a chronic imbalance between energy intake and expenditure. Leptin resistance, or its inability to modulate energy intake and expenditure is common in obesity. Leptin is an adipokine secreted primarily by adipocytes, and it inhibits energy intake and regulates energy homeostasis by acting via hypothalamic receptors (1, 2). Research shows that leptin is a marker of obesity and reflects the degree of adiposity. Circulating leptin concentrations are determined by body fat mass (BFM), body mass index (BMI), and sex. In fact, leptin concentration is higher in females than in males regardless of BFM (1, 3, 4). In addition, leptin levels change significantly during progressive pubertal stages, with girls having higher serum leptin levels than boys which rise throughout puberty, concomitant with an increase in estrogen levels (4). Furthermore, serum leptin concentrations are higher in early adolescence than in childhood and may play a role in pubertal development (4). Body fat percentage (%BF), basal metabolic rate, muscle mass (MM), bone mass, and serum 25-hydroxyvitamin D (25(OH)D) had an impact on serum leptin (5).
In obesity, there is not only an imbalance of adipokines, but also a decrease in vitamin D bioavailability (6, 7). Adipose tissue is a target for vitamin D and the main storage depot for vitamin D and its metabolites (8, 9). Leptin exerts an autocrine–paracrine lipolytic effect on adipocytes by interacting with the vitamin D receptor, and it inhibits the enzyme that converts 25(OH)D to 1,25 dihydroxyvitamin D (10). Thus, vitamin D depletion might increase appetite and lead to obesity by directly regulating leptin expression (6, 11). Previous studies reported that there was a negative association between %BF and vitamin D and a positive correlation between %BF and leptin that confirmed excess of %BF, leading to decreased vitamin D and raised leptin (12). The distribution of fat in adolescents with BMI of 36 ± 5 and %BF of 40 ± 5 might be associated with vitamin D status with decreased 25(OH)D (13). A recent study in young adults aged 20–21 years found that males and females demonstrated positive relationships between serum leptin and BMI, waist circumference, and %BF; however, males showed inverse correlations between serum leptin, MM, and 25(OH)D (5). In adults, the relationship between total body fat and 25(OH)D levels also varied by sex (14). In addition, the relationship between serum 25(OH)D and leptin is largely explained by the presence of adiposity or the amount of body fat, which disappeared after adjustment for total body fat and waist circumference (15).
Although the inverse relationship between leptin and 25(OH)D has been reported with respect to BMI and %BF, this association has not been fully explored in young adolescents. Moreover, there was a report in Thai school children aged 6–14 years that dietary calcium intake was low, vitamin D status was sufficient, and girls experienced a decline in 25(OH)D levels with increasing age (16). Thus, this study investigated the correlations between body composition and leptin and 25(OH)D levels in boys and girls stratified degree of obesity by %BF.
Students with ages 12–14 years and BMI-for-age > 50th percentile (17) were selected from high schools in southern Thailand. Those with chronic diseases, other conditions such as asthma, allergies, or gastritis, and those using steroids were excluded. Participants and their parents received the information regarding the purposes and methods of the study, and they were required to sign the informed consent. Participants (n = 205) were grouped by sexes (107 boys and 98 girls), and after body composition measurement, they were classified by %BF into three groups, group 1, %BF < 30 (28 boys and 12 girls); group 2, %BF ≥ 30 and < 40 (38 boys and 43 girls); group 3, %BF ≥ 40 (41 boys and 43 girls), according to the %BF cutoff values at 95th percentile for age, 30% (18, 19) and 40% (20). Participants were asked about their lifestyle habits, and they did not exercise regularly; during the day they spent most of their time sitting in class and ate three meals a day with snacks and no calcium and vitamin D supplementation (21).
This study was reviewed and approved by the Human Research Ethics Committee of Walailak University, Thailand (Approval Number: WUEC-19-102-21). A consent form was obtained from all participants or their legal representative before enrollment.
Body composition, including body weight (BW, kg), BMI (kg/m2), %BF, BFM (kg), free fat mass (FFM, kg), and MM (kg), were analyzed by bioelectrical impedance analysis (22, 23) using a TANITA SC-330ST series body composition analyzer (Tanita Corporation, Tokyo, Japan). To increase measurement accuracy, participants wore light clothes and no shoes. Since the level of hydration, the presence of edema, and the daily weight variability affected the total body weight, 0.5 kg was subtracted from the obtained weight values (23). Standing heights (m) were measured without shoes using a locally made stadiometer and were recorded to the nearest 0.1 cm (21).
After an overnight fast, participants were collected venous blood samples in clotted blood tubes. The blood samples were centrifuged at 2000 revolutions per minute for 10 minutes, and the serum was harvested into 1.5 mL microcentrifuge tubes and stored at -70°C until used to measure leptin and 25(OH)D.
Leptin and 25(OH)D levels were determined by an immunometric sandwich ELISA using commercial ELISA kits leptin (R&D Systems, Inc., Minneapolis, MN, USA, sensitivity to 7.8 pg/mL and intra-assay coefficient of variability < 10%), and 25(OH)D (DRG Diagnostics, Frauenbergstrasse, Germany, sensitivity to 2.89 ng/mL and intra-assay coefficient of variability < 10%), per the manufacturer’s instructions. Standard or diluted serum samples were prepared and incubated in coated microplates at room temperature. Immunoassays were performed in duplicates. After washing, a mixture of capture and detector antibodies was added to the plate and incubated at room temperature. A 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added to each well and incubated for 10 minutes to detect the antigen-antibody complex reaction. Finally, a stop solution was added to each well, and the optical density (OD) was measured at 450 nm using a microplate reader.
Descriptive data of all variables were presented as mean ± standard deviation (S.D). Differences in variables of body composition, leptin, and 25(OH)D among sexes and %BF groups were compared by independent-samples T-test. The correlations between those variables were calculated by Pearson’s correlation coefficient (r). The P values less than 0.05, 0.01, or 0.001 were considered statistically significant. Data analysis was performed using IBM SPSS statistics version 22.0 software license authorization wizard.
Table 1 shows that for all participants, BMI, %BF, BFM, and 25(OH)D levels were not different between boys and girls, whereas boys had BW, height, FFM, and MM greater than girls, and girls had higher leptin levels than boys. When participants were divided into three groups, similar results were obtained for all groups: the BW, height, and MM of boys were higher than those of girls. In group 1, girls had higher %BF and leptin levels, and lower FFM than boys. In group 2, boys had more BFM and FFM than girls. In group 3, the BFM of boys was greater than that of girls. Although, serum 25(OH)D levels were not different between boys and girls in all groups, they appeared to be slightly less than 20 ng/mL in groups 1 and 2 of girls. Furthermore, Table 2 shows that BW, BMI, %BF, and BFM of both sexes increased with increasing %BF, but their heights did not differ. Boys and girls in group 3 had higher 25(OH)D levels than those in group 1 and 2. The FFM and MM of group 1 were greater than those of groups 2 and 3 in girls, while leptin levels in group 3 were higher than those in group 1 in boys.
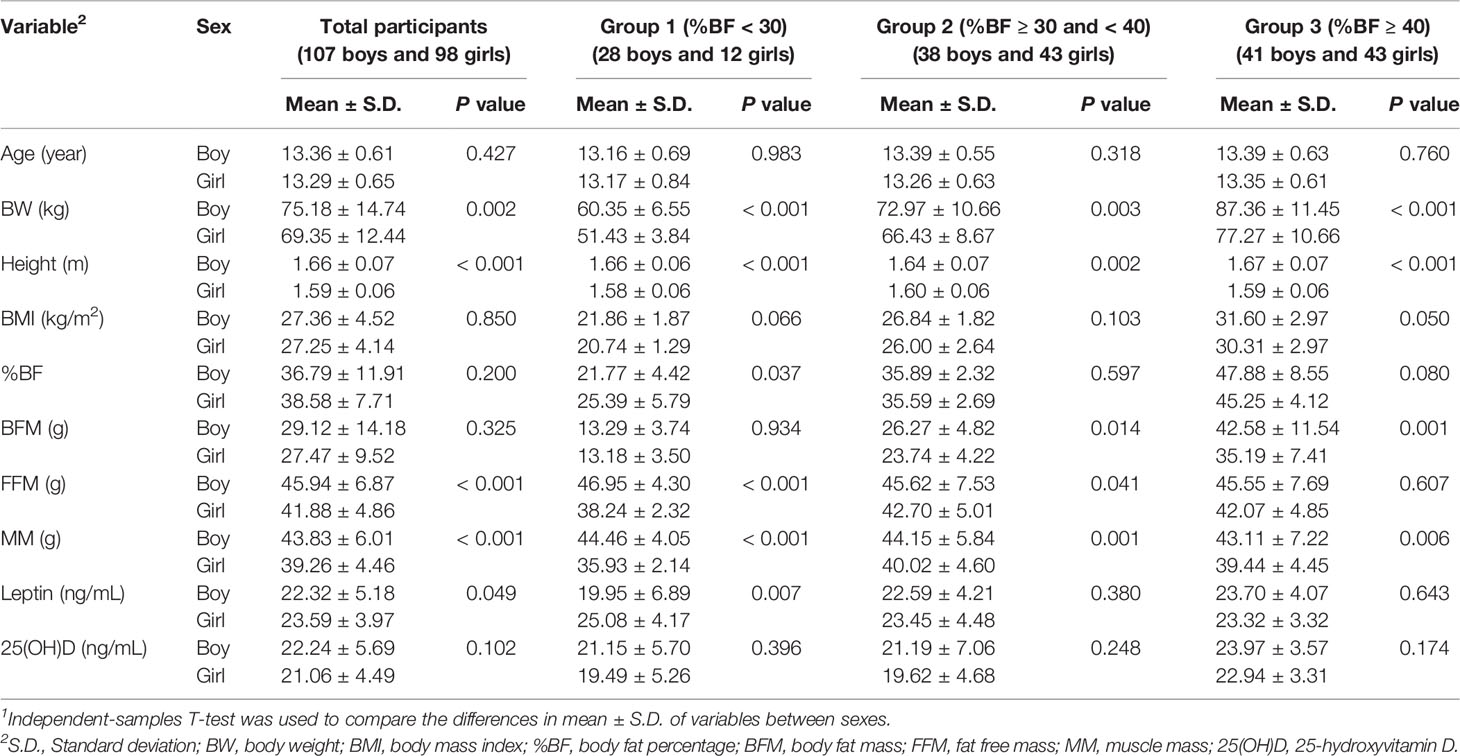
Table 1 Characteristics of study participants and comparisons1 of the variables between boy and girl.
Table 3 shows that in the group made up of only boys, BW and height were positively correlated with 25(OH)D as well as all variables of body composition (except between height and %BF), and that BMI was positively correlated with %BF (r = 0.938, p < 0.001), BFM (r = 0.963, p < 0.001), leptin (r = 0.195, p = 0.044), and 25(OH)D (r = 0.252, p = 0.009). Leptin and 25(OH)D levels positively correlated with %BF (r = 0.212, p = 0.029 and r = 0.212, p = 0.028, respectively), leptin levels negatively correlated with 25(OH)D levels (r = -0.35, p < 0.001), and 25(OH)D levels positively correlated with BFM (r = 0.262, p = 0.006) and FFM (r = 0.193, p = 0.046). In group 1, BW and BMI positively correlated with all variables of body composition. Height positively correlated with FFM (r = 0.885, p < 0.001) and MM (r = 0.885, p < 0.001), while leptin negatively correlated with 25(OH)D (r = -0.64, p < 0.001). In group 2, BW, height, and BMI positively correlated with all body composition variables; BW and height negatively correlated with leptin (r = -0.34, p = 0.037 and r = -0.388, p = 0.016, respectively); while height positively correlated with 25(OH)D (r = 0.334, p = 0.04). Leptin levels in group 2 negatively correlated with all variables of body composition (except %BF), and 25(OH)D (r = -0.5, p = 0.001). Additionally, 25(OH)D levels and BFM were positively correlated (r = 0.334, p = 0.04). In group 3, there were positive correlations between BW and %BF (r = 0.45, p = 0.003), BFM (r = 0.731, p < 0.001), as well as height with FFM and MM (r = 0.719, p < 0.001), while BMI was positively correlated with %BF (r = 0.799, p < 0.001) and BFM (r = 0.899, p < 0.001), but negatively correlated with FFM (r = -0.328, p = 0.036) and MM (r = -0.326, p = 0.037). However, there were no correlations between body composition, and leptin and 25(OH)D levels in group 3.
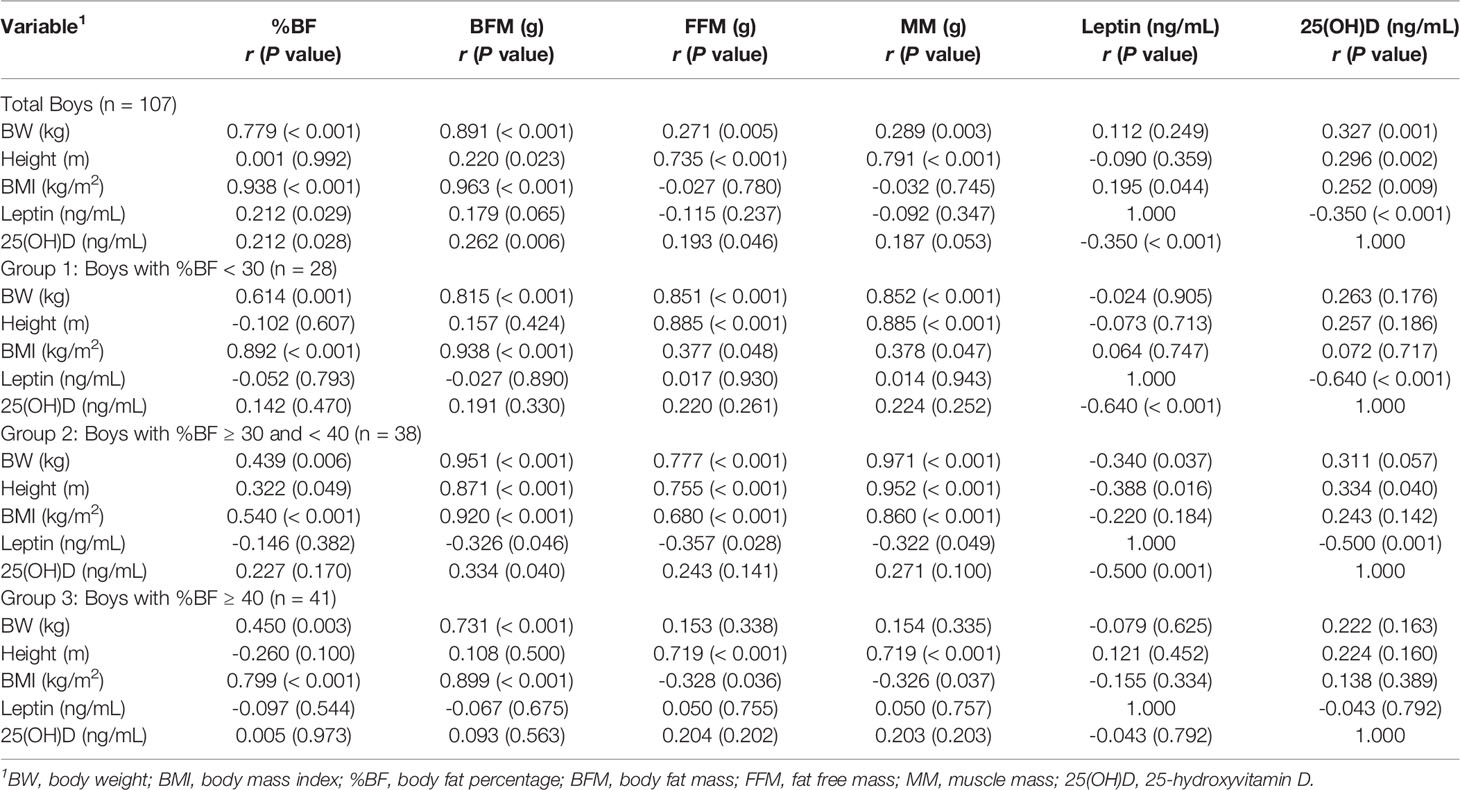
Table 3 Correlation coefficient, r (P value), between BMI parameters, body composition, leptin, and 25(OH)D in boys.
Table 4 shows that in a group of total girls, BW, height, and BMI were positively correlated with all variables of body composition, while %BF and BFM were positively correlated with 25(OH)D levels (r = 0.302, p = 0.002 and r = 0.279, p = 0.005). In group 1, there were positive correlations of BW and height with %BF (r = 0.630, p = 0.028 and r = 0.591, p = 0.043) and BFM (r = 0.804, p = 0.002 and r = 0.661, p = 0.019), and a negative correlation between leptin and 25(OH)D (r = -0.784, p = 0.003). In group 2, BW and BMI were positively correlated with all variables of body composition and negatively correlated with 25(OH)D (r = -0.329, p = 0.031 and r = -0.373, p = 0.014), while height positively correlated with BFM (r = 0.511, p < 0.001), FFM (r = 0.711, p < 0.001) and MM (r = 0.711, p < 0.001). FFM and MM were negatively correlated with 25(OH)D (r = -0.353, p = 0.02). In group 3, there were strong positive correlations between BW, height, BMI, and all variables of body composition except between height and %BF, but no correlations between body composition variables, leptin, and 25(OH)D.
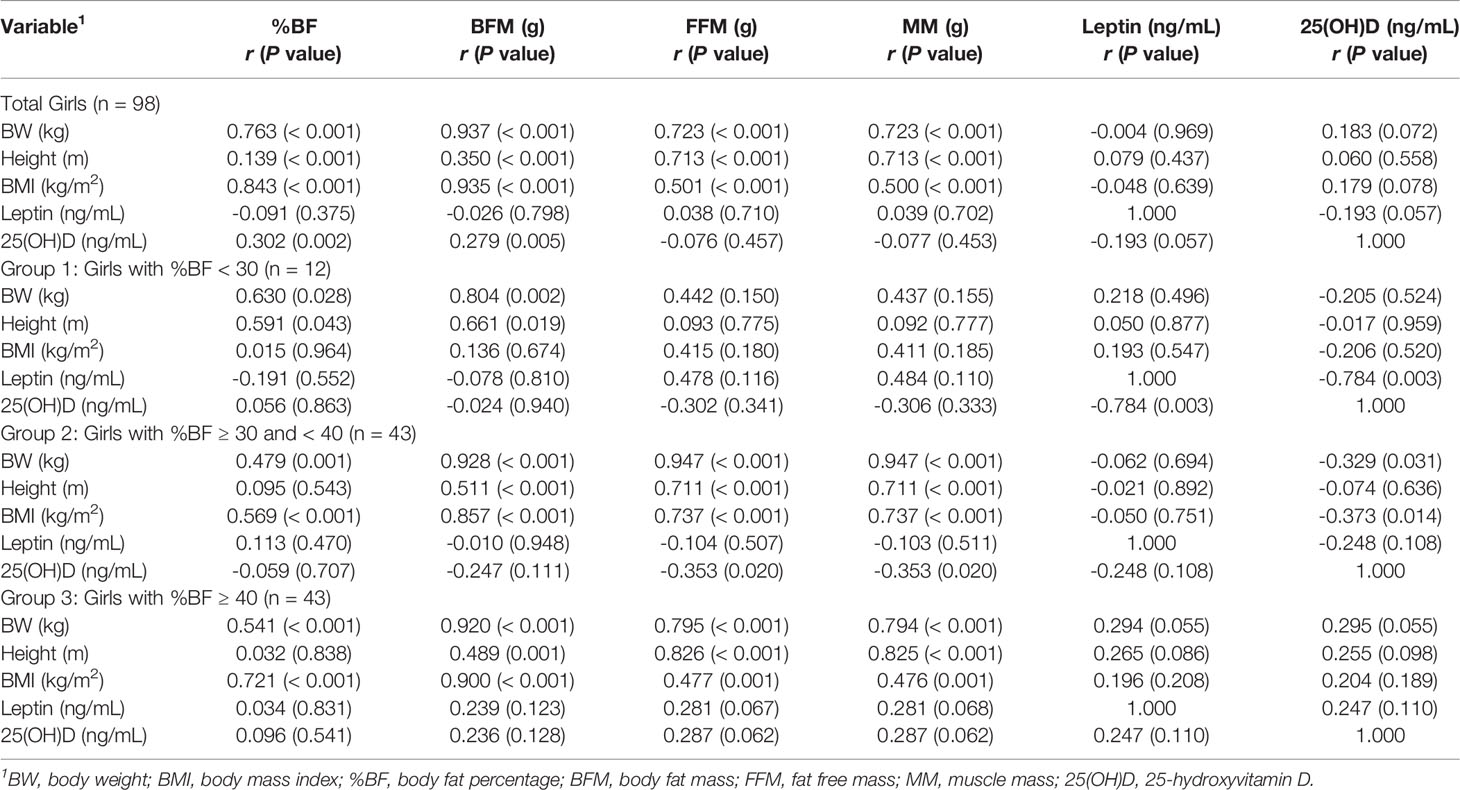
Table 4 Correlation coefficient, r (P value), between BMI parameters, body composition, leptin, and 25(OH)D in girls.
This study included participants aged 13–14 years, which is the period of age when growth spurt occurs (24). The BMIs for total boys and girls were more than the 97th percentile for age (17), which classified them as obese. Obesity is defined by the WHO as a condition of abnormal or excessive fat accumulation in adipose tissue. However classifying obesity during childhood or adolescence has the added complication of height still increasing, and body composition continually changing (25). In this study, participants in group 1 were defined as having low excess %BF due to %BF < 50th percentile (20), whereas groups 2 and 3 consisted of participants with moderate and high excess %BF, because %BF was above the 95th percentile of %BF-for-age (18, 19). When referring to another percentile curve (20), the mean %BF of girls in groups 2 and 3 was at the 90th and > 95th percentiles, respectively. In each group, BMI did not discern the differences in body composition based on sex (26), and high BMI did not distinguish excess fat mass from lean mass (27). In addition, most variables of body composition varied with the increase in %BF in both sexes, while height, FFM, and MM of boys remained unchanged, which corresponded to growth development during puberty in boys (28). Contrarily, decreases in FFM and MM were observed in girls with the highest excess %BF. However, these characteristics may be related to leptin and 25(OH)D levels which are discussed later.
Furthermore, this study did not find a decrease in 25(OH)D levels due to an increase in %BF as reported in previous studies (13, 29), and 25(OH)D levels appeared to be slightly less than 20 ng/mL in girls with %BF < 40, whereas participants with high excess %BF (≥ 40) had higher serum 25(OH)D levels than the other groups. This finding may be consistent with a previous study reporting that 25(OH)D levels are associated with BMI, sex, puberty, and age (30). The highest 25(OH)D levels were observed in groups with %BF ≥ 40, which might be due to unchanged leptin levels according to the increased %BF, and seemed to be the lowest leptin levels compared to other groups. These results were in contrast to previous findings that serum leptin was increased and that serum vitamin D might be decreased in obese individuals (3, 4, 6, 31). Thus, sex and the amount of %BF are likely the important factors in determining changes in serum leptin and 25(OH)D levels in adolescents.
In this study, boys and girls showed differences in the correlations between leptin, and 25(OH)D levels, likely due to differences in body composition and their relationships in each %BF group. The negative relationship between serum leptin and 25(OH)D was clearly demonstrated in boys with %BF < 40, which was evident in the positive relationships between BMI and body composition and in the inverse relationships with leptin levels. Conversely, the relationship between serum leptin and 25(OH)D was not observed in boys when BMI and excess %BF (> 40) increased, which was likely explained by the change in the lean proportion and the negative relationships between BMI, FFM, and MM. Thus, in boys, lean mass (FFM and MM) and excess BF were likely key variables that indicated a correlation between leptin and 25(OH)D. Although, there are a few studies reporting the relationship between leptin and 25(OH)D in adolescents with obesity, previous studies in adults revealed that measures of adiposity largely explained the negative association of serum 25(OH)D with leptin, and that MM, %BF, and serum 25(OH)D had an impact on serum leptin (5, 32). Furthermore, %BF explained all sex differences in leptin concentrations, and lean body mass was inversely related to leptin concentrations (32).
In girls, whether the inverse relationship between leptin and 25(OH)D was modulated by excess %BF because it appeared in a group with %BF < 30 remained unclear, whereas strong correlations between BMI and body composition were not observed. However, this relationship might be a result of the highest leptin levels and the lowest 25(OH)D levels in girls in this group when compared to other groups, and explained by the excess %BF, thus leading to decreased vitamin D and increased leptin levels (12). In contrast to boys, 25(OH)D levels were inversely related to lean mass (FFM and MM), BW, and BMI in girls with moderately excess %BF. Although, strong correlations between variables of BMI and body composition appeared clearly in girls with high excess %BF, they did not seem to contribute to the relationship between leptin and 25(OH)D. This might be because the 25(OH)D levels in girls did not decrease with increasing %BF, as found in previous studies (12, 13). In addition, the relationship between fat distribution and vitamin D status may be dependent on metabolic factors as well as parathyroid hormone, which is released in response to low 25(OH)D (13). However, this study suggests that BW, BMI, and MM should be considered when interpreting serum 25(OH)D levels as markers of vitamin D status (33).
This study is limited by the small number of participants; thus, it may not be sufficient in powered to be generalizable to Thai early adolescents and to allow comparisons between sexes and groups of excess %BF. Further research is needed to explore the relationship between height, leptin, and 25(OH)D in boys with approximately 30-40%BF, including the inverse relationship between leptin and 25(OH)D in girls.
Negative correlations between leptin, 25(OH)D, and body composition appeared clearly in boys and girls when using %BF at 30 and 40 to classify their degrees of obesity. The relationship between leptin and 25(OH)D is sex-specific and differ depending on body composition, and the degree of excess body fat may be a determinant.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The Human Research Ethics Committee of Walailak University, Thailand (Approval Number: WUEC-19-102-21). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
RK conceived and carried out study design, data collection, data analysis, data interpretation, literature search, and writing of the manuscript. CP carried out ELISA measurement, and involved in writing of the manuscript. All authors contributed to the article and approved the submitted version.
The Research Institute for Health Sciences, Walailak University, Thailand under the contract no. WU-IRG-62-018.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all participants for their cooperation, teachers and staff of high schools in Nakhon Si Thammarat for the help and collaboration, and staff of The Center for Scientific and Technological Equipment, Walailak University, for the help with body composition measurement.
BFM, body fat mass; BMI, body fat index; %BF, body fat percentage; MM, muscle mass; 25(OH)D, 25-hydroxyvitamin D; BW, body weight; FFM, free fat mass; ELISA, Enzyme-Linked Immunosorbent Assay.
1. Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients (2019) 11(11):2704. doi: 10.3390/nu11112704
2. Reyes M, Quintanilla C, Burrows R, Blanco E, Cifuentes M, Gahagan S. Obesity Is Associated With Acute Inflammation in A Sample of Adolescents. Pediatr Diabetes (2015) 16:109–16. doi: 10.1111/pedi.12129
3. Jéquier E. Leptin Signaling, Adiposity, and Energy Balance. Ann New York Acad Sci (2002) 967:379–88. doi: 10.1111/j.1749-6632.2002.tb04293.x
4. Soliman AT, Yasin M, Kassem A. Leptin in Pediatrics: A Hormone From Adipocyte That Wheels Several Functions in Children. Indian J Endocrinol Metab (2012) 16:S577–87. doi: 10.4103/2230-8210.105575
5. Khan AH, Fatima SS, Raheem A, Jafri L. Are Serum Leptin Levels Predicted by Lipoproteins, Vitamin D and Body Composition? World J Diabetes (2019) 10:260–8. doi: 10.4239/wjd.v10.i4.260
6. Savastano S, Barrea L, Savanelli MC, Nappi F, Di Somma C, Orio F, et al. Low Vitamin D Status and Obesity: Role of Nutritionist. Rev Endocr Metab Disord (2017) 18:215–25. doi: 10.1007/s11154-017-9410-7
7. Vranić L, Mikolašević I, Milić S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina (Kaunas) (2019) 55:541. doi: 10.3390/medicina55090541
8. Avtanski D, Garcia A, Liao EP. Vitamin D and Obesity. In: Liao EP, editor. Extraskeletal Effects of Vitamin D: A Clinical Guide. Cham: Springer International Publishing (2018). p. 165–81.
9. Abbas MA. Physiological Functions of Vitamin D in Adipose Tissue. J Steroid Biochem Mol Biol (2017) 165:369–81. doi: 10.1016/j.jsbmb.2016.08.004
10. Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin Corrects Increased Gene Expression of Renal 25-Hydroxyvitamin D3-1 Alpha-Hydroxylase and -24-Hydroxylase in Leptin-Deficient, Ob/Ob Mice. Endocrinology (2004) 145:1367–75. doi: 10.1210/en.2003-1010
11. Mehmood Z-T-NH, Papandreou D. An Updated Mini Review of Vitamin D and Obesity: Adipogenesis and Inflammation State. Open Access Maced J Med Sci (2016) 4:526–32. doi: 10.3889/oamjms.2016.103
12. Fatima SS, Farooq S, Tauni MA, Irfan O, Alam F. Effect of Raised Body Fat on Vitamin D, Leptin and Bone Mass. J Pak Med Assoc (2015) 65(12):1315–9.
13. Lenders CM, Feldman HA, Von Scheven E, Merewood A, Sweeney C, Wilson DM, et al. Relation of Body Fat Indexes to Vitamin D Status and Deficiency Among Obese Adolescents. Am J Clin Nutr (2009) 90:459–67. doi: 10.3945/ajcn.2008.27275
14. Rafiq R, Walschot F, Lips P, Lamb HJ, de Roos A, Rosendaal FR, et al. Associations of Different Body Fat Deposits With Serum 25-Hydroxyvitamin D Concentrations. Clin Nutr (2019) 38:2851–7. doi: 10.1016/j.clnu.2018.12.018
15. Rafiq R, El Haddaoui H, de Mutsert R, Rosendaal FR, Hiemstra PS, Cobbaert CM, et al. Adiposity Is A Confounding Factor Which Largely Explains the Association of Serum Vitamin D Concentrations With C-Reactive Protein, Leptin and Adiponectin. Cytokine (2020) 131:155104. doi: 10.1016/j.cyto.2020.155104
16. Houghton LA, Gray AR, Harper MJ, Winichagoon P, Pongcharoen T, Gowachirapant S, et al. Vitamin D Status Among Thai School Children and the Association With 1,25-Dihydroxyvitamin D and Parathyroid Hormone Levels. PloS One (2014) 9:e104825. doi: 10.1371/journal.pone.0104825
17. World Health Organization (WHO). Growth Reference Data for 5-19 Years . Available at: https://www.who.int/tools/growth-reference-data-for-5to19-years/indicators/bmi-for-age (Accessed January 18, 2022).
18. Sung RY, So HK, Choi KC, Li AM, Yin J, Nelson EA. Body Fat Measured by Bioelectrical Impedance in Hong Kong Chinese Children. Hong Kong Med (2009) 15(2):110–7.
19. Trang LT, Trung NN, Chu DT, Hanh NTH. Percentage Body Fat is As a Good Indicator for Determining Adolescents Who Are Overweight or Obese: A Cross-Sectional Study in Vietnam. Osong Public Health Res Perspect (2019) 10(2):108–14. doi: 10.24171/j.phrp.2019.10.2.10
20. Dong H, Yan Y, Liu J, Cheng H, Zhao X, Shan X, et al. Reference Centiles for Evaluating Total Body Fat Development and Fat Distribution by Dual-Energy X-Ray Absorptiometry Among Children and Adolescents Aged 3-18 Years. Clin Nutr (2021) 40(3):1289–95. doi: 10.1016/j.clnu.2020.08.012
21. Khwanchuea R, Punsawad C. Sex Differences in the Relationship Between Body Composition and Biomarkers of Bone and Fat Metabolism in Obese Boys and Girls. Bone Rep (2021) 14:101087. doi: 10.1016/j.bonr.2021.101087
22. Talma H, Chinapaw MJ, Bakker B, HiraSing RA, Terwee CB, Altenburg TM. Bioelectrical Impedance Analysis to Estimate Body Composition in Children and Adolescents: A Systematic Review and Evidence Appraisal of Validity, Responsiveness, Reliability and Measurement Error. Obes Rev (2013) 14:895–905. doi: 10.1111/obr.12061
23. Dobroch J, Cieśluk K, Sawicka-Żukowska M, Krawczuk-Rybak M. Body Composition Measurements in Paediatrics - A Review. Part 1. Pediatr Endocrinol Diabetes Metab (2018) 24:185–90. doi: 10.5114/pedm.2018.83365
24. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull World Health Organ (2007) 85(9):660–7. doi: 10.2471/blt.07.043497
25. WHO. Obesity. Preventing and Managing the Global Epidemic. World Health Organ Tech Rep Ser (2000) 894:i–xii, 1–253.
26. Chung S. Body Mass Index and Body Composition Scaling to Height in Children and Adolescent. Ann Pediatr Endocrinol Metab (2015) 20(3):125–9. doi: 10.6065/apem.2015.20.3.125
27. Must A, Anderson SE. Body Mass Index in Children and Adolescents: Considerations for Population-Based Applications. Int J Obes (2006) 30(4):590–4. doi: 10.1038/sj.ijo.0803300
28. Shalitin S, Gat-Yablonski G. Associations of Obesity With Linear Growth and Puberty. Horm Res Paediatr (2021) 15:279–95. doi: 10.1159/000516171
29. Vranić L, Mikolašević I, Milić S. Vitamin D Deficiency: Consequence or Cause of Obesity? Medicina (Kaunas) (2019) 55(9):541. doi: 10.3390/medicina55090541
30. Barja-Fernández S, Aguilera CM, Martínez-Silva I, Vazquez R, Gil-Campos M, Olza J, et al. 25-Hydroxyvitamin D Levels of Children Are Inversely Related to Adiposity Assessed by Body Mass Index. J Physio Biochem (2018) 74(1):111–8. doi: 10.1007/s13105-017-0581-1
31. Zakharova I, Klimov L, Kuryaninova V, Nikitina I, Malyavskaya S, Dolbnya S, et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front Endocrinol (2019) 10:103. doi: 10.3389/fendo.2019.00103
32. Marshall JA, Grunwald GK, Donahoo WT, Scarbro S, Shetterly SM. Percent Body Fat and Lean Mass Explain the Gender Difference in Leptin: Analysis and Interpretation of Leptin in Hispanic and Non-Hispanic White Adults. Obes Res (2000) 8(8):543–52. doi: 10.1038/oby.2000.70
Keywords: body fat percentage (BF%), leptin, 25-hydroxyvitamin D, adolescents, body composition
Citation: Khwanchuea R and Punsawad C (2022) Associations Between Body Composition, Leptin, and Vitamin D Varied by the Body Fat Percentage in Adolescents. Front. Endocrinol. 13:876231. doi: 10.3389/fendo.2022.876231
Received: 15 February 2022; Accepted: 05 May 2022;
Published: 03 June 2022.
Edited by:
Dénes Molnár, University of Pécs, HungaryReviewed by:
Deborah Masquio, Centro Universitário São Camilo, BrazilCopyright © 2022 Khwanchuea and Punsawad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rapheeporn Khwanchuea, a3JhcGhlZXBAbWFpbC53dS5hYy50aA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.