
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 22 July 2022
Sec. Bone Research
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.875678
 XinChao Lin1
XinChao Lin1 HongTao Guo2
HongTao Guo2 YiGang Lian1
YiGang Lian1 Jiajing Kou1
Jiajing Kou1 GuangLei Wang1
GuangLei Wang1 YiYun Chen1
YiYun Chen1 Juan Wang3
Juan Wang3 Xu Han3
Xu Han3 Miao Jiang3*
Miao Jiang3* QiaoHui Yang1*
QiaoHui Yang1*Background: Identification of the high risk population for osteoporosis and timely prevention are the best strategies at present. Detailed epidemiological investigation in a well-defined population is necessary to explore the population-based characteristics and risk factors of osteoporosis, thus to facilitate better prevention programs.
Method: In this prospective cross-sectional study, 1423 questionnaires were given out to the urban residents (female ≥ 40 years of age, male ≥50 years) who lived in the 27 Elderly-Care Inns interspersed among the seven central urban areas of Beijing. All participants were voluntary and underwent routine physical examination and spine and hip BMD measurements using the DXA instrument. The study protocols were approved by the Medical Ethics Committee of Dongzhimen Hospital, Beijing University of Chinese Medicine (JDZX2015079).
Results: Altogether 1407 participants fulfilled the survey. Among 359 men, the prevalence of osteoporosis, osteopenia, and normal BMD were 18.1%, 56.6%, and 25.3%, respectively; among 1048 women, the corresponding figures were 40.3%, 42.8%, and 16.9%, respectively. After adjustment of age and BMI, both hands grip strength, height loss over 3 cm, serum levels of β-CTx, PINP, and OST were the independent risk factors for osteoporosis in both men and women; besides, familial Alzheimer’s disease history in men; and history of steatohepatitis and fracture, serum levels of PTH and ALT, age of menarche, age of menopause, and duration of menstruation in women were also risk factors of osteoporosis. In both genders, the cost-effective method, which adopted both hands grip strength, height loss over 3 cm, and medical history, indicated a good predictive ability to evaluate the risk of osteoporosis (in men AUC=0.730, 95%CI=0.642~0.817; in women AUC=0.769, 95%CI=0.724~0.813).
Conclusions: In the population of elderly Beijing urban residents in Elderly-Care Inns, the prevalence of osteoporosis in women is higher than that in men and increases with aging more rapidly; the prevalence of osteopenia in men is higher than in women. The cost-effective method, including both hands grip strength, height loss over 3 cm, and familial Alzheimer’s disease history in men; fracture and steatohepatitis history as well as menstrual history in women is recommended in identifying the high-risk subjects for osteoporosis.
Osteoporosis is a kind of systemic bone disease characterized by decreased bone mass and bone microstructure damage, resulting in increased bone fragility and easy fracture (1). Osteopenia is a term to define bone density that is not normal but also not as low as osteoporosis (2). The prevalence rate of osteoporosis increases with age (3). In China, the prevalence rate of osteoporosis among the population above 60 years old was reported to be 36%; 23% in male population and 49% in female (4).
The most serious consequence of osteoporosis is osteoporotic fracture, which has been regarded as one of the most severe public health issues in middle-aged and elderly people concerned with the growth in the aging population throughout the world. It has been estimated that approximately 50% of women and 20% of men over 50 years old will suffer an osteoporosis-related fracture; hip fracture is the most devastating due to the consequent disability, mortality, and costs (5). The number of patients with osteoporosis fractures in China reached 2.33 million in 2010, including 360,000 hip fractures, 1.11 million vertebral fractures, and 860,000 other osteoporotic fractures (6). Among them, 20% of patients die of complications within one year after a hip fracture, and about 50% become disabled, with a significantly reduced quality of life (7). This brings a heavy economic burden on both the families and society, the medical expenditure of fractures in China in 2010 reached $10.2 billion USD, and this figure will be as high as $275 billion USD led by the estimated 5.99 million patients with osteoporotic fracture by the year 2050 (6).
Osteoporosis is preventable and treatable. Proper treatment can effectively reduce the risk of fracture and refracture even for those who have already suffered from brittle fracture (8). Thus, early screening and identification of high-risk populations so as to take timely preventive measures should never be over strengthened (9).
However, the fact is that osteoporosis is underdiagnosed and undertreated, especially in those over 75 years old, in whom treatment is probably most beneficial and cost-effective, and even among the highest-risk populations who have already suffered fractures in big cities such as Beijing (10), although many researchers and clinicians have already concentrated on these issues and made remarkable achievements, such as the publication of the guidelines for the diagnosis and treatment of osteoporosis both in modern medicine and in Chinese medicine (11–16).
Besides the efforts to improve the accessibility to bone densitometry, the awareness of the disease by professionals and the public, and the use and reimbursement of drugs, the first gap that needs to be closed in the structured care pathways for osteoporosis care is how to determine the risk factors so as to identify the high-risk subjects.
As a complex disease, osteoporosis is affected by multiple risk factors, including environmental factors and genetic factors. Genetic factors (uncontrollable factors), include race, aging, female menopause, and family history of brittle fracture. Environmental factors (controllable factors) include mainly unhealthy lifestyle such as smoking, alcohol consumption, nutritional imbalance, improper diet, low physical activity, and so on. These risk factors can individually or synergistically cause the loss of bone mineral density and lead to osteoporosis (17).
Notably, there exist significant differences in the lifestyle between different regions, countries, or ethnicities, for example, even the lifestyles between urban and rural areas and among different regions in China vary greatly, which leads to great differences in the prevalence rate of osteoporosis, the major risk factors, and the proper prevention measures among regions and populations (18).
The accurate and cost-effective clinical risk factor models for the assessment of osteoporosis probability should be based on the highly population-specific data. Unfortunately, work in this area is currently seriously insufficient in China, it has been pointed out that cost-effective analysis on screening strategy and intervention thresholds based on local epidemiology data and economic status are available only in Japan throughout the east Asia area (10). Therefore, the next key step should be focused on the establishment of local data to develop a cost-effective risk assessment strategy to identify high risk individuals for screening and treatment.
Regarding these considerations, the Chinese Society for Health Management of the Chinese Medical Association collaborated with the Chinese Geriatrics Society for Osteoporosis and Bone Mineral Research, for the first time, developed a multicenter epidemiological survey in a large-scale Chinese population for determining the prevalence of osteoporosis and establishing a BMD reference database with a unified DXA system (19).
This study was a sub-project of the above survey, undertaken by Dongzhimen Hospital, Beijing University of Chinese Medicine, the target population was about 1500 elderly urban residents in Beijing, China, in order to obtain the prevalence and risk factors of osteoporosis among them. The data from this survey were included in the first epidemiological study of osteoporosis among Chinese residents developed by the Chronic Disease Center of the Chinese Center for Disease Control and Prevention and the Osteoporosis and Bone Mineral Salt Diseases Branch of the Chinese Medical Association in 2018 organized by the National Health Commission.
This was a prospective cross-sectional study. All participants were recruited from Beijing urban area and were voluntary and underwent routine physical examination carried out by physicians in the Health Management unnormal Center of Dongzhimen Hospital, Beijing University of Chinese Medicine.
Eligible participants must meet the inclusion criteria: female ≥ 40 years of age, male ≥50 years, and with spine and hip BMD measurements. Pregnant or lactating women; participants with a history of chronic diseases, such as metabolic bone disease, malignant tumors, calcium absorption; participants with acute infectious diseases; participants with mental health problems; and participants with a history of use of any drugs affecting bone metabolism were excluded. Each participant signed written informed consent.
The study protocols were approved by the Medical Ethics Committee of Dongzhimen Hospital, Beijing University of Chinese Medicine.(JDZX2015079). The registration number of the study was NCT02958020.
From July 26, 2017 to May 4, 2018, altogether 1423 questionnaires were given out to the middle-aged and elderly urban residents in Beijing, China. These residents were recruited from 27 Elderly-Care Inns interspersed among the seven central urban areas of Beijing. The distribution of the Elderly-Care Inns and corresponding population is shown in Table 1.
Age, gender, address, height, weight, waistline, hipline, education level, marital status, occupation information, personal history (smoke, alcohol, dietary favor, pregnancy and parity history, menstrual history, etc.), medical history (chronic diseases, fracture history, medication use, sleep quality, etc.), were collected by specific designed form and face-to-face interviews.
International Physical Activity Scale (IPAQ) short form was applied to evaluate the physical activity (20); International Osteoporosis Foundation (IOF) was adopted for evaluating the osteoporosis risk (21); EuroQol Health Index Scale EQ-5D (EuroQol-5) dimension [17] was used to assess life quality. Questionnaires were carried out by physicians from the Health Management Center of Dongzhimen Hospital, Beijing University of Chinese Medicine.
Fasting peripheral blood was collected from each participant. Levels of serum total calcium, serum phosphorus, serum magnesium, serum creatinine, alkaline phosphatase (ALP), thyrotropin (TPH), and 25-Hydroxyvitamin D were tested. Serum procollagen type 1 N-terminal propeptide (PINP), serum osteocalcin (OST), and β cross-linked C-telopeptide of type 1 collagen (β-CTx) were tested using radioimmunoassay. Blood samples were collected and tested by researchers from Guangzhou KingMed Diagnostics Group Co. Ltd.
Each participant underwent a BMD measurement using the DXA instrument (Hologic, American Hologic WI), which was equipped in a BMD examination car, sponsored by Guangzhou KingMed Diagnostics Group Co. Ltd. The BMD examination car entered each community and provided BMD examination service to each participant, the lumbar spine (L1-L4) and the left proximal femur (Neck), the Wards triangle, the Troch, the Shaft, and the Total femur (Total) were measured.
Diagnostic criteria of osteoporosis were based on DXA bone mineral density T-score/Z-score (16). BMD values (g/cm2) were expressed as T- scores for men above 50 years old and postmenopausal women (number of SD above/below the mean for healthy 30-year-old adults); or as Z-scores for premenopausal women (number of SD above/below the mean for the patient’s age). The gender-specific mean BMD and standard deviation (SD) were calculated. The maximal gender-specific mean BMD was defined as the peak BMD. In accordance with the Chinese society of osteoporosis and mineral research (CSOBMR) criteria, osteoporosis was diagnosed using the following criteria:

Continuous variables were expressed as mean ± SD and categorical variables as numbers (percentages). All analyses were stratified by gender. Descriptive statistics were performed using one-way analysis of variance and LSD methods were adopted in the post hoc test. For the variables that did not conform to the normal distribution, the Kruskal-Wallis H test was applied. Multivariate logistic regression analysis was conducted to evaluate the risk factors of osteoporosis including demographic variables, medical history, and physical and chemical examination variables. The interaction of age and BMI was included; OR value and 95%CI were calculated. The Youden’s index from the ROC was used to determine the cutoff values of the risk factors in predicting osteoporosis and osteopenia. A two-sided P value <0.05 was considered statistically significant. All analyses were conducted using SPSS24.0 software (IBM Corp., Armonk, NY, USA).
The baseline characteristics of the participants are listed in Table 2. Altogether 1048 female and 359 male participants were involved in the analysis, the average age of men was 67.14 ± 7.44 (range from 50 to 87), of women was 64.33 ± 8.55 (range from 41 to 96). There were no significant differences among the three groups (normal, osteopenia, and osteoporosis population) in the distribution of monthly household income, working condition, history of smoke and alcohol intake in both genders, job category, and working condition in men.
Among the three groups, the distributions of age, education level, and marital status in both genders; job category and working condition in women, showed statistical differences (P<0.05). Subjects with elder age, physical worker, single status, and those with lower education levels tended to have lower BMD.
The overall prevalence rate of osteoporosis was 34.6% and osteopenia was 46.3% as listed in Table 4. In male population, the prevalence of osteoporosis and osteopenia was 18.1% and 56.6%, respectively; in the female population it was 40.3% and 42.8%, respectively. The prevalence of both osteoporosis and osteopenia in senile women were much higher than those in senile men. Figure 1 shows the prevalence of osteoporosis and osteopenia by age and gender. With the increase of age, the prevalence of osteoporosis elevates more significantly in women.
After the age of 50, the prevalence of osteoporosis in men increased slowly, but the prevalence of osteopenia was always higher than that in women in the same age period. Therefore, the issue of BMD declining in elderly men also needs to be paid high attention. In women, the prevalence of osteoporosis after the age of 50 increased quickly with age. After 80 years old, all women indicated abnormal BMD; the prevalence of osteopenia kept relatively stable. The characteristics of BMD decline between men and women were different.
The results of physical examination, biochemical test, and bone metabolic examination are listed in Table 5.
TCM symptoms and constitution differentiation, International Physical Activity Scale (IPAQ) short form, International Osteoporosis Foundation (IOF), and EuroQol Health Index Scale EQ-5D (EuroQol-5) were also adopted and the information were analyzed, since no positive result was detected, the relative results were ignored.
Univariate analysis showed that in the male population, height, weight, grip strength of each hand, OST, β-CTx, and PINP presented significant differences among groups (P<0.05), the P values in post analysis by LSD method are listed as well. In the female population, height, weight, BMI, grip strength of each hand, sit test, ALP, PTH, OST, serum magnesium, β-CTx, and PINP show differences among groups (P<0.05).
Multivariate analysis was adopted to determine the risk factors of osteoporosis and the results are displayed and ordered by OR value in Table 6. For men, β-CTx, familial Alzheimer disease, OST, height loss, and PINP were risk factors for osteoporosis after adjustment of age and BMI. Grip strength of each hand showed slight negative correlation with osteoporosis. Men with familial Alzheimer’s disease history, height loss (>3cm), and higher levers of serum β-CTx, PINP, OST, and lower hands grip strength had a higher risk of osteoporosis. More risk factors were screened in women after adjustment of age and BMI, including β-CTx, fracture history, height loss (>3cm), PTH, OST, age of menarche, ALP, PINP; while grip strength of each hand, age of menopause, duration of menstruation, and steatohepatitis history were negatively correlated with osteoporosis.
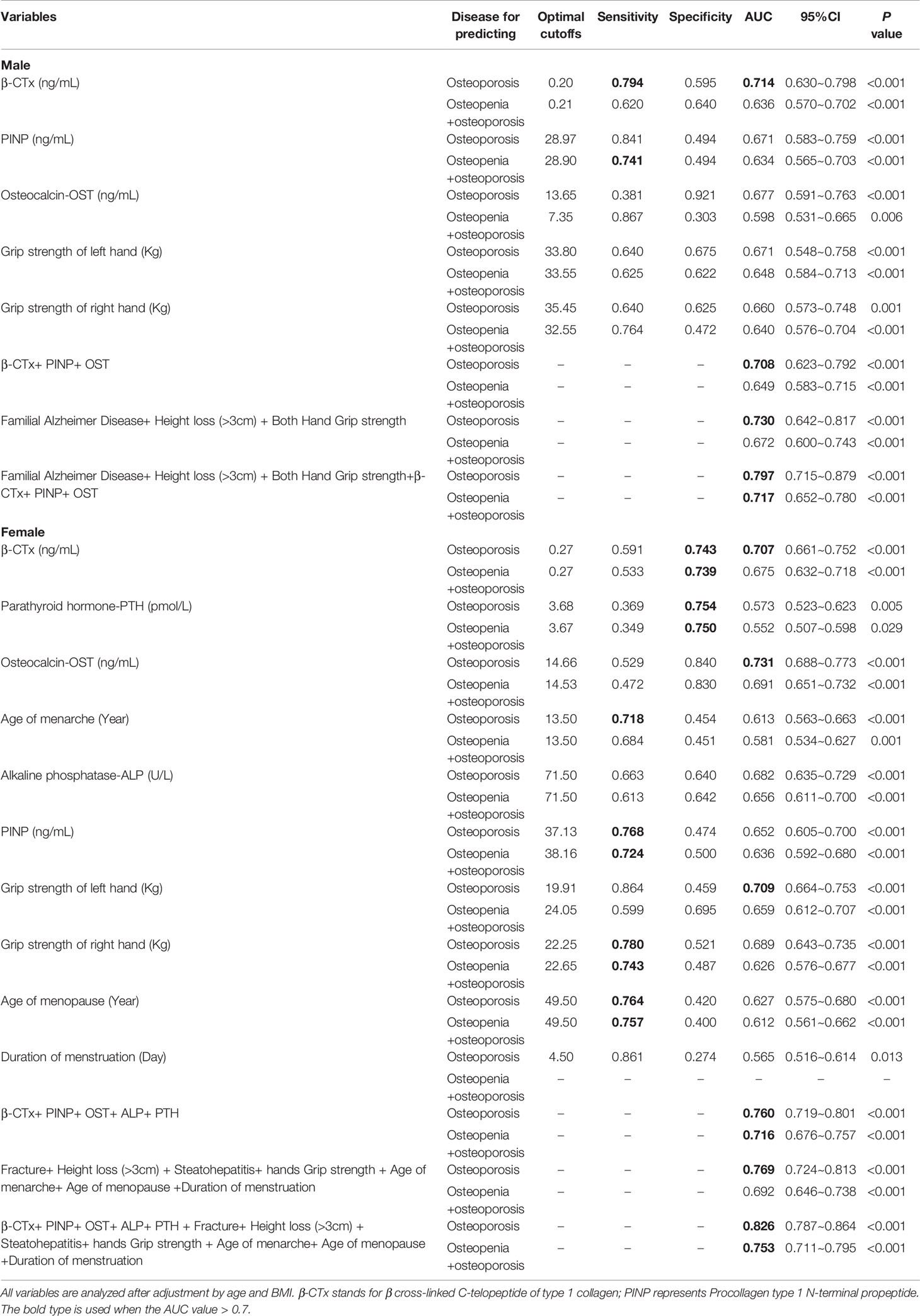
Table 7 Predictive ability of the risk factors for predicting osteoporosis and osteopenia by gender.
To our best knowledge, this is the first epidemiological investigation for osteoporosis based on a well-defined population in the Beijing urban area. In this group of subjects, it has been revealed that the cost-effective method could be used as a predictive factor for early identification of high-risk subjects for osteoporosis and osteopenia.
Previous studies usually concentrated on a certain group of subjects, such as the elderly (22), postmenopausal women (23, 24), persons with physical disabilities (25), or people in some region or a country (26–28). These studies facilitated to deepen our understanding of the role of human factors in the development of osteoporosis and promoted the guidelines for the prevention and treatment of osteoporosis. However, the controllable factors, or lifestyle factors, should receive more attention because they also play an undeniably important role on the occurrence of osteoporosis. The diagnosis and treatment rate of osteoporosis still varied significantly among regions and between urban and rural areas, therefore, epidemiological investigation of osteoporosis in specific populations with similar lifestyles in the same living area could help to find more effective prevention and treatment methods for this population.
Aging and urbanization have been the two major issues in China, which make lifestyle changes significant, especially in the big cities such as Beijing, Shanghai, and so on. In the year 2020, the average life expectancy of registered residents in Beijing was 82.43 years. In 2020 there were 4.299 million registered elderly residents (aged 60 and above) in Beijing, accounting for 19.6% of registered residents. These numbers keep increasing; it is expected that by 2025, this figure will be close to 24%. The distribution of the elderly population in Beijing is uneven. In the central urban area, the aging population is the highest, with 65% of the elderly population in Beijing living in the six central urban districts (Dongcheng, Xicheng, Chaoyang, Haidian, Fengtai, and Shijingshan District).
The consequent pension problem has become an urgent social problem. Given China’s traditional culture, most elderly residents tend to live in their own homes rather than going to the elder nursing institutions. Therefore, the government tried to put forward the mode of combining home-based, community-based, and institutional care, and increased government investment and policy support for the elderly. The Elderly-Care Inns, as the main functional unit of elderly care service, have been widely established in urban communities in Beijing, as well as other big cities in China, aiming to provide home-based elderly care services for the surrounding community residents: meal, assistant, medical aid, day care, full care services, etc.
This home-based pension service mode is suitable to be the main pension model in China’s big cities. By the end of 2017, Beijing had established 380 community Elderly-Care Inns in the urban area. These Elderly-Care Inns have greatly alleviated the increasingly urgent demand for urban elderly care in Beijing, however, as a new thing, there is not accurate survey data about the pension needs, population characteristics, and health conditions of the urban elderly in China.
Thus, our study was designed to develop a survey among the elder population who lived in Beijing urban area for at least 5 years, and all received the service from the Elderly-Care Inns, to investigate the prevalence of osteoporosis, and health status of these population, in order to provide assistance for the construction of the hardware and the service contents, as well to provide data support and theoretical guidance for the combination of medical care and rehabilitation for the Elderly-Care institutions.
In this study, a cost-effective method to evaluate the risk of abnormal BMD level (including osteoporosis and osteopenia) was established, based on hands grip strength, height loss, and familial Alzheimer’s history in men; and hands grip strength, height loss, history of fracture and steatohepatitis, and menstrual history in women. The predictive ability for osteoporosis is desirable in both men and women.
Hand grip strength was independently associated with increased fall risk score in osteoporotic elderly women (29), and low hand-grip strength was specifically associated with the risk of distal forearm fractures within 10 years and clinical vertebral fractures within 15 years or more in Japanese postmenopausal women (30). Since hand grip strength is easy and cost-effective to measure, it is suitable to be used as an easy assessment method for identifying individuals at a high risk of osteoporosis. The cutoff strength for evaluating osteoporosis in adults is age and sex specific (31). The results in this study were consistent with the previous studies.
Height loss is also simple to evaluate in the clinical setting, and it is a frequent manifestation of vertebral osteoporosis or fracture. The degree of height loss varied among individuals, and excessive height loss, of 3-4cm or more can be considered a simple indicator of increasing fracture risk (32, 33). In our study, height loss over 3cm was an independent risk factor for osteoporosis in both men (OR =1.613 and 95% CI= 1.028~2.527) and women (OR = 1.316 and 95% CI =1.042~1.786), which was consistent with the previous studies, in which the magnitude of the association translated to a 19% increase in odds for 1/2 in. and 177% for 3 in (34). Thus, height loss was suggested as routine evaluation in the outpatient setting for its ability in detecting osteoporosis of the hip (32, 35).
Steatohepatitis history was negatively associated with the risk of osteoporosis based on this study (OR = 0.677 and 95% CI =0.498~0.922). There existed controversy regarding the relationship between BMD and nonalcoholic fatty liver disease (36–38). As study concluded that the incidence of osteoporosis was significantly higher in the nonalcoholic fatty liver disease group (39). In addition, contradictory results have also been reported regarding a relationship between serum lipid levels and BMD. Some researchers believed that serum lipid levels could be potentially useful indicators to reflect the process of osteoporosis (40), some think that hyperlipidemia was associated with decreased BMD (41), and some found serum lipids did not directly and correlatively influence BMD (42, 43). Given that in the present study, we did not distinguish between alcoholic fatty liver and nonalcoholic fatty liver, and the serum lipid levels were not available, further studies are needed to clarify the relationship between steatohepatitis history or lipid parameters and osteoporosis.
In men, the cost-effective method adopts both hands grip strength, height loss over 3 cm, and familial Alzheimer’s disease history indicated a good predictive ability to evaluate the risk of osteoporosis (AUC=0.730, 95%CI=0.642~0.817) and abnormal BMD (AUC=0.672, 95%CI=0.600~0.743). In women, the method adopted both hands grip strength, height loss over 3 cm, Steatohepatitis history, and menstrual history (age of menarche, age of menopause, and duration of menstruation) achieved similar predictive ability (AUC=0.769, 95%CI=0.724~0.813).
The predictive abilities of serum testing variables, including serum levels of β-CTx, OST, and PINP in men and PTH and ALT in women, were close to those of the cost-effective method. β-CTx and PINP were designated as reference bone turnover markers in osteoporosis by the International Osteoporosis Foundation (IOF) and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) (44). The IOF-IFCC Joint Working Group on Bone Marker Standards (WG-BMS) recommended PINP and β-CTX be used as blood reference markers for bone formation and bone resorption, respectively, in osteoporosis (45, 46). Serum β-CTx and PINP distributed abnormally and stably in healthy men and women of Han nationality, the serum β-CTx and PINP reference interval were provided by several studies (47, 48).
Although the combination predictor including both cost-effective and serum testing factors achieved better predictive ability (in men AUC=0.797 with 95%CI=0.715~0.879; in women AUC=0.826 with 95%CI=0.787~0.864), considering the simplicity and accessibility of operation, the cost-effective factors were strongly recommended in the identification of high risk subjects in the elderly urban residents in Elderly-Care Inns in Beijing.
There were still some limitations in this study. Firstly, the number of male subjects was relatively small, which might weaken the power of the conclusion. Secondly, there was too much missing data of some important indexes, such as the use of vitamin D and calcium, physical activity, and the determination of TCM symptoms and constitution, which made it difficult to conduct further in-depth analysis in the risk factor screening. In addition, as a cross-sectional study, the evidence that this study could provide was relatively limited, so long-term follow-up of this population is necessary to provide more evidence.
In the population of elderly urban residents in Elderly-Care Inns in Beijing, the prevalence of osteoporosis in women was higher than that in men, and increased with aging more rapidly; yet, the prevalence of osteopenia in men was higher than that in women. After adjustment of age and BMI, both hands grip strength, height loss over 3 cm, serum levels of β-CTx, PINP, and OST were the independent risk factors for osteoporosis in both men and women; besides, familial Alzheimer’s disease history in men; and history of steatohepatitis and fracture, serum levels of PTH and ALT, age of menarche, age of menopause, duration of menstruation in women, were also risk factors of osteoporosis. The cost-effective method, including both hands grip strength, height loss over 3 cm, and familial Alzheimer’s disease history in men; fracture and steatohepatitis history and menstrual history in women, indicated good predictive ability to evaluate the risk of osteoporosis, thus was recommended in identifying the high risk subjects for osteoporosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of Dongzhimen Hospital, Beijing University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
XL designed the study protocol and drafted the manuscript; HG, YL, JK, GW, and YC developed the questionnaires and data collection; JW and XH performed the data analysis; MJ and QY took part in the design of the study and MJ made revision of the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the National Clinical Research Base of TCM Project (JDZX2015079), the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021A05404), and the National Natural Science Foundation of China (81873181).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Xu W, Wu W, Yang S, Chen T, Teng X, Gao D, et al. Risk of Osteoporosis and Fracture After Hysterectomies Without Oophorectomies: A Systematic Review and Pooled Analysis. Osteoporos Int (2022). doi: 10.1007/s00198-022-06383-1 (Online ahead of print)
2. Varacallo M, Seaman TJ, Jandu JS, Pizzutillo P. Osteopenia. In: StatPearls. Treasure Island (FL: StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC (2022).
3. Kim D, Pirshahid AA, Li Y, Varghese T, Pope JE. Prevalence of Osteoporosis in Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoporos Int (2022). doi: 10.1007/s00198-022-06376-0 (Online ahead of print)
4. Liying H, Yun S, Wenjuan Y, Keqin P. The Prevalence Rate of Osteoporosis in the Elderly in China Between 2010 and 2016: A Meta-Analysis of Single Rate. Chin J Osteoporos (2016) 22:1590–5. doi: 10.3969/j.issn.1006-7108.2016.12.019
5. Coughlan T, Dockery F. Osteoporosis and Fracture Risk in Older People. Clin Med (Lond) (2014) 14:187–91. doi: 10.7861/clinmedicine.14-2-187
6. Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of Osteoporosis-Related Fractures and Costs in China: 2010-2050. Osteoporos Int (2015) 26:1929–37. doi: 10.1007/s00198-015-3093-2
7. Lahtinen A, Leppilahti J, Vähänikkilä H, Kujala S, Ristiniemi J, Jalovaara P. No Major Differences in Recovery After Hip Fracture Between Home-Dwelling Female and Male Patients. Scandinavian J Surg SJS Off Organ Finnish Surg Soc Scandinavian Surg Society (2020) 109:250–64. doi: 10.1177/1457496919847932
8. Disease. TCMAoOaBM. Guidelines for the Diagnosis and Treatment of Primary Osteoporosis (2017). Chin J Pract Internal Med (2018) 38:127–50. doi: 10.19538/j.nk201802109
9. Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int (2014) 25:2359–81. doi: 10.1007/s00198-014-2794-2
10. Cheung EYN, Tan KCB, Cheung CL, Kung AWC. Osteoporosis in East Asia: Current Issues in Assessment and Management. Osteoporos Sarcopenia (2016) 2:118–33. doi: 10.1016/j.afos.2016.07.001
11. Microsurgery Department of the Orthopedics Branch of the Chinese Medical Doctor Association; Group from the Osteonecrosis and Bone Defect Branch of the Chinese Association of Reparative and Reconstructive Surgery; Microsurgery and Reconstructive Surgery Group of the Orthopedics Branch of the Chinese Medical Association. Chinese Guideline for the Diagnosis and Treatment of Osteonecrosis of the Femoral Head in Adults. Orthopaedic Surg (2017) 9:3–12. doi: 10.1111/os.12302
12. Briot K, Roux C, Thomas T, Blain H, Buchon D, Chapurlat R, et al. 2018 Update of French Recommendations on the Management of Postmenopausal Osteoporosis. Joint Bone Spine (2018) 85:519–30. doi: 10.1016/j.jbspin.2018.02.009
13. Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK Clinical Guideline for the Prevention and Treatment of Osteoporosis. Arch Osteoporos (2017) 12:43. doi: 10.1007/s11657-017-0324-5
14. Kanis JA, Cooper C, Rizzoli R, Reginster JY. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos Int (2019) 30:3–44. doi: 10.1007/s00198-018-4704-5
15. Qaseem A, Forciea MA, McLean RM, Denberg TD. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann Internal Med (2017) 166:818–39. doi: 10.7326/m15-1361
16. Yuanzheng M, Yipeng W, Qiang L, Chunlin L, Xun M, Yongjun W, et al. 2018 China Guideline for Diagnosis and Treatment of Senile Osteoporosis. Chin J Osteoporos (2018) 24:1541–67. doi: 10.3969/j.issn.1006-7108.2018.10.001
17. Elonheimo H, Lange R, Tolonen H, Kolossa-Gehring M. Environmental Substances Associated With Osteoporosis-A Scoping Review. Int J Environ Res Public Health (2021) 18:738. doi: 10.3390/ijerph18020738
18. Chen P, Li Z, Hu Y. Prevalence of Osteoporosis in China: A Meta-Analysis and Systematic Review. BMC Public Health (2016) 16:1039. doi: 10.1186/s12889-016-3712-7
19. Zeng Q, Li N, Wang Q, Feng J, Sun D, Zhang Q, et al. The Prevalence of Osteoporosis in China, a Nationwide, Multicenter DXA Survey. J Bone Mineral Res Off J Am Soc Bone Mineral Res (2019) 34:1789–97. doi: 10.1002/jbmr.3757
20. Craig C, Marshall A, Sjstrm M, Bauman A, Booth M, Ainsworth B, et al. International Physical Activity Questionnaire: 12- Country Reliability and Validity. Med Sci Sports Exercise (2003) 35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB
21. Kharroubi A, Saba E, Ghannam I, Darwish H. Evaluation of the Validity of Osteoporosis and Fracture Risk Assessment Tools (IOF One Minute Test, SCORE, and FRAX) in Postmenopausal Palestinian Women. Arch Osteoporos (2017) 12:6. doi: 10.1007/s11657-016-0298-8
22. Choi E, Park Y. The Association Between the Consumption of Fish/Shellfish and the Risk of Osteoporosis in Men and Postmenopausal Women Aged 50 Years or Older. Nutrients (2016) 8:113. doi: 10.3390/nu8030113
23. Rossi LMM, Copes RM, Dal Osto LC, Flores C, Comim FV, Premaor MO. Factors Related With Osteoporosis Treatment in Postmenopausal Women. Medicine (2018) 97:e11524. doi: 10.1097/md.0000000000011524
24. Langer FW, da Silveira Codevilla AA, Bringhenti R, Dal Osto LC, Campos TR, Martins TT, et al. Low Self-Awareness of Osteoporosis and Fracture Risk Among Postmenopausal Women. Arch Osteoporos (2016) 11:27. doi: 10.1007/s11657-016-0266-3
25. Smeltzer SC, Zimmerman V, Capriotti T. Osteoporosis Risk and Low Bone Mineral Density in Women With Physical Disabilities. Arch Phys Med rehabilitation (2005) 86:582–6. doi: 10.1016/j.apmr.2004.09.002
26. Modi A, Sen S, Adachi JD, Adami S, Cortet B, Cooper AL, et al. Rationale and Design of MUSIC Os-EU: An International Observational Study of the Treatment of Postmenopausal Women for Osteoporosis in Europe and Canada. Clin Exp Rheumatol (2015) 33:537–44
27. Kim SW, Bae KH, Seo JB, Jeon JH, Lee WK, Lee IK, et al. Association Between Household Size, Residential Area, and Osteoporosis: Analysis of 2008 to 2011 Korea National Health and Nutrition Examination Survey. Korean J Internal Med (2016) 31:712–21. doi: 10.3904/kjim.2015.274
28. Zhang J, Yang M, Zhang X, He J, Wen L, Wang X, et al. The Effectiveness of a Co-Management Care Model on Older Hip Fracture Patients in China - A Multicentre Non-Randomised Controlled Study. Lancet regional Health Western Pacific (2022) 19:100348. doi: 10.1016/j.lanwpc.2021.100348
29. Nagai T, Okano I, Ishikawa K, Kuroda T, Oshita Y, Tsuchiya K, et al. The Serum 25(OH)D Level and Hand Grip Strength for Fall Risk Assessment Among Osteoporotic Elderly Japanese Women. Arch Osteoporos (2021) 16:42. doi: 10.1007/s11657-021-00901-0
30. Kamiya K, Kajita E, Tachiki T, Ikehara S, Kouda K, Sato Y, et al. Association Between Hand-Grip Strength and Site-Specific Risks of Major Osteoporotic Fracture: Results From the Japanese Population-Based Osteoporosis Cohort Study. Maturitas (2019) 130:13–20. doi: 10.1016/j.maturitas.2019.09.008
31. Lin YH, Chen HC, Hsu NW, Chou P, Teng MMH. Hand Grip Strength in Predicting the Risk of Osteoporosis in Asian Adults. J Bone Miner Metab (2021) 39:289–94. doi: 10.1007/s00774-020-01150-w
32. Pluskiewicz W, Adamczyk P, Drozdzowska B. Height Loss in Postmenopausal Women-Do We Need More for Fracture Risk Assessment? Results from the GO Study. Osteoporos Int (2021) 32:2043–9. doi: 10.1007/s00198-021-05941-3
33. Ahn KS, Kang CH, Cho SB, Cho KH, Han KD, Park YG, et al. Height Loss was Associated With Osteoporosis in Korean Elderly Men, Not in Women: The Korea National Health and Nutrition Examination Survey 2008-2010. J Clin Densitom (2019) 22:59–66. doi: 10.1016/j.jocd.2017.07.001
34. Xu W, Perera S, Medich D, Fiorito G, Wagner J, Berger LK, et al. Height Loss, Vertebral Fractures, and the Misclassification of Osteoporosis. Bone (2011) 48:307–11. doi: 10.1016/j.bone.2010.09.027
35. Wáng YXJ, Diacinti D, Leung JCS, Iannacone A, Kripa E, Kwok TCY, et al. Much Lower Prevalence and Severity of Radiographic Osteoporotic Vertebral Fracture in Elderly Hong Kong Chinese Women Than in Age-Matched Rome Caucasian Women: A Cross-Sectional Study. Arch Osteoporos (2021) 16:174. doi: 10.1007/s11657-021-00987-6
36. Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Nonalcoholic Fatty Liver Disease and Osteoporosis: A Systematic Review and Meta-Analysis. J Bone Miner Metab (2017) 35:685–93. doi: 10.1007/s00774-016-0807-2
37. Filip R, Radzki RP, Bieńko M. Novel Insights Into the Relationship Between Nonalcoholic Fatty Liver Disease and Osteoporosis. Clin Interventions Aging (2018) 13:1879–91. doi: 10.2147/cia.s170533
38. Yilmaz Y. Review Article: Non-Alcoholic Fatty Liver Disease and Osteoporosis–Clinical and Molecular Crosstalk. Alimentary Pharmacol Ther (2012) 36:345–52. doi: 10.1111/j.1365-2036.2012.05196.x
39. Loosen SH, Roderburg C, Demir M, Qvartskhava N, Keitel V, Kostev K, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) Is Associated With an Increased Incidence of Osteoporosis and Bone Fractures. Z fur Gastroenterologie (2021). doi: 10.1055/a-1482-9236 (Online ahead of print)
40. Chen YY, Wang WW, Yang L, Chen WW, Zhang HX. Association Between Lipid Profiles and Osteoporosis In Postmenopausal Women: A Meta-Analysis. Eur Rev Med Pharmacol Sci (2018) 22:1–9. doi: 10.26355/eurrev_201801_14093
41. Alay I, Kaya C, Cengiz H, Yildiz S, Ekin M, Yasar L. The Relation of Body Mass Index, Menopausal Symptoms, and Lipid Profile With Bone Mineral Density in Postmenopausal Women. Taiwanese J Obstetrics Gynecology (2020) 59:61–6. doi: 10.1016/j.tjog.2019.11.009
42. Arikan DC, Coskun A, Ozer A, Kilinc M, Atalay F, Arikan T. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation With Osteoporosis. Biol Trace Element Res (2011) 144:407–17. doi: 10.1007/s12011-011-9109-7
43. Pliatsika P, Antoniou A, Alexandrou A, Panoulis C, Kouskouni E, Augoulea A, et al. Serum Lipid Levels and Bone Mineral Density in Greek Postmenopausal Women. Gynecological Endocrinol Off J Int Soc Gynecological Endocrinology (2012) 28:655–60. doi: 10.3109/09513590.2011.650766
44. Bhattoa HP, Cavalier E, Eastell R, Heijboer AC, Jørgensen NR, Makris K, et al. Analytical Considerations and Plans to Standardize or Harmonize Assays for the Reference Bone Turnover Markers PINP and β-CTX in Blood. Clin Chim Acta (2021) 515:16–20. doi: 10.1016/j.cca.2020.12.023
45. Vasikaran S, Eastell R, Bruyere O, Foldes AJ, Garnero P, Griesmacher A, et al. Markers of Bone Turnover for the Prediction of Fracture Risk and Monitoring of Osteoporosis Treatment: A Need for International Reference Standards. Osteoporos Int (2011) 22:391–420. doi: 10.1007/s00198-010-1501-1
46. Diemar SS, Lylloff L, Rønne MS, Møllehave LT, Heidemann M, Thuesen BH, et al. Reference Intervals in Danish Children and Adolescents for Bone Turnover Markers Carboxy-Terminal Cross-Linked Telopeptide of Type I Collagen (β-CTX), Pro-Collagen Type I N-Terminal Propeptide (PINP), Osteocalcin (OC) and Bone-Specific Alkaline Phosphatase (Bone ALP). Bone (2021) 146:115879. doi: 10.1016/j.bone.2021.115879
47. Ranxing Z, Hanlian L, Xu W, Jinghua G, Yanming X, Yan H, et al. Reference Interval Analysis of Procollagen Type 1 N-terminal Propeptide and β Cross-Linked C- Telopeptide of Type 1 Collagen for Healthy Premenopausal Women in Beijing. Chin J Osteoporos (2018) 24:769–75 [in Chinese].
48. Mei L, ZhenLin Z, Yan L, WeiMin D, ZhongLiang D, YingYng H, et al. Re-Analysis of Serum Procollagen Type 1 N-terminal Propeptide and β Cross-Linked C-telopeptide of Type 1 Collagen Concentrations in Healthy Men and Women of Han Nationality. Chin J Osteoporosis Bone Miner Res (2016) 9:7–13 [in Chinese].
Keywords: osteoporosis, osteopenia, DXA, urban area in Beijing, survey, risk factor
Citation: Lin X, Guo H, Lian Y, Kou J, Wang G, Chen Y, Wang J, Han X, Jiang M and Yang Q (2022) Osteoporosis and Related Health Status Among the Elderly Urban Residents in Elderly-Care Inns in Beijing, a Multicenter DXA Survey. Front. Endocrinol. 13:875678. doi: 10.3389/fendo.2022.875678
Received: 14 February 2022; Accepted: 19 April 2022;
Published: 22 July 2022.
Edited by:
Melissa Orlandin Premaor, Federal University of Minas Gerais, BrazilReviewed by:
Alfredo Maria Lurati, Fornaroli hospital Magenta, ItalyCopyright © 2022 Lin, Guo, Lian, Kou, Wang, Chen, Wang, Han, Jiang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: QiaoHui Yang, OTA5NzA4MzkxQHFxLmNvbQ==; Miao Jiang, bWlhb19qbUB2aXAuMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.