- 1Department of Thyroid and Hernia Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Shantou University Medical College, Shantou, China
- 3The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
Background: Prediction of central lymph node metastasis (CLNM) is vital for clinical decision-making processes in clinically N0 (cN0) unifocal papillary thyroid carcinoma (PTC), but the sensitivity of preoperative detection of CLNM is limited. The aim of the present study was to determine whether there are ultrasonic (US) characteristics associated with CLNM.
Methods: In total, 1657 PTC patients (514 men and 1143 women) were enrolled in the present study between January 2018 and May 2021. The patients met the following inclusion criteria based on preoperative detection: suspected nodule confirmed as PTC by biopsy; the nodule was unifocal and less than 4 cm in diameter; no prior neck radiation exposure; no extrathyroidal extension; and no CLNM or distant metastases on imaging. All the enrolled patients underwent total thyroidectomy with prophylactic central lymph node dissection (CLND). A postoperative pathological diagnosis was made.
Results: CLNM was found in 58.4% of male patients and 36.9% of female patients. In univariate analysis, size, adjacent anterior capsule, distance to the lower pole and color Doppler flow imaging (CDFI) were considered risk factors for the male and female groups (p < 0.05). In multivariate analyses, size, adjacent anterior capsule, distance to the lower pole and CDFI were independent risk factors for male patients. For females, the independent risk factors included size, adjacent anterior capsule, distance to the lower pole and CDFI.
Conclusion: In the present cohort, US imaging characteristics, including size, adjacent anterior capsule, distance to the lower pole and CDFI, were identified to be potentially beneficial in preoperative clinical decision-making processes for cN0 unifocal PTC patients.
Introduction
Thyroid carcinoma is one of the most common neoplastic diseases (1), and its morbidity is increasing worldwide (2). The age-standardized incidence of thyroid carcinoma is over 5% in some Asian countries (3), and this carcinoma occurs approximately three times more often in women than in men (4). Papillary thyroid carcinoma (PTC) accounts for approximately 95% of thyroid carcinomas and generally has an excellent prognosis with 10-year survival rates approaching 90-95% (5). Nevertheless, because PTC represents approximately 95% of all cases, most cancer related mortality is due to PTC.
The primary treatment method of PTC is surgical ablation. The objectives of initial surgical therapy include removing the primary tumor and clinically significant cervical lymph nodes as well as minimizing treatment-related morbidity and the risk of recurrence or metastasis. Recent American Thyroid Association (ATA) guidelines and National Comprehensive Cancer Network (NCCN) guidelines note primary risk factors for preoperative determination of the thyroid resection extent (6, 7). Lobectomy is indicated if the following criteria are met: no prior radiation exposure, no cervical lymph node metastases, no extrathyroidal extension, no distant metastases and tumor size less than 4 cm in diameter. For these criteria-matched clinically N0 (cN0) unifocal PTCs, routine prophylactic central compartment lymph node dissection (CLND) is not recommended by both guidelines. However, central compartment lymph node metastasis (CLNM) is relevant to risk stratification and prognosis (8, 9). Currently, there are no non- or minimally-invasive methods that are completely reliable for detecting all of the potential metastases (10). Thus, an accurate preoperative evaluation of CLNM is vital for the management of PTC patients.
For cN0 unifocal PTCs, the accurate identification of CLNM is crucial. Nonetheless, CLNM is difficult to detect preoperatively, and the current assessment methods have limited power. Approximately 30–80% of PTCs are associated with CLNM (11–13), and some studies have suggested that CLNM is related to disease relapse and distant metastases (14–16). As CLNM is difficult to detect preoperatively and CLND is related to morbidity (17), the clinical decisions for treatment are controversial (18). Previous medical studies have suggested that CLND may reduce the recurrence of PTC, indicating a risk stratification for recurrence and distant metastases. In addition, the treatment procedure, such as radioactive iodine therapy, may be altered accordingly. For those criteria-matched cases, prophylactic CLND permits patients to obtain more active medical treatment and less hazardous reoperative surgical treatment (19, 20). However, prophylactic CLND increases the morbidity, such as hypoparathyroidism and recurrent laryngeal nerve injury (21, 22). The ATA and NCCN guidelines do not suggest prophylactic CLND, stating that prophylactic CLND may be considered in specific patients who have advanced primary tumors. There is also a viewpoint that more evidence is needed to support that prophylactic CLND is beneficial to reduce recurrence rates (23). Above all, a more accurate evaluation of CLNM is necessary for cN0 unifocal PTC patients to obtain better clinical decisions.
Ultrasonic (US) detection is the preferred diagnostic method for CNLM. Although it has many advantages, there are limitations. For example, the sensitivity of US detection in evaluating CLNM ranges from 20 to 60% (24–26). Although many studies have reported high-risk factors related to clinical and US characteristics predictive of CLNM of PTC patients, the conclusions are controversial. Some of the identified risk factors, such as tumor differentiation, extrathyroidal invasion and gene type, are only available postoperatively (27), indicating that they cannot provide reliable information for preoperative clinical decision-making processes. Consequently, the research on a noninvasive and valuable approach based on US detection for evaluating CLNM is essential but challenging.
The present study aimed to evaluate the US imaging characteristics of nodules associated with CLNM in cN0 unifocal PTC patients. The present conclusions may be useful in preoperative clinical decision-making processes.
Materials and Methods
Patient Data and Ethical Approval
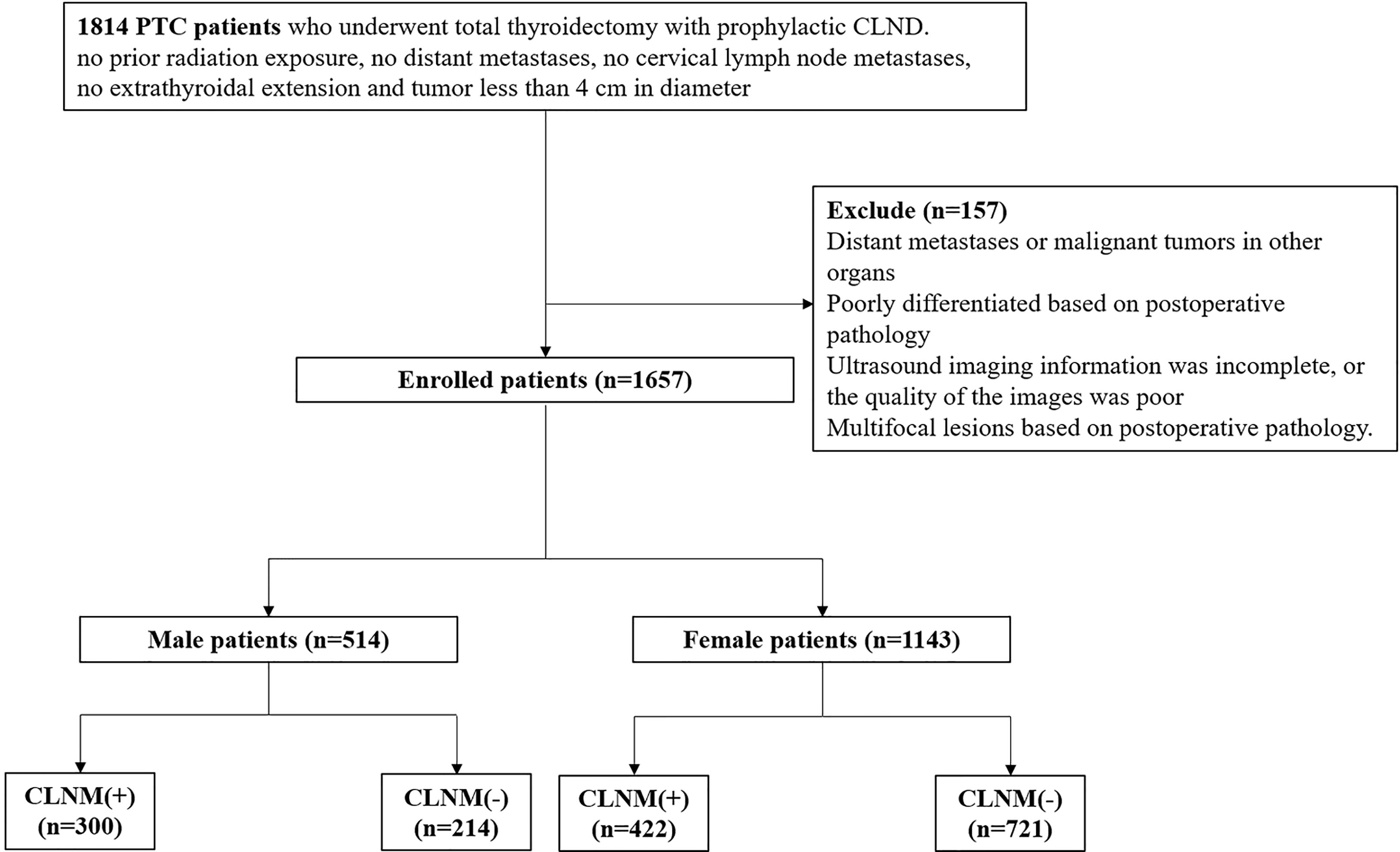
The studies involving human participants were reviewed and approved by the Ethics Committee of the Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (Guangzhou, Guangdong Province, People’s Republic of China), and they conformed to the provisions of the Declaration of Helsinki. Written informed consent from the patients was not required to participate in this study in accordance with national legislation and the institutional requirements. We evaluated the patients retrospectively with histologically confirmed PTC in our hospital between January 2018 and May 2021. The patients were enrolled according to the following criteria: (1) the suspected thyroid nodule was unifocal and less than 4 cm in diameter based on US examination; (2) the suspected nodule was confirmed to be malignant by ultrasound-guided puncture biopsy; (3) no extrathyroidal extension and no cervical lymph node metastases based on US examination; (4) patients were subjected to an initial thyroid surgery with CLND and were histologically confirmed as having PTC; and (5) no prior neck radiation exposure. Patients were excluded based on the following criteria: (1) having distant metastases or malignant tumors in other organs; (2) poorly differentiated based on postoperative pathology; (3) the US imaging information was incomplete, or the quality of the images was unclear; or (4) multifocal lesions based on postoperative pathology. Figure 1 shows the patient recruitment process. Ultimately, 1657 patients (514 men and 1143 women) were included in the present study. The data were divided into CLNM-negative and CLNM-positive groups according to the pathology results.
US Equipment and Evaluation of US Characteristics
US examinations were performed using HI Vision 900, HI Vision Ascendus and HI Vision Preirus color US units (with US elasticity imaging capability) from Hitachi, and the probe frequency was 6.0–13.0 MHz. The US imaging features of every patient were retrospectively re-examined by two independent radiologists with more than 10 years of experience in thyroid US imaging; neither observer knew the clinical nor the pathological outcomes. If the radiologists faced a dilemma, they would determine their final decisions by a consensus. The imaging characteristics of each nodule were as follows: tumor size; multifocality; aspect ratio (height divided by width on transverse views, A/T); tumor location; distance between the nodules and the adjacent capsule; microcalcification situation; border; US halo; tumor internal vascularity; and Hashimoto’s thyroiditis. Many images of the longitudinal and transverse axes were fully evaluated. The tumor size refers to the maximum diameter (D) of the nodule, which was classified as follows: D ≤ 0.5 cm, 0.5 < D ≤ 1.0 cm, 1.0 < D ≤ 1.5 cm and D > 1.5 cm. The A/T was classified as ≤ 1 or > 1. The location of the tumor was evaluated according to the following three aspects: location (left lobe, right lobe and isthmic), distance to the upper pole and distance to the lower pole. The distance between the tumor and adjacent capsule (anterior and posterior) was classified into three categories as follows: < 1 mm and not protruding outside the thyroid capsule; 1 ≤ and < 2 mm; and ≥ 2 mm. Tumor vascularity was classified from 0 to 3 and evaluated by color Doppler flow imaging (CDFI). Hashimoto’s thyroiditis was diagnosed on the basis of US characteristics. Because the diagnostic performance of the present study depended on the accuracy of operator-reported imaging features, the interobserver reproducibility for US features was assessed. Regarding the preoperative identification of cervical lymph nodes (LNs), a LN was considered suspicious if it had one of the following characteristics: microcalcifications, hyperechoic change, loss of fatty hilum; round shape; and necrosis (28).
Statistical Analysis
Statistical analysis was performed with SPSS Statistics version 24.0 (IBM Corp.). Categorical variables are presented as numbers and percentages. A chi-square test or Fisher’s exact test was used to assess differences between groups. A logistic regression model was used to evaluate the risk factors. The reported statistical significance levels were all two-sided with statistical significance set at 0.05.
Results
Characteristics of Patients
Among the 1657 patients, there were 514 (31.0%) male patients and 1143 (69.0%) female patients. A significant difference was found in gender between CLNM-positive and CLNM-negative patients; 58.4% of males and 36.9% of females were CLNM-positive patients (p < 0.05). The gender disparities in incidence, prognosis and aggressiveness are well established for PTC, but the underlying causes remain poorly understood. Population-based studies have shown that reduced estrogen exposure favors PTC malignancy (29, 30). To adjust for the gender factor, we arranged two separate groups for these patients.
Risk Factors for Male and Female Patients With cN0 Unifocal PTC
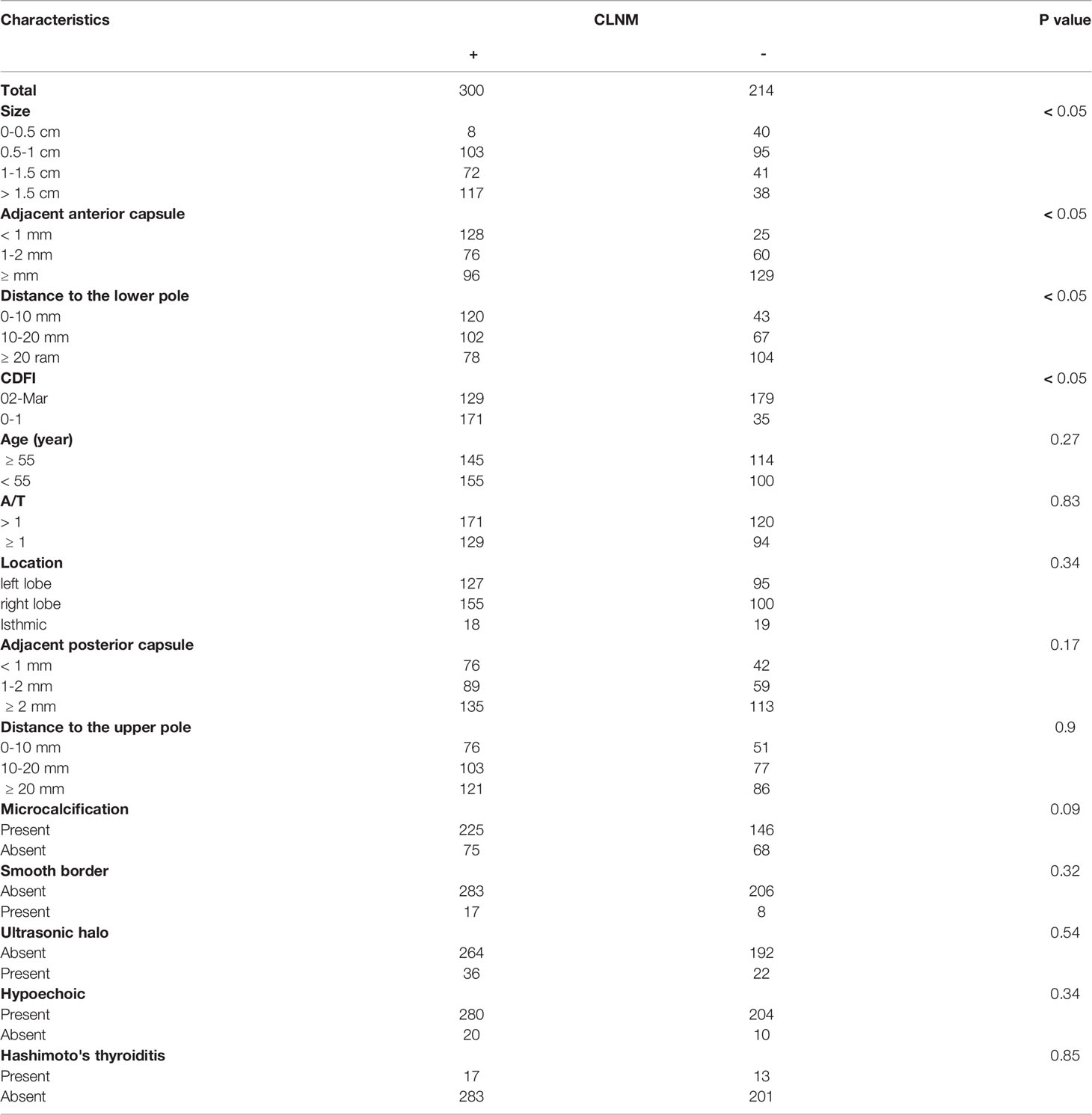
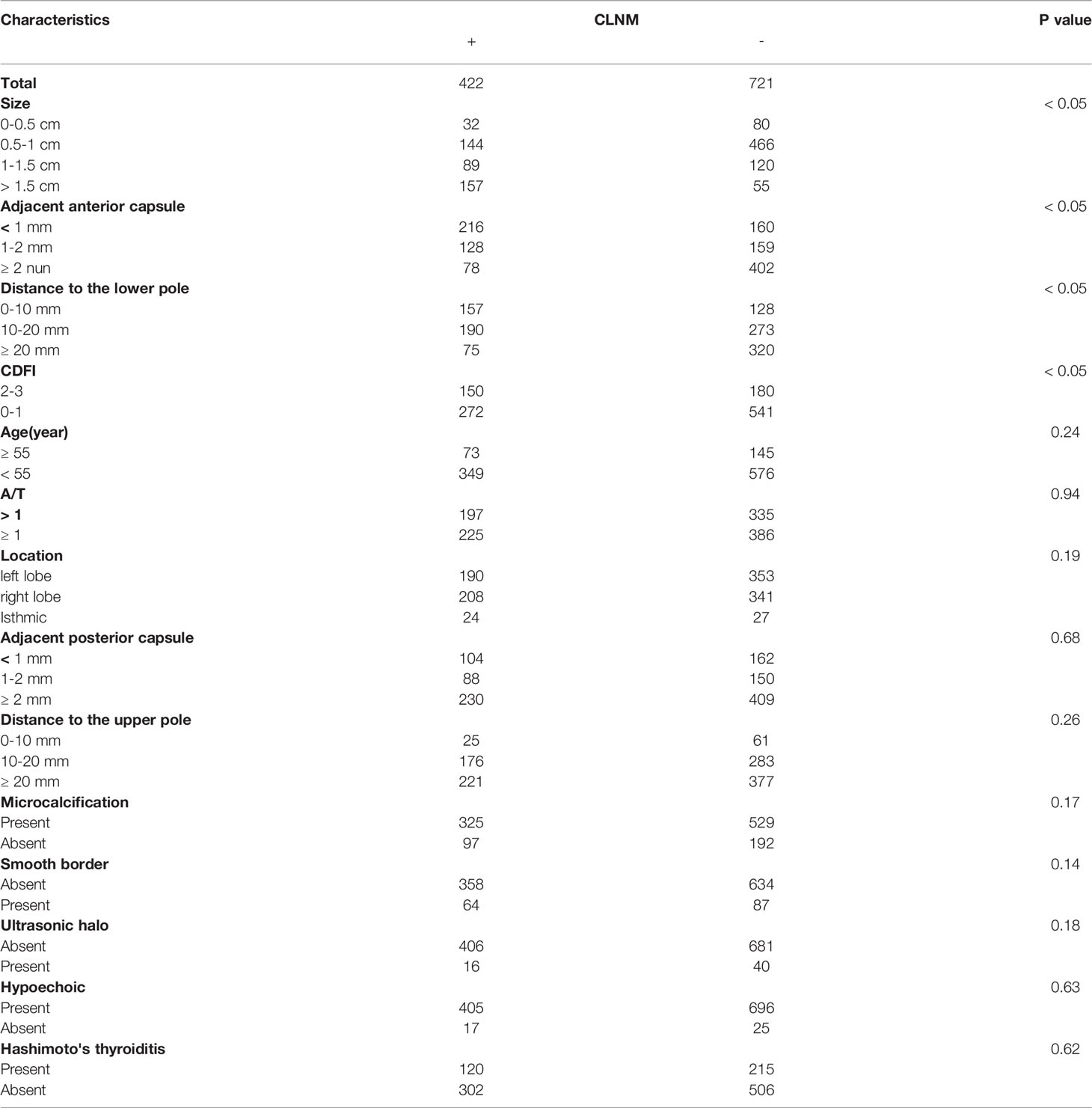
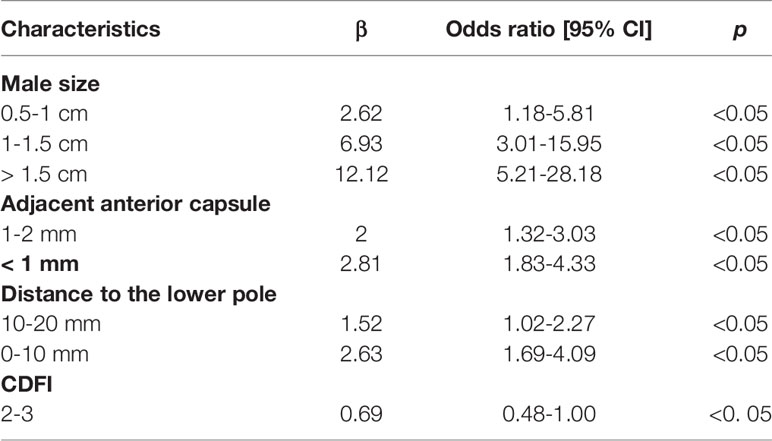
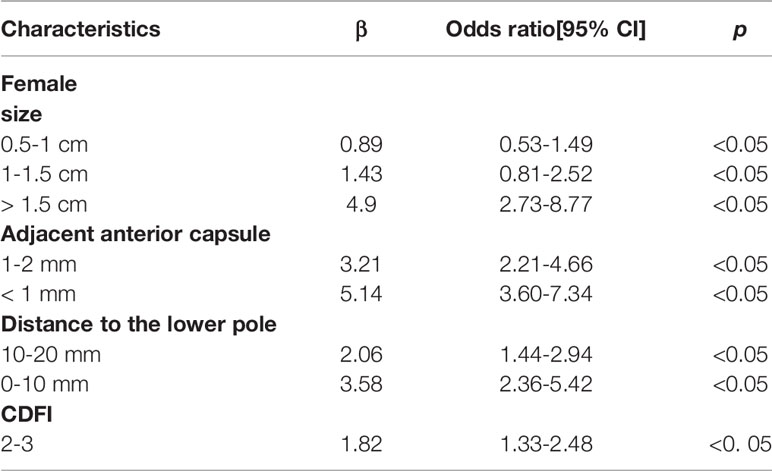
The patient features and US imaging characteristics of thyroid nodules in the male and female cohorts are shown in Tables 1 and 2. Univariate and multivariate analyses were conducted to determine the differences in clinical and US imaging features between CLNM-positive and CLNM-negative groups. In univariate analysis, size (p < 0.05), adjacent anterior capsule (p < 0.05), distance to the lower pole (p < 0.05) and CDFI (p < 0.05) were considered risk factors for both male and female groups (Tables 1 and 2). In multivariate analyses, size, adjacent anterior capsule, distance to the lower pole and CDFI were considered independent risk factors (Tables 3 and 4). For male patients, size (0.5-1 cm, OR 2.62, 95% CI 1.18-5.81; 1-1.5 cm, OR 6.93, 95% CI 3.01-15.95; >1.5 cm, OR 12.12, 95% CI 5.21-28.18), adjacent anterior capsule (1-2 mm, OR 2.00, 95% CI 1.32-3.03; <1 mm, OR 2.81, 95% CI 1.83-4.33), distance to the lower pole (10-20 mm, OR 1.52, 95% CI 1.02-2.27; 0-10 mm, OR 2.63, 95% CI 1.69-4.09) and CDFI (2-3, OR 0.69, 95% CI 0.48-1.00) were considered independent risk factors. For female patients, size (0.5-1 cm, OR 0.89, 95% CI 0.53-1.49; 1-1.5 cm, OR 1.43, 95% CI 0.81-2.52; >1.5 cm, OR 4.90, 95% CI 2.73-8.77), adjacent anterior capsule (1-2 mm, OR 3.21, 95% CI 2.21-4.46; <1 mm, OR 5.14, 95% CI 3.60-7.34), distance to the lower pole (10-20 mm, OR 2.06, 95% CI 1.44-2.94; 0-10 mm, OR 3.58, 95% CI 2.36-5.42) and CDFI (2-3, OR 1.82, 95% CI 1.33-2.48) were considered independent risk factors.
Discussion
In the present study, we found and validated several US-based characteristics for predicting the probability of CLNM in cN0 unifocal PTC patients. The patients in the present study met the following criteria based on preoperative detection: the suspected nodule was confirmed to be PTC by biopsy; the nodule was unifocal and less than 4 cm in diameter; no prior neck radiation exposure; no extrathyroidal extension; no CLNM; and no distant metastases. The present findings indicated that these risk factors may improve the preoperative prediction of CLNM in a noninvasive manner. The sensitivity for detecting CLNM using preoperative neck US imaging is low (31, 32) due to air in the trachea, complex structures in the sternum and clavicle, which make it difficult for US imaging to detect CLNM.
For clinically N0 unifocal PTCs, the precise evaluation of CLNM is important. Barczynski et al. acknowledged that CLND promotes both a locoregional situation and 10-year disease-specific survival without increasing the risk of permanent morbidity (33). Hartl et al. reported that CLND does not enhance the incidence of morbidity, especially the permanent dissections (34), which may be due to the surgical skills of the surgeons reducing complications. In our study, all of the complications and side effects were documented and treated. The complications of surgery included hypocalcemia, hoarseness, seroma, pain and choke. The complications were totally under control and there were no permanent injury caused. It is well-known that revision surgery in scarred areas promotes a high risk for recurrent laryngeal nerve (RLN) injury and parathyroid gland injury. Zhao et al. indicated that CLND significantly lowers LN recurrence (35). In addition, CLND helps surgeons assess the tumor-node-metastasis (TNM) stage of patients with PTC to determine the subsequent radioactive iodine (RAI) therapy (36). However, ATA and NCCN guidelines do not recommend prophylactic CLND. Nixon et al. found that the 5- and 10-year disease-free survival of patients with PTC who did not undergo prophylactic CLND is 100%; they considered an active observation of CLN safe and that it should be suggested for all patients with PTC considered before and during surgery without central neck metastasis (37). Furthermore, many researchers have suggested that prophylactic CLND may promote the complication rate of RLN and parathyroid gland permanent injury by approximately 2-fold (38, 39). Above all, CLND is important but should be implemented with care. Further, it is imperative to diagnose CLNM preoperatively for clinical decision-making processes.
In the present study, an US feature was generated using risk factors, including tumor size, for the prediction of CLNM. In the present study, tumors with a larger size on US examination were more likely to be related to CLNM, which was consistent with other reports (40). Tumor size is widely analyzed in many staging systems, including the American Joint Committee on Cancer (AJCC) staging system. The most used cutoff in risk stratification is 1 cm, which is widely accepted as a risk factor for CLNM and is associated with higher mortality (41). Many studies have utilized the largest diameter of the tumor as the tumor size, but there is no definitive conclusion at present (42). However, some studies have reported that tumor size is not an adequate independent predictor of CLNM (43). Several previous studies have set the size threshold between 5 and 10 mm; however, these studies have reported that when the tumor is less than this threshold, the rate of CLNM is still high, ranging from 26% to 55% (44, 45).
The location features of the nodule may also be important risk factors. A tumor adjacent to the anterior capsule and that has a short distance to the lower pole has a close association with CLNM (46). The thyroid gland is encapsulated by a thin fibroelastic (true) capsule, and this capsule is covered by a pretracheal fascia from the outside and is called a false capsule. The true capsule gives rise to septa deep into the parenchyma, dividing the thyroid gland into lobules. The septa makes room for blood vessels, nerves and lymphatics in the gland. The thyroid gland and its neighboring structures have many lymphatics, which drain the thyroid in almost every direction. Within the thyroid gland, lymphatic channels are present beneath the capsule and connect lobes through the isthmus. Most thyroid neoplasms drain directly to CLN basins, except for cancers in the superior third of the gland, which may drain to the lateral compartment (known as skip metastases) (47). This may be the reason for the association of a closer distance to the capsule and lower pole on US imaging with CLNM. In the present study, the CDFI was significantly different between the CLNM-positive and CLNM-negative groups; richer blood supply was correlated with a higher probability of CLNM (48).
The present study had several limitations, including those inherent to a retrospective study design. The present study was also a single-center historical cohort study, and our results may have been biased accordingly. Stringent external validation needs to be performed in larger, prospective multicenter clinical trials to obtain a more objective conclusion. In addition, a relatively small number of patients had large-volume CLNM, which did not allow us to demonstrate the key predictive factor of occult large-volume CLNM. The performance of our prediction depends on the accuracy of operator-reported imaging characteristics. The criteria used to evaluate the US features were subjective. However, the interobserver agreement for each feature in the present study was good. Although we did not evaluate the recurrence of PTC according to different clinical factors, our findings are still important for clinicians to make decisions on management strategies for cN0 unifocal PTC. The CLNM status is an indicator of aggressive behavior in PTC, but its evaluation has been limited in imaging studies. The present study suggested that the size, adjacent anterior capsule, distance to the lower pole and CDFI of cN0 unifocal PTC patients are good preoperative clinical factors that predict the occult CLNM status. Thus, it may be appropriate to perform more precise inspection or surgical intervention rather than active surveillance for those patients.
In summary, the present study revealed several risk factors based on US imaging characteristics, suggesting that this easy-to-use method can be applied to facilitate preoperative individualized prediction of occult CLNM in cN0 unifocal PTC patients, which is in line with the current trend towards precision medicine.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (Guangzhou, Guangdong Province, People’s Republic of China). Written informed consent was not required for this study, in accordance with the local legislation and institutional requirements.
Author Contributions
YL, JH, and ZZ, have contributed equally to this work and share first authorship. They are responsible for research design, data collecting, analysis and writing. YH and JD are responsible for research design and data collecting. SW and ZW are responsible for research design, data analysis. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Guangdong Province (No. 2020A1515010127) and Guangdong Provincial People’s Hospital Scientific Foundation for Distinguished Young Scholars of Guangdong Province (No. KJ012019441).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Giani F, Masto R, Trovato MA, Malandrino P, Russo M, Pellegriti G, et al, et al. Heavy Metals in the Environment and Thyroid Cancer. Cancers (2021) 13(16):4052. doi: 10.3390/cancers13164052
3. Kim J, Gosnell JE, Roman SA. Geographic Influences in the Global Rise of Thyroid Cancer. Nat Rev Endocrinol (2020) 16(1):17–29. doi: 10.1038/s41574-019-0263-x
4. Costa AR, Lanca de Oliveira M, Cruz I, Goncalves I, Cascalheira JF, Santos CRA. The Sex Bias of Cancer. Trends Endocrinol Metab (2020) 31(10):785–99. doi: 10.1016/j.tem.2020.07.002
5. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More "Personalized" Approach to Cancer Staging. CA: Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
6. Gulec SA, Ahuja S, Avram AM, Bernet VJ, Bourguet P, Draganescu C, et al. A Joint Statement From the American Thyroid Association, the European Association of Nuclear Medicine, the European Thyroid Association, the Society of Nuclear Medicine and Molecular Imaging on Current Diagnostic and Theranostic Approaches in the Management of Thyroid Cancer. Thyroid (2021) 31(7):1009–19. doi: 10.1089/thy.2020.0826
7. Haddad RI, Nasr C, Bischoff L, Busaidy NL, Byrd D, Callender G, et al. NCCN Guidelines Insights: Thyroid Carcinoma, Version 2.2018. J Natl Compr Cancer Netw (2018) 16(12):1429–40. doi: 10.6004/jnccn.2018.0089
8. Gambardella C, Tartaglia E, Nunziata A, Izzo G, Siciliano G, Cavallo F, et al. Clinical Significance of Prophylactic Central Compartment Neck Dissection in the Treatment of Clinically Node-Negative Papillary Thyroid Cancer Patients. World J Surg Oncol (2016) 14(1):247. doi: 10.1186/s12957-016-1003-5
9. Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In Papillary Thyroid Cancer, Preoperative Central Neck Ultrasonic Detects Only Macroscopic Surgical Disease, But Negative Findings Predict Excellent Long-Term Regional Control and Survival. Thyroid (2012) 22(4):347–55. doi: 10.1089/thy.2011.0121
10. Mazzaferri EL, Doherty GM, Steward DL. The Pros and Cons of Prophylactic Central Compartment Lymph Node Dissection for Papillary Thyroid Carcinoma. Thyroid (2009) 19(7):683–9. doi: 10.1089/thy.2009.1578
11. Wu X, Li B, Zheng C, He X. Risk Factors for Central Lymph Node Metastases in Patients With Papillary Thyroid Microcarcinoma. Endocr Pract (2018) 24(12):1057–62. doi: 10.4158/EP-2018-0305
12. Xu Y, Xu L, Wang J. Clinical Predictors of Lymph Node Metastasis and Survival Rate in Papillary Thyroid Microcarcinoma: Analysis of 3607 Patients at a Single Institution. J Surg Res (2018) 221:128–34. doi: 10.1016/j.jss.2017.08.007
13. Feng JW, Yang XH, Wu BQ, Sun DL, Jiang Y, Qu Z. Predictive Factors for Central Lymph Node and Lateral Cervical Lymph Node Metastases in Papillary Thyroid Carcinoma. Clin Trans Oncol (2019) 21(11):1482–91. doi: 10.1007/s12094-019-02076-0
14. Zhu Y, Zheng K, Zhang H, Chen L, Xue J, Ding M, et al. The Clinicopathologic Differences of Central Lymph Node Metastasis in Predicting Lateral Lymph Node Metastasis and Prognosis in Papillary Thyroid Cancer Associated With or Without Hashimoto's Thyroiditis. Tumour Biol (2016) 37(6):8037–45. doi: 10.1007/s13277-015-4706-2
15. Chen Q, Wei T, Wang XL, Li ZH, Du ZH, Zhu JQ. The Total Number of Prelaryngeal and Pretracheal Lymph Node Metastases: Is it a Reliable Predictor of Contralateral Central Lymph Node Metastasis in Papillary Thyroid Carcinoma? J Surg Res (2017) 214:162–7. doi: 10.1016/j.jss.2015.02.056
16. Zhu Y, Lin J, Yan Y, Zheng K, Zhang H, Wu K, et al. Delphian Lymph Node Metastasis is a Novel Indicator of Tumor Aggressiveness and Poor Prognosis in Papillary Thyroid Cancer. J Surg Oncol (2021) 123(7):1521–8. doi: 10.1002/jso.26380
17. Calo PG, Conzo G, Raffaelli M, Medas F, Gambardella C, De Crea C, et al. Total Thyroidectomy Alone Versus Ipsilateral Versus Bilateral Prophylactic Central Neck Dissection in Clinically Node-Negative Differentiated Thyroid Carcinoma. A retrospective Multicenter Study. Eur J Surg Oncol (2017) 43(1):126–32. doi: 10.1016/j.ejso.2016.09.017
18. Conzo G, Tartaglia E, Avenia N, Calo PG, de Bellis A, Esposito K, et al. Role of Prophylactic Central Compartment Lymph Node Dissection in Clinically N0 Differentiated Thyroid Cancer Patients: Analysis of Risk Factors and Review of Modern Trends. World J Surg Oncol (2016) 14:149. doi: 10.1186/s12957-016-0879-4
19. Nylen C, Eriksson FB, Yang A, Aniss A, Turchini J, Learoyd D, et al. Prophylactic Central Lymph Node Dissection Informs the Decision of Radioactive Iodine Ablation in Papillary Thyroid Cancer. Am J Surg (2021) 221(5):886–92. doi: 10.1016/j.amjsurg.2020.08.012
20. Lee YC, Na SY, Park GC, Han JH, Kim SW, Eun YG. Occult Lymph Node Metastasis and Risk of Regional Recurrence in Papillary Thyroid Cancer After Bilateral Prophylactic Central Neck Dissection: A Multi-Institutional Study. Surgery (2017) 161(2):465–71. doi: 10.1016/j.surg.2016.07.031
21. Ahn JH, Kwak JH, Yoon SG, Yi JW, Yu HW, Kwon H, et al. A Prospective Randomized Controlled Trial to Assess the Efficacy and Safety of Prophylactic Central Compartment Lymph Node Dissection in Papillary Thyroid Carcinoma. Surgery (2022) 171(1):182–9. doi: 10.1016/j.surg.2021.03.071
22. De Napoli L, Matrone A, Favilla K, Piaggi P, Galleri D, Ambrosini CE, et al. Role of Prophylactic Central Compartment Lymph Node Dissection on the Outcome Of Patients With Papillary Thyroid Carcinoma and Synchronous Ipsilateral Cervical Lymph Node Metastases. Endocr Pract (2020) 26(8):807–17. doi: 10.4158/EP-2019-0532
23. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The Prognostic Significance of Nodal Metastases From Papillary Thyroid Carcinoma can be Stratified Based on the Size and Number of Metastatic Lymph Nodes, as Well as the Presence of Extranodal Extension. Thyroid (2012) 22(11):1144–52. doi: 10.1089/thy.2012.0043
24. Liu Z, Xun X, Wang Y, Mei L, He L, Zeng W, et al. MRI and Ultrasonography Detection of Cervical Lymph Node Metastases in Differentiated Thyroid Carcinoma Before Reoperation. Am J Trans Res (2014) 6(2):147–54.
25. Stulak JM, Grant CS, Farley DR, Thompson GB, van Heerden JA, Hay ID, et al. Value of Preoperative Ultrasonography in the Surgical Management of Initial and Reoperative Papillary Thyroid Cancer. Arch Surg (2006) 141(5):489–94. doi: 10.1001/archsurg.141.5.489
26. Yeh MW, Bauer AJ, Bernet VA, Ferris RL, Loevner LA, Mandel SJ, et al. American Thyroid Association Statement on Preoperative Imaging for Thyroid Cancer Surgery. Thyroid (2015) 25(1):3–14. doi: 10.1089/thy.2014.0096
27. Wang Y, Guan Q, Xiang J. Nomogram for Predicting Central Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Retrospective Cohort Study of 8668 Patients. Int J Surg (2018) 55:98–102. doi: 10.1016/j.ijsu.2018.05.023
28. Wu LM, Gu HY, Qu XH, Zheng J, Zhang W, Yin Y, et al. The Accuracy of Ultrasonography in the Preoperative Diagnosis of Cervical Lymph Node Metastasis in Patients With Papillary Thyroid Carcinoma: A Meta-Analysis. Eur J Radiol (2012) 81(8):1798–805. doi: 10.1016/j.ejrad.2011.04.028
29. Zane M, Parello C, Pennelli G, Townsend DM, Merigliano S, Boscaro M, et al. Estrogen and Thyroid Cancer is a Stem Affair: A Preliminary Study. BioMed Pharmacother (2017) 85:399–411. doi: 10.1016/j.biopha.2016.11.043
30. Rubio GA, Catanuto P, Glassberg MK, Lew JI, Elliot SJ. Estrogen Receptor Subtype Expression and Regulation is Altered in Papillary Thyroid Cancer After Menopause. Surgery (2018) 163(1):143–9. doi: 10.1016/j.surg.2017.04.031
31. Khokhar MT, Day KM, Sangal RB, Ahmedli NN, Pisharodi LR, Beland MD, et al. Preoperative High-Resolution Ultrasonic for the Assessment of Malignant Central Compartment Lymph Nodes in Papillary Thyroid Cancer. Thyroid (2015) 25(12):1351–4. doi: 10.1089/thy.2015.0176
32. Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasonic Criteria of Malignancy for Cervical Lymph Nodes in Patients Followed Up for Differentiated Thyroid Cancer. J Clin Endocrinol Metab (2007) 92(9):3590–4. doi: 10.1210/jc.2007-0444
33. Barczynski M, Konturek A, Stopa M, Nowak W. Prophylactic Central Neck Dissection for Papillary Thyroid Cancer. Br J Surg (2013) 100(3):410–8. doi: 10.1002/bjs.8985
34. Hartl DM, Mamelle E, Borget I, Leboulleux S, Mirghani H, Schlumberger M. Influence of Prophylactic Neck Dissection on Rate of Retreatment for Papillary Thyroid Carcinoma. World J Surg (2013) 37(8):1951–8. doi: 10.1007/s00268-013-2089-3
35. Zhao W, You L, Hou X, Chen S, Ren X, Chen G, et al. The Effect of Prophylactic Central Neck Dissection on Locoregional Recurrence in Papillary Thyroid Cancer After Total Thyroidectomy: A Systematic Review and Meta-Analysis: pCND for the Locoregional Recurrence of Papillary Thyroid Cancer. Ann Surg Oncol (2017) 24(8):2189–98. doi: 10.1245/s10434-016-5691-4
36. Wang TS, Evans DB, Fareau GG, Carroll T, Yen TW. Effect of Prophylactic Central Compartment Neck Dissection on Serum Thyroglobulin and Recommendations for Adjuvant Radioactive Iodine in Patients With Differentiated Thyroid Cancer. Ann Surg Oncol (2012) 19(13):4217–22. doi: 10.1245/s10434-012-2594-x
37. Nixon IJ, Wang LY, Ganly I, Patel SG, Morris LG, Migliacci JC, et al. Outcomes for Patients With Papillary Thyroid Cancer Who do Not Undergo Prophylactic Central Neck Dissection. Br J Surg (2016) 103(3):218–25. doi: 10.1002/bjs.10036
38. Conzo G, Mauriello C, Docimo G, Gambardella C, Thomas G, Cavallo F, et al. Clinicopathological Pattern of Lymph Node Recurrence of Papillary Thyroid Cancer. Implications for Surgery. Int J Surg (2014) 12 Suppl 1:S194–7.doi: 10.1016/j.ijsu.2014.05.010
39. Kim JW, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Recurrence in Patients With Clinically Early-Stage Papillary Thyroid Carcinoma According to Tumor Size and Surgical Extent. Am J Surg (2016) 212(3):419–25.e411. doi: 10.1016/j.amjsurg.2015.12.015
40. Huang XP, Ye TT, Zhang L, Liu RF, Lai XJ, Wang L, et al. Sonographic Features of Papillary Thyroid Microcarcinoma Predicting High-Volume Central Neck Lymph Node Metastasis. Surg Oncol (2018) 27(2):172–6. doi: 10.1016/j.suronc.2018.03.004
41. Lang BH, Chow SM, Lo CY, Law SC, Lam KY. Staging Systems for Papillary Thyroid Carcinoma: A Study of 2 Tertiary Referral Centers. Ann Surg (2007) 246(1):114–21. doi: 10.1097/01.sla.0000262785.46403.9b
42. Zhu M, Zheng W, Xiang Y, Gu J, Wang K, Shang J. The Relationship Between Central Lymph Node Metastasis and the Distance From Tumor to Thyroid Capsule in Papillary Thyroid Microcarcinoma Without Capsule Invasion. Gland Surg (2020) 9(3):727–36. doi: 10.21037/gs-20-478
43. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Study of 551 Resections. Surgery (2010) 148(3):526–31. doi: 10.1016/j.surg.2010.01.003
44. Seo GH, Chai YJ, Choi HJ, Lee KE. Incidence of Permanent Hypocalcaemia After Total Thyroidectomy With or Without Central Neck Dissection for Thyroid Carcinoma: A Nationwide Claim Study. Clin Endocrinol (2016) 85(3):483–7. doi: 10.1111/cen.13082
45. Roh JL, Kim JM, Park CI. Central Lymph Node Metastasis of Unilateral Papillary Thyroid Carcinoma: Patterns and Factors Predictive of Nodal Metastasis, Morbidity, and Recurrence. Ann Surg Oncol (2011) 18(8):2245–50. doi: 10.1245/s10434-011-1600-z
46. Xu SY, Yao JJ, Zhou W, Chen L, Zhan WW. Clinical Characteristics and Ultrasonographic Features for Predicting Central Lymph Node Metastasis in Clinically Node-Negative Papillary Thyroid Carcinoma Without Capsule Invasion. Head Neck (2019) 41(11):3984–91. doi: 10.1002/hed.25941
Keywords: ultrasonic characteristics, CLNM, cN0 PTC, unifocal, predictor
Citation: Liu Y, Huang J, Zhang Z, Huang Y, Du J, Wang S and Wu Z (2022) Ultrasonic Characteristics Improve Prediction of Central Lymph Node Metastasis in cN0 Unifocal Papillary Thyroid Cancer. Front. Endocrinol. 13:870813. doi: 10.3389/fendo.2022.870813
Received: 07 February 2022; Accepted: 16 May 2022;
Published: 20 June 2022.
Edited by:
T Metin Onerci, Hacettepe University, TurkeyReviewed by:
Eliana Piantanida, University of Insubria, ItalyXinying Li, Central South University, China
Copyright © 2022 Liu, Huang, Zhang, Huang, Du, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanming Wang, V2lud3NtQDEyNi5jb20=; Zeyu Wu, d3UuemV5dUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yongchen Liu
Yongchen Liu Jianhao Huang
Jianhao Huang Zhiyuan Zhang1,3†
Zhiyuan Zhang1,3† Yijie Huang
Yijie Huang Zeyu Wu
Zeyu Wu