
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 13 June 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.873726
Objective: Insulin resistance (IR) is an important determinant of the phenotype and morbidity of the polycystic ovary syndrome (PCOS). In this study, we aimed to figure out the association between the degree of menstrual disturbance and the severity of IR in women with PCOS.
Design: It is a cross-sectional study conducted in an academic tertiary setting.
Patients: The patients comprised five hundred twenty-seven women diagnosed with PCOS by the 2003 Rotterdam criteria and 565 controls with regular vaginal bleeding.
Interventions: The interventions done for this study are medical history collection, physical examination, and blood sampling.
Main outcome measures: The main outcome measures are body mass index (BMI), fasting glucose, fasting insulin, homeostatic model assessment for IR (HOMA-IR), and hormonal parameters.
Results: Women with PCOS had a higher level of BMI, HOMA-IR, and HOMA-β than controls, with a decreased level of sex hormone-binding globulin and QUICK I index. The luteinizing hormone (LH)/follicle-stimulating hormone (FSH), testosterone (T), antral follicle count (AFC), dehydroepiandrosterone sulfate, free androgen index, modified Ferriman–Gallwey score, and the incidence of delayed insulin peak increased with the degree of menstrual disturbance, although there was no significance for the latter four parameters. Women with vaginal bleeding intervals of 45–90 days had a relatively higher level of HOMA-IR and HOMA-β, although it was adjusted with age and BMI than the other two groups. Similar results were observed in AUCI (area under the curve of insulin) and I/G [the ratio of AUCI and AUCG (area under the curve of glucose)]. Anovulatory women with vaginal bleeding episodes of less than 45 days tended to have higher glucose and insulin levels, area under the curve of glucose (AUCG), area under the curve of insulin (AUCI), HOMA-IR, and HOMA-β but decreased QUICK I and Matsuda index than those who were ovulatory. Women with vaginal bleeding intervals of longer than 45 days who had hyperandrogenism (HA) showed a higher level of glucose, insulin, HOMA-IR, and HOMA-β but lower QUICK I and Matsuda Index.
Conclusions: In women with PCOS, the severity of IR, the LH/FSH ratio, and androgen level increased with a higher degree of disturbance in menstrual cyclicity (i.e., the vaginal bleeding intervals). Subgroup analysis indicated that the situation of HA may aggravate the disorder of glucose metabolism in women with PCOS. Overall, the interval between episodes of vaginal bleeding may be useful as a ready measure for predicting the severity of IR in PCOS.
Polycystic ovary syndrome (PCOS), characterized by hyperandrogenism (HA), ovulation dysfunction, and polycystic ovarian changes (1), is one of the most common endocrine and metabolic diseases of reproductive-aged women (2). Approximately 70% of women with PCOS are reported to be accompanied with insulin resistance (IR), which will further result in reproductive and metabolic complications in the long term (3–8). Therefore, identifying clinical and/or biological indicators to detect the early IR in women with PCOS will, to some extent, reduce the incidence of diabetes and metabolic syndrome and improve the life quality and long-term prognosis.
Oligomenorrhea and irregular menstrual cycle are important characteristics and criteria of PCOS, regardless of how PCOS is defined (9). Approximately 85%–90% of women with PCOS demonstrated oligoovulation and a prolonged interval between episodes of vaginal bleeding (9, 10). A cohort study indicated that irregular and long menstrual cycles have a strong correlation with hyperinsulinemia (8); these observations are in accord with the results from a cross-sectional study, in which the authors found that women with 35-day-longer bleeding intervals had a higher level of homeostatic model assessment for IR (HOMA-IR) than healthy controls, suggesting that the severity of oligomenorrhea is likely to be positively correlated with IR in PCOS (11). These studies revealed that menstrual dysfunction could be applied as an effective clinical marker to evaluate the potential metabolic disorders in women with PCOS. However, these studies are focused on the association between irregular menstrual cycles and the risk of type 2 diabetes mellitus in women from Spain and the United States. There are limited studies exploring the correlation between menstrual disturbance and the severity of IR in PCOS subjects who are anovulatory and with hyperandrogenism (HA). Given the heterogeneity of PCOS and racial difference, a cross-sectional study was conducted to evaluate the relationship between the degree of menstrual irregularity and glucose metabolic dysfunction in women with PCOS of Asian populations. The influence of the ovulatory status and serum androgen level on glucose metabolism was also considered.
In this study, a total of 527 women with PCOS and 565 controls were recruited. We evaluated the correlation between the menstrual cycle and body mass index (BMI), fasting glucose, fasting insulin, HOMA-IR, triglycerides, and other indicators. Our results revealed that the degree of menstrual dysfunction could act as a robust clinical marker to predict the severity of IR in women with PCOS.
Five hundred twenty-seven women with PCOS and 565 controls were recruited from patients presented to the Reproductive Medicine Center of Sun Yat-sen Memorial Hospital, Sun Yat-sen University between 2009 and 2015. PCOS was diagnosed by the Rotterdam 2003 criteria and was defined by the presence of either two of the following three features: 1) oligo- or anovulation, 2) clinical and/or biochemical signs of hyperandrogenism, and 3) the presence of polycystic ovarian morphology under ultrasound (12). PCOS was diagnosed only after other related disorders had been excluded.
Women with infertility caused by fallopian tube obstruction or male factors (asthenozoospermia or azoospermia) were recruited as control. All control subjects had a long-term history of regular vaginal bleeding (26–35 days) consistent with ovulatory cycles, did not have polycystic ovarian morphology on ultrasonography, and were non-hirsute [modified Ferriman–Gallwey (mFG) score ≤ 3] (13). Controls were excluded if the detailed information of the vaginal bleeding interval was unavailable or if they received a hormonal medication within 3 months of evaluation.
Related disorders were excluded by assessing thyroid stimulating hormone (TSH), prolactin, 17-hydroxyprogesterone, and so on. Screening for Cushing’s syndrome and androgen-secreting neoplasms was performed if clinically indicated.
Subjects were eligible for inclusion with data available for the main outcome measurement if they could be categorized as either PCOS or controls. This study was approved by the Ethics Committee of Sun-Yat Sun Memorial Hospital of Sun Yat-Sen University, under the Chinese Clinical Trial Registry (https://www.chictr.org.cn/enIndex.aspx) number ChiCTR‐DDT‐14005186. All subjects signed written informed consent.
All subjects completed a questionnaire for personal information, menstrual history, relative family history, skin problems (hirsutism, acne, and premature alopecia) associated with hyperandrogenism, and metabolic diseases. Then, participants underwent a thorough medical evaluation including a physical exam for height, weight, waist and hip measurements, and transvaginal pelvic ultrasound (Philips EnVisor C HD Ultrasound) for antral follicle counting. Polycystic ovarian morphology (PCOM) was defined as 12 or more cysts measuring 2–9 mm in one side of the ovary and/or over 10 ml of either ovary volume (14). The mFG score was used to assess hair growth, and hirsutism was defined as an mFG score of ≥6 (15). All patients were examined and assigned an mFG score by XZ.
Women with PCOS were divided into three groups according to the vaginal bleeding intervals and classified as less than 45 days, 45–90 days, and longer than 90 days. Part of women with less than 45 days of bleeding intervals were eumenorrheic, and the ovulatory function was evaluated by the progesterone level in days 22–24 of menstrual cycle. Less than 4 ng/ml was considered as anovulatory, and the remainder were considered ovulatory (11). To study the correlation between ovulatory function and IR, women with vaginal bleeding intervals of less than 45 days were further divided as ovulatory and anovulatory groups. Fasting baseline blood samples for biochemical and hormone testing were obtained at the follicular or preovulatory phase of the cycle (days 2–4 of menstrual cycle) or at random for those who have amenorrhea. Participants were asked not to take any food except water for at least 8 h before testing. Glucose and total cholesterol (CHOL), triglyceride (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were measured by a Beckman AU5800 automatic biochemical analyzer (Beckman Coulter, California, United States). The insulin level, dehydroepiandrosterone sulfate (DHEAS), and sex hormone-binding globulin (SHBG) were assessed by the automatic chemical luminescence immunoassay (Immulite 1000; Siemens, China Medical Solution Group). Serum hormones including the follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), total testosterone (TT), and free testosterone (FT) were measured using Access 2 chemiluminescence immunoassays (Beckman, Chaska, MN, USA) according to the manufacturer’s protocols. The free androgen index (FAI) was calculated using the following formula: [TT (nmol/L) × 100/SHBG (nmol/L)].
The oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) are currently the gold standard to evaluate glucose metabolism by assessing venous plasma glucose and the insulin level in a fasting state and 1 and 2 h after administering a glucose solution. The following mathematical models were used to evaluate IR. The homeostatic model for the assessment of insulin resistance (HOMA-IR) was calculated as follows (16): fasting glucose (mmol/L) × fasting insulin (μU/ml)/22.5. The homeostatic model for the assessment of β-cell function (HOMA-β) was calculated using the following formula (16): 20 × fasting insulin (μU/ml)/[fasting glucose (mmol/L) − 3.5]. The quantitative insulin sensitivity index (QUICK I) was calculated as follows (17): 1/[log(fasting insulin)(μU/ml) + log(fasting glucose)(mg/dl)]. The Matsuda insulin sensitivity index (Matsuda index) was calculated using the following formula (18): 10,000/√(fasting glucose (mg/dl) × fasting insulin (μU/ml) × mean glucose concentration (mg/dl) × mean insulin concentration (μU/ml).
Descriptive statistics were expressed as mean ± standard error. The ANOVA test was used to compare mean values among different groups. The χ2 test and Fisher’s exact test were used to compare categorical variables. A stepwise multiple regression analysis was used to compare the mean HOMA-IR, HOMA-β, QUICK I, and Matsuda index of controls with each cycle length group while controlling for BMI and age. Statistical analysis was performed using SPSS ver. 25.0 (SPSS, Inc., Chicago, IL, USA). P <0.05 was considered statistically significant.
A total of 527 PCOS patients and 565 controls were included in our analysis. The basic characteristics of the two groups are shown in Table 1. As a whole, women with PCOS had significantly higher BMI, LH/FSH, T, AFC, mFG, and FAI than those of controls, while the level of SHBG was significantly decreased in women with PCOS. Compared with the control group, PCOS subjects also had higher levels of fasting insulin, HOMA-IR, HOMA-β, CHOL, TGs, HDL, and LDL but lower levels of fasting glucose and QUICK I. These results suggested that PCOS is a heterogeneous disorder with multiple phenotypes, but the core pathogenesis of PCOS was IR and hyperandrogenism (HA).
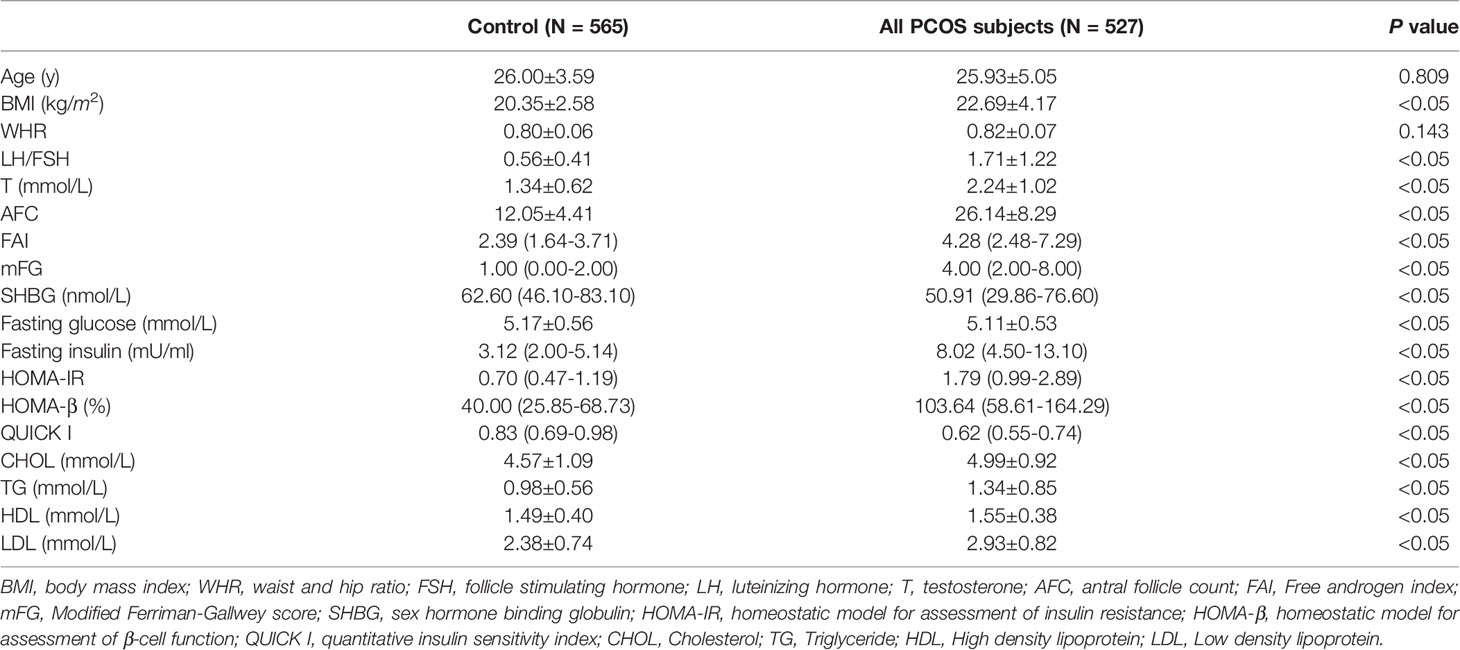
Table 1 Basic characteristic of patients, glucose and lipid metabolism parameters between control and PCOS group.
Women with PCOS were then grouped based on the vaginal bleeding interval (Table 2). Seventy-three percent of PCOS subjects had apparent oligomenorrhea with the interval between episodes of vaginal bleeding of at least 45 days. Women with 45–90-day bleeding intervals comprised 50% of the total, 27% had bleeding intervals of less than 45 days, and the remaining 23% had longer than 90 days. As the intervals of vaginal bleeding were prolonged, the values of LH/FSH, T, DHEAs, AFC, FAI, and mFG tended to increase, although no statistically significant differences were found among groups for DHEAs, FAI, and mFG. There were no differences in age, BMI, waist-to-hip ratio, fasting glucose, and 1 and 2 h blood glucose values among groups. In contrast, both the fasting insulin level and 1 h blood insulin value increased in women with 45–90-day bleeding intervals but insulin value went down to a similar level to the other groups after 2 h of glucose load. Moreover, we noticed a phenomenon that the incidence of insulin peak delay tended to increase as vaginal bleeding intervals were prolonged. After adjusting for age and the BMI, PCOS subjects with a menstrual cycle of 45–90 days showed the highest HOMA-IR and HOMA-β but lowest Matsuda index; meanwhile, these women showed the highest levels of CHOL and LDL and the lowest levels of TG and HDL, although there was no statistical significance among groups (Table 2).
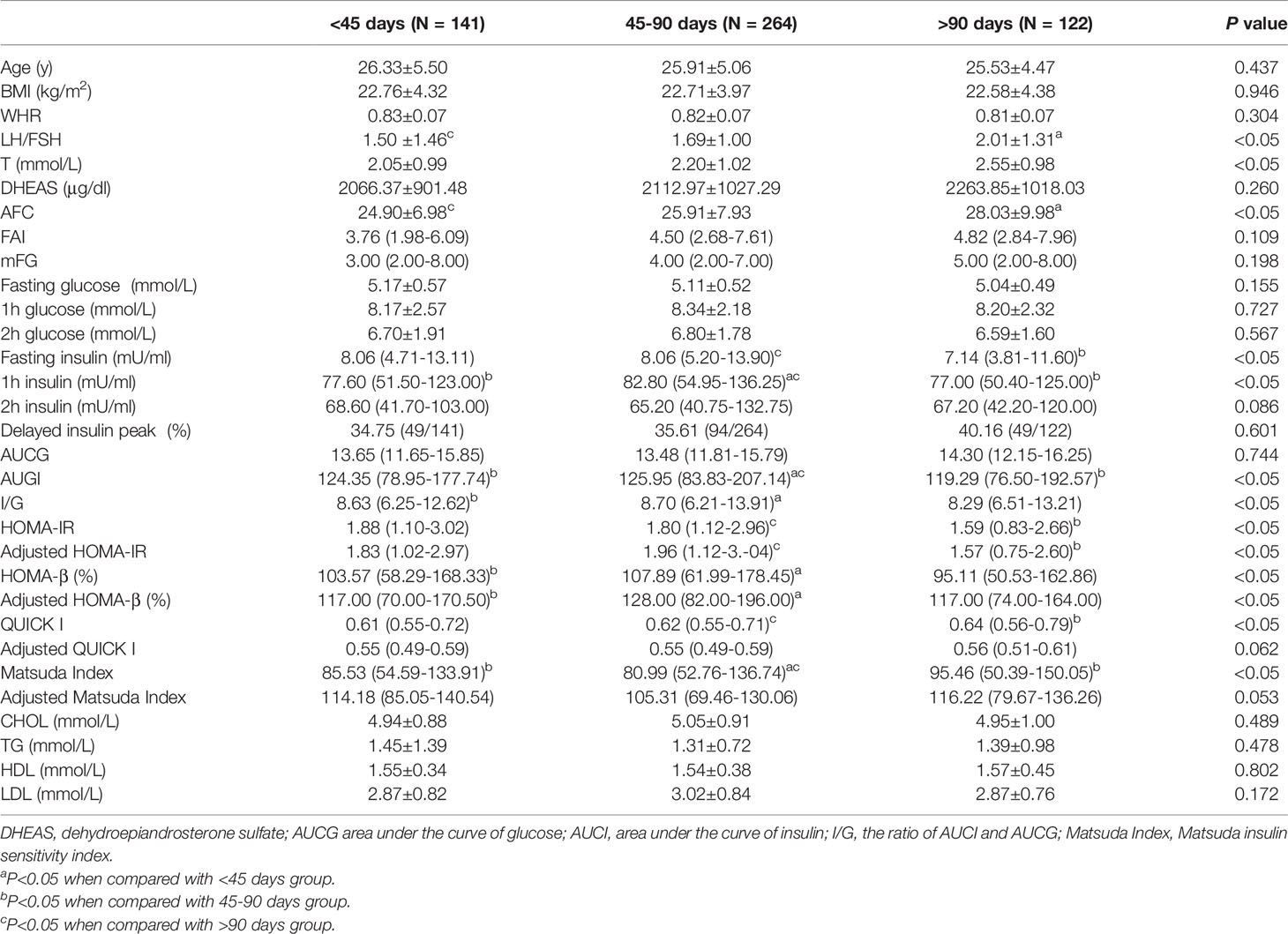
Table 2 Basic characteristic of patients, glucose and lipid metabolism parameters among groups of different menstrual cycle length.
A previous study found that approximately 10% of women with PCOS were ovulatory, although they had an irregular menstrual cycle (11). We further grouped women with less than 45 days of bleeding interval according to the ovulatory function and classified as less than 45 days ovulatory and less than 45 days anovulatory (Table 3). Compared with ovulatory women, those who were anovulatory showed a higher level of BMI and an increasing trend of LH/FSH, mFG, the glucose level of fasting, 1 and 2 h, fasting insulin, and 1 h blood insulin value, resulting in a relatively higher level of HOMA-IR, HOMA-β, and QUICK I but a decreasing trend of SHBG and Matsuda index (Table 3). Meanwhile, CHOL, TG, and LDL also tended to increase in anovulatory women but the level of HDL was decreased, suggesting that the ovulatory function of women with PCOS not only had an effect on glucose but also lipid metabolism.
Apart from IR, hyperandrogenism is considered as another important pathological mechanism of PCOS. We further divided women with 45 days longer bleeding interval into the non-HA group and HA group (Table 4). Women with HA of both 45–90-day and 90-day-longer vaginal bleeding intervals showed a higher BMI, although there was no significance in the latter group. Similar results were also observed in women with HA with higher levels of T, DHEAS, FAI, mFG, glucose and insulin level of fasting, 1 and 2 h blood glucose and insulin values, HOMA-IR, and HOMA-β. Meanwhile, QUICK I and the Matsuda index showed a decreasing trend under the situation of HA. However, the change of lipid metabolism parameters was not as obvious as glucose, suggesting that the situation of HA may aggravate the disorder of glucose metabolism in women with PCOS (Table 4).
In this study, we validated the severity of IR and the level of androgen was significantly higher in women with PCOS when compared with controls. Further analysis indicated a significant correlation between the degree of menstrual disturbance and the severity of IR by grouped women with PCOS according to the intervals between episodes of vaginal bleeding. A similar relationship between the LH/FSH ratio and the severity of IR was also observed. Meanwhile, HA may aggravate the disorder of glucose metabolism in PCOS.
A growing number of studies have shown that PCOS is often accompanied by IR, and obese PCOS subjects have a higher risk of developing IR (13, 19). Studies revealed that the incidence of IR in normal-weight PCOS patients is approximately 65%, while the incidence of IR is as high as 95% in obese PCOS subjects (14, 20). In our study, we found that women with PCOS had a significantly higher BMI than controls although the diagnostic criteria for obesity were not met (Table 1). Hyperinsulinemia caused by IR, HA, and changes in the paracrine signal of the follicle can interfere with the activation, growth, and selection of the follicle, and damage the normal development and ovulation of the follicle (21). Many studies have shown that IR may damage the development of oocytes and embryo quality, and is related to the low fertilization rate and implantation rate of women with PCOS (22–24). Identifying clinical markers that can effectively predict the occurrence of IR may help to improve the pregnancy outcome of women with PCOS. In our study, menstrual dysfunction, reflected by vaginal bleeding interval, was found to be correlated with the severity of IR, these results were consistent with previous studies (11, 25). Interestingly, women with PCOS and vaginal bleeding episodes of 45–90-day intervals had higher HOMA-IR and HOMA-β but lower QUICK I and Matsuda index than those with a 90-day-longer cycle, whereas the prevalence of delayed insulin peak went up with the prolongation of vaginal bleeding intervals. These observations may be related with the worse function of the islet B cell in PCOS subjects with vaginal bleeding intervals longer than 90 days.
Oligomenorrhea or an irregular menstrual cycle is reported to be associated with higher androgen levels and a lower level of SHBG (9). We found that with the prolongation of the vaginal bleeding intervals, the testosterone levels in PCOS patients gradually increased. Previous studies have shown that the serum androgen levels in both healthy women and those with PCOS are positively correlated with the number of follicles, and anti-androgen therapy in women with PCOS can effectively improve the situation of polycystic ovarian morphology (26, 27). There is a hypothesis that HA is the first hit to the development of PCOS follicles. High androgen levels in follicles promote the recruitment of small follicles, and excessively recruited small follicles will inhibit the selection process of dominant follicles (28, 29). These studies suggest that HA in PCOS patients may disrupt the menstrual cycle by impairing the normal development of follicles. In the subgroup analysis of this study, we noticed that women with HA of both 45–90 days and 90 days longer vaginal bleeding intervals had a worse situation of IR, indicating that HA may aggravate the severity of IR in PCOS. These results were similar to our previous study, in which HA was proven to be independently associated with the risk for obesity and type 2 diabetes (30). Then, a more severe IR induced a continuous excessive production of androgen (31, 32).
Although there are several studies that proved the positive relationship between menstrual disturbance and the severity of IR, the internal mechanism needs further study. PCOS subjects with IR were reported to exacerbate abnormal steroid production in granulosa cells, which is related with anovulation (33). A systemic glucose uptake disorder is considered as one of the main features of IR (34). GLUT4, expressed in insulin-sensitive tissues, is the main protein responsible for insulin-mediated glucose transport to adipocytes (35, 36), playing an important role in regulating glucose tolerance, glucose metabolism, and insulin sensitivity (37). A previous study found that the expression level of GLUT4 in the endometrial epithelial cells of PCOS subjects with HA was significantly lower than that of the control group (38), and GLUT4 dysfunction may lead to the occurrence of IR in PCOS (39, 40). The dysregulation of GLUT4 expression in granulosa cells may be the inner link between IR and oligomenorrhea, which is an underlying mechanism worthy to explore.
In summary, we found that the abnormality of androgen metabolism in women with PCOS changed with the severity of oligomenorrhea, and the severity of IR in women with PCOS is positively correlated with the vaginal bleeding interval. The menstrual disturbance reflected by the length of a menstrual cycle may be an effective indicator to predict IR in women with PCOS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Sun-Yat Sun Memorial Hospital of Sun Yat-Sen University. The patients/participants provided their written informed consent to participate in this study.
XL: data collection, data analysis, and manuscript writing. DYY: manuscript writing. PP: data collection. RA: project development. DZY: project development. YC: project development. XZ: project development and manuscript writing. All authors contributed to the article and approved the submitted version.
This work was supported by the GDPH Supporting Fund for Talent Program (KY012021439), the National Key Research and Development Program of China (2017YFC1001004), National Natural Science Foundation of China (81771545), the Guangdong Basic and Applied Basic Research Foundation (2020B1515020001), and the Special Fund for Clinical Research of Chinese Medical Association (18010180747).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ezeh U, Chen IY, Chen YH, Azziz R. Adipocyte Insulin Resistance in PCOS: Relationship With GLUT-4 Expression and Whole-Body Glucose Disposal and β-Cell Function. J Clin Endocrinol Metab (2020) 105(7):e2408–20. doi: 10.1210/clinem/dgaa235
2. Ezeh U, Huang A, Landay M, Azziz R. Long-Term Response of Hirsutism and Other Hyperandrogenic Symptoms to Combination Therapy in Polycystic Ovary Syndrome. J Womens Health (Larchmt) (2018) 27(7):892–902. doi: 10.1089/jwh.2017.6833
3. Jeanes Y, Reeves S. Metabolic Consequences of Obesity and Insulin Resistance in Polycystic Ovary Syndrome: Diagnostic and Methodological Challenges. Nutr Res Rev (2017) 30(1):97–105. doi: 10.1017/s0954422416000287
4. Yang Y, Jiang H, Xiao L, Yang X. MicroRNA-33b-5p is Overexpressed and Inhibits GLUT4 by Targeting HMGA2 in Polycystic Ovarian Syndrome: An In Vivo and In Vitro Study. Oncol Rep (2018) 39(6):3073–85. doi: 10.3892/or.2018.6375
5. Marciniak A, Nawrocka Rutkowska J, Brodowska A, Wiśniewska B, Starczewski A. Cardiovascular System Diseases in Patients With Polycystic Ovary Syndrome - the Role of Inflammation Process in This Pathology and Possibility of Early Diagnosis and Prevention. Ann Agric Environ Med AAEM (2016) 23(4):537–41. doi: 10.5604/12321966.1226842
6. Merz CN, Shaw LJ, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, et al. Cardiovascular Disease and 10-Year Mortality in Postmenopausal Women With Clinical Features of Polycystic Ovary Syndrome. J Women's Health (2002) (2016) 25(9):875–81. doi: 10.1089/jwh.2015.5441
7. Velija-Asimi Z, Burekovic A, Dujic T, Dizdarevic-Bostandzic A, Semiz S. Incidence of Prediabetes and Risk of Developing Cardiovascular Disease in Women With Polycystic Ovary Syndrome. Bosnian J Basic Med Sci (2016) 16(4):298–306. doi: 10.17305/bjbms.2016.1428
8. Wang YX, Shan Z, Arvizu M, Pan A, Manson JE, Missmer SA, et al. Associations of Menstrual Cycle Characteristics Across the Reproductive Life Span and Lifestyle Factors With Risk of Type 2 Diabetes. JAMA Netw Open (2020) 3(12):e2027928. doi: 10.1001/jamanetworkopen.2020.27928
9. Harris HR, Titus LJ, Cramer DW, Terry KL. Long and Irregular Menstrual Cycles, Polycystic Ovary Syndrome, and Ovarian Cancer Risk in a Population-Based Case-Control Study. Int J Cancer (2017) 140(2):285–91. doi: 10.1002/ijc.30441
10. Hart R, Hickey M, Franks S. Definitions, Prevalence and Symptoms of Polycystic Ovaries and Polycystic Ovary Syndrome. Best Pract Res Clin Obstetrics Gynaecol (2004) 18(5):671–83. doi: 10.1016/j.bpobgyn.2004.05.001
11. Brower M, Brennan K, Pall M, Azziz R. The Severity of Menstrual Dysfunction as a Predictor of Insulin Resistance in PCOS. J Clin Endocrinol Metab (2013) 98(12):E1967–71. doi: 10.1210/jc.2013-2815
12. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum Reprod (2004) 19(1):41–7. doi: 10.1093/humrep/deh098
13. Wu XK, Zhou SY, Liu JX, Pöllänen P, Sallinen K, Mäkinen M, et al. Selective Ovary Resistance to Insulin Signaling in Women With Polycystic Ovary Syndrome. Fertil Steril (2003) 80(4):954–65. doi: 10.1016/s0015-0282(03)01007-0
14. Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and Predictors of Risk for Type 2 Diabetes Mellitus and Impaired Glucose Tolerance in Polycystic Ovary Syndrome: A Prospective, Controlled Study in 254 Affected Women. J Clin Endocrinol Metab (1999) 84(1):165–9. doi: 10.1210/jcem.84.1.5393
15. Kim JJ, Chae SJ, Choi YM, Hwang SS, Hwang KR, Kim SM, et al. Assessment of Hirsutism Among Korean Women: Results of a Randomly Selected Sample of Women Seeking Pre-Employment Physical Check-Up. Hum Reprod (Oxford England) (2011) 26(1):214–20. doi: 10.1093/humrep/deq303
16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/bf00280883
17. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity in Humans. J Clin Endocrinol Metab (2000) 85(7):2402–10. doi: 10.1210/jcem.85.7.6661
18. Matsuda M, DeFronzo RA. Insulin Sensitivity Indices Obtained From Oral Glucose Tolerance Testing: Comparison With the Euglycemic Insulin Clamp. Diabetes Care (1999) 22(9):1462–70. doi: 10.2337/diacare.22.9.1462
19. Book CB, Dunaif A. Selective Insulin Resistance in the Polycystic Ovary Syndrome. J Clin Endocrinol Metab (1999) 84(9):3110–6. doi: 10.1210/jcem.84.9.6010
20. Carmina E, Lobo RA. Use of Fasting Blood to Assess the Prevalence of Insulin Resistance in Women With Polycystic Ovary Syndrome. Fertil Steril (2004) 82(3):661–5. doi: 10.1016/j.fertnstert.2004.01.041
21. Dumesic DA, Richards JS. Ontogeny of the Ovary in Polycystic Ovary Syndrome. Fertil Steril (2013) 100(1):23–38. doi: 10.1016/j.fertnstert.2013.02.011
22. Cano F, Garcia-Velasco JA, Millet A, Remohi J, Simon C, Pellicer A. Oocyte Quality in Polycystic Ovaries Revisited: Identification of a Particular Subgroup of Women. J Assist Reprod Genet (1997) 14(5):254–61. doi: 10.1007/BF02765826
23. Samoto T, Maruo T, Ladines-Llave CA, Matsuo H, Deguchi J, Barnea ER, et al. Insulin Receptor Expression in Follicular and Stromal Compartments of the Human Ovary Over the Course of Follicular Growth, Regression and Atresia. Endocr J (1993) 40(6):715–26. doi: 10.1507/endocrj.40.715
24. Ou XH, Li S, Wang ZB, Li M, Quan S, Xing F, et al. Maternal Insulin Resistance Causes Oxidative Stress and Mitochondrial Dysfunction in Mouse Oocytes. Hum Reprod (2012) 27(7):2130–45. doi: 10.1093/humrep/des137
25. Ezeh U, Ezeh C, Pisarska MD, Azziz R. Menstrual Dysfunction in Polycystic Ovary Syndrome: Association With Dynamic State Insulin Resistance Rather Than Hyperandrogenism. Fertil Steril (2021) 115(6):1557–68. doi: 10.1016/j.fertnstert.2020.12.015
26. Dumesic DA, Damario MA, Session DR, Famuyide A, Lesnick TG, Thornhill AR, et al. Ovarian Morphology and Serum Hormone Markers as Predictors of Ovarian Follicle Recruitment by Gonadotropins for In Vitro Fertilization. J Clin Endocrinol Metab (2001) 86(6):2538–43. doi: 10.1210/jcem.86.6.7605
27. De Leo V, Lanzetta D, D'Antona D, la Marca A, Morgante G. Hormonal Effects of Flutamide in Young Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (1998) 83(1):99–102. doi: 10.1210/jcem.83.1.4500
28. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens Stimulate Early Stages of Follicular Growth in the Primate Ovary. J Clin Invest (1998) 101(12):2622–9. doi: 10.1172/JCI2081
29. Jonard S, Dewailly D. The Follicular Excess in Polycystic Ovaries, Due to Intra-Ovarian Hyperandrogenism, may be the Main Culprit for the Follicular Arrest. Hum Reprod Update (2004) 10(2):107–17. doi: 10.1093/humupd/dmh010
30. Zhao X, Zhong J, Mo Y, Chen X, Chen Y, Yang D. Association of Biochemical Hyperandrogenism With Type 2 Diabetes and Obesity in Chinese Women With Polycystic Ovary Syndrome. Int J Gynaecol Obstetrics: Off Organ Int Fed Gynaecol Obstetrics (2010) 108(2):148–51. doi: 10.1016/j.ijgo.2009.09.021
31. Diamanti-Kandarakis E, Dunaif A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr Rev (2012) 33(6):981–1030. doi: 10.1210/er.2011-1034
32. Garzia E, Galiano V, Marfia G, Navone S, Grossi E, Marconi AM. Hyperandrogenism and Menstrual Imbalance are the Best Predictors of Metformin Response in PCOS Patients. Reprod Biol Endocrinol RB&E (2022) 20(1):6. doi: 10.1186/s12958-021-00876-0
33. Li X, Zhu Q, Wang W, Qi J, He Y, Wang Y, et al. Elevated Chemerin Induces Insulin Resistance in Human Granulosa-Lutein Cells From Polycystic Ovary Syndrome Patients. FASEB J (2019) 33(10):11303–13. doi: 10.1096/fj.201802829R
34. Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, et al. Effects of Endogenous Androgens and Abdominal Fat Distribution on the Interrelationship Between Insulin and Non-Insulin-Mediated Glucose Uptake in Females. J Clin Endocrinol Metab (2013) 98(4):1541–8. doi: 10.1210/jc.2012-2937
35. Shepherd PR, Kahn BB. Glucose Transporters and Insulin Action–Implications for Insulin Resistance and Diabetes Mellitus. N Engl J Med (1999) 341(4):248–57. doi: 10.1056/NEJM199907223410406
36. Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, et al. miRNA-93 Inhibits GLUT4 and is Overexpressed in Adipose Tissue of Polycystic Ovary Syndrome Patients and Women With Insulin Resistance. Diabetes (2013) 62(7):2278–86. doi: 10.2337/db12-0963
37. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-Selective Targeting of the GLUT4 Gene Impairs Insulin Action in Muscle and Liver. Nature (2001) 409(6821):729–33. doi: 10.1038/35055575
38. Mioni R, Chiarelli S, Xamin N, Zuliani L, Granzotto M, Mozzanega B, et al. Evidence for the Presence of Glucose Transporter 4 in the Endometrium and its Regulation in Polycystic Ovary Syndrome Patients. J Clin Endocrinol Metab (2004) 89(8):4089–96. doi: 10.1210/jc.2003-032028
39. Johansson J, Feng Y, Shao R, Lonn M, Billig H, Stener-Victorin E. Intense Electroacupuncture Normalizes Insulin Sensitivity, Increases Muscle GLUT4 Content, and Improves Lipid Profile in a Rat Model of Polycystic Ovary Syndrome. Am J Physiol Endocrinol Metab (2010) 299(4):E551–9. doi: 10.1152/ajpendo.00323.2010
Keywords: polycystic ovary syndrome, glucose metabolism, insulin resistance, vaginal bleeding intervals, hyperandrogenism
Citation: Li X, Yang D, Pan P, Azziz R, Yang D, Cheng Y and Zhao X (2022) The Degree of Menstrual Disturbance Is Associated With the Severity of Insulin Resistance in PCOS. Front. Endocrinol. 13:873726. doi: 10.3389/fendo.2022.873726
Received: 11 February 2022; Accepted: 15 April 2022;
Published: 13 June 2022.
Edited by:
Giovanna Muscogiuri, University of Naples Federico II, ItalyReviewed by:
Sarantis Livadas, Metropolitan Hospital, GreeceCopyright © 2022 Li, Yang, Pan, Azziz, Yang, Cheng and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomiao Zhao, emhhb3htaWFvQDE2My5jb20=; Yanxiang Cheng, eWFueGlhbmdDaGVuZ0B3aHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.