- 1School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Shenzhen, China
- 2Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, United States
- 3Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China
- 4Chronic Disease Epidemiology Laboratory, Pennington Biomedical Research Center, Baton Rouge, LA, United States
- 5Department of Public Health Laboratory Sciences, West China School of Public Health, Sichuan University, Chengdu, China
- 6Department of Pediatrics, Children’s Hospital New Orleans, New Orleans, LA, United States
- 7Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, United States
- 8Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
The prevalence of gestational diabetes mellitus (GDM) has increased with the increasing rate of obesity. However, national data on the prevalence and secular trends of GDM during the past decade in the United States are lacking. This study included 37,357 women aged more than 18 years and who had ever been pregnant from the National Health Interview Survey (NHIS). We examined GDM prevalence in 2006, 2016, and 2017, with age-standardized to the US population in 2000. We found that the prevalence of GDM per 100 people increased from 4.6 (95% CI, 4.1–5.1) in 2006 to 8.2 (95% CI, 7.5–8.9) in 2016 (test for difference; P <0.001), with a relatively increased rate of 78%. Non-Hispanic white women tended to have a lower increase (2.8%) than non-Hispanic black women (3.8%), Hispanic women (4.1%), and women of other race/ethnicity (8.4%). The prevalence of GDM in non-Hispanic white women was higher than that in non-Hispanic black women in 2006 (4.8% vs 3.5%, P = 0.006); such differences became non-significant in 2016 (P = 0.72). Additionally, the increase of GDM from 2006 to 2016 tended to be more evident among women who were overweight (25≤ BMI ≤30 kg/m2), physically inactive, and with family income below the poverty threshold than women in other BMI ranges, with more physical activity, and with higher incomes. The prevalence of GDM per 100 people in 2017 was 8.4 (7.6–9.2), and there was no significant change in the overall and subgroup prevalence compared with 2016. Collectively, in the United States, the prevalence of GDM continuously increased, nearly doubled, from 2006 to 2016, and then leveled off in 2017. The increase appeared more marked among the minority populations and subpopulations with overweight people, insufficient activity, and family incomes below the poverty threshold.
Introduction
Gestational diabetes mellitus (GDM) is a condition in women with impaired glucose tolerance with the onset or first recognition during pregnancy (1). In recent years, GDM has become an increasing public health concern due to its adverse implications for maternal and child health (2–6). In the short-term, GDM increases adverse pregnancy outcomes (2); and in the long-term, GDM carries an increased risk of developing type 2 diabetes for the mothers and an elevated risk of various cardiometabolic disorders in the offspring (7–9).
The prevalence of GDM has increased during recent decades in the United States. In a study using the National Hospital Discharge Survey database, the estimated prevalence of GDM in the United States was 5.8% in 2008–2010, with an absolute increase of 5.5% and a relative increase of 23 folds since 1979–1980 (10). More specifically, from 1989–1990 to 2003–2004, the prevalence of GDM increased from 1.9 to 4.2%, with a relative increase of 122% (11). This increasing trend was also observed in regional data (12–15). However, inconsistent data were also reported; for example, no significant change in GDM prevalence from 2007 (8.1%) to 2010 (8.5%) was observed in the Pregnancy Risk Assessment Monitoring System (PRAMS) including 21 states of the United States (5). Additionally, national data on the most recent prevalence and secular trend of GDM prevalence in the United States are lacking. Moreover, little is known about whether GDM prevalence and changing trends differ with race/ethnicity and other population characteristics.
The National Health Interview Survey (NHIS) is a national cross-sectional survey that collects health and lifestyle information from sample participants representing the U.S. population. This study aimed to determine the temporal trend of GDM prevalence among pregnant women from 2006 to 2016, and 2017 using data from NHIS. We particularly analyzed the secular trend of GDM prevalence and compared the differences in subgroups categorized by race/ethnicity, Body Mass Index (BMI), physical activity, and socioeconomic status.
Materials and Methods
Study Design and Participants
The NHIS is an ongoing national cross-sectional survey that monitors the health of the U.S. population. Using a stratified, multistage sampling design, NHIS conducts personal household interviews to collect health and lifestyle information from sample participants who represent the U.S. population. One adult was randomly selected from each household to answer the questionnaire. The annual response rate of NHIS was nearly 90% of the eligible households in the sample.
GDM Assessment
We examined GDM prevalence in 2006, 2016, and 2017. A total of 37, 357 women aged more than 18 years and who had ever been pregnant were included in the current study. In 2006, GDM was asked among 13,525 women aged more than 18 years and who had ever been pregnant, in response to the question “Before you were told you had diabetes, were you ever told that you had diabetes or gestational diabetes while you were pregnant” (cases with diabetes, N = 138) and “Have you ever been told that you had diabetes or gestational diabetes while you were pregnant?” (cases without diabetes, N = 430). In 2016 and 2017, GDM prevalence was measured in 13,650 and 11,041 women by responding to the questions: “Were you ever told by a doctor or other health professional that you had diabetes, sugar diabetes, or gestational diabetes during pregnancy?” (N = 974 and 799, respectively). Related variables with values “Never been pregnant”, “Refused to answer”, “Not ascertained” or “Don’t know” were set as missing, leaving 12,728 participants in 2006, 13,612 in 2016, and 11,071 in 2017. Though GDM was self-reported and not been objectively validated in this study, previous studies suggested high validity of self-reported diagnosis of GDM (16).
Data Assessment
A standardized questionnaire was used to collect information on age, sex, race/ethnicity family income, physical activity, body weight, and height. Stratified analyses were performed to assess the prevalence in subgroups according to different race/ethnicity, BMI categories, physical activity level, and family income.
Race/ethnicity was categorized as Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic for all other race/ethnicity groups. BMI was calculated as weight in kilograms divided by height squared. Normal-weight, overweight, and obesity groups were defined by BMI levels (<25 kg/m2, 25–30 kg/m2, and >30 kg/m2, respectively). Based on imputed household income, income was categorized by the ratio of family income to the poverty threshold (<100%, 100–190%, 200–399%, and >400%). Total minutes of physical activity (TPA) was calculated as the sum of the light-moderate PA min and vigorous PA min multiplied by 2. Then, insufficiently active was defined as (TPA) <150 min/wk, sufficiently active as 150≤ TPA ≤300, and highly active as TPA >300 min/wk.
Statistical Analysis
Characteristics of study participants in 2006 and 2016 were reported in unweighted and sample-weighted mean and standard error (SE) for continuous variables and numbers and percentages for categorical variables. We used χ2 tests to test differences in the frequency of stratification factors. The SURVEYREG procedure in SAS was used to test differences between continuous variables and the prevalence of GDM. We first compared the prevalence between 2006 and 2016, and then 2016 and 2017. A Z-test was used to compare the two prevalence estimates. All calculations were weighted to represent the general female adult population aged 18 years or older in the US. We examined GDM prevalence age-standardized to the U.S. population in 2000. The imputation of family income was conducted by CDC using multiple-imputation methodology. For all analyses, weights, strata, and clusters in the NHIS design were taken into account as recommended by the CDC. All statistical analyses were performed with the use of SAS version 9.4 (SAS Institute, Inc., Cary, NC). Two-sided p-values of <0.05 were considered significant.
Results
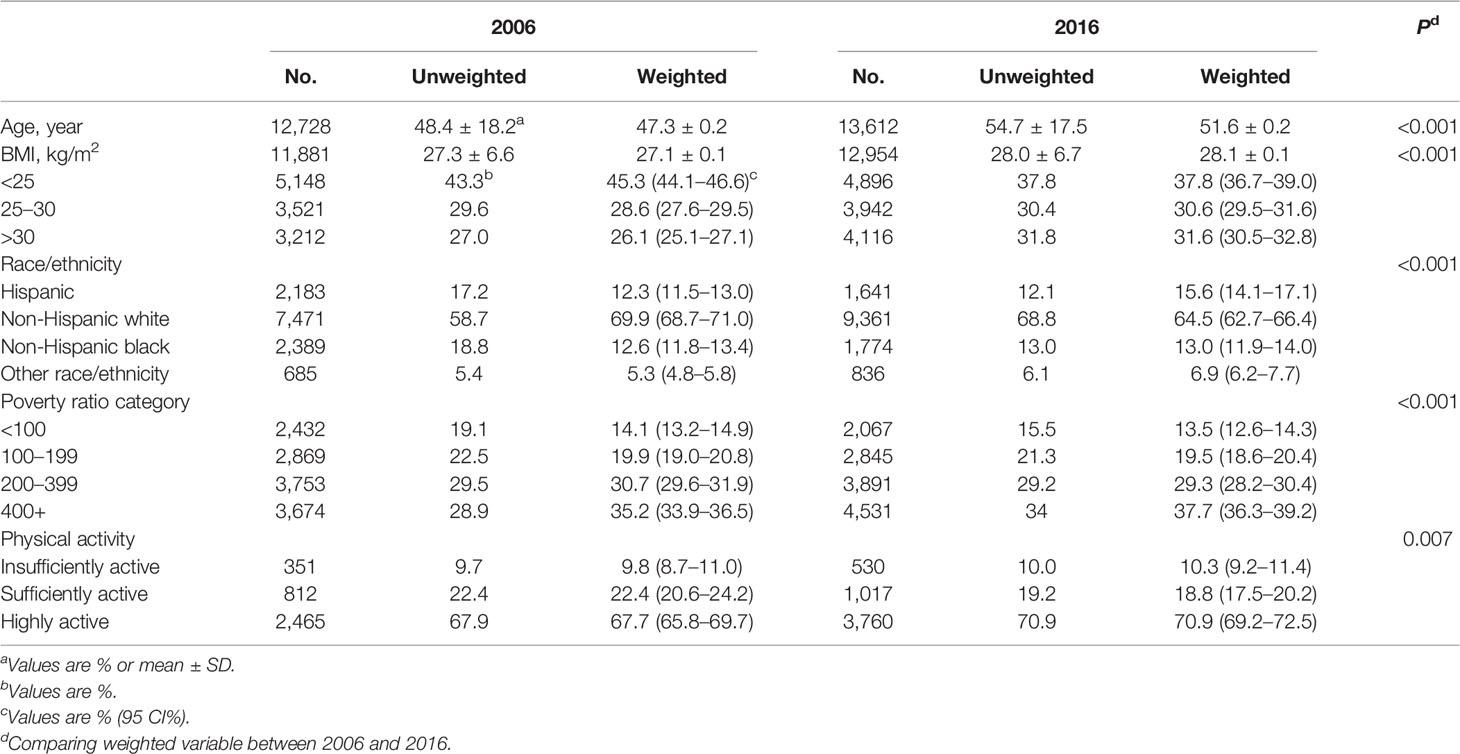
Table 1 shows the characteristics of the participants in 2006 and 2016, respectively. A total of 26,340 women were included in the two years. The mean age of participants was 47.3 ± 0.2 years in 2006 and 51.6 ± 0.2 years in 2016 (P <0.001). The mean BMI increased from 27.1 ± 0.1 kg/cm2 in 2006 to 28.1 ± 0.1 kg/cm2 in 2016 (P <0.001). The composition of race/ethnicity significantly differed between the two surveys, with more Hispanic and minority populations and less non-Hispanic white in 2016 compared with that in 2006 (P <0.001). Differences in the composition of family income and physical activity were also observed (P <0.001 and P = 0.007, respectively).
Among the whole study populations, the age-standardized prevalence of GDM per 100 people increased from 4.6 (95% CI, 4.1–5.1) in 2006 to 8.2 (95% CI, 7.5–8.9) in 2016, with an absolute increase of 3.6% and a relative increase of 78% (P <0.001) (Figure 1). When populations with various race/ethnicity were compared, non-Hispanic white women showed less increase (2.8%) than non-Hispanic black women (3.8%), Hispanic women (4.1%), and women of other race/ethnicity (8.5%). Notably, the prevalence of GDM in non-Hispanic white women was higher than in non-Hispanic black women in 2006 (P = 0.001). However, such differences became non-significant in 2016 (P = 0.72) (Table 2 and Figure 2).
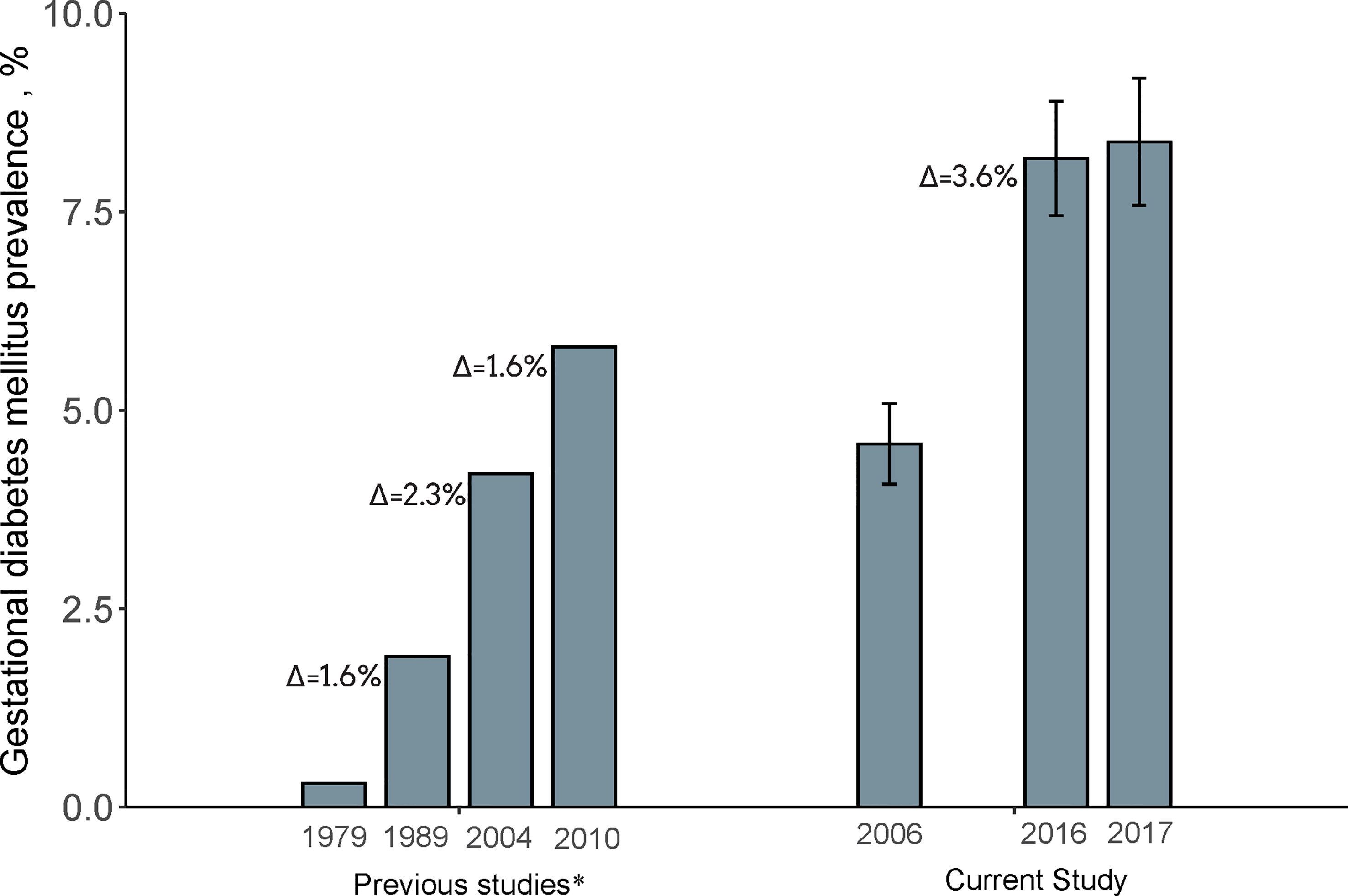
Figure 1 Prevalence of Gestational diabetes mellitus in 2006, 2016, and 2017. Data of the current study were expressed as Estimate ± 95% confidence interval. GDM prevalence was calculated with age-standardized to the U.S. population in 2000. N = 12,728 in 2006, 13,612 in 2016, and 11,071 in 2017. aDate for comparisons were obtained from previous studies with the use of National Hospital Discharge Survey database1–2. Δ indicated absolute increase compared with the prevalence of last time period. 1. Getahun et al., Am J Obstet Gynecol. 2008. 2. Lavery et al., BJOG An Int J Obstet Gynaecol. 2017.
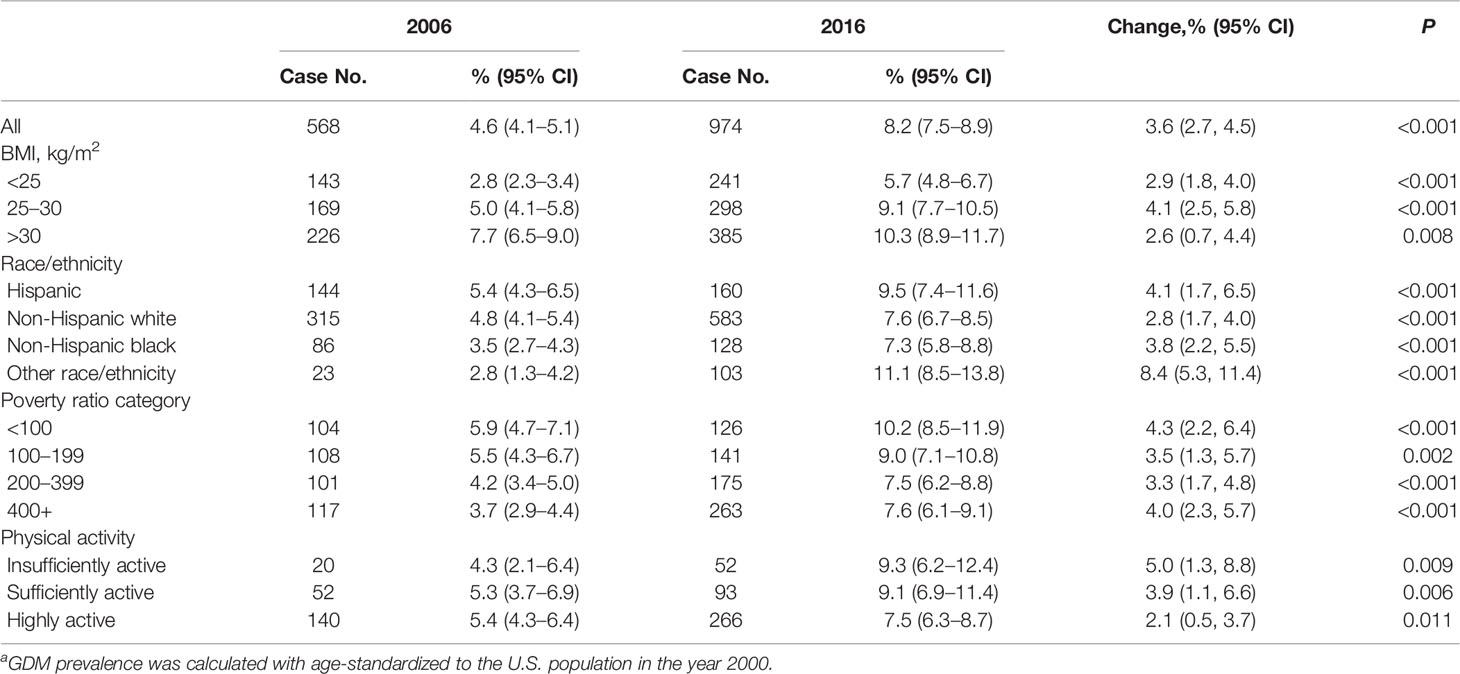
Table 2 Trend in diagnosed Gestational diabetes mellitus of participants in 2006 and 2016a.
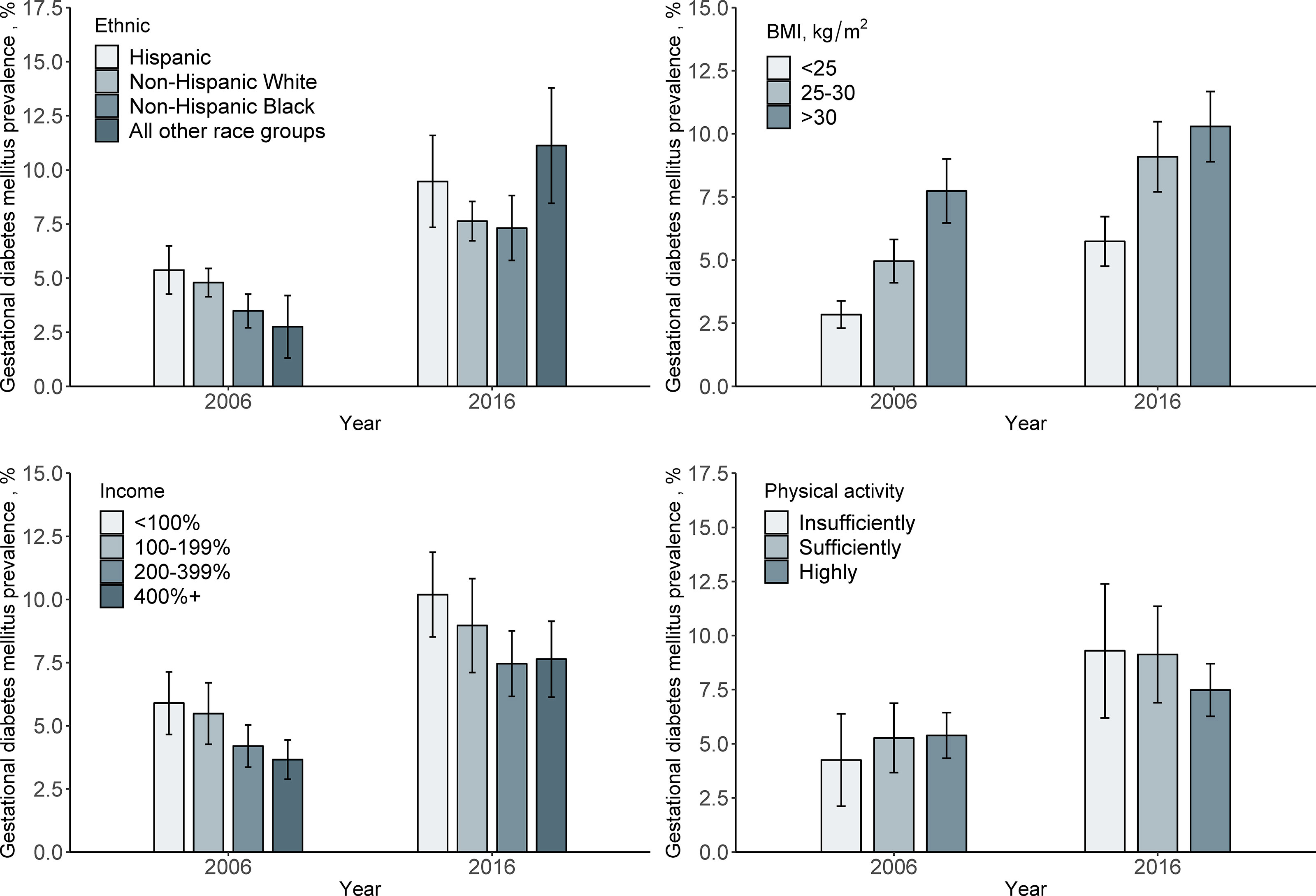
Figure 2 Prevalence of Gestational diabetes mellitus in 2006 and 2016 by demographic variables. Data were expressed as Estimate ± 95% confidence interval. GDM prevalence was calculated with age-standardized to the U.S. population in 2000.
We also analyzed the GDM prevalence according to the major risk factors. We found that the prevalence was higher in obese women than in those who were overweight in 2006 (P <0.001), whereas in 2016, the prevalence did not differ significantly (P = 0.23). From 2006 to 2016, the GDM prevalence increased by 4.1% (95% CI, 5.0 to 9.1%) in overweight women, and the corresponding increase tended to be less evident in obese women and women with BMI <25 kg/m2, with a change from 7.7 to 10.3% (increased by 2.6%) and 2.8 to 5.7% (increased by 2-9%), respectively (Table 2 and Figure 2).
For the changes in GDM prevalence from 2006 to 2016, women with income below poverty threshold <100% tended to have more increase (4.3%) than those within other income categories including 100–190% (3.5%), 200–399% (3.3%), and >400% (4.0%) (Table 2 and Figure 2).
The GDM prevalence also showed different secular trends from 2006 to 2016 according to physical activity levels. The increase in GDM prevalence appeared more pronounced among women who had insufficient physical activity (5.0%) than among those who had sufficient physical activity (2.1%) (Table 2 and Figure 1).
The prevalence calculated from 11,071 women in 2017 was 8.4 (7.6–9.2) per 100 people, and there was no significant change in the overall and subgroup prevalence compared to that in 2016 (Table 3).
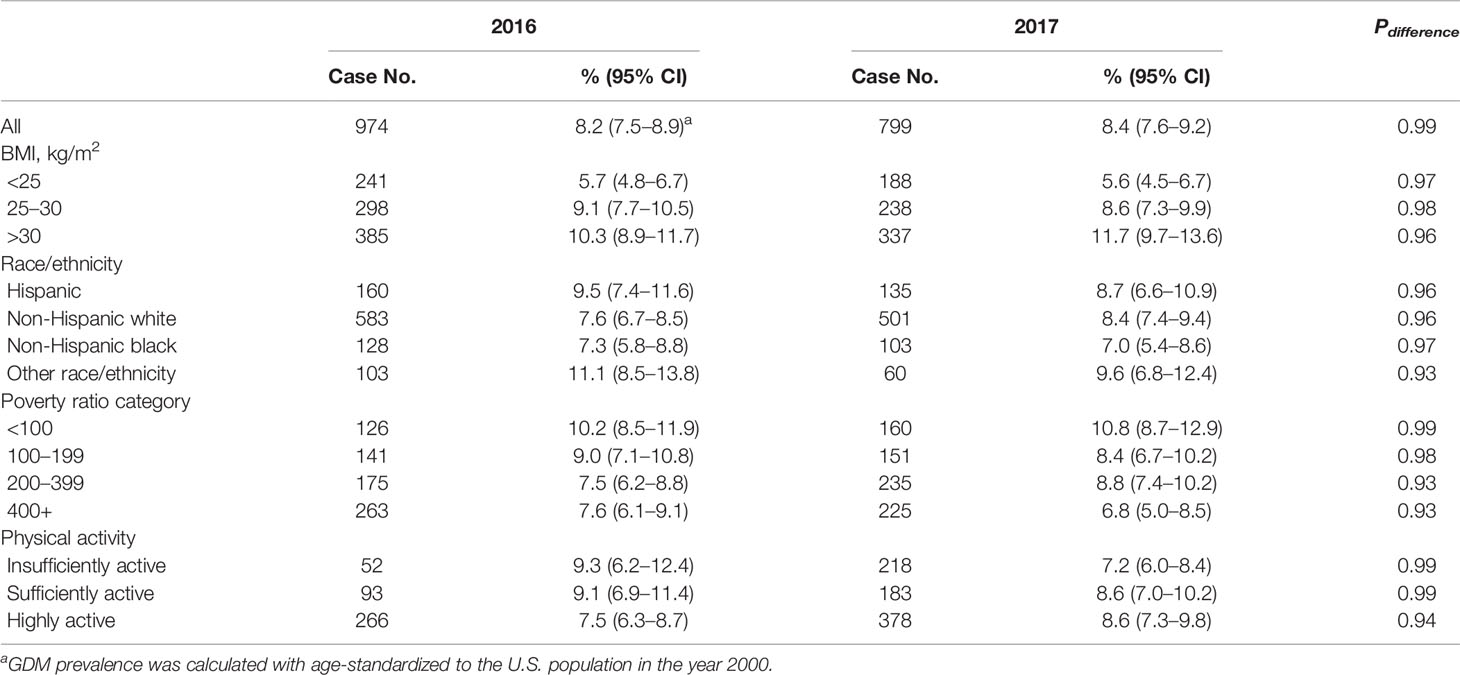
Table 3 Difference of prevalence in diagnosed Gestational diabetes mellitus of participants in 2016 and 2017.
Discussion
In this study of the nationally representative data of U.S. populations, we found that the prevalence of GDM increased from 4.6% in 2006 to 8.2% in 2016, with a relative increase rate of 78%. Our stratified analysis revealed that the increase tended to be more pronounced among women who were non-white, overweight, had insufficient activity, and had lower socioeconomic status. The prevalence of GDM has reached a steady rate in 2017 since 2016.
Several previous studies have reported an increasing trend of GDM prevalence in the United States between 1979 and 2010 (5, 10, 13, 17). In a national survey among hospitalized women, the prevalence of GDM increased from 1.9% in 1989–1990 to 4.2% in 2003–2004 (11). Another regional study, the Kaiser Permanente of Colorado (KPCO) study, showed a similar trend, with the prevalence of GDM increasing from 2.1% in 1994 to 4.1% in 2002 (13). An increasing trend of GDM prevalence was also observed in other studies (17, 18), while inconsistent observations were also reported. For example, in PRAMS, no significant change was observed between 2007–2008 (8.1%) and 2009–2010 (8.5%) (5). Compared with previous national studies, in which the GDM prevalence increased from 1979 to 2010 with an absolute increase of ~1.8% per decade (10, 11), our data indicated that the GDM prevalence continuously increased from 2006 to 2016, and the absolute increase rate (3.6% per 10 years) appeared to be accelerated as compared with previous years.
The significant increase in the prevalence of GDM in the past 10 years might be attributed to the concurrent changes in multiple risk factors, such as increased prevalence of overweight and obesity (19, 20), advanced maternal age (21) and the growth of minority populations that had a higher risk of GDM (22). Overweight and obesity are major risk factors for developing GDM (23). Between 2006 and 2016, the increased prevalence of overweight and obesity persisted among adult women in the United States (20, 24), and overweight and obesity were considered to be the major driving forces for the increase in GDM prevalence (12, 25). Intriguingly, we found that the increase in GDM prevalence was more pronounced among overweight rather than obese women. We assumed that changes in certain risk factors might more likely increase GDM risk among overweight women than obese women. For example, several studies showed that the associations of gestational weight gain (GWG) with gestational impaired glucose tolerance and GDM were stronger among overweight women than obese women (26, 27). Therefore, the greater increase in GDM prevalence among overweight women than obese women might be partly due to the increasing excessive GWG over the past decades (28, 29). Even though the increasing prevalence of GDM was observed in all racial/ethnic groups, we noted that non-Hispanic whites showed the least increase, while the increase was most evident among women of other race/ethnicity (more than 72% were Asian). Given the growth of minority populations in the past decade, both observed in the present study and reported previously (22), our data suggest the changing racial/ethnic profiles might partly explain the increase in GDM prevalence. Additionally, we found that women who had insufficient physical activity tended to a greater increase in GDM. Our results are in agreement with findings from several prospective studies in which regular physical activity before pregnancy was related to a reduced GDM risk (30–32). Moreover, we found that women with low socioeconomic status had a greater increase in GDM prevalence than those with high socioeconomic status. Socioeconomic status has been inversely correlated with the risk of GDM. It was reported that the risk of GDM among women living in the lowest socioeconomic regions was approximately two-thirds higher than that of women living in the highest socioeconomic regions (33). Women with low socioeconomic status have limited access to effective medical care (34), and low socioeconomic status could be considered as a marker for other established risk factors for GDM, such as obesity (35).
Changed diagnostic criteria or screening strategies might also partly account for the observed increase in GDM prevalence (1). The GDM diagnosis in 2006 was based on the World Health Organization diagnostic criteria with 1-h 50 H GCT plus 3-h 100 g OGTT (two-step) (36). After 2011, the year when the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria (with only one step: 2-h 75G OGTT) (1) for screening and diagnosis of GDM were recommended by ADA guidelines, leading to an increase in the prevalence based on change criteria in some areas (37–39). Since a previous study also showed the GDM prevalence increased from 1998 to 2010 using consistent diagnostic criteria (10); and not all women in 2016 had their first pregnant after 2011, this change in diagnostic criteria alone might not explain the observed increase in GDM prevalence.
We found no change in the prevalence of GDM between 2016 and 2017. The potential slowing of the increase in GDM prevalence may relate to the slowing of BMI (40), which is a major risk factor for GDM. Overall, the prevalence of obesity increased from 35.7% in 2005–2006 to 40.5% in 2013–2014 among women (41). But the increase might slow down in certain years. For example, there was no significant change in obesity prevalence between 2009–2010 and 2011–2012 (42). Another explanation may be policy and advocacy for healthier lifestyles that could attenuate the adverse effects of other GDM-related risk factors may be another explanation. The growing number of noncommunicable diseases (NCDs) and related risk factors might also impact the GDM prevalence (43). Recognizing the burden of NCDs, the WHO Global NCD Action Plan 2013–2020 has been developed to prevent and control NCDs and their risk factors and determinants, which might to a certain extent decrease the prevalence of GDM. Additionally, the relatively short period between 2016 and 2017 may also account for the non-significant change.
In the short and long term, GDM has been linked with a wide range of adverse health consequences for women and their offspring. For example, GDM has been related to a higher risk of type 2 diabetes and cardiovascular disease in women. Additionally, offspring of mothers with GDM are prone to various adverse outcomes such as macrosomia, hypoglycemia, and type 2 diabetes later in life (44). Our study identified the subgroups at high GDM risk, namely, women of the minority (e.g., Hispanic women) or those who are overweight, have insufficient activity, and low family income; and these findings call for more attention and intervention by healthcare workers to prevent the development of GDM or its adverse outcomes in these high-risk women.
Strengths and Limitations of the Study
As far as we are aware, this study is the first to report the nationally representative data of GDM prevalence and trends in the past decade in the United States. A major strength lies in the ability of our comprehensive analysis to display the trend of GDM prevalence by race/ethnicity and other demographic and socioeconomic characteristics. Our study has several limitations. A major limitation is that self-reported physician-diagnosed GDM may under or overestimate the true prevalence of diagnosed GDM. However, the sensitivity and specificity of self-reported GDM have been reported in previous studies (16), and the self-reported GDM in NHIS has been widely used in other studies (45, 46). Another potential limitation of this study is that information was limited on which criteria were used for the diagnosis of GDM. Thus, we could not determine to what extent the changed criteria might account for the observed increase. Additionally, a new sample design was implemented for the 2016 and 2017 NHIS and sample areas were reselected to consider changes in the distribution of the U.S. population since 2006. This might also affect the estimate of the GDM prevalence. Moreover, data on institutionalized people, for whom the GDM prevalence might differ from those in the general population, was not available in the NHIS. Lastly, the relatively small sample size of a subgroup decreased the power to test the differences among the changes in subgroups.
Conclusions
Our study provides evidence that the prevalence of GDM has continuously increased among U.S. women in the past decade, and the increase tended to be more marked among the minority populations and those who were overweight, had insufficient activity, and had an income below the poverty threshold.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhis/index.htm.
Author Contributions
LQ conceptualized and designed the study, critically reviewed the manuscript, and approved the final submission. TZ conceptualized and designed the study, contributed to data cleaning and the statistical analysis, and drafted the initial manuscript. SD, DS, YH, GH, LS, XP, and XS contributed to data cleaning and the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, and HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK115679, and DK078616), the Fogarty International Center (TW010790), the Boston Obesity Nutrition Research Center (DK46200), the United States–Israel Binational Science Foundation Grant 2011036, the National Natural Science Foundation of China (No. 31971147), the Guangdong Basic and Applied Basic Research Foundation (No. 2021B1515020047), and the Shenzhen Science and Technology Innovation Commission (JCYJ20200109142446804). LQ was a recipient of the American Heart Association Scientist Development Award (0730094N). All investigators are independent from funders.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Parts of this study were presented in the American Diabetes Association’s 78th Scientific Sessions, June 22-26, 2018, Orlando, Florida. All data used in this study were collected by the National Center for Health Statistics, Centers for Disease Control and Prevention (CDC).
References
1. Association AD. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care (2014) 37:S81–90. doi: 10.2337/dc14-S081
2. Group HSCR. Hyperglycemia and Adverse Pregnancy Outcomes. N Engl J Med (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
3. The HAPO Study Cooperative Research Group. Associations With Neonatal Anthropometrics. Diabetes (2009) 58:453–9. doi: 10.2337/db08-1112
4. Lowe LP, Coustan DR, Metzger BE, Hadden DR, Dyer AR, Hod M, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations of Maternal A1C and Glucose With Pregnancy Outcomes. Diabetes Care (2012) 35:574–80. doi: 10.2337/dc11-1687
5. DeSisto CL, Kim SY, Sharma AJ. Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Preventing chronic Dis (2014) 11:E104. doi: 10.5888/pcd11.130415
6. Lowe JrWL, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA (2018) 320:1005–16. doi: 10.1001/jama.2018.11628
7. Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 Diabetes Mellitus After Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5
8. Dabelea D, Mayer-Davis EJ, Lamichhane A, D’Agostino RB, Liese AD, Vehik KS, et al. Association of Intrauterine Exposure to Maternal Diabetes and Obesity With Type 2 Diabetes in Youth The SEARCH Case-Control Study. Diabetes Care (2008) 31:1422–6. doi: 10.2337/dc07-2417
9. Karagiannis T, Bekiari E, Manolopoulos K, Paletas K, Tsapas A. Gestational Diabetes Mellitus: Why Screen and How to Diagnose. Hippokratia (2010) 14:151–4.
10. Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational Diabetes in the United States: Temporal Changes in Prevalence Rates Between 1979 and 2010. BJOG: Int J Obstet Gynaecol (2017) 124:804–13. doi: 10.1111/1471-0528.14236
11. Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational Diabetes in the United States: Temporal Trends 1989 Through 2004. Am J Obstet Gynecol (2008) 198:1–5. doi: 10.1016/j.ajog.2007.11.017
12. Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An Increase in the Incidence of Gestational Diabetes Mellitus: Northern California, 1991-2000. Obstet Gynecol (2004) 103:526–33. doi: 10.1097/01.AOG.0000113623.18286.20
13. Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing Prevalence of Gestational Diabetes Mellitus (GDM) Over Time and by Birth Cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care (2005) 28:579–84. doi: 10.2337/diacare.28.3.579
14. Ferrara A. Increasing Prevalence of Gestational Diabetes Mellitus: A Public Health Perspective. Diabetes Care (2007) 30(Supplement_2): S141-6. doi: 10.2337/dc07-s206
15. Wang Y, Chen L, Xiao K, Horswell R, Besse J, Johnson J, et al. Increasing Incidence of Gestational Diabetes Mellitus in Louisiana, 1997–2009. J Women’s Health (2012) 21:319–25. doi: 10.1089/jwh.2011.2838
16. Zhang C, Liu S, Solomon CG, Hu FB. Dietary Fiber Intake, Dietary Glycemic Load, and the Risk for Gestational Diabetes Mellitus. Diabetes Care (2006) 29:2223–30. doi: 10.2337/dc06-0266
17. Bardenheier BH, Imperatore G, Gilboa SM, Geiss LS, Saydah SH, Devlin HM, et al. Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000-2010. Am J Prev Med (2015) 49:12–9. doi: 10.1016/j.amepre.2015.01.026
18. Thorpe LE, Berger D, Ellis JA, Bettegowda VR, Brown G, Matte T, et al. Trends and Racial/Ethnic Disparities in Gestational Diabetes Among Pregnant Women in New York City, 1990-2001. Am J Public Health (2005) 95:1536–9. doi: 10.2105/AJPH.2005.066100
19. Yang L, Colditz G. Prevalence of Overweight and Obesity in the United States, 2007-2012. JAMA Internal Med (2015) 175:1412–3. doi: 10.1001/jamainternmed.2015.2405
20. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. JAMA (2018) 319:4–6. doi: 10.1001/jama.2018.3060
21. Mathews TJ, Hamilton BE. Mean Age of Mothers is on the Rise: United States, 2000-2014. NCHS Data Brief (2016) 232:1–8.
22. Heisler EJ, Shrestha LB. The Changing Demographic Profile of the United States. Congress Res Service Rep Congress (2011).
23. Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A Prospective Study of Pregravid Determinants of Gestational Diabetes Mellitus. JAMA: J Am Med Assoc (1997) 278:1078. doi: 10.1001/jama.1997.03550130052036
24. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief (2017) 288:1–8. doi: 10.1017/S1368980017000088
25. Jovanovic L, Pettitt DJ. Gestational Diabetes Mellitus. Jama (2001) 286:2516–8. doi: 10.1001/jama.286.20.2516
26. Saldana TM, Siega-Riz AM, Adair LS, Suchindran C. The Relationship Between Pregnancy Weight Gain and Glucose Tolerance Status Among Black and White Women in Central North Carolina. Am J Obstet Gynecol (2006) 195:1629–35. doi: 10.1016/j.ajog.2006.05.017
27. Hedderson MM, Gunderson EP, Ferrara A. Gestational Weight Gain and Risk of Gestational Diabetes Mellitus. Obstet Gynecol (2010) 115:597–604. doi: 10.1097/AOG.0b013e3181cfce4f
28. IOM (Institute of Medicine) and NRC (National Research Council). Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): Natl Academies Press (2009). doi: 10.17226/12584
29. Bloomgarden ZT. Gestational Diabetes Mellitus and Obesity. Diabetes Care (2010) 33:5–9. doi: 10.2337/dc10-zb05
30. Dempsey JC. Prospective Study of Gestational Diabetes Mellitus Risk in Relation to Maternal Recreational Physical Activity Before and During Pregnancy. Am J Epidemiol (2004) 159:663–70. doi: 10.1093/aje/kwh091
31. Zhang C, Solomon CG, Manson JE, Hu FB. A Prospective Study of Pregravid Physical Activity and Sedentary Behaviors in Relation to the Risk for Gestational Diabetes Mellitus. Arch Internal Med (2006) 166:543. doi: 10.1001/archinte.166.5.543
32. Deidre KT, Zhang C, Van Dam RM, Bowers K, Hu FB. Physical Activity Before and During Pregnancy and Risk of Gestational. Diabetes Care (2011) 34:223–9. doi: 10.2337/dc10-1368
33. Anna V, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic Correlates of the Increasing Trend in Prevalence of Gestational Diabetes Mellitus in a Large Population of Women Between 1995 and 2005. Diabetes Care (2008) 31:2288–93. doi: 10.2337/dc08-1038
34. Fiscella K, Franks P, Gold MR, Clancy CM. Inequality in Quality: Addressing Socioeconomic, Racial, and Ethnic Disparities in Health Care. Jama (2000) 283:2579–84. doi: 10.1001/jama.283.19.2579
35. Ogden CL, Lamb MM, Carroll MD, Flegal KM. Obesity and Socioeconomic Status in Adults: United States, 2005-2008. NCHS Data Brief (2010) 127:1–8.
36. Alberti KGMM, Zimmet PZ. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabetic Med (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
37. Kalter-Leibovici O, Freedman LS, Olmer L, Liebermann N, Heymann A, Tal O, et al. Screening and Diagnosis of Gestational Diabetes Mellitus: Critical Appraisal of the New International Association of Diabetes in Pregnancy Study Group Recommendations on a National Level. Diabetes Care (2012) 35:1894–6. doi: 10.2337/dc12-0041
38. Agarwal MM, Dhatt GS, Shah SM. Gestational Diabetes Mellitus Simplifying the International Association of Diabetes and Pregnancy Diagnostic Algorithm Using Fasting Plasma Glucose. Diabetes Care (2010) 33:2018–20. doi: 10.2337/dc10-0572
39. Brown FM, Wyckoff J. Application of One-Step IADPSG Versus Two-Step Diagnostic Criteria for Gestational Diabetes in the Real World: Impact on Health Services, Clinical Care, and Outcomes. Curr Diabetes Rep (2017) 17:1–13. doi: 10.1007/s11892-017-0922-z
40. Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, et al. Prevalence and Incidence Trends for Diagnosed Diabetes Among Adults Aged 20 to 79 Years, United States, 1980-2012. JAMA - J Am Med Assoc (2014) 312:1218–26. doi: 10.1001/jama.2014.11494
41. Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA (2016) 315:2284–91. doi: 10.1001/jama.2016.6458
42. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity Among Adults: United States, 2011-2012. NCHS Data Brief. (2013) 131:1–8.
43. Mazumder T, Akter E, Rahman SM, Islam MT, Talukder MR. Prevalence and Risk Factors of Gestational Diabetes Mellitus in Bangladesh: Findings From Demographic Health Survey 2017–2018. Int J Environ Res Public Health (2022) 19:2583. doi: 10.3390/ijerph19052583
44. Mitanchez D, Yzydorczyk C, Simeoni U. What Neonatal Complications Should the Pediatrician be Aware of in Case of Maternal Gestational Diabetes? World J Diabetes (2015) 6:734–43. doi: 10.4239/wjd.v6.i5.734
45. Kim C, Vahratian A. Self-Rated Health and Health Care Use Among Women With Histories of Gestational Diabetes Mellitus. Diabetes Care (2010) 33:41–2. doi: 10.2337/dc09-1760
46. Ogunwole SM, Turkson-Ocran R-AN, Boakye E, Creanga AA, Wang X, Bennett WL, et al. Disparities in Cardiometabolic Risk Profiles and Gestational Diabetes Mellitus by Nativity and Acculturation: Findings From 2016–2017 National Health Interview Survey. BMJ Open Diabetes Res Care (2022) 10:e002329. doi: 10.1136/bmjdrc-2021-002329
Keywords: gestational diabetes, trend, prevalence, risk factors, National Health Interview Survey
Citation: Zhou T, Du S, Sun D, Li X, Heianza Y, Hu G, Sun L, Pei X, Shang X and Qi L (2022) Prevalence and Trends in Gestational Diabetes Mellitus Among Women in the United States, 2006–2017: A Population-Based Study. Front. Endocrinol. 13:868094. doi: 10.3389/fendo.2022.868094
Received: 02 February 2022; Accepted: 04 May 2022;
Published: 06 June 2022.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenCopyright © 2022 Zhou, Du, Sun, Li, Heianza, Hu, Sun, Pei, Shang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Qi, bHFpMUB0dWxhbmUuZWR1
†These authors have contributed equally to this work
 Tao Zhou
Tao Zhou Shan Du
Shan Du Dianjianyi Sun2,3
Dianjianyi Sun2,3 Xiang Li
Xiang Li Gang Hu
Gang Hu Litao Sun
Litao Sun