- 1Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy
- 2Endocrinology Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 3Pathology Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Otolaryngology and Head and Neck Surgery Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Endocrine Surgery Unit, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
Context: Medullary thyroid carcinoma (MTC) is a malignant neuroendocrine neoplasm that may spread to lymph nodes before the primary tumor is diagnosed; moreover, distant metastases are already present in about 10% of patients at diagnosis. Serum calcitonin (Ctn) usually reflects the spread of disease, thus orienting the extent of surgery and predicting the possibility of biochemical remission. Tumor size and vascular invasion are important prognostic factors, but little is known on the relationship between other histopathological features, such as the presence of a tumor capsule, and long term outcome of MTC.
Purpose: To evaluate the prevalence of encapsulated tumors among MTCs and the association of tumor capsule with a favorable outcome after surgery.
Methods: A retrospective observational single-center study was conducted together with a narrative review of the available literature.
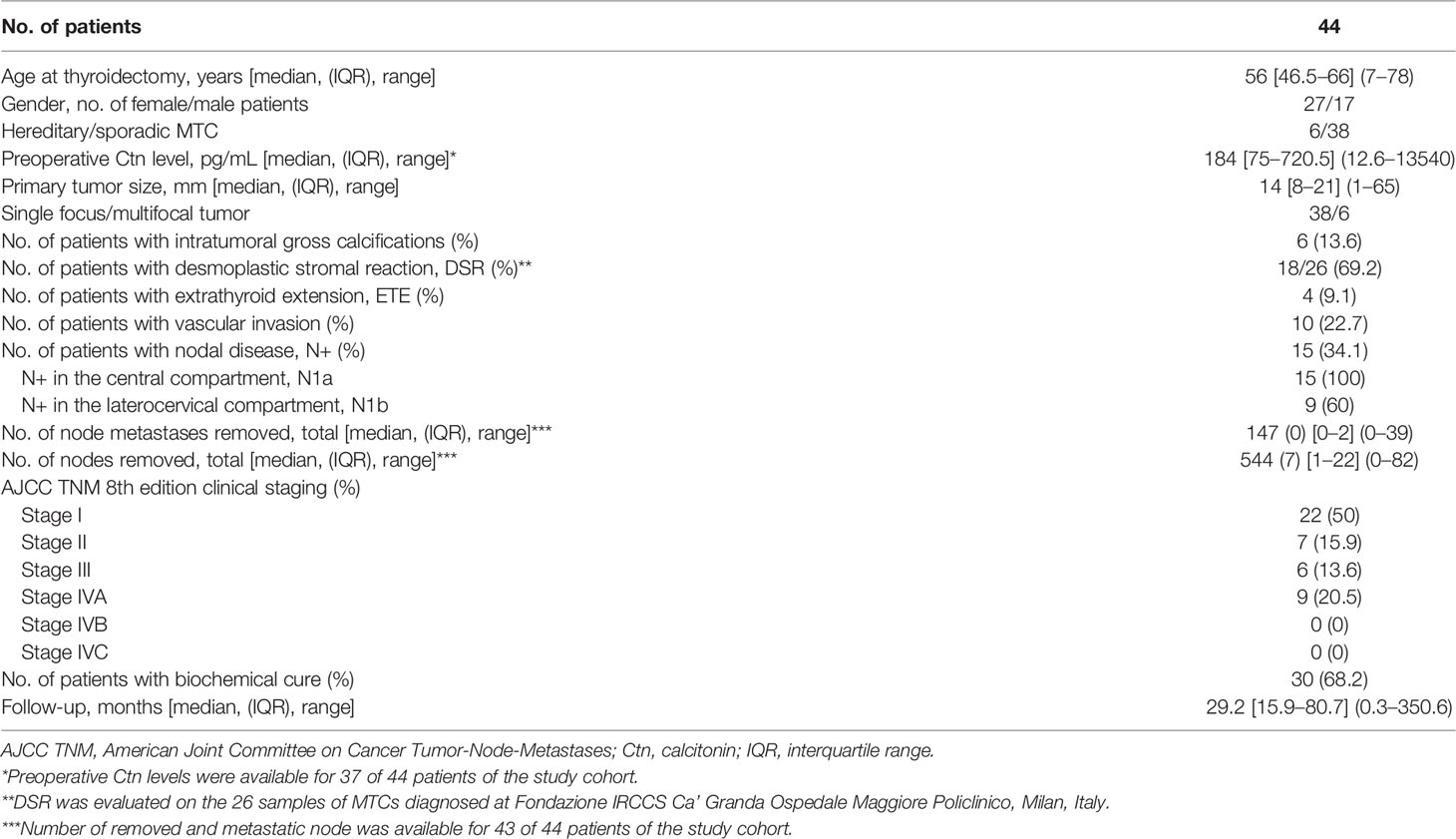
Results: Among 44 patients (27 female, 17 male; median age: 56 years) with MTC (6 hereditary, 37 sporadic) followed up at our center in the last four years (median follow-up: 29.2 months), seven (15.9%) showed an encapsulated tumor at histology and a clinical remission after surgery. None of them had nodal metastases and median preoperative Ctn (398 pg/mL, IQR 126.5–7336) did not differ significantly from that of the 14 patients (31.8%) with persistent disease after surgery (787 pg/mL, IQR 340.5–2905.5; p=0.633), although their tumor size was significantly higher (median 33 mm versus 16 mm respectively, p=0.036). Among patients with preoperative Ctn levels above 500 pg/mL (n=11), only two (18.2%) showed undetectable Ctn levels during follow-up, both having an encapsulated MTC (OR 0.000, p=0.02). Notably, they were two similar cases of large MTC (> 3 cm) with extensive hyalinization and calcification, associated with very high Ctn levels (> 13’500 and 1’100 pg/mL, respectively) but no nodal nor distant metastases, in complete remission after surgery although one of them carried the aggressive M918T somatic RET mutation.
Conclusion: MTC rarely shows a tumor capsule, which seems to correlate with a better prognosis and absence of nodal metastases, regardless of RET or RAS mutational status. Among encapsulated MTCs (E-MTC), Ctn levels and tumor size are not predictive of persistence of disease after surgery.
Introduction
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor originating from calcitonin-secreting thyroid C-cells (1). It accounts for 3-5% of all primary thyroid malignancies and occurs sporadically in 75-80% of cases. Activating germline mutations of the RET proto-oncogene are responsible for remaining hereditary forms, which include multiple endocrine neoplasia (MEN) syndromes type 2A and 2B (2). MTC shows variable clinical course but an overall more aggressive behavior, for different tumor cell lymphovascular dissemination compared to well-differentiated papillary and follicular thyroid carcinomas, and it is more prone to have lymph node and distant metastases at diagnosis (50-75% and 10%, respectively) (3).
Calcitonin (Ctn) serum concentration is a sensitive and specific biomarker useful for early detection of MTC (4). Furthermore, preoperative Ctn levels in MTC may be indicative of tumor burden, as with every increment of basal Ctn levels (above 20, 50 and 200 pg/mL, respectively) there is a successive involvement of the ipsilateral, contralateral paratracheal and bilateral laterocervical lymph node compartments, with upper mediastinal and distant metastases becoming more common above a basal Ctn threshold of 500 pg/mL (5). Therefore, Ctn is very useful for orienting the locoregional extent of surgery (after proper preoperative radiological staging) and predicting postoperative biochemical cure of patients with MTC (6). A similar relation with tumor size, number of lymph nodes metastases and outcome was seen for carcinoembryonic antigen (CEA) levels and, recently, also for procalcitonin (PCT) levels (5, 7).
Patients with intrathyroidal disease have a 10-year survival rate of 95.6%, whereas the presence of locoregional involvement or distant metastases at the time of diagnosis are associated with overall survival rates of 75.5% and 40%, respectively (8). Therefore, radical neck (thyroid and involved cervical lymph nodes) surgery represents the first-line therapy to achieve MTC cure (9). Systemic treatment is to be considered for those patients with progressive advanced disease (10). Current available drugs for MTC include multikinase inhibitors (MKIs) Vandetanib and Cabozantinib and new selective RET inhibitors Selpercatinib and Pralsetinib, but none of these have been shown to improve patients’ overall survival (OS) (11). Moreover, numerous side effects have frequently been reported and primary or acquired resistance mechanisms may be present (12).
Some histological features of the primary tumor have been proposed for predicting the outcome of MTC (13). Among these, lymphovascular invasion, intense desmoplastic stromal reaction (DSR), evidence of infiltrative tumor margins and extrathyroidal extension (ETE) (14, 15) significantly correlate with the presence of node metastases which is, in turn, the most relevant predictor of distant metastatic disease in MTC (16). On the contrary, the presence of a complete tumor capsule is a strong predictor of the absence of lymphatic spreading of the disease (17).
To date, just over a dozen published studies have described patients with encapsulated MTC (E-MTC) and correlated the presence and infiltration of the tumor capsule with the presence of lymph node metastases or disease remission after surgery, respectively. Very recently, Machens et al. (18) proposed the possibility of avoiding lymph node dissection in the case of well-encapsulated tumors without associated desmoplastic reaction, tested at intraoperative examination. Considering this interesting hypothesis, in the present article we have retrospectively researched cases of encapsulated tumors within the series of MTC patients in follow-up at our Institution to assess their staging at diagnosis and subsequent response to therapy. In addition, previously published research studies focusing on this histopathological issue have been reviewed and discussed.
Material and Methods
A total of 53 consecutive patients with MTC underwent one or more follow-up visits at our Institution between January 2017 and January 2022. From this cohort, 44 patients with sufficiently detailed histopathological examination were selected, and samples of 26 MTCs diagnosed at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy between 2010 and 2021 were identified and independently reviewed by three pathologists with experience of endocrine pathology, blinded to the patient lymph nodes status and clinical outcome. The study was conducted in accordance with the World Medical Association’s Declaration of Helsinki and approved by the local ethics committee.
All patients with a preoperative diagnosis of MTC underwent total thyroidectomy (except for one subject who was submitted to lobo-isthmectomy for surgical contraindications) and systematic central neck compartment lymphadenectomy. Patients with clinical evidence or suspicion of laterocervical metastases also underwent dissection of lateral neck compartments (ipsilateral or bilateral, as appropriate). Preoperative assessment of distant metastases was performed with total body CT or CT/PET in patients with Ctn levels above 500 pg/mL. The diagnosis of MTC was confirmed histologically and the following common pathological features were assessed: primary tumor size and extension (single focus or multifocal tumor), tumor margins, intratumoral gross calcifications, extrathyroid extension (ETE), vascular invasion, number of removed lymph nodes metastases, total removed lymph nodes. Tumoral encapsulation was defined as the presence of a fibrous rim of tissue enveloping the tumor and capsular invasion was defined as full-thickness tumor infiltration of the capsule into the adjacent thyroid tissue. DSR was defined as newly formed collagen-rich stroma into a peritumoral circumferential area of 0.5 cm from the tumor margins; it was evaluated semi-quantitatively by visually estimating the presence or absence of fibrosis. The TNM classification and tumor staging were performed according to the criteria described in the 8th Edition of American Joint Committee on Cancer (AJCC) TNM Classification of MTC (19).
The selected patients’ medical records were retrospectively assessed up until the last follow-up (January 2022). Pre- and postoperative serum levels of Ctn and CEA, when available, were determined using chemoluminescent (CLIA) and electrochemiluinescent (ECLIA) assays. Patients diagnosed at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy between 2018 and 2021 also performed PCT dosage with ECLIA method (Elecsys BRAHMS PCT, normal values between 0.02 and 0.06 ng/mL). The follow-up was based on regular clinical examination, neck ultrasound imaging and serum Ctn and CEA measurement every 3 to 12 months, depending on the patient’s response to treatment. Further radiological investigations, such as neck-torax-abdomen CT and total body CT/PET were performed to assess any distant metastases in patients with permanently elevated or progressively increasing Ctn values after surgery. Of the patients with persistent disease after the first surgery, three underwent a second surgery on the neck lymph nodes. At last control, patients were considered in remission when there was neither biochemical (basal Ctn levels < 2 pg/mL) nor structural evidence of disease.
Molecular genetics investigations to discover the presence of germline RET mutations on peripheral blood of all MTC patients were performed by targeted Sanger or NGS (in the last 5 years) sequencing. Somatic RET and RAS pathogenetic variants were tested on the genomic DNA extracted from FFPE samples of surgically resected E-MTCs. DNA was obtained with the MagMAX FFPE DNA/RNA Ultra Kit (Applied Biosystems, US) according to the manufacturer’s instructions and the analysis was performed by targeted NGS sequencing on the Illumina MiSeq platform, using the HaloPlex Target Enrichment System kit (Agilent Technologies, Santa Clara, CA) for the library preparation; data analysis, including alignment, categorization and annotation of variants, was done with the SureCall application (Agilent Technologies, Santa Clara, CA). Some extremely degraded FFPE-derived DNAs were pre-amplified with the SsoAdvanced PreAmp Supermix (BioRad, US) to obtain amplicons of sufficient quality for subsequent Sanger sequencing (BigDye Terminator v3.1, Applied Biosystems, US); target sequences were previously amplified with the high fidelity polymerase Takara Taq HS polymerase (Takara, Japan).
Statistical analysis was performed with GraphPad Prism (version 9.3.1). Quantitative variables were expressed as medians with interquartile ranges (IQR) and complete ranges (from minimum to maximum) and were compared with the two-tailed Mann-Whitney U test. Qualitative variables were presented as absolute and relative (percentage) frequencies and were tested with a Chi-square test or Fisher’s exact test. Odds ratios (OR) were expressed together with their 95% confidence interval (95%CI). Correlations between quantitative variables were assessed by calculating Pearson’s correlation coefficient. The level of statistical significance (two-tailed) was set at p < 0.05.
Results
Between January 2017 and January 2022, a total of 53 patients with MTC underwent a follow-up visit at our Institution. Among them, we retrieved complete pre- and postoperative medical records for 44 patients (female to male ratio: 27/17) who were diagnosed with MTC between February 1996 and August 2021 (median age at thyroidectomy of 56 years, IQR 46.5–66 years).
As shown in Table 1, preoperative serum Ctn was available for 37 of 44 patients of the study cohort and its median level was 184 pg/mL (IQR 75 – 720.5 pg/mL). Ctn was significantly correlated with tumor size (r = 0.723, 95%CI 0.521–0.848, p < 0.001) but not with the number of positive lymph nodes (r = 0.272, p = 0.109). DNA analysis for germline RET mutations showed 4 cases of hereditary MTC in the context of a MEN2A syndrome and 2 cases of FMTC (belonging to four different families).
After histopathological examination, median primary tumor size was 14 mm (IQR 8–21 mm) and multifocality was present in 6 (13.6%) cases, five of whom showed bilateral tumor foci. We found that 7 of 44 MTCs had a tumor capsule (Figure 1). Among the non-encapsulated (NE-MTC) tumors (84.1%), defined by the total absence of a capsule surrounding the tumor, infiltrative margins were reported in 14 and expansive or well-defined margins in 14 out of 28 cases. Concerning other histopathological findings, 6 out of 44 (13.6%) exhibited diffuse (50%) or focal (50%) intratumoral gross calcifications, vascular invasion was observed in 10 (22.7%) and ETE in 4 (9.1%) of 44 cases. Fifteen (34.1%) patients had histologically confirmed lymph node metastases (pN1) at initial surgery, in all cases involving the central compartment (VI level) of the neck and in 60% of cases also the laterocervical compartment. Peritumoral desmoplasia was present in 18 of the 26 (69.2%) reviewed MTC specimen at our Institution. Half of these were associated with lymph node metastases (positive predictive value of 50%), whereas no DSR-negative cases had lymph node metastases (negative predictive value of 100%, p = 0.023) neither at primary surgery nor during the follow-up. According to the 8th edition AJCC TNM Staging System, 22 (50%) patients had a Stage I tumor after surgery, 7 (15.9%) patients were at Stage II, 6 (13.6%) at Stage III and 9 (20.5%) at stage IVA. At diagnosis, as well as at the last outpatient visit, no radiologically proven distant metastases were detected.
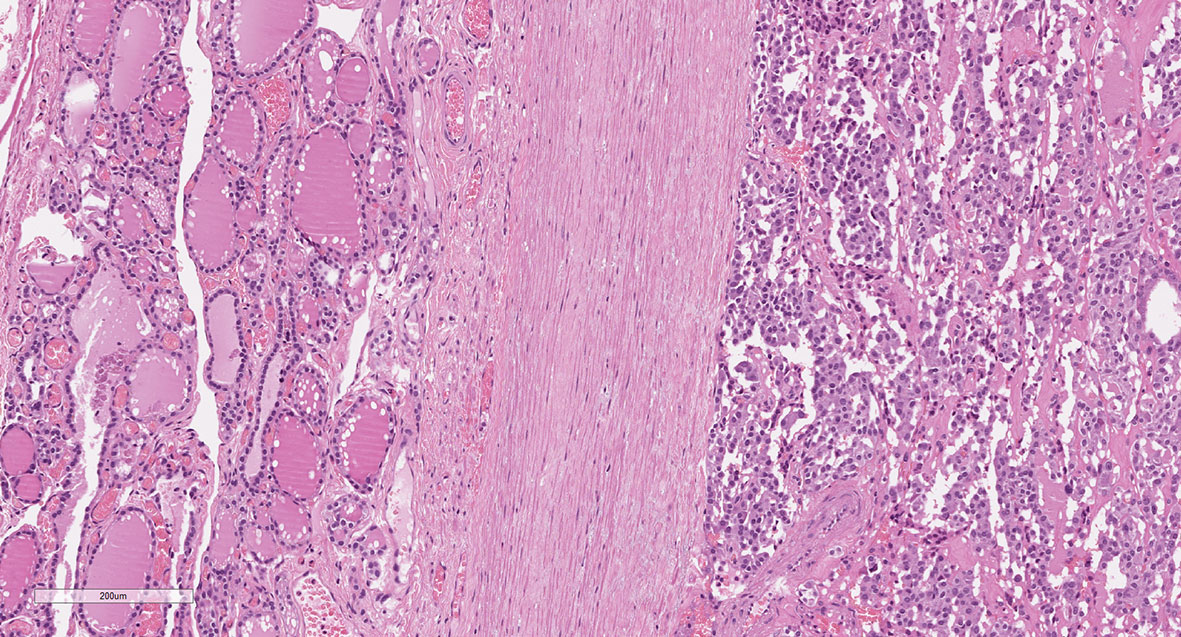
Figure 1 Medullary thyroid carcinoma showing a tumor capsule. The neoplastic cells (right) are demarcated from the normal thyroid parenchima (left) by a fibrous capsule (center). (Hematoxylin and eosin, original magnification 10x).
At the last follow-up (median follow-up period of 29.2 months, IQR 15.9–80.7), 30 (68.2%) patients (including 3 of the 15 pN1 patients) achieved biochemical cure, 13 (29.5%) showed a biochemical incomplete response and a single patient (2.3%) had a structural incomplete response with stable disease. Statistically significant predictive factors of persistence of disease after primary surgery were vascular invasion (OR infinity, 95%CI 12.3–infinity, p < 0.0001), lymph nodes involvement (OR 54, 95%CI 6.99–279.2, p < 0.0001) and ETE (OR infinity, 95%CI 2.29–infinity, p = 0.007).
Non-Encapsulated MTCs
Clinical and pathological features of NE-MTC patients (84.1%) were reported in the right side of Table 2, subgrouping them according to the presence or absence of biochemical remission at last visit (23 ‘cured’ and 14 ‘non-cured’ patients).
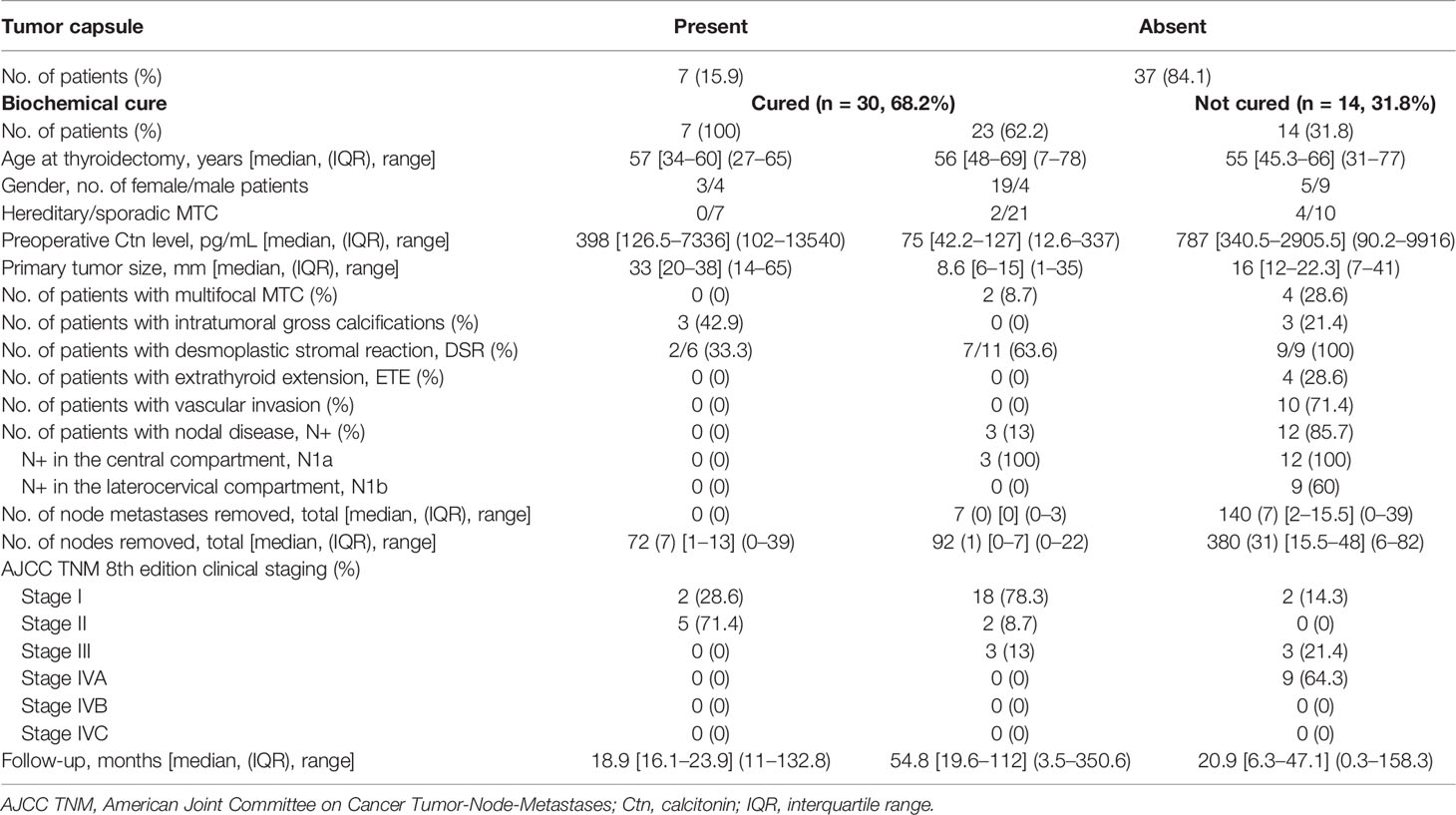
Table 2 Clinical and histopathological characteristics of encapsulated (all cured) and non-encapsulated MTC (cured and not cured).
There were no significant differences in term of germline RET mutations (p = 0.174) and patients’ age when comparing patients in remission and those not in remission, whereas a statistically significant difference was noted concerning the preponderance of males among those non-cured (64.3% versus 17.4% among cured patients, p = 0.006).
Higher preoperative serum Ctn (p < 0.0001), greater tumor size (p = 0.008) and presence of ETE (p = 0.015) were significantly associated with lack of biochemical cure after surgery. Multifocality was not different between the two groups of NE-MTC patients. Vascular invasion was observed only in non-cured MTCs (p < 0.0001) and lymph nodes involvement was significantly more frequent in this subgroup of patients (12/15 positive nodes in non-cured versus 3/25 in cured patients, p < 0.0001). DSR was detected in 9 out of 9 (100%) non-cured NE-MTCs, almost always associated with lymph node metastases (88.9%), and in 63.6% (7/11) of cured NE-MTCs, only in one case (14.3%) associated with lymph node metastases.
Among all NE-MTCs, a significant correlation was found between serum Ctn at diagnosis and the number of node metastases removed (r = 0.515, 95%CI 0.197–0.735, p = 0.003).
Encapsulated MTCs
Prevalence of encapsulated tumors in the present cohort of patients with histologically proven MTC was 15.9% (95%CI 5.1– 26.7%). Full-thickness invasion of the capsule was detected in 3 (42.9%) cases but such invasion was observed only in a single focus per case. No E-MTC was associated with nodal or distant metastases and all seven patients achieved biochemical and structural remission after neck surgery (excellent response).
All E-MTCs were sporadic tumors and concerned male patients in 57.1% and female in 42.9%, with a median age at thyroidectomy comparable with that of the entire study cohort (57 years, IQR 34–60 years).
Comparing E-MTCs with non-cured NE-MTCs (Table 2), there were no significant differences in term of preoperative Ctn levels (median 398 pg/mL, IQR 126.5–7336 pg/mL versus median 787 pg/mL, IQR 340.5–2905.5 pg/mL respectively, p = 0.633) but there were for primary tumor size (median 33 mm, IQR 20–38 mm versus median 16 mm, IQR 12–22.3 mm respectively, p = 0.036), as shown in Figure 2. However, E-MTCs did not show multifocality, extrathyroidal extension nor vascular invasion (p = 0.004). One case had a large central cystic component, whereas intratumoral gross calcifications were detected in 3 out of 7 E-MTCs (42.9%). DSR was significantly more present among the NE-MTCs examined (76.9%) compared to the E-MTCs (23.1%, p = 0.029). Among the latter, the two cases associated with peritumoral desmoplasia (only mild grade) both showed focal capsular invasion. Unlike non-cured NE-MTCs, in which AJCC/TNM stages III and IV prevailed (p = 0.003), E-MTCs were only stage I (28.6%) and Stage II (71.4%) tumors, depending exclusively on their size.
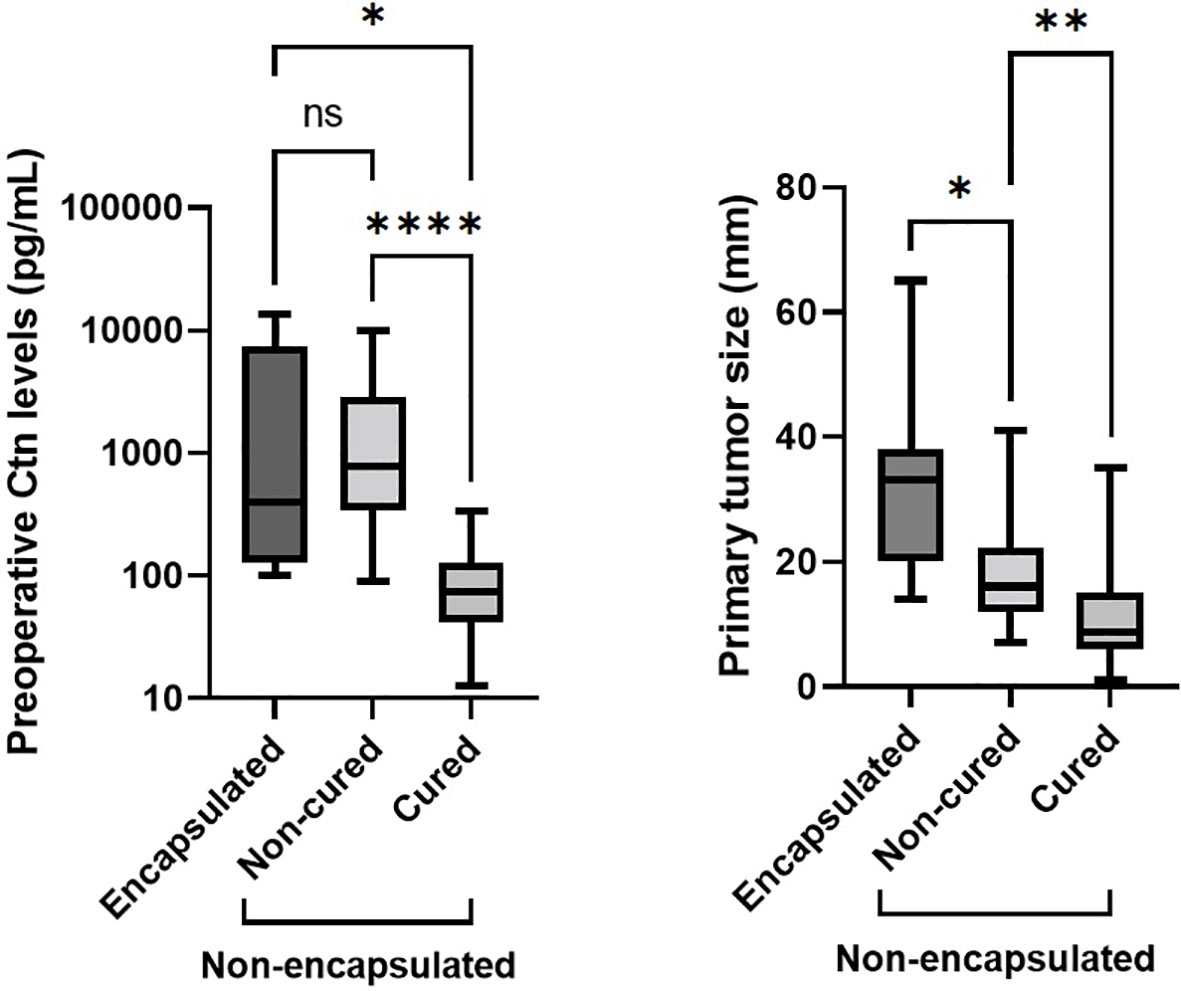
Figure 2 Comparing E-MTCs with non-cured NE-MTCs (Table 2), there were no significant differences in term of preoperative Ctn levels (p = 0.633, left side) but there were for primary tumor size (p = 0.036, right side), which was higher in the former (median 33 mm versus median 16 mm, respectively). Among NE-MTCs, higher preoperative serum Ctn (p < 0.0001) and greater tumor size (p = 0.008) were seen in non-cured patients than those cured. Ctn, calcitonin; ns, not significant. The asterisks refer to the different p values indicated in the caption of the figure (*p = 0.036, **p = 0.008, ****p < 0.0001).
Tumor tissue mutational status was known for four (57.1%) of the E-MTCs: one case was positive for the two RET polymorphisms Gly691Ser (rs1799939) and Arg982Cys (rs17158558), other two cases showed the previous two RET variants combined with the somatic Met918Thr mutation (p.G691S/M918T/R982C compound genotype), whereas the last one was both RET and RAS wild-type. The remaining samples were not analyzable despite appropriate DNA amplification techniques.
MTCs With Preoperative Ctn > 500 pg/mL
Eleven of 37 (29.7%) MTCs of the present study were associated to preoperative serum Ctn levels higher than 500 pg/mL (median 1730 pg/mL, IQR 787–3145 pg/mL), with a median CEA of 44.2 ng/mL (IQR 29–120 ng/mL) and a median PCT of 45.9 ng/mL (IQR 10.9–65.9 ng/mL). Their clinical and histopathological characteristics as well as response to surgery were reported in Table 3.
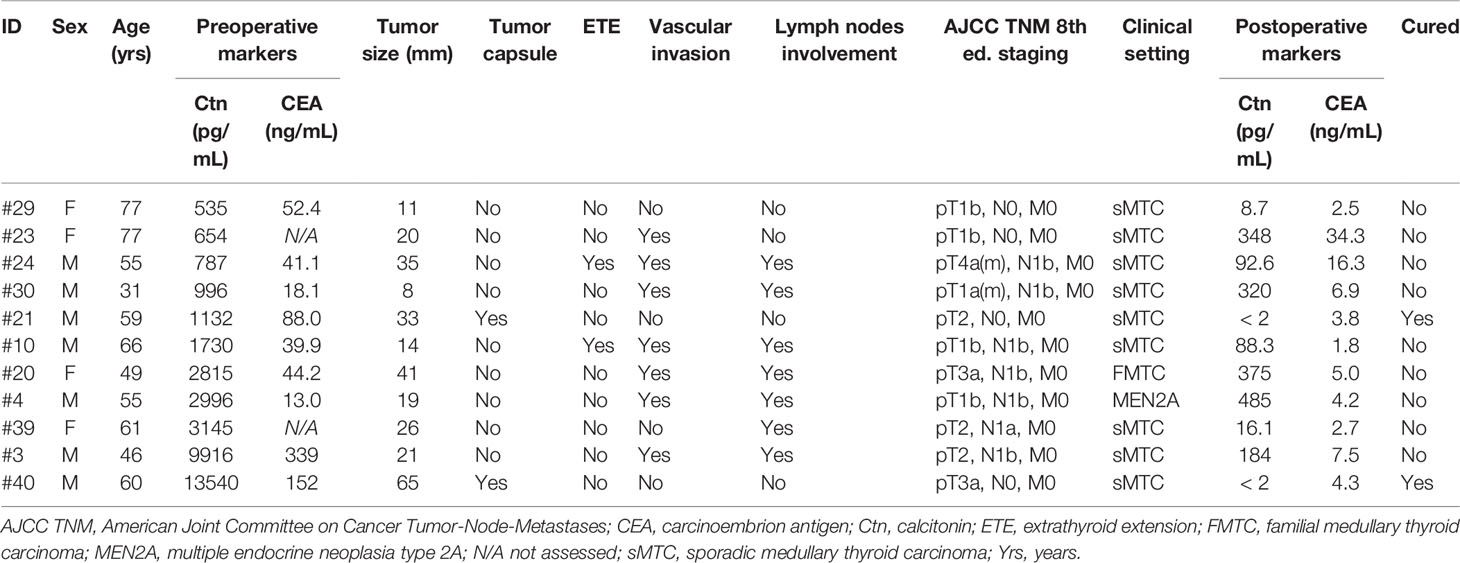
Table 3 Clinical and histopathological characteristics and response to surgery of MTCs presenting with preoperative serum Ctn levels above 500 pg/mL.
Median primary tumor size was 21 mm (IQR 14–35 mm), vascular invasion and lymph node metastases were observed in 7 (63.6%) and ETE in 2 (18.2%) of 11 cases. Biochemical cure was reported only in two of the high preoperative Ctn-associated MTCs and both were encapsulated, so the presence of a tumor capsule was predictive of disease remission in this specific subgroup (OR 0.000, 95%CI 0–0.3, p = 0.02) but not in the entire MTC cohort (OR 0.000, 95%CI 0–1.185, p = 0.078). Notably, both E-MTCs were largely replaced by sclero-hyaline tissue with abundant intratumoral calcifications at the histological examination.
Discussion
Tumoral encapsulation is a well-known important, if not fundamental, element for the pathological definition of follicular cell-derived thyroid tumors. According to the 4th edition WHO Classification of Tumors of Endocrine Organs, published in 2017 (20), the detection of a whole and preserved tumor capsule in thyroid lesions allows to define benign tumors such as follicular adenoma and new borderline tumors such as the “follicular tumor of uncertain malignant potential” (FT-UMP) and the “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” (NIFTP), which share a more indolent behavior and a favorable prognosis (21). Among malignant tumors, the presence of an encapsulated nodule, without evidence of the invasion of the tumor capsule, seems to be an independent prognostic factor for a good prognosis also in the classical variant of papillary thyroid carcinomas (encapsulated non-invasive CV-PTC), according to recent studies (22). Furthermore, it is known that follicular thyroid carcinomas (FTC) showing limited capsular infiltration, but not foci of vascular invasion (so called minimally invasive FTC), have a low recurrence risk after surgery (23).
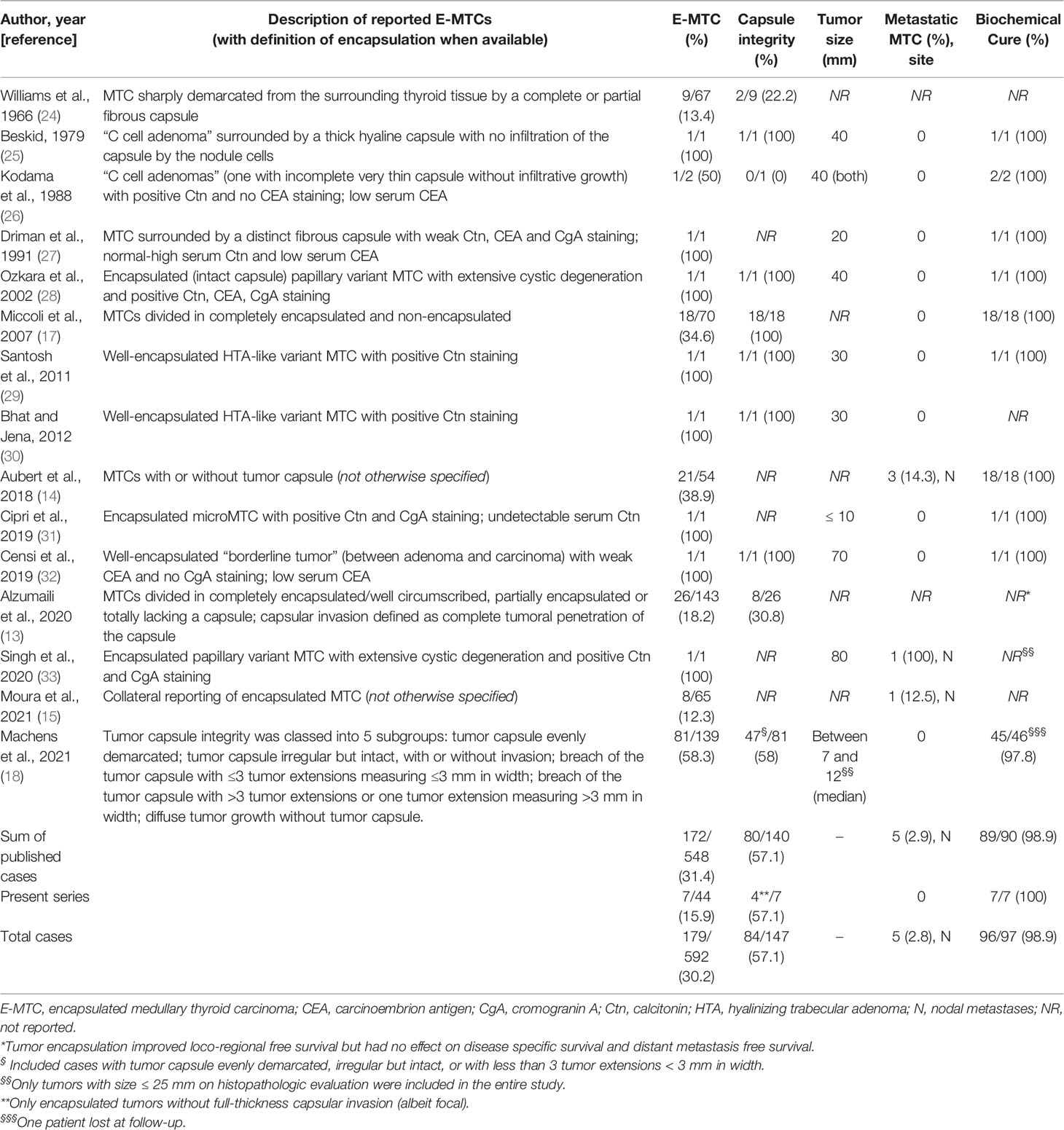
Against so many evidences regarding both the diagnostic and prognostic value of tumoral encapsulation in differentiated thyroid tumors of follicular origin, only a limited number of studies (including several not recent case reports) have investigated the possible correlation between the presence of a complete tumor capsule with the clinical behavior and outcome in the setting of MTC. As shown in Table 4, so far fifteen studies on the pathological features of MTCs have described the presence of about 30% grossly and/or microscopically encapsulated tumors, of which at least half surrounded by a continuous and/or invasion-free capsule. Five (2.9%) cases were reported to have an extra-thyroidal involvement, exclusively limited to neck lymph nodes, but it was not known whether tumor capsule was intact or infiltrated or neither. Only ten (66.7%) of the 15 selected studies also analyzed clinical data on the follow-up and treatment response of patients with E-MTC. Where available, these studies showed biochemical remission of disease in almost all cases (98.9%).
According to their apparent more benign prognosis (than typical MTC), these encapsulated thyroid lesions have been named “C-cell adenomas” by some Authors (25, 26, 32) for the lack of malignant morphological features commonly found in MTC (mainly infiltrative growth pattern). However, they did not better define a reproducible pathological or molecular profile typical of this entity. A possible shared element between these cases could be the association with low serum CEA levels. Although less sensitive and specific than Ctn, CEA levels tend to increase with the disease stage in MTC. However, MTC associated with normal CEA values have been described both at diagnosis (some of which with ascertained lymph node metastases) and at the time of relapse (34), so that negativity to CEA can hardly be considered as a marker of benign behavior.
These promising albeit limited data have led some Authors (Miccoli et al.) to hypothesize the possibility of reconsider the extension of MTC surgical treatment on the basis of the lack of a preserved tumor capsule, that may be intraoperatively revealed by a frozen section analysis (17).
Other morphological parameters have been proposed as useful intraoperative markers to exclude node involvement and thereby to modulate the extent of the surgery in MTC patients, such as DSR (35). Peritumoral desmoplasia is defined as the presence of a newly formed fibrotic stroma surrounding the invasive epithelial tumor cells and can be demonstrated in the majority of MTCs (approximately 80%). In the remaining 20% of sporadic MTCs, as well as in a number of hereditary MTCs, DSR is completely lacking; these cases, which are usually well circumscribed but not necessarily enveloped by a tumor capsule, are typically associated with a very low metastatic potential (36). Combining both the favorable features of tumor encapsulation and absence of peritumoral desmoplasia, very recently Machens et al. proved that patients with E-MTC without associated DSR (or minimal/low desmoplasia), if confirmed on frozen section analysis by experienced pathologists, could avoid even routine central compartment lymph node dissection (18).
In the present study, we have retrospectively researched cases of encapsulated tumors within a series of MTC patients in follow-up at our Institution to assess their staging at diagnosis and subsequent response to therapy. To this purpose, we studied 44 cases of MTC, both hereditary (13.6%) and sporadic (86.4%) cases, and found that prevalence of E-MTC was 15.9%.
Within the entire cohort, preoperative Ctn significantly correlated with tumor size but not with the number of positive lymph nodes; excluding encapsulated tumors from these, however, a significant correlation was found between serum Ctn levels and the number of node metastases. This is because, although Ctn levels of E-MTCs were not significantly different from those of non-cured NE-MTCs (Table 2), none of the E-MTCs was associated with nodal or distant metastases, so they were only Stage I-II tumors. Furthermore, they did not show ETE nor vascular invasion, both histological features known to be associated with lymph node involvement in MTC, and all affected patients achieved clinical remission after the first surgical treatment. In the present study (Table 2), vascular invasion, lymph nodes involvement and ETE were statistically significant predictive factors of persistence of disease after primary surgery.
Peritumoral desmoplasia was a frequent finding among the reviewed MTC of the present study, always but not exclusively detected in non-cured NE-MTC, where it was almost invariably associated with lymph node metastases. DSR-negative MTCs did not involve lymph nodes neither at primary surgery nor during the follow-up, confirming the negative predictive value already described by various Authors. In support of this evidence, in a large retrospective study of 360 patients with MTC who underwent intraoperative frozen-section analysis before surgery, patients with DSR-negative tumor (18%) did not undergo lateral lymph nodes dissection and all maintained biochemical remission for up to 100 months after surgery. Patients with an intraoperative diagnosis of a DSR in the MTC specimen (82%) underwent total thyroidectomy and bilateral central and functional lateral neck dissection; in this group, lymph node and distant metastases were present in 31% and 6% of patients, respectively. As no patient in the DSR-negative group presented with LN metastases in any compartment (negative predictive value of 100%) and each of them had an excellent long-term prognosis, Authors proposed to avoid lateral neck surgery in MTC patients with intraoperative frozen-section negative for DSR (37). Among the E-MTCs of the present study, only two cases showed a mild grade DSR, and both were associated with focal capsular invasion.
Among NE-MTCs, male gender was significantly associated with lymph nodes metastases (but not with larger primary tumor size) and with persistence of disease after surgery. According to the current literature, men with MTC present with larger tumors and are less likely to have localized disease (38), since male sex was recognized as a possible risk factor for lateral neck lymph node metastasis (39). Male gender independently predicts worse overall survival in MTC, even if both disease burden at initial surgery and biochemical response to surgery appear to be stronger prognostic factors (40). Gender differences in term of MTC presentation and outcome could be attributed to a later diagnosis in men, for behavioral reasons and possibly for the lower tendency to perform thyroid tests compared to women, although an underlying biological explanation has been proposed recently but not yet confirmed in larger studies (41). Notably, men and women were almost numerically equal among E-MTC patients in the present study.
Primary tumor size was higher for E-MTCs than non-cured NE-MTCs, so neither serum Ctn nor tumor diameter were predictive of persistence of disease when tumor encapsulation was present (Figure 2). It was hypothesized that tumor lymphatic dissemination might be correlated with an infiltrative behavior, depending on the ability of the tumor cells to invade lymphatic vessels located in surrounding normal thyroid tissue (42). Conversely, encapsulated tumors are prevented from loco-regional dissemination due to the containing effect exerted by the capsule itself, and tend to reach much larger size as a result of their expansive growth pattern.
The different predictive value of preoperative Ctn between E- and NE-MTC is particularly evident in the patients subgroup with serum Ctn levels higher than 500 pg/mL (Table 3). Above this threshold, distant metastases become very common while biochemical cure rate is progressively reduced (6). No distant metastases were detected in the present series and tumor encapsulation was a statistically significant predictive factor of biochemical remission in this specific subgroup, but not in the entire MTC cohort. Among high preoperative Ctn-associated MTCs, only the two E-MTCs showed undetectable postoperative Ctn levels during a mean follow-up period of 17.4 months (versus 20.1 months in MTCs with persistent disease). It is noteworthy that they were two similar cases of large MTC characterized by extensive tumoral hyalinization and calcification, associated with very high preoperative Ctn and CEA levels, in complete remission after surgery although one of them carried the aggressive M918T somatic RET mutation.
Somatic RET mutations are present in 40-50% of sporadic MTC (sMTC), with the most common occurring in codon M918 (which is present in up to 90% of RET-positive cases) and in codon C634 (43). Recently, activating point mutations in RAS genes (H-, K-, and NRAS) have been described predominantly in RET-negative sMTC, with a percentage ranging from 10 to 60% depending on the different series (44) but a better prognosis than those harboring RET mutations or presenting no mutations. Trying to define a genotype–phenotype correlation in MTC, tumors with somatic p.Met918Thr RET mutation and those having no detectable RET or RAS mutations have been typically associated with lymphovascular invasion, extrathyroidal extension and more advanced stages of disease (15). It is not known whether this more aggressive behavior is maintained even in the presence of more favorable pathological features, such as the tumor capsule. So far only two studies (15, 32), in addition to the present one, have analyzed the mutational status of sporadic E-MTC (Table 5). Overall, four cases (30.8%) carried pathogenic mutations in RET exons 10 and 11 (C620S and C630S) and three (23.1%) the aggressive somatic M918T mutation (isolated or in combination with other polymorphic variants), while in two cases (15.4%) there were only RET polymorphisms (G691S variant alone or combined G619S/R982C variant). RAS mutations emerged in two RET wild-type cases and no mutations of either RET or RAS were detected in other two. Therefore, E-MTCs seem to be genetically heterogeneous, with a relative low prevalence of M918T somatic mutations (3 out of 7 RET mutated E-MTCs), but retain a more benign behavior regardless of the presence and type of underlying driver molecular alteration.
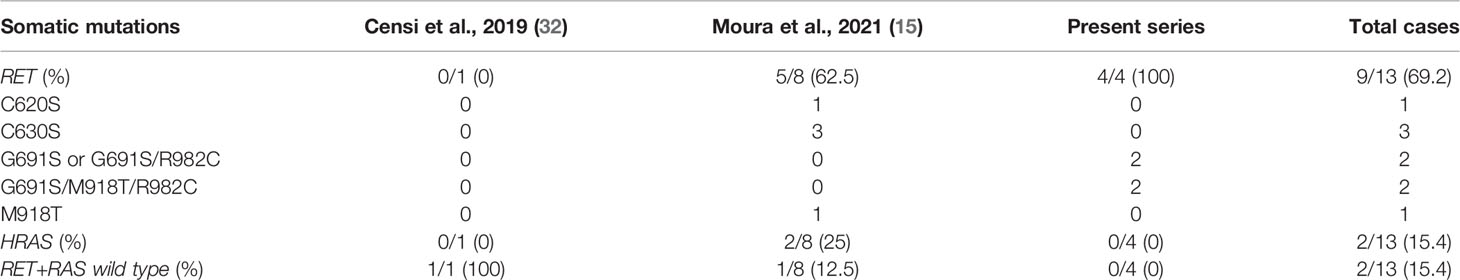
Table 5 RET and RAS genes mutational status in encapsulated MTC samples from previously published studies and present series.
In conclusion, the current research, along with previously published findings here reviewed, provides support for the idea that tumor encapsulation may represent a valid tissue biomarker of node-negative MTC, even in the setting of focal full-thickness capsular invasion, and thus be predictive of a better prognosis, similar to what is well established in follicular-derived thyroid carcinomas. We cannot say how much and in what way this can change the surgical approach to MTC, with the aim of limiting the number of unnecessary lymph node dissections for achieving disease remission with fewer side effects, as proposed by Machens and colleagues (18). From the literature review presented here, some E-MTCs with lymph node metastases have been described, although univocal definitions and shared criteria for the evaluation of tumor capsule, its continuity and integrity have not been provided.
Further studies would be also necessary to clarify the possible correlation of the presence of a complete capsule with other histological characteristics and with the molecular profile of the tumor, as well as larger longitudinal studies to better understand the outcome of patients with E-MTC on longer follow-up periods. Therefore, our proposal is to always describe the tumor capsule when present at the histopathological examination of a MTC, specifying its integrity and possible tumor invasion. Although this is not a necessary element for the diagnosis, as in other histotypes of thyroid cancer, this could be the first step in recognizing a standalone variant of MTC.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Milan Area 2 ethics committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MA and AC designed the present study. AC conducted the literature research, collected clinical data and prepared the manuscript. MA, AD, UV, GC, EI, and AC performed clinical diagnosis and patient treatment and follow-up. MM, GL, and FP performed histopathological review of available samples at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. FE and GM were responsible for DNA analysis and RET and RAS mutations detection. MA, GM, and AD performed the critical revision of the manuscript. All authors read and approved the submitted version.
Funding
This work was supported by Ricerca Corrente Funds from the Italian Ministry of Health to Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chernock RD, Hagemann IS. Molecular Pathology of Hereditary and Sporadic Medullary Thyroid Carcinomas. Am J Clin Pathol (2015) 143(6):768–77. doi: 10.1309/AJCPHWACTTUYJ7DD
2. Elisei R, Tacito A, Ramone T, Ciampi R, Bottici V, Cappagli V, et al. Twenty-Five Years Experience on RET Genetic Screening on Hereditary MTC: An Update on The Prevalence of Germline RET Mutations. Genes (Basel) (2019) 10(9):698. doi: 10.3390/genes10090698
3. Machens A, Lorenz K, Dralle H. Prediction of Biochemical Cure in Patients With Medullary Thyroid Cancer. Br J Surg (2020) 107(6):695–704. doi: 10.1002/bjs.11444
4. Costante G, Meringolo D. Calcitonin as a Biomarker of C Cell Disease: Recent Achievements and Current Challenges. Endocrine (2020) 67(2):273–80. doi: 10.1007/s12020-019-02183-6
5. Machens A, Dralle H. Biomarker-Based Risk Stratification for Previously Untreated Medullary Thyroid Cancer. J Clin Endocrinol Metab (2010) 95(6):2655–63. doi: 10.1210/jc.2009-2368
6. Machens A, Schneyer U, Holzhausen HJ, Dralle H. Prospects of Remission in Medullary Thyroid Carcinoma According to Basal Calcitonin Level. J Clin Endocrinol Metab (2005) 90(4):2029–34. doi: 10.1210/jc.2004-1836
7. Machens A, Lorenz K, Dralle H. Utility of Serum Procalcitonin for Screening and Risk Stratification of Medullary Thyroid Cancer. J Clin Endocrinol Metab (2014) 99(8):2986–94. doi: 10.1210/jc.2014-1278
8. Roman S, Lin R, Sosa JA. Prognosis of Medullary Thyroid Carcinoma: Demographic, Clinical, and Pathologic Predictors of Survival in 1252 Cases. Cancer (2006) 107(9):2134–42. doi: 10.1002/cncr.22244
9. Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid (2015) 25(6):567–610. doi: 10.1089/thy.2014.0335
10. Schlumberger M, Bastholt L, Dralle H, Jarzab B, Pacini F, Smit JW, et al. 2012 European Thyroid Association Guidelines for Metastatic Medullary Thyroid Cancer. Eur Thyroid J (2012) 1(1):5–14. doi: 10.1159/000336977
11. Koehler VF, Adam P, Frank-Raue K, Raue F, Berg E, Hoster E, et al. Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid (2021) 31(3):459–69. doi: 10.1089/thy.2020.0206
12. Jager EC, Broekman KE, Kruijff S, Links TP. State of the Art and Future Directions in the Systemic Treatment of Medullary Thyroid Cancer. Curr Opin Oncol (2022) 34(1):1–8. doi: 10.1097/CCO.0000000000000798
13. Alzumaili B, Xu B, Spanheimer PM, Tuttle RM, Sherman E, Katabi N, et al. Grading of Medullary Thyroid Carcinoma on the Basis of Tumor Necrosis and High Mitotic Rate is an Independent Predictor of Poor Outcome. Mod Pathol (2020) 33(9):1690–701. doi: 10.1038/s41379-020-0532-1
14. Aubert S, Berdelou A, Gnemmi V, Behal H, Caiazzo R, D’herbomez M, et al. Large Sporadic Thyroid Medullary Carcinomas: Predictive Factors for Lymph Node Involvement. Virchows Arch (2018) 472(3):461–68. doi: 10.1007/s00428-018-2303-7
15. Moura MM, Cabrera RA, Esteves S, Cavaco BM, Soares P, Leite V. Correlation of Molecular Data With Histopathological and Clinical Features in a Series of 66 Patients With Medullary Thyroid Carcinoma. J Endocrinol Invest (2021) 44(9):1837–46. doi: 10.1007/s40618-020-01456-6
16. Machens A, Lorenz K, Weber F, Dralle H. Exceptionality of Distant Metastasis in Node-Negative Hereditary and Sporadic Medullary Thyroid Cancer: Lessons Learned. J Clin Endocrinol Metab (2021) 106(8):e2968–79. doi: 10.1210/clinem/dgab214
17. Miccoli P, Minuto MN, Ugolini C, Molinaro E, Basolo F, Berti P, et al. Clinically Unpredictable Prognostic Factors in the Outcome of Medullary Thyroid Cancer. Endocr Relat Cancer (2007) 14(4):1099–105. doi: 10.1677/ERC-07-0128
18. Machens A, Kaatzsch P, Lorenz K, Horn LC, Wickenhauser C, Schmid KW, et al. Abandoning Node Dissection for Desmoplasia-Negative Encapsulated Unifocal Sporadic Medullary Thyroid Cancer. Surgery (2022) 171(2):360–67. doi: 10.1016/j.surg.2021.07.035
19. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. eds. AJCC Cancer Staging Manual. 8th ed. Cham, Switzerland: Springer (2017) 1032 p.
20. Lloyd RV, Osamura RY, Klöppel GRJ. WHO Classification of Tumours of Endocrine Organs (2017). WHO–OMS. Available at: https://apps-who-int.pros2.lib.unimi.it/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4010#%0Ahttp://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4010 (Accessed February 15, 2021).
21. Ito Y, Hirokawa M, Hayashi T, Kihara M, Onoda N, Miya A, et al. Clinical Outcomes of Follicular Tumor of Uncertain Malignant Potential of the Thyroid: Real-World Data. Endocr J (2022). doi: 10.1507/endocrj.EJ21-0723
22. Giani C, Torregrossa L, Ramone T, Romei C, Matrone A, Molinaro E, et al. Whole Tumor Capsule Is Prognostic of Very Good Outcome in the Classical Variant of Papillary Thyroid Cancer. J Clin Endocrinol Metab (2021) 106(10):e4072–83. doi: 10.1210/clinem/dgab396
23. Xu B, Ghossein R. Encapsulated Thyroid Carcinoma of Follicular Cell Origin. Endocr Pathol (2015) 26(3):191–9. doi: 10.1007/s12022-015-9376-5
24. Williams ED, Brown CL, Doniach I. Pathological and Clinical Findings in a Series of 67 Cases of Medullary Carcinoma of the Thyroid. J Clin Pathol (1966) 19(2):103–13. doi: 10.1136/jcp.19.2.103
25. Beskid M. C Cell Adenoma of the Human Thyroid Gland. Oncology (1979) 36(1):19–22. doi: 10.1159/000225312
26. Kodama T, Okamoto T, Fujimoto Y, Obara T, Ito Y, Aiba M, et al. C Cell Adenoma of the Thyroid: A Rare But Distinct Clinical Entity. Surgery (1988) 104(6):997–1003. doi: 10.5555/uri:pii:0039606088901602
27. Driman D, Murray D, Kovacs K, Stefaneanu L, Higgins HP. Encapsulated Medullary Carcinoma of the Thyroid. A Morphologic Study Including Immunocytochemistry, Electron Microscopy, Flow Cytometry, and in Situ Hybridization. Am J Surg Pathol (1991) 15(11):1089–95. doi: 10.1097/00000478-199111000-00009
28. Ozkara SK, Gürbüz Y, Müezzinoğlu B, Yumbal Z. Encapsulated Cystic Papillary Variant of Medullary Carcinoma of Thyroid Gland. Endocr Pathol (2002) 13(2):167–71. doi: 10.1385/EP:13:2:167
29. Santosh KV, Raychaudhuri S, Subramanya H, Naveen Kumar BJ. Cytology of Hyalinising Trabecular Adenoma-Like Variant of Medullary Thyroid Carcinoma. J Cancer Res Ther (2011) 7(2):189–91. doi: 10.4103/0973-1482.82916
30. Bhat V, Jena M. Medullary Carcinoma of the Thyroid - An Unusual Case of Hyalinizing Trabecular Adenoma-Like Variant (Encapsulated). Internet J Med Update (2012) 7(2):54–6.
31. Cipri C, Vescini F, Torresan F, Pennelli G, Pelizzo MR, Triggiani V, et al. An Unusual Case of Medullary Thyroid Carcinoma and A Revision of Current Literature. Endocr Metab Immune Disord Drug Targets (2019) 19(2):226–29. doi: 10.2174/1871530319666181220165350
32. Censi S, Cavedon E, Watutantrige-Fernando S, Barollo S, Bertazza L, Manso J, et al. Unique Case of a Large Indolent Medullary Thyroid Carcinoma: Time to Reconsider the Medullary Thyroid Adenoma Entity? Eur Thyroid J (2019) 8(2):108–12. doi: 10.1159/000494675
33. Singh M, Ahuja A, Rahar S, Bhardwaj M. Encapsulated Papillary Variant of Medullary Carcinoma of Thyroid With Extensive Cystic Change: An Extremely Rare Presentation. Med Pharm Rep (2021) 94(3):372–76. doi: 10.15386/mpr-1570
34. Kim J, Park H, Choi MS, Park J, Jang HW, Kim TH, et al. Serum Carcinoembryonic Antigen as a Biomarker for Medullary Thyroid Cancer. Int J Thyroidol (2021) 14(2):143–51. doi: 10.11106/ijt.2021.14.2.143
35. Scheuba C, Kaserer K, Kaczirek K, Asari R, Niederle B. Desmoplastic Stromal Reaction in Medullary Thyroid Cancer-an Intraoperative “Marker” for Lymph Node Metastases. World J Surg (2006) 30(5):853–9. doi: 10.1007/s00268-005-0391-4
36. Koperek O, Scheuba C, Cherenko M, Neuhold N, De Micco C, Schmid KW, et al. Desmoplasia in Medullary Thyroid Carcinoma: A Reliable Indicator of Metastatic Potential. Histopathology (2008) 52(5):623–30. doi: 10.1111/j.1365-2559.2008.03002.x
37. Niederle MB, Riss P, Selberherr A, Koperek O, Kaserer K, Niederle B, et al. Omission of Lateral Lymph Node Dissection in Medullary Thyroid Cancer Without a Desmoplastic Stromal Reaction. Br J Surg (2021) 108(2):174–81. doi: 10.1093/bjs/znaa047
38. Cox C, Chen Y, Cress R, Semrad AM, Semrad T, Gosnell JE, et al. Are There Disparities in the Presentation, Treatment and Outcomes of Patients Diagnosed With Medullary Thyroid Cancer? An Analysis of 634 Patients From the California Cancer Registry. Gland Surg (2016) 5(4):398–404. doi: 10.21037/gs.2016.04.02
39. Zhou TH, Zhao LQ, Zhang Y, Wu F, Lu KN, Mao LL, et al. The Prediction of Metastases of Lateral Cervical Lymph Node in Medullary Thyroid Carcinoma. Front Endocrinol (Lausanne) (2021) 12:741289. doi: 10.3389/fendo.2021.741289
40. Kotwal A, Erickson D, Geske JR, Hay ID, Castro MR. Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma Over Two Decades. Thyroid (2021) 31(4):616–26. doi: 10.1089/thy.2020.0167
41. Costa C, Souteiro P, Paredes S, Bettencourt-Silva R, Pedro J, Ferreira MJ, et al. Male Gender as a Poor Prognostic Factor in Medullary Thyroid Carcinoma: Behaviour or Biological Difference? Minerva Endocrinol (Torino) (2022). doi: 10.23736/S2724-6507.22.03692-2
42. Pereira F, Pereira SS, Mesquita M, Morais T, Costa MM, Quelhas P, et al. Lymph Node Metastases in Papillary and Medullary Thyroid Carcinoma Are Independent of Intratumoral Lymphatic Vessel Density. Eur Thyroid J (2017) 6(2):57–64. doi: 10.1159/000457794
43. Ciampi R, Romei C, Ramone T, Prete A, Tacito A, Cappagli V, et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience (2019) 20:324–36. doi: 10.1016/j.isci.2019.09.030
Keywords: thyroid tumors, medullary thyroid carcinoma, tumor encapsulation, capsular invasion, calcitonin, desmoplastic stromal reaction
Citation: Contarino A, Dolci A, Maggioni M, Porta FM, Lopez G, Verga U, Elli FM, Iofrida EF, Cantoni G, Mantovani G and Arosio M (2022) Is Encapsulated Medullary Thyroid Carcinoma Associated With a Better Prognosis? A Case Series and a Review of the Literature. Front. Endocrinol. 13:866572. doi: 10.3389/fendo.2022.866572
Received: 31 January 2022; Accepted: 23 March 2022;
Published: 27 April 2022.
Edited by:
Antonino Belfiore, University of Catania, ItalyReviewed by:
Friedhelm Raue, University of Heidelberg, GermanyValeriano Leite, Instituto Português de Oncologia Francisco Gentil, Portugal
Maria Alevizaki, National and Kapodistrian University of Athens, Greece
Takahiro Okamoto, Tokyo Women’s Medical University, Japan
Copyright © 2022 Contarino, Dolci, Maggioni, Porta, Lopez, Verga, Elli, Iofrida, Cantoni, Mantovani and Arosio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Contarino, YW5kcmVhLmNvbnRhcmlub0B1bmltaS5pdA==
 Andrea Contarino
Andrea Contarino Alessia Dolci2
Alessia Dolci2 Gianluca Lopez
Gianluca Lopez Giovanna Mantovani
Giovanna Mantovani Maura Arosio
Maura Arosio